Multifaceted Sulfonamide-Derived Thiosemicarbazones: Combining Metal Chelation and Carbonic Anhydrases Inhibition in Anticancer Therapy
Abstract
1. Introduction
2. Results and Discussion
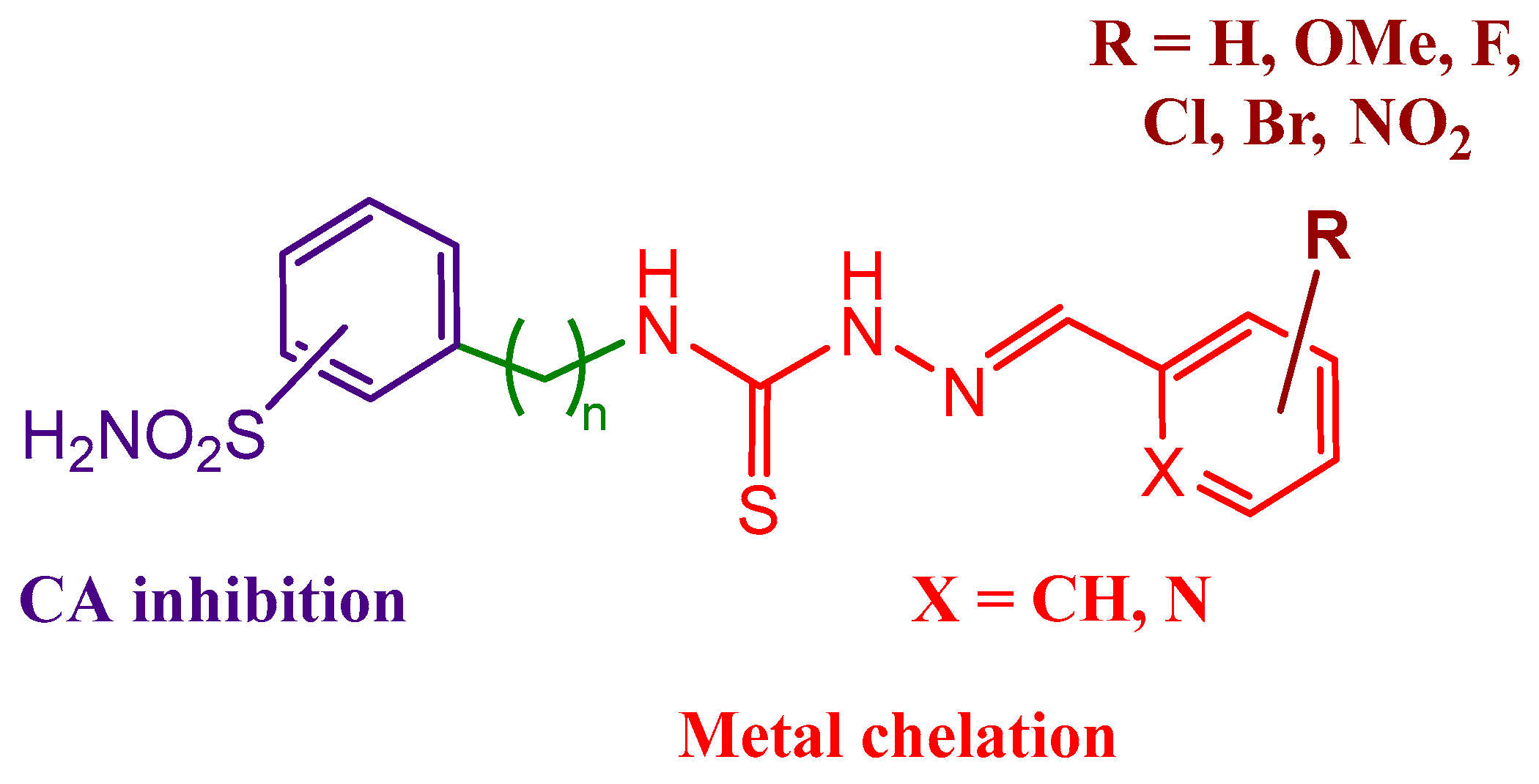
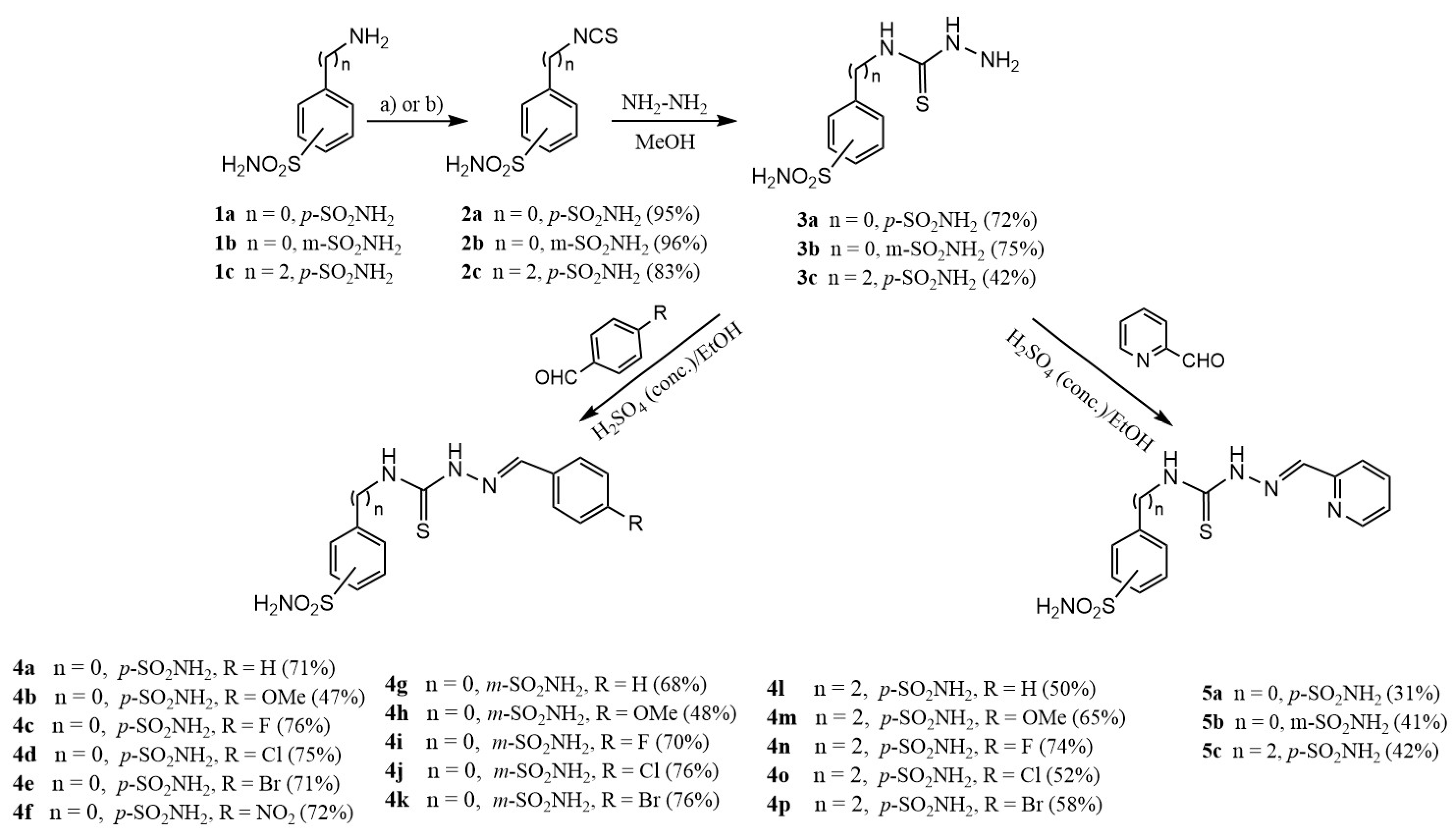
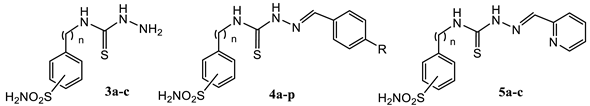
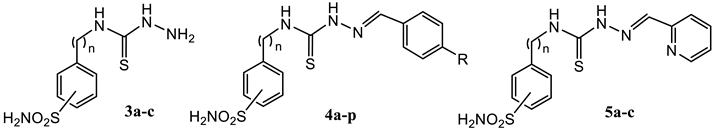
2.1. Drug Design and Chemistry
2.2. Biological Assessments
2.2.1. CA Inhibition
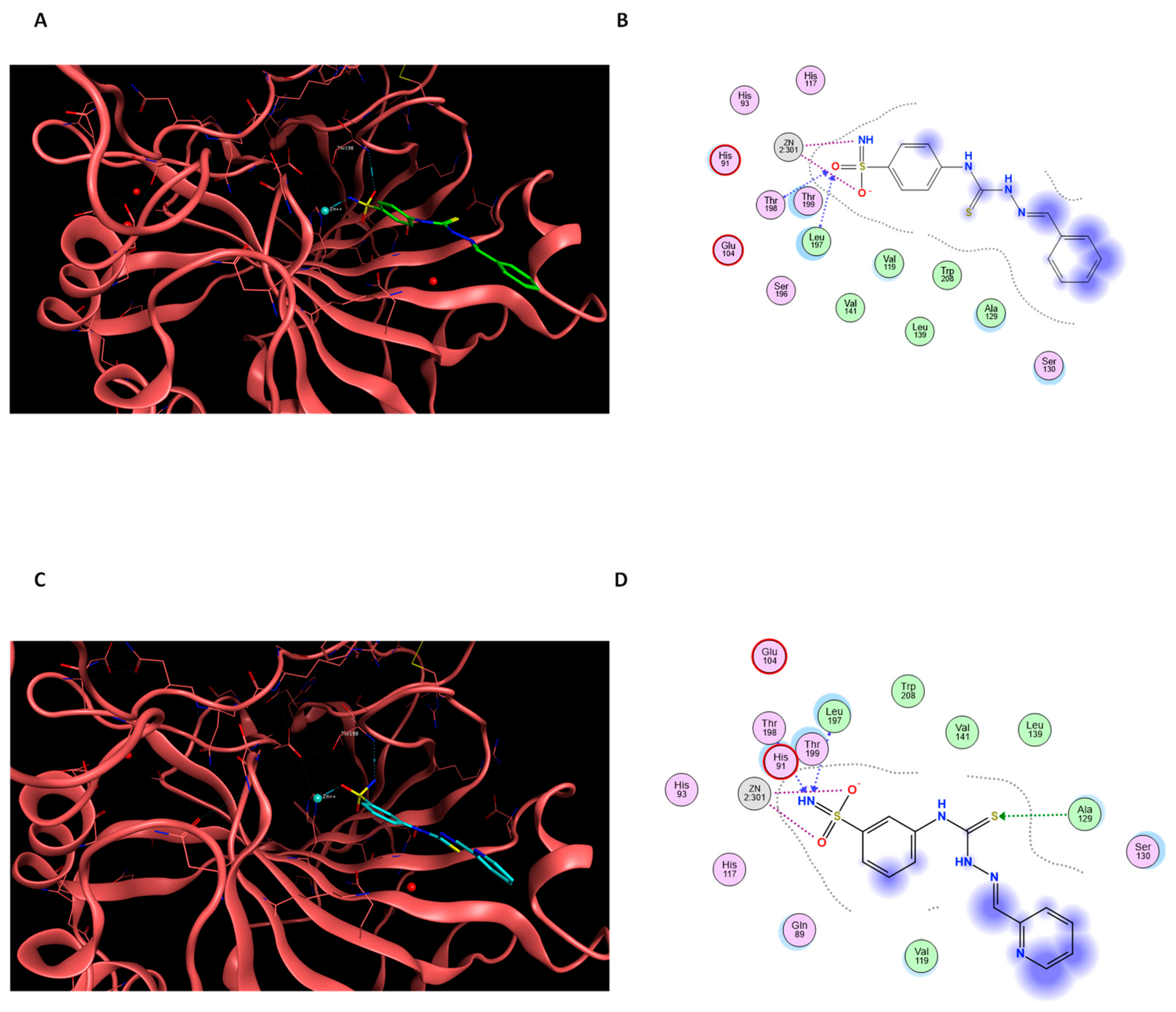
2.2.2. Docking Simulations
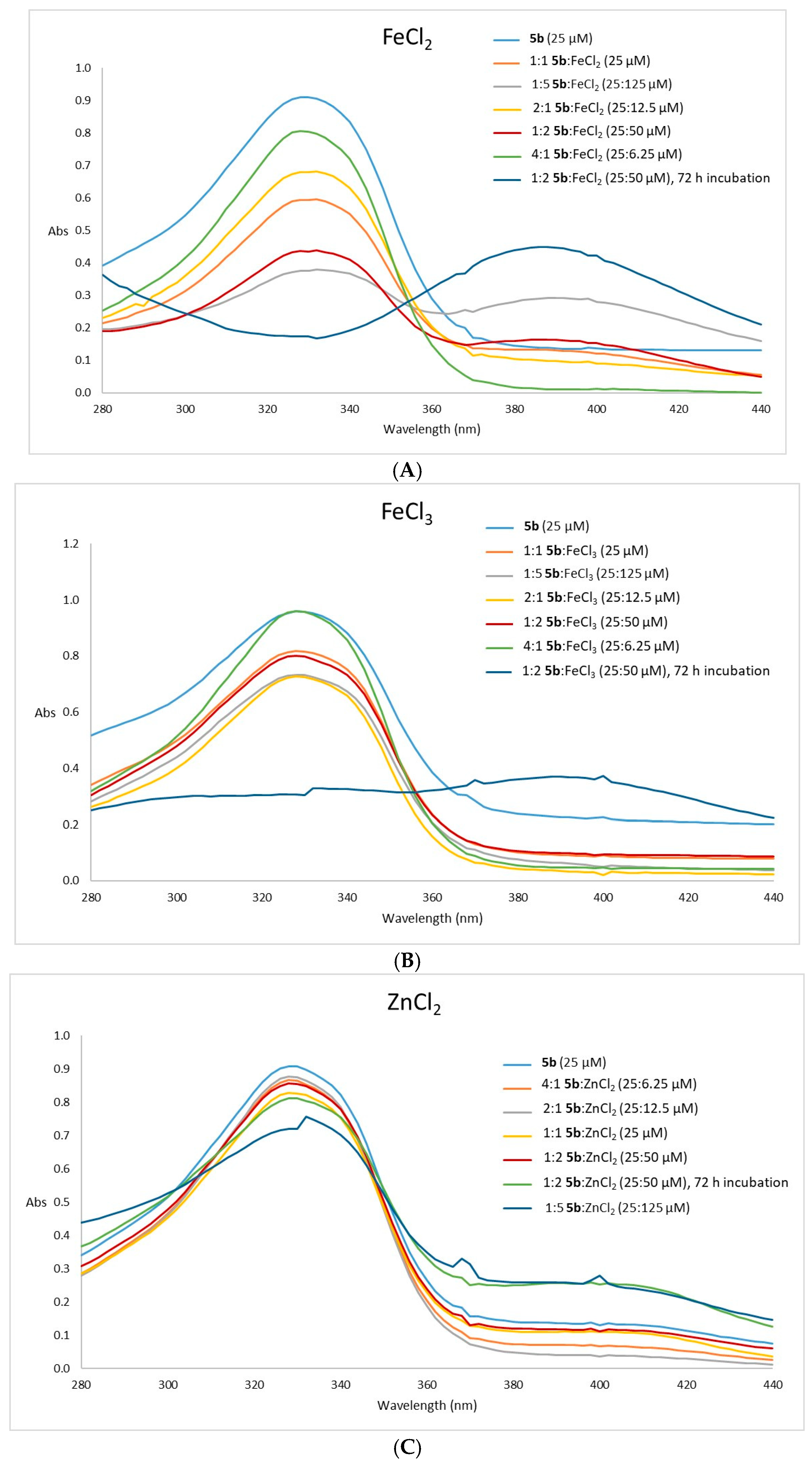
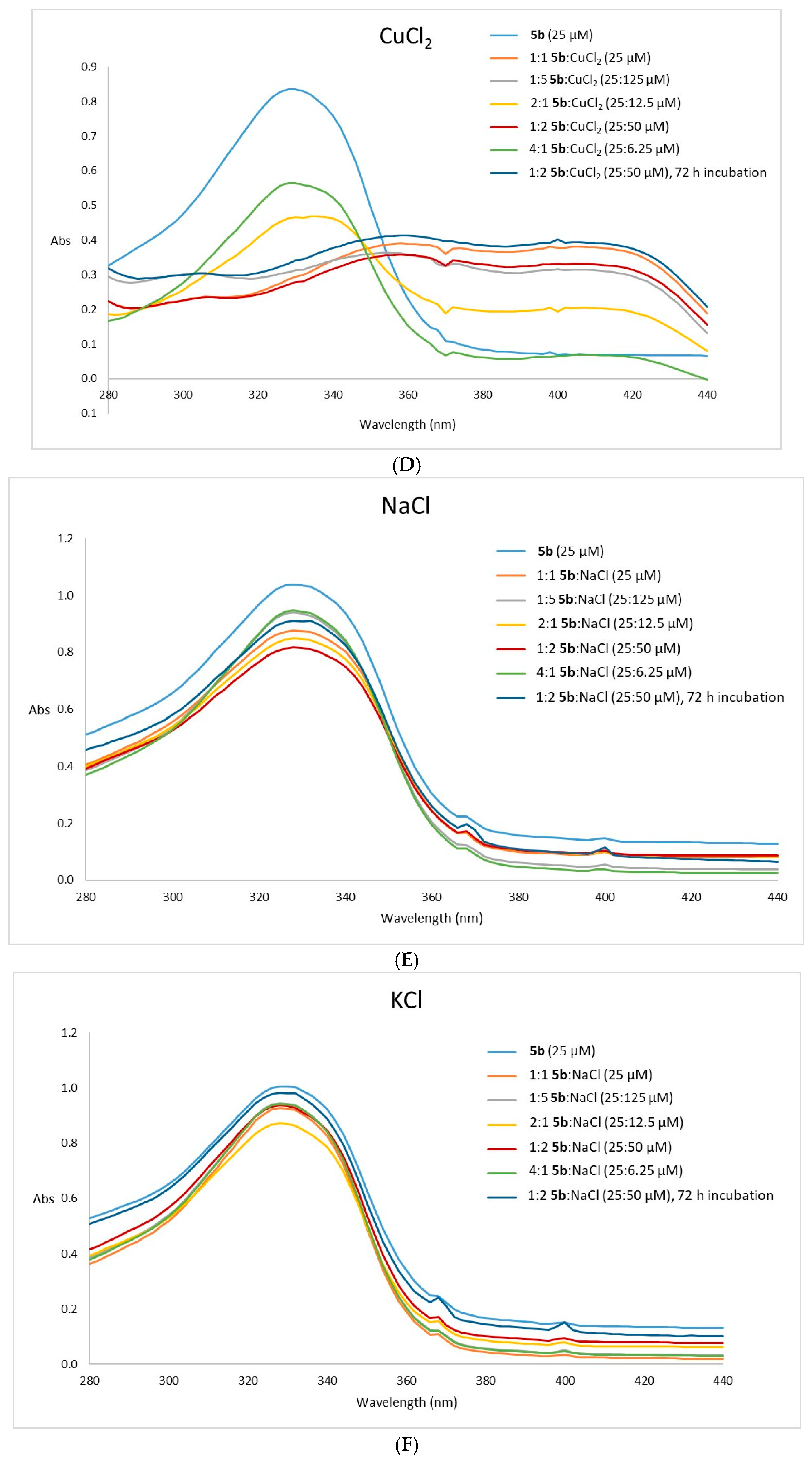
2.2.3. Metal Complexation Assays
2.2.4. Antiproliferative Assay
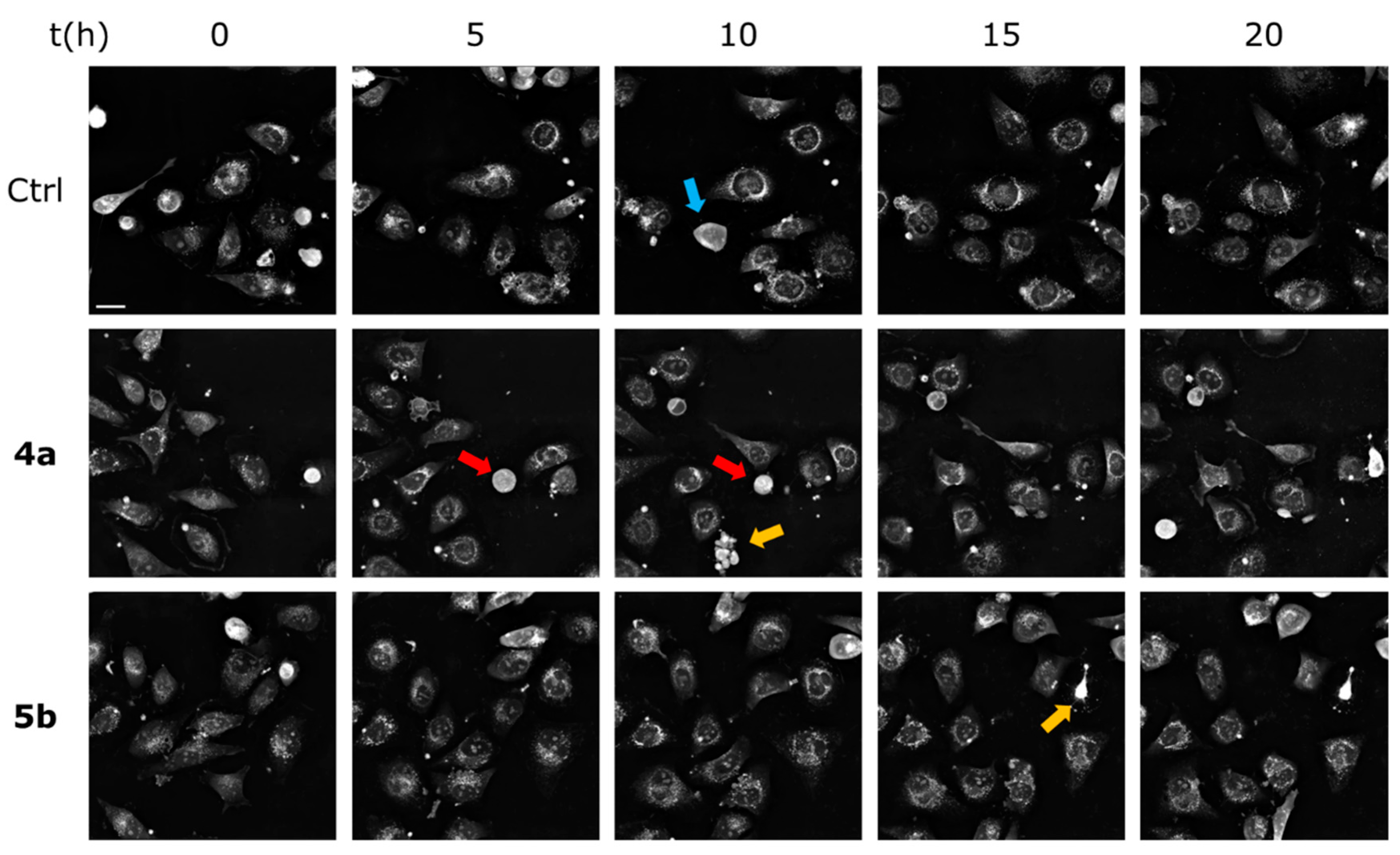
2.2.5. Label-Free Continuous Live Cell Imaging
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
3.1.2. General Procedure for the Preparation of Isothiocyanates 2a,b [43]
3.1.3. 4-(2′-Isothiocyanatoethyl)Benzenesulfonamide (2c) [44]
3.1.4. General Procedure for the Preparation of Thiosemicarbazides 3a–c
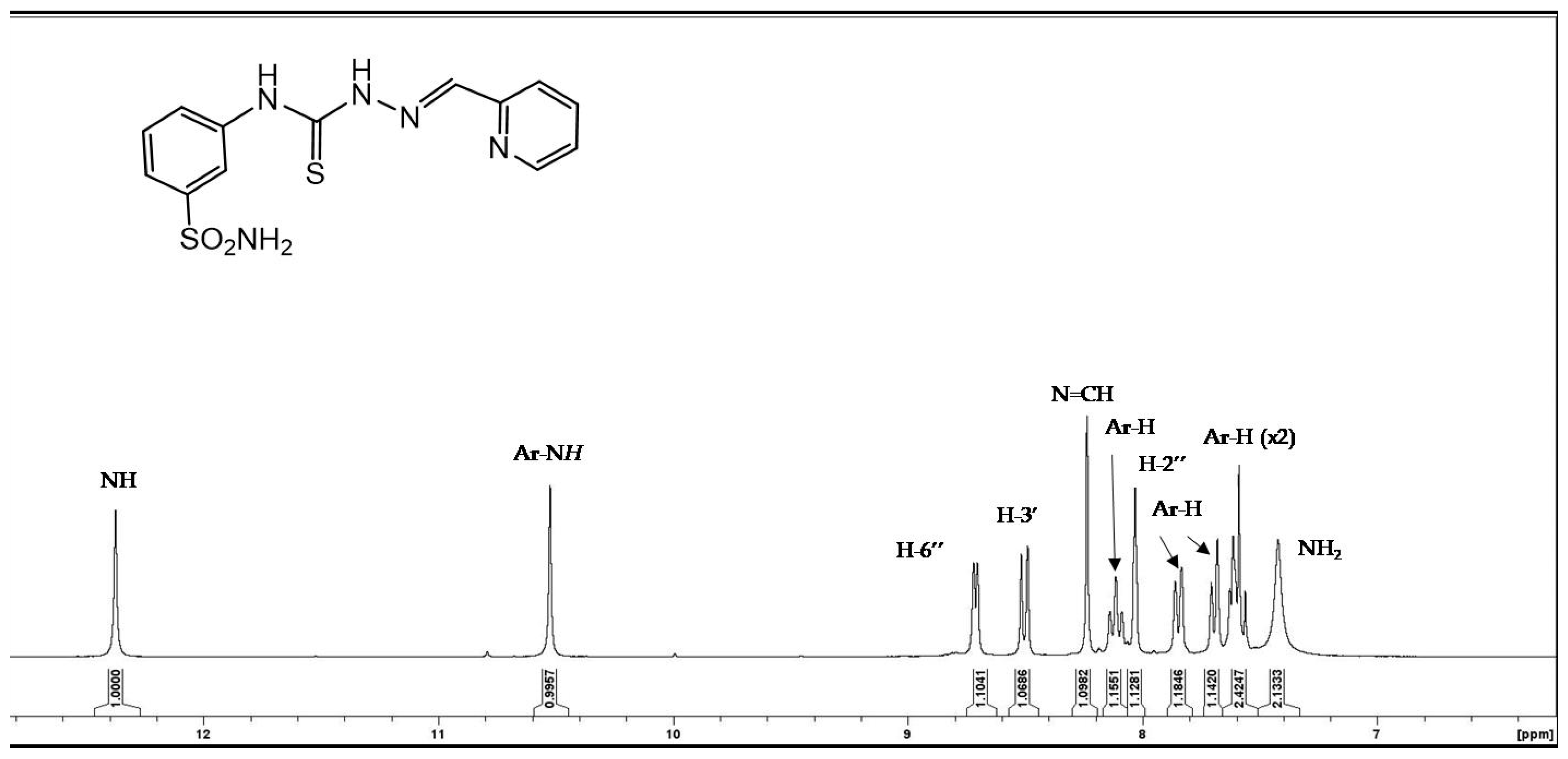
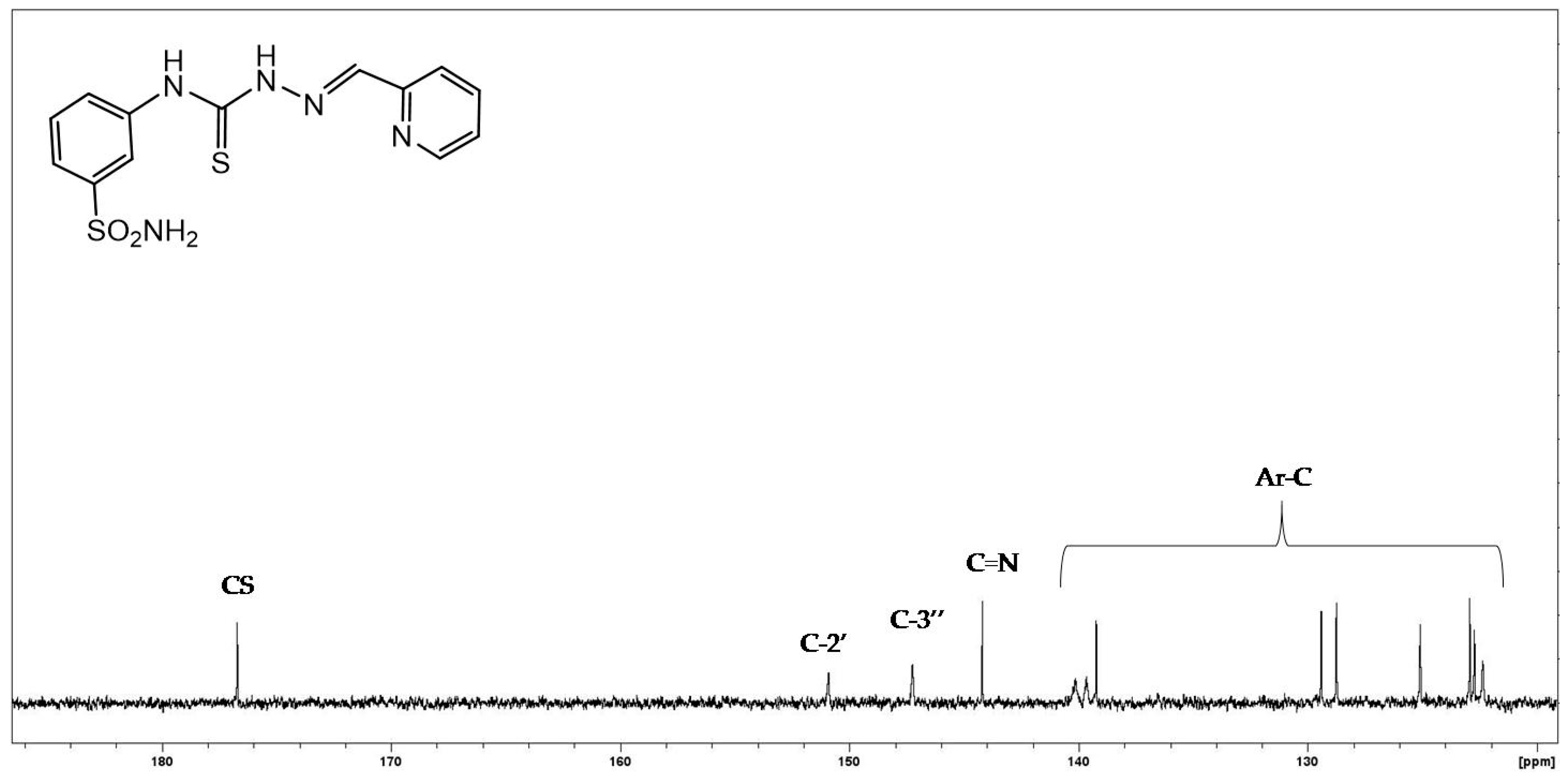
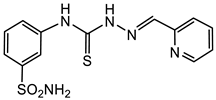
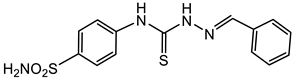
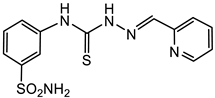
3.1.5. General Procedure for the Preparation of Thiosemicarbazones 4a–p, 5a–c
3.2. CA Inhibition Assays
3.3. Docking Simulations
3.4. Metal Complexation Assays
3.5. Antiproliferative Assays
3.5.1. Cell Lines and Culture
3.5.2. Antiproliferative Tests
3.5.3. Cell Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 3 November 2024).
- Nguyen, O.T. The global challenge of cancer. Nat. Cancer 2020, 1, 1–2. [Google Scholar]
- Grunt, T.W.; Valent, P. Cancer—A devastating disease, but also an eye-opener and window into the deep mysteries of life and its origins. Progress Biophys. Mol. Biol. 2022, 175, 131–139. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Acher, A.W.; Bleicher, J.; Cannon, A.; Scaife, C. Advances in surgery for pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 1037–1043. [Google Scholar] [CrossRef]
- Nuwer, R. Advances in highly targeted radiation treatment for cancer have ignited interest in a once obscure field. Nature 2024, 629, S7–S9. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, P.; Lei, J.; Bai, P.; Zhong, P.; Yang, M.; Wei, P. Old concepts, new tricks: How peptide vaccines are reshaping cancer immunotherapy? Int. J. Biol. Macromol. 2025, 279, 135541. [Google Scholar] [CrossRef]
- Dash, P.; Panda, P.K.; Su, C.; Lin, Y.-C.; Sakthivel, R.; Chena, S.-L.; Chung, R.-J. Near-infrared-driven upconversion nanoparticles with photocatalysts through water-splitting towards cancer treatment. J. Mater. Chem. B 2024, 12, 3881–3907. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Liu, X.-J.; Zhao, H.-C.; Hou, S.-J.; Zhang, H.-J.; Cheng, L.; Yuan, S.; Zhang, L.-R.; Song, J.; Zhang, S.-Y.; Chen, S.-W. Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy. Bioorg. Chem. 2023, 133, 106425. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, R.; Boron, W.F. Role of carbonic anhydrases and inhibitors in acid–base physiology: Insights from mathematical modelling. Int. J. Mol. Sci. 2019, 20, 3841. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, C.; Lim, S.W.; Adhikari, A.; Andring, J.T.; McKenna, R.; Ghim, C.-M.; Kim, C.U. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 2020, 11, 4557. [Google Scholar] [CrossRef]
- Supuran, C.T. Multi- and poly-pharmacology of carbonic anhydrase inhibitors. Pharmacol. Rev 2025, 77, 100004. [Google Scholar] [CrossRef]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef]
- Mboge, M.Y.; McKenna, R.; Frost, S.C. Advances in anti-cancer drug development targeting carbonic anhydrase IX and XII. Top. Anticancer Res. 2015, 5, 3–42. [Google Scholar]
- Higazy, S.; Samir, N.; El-Khouly, A.; Giovannuzzi, S.; Begines, P.; Gaber, H.M.; Supuran, C.T.; Abouzid, K.A.M. Identification of thienopyrimidine derivatives tethered with sulfonamide and other moieties as carbonic anhydrase inhibitors: Design, synthesis and anti-proliferative activity. Bioorg. Chem. 2024, 144, 107089. [Google Scholar] [CrossRef]
- Elsayad, K.A.; Elmasry, G.F.; Mahmoud, S.T.; Awadallah, F.M. Sulfonamides as anticancer agents: A brief review on sulfonamide derivatives as inhibitors of various proteins overexpressed in cancer. Bioorg. Chem. 2024, 147, 107409. [Google Scholar] [CrossRef]
- Sarnella, A.; Ferrara, Y.; Auletta, L.; Albanese, S.; Cerchia, L.; Alterio, V.; De Simone, G.; Supuran, C.T.; Zannetti, A. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J. Exp. Clin. Cancer Res. 2022, 41, 122. [Google Scholar] [CrossRef]
- Eloranta, K.; Pihlajoki, M.; Liljeström, E.; Nousiainen, R.; Soini, T.; Lohi, J.; Cairo, S.; Wilson, D.B.; Parkkila, S.; Heikinheimo, M. SLC-0111, an inhibitor of carbonic anhydrase IX, attenuates hepatoblastoma cell viability and migration. Front. Oncol. 2023, 13, 1118268. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Krzywoszyńska, K.; Witkowska, D.; Świątek-Kozłowska, J.; Szebesczyk, A.; Kozłowski, H. General aspects of metal ions as signaling agents in health and disease. Biomolecules 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Columbano, A.; Ammarah, U.; Mazzone, M.; Menga, A. Understanding metal dynamics between cancer cells and macrophages: Competition or synergism? Front. Oncol. 2020, 10, 646. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, H.; Guo, Z. Modulation of metal homeostasis for cancer therapy. ChemPlusChem 2024, 89, e202300624. [Google Scholar] [CrossRef]
- Sousa, L.; Oliveira, M.M.; Pessôa, M.T.C.; Barbosa, L.A. Iron overload: Effects on cellular biochemistry. Clin. Chim. Acta 2000, 504, 180–189. [Google Scholar] [CrossRef]
- Guan, D.; Zhao, L.; Shi, X.; Ma, X.; Chen, Z. Copper in cancer: From pathogenesis to therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New iron metabolic pathways and chelation targeting strategies affecting the treatment of all types and stages of cancer. Int. J. Mol. Sci. 2022, 23, 13990. [Google Scholar] [CrossRef]
- Hassan, A.E.; Albohy, S.A.H.; Elzaref, A.S.; Elfeky, A.S.; El-Fakharany, E.M.; Saleh, A.K.; Mahmoud, A.M.; Elgammal, W.E. Metal complexes with thiosemicarbazone derivative and isatine: A promising new class of materials for biomedical and environmental applications. J. Photochem. Photobiol. A Chem. 2024, 455, 115764. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Rabee, M.M.; Bräse, S. Transition metal complexes of thiosemicarbazides, thiocarbohydrazides, and their corresponding carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A review. Molecules 2023, 28, 1808. [Google Scholar] [CrossRef]
- Dömötör, O.; May, N.V.; Pelivan, K.; Kiss, T.; Keppler, B.K.; Kowol, C.R.; Enyedy, E.A. A comparative study of α-N-pyridyl thiosemicarbazones: Spectroscopic properties, solution stability and copper(II) complexation. Inorg. Chim. Acta 2018, 472, 264–275. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Gašparović, A.Č.; Valente, A.; López, Ó.; Nunes, J.H.B.; Kowol, C.R.; Heffeter, P.; Filipović, N.R. Schiff bases and their metal complexes to target and overcome (multidrug) resistance in cancer. Eur. J. Med. Chem. 2024, 270, 116363. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; Khairy, M. Synthesis, characterization, optical, DFT, TD DFT studies and in silico ADME predictions of thiosemicarbazone ligand and its Au(III) complex. Orient. J. Chem. 2022, 38, 537–546. [Google Scholar] [CrossRef]
- Yousef, T.A.; El-Reash, G.M.A. Synthesis, and biological evaluation of complexes based on thiosemicarbazone ligand. J. Mol. Struct. 2020, 1201, 127180. [Google Scholar] [CrossRef]
- de Siqueira, L.R.P.; de Moraes Gomes, P.A.T.; de Lima Ferreira, L.P.; de Melo Rêgo, M.J.B.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as potent anticancer agents and their modes of action. Mini-Rev. Med. Chem. 2020, 20, 638–661. [Google Scholar] [CrossRef]
- de Almeida, S.M.V.; Almeida Lafayette, E.; Gomes da Silva, L.P.B.; da Cruz Amorim, C.A.; de Oliveira, T.B.; Gois Ruiz, A.L.T.; de Carvalho, J.E.; de Moura, R.O.; Carneiro Beltrão, E.I.; Alves de Lima, M.d.C.; et al. Synthesis, DNA binding, and antiproliferative activity of novel acridine-thiosemicarbazone derivatives. Int. J. Mol. Sci. 2015, 16, 13023–13042. [Google Scholar] [CrossRef]
- de Almeida, S.M.V.; Ribeiro, A.G.; de Lima Silva, G.C.; Ferreira Alves, J.E.; Carneiro Beltrão, E.I.; Ferreira de Oliveira, J.; de Carvalho, L.B., Jr.; Alves de Lima, M.d.C. DNA binding and topoisomerase inhibition: How can these mechanisms be explored to design more specific anticancer agents? Biomed. Pharmacother. 2017, 96, 1538–1556. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The new wave in cancer treatment. Fut. Med. Chem. 2009, 1, 1143–1151. [Google Scholar] [CrossRef]
- Schaier, M.; Falcone, E.; Prstek, T.; Vileno, B.; Hager, S.; Keppler, B.K.; Heffeter, P.; Koellensperger, G.; Faller, P.; Kowol, C.R. Human serum albumin as a copper source for anticancer thiosemicarbazones. Metallomics 2023, 15, mfad046. [Google Scholar] [CrossRef]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer thiosemicarbazones: Chemical properties, interaction with iron metabolism, and resistance development. Antioxid. Redox Signal. 2019, 30, 1062–1082. [Google Scholar] [CrossRef]
- Ohui, K.; Stepanenko, I.; Besleaga, I.; Babak, M.V.; Stafi, R.; Darvasiova, D.; Giester, G.; Pósa, V.; Enyedy, E.A.; Vegh, D.; et al. Triapine derivatives act as copper delivery vehicles to induce deadly metal overload in cancer cells. Biomolecules 2010, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; El Ella, D.A.A.; Heiba, H.I.; Soliman, A.M. Synthesis of certain new thiazole derivatives bearing a sulfonamide moiety with expected anticancer and radiosensitizing activities. J. Mat. Sci. Eng. A 2011, 1, 684–691. [Google Scholar]
- Khobzaoui, M.; Tillekeratne, L.M.V.; Hudson, R.A. Potent isothiocyanate inhibitors of carbonic anhydrase: Synthesis and evaluation. Biochem. Biophys. Res. Commun. 2004, 318, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Mujundar, P.; Annovazzi, L.; Kopecha, J.; Mellai, M.; Schiffer, D.; Poulsen, S.A.; Riganti, C. Carbonic anhydrase XII inhibitors overcome P-glycoprotein-mediated resistance to temozolomide in glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef]
- Supuran, C.T.; Altamimi, A.S.A.; Carta, F. Carbonic anhydrase inhibition and the management of glaucoma: A literature and patent review 2013–2019. Expert Opin. Ther. Pat. 2019, 29, 781–792. [Google Scholar] [CrossRef]
- Martínez-Montiel, M.; Romero-Hernández, L.L.; Giovannuzzi, S.; Begines, P.; Puerta, A.; Ahuja-Casarín, A.I.; Fernandes, M.X.; Merino-Montiel, P.; Montiel-Smith, S.; Nocentini, A.; et al. Conformationally restricted glycoconjugates derived from arylsulfonamides and coumarins: New families of tumour-associated carbonic anhydrase inhibitors. Int. J. Mol. Sci. 2023, 24, 9401. [Google Scholar] [CrossRef]
- Argibay-Otero, S.; Carballo, R.; Vázquez-López, E.M. Coordination chemistry of potentially S,N,Npy-tridentate thiosemicarbazones with the {Re(CO)3}+ fragment and formation of hemiaminal derivatives. Inorg. Chem. 2023, 62, 224–237. [Google Scholar] [CrossRef]
- Deng, J.; Yu, P.; Zhang, Z.; Wang, J.; Cai, J.; Wu, N.; Sun, H.; Liang, H.; Yang, F. Designing anticancer copper(II) complexes by optimizing 2-pyridine-thiosemicarbazone ligands. Eur. J. Med. Chem. 2018, 158, 442–452. [Google Scholar] [CrossRef]
- Bai, X.-G.; Zheng, Y.; Qi, J. Advances in thiosemicarbazone metal complexes as anti-lung cancer agents. Front. Pharmacol. 2022, 13, 1018951. [Google Scholar]
- Zhang, X.-H.; Wang, B.; Tao, Y.-Y.; Ma, Q.; Wang, H.-J.; He, Z.-X.; Wu, H.-P.; Li, Y.-H.; Zhao, B.; Ma, L.-Y.; et al. Thiosemicarbazone-based lead optimization to discover high-efficiency and low-toxicity anti-gastric cancer agents. Eur. J. Med. Chem. 2020, 199, 112349. [Google Scholar] [CrossRef]
- Pape, V.F.S.; Tóth, S.; Füredi, A.; Szebényi, K.; Lovrics, A.; Szabó, P.; Wiese, M.; Szakacs, G. Design, synthesis and biological evaluation of thiosemicarbazones, hydrazinobenzothiazoles and arylhydrazones as anticancer agents with a potential to overcome multidrug resistance. Eur. J. Med. Chem. 2016, 117, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Peña, J.M.; Puerta, A.; Dinić, J.; Jovanović Stojanov, S.; González-Bakker, A.; Hicke, F.J.; Mishra, A.; Piyasaengthong, A.; Maya, I.; Walton, J.W.; et al. Biotinylated selenocyanates: Potent and selective cytostatic agents. Bioorg. Chem. 2023, 133, 106410. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Pérez, M.C.; Sánchez-Fernández, E.M.; González-Bakker, A.; Puerta, A.; Padrón, J.M.; Martín-Loro, F.; Arroba, A.I.; García Fernández, J.M.; Mellet, C.O. Fluoro-labelled sp2-iminoglycolipids with immunomodulatory properties. Eur. J. Med. Chem. 2023, 255, 115390. [Google Scholar] [CrossRef] [PubMed]
- Puerta, A.; González-Bakker, A.; Santos, G.; Padrón, J.M. Early pharmacological profiling of antiproliferative compounds by live cell Imaging. Molecules 2022, 27, 5261. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical shifts of traceimpurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Karalı, N.; Akdemir, A.; Göktaş, F.; Elma, P.E.; Angeli, A.; Kızılırmak, M.; Supuran, C.T. Novel sulfonamide-containing 2-indolinones that selectively inhibit tumor-associated alpha carbonic anhydrases. Bioorg. Med. Chem. 2017, 25, 3714–3718. [Google Scholar] [CrossRef]
- Demir-Yazici, K.; Apaydin, C.B.; Soylu-Eter, O.; Oezsoy, N.; Karali, N. Synthesis, molecular modeling and cholinesterase inhibitory effects of 2-indolinone-based hydrazinecarbothioamides. Fut. Med. Chem. 2021, 13, 2133–2151. [Google Scholar] [CrossRef]
- Trawally, M.; Demir-Yazıcı, K.; Angeli, A.; Kaya, K.; Akdemir, A.; Supuran, C.T.; Güzel-Akdemir, Ö. Thiosemicarbazone-benzenesulfonamide derivatives as human carbonic anhydrases inhibitors: Synthesis, characterization, and in silico studies. Anti-Cancer Agents Med. Chem. 2024, 24, 649–667. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]


 | ||||||
|---|---|---|---|---|---|---|
| Compound | CA I | CA II | CA IX | CA XII | Selectivity Ratio I/IX//II/IX | Selectivity Ratio I/XII//II/XII |
| 3a (n = 0, p-) | 327 | 7600 | 133 | 68 | 2.5//57.1 | 4.8//111.8 |
| 3b (n = 0, m-) | 1457 | 439 | 185 | 116 | 7.9//2.4 | 12.6//3.8 |
| 3c (n = 2, p-) | 79.0 | 8.51 | 13.0 | 5.02 | 6.1//0.65 | 15.7//1.7 |
| 4a (n = 0, p-, R = H) | 504 | 23.7 | 16.1 | 8.38 | 31.3//1.5 | 60.1//2.8 |
| 4b (n = 0, p-, R = OMe) | 215 | 78 | 91 | 49 | 2.4//0.86 | 4.4//1.6 |
| 4c (n = 0, p-, R = F) | 196 | 44.6 | 147 | 51.8 | 1.3//0.30 | 3.8//0.86 |
| 4d (n = 0, p-, R = Cl) | 485 | 48.8 | 27.4 | 12.1 | 17.7//1.8 | 40.1//4.0 |
| 4e (n = 0, p-, R = Br) | 574 | 46.8 | 23.3 | 13.6 | 24.6//2.0 | 42.2//3.4 |
| 4f (n = 0, p-, R = NO2) | 845 | 820 | 477 | 288 | 1.8//1.7 | 2.9//2.8 |
| 4g (n = 0, m-, R = H) | 876 | 60.5 | 74.3 | 50.9 | 11.8//0.81 | 17.2//1.2 |
| 4h (n = 0, m-, R = OMe) | 748 | 39.4 | 387 | 2.3 | 1.9//0.10 | 325//17.1 |
| 4i (n = 0, m-, R = F) | 496 | 97.1 | 89.3 | 49.3 | 5.6//1.1 | 10.1//2.0 |
| 4j (n = 0, m-, R = Cl) | 5186 | 312 | 217 | 85.4 | 23.9//1.4 | 60.7//3.7 |
| 4k (n = 0, m-, R = Br) | 5337 | 531 | 206 | 91.3 | 25.9//2.6 | 58.5//5.8 |
| 4l (n = 2, p-, R = H) | 568 | 70.2 | 30.8 | 9.12 | 18.4//2.3 | 62.3//7.7 |
| 4m (n = 2, p-, R = OMe) | 3479 | 321 | 164 | 35.0 | 21.2//2.0 | 99.4//9.2 |
| 4n (n = 2, p-, R = F) | 2485 | 590 | 300 | 48.5 | 8.3//2.0 | 51.2//12.2 |
| 4o (n = 2, p-, R = Cl) | 6217 | 773 | 408 | 143 | 15.2//1.9 | 43.4//2.9 |
| 4p (n = 2, p-, R = Br) | 6092 | 813 | 499 | 123 | 12.2//1.6 | 49.5//6.6 |
| 5a (n = 0, p-) | 207 | 156.6 | 351.9 | 4.9 | 0.59//0.45 | 42.2//31.9 |
| 5b (n = 0, m-) | 538 | 2.5 | 4.9 | 5.6 | 109.8//0.49 | 96.1//0.43 |
| 5c (n = 2, p-) | 270 | 83.9 | 28.5 | 9.3 | 9.5//2.9 | 29.0//9.0 |
| AAZ | 250.0 | 12.0 | 25.0 | 5.7 | 10.0//0.48 | 43.9//2.1 |
| Compound | Binding Energy (kcal/mol) |
|---|---|
 4a | −7.93 |
 5b | −7.45 |
| Compound | Binding Energy (kcal/mol) |
|---|---|
 4a | −8.20 |
 5b | −7.68 |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | A549 (Lung) | HBL-100 (Breast) | HeLa (Cervix) | SW1573 (Lung) | T-47D (Breast) | WiDr (Colon) |
| 3a (n = 0, p-) | >100 | |||||
| 3b (n = 0, m-) | ||||||
| 3c (n = 2, p-) | ||||||
| 4a (n = 0, p-, R = H) | 5.1 ± 3.7 | 3.0 ± 1.6 | 2.3 ± 0.2 | 2.9 ± 1.4 | 2.6 ± 0.1 | 4.5 ± 1.4 |
| 4b (n = 0, p-, R = OMe) | >100 | 64 ± 23 | >100 | |||
| 4c (n = 0, p-, R = F) | 25 ± 5 | 28 ± 1 | 7.9 ± 1.1 | 6.0 ± 0.4 | 17 ± 1 | 24 ± 4 |
| 4d (n = 0, p-, R = Cl) | >100 | |||||
| 4e (n = 0, p-, R = Br) | 23 ± 6 | 24 ± 2 | 18 ± 1 | 22 ± 1 | 36 ± 1 | 62 ± 12 |
| 4f (n = 0, p-, R = NO2) | >100 | |||||
| 4g (n = 0, m-, R = H) | 51 ± 10 | >100 | 61 ± 6 | 70 ± 1 | 83 ± 10 | 62 ± 10 |
| 4h (n = 0, m-, R = OMe) | >100 | |||||
| 4i (n = 0, m-, R = F) | 83 ± 16 | >100 | 79 ± 29 | |||
| 4j (n = 0, m-, R = Cl) | 22 ± 4 | 42 ± 15 | 38 ± 3 | 36 ± 4 | 59 ± 16 | 24 ± 8 |
| 4k (n = 0, m-, R = Br) | 19 ± 6 | 40 ± 1 | 45 ± 5 | 46 ± 1 | >100 | |
| 4l (n = 2, p-, R = H) | 90 ± 14 | >100 | 99 ± 1 | >100 | 90 ±14 | |
| 4m (n = 2, p-, R = OMe) | 97 ± 4 | >100 | ||||
| 4n (n = 2, p-, R = F) | >100 | |||||
| 4o (n = 2, p-, R = Cl) | 62 ± 6 | 76 ± 32 | 45 ±16 | 73 ± 38 | >100 | >100 |
| 4p (n = 2, p-, R = Br) | 21 ± 4 | 42 ± 8 | 31 ± 9 | 53 ± 17 | 37 ± 8 | 58 ± 9 |
| 5a (n = 0, p-) | 18 ± 2 | 5.2 ± 2.2 | 5.2 ± 0.3 | 4.2 ± 0.1 | 5.7 ± 2.1 | 3.8 ± 0.3 |
| 5b (n = 0, m-) | 10 ± 2 | 7.8 ± 2 | 5.8 ± 0.4 | 7.1 ± 0.4 | 5.3 ± 0.7 | 4.5 ± 0.6 |
| 5c (n = 2, p-) | 37 ± 11 | >100 | ||||
| 5-Fluorouracil | 2.2 ± 0.3 | 4.4 ± 0.7 | 16 ± 5 | 3.3 ± 1.2 | 43 ± 16 | 49 ± 7 |
| CDDP | 4.9 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.5 | 2.7 ± 0.4 | 17 ± 3 | 26 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Montiel, M.; Arrighi, G.; Begines, P.; González-Bakker, A.; Puerta, A.; Fernandes, M.X.; Merino-Montiel, P.; Montiel-Smith, S.; Nocentini, A.; Supuran, C.T.; et al. Multifaceted Sulfonamide-Derived Thiosemicarbazones: Combining Metal Chelation and Carbonic Anhydrases Inhibition in Anticancer Therapy. Int. J. Mol. Sci. 2025, 26, 1225. https://doi.org/10.3390/ijms26031225
Martínez-Montiel M, Arrighi G, Begines P, González-Bakker A, Puerta A, Fernandes MX, Merino-Montiel P, Montiel-Smith S, Nocentini A, Supuran CT, et al. Multifaceted Sulfonamide-Derived Thiosemicarbazones: Combining Metal Chelation and Carbonic Anhydrases Inhibition in Anticancer Therapy. International Journal of Molecular Sciences. 2025; 26(3):1225. https://doi.org/10.3390/ijms26031225
Chicago/Turabian StyleMartínez-Montiel, Mónica, Giulia Arrighi, Paloma Begines, Aday González-Bakker, Adrián Puerta, Miguel X. Fernandes, Penélope Merino-Montiel, Sara Montiel-Smith, Alessio Nocentini, Claudiu T. Supuran, and et al. 2025. "Multifaceted Sulfonamide-Derived Thiosemicarbazones: Combining Metal Chelation and Carbonic Anhydrases Inhibition in Anticancer Therapy" International Journal of Molecular Sciences 26, no. 3: 1225. https://doi.org/10.3390/ijms26031225
APA StyleMartínez-Montiel, M., Arrighi, G., Begines, P., González-Bakker, A., Puerta, A., Fernandes, M. X., Merino-Montiel, P., Montiel-Smith, S., Nocentini, A., Supuran, C. T., Padrón, J. M., Fernández-Bolaños, J. G., & López, Ó. (2025). Multifaceted Sulfonamide-Derived Thiosemicarbazones: Combining Metal Chelation and Carbonic Anhydrases Inhibition in Anticancer Therapy. International Journal of Molecular Sciences, 26(3), 1225. https://doi.org/10.3390/ijms26031225










