Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches
Abstract
1. Introduction
2. Nitric Oxide Synthase and Inflammation
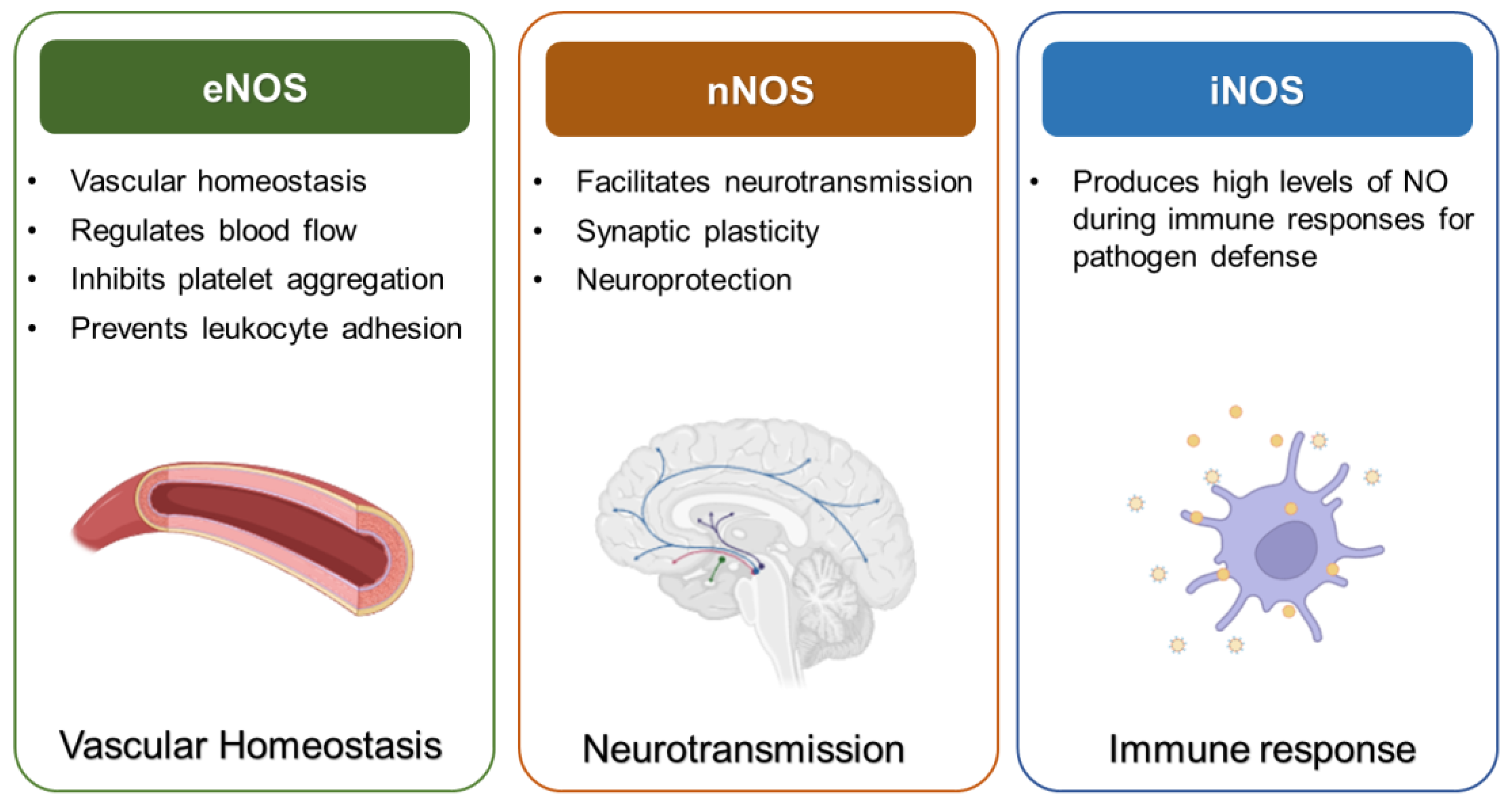
2.1. Types and Functions of NOS
2.2. Mechanisms of Inflammatory Regulation of NOS
2.3. Dual Role of NOS in Inflammation
3. Key Inflammatory Mediators in NOS Regulation
3.1. Cytokines
3.2. Lipid Mediators
3.3. MicroRNAs (miRNAs)
4. Role of NOS in Disease Pathogenesis
4.1. Neurological Disorders
4.2. Cancer
5. Therapeutic Approaches Targeting NOS
5.1. NOS Modulators
5.2. Targeting Upstream Regulators of NOS
5.3. Nanotechnology-Based Delivery Systems
5.4. Personalized Approaches in NOS-Targeted Therapies
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Black, S.M.; Catravas, J.D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul. Pharmacol. 2008, 49, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Krol, M.; Kepinska, M. Human Nitric Oxide Synthase-Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 10056. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodriguez Guzman, R.; Centofanti, F.; Doldo, E.; Cespedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Iova, O.M.; Marin, G.E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboaca, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders—An Overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Huang, J.B.; Chen, Z.R.; Yang, S.L.; Hong, F.F. Nitric Oxide Synthases in Rheumatoid Arthritis. Molecules 2023, 28, 4414. [Google Scholar] [CrossRef]
- Deeb, R.S.; Hajjar, D.P. Repair Mechanisms in Oxidant-Driven Chronic Inflammatory Disease. Am. J. Pathol. 2016, 186, 1736–1749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, H.; Zou, M.; Yuan, Q.; Huang, Z.; Pan, X.; Zhang, W. Nicotine in Inflammatory Diseases: Anti-Inflammatory and Pro-Inflammatory Effects. Front. Immunol. 2022, 13, 826889. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Eichwald, T.; da Silva, L.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espindola, G.; Huang, W.L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor. Antioxidants 2023, 12, 1037. [Google Scholar] [CrossRef]
- Nakazawa, H.; Chang, K.; Shinozaki, S.; Yasukawa, T.; Ishimaru, K.; Yasuhara, S.; Yu, Y.M.; Martyn, J.A.; Tompkins, R.G.; Shimokado, K.; et al. iNOS as a Driver of Inflammation and Apoptosis in Mouse Skeletal Muscle after Burn Injury: Possible Involvement of Sirt1 S-Nitrosylation-Mediated Acetylation of p65 NF-kappaB and p53. PLoS ONE 2017, 12, e0170391. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Edgar, K.S.; Galvin, O.M.; Collins, A.; Katusic, Z.S.; McDonald, D.M. BH4-Mediated Enhancement of Endothelial Nitric Oxide Synthase Activity Reduces Hyperoxia-Induced Endothelial Damage and Preserves Vascular Integrity in the Neonate. Investig. Ophthalmol. Vis. Sci. 2017, 58, 230–241. [Google Scholar] [CrossRef]
- Bi, X.Y.; He, X.; Zhao, M.; Yu, X.J.; Zang, W.J. Role of endothelial nitric oxide synthase and vagal activity in the endothelial protection of atorvastatin in ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. 2013, 61, 391–400. [Google Scholar] [CrossRef]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int J Mol Sci 2024, 25, 3007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Li, F.; Zhu, D.Y. nNOS and Neurological, Neuropsychiatric Disorders: A 20-Year Story. Neurosci. Bull. 2023, 39, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.; Kunert, A.T.; Reinmuth-Selzle, K.; Leifke, A.L.; Widera, D.; Weller, M.G.; Schuppan, D.; Frohlich-Nowoisky, J.; Lucas, K.; Poschl, U. Chemical modification of pro-inflammatory proteins by peroxynitrite increases activation of TLR4 and NF-kappaB: Implications for the health effects of air pollution and oxidative stress. Redox Biol. 2020, 37, 101581. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Ramdial, K.; Franco, M.C.; Estevez, A.G. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Res. Bull. 2017, 133, 4–11. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A.; American Heart Association Council on Basic Cardiovascular Sciences. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Manzano-Pech, L.; Rubio-Ruiz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Konya, V.; Marsche, G.; Schuligoi, R.; Heinemann, A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol. Ther. 2013, 138, 485–502. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Mao, W.; Gao, R.; Wu, J.; Deng, Y.; Shen, Y.; Liu, K.; Cao, J. Prostaglandin E(2) promotes nitric oxide synthase 2, platelet-activating factor receptor, and matrix metalloproteinase-2 expression in Escherichia coli-challenged ex vivo endometrial explants via the prostaglandin E(2) receptor 4/protein kinase a signaling pathway. Theriogenology 2019, 134, 65–73. [Google Scholar] [CrossRef]
- Luo, M.; He, N.; Xu, Q.; Wen, Z.; Wang, Z.; Zhao, J.; Liu, Y. Roles of prostaglandins in immunosuppression. Clin. Immunol. 2024, 265, 110298. [Google Scholar] [CrossRef]
- Ermis, E.; Nargis, T.; Webster, K.; Tersey, S.A.; Anderson, R.M.; Mirmira, R.G. Leukotriene B4 receptor 2 governs macrophage migration during tissue inflammation. J. Biol. Chem. 2024, 300, 105561. [Google Scholar] [CrossRef]
- Filgueiras, L.R.; Brandt, S.L.; Wang, S.; Wang, Z.; Morris, D.L.; Evans-Molina, C.; Mirmira, R.G.; Jancar, S.; Serezani, C.H. Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci. Signal. 2015, 8, ra10. [Google Scholar] [CrossRef]
- Miyabe, Y.; Miyabe, C.; Luster, A.D. LTB4 and BLT1 in inflammatory arthritis. Semin. Immunol. 2017, 33, 52–57. [Google Scholar] [CrossRef]
- Lavy, M.; Gauttier, V.; Poirier, N.; Barille-Nion, S.; Blanquart, C. Specialized Pro-Resolving Mediators Mitigate Cancer-Related Inflammation: Role of Tumor-Associated Macrophages and Therapeutic Opportunities. Front. Immunol. 2021, 12, 702785. [Google Scholar] [CrossRef]
- Sun, T.W.; Wu, Z.H.; Weng, X.S. Celecoxib can suppress expression of genes associated with PGE2 pathway in chondrocytes under inflammatory conditions. Int. J. Clin. Exp. Med. 2015, 8, 10902–10910. [Google Scholar] [PubMed]
- Maghsoudi, H.; Sheikhnia, F.; Sitarek, P.; Hajmalek, N.; Hassani, S.; Rashidi, V.; Khodagholi, S.; Mir, S.M.; Malekinejad, F.; Kheradmand, F.; et al. The Potential Preventive and Therapeutic Roles of NSAIDs in Prostate Cancer. Cancers 2023, 15, 5435. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Chen, Y.; Cai, Q. The role of the LTB4-BLT1 axis in health and disease. Pharmacol. Res. 2020, 158, 104857. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, M.C.; Juhn, Y.J.; Wi, C.I.; Sheen, Y.H. Asthma and the Risk of Rheumatoid Arthritis: An Insight into the Heterogeneity and Phenotypes of Asthma. Tuberc. Respir. Dis. 2017, 80, 113–135. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Pashangzadeh, S.; Motallebnezhad, M.; Vafashoar, F.; Khalvandi, A.; Mojtabavi, N. Implications the Role of miR-155 in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 669382. [Google Scholar] [CrossRef]
- Seddiki, N.; Brezar, V.; Ruffin, N.; Levy, Y.; Swaminathan, S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 2014, 142, 32–38. [Google Scholar] [CrossRef]
- Lv, F.; Huang, Y.; Lv, W.; Yang, L.; Li, F.; Fan, J.; Sun, J. MicroRNA-146a Ameliorates Inflammation via TRAF6/NF-kappaB Pathway in Intervertebral Disc Cells. Med. Sci. Monit. 2017, 23, 659–664. [Google Scholar] [CrossRef]
- Artimovic, P.; Spakova, I.; Macejkova, E.; Pribulova, T.; Rabajdova, M.; Marekova, M.; Zavacka, M. The ability of microRNAs to regulate the immune response in ischemia/reperfusion inflammatory pathways. Genes Immun. 2024, 25, 277–296. [Google Scholar] [CrossRef]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and application strategies of miRNA-146a regulating inflammation and fibrosis at molecular and cellular levels (Review). Int. J. Mol. Med. 2023, 51, 1–16. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist 2018, 24, 221–245. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells. Redox Biol. 2023, 62, 102681. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diazcouder, A.; Romero-Nava, R.; Del-Rio-Navarro, B.E.; Sanchez-Munoz, F.; Guzman-Martin, C.A.; Reyes-Noriega, N.; Rodriguez-Cortes, O.; Leija-Martinez, J.J.; Velez-Resendiz, J.M.; Villafana, S.; et al. The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients 2024, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Navarro, I.; Botana, L.; Diez-Mata, J.; Tesoro, L.; Jimenez-Guirado, B.; Gonzalez-Cucharero, C.; Alcharani, N.; Zamorano, J.L.; Saura, M.; Zaragoza, C. Replicative Endothelial Cell Senescence May Lead to Endothelial Dysfunction by Increasing the BH2/BH4 Ratio Induced by Oxidative Stress, Reducing BH4 Availability, and Decreasing the Expression of eNOS. Int. J. Mol. Sci. 2024, 25, 9890. [Google Scholar] [CrossRef]
- Tian, M.; Yuan, Y.C.; Li, J.Y.; Gionfriddo, M.R.; Huang, R.C. Tumor necrosis factor-alpha and its role as a mediator in myocardial infarction: A brief review. Chronic Dis. Transl. Med. 2015, 1, 18–26. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H.; Kang, G. Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. Int. J. Mol. Sci. 2021, 22, 9794. [Google Scholar] [CrossRef]
- Stykel, M.G.; Ryan, S.D. Nitrosative stress in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 104. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Ren, H.; Han, R.; Chen, X.; Liu, X.; Wan, J.; Wang, L.; Yang, X.; Wang, J. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J. Cereb. Blood Flow. Metab. 2020, 40, 1752–1768. [Google Scholar] [CrossRef] [PubMed]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2020, 98, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Okumura, T.; Matsuo, Y.; Okuyama, T.; Michiura, T.; Kaibori, M.; Umezaki, N.; Bono, H.; Hirota, K.; Sekimoto, M. Activation of transcription factor HIF inhibits IL-1beta-induced NO production in primary cultured rat hepatocytes. Nitric Oxide 2022, 124, 1–14. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ain, R. Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling. J. Biol. Chem. 2017, 292, 6600–6620. [Google Scholar] [CrossRef]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.S.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; et al. Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid. Redox Signal 2019, 30, 1124–1143. [Google Scholar] [CrossRef]
- Gao, D.; Asghar, S.; Hu, R.; Chen, S.; Niu, R.; Liu, J.; Chen, Z.; Xiao, Y. Recent advances in diverse nanosystems for nitric oxide delivery in cancer therapy. Acta Pharm. Sin. B 2023, 13, 1498–1521. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Munzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Carlstrom, M.; Weitzberg, E.; Lundberg, J.O. Nitric Oxide Signaling and Regulation in the Cardiovascular System: Recent Advances. Pharmacol. Rev. 2024, 76, 1038–1062. [Google Scholar] [CrossRef]
- Mikrut, K.; Kupsz, J.; Kozlik, J.; Krauss, H.; Pruszynska-Oszmalek, E.; Gibas-Dorna, M. Angiotensin-converting enzyme inhibitors reduce oxidative stress intensity in hyperglicemic conditions in rats independently from bradykinin receptor inhibitors. Croat. Med. J. 2016, 57, 371–380. [Google Scholar] [CrossRef]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-kappaB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Ahmed, R.; Augustine, R.; Chaudhry, M.; Akhtar, U.A.; Zahid, A.A.; Tariq, M.; Falahati, M.; Ahmad, I.S.; Hasan, A. Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: State of the art and recent trends. Biomed. Pharmacother. 2022, 149, 112707. [Google Scholar] [CrossRef]
- Tabish, T.A.; Crabtree, M.J.; Townley, H.E.; Winyard, P.G.; Lygate, C.A. Nitric Oxide Releasing Nanomaterials for Cardiovascular Applications. JACC Basic Transl. Sci. 2024, 9, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, Q.; Li, X.; Chen, C.; Liu, J.; Ye, Y.; Ruan, Y.; Hei, Z. Asymmetric dimethylarginine and all-cause mortality: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44692. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Zhang, Z.Y.; Wei, L.H.; Li, R.; Yu, J. The Endothelial Nitric Oxide Synthase Gene T-786C Polymorphism Increases Myocardial Infarction Risk: A Meta-Analysis. Med. Sci. Monit. 2017, 23, 759–766. [Google Scholar] [CrossRef]
| Regulatory Mechanism | Effect | Examples |
|---|---|---|
| Cytokines | Pro-inflammatory cytokines (e.g., TNF-α, IL-1β) induce iNOS; anti-inflammatory cytokines (e.g., IL-10) suppress iNOS | TNF-α promotes NF-κB activation; IL-10 inhibits NF-κB and reduces NO production |
| Lipid Mediators | Prostaglandins (e.g., PGE2) and leukotrienes (e.g., LTB4) modulate NOS through receptor-mediated signaling pathways | PGE2 enhances iNOS expression; LTB4 promotes inflammation in asthma and arthritis |
| MicroRNAs | miRNAs like miR-155 enhance iNOS, while miR-146a suppresses NF-κB signaling, reducing iNOS expression | miR-155 linked to autoimmune diseases; miR-146a protective in neuroinflammation |
| Disease | Pathological Role of NOS | Therapeutic Strategy |
|---|---|---|
| Cardiovascular Diseases | eNOS dysfunction leads to reduced NO, endothelial damage, and vascular inflammation | BH4 supplementation, statins, and ACE inhibitors to restore NO bioavailability and vascular function |
| Neurodegenerative Disorders | Overactive nNOS and iNOS contribute to oxidative stress and neuroinflammation | Selective nNOS inhibitors (e.g., 7-NI), siRNA targeting iNOS to reduce nitrosative stress |
| Cancer | iNOS promotes angiogenesis and immune evasion; nNOS/eNOS have dual roles in tumor growth and immune responses | iNOS inhibitors to suppress tumor progression; leveraging nNOS/eNOS for enhancing immunotherapy |
| Inflammatory Diseases | iNOS overactivation exacerbates chronic inflammation and tissue damage | NF-κB inhibitors and RNA-based therapies to downregulate iNOS expression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. https://doi.org/10.3390/ijms26031204
Kim ME, Lee JS. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. International Journal of Molecular Sciences. 2025; 26(3):1204. https://doi.org/10.3390/ijms26031204
Chicago/Turabian StyleKim, Mi Eun, and Jun Sik Lee. 2025. "Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches" International Journal of Molecular Sciences 26, no. 3: 1204. https://doi.org/10.3390/ijms26031204
APA StyleKim, M. E., & Lee, J. S. (2025). Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. International Journal of Molecular Sciences, 26(3), 1204. https://doi.org/10.3390/ijms26031204







