Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure
Abstract
1. Introduction
2. Innate Immune Response in RPL and RIF
2.1. Natural Killer (NK) Cells
2.2. Macrophages and Dendritic Cells
2.3. Polymorphonuclear Cells
2.4. T Cells
2.5. B Cells
2.6. Myeloid Suppressor Cells
| Cell Type | Physiological Function | Described Dysfunction in RPL | Ref. |
|---|---|---|---|
| Innate immunity | The response does not involve antigen presentation. | [36] | |
| NK cells | Elimination of abnormal cells and pathogens. The tolerogenic response to fetus uterine and decidual NK cells differs from that of peripheral NK cells. Elimination of abnormal cells and pathogens. | Decrease in tolerogenic role and increase in cytotoxic response. | [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] |
| NKT cells | Elimination of abnormal cells and pathogens. | Increased cytotoxic function and involvement in local inflammation. | [67,69] |
| Tγδ cells | Control tissue homeostasis, phagocytosis of pathogens, and antigen presentation. | Increased cytotoxicity and involvement in local inflammation. | [70,71] |
| Macrophages | Present in the uterus. Involvement in tolerogenic responses. | Proinflammatory response and secretion of cytotoxic cytokines increase reactive oxygen and nitrogen species. | [72,73,74,75,76,77,78,79] |
| Dendritic cells | Efficient antigen presentation. | Abnormal antigen expression. | [80,81,82,83,84,85] |
| Mast cells | Present in the endometrium | Abnormal activation and proinflammatory role. | [86,87,88,89,90] |
| Eosinophils | Present in endometrium in part of the hormonal cycle. | Unknown. | [92,93] |
| Adaptative immunity | Requires antigen presentation. Highly selective. | [36] | |
| T cytotoxic cells Th-1 | Elimination of unwanted cells. Proinflammatory response. Activation of B cells. IgG production. | Involved in fetal rejection. Involved in fetal rejection. | [97,98,99] [101,102] |
| Th-2 | Pro allergen response. Activation of B cells. IgE production. | Antagonism of Th1. | [101,102] |
| Th-17 | Proinflammatory response. | Fetal rejection. Induces neutrophil migration. | [106,107] |
| T regulatory cells | Tolerogenic role to the fetus. | A decrease in these cells facilitates Th1 and cytotoxic functions. | [103,108,119] |
| B cells | B1 cells produce IgM against pathogens and protect tissue. | Decrease in B1 cells and increase in B2 cells in endometrium. Autoantibody production? | [121,122,123,124,125,126] |
| Myeloid suppressive cells | M-MDSC and PM-MDSC are involved in tissue tolerogenic response. | Impaired amounts of these cells in the endometrium. | [127,128,129,130,131,132,133,134] |
3. Cytokines
4. HLA in RPL and RIF
5. Immune Checkpoints in RPL and RIF
6. Autoimmunity
6.1. Antiphospholipid Antibodies (aPL) and Antiphospholipid Syndrome
6.2. Systemic Lupus Erythematosus and Other Autoimmune Diseases
6.3. Celiac Disease
6.4. Thyroid Autoimmunity
7. MicroRNAs (miRNAs) and RPL
8. Microbiota in RPL and RIF
9. Immunological Treatment of RPL and RIF
9.1. Corticosteroids
9.2. Hydroxychloroquine
9.3. Calcineurin Inhibitors
9.4. Intravenous Immunoglobulins (IVIGs)
9.5. Granulocyte Colony-Stimulating Factor (G-CSF)
9.6. Tumor Necrosis Factor (TNF)-α Inhibitors
9.7. Allogenic Peripheral Blood Mononuclear Cell (PBMC) Immunotherapy
9.8. Intrauterine Peripheral Blood Mononuclear Cells
9.9. Intrauterine Autologous Platelet-Rich Plasma (PRP)
9.10. Lipid Emulsion (Intralipid) Intravenous Therapy
9.11. Omega 3 Fatty Acid Supplementation
9.12. Low-Molecular-Weight Heparin (LMWH)
9.13. Low-Dose Acetylsalicylic Acid
9.14. Vitamin D
9.15. Progesterone
9.16. Intrauterine Human Chorionic Gonadotropin (hCG) Infusion
9.17. Anti-Obesity Drugs to Increase Fertility
- Glucocorticoids are not recommended for treating unexplained RPL or RPL exhibiting specific immunological biomarkers. There is insufficient evidence to endorse the use of progesterone for enhancing live birth rates in women with RPL and luteal phase insufficiency. However, vaginal progesterone may have a positive impact on live birth rates for women with three or more pregnancy losses combined with vaginal bleeding in subsequent pregnancies.
- The use of heparin or low-dose aspirin is not advised in RPL patients without antiphospholipid syndrome, as evidence indicates that these interventions do not improve live birth rates in women with unexplained RPL.
- There is also insufficient evidence for the effectiveness of human hCG in improving live birth rates among women with RPL and luteal phase insufficiency. Additionally, there is inadequate support for the use of metformin supplementation during pregnancy to prevent pregnancy loss in women with RPL and glucose metabolism anomalies.
- Counseling women with RPL about the general recommendation to consider prophylactic vitamin D supplementation before conception may be beneficial. Low-dose folic acid is routinely initiated preconceptionally to prevent neural tube defects; however, it has not been demonstrated to avoid pregnancy loss in women with unexplained RPL. Due to inconclusive evidence, current guidelines neither endorse nor recommend using vitamin supplements as treatment. Patients should receive appropriate advice regarding the potential harms of vitamin supplements, notably vitamins E and A.
- No evidence supports the recommendation of G-CSF in unexplained RPL.
- Lymphocyte immunization therapy is not advised to treat unexplained RPL due to its lack of significant efficacy and potential for serious adverse effects. However, the administration of repeated and high doses of IVIGs early in pregnancy may increase live birth rates in women who have experienced four or more instances of unexplained RPL.
- There is insufficient evidence to support intralipid therapy as a means of improving live birth rates in women with unexplained RPL.
- According to the European Society of Human Reproduction and Embryology (ESHE), substantial studies on alternative therapies for couples experiencing RPL, including homeopathy, bioresonance therapy, and NaPro technology, are lacking.
| Treatment | Rationale | Effect | References |
|---|---|---|---|
| Corticosteroids (Treatment Level I) | Decrease in peripheral NK cells and increase tolerogenic activity. Combined with aspirin in patients with autoimmune antibodies. | Decreased cytotoxic function. | [244,245,249] |
| No suppressive effect | [246] | ||
| Increased implantation rate in IVF. | [250,251] | ||
| Increased implantation rate and pregnancy success. | [253,254] | ||
| No increase in live birth rates. | [255] | ||
| Combined with aspirin and heparin in antiphospholipid syndrome. | Increased implantation and pregnancy success. | [256,257,258,259,260] | |
| Hydroxy- Chloroquine (Treatment Level I) | Anti-thrombotic and immunomodulatory properties. | Decreased pregnancy loss. | [261,262,263,264,265] |
| Effect dependent on dose. | [265,266] | ||
| Combined with conventional treatment in antiphospholipid syndrome. | Enhanced Tregs, diminished Th17. | [267] | |
| Does not prevent further miscarriage. | [268] | ||
| Calcineurin inhibitors (Treatment Level II) | Cyclosporine and Tacrolimus. Immunosuppressive agents with risk of birth defects [264,265]. | Increased implantation and pregnancy rate. | [272,273,274,275] |
| Hypertensive disorders with treatment | [276] | ||
| No increase in implantation rate. | [277] | ||
| Increased implantation success and pregnancy outcome. | [278,279,280] | ||
| Low-dose tacrolimus in women with immune disorders alone or combined with heparin. Low side effects. | Decreased Th1/Th2 ratio. | [281,282] | |
| Risk-benefit effect in endometriosis | [283,284] | ||
| Sirolimus (rapamycin) inhibits the mTOR pathway that is altered in some RIF and RPL patients [279,280] | Phase II clinical in altered Th17/Treg patients. Increased implantation and pregnancy success. | [285] | |
| Intravenous immunoglobulins (Treatment Level I) | Inhibition of HLA antibodies decreases Fc receptor expression and modulates NK cells. | Increased pregnancy success. Better efficiency at high doses. | [286,287,288,289,290,291,292,293,294,295,296] |
| Effective in women with immunological problems | [297,298,299,300,301,302,303,304,305,306,309,310] | ||
| Granulocyte colony-stimulating factor (G-CSF) (Treatment Level II) | Tolerogenic response. Increase in Tregs/IL-10 [305,306]. | Increased pregnancy success. | [313] |
| There is no difference compared to placebo. | [314] | ||
| Subcutaneous injections have a better effect on women’s ongoing procedures. | [315] | ||
| Subcutaneous G-CSF increased implantation success in RIF patients. | [316,317,318,319] | ||
| Anti-TNFα (Treatment Level II) | Inhibition of TNFα decreases local inflammatory milieu. | Benefit for RPL and RIF patients with autoimmune spectrum. | [320,321] |
| Combined with IVIG, it increased pregnancy success. | [324] | ||
| Allogenic peripheral blood mononuclear cell (PBMC) immunotherapy. (Treatment level II) | Generation of tolerogenic response to HLA antigens from the father and fetus [319,320,321]. | Increased successful pregnancies in some trials. | [328,329,330,333,334,336,337] |
| Benefit in primary RPL only. | [331] | ||
| No beneficial effect. | [334,335] | ||
| Therapy may have complications. | [19,338,339] | ||
| Autologous Intrauterine (PBMC) (Treatment Level II) | PBMC is activated by human chorionic gonadotropin to generate a local tolerogenic response. | Increased successful pregnancies in RPL patients. | [340,341,342,343,344] |
| Increased Tregs in patients with low endometrial Treg. | [345] | ||
| Intrauterine autologous platelet-rich plasma (PRP) (Treatment Level II) | Decrease in local inflammatory response. | No significant effects. | [346] |
| Improved live pregnancies in RIF patients. | [347,348] | ||
| PRP therapy was superior to G-CSF infusion. | [349] | ||
| Intralipid/Intravenous lipid emulsions (treatment Level II) | Suppression of NK cytotoxic function [344,345] and probably T CD8 cells. | Increased pregnancy rate in previously failed IVF. | [350,352,355] |
| No effect on pregnancy rate. | [353,354,356] | ||
| Effective in patients with high Th1 in endometrial biopsy. | [357] | ||
| No effect in patients with high endometrial NK cells | [359] | ||
| Omega-3 fatty acid oral supplementation (Treatment Level II) | Decreases peroxide formation—generation of resolvins to decrease the inflammatory response. | Positive effect in antiphospholipid syndrome RPL patients with conventional treatment. | [360,361,362] |
| Low molecular weight heparin (LMWH). (Treatment Level IV) | Decreases thrombotic risk in patients with antiphospholipid syndrome. Used as a guideline for antiphospholipid patients [357,358]. | Increased live birth rate in RPL patients with persistent antiphospholipid antibodies. | [365,366,369,370,371] |
| Increased live birth rates in patients with thrombophilia and RPL. | [367,368,379,380] | ||
| There are no significant differences in patients with inherited thrombophilia and heterogeneous pregnancy morbidity. No beneficial effects. | [372,376,377,378] | ||
| Low-dose acetylsalicylic acid. (Treatment Level IV) | A co-treatment in antiphospholipid syndrome. | Combination treatment with LMWH enhanced birth rates compared to aspirin monotherapy. | [381,382,383,384,385,386] |
| Low success rate with monotherapy | [387,388] | ||
| Vitamin D (Treatment Level II) | Deficiency in vitamin D is related to impaired immune response. Decreases the Th17 cell population | Vitamin D deficiency is observed in RPL patients. | [391] |
| Decreased vitamin D in antiphospholipid syndrome | [324,393] | ||
| Progesterone (Treatment Level I) | Decreases the inflammatory response—decreases macrophages, NKs, and T cell activation [388,389,390]. Suppresses mTOR pathway. | Increased pregnancy rate (vaginal). | [397,399] |
| No effect. | [398] | ||
| Intrauterine human chorionic gonadotropin (hCG) (Treatment Level II) | Induces tolerogenic milieu | Increased fertility rate, but not live birth rate. | [400,401] |
| Lower effect than GM-CSF | [401] | ||
| Intrauterine GCSF administration simultaneously with hCG injection may increase pregnancy outcome | [402] | ||
| Anti-obesity drugs (Treatment Level V) | Obesity decreases fertility rates. Subclinical inflammation may be responsible for reduced implantation rate and pregnancy success [403,404,405]. | Metformin increases pregnancy success in polycystic ovary syndrome patients. | [406,407,408] |
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomkiewicz, J.; Darmochwał-Kolarz, D. The Diagnostics and Treatment of Recurrent Pregnancy Loss. J. Clin. Med. 2023, 12, 4768. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Who: Recommended Definitions, Terminology, and Format for Statistical Tables Related to The Perinatal Period And Use of A New Certificate For the Cause of Perinatal Deaths. Acta Obstet. Gynecol. Scand. 1977, 56, 247–256. [CrossRef]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020, 6, 98. [Google Scholar] [CrossRef]
- Dong, P.; Wen, X.Z.; Liu, J.; Yan, C.; Yuan, J.; Luo, L.; Hu, Q.F.; Li, J. Simultaneous detection of decidual Th1/Th2 and NK1/NK2 immunophenotyping in unknown recurrent miscarriage using 8-color flow cytometry with FSC/Vt extended strategy. Biosci. Rep. 2017, 37, BSR20170150. [Google Scholar] [CrossRef]
- Kohl Schwartz, A.S.; Wölfler, M.M.; Mitter, V.; Rauchfuss, M.; Haeberlin, F.; Eberhard, M.; von Orelli, S.; Imthurn, B.; Imesch, P.; Fink, D.; et al. Endometriosis, especially mild disease: A risk factor for miscarriages. Fertil. Steril. 2017, 108, 806–814.e2. [Google Scholar] [CrossRef]
- Harb, H.M.; Ghosh, J.; Al-Rshoud, F.; Karunakaran, B.; Gallos, I.D.; Coomarasamy, A. Hydrosalpinx and pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 427–441. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Han, L.; Zhang, L.; Zu, Z.; Zhang, J. Association between semen parameters and recurrent pregnancy loss: An umbrella review of meta-analyses. J. Obstet. Gynaecol. Res. 2024, 50, 545–556. [Google Scholar] [CrossRef]
- Deshmukh, H.; Way, S.S. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu. Rev. Pathol. 2019, 14, 185–210. [Google Scholar] [CrossRef]
- Bagkou Dimakou, D.; Lissauer, D.; Tamblyn, J.; Coomarasamy, A.; Richter, A. Understanding human immunity in idiopathic recurrent pregnancy loss. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Comins Boo, A.; Segovia, A.G.; del Prado, N.N.; de la Fuente, L.; Alonso, J.; Ramon, S.S. Evidence-based Update: Immunological Evaluation of Recurrent Implantation Failure. Reprod. Immunol. Open Access. 2016, 1, 24. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Li, L.; Liu, L.; Yang, X.; Yan, P.; Yang, K.; Zhang, X. Autologous peripheral blood mononuclear cells intrauterine instillation to improve pregnancy outcomes after recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 1445–1459. [Google Scholar] [CrossRef]
- Wu, H.; You, Q.; Jiang, Y.; Mu, F. Tumor necrosis factor inhibitors as therapeutic agents for recurrent spontaneous abortion. Mol. Med. Rep. 2021, 24, 847. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Ortashi, O.; Al Khodor, S. Role of the vaginal microbiome in miscarriage: Exploring the relationship. Front. Cell. Infect. Microbiol. 2023, 13, 1232825. [Google Scholar] [CrossRef]
- Mrozikiewicz, A.E.; Ożarowski, M.; Jędrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Fan, R.; Wang, M.; Ren, C.; Jiang, A.; Yang, T. Role of inflammatory factors in the etiology and treatment of recurrent implantation failure. Reprod. Biol. 2022, 22, 100698. [Google Scholar] [CrossRef]
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2023, 13, 1061766. [Google Scholar] [CrossRef]
- Fathi, M.; Omrani, M.A.; Kadkhoda, S.; Ghahghaei-Nezamabadi, A.; Ghafouri-Fard, S. Impact of miRNAs in the pathoetiology of recurrent implantation failure. Mol. Cell Probes 2024, 74, 101955. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Tian, Y.; Cao, Y.; Wang, T.; Mi, S.; Yang, R.; Liu, S.; Ma, X.; Wang, J. Identification of Differentially Expressed mRNAs and lncRNAs Contributes to Elucidation of Underlying Pathogenesis and Therapeutic Strategy of Recurrent Implantation Failure. Reprod. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zahir, M.; Tavakoli, B.; Zaki-Dizaji, M.; Hantoushzadeh, S.; Majidi Zolbin, M. Non-coding RNAs in Recurrent implantation failure. Clin. Chim. Acta 2024, 553, 117731. [Google Scholar] [CrossRef] [PubMed]
- Colamatteo, A.; Fusco, C.; Micillo, T.; D’Hooghe, T.; de Candia, P.; Alviggi, C.; Longobardi, S.; Matarese, G. Immunobiology of pregnancy: From basic science to translational medicine. Trends Mol. Med. 2023, 29, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Hu, X.; Ying, C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms. 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms. 2024, 12, 1641. [Google Scholar] [CrossRef]
- Jia, D.; Sun, F.; Han, S.; Lu, L.; Sun, Y.; Song, Q. Adverse outcomes in subsequent pregnancies in women with a history of recurrent spontaneous abortion: A meta-analysis. J. Obstet. Gynaecol. Res. 2024, 50, 281–297. [Google Scholar] [CrossRef]
- Field, K.; Murphy, D.J. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: A retrospective cohort study. Hum. Reprod. 2015, 30, 1239–1245. [Google Scholar] [CrossRef]
- Fang, Y.; Jingjing, F.; Tiantain, C.; Huanhuan, X.; Qiaohua, H. Impact of the number of previous embryo implantation failures on IVF/ICSI-ET pregnancy outcomes in patients younger than 40 years: A retrospective cohort study. Front. Endocrinol. 2023, 14, 1243402. [Google Scholar] [CrossRef]
- Cimadomo, D.; Rienzi, L.; Conforti, A.; Forman, E.; Canosa, S.; Innocenti, F.; Poli, M.; Hynes, J.; Gemmell, L.; Vaiarelli, A.; et al. Opening the black box: Why do euploid blastocysts fail to implant? A systematic review and meta-analysis. Hum. Reprod. Update 2023, 29, 570–633. [Google Scholar] [CrossRef]
- Nitu, R.; Neamtu, R.; Lordache, O.; Stelea, L.; Dahma, G.; Sacarin, G.; Socol, G.; Boarta, A.; Silaghi, C.; Puichita, D.; et al. Cross-Sectional Analysis of Intimacy Problems, Stress Levels, and Couple Satisfaction among Women with Thrombophilia Affected by Recurrent Pregnancy Loss. Int J Environ Res Public Health. 2023, 20, 1208. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chang, S.; Kuo, P.; Chen, C. Stress, anxiety and depression perceived by couples with recurrent miscarriage. Int J Nurs Pract. 2020, 26, e12796. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021, 397, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Voss, P.; Schick, M.; Langer, L.; Ainsworth, A.; Ditzen, B.; Strowitzki, T.; Wischmann, T.; Kuon, R.J. Recurrent pregnancy loss: A shared stressor---couple-orientated psychological research findings. Fertil Steril. 2020, 114, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Williams, P.L.; Souter, I.; Ford, J.B.; Hauser, R.; Chavarro, J.E. Women’s preconception psychological stress and birth outcomes in a fertility clinic: The EARTH study. Front Glob Womens Health. 2024, 5, 1293255. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, J.B. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno. 2021, 1, 174–193. [Google Scholar] [CrossRef]
- Lanier, L.L. Five decades of natural killer cell discovery. J. Exp. Med. 2024, 221, e20231222. [Google Scholar] [CrossRef]
- Rao, V.A.; Kurian, N.K.; Rao, K.A. Cytokines, NK cells, and regulatory T cell functions in normal pregnancy and reproductive failures. Am. J. Reprod. Immunol. 2023, 89, e13667. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; da Silva, P.H.A.; Carvalho, T.R.; Sampaio, O.G.M.; Câmara, F.E.A.; Cavalcante, C.T.M.B.; Barini, R.; Kwak-Kim, J. Peripheral blood natural killer cell cytotoxicity in recurrent miscarriage: A systematic review and meta-analysis. J. Reprod. Immunol. 2023, 158, 103956. [Google Scholar] [CrossRef]
- Sacks, G.; Yang, Y.; Gowen, E.; Smith, S.; Fay, L.; Chapman, M. Detailed Analysis of Peripheral Blood Natural Killer Cells in Women with Repeated IVF Failure. Am. J. Reprod. Immunol. 2012, 67, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Y.; Tang, Y.Y.; Deng, X.H.; Li, Y.J.; Liang, G.; Meng, Y.Q.; Zhou, H. Recurrent Implantation Failure May Be Identified by a Combination of Diagnostic Biomarkers: An Analysis of Peripheral Blood Lymphocyte Subsets. Front. Endocrinol. 2024, 13, 865807. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G. Enough! Stop the arguments and get on with the science of natural killer cell testing. Hum. Reprod. 2015, 30, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Dons’koi, B.V. Accentuated hypo- and hyper-NK lymphocyte CD8 expression is a marker of NK subsets’ misbalance and is predictive for reproductive failures. Immunobiology 2015, 220, 649–655. [Google Scholar] [CrossRef]
- Dons’koi, B.V.; Chernyshov, V.P.; Sirenko, V.Y.; Strelko, G.V.; Osypchuk, D.V. Peripheral blood natural killer cells activation status determined by CD69 upregulation predicts implantation outcome in IVF. Immunobiology 2014, 219, 167–171. [Google Scholar] [CrossRef]
- Gothe, J.P.; de Mattos, A.C.; Silveira, C.F.; Malavazi, K.C. Exploring Natural Killer Cell Testing in Embryo Implantation and Reproductive Failure: An Overview of Techniques and Controversies. Reprod. Sci. 2024, 31, 603–632. [Google Scholar] [CrossRef]
- Zhang, J.; Lye, S.J. The immune potential of decidua-resident CD16+CD56+ NK cells in human pregnancy. Hum. Immunol. 2021, 82, 332–339. [Google Scholar] [CrossRef]
- Salazar, M.D.; Wang, W.J.; Skariah, A.; He, Q.; Field, K.; Nixon, M.; Reed, R.; Dambaeva, S.; Beaman, K.; Gilman-Sachs, A.; et al. Post-hoc evaluation of peripheral blood natural killer cell cytotoxicity in predicting the risk of recurrent pregnancy losses and repeated implantation failures. J. Reprod. Immunol. 2022, 150, 103487. [Google Scholar] [CrossRef]
- Singh, N.; Dogra, Y.; Kumar, P.; Mathur, S.; Sharma, A.; Patel, G. Establishment of Cut-off Values for Uterine and Peripheral Blood Natural Killer Cells During the Peri-implantation Period in Fertile Controls and Women with Unexplained Recurrent Implantation Failure. J. Reprod. Infert. 2023, 24, 248–256. [Google Scholar] [CrossRef]
- Santillán, I.; Fernández Lozano, I.; Illán, J.; Verdú, V.; Coca, S.; Bajo-Arenas, J.; Martinez, F. Where and when should natural killer cells be tested in women with repeated implantation failure? J. Reprod. Immunol. 2015, 108, 142–148. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Bagkou Dimakou, D.; Tamblyn, J.; Justin, C.; Coomarasamy, A.; Richter, A. Diagnosis and management of idiopathic recurrent pregnancy loss (RPL): Current immune testing and immunomodulatory treatment practice in the United Kingdom. J. Reprod. Immunol. 2022, 153, 103662. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, M.; Miron, P.; Hemmings, R.; Roy, D. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J. Immunol. 1996, 156, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Chen, H.H.; Huang, C.C.; Lee, C.I.; Lin, P.Y.; Lee, M.S.; Lee, T.H. Peripheral CD56+CD16+ NK Cell Populations in the Early Follicular Phase Are Associated With Successful Clinical Outcomes of Intravenous Immunoglobulin Treatment in Women With Repeated Implantation Failure. Front. Endocrinol. 2020, 10, 937. [Google Scholar] [CrossRef]
- Fukui, A.; Fujii, S.; Yamaguchi, E.; Kimura, H.; Sato, S.; Saito, Y. Natural Killer Cell Subpopulations and Cytotoxicity for Infertile Patients Undergoing In Vitro Fertilization. Am. J. Reprod. Immunol. 1999, 41, 413–422. [Google Scholar] [CrossRef]
- Strobel, L.; Vomstein, K.; Kyvelidou, C.; Hofer-Tollinger, S.; Feil, K.; Kuon, R.J.; Ebner, S.; Troppmair, J.; Toth, B. Different Background: Natural Killer Cell Profiles in Secondary versus Primary Recurrent Pregnancy Loss. J. Clin. Med. 2021, 10, 194. [Google Scholar] [CrossRef]
- Fukui, A.; Kwak-Kim, J.; Ntrivalas, E.; Gilman-Sachs, A.; Lee, S.K.; Beaman, K. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil. Steril. 2008, 89, 157–165. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; de Los Santos, M.J.; Lucia, A.; Castro-Santos, P. Understanding the role of killer cell immunoglobulin-like receptors in pregnancy complications. J. Assist. Reprod. Genet. 2019, 36, 827–835. [Google Scholar] [CrossRef]
- Lin, Q.D.; Qiu, L.H. Pathogenesis, diagnosis, and treatment of recurrent spontaneous abortion with immune type. Front. Med. China 2010, 4, 275–279. [Google Scholar] [CrossRef]
- Dambaeva, S.V.; Lee, D.H.; Sung, N.; Chen, C.Y.; Bao, S.; Gilman-Sachs, A.; Kwak-Kim, J.; Beaman, K.D. Recurrent Pregnancy Loss in Women with Killer Cell Immunoglobulin-Like Receptor KIR2DS1 is Associated with an Increased HLA-C2 Allelic Frequency. Am. J. Reprod. Immunol. 2016, 75, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Shahsavar, F.; Karami, R.; Yari, F.; Anbari, K.; Ahmadi, S.A.Y. Recurrent Spontaneous Abortion (RPL) and Maternal KIR Genes: A Comprehensive Meta-Analysis. JBRA Assist. Reprod. 2020, 24, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, E.; Wang, W.; He, Q.; Jubiz, G.; Katukurundage, D.; Dambaeva, S.; Beaman, K.D.; Kwak-Kim, J. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J. Reprod. Immunol. 2020, 142, 103186. [Google Scholar] [CrossRef]
- Feyaerts, D.; Benner, M.; Comitini, G.; Shadmanfar, W.; van der Heijden, O.W.H.; Joosten, I.; van der Molen, R.G. NK cell receptor profiling of endometrial and decidual NK cells reveals pregnancy-induced adaptations. Front. Immunol. 2024, 15, 1353556. [Google Scholar] [CrossRef] [PubMed]
- Maftei, R.; Doroftei, B.; Popa, R.; Harabor, V.; Adam, A.M.; Popa, C.; Harabor, A.; Adam, G.; Nechita, A.; Vasilache, I.A.; et al. The Influence of Maternal KIR Haplotype on the Reproductive Outcomes after Single Embryo Transfer in IVF Cycles in Patients with Recurrent Pregnancy Loss and Implantation Failure—A Single Center Experience. J. Clin. Med. 2023, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Wilczyńska, K.; Wilczyński, J.R.; Malinowski, A.; Radwan, P.; Radwan, M.; Kuśnierczyk, P. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch. Immunol. Ther. Exp. 2017, 65, 391–399. [Google Scholar] [CrossRef]
- Braun, A.S.; Vomstein, K.; Reiser, E.; Tollinger, S.; Kyvelidou, C.; Feil, K.; Toth, B. NK and T Cell Subtypes in the Endometrium of Patients with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Implications for Pregnancy Success. J. Clin. Med. 2023, 12, 5585. [Google Scholar] [CrossRef]
- Morin, S.J.; Treff, N.R.; Tao, X.; Scott, R.T., 3rd; Franasiak, J.M.; Juneau, C.R.; Maguire, M.; Scott, R.T. Combination of uterine natural killer cell immunoglobulin receptor haplotype and trophoblastic HLA-C ligand influences the risk of pregnancy loss: A retrospective cohort analysis of direct embryo genotyping data from euploid transfers. Fertil. Steril. 2017, 107, 677–683.e2. [Google Scholar] [CrossRef]
- Khalaf, W.S.; Mahmoud, M.R.A.; Elkhatib, W.F.; Hashem, H.R.; Soliman, W.E. Phenotypic characterization of NKT-like cells and evaluation of specifically related cytokines for the prediction of unexplained recurrent miscarriage. Heliyon 2021, 7, e08409. [Google Scholar] [CrossRef]
- Xu, Q.H.; Liu, H.; Wang, L.L.; Zhu, Q.; Zhang, Y.J.; Muyayalo, K.P.; Liao, A.H. Roles of γδT cells in pregnancy and pregnancy-related complications. Am. J. Reprod. Immunol. 2021, 86, e13487. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Zhou, W.; Yang, C.; Feng, T.; Li, H. Human chorionic gonadotrophin indirectly activates peripheral γδT cells to produce interleukin-10 during early pregnancy. Immun. Inflamm. Dis. 2024, 12, e1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Y.; Duan, T.; Zhou, Q. The role of macrophages in reproductive-related diseases. Heliyon 2022, 8, e11686. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, T.; Schust, D.J. The Contribution of Macrophages to Normal and Pathological Pregnancies. Am. J. Reprod. Immunol. 2010, 63, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho, H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Moldenhauer, L.M.; Green, E.S.; Care, A.S.; Hull, M.L. Immune determinants of endometrial receptivity: A biological perspective. Fertil. Steril. 2022, 117, 1107–1120. [Google Scholar] [CrossRef]
- Wang, W.J.; Hao, C.F.; Lin, Q.D. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J. Reprod. Immunol. 2011, 92, 97–102. [Google Scholar] [CrossRef]
- Quenby, S.; Bates, M.; Doig, T.; Brewster, J.; Lewis-Jones, D.I.; Johnson, P.M.; Vince, G. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum. Reprod. 1999, 14, 2386–2391. [Google Scholar] [CrossRef]
- Krop, J.; Tian, X.; van der Hoorn, M.L.; Eikmans, M. The Mac Is Back: The Role of Macrophages in Human Healthy and Complicated Pregnancies. Int. J. Mol. Sci. 2023, 24, 5300. [Google Scholar] [CrossRef]
- Tremellen, K.P.; Russell, P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: Adenomyosis and macrophages. J. Reprod. Immunol. 2012, 93, 58–63. [Google Scholar]
- Wei, R.; Lai, N.; Zhao, L.; Zhang, Z.; Zhu, X.; Guo, Q.; Chu, C.; Fu, X.; Li, X. Dendritic cells in pregnancy and pregnancy-associated diseases. Biomed. Pharmacother. 2021, 133, 110921. [Google Scholar] [CrossRef]
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod. Med. Biol. 2024, 23, e12600. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, H.; Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4+dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Yin, X.Q.; Hei, G.Z.; Wei, R.; Guo, Q.; Zhao, L.; Zhang, Z.; Chu, C.; Fu, X.X.; Xu, K.; et al. Increased miR-6875-5p inhibits plasmacytoid dendritic cell differentiation via the STAT3/E2-2 pathway in recurrent spontaneous abortion. Mol. Hum. Reprod. 2021, 27, gaab044. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Chen, X.; Diao, L.; Lian, R.; Zhang, X.; Hu, L.; Zeng, Y. Association of peripheral blood dendritic cells with recurrent pregnancy loss: A case-controlled study. Am. J. Reprod. Immunol. 2016, 76, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, M.; Gęca, T.; Krzyżanowski, A.; Malec, A.; Kwaśniewska, A. Peripheral Dendritic Cells and CD4+CD25+Foxp3+ Regulatory T Cells in the First Trimester of Normal Pregnancy and in Women with Recurrent Miscarriage. PLoS ONE. 2015, 10, e0124747. [Google Scholar] [CrossRef]
- Sivridis, E.; Giatromanolaki, A.; Agnantis, N.; Anastasiadis, P. Mast cell distribution and density in the normal uterus--metachromatic staining using lectins. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 109–113. [Google Scholar] [CrossRef]
- Norrby, K. On Connective Tissue Mast Cells as Protectors of Life, Reproduction, and Progeny. Int. J. Mol. Sci. 2024, 25, 4499. [Google Scholar] [CrossRef]
- Lampiasi, N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. Int. J. Mol. Sci. 2022, 23, 5414. [Google Scholar] [CrossRef]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed, R.; Salazar Garcia, M.D.; Skariah, A.; Dambaeva, S.; Fernandez, E.; Germain, A.; Gilman-Sachs, A.; et al. Mast cell-induced immunopathology in recurrent pregnancy losses. Am J Reprod Immunol. 2019, 82, e13128. [Google Scholar] [CrossRef]
- McCallion, A.; Nasirzadeh, Y.; Lingegowda, H.; Miller, J.E.; Khalaj, K.; Ahn, S.; Monsanto, S.P.; Bidarimath, M.; Sisnett, D.J.; Craig, A.W.; et al. Estrogen mediates inflammatory role of mast cells in endometriosis pathophysiology. Front Immunol. 2022, 13, 961599. [Google Scholar] [CrossRef]
- Dunn, T.N.; Cope, D.I.; Tang, S.; Sirupangi, T.; Parks, S.E.; Liao, Z.; Yuan, F.; Creighton, C.J.; Masand, R.P.; Alpuing Radilla, L.; et al. Inhibition of CSF1R and KIT With Pexidartinib Reduces Inflammatory Signaling and Cell Viability in Endometriosis. Endocrinology 2024, 165, bqae003. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, R.D.; Samoszuk, M.; Taylor, A.P.; Brown, G.; Alisauskas, R.; Goldenberg, D.M. Degranulating eosinophils in human endometriosis. Am. J. Pathol. 2020, 156, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Hornung, D.; Dohrn, K.; Sotlar, K.; Greb, R.R.; Wallwiener, D.; Kiesel, L.; Taylor, R.N. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2604–2608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naseri, S.; Rosenberg-Hasson, Y.; Maecker, H.T.; Avrutsky, M.I.; Blumenthal, P.D. A cross-sectional study comparing the inflammatory profile of menstrual effluent vs. peripheral blood. Health Sci. Rep. 2023, 6, e1038. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Li, D.; Guo, X.; Zhou, Z.; Qi, M.; Wang, G.; Wang, F. The Abundance and Function of Neutrophils in the Endometriosis Systemic and Pelvic Microenvironment. Mediat. Inflamm. 2023, 2023, 1481489. [Google Scholar] [CrossRef]
- Hebeda, C.B.; Savioli, A.C.; Scharf, P.; de Paula-Silva, M.; Gil, C.D.; Farsky, S.H.P.; Sandri, S. Neutrophil depletion in the pre-implantation phase impairs pregnancy index, placenta and fetus development. Front. Immunol. 2022, 13, 969336. [Google Scholar] [CrossRef]
- Ghafourian, M.; Abuhamidy, A.; Karami, N. Increase of peripheral blood TCD8+cells in women with recurrent miscarriage. J. Obstet. Gynaecol. 2013, 34, 36–39. [Google Scholar] [CrossRef]
- Morita, K.; Tsuda, S.; Kobayashi, E.; Hamana, H.; Tsuda, K.; Shima, T.; Nakashima, A.; Ushijima, A.; Kishi, H.; Saito, S. Analysis of TCR Repertoire and PD-1 Expression in Decidual and Peripheral CD8+ T Cells Reveals Distinct Immune Mechanisms in Miscarriage and Preeclampsia. Front. Immunol. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Carbone, J.; Sarmiento, E.; Gallego, A.; Lanio, N.; Navarro, J.; Garcia, S.; Fernández-Cruz, E. Peripheral blood T- and B-cell immunophenotypic abnormalities in selected women with unexplained recurrent miscarriage. J. Reprod. Immunol. 2016, 113, 50–53. [Google Scholar] [CrossRef]
- Huang, C.; Xiang, Z.; Zhang, Y.; Li, Y.; Xu, J.; Zhang, H.; Zeng, Y.; Tu, W. NKG2D as a Cell Surface Marker on γδ-T Cells for Predicting Pregnancy Outcomes in Patients With Unexplained Repeated Implantation Failure. Front. Immunol. 2021, 12, 631077. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Wang, L.; Yan, S.; Chen, S.; Xu, Q.; Su, D.; Wang, X. Identification and validation of immune cells and hub genes alterations in recurrent implantation failure: A GEO data mining study. Front. Genet. 2023, 13, 1094978. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Z.; Hong, Y.; Zhao, A.; Qiu, L.; Lu, P.; Lin, Q. The Skewed TCR-BV Repertoire Displayed at the Maternal-Fetal Interface of Women with Unexplained Pregnancy Loss. Am. J. Reprod. Immunol. 2005, 54, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qiu, L.; Chen, G.; Ye, Z.; Lü, C.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil. Steril. 2008, 89, 656–661. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhao, Q.Y.; Yang, W.J.; Jiang, A.F.; Ren, C.E.; Meng, Y.H. Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion. J. Inflamm. Res. 2024, 17, 2697–2710. [Google Scholar] [CrossRef]
- Wang, W.J.; Hao, C.F.; Qu, Q.L.; Wang, X.; Qiu, L.H.; Lin, Q.D. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum. Reprod. 2010, 25, 2591–2596. [Google Scholar] [CrossRef]
- Garmendia, J.V.; Blanca, I.; Peña, M.J.; De Sanctis, C.V.; De Sanctis, J.B. Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss. Immuno 2024, 4, 301–311. [Google Scholar] [CrossRef]
- Heitmann, R.J.; Weitzel, R.P.; Feng, Y.; Segars, J.H.; Tisdale, J.F.; Wolff, E.F. Maternal T Regulatory Cell Depletion Impairs Embryo Implantation Which Can Be Corrected With Adoptive T Regulatory Cell Transfer. Reprod. Sci. 2017, 24, 1014–1024. [Google Scholar] [CrossRef]
- Granne, I.; Shen, M.; Rodriguez-Caro, H.; Chadha, G.; O’Donnell, E.; Brosens, J.J.; Quenby, S.; Child, T.; Southcombe, J.H. Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss. Mucosal Immunol. 2022, 15, 120–129. [Google Scholar] [CrossRef]
- Moldenhauer, L.M.; Foyle, K.L.; Wilson, J.J.; Wong, Y.Y.; Sharkey, D.J.; Green, E.S.; Barry, S.C.; Hull, M.L.; Robertson, S.A. A disrupted FOXP3 transcriptional signature underpins systemic regulatory T cell insufficiency in early pregnancy failure. iScience. 2024, 27, 108994. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L. Low Circulating CD4+ CD25+ Foxp3+ T Regulatory Cell Levels Predict Miscarriage Risk in Newly Pregnant Women with a History of Failure. Am. J. Reprod. Immunol. 2011, 66, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.P.; Chen, Q.Y.; Zhang, T.; Guo, P.F.; Li, D.J. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin. Immunol. 2009, 133, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hu, W. The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms. Placenta 2023, 142, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; Fallahi, S.; Rostami, S.; Rostami, P.; Rafatpanah, H.; Habibagahi, M. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J. Clin. Transl. Res. 2022, 8, 256–265. [Google Scholar] [PubMed]
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Vergadi, E.; Makrygiannakis, F.; Vrekoussis, T.; Makrigiannakis, A. Repeated implantation failure is associated with increased Th17/Treg cell ratio, during the secretory phase of the human endometrium. J. Reprod. Immunol. 2024, 161, 104170. [Google Scholar] [CrossRef]
- Niafar, M.; Samaie, V.; Soltani-Zangbar, M.S.; Motavalli, R.; Dolati, S.; Danaii, S.; Mehdizadeh, A.; Yousefi, M. The association of Treg and Th17 cells development factors and anti-TPO autoantibodies in patients with recurrent pregnancy loss. BMC Res. Notes 2023, 16, 302. [Google Scholar] [CrossRef]
- Wang, W.J.; Salazar Garcia, M.D.; Deutsch, G.; Sung, N.; Yang, X.; He, Q.; Jubiz, G.; Bilal, M.; Dambaeva, S.; Gilman-Sachs, A.; et al. PD-1 and PD-L1 expression on T-cell subsets in women with unexplained recurrent pregnancy losses. Am. J. Reprod. Immunol. 2020, 83, e13230. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef]
- Muzzio, D.; Zenclussen, A.C.; Jensen, F. The Role of B Cells in Pregnancy: The Good and the Bad. Am. J. Reprod. Immunol. 2013, 69, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Eblen, A.C.; Gercel-Taylor, C.; Shields, L.B.E.; Sanfilippo, J.S.; Nakajima, S.T.; Taylor, D.D. Alterations in humoral immune responses associated with recurrent pregnancy loss. Fertil. Steril. 2000, 73, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Marron, K.; Walsh, D.; Harrity, C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J. Assist. Reprod. Genet. 2019, 36, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Vujisić, S.; Lepej, S.Ž.; Akšamija, A.; Jerković, L.; Sokolić, B.; Kupešić, S.; Vince, A. B- and T-cells in the Follicular Fluid and Peripheral Blood of Patients Undergoing IVF/ET Procedures. Am. J. Reprod. Immunol. 2004, 52, 379–385. [Google Scholar] [CrossRef]
- Liu, J.C.; Zeng, Q.; Duan, Y.G.; Yeung, W.S.B.; Li, R.H.W.; Ng, E.H.Y.; Cheung, K.W.; Zhang, Q.; Chiu, P.C.N. B cells: Roles in physiology and pathology of pregnancy. Front. Immunol. 2024, 15, 1456171. [Google Scholar] [CrossRef]
- Danaii, S.; Ghorbani, F.; Ahmadi, M.; Abbaszadeh, H.; Koushaeian, L.; Soltani-Zangbar, M.S.; Mehdizadeh, A.; Hojjat-Farsangi, M.; Kafil, H.S.; Aghebati-Maleki, L.; et al. IL-10-producing B cells play important role in the pathogenesis of recurrent pregnancy loss. Int. Immunopharmacol. 2020, 87, 106806. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Figley, C.; Long, R.; Park, D.; Carter, D.; Clements, V.K. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal–fetal tolerance in mice. J. Leukoc. Biol. 2016, 101, 1091–1101. [Google Scholar] [CrossRef]
- Köstlin, N.; Hofstädter, K.; Ostermeir, A.L.; Spring, B.; Leiber, A.; Haen, S.; Abele, H.; Bauer, P.; Pollheimer, J.; Hartl, D.; et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J. Immunol. 2016, 196, 1132–1145. [Google Scholar] [CrossRef]
- Bartmann, C.; Junker, M.; Segerer, S.E.; Häusler, S.F.; Krockenberger, M.; Kämmerer, U. CD33+/HLA-DRnegand CD33+/HLA-DR+/−Cells: Rare Populations in the Human Decidua with Characteristics of MDSC. Am. J. Reprod. Immunol. 2016, 75, 539–556. [Google Scholar] [CrossRef]
- Pan, T.; Zhong, L.; Wu, S.; Cao, Y.; Yang, Q.; Cai, Z.; Cai, X.; Zhao, W.; Ma, N.; Zhang, W.; et al. 17β-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy. Clin. Exp. Immunol. 2016, 185, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Kang, X.; Chen, C.; Guo, F.; Wang, Q.; Zhao, A. Upregulated TRAIL and Reduced DcR2 Mediate Apoptosis of Decidual PMN-MDSC in Unexplained Recurrent Pregnancy Loss. Front. Immunol. 2020, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhu, M.; Guo, P.; Bi, K.; Lu, Z.; Li, C.; Zhai, M.; Wang, K.; Cao, Y. Impaired myeloid-derived suppressor cells are associated with recurrent implantation failure: A case-control study. J. Reprod. Immunol. 2021, 145, 103316. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.S.; Fuente-Muñoz, E.; Gil-Laborda, R.; Villegas, Á.; Alonso-Arenilla, B.; Cristóbal, I.; Pilar-Suárez, L.; Jiménez-Huete, A.; Calvo, M.; Sarria, B.; et al. Myeloid-derived suppressor cells as a potential biomarker for recurrent pregnancy loss and recurrent implantation failure. Am. J. Reprod. Immunol. 2023, 90, e13783. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, M.; Li, X.; Huang, H.; Sun, Y. Gene profiling reveals the role of inflammation, abnormal uterine muscle contraction and vascularity in recurrent implantation failure. Front. Genet. 2023, 14, 1108805. [Google Scholar] [CrossRef]
- Kalu, E.; Bhaskaran, S.; Thum, M.Y.; Vishwanatha, R.; Croucher, C.; Sherriff, E.; Ford, B.; Bansal, A.S. Serial Estimation of Th1:Th2 Cytokines Profile in Women Undergoing In-Vitro Fertilization-Embryo Transfer. Am. J. Reprod. Immunol. 2008, 59, 206–211. [Google Scholar] [CrossRef]
- Piekarska, K.; Dratwa, M.; Radwan, P.; Radwan, M.; Bogunia-Kubik, K.; Nowak, I. Pro- and anti-inflammatory cytokines and growth factors in patients undergoing in vitro fertilization procedure treated with prednisone. Front. Immunol. 2023, 14, 1250488. [Google Scholar] [CrossRef]
- Mukherjee, N.; Sharma, R.; Modi, D. Immune alterations in recurrent implantation failure. Am. J. Reprod. Immunol. 2022, 89, e13563. [Google Scholar] [CrossRef]
- Guo, L.; Guo, A.; Yang, F.; Li, L.; Yan, J.; Deng, X.; Dai, C.; Li, Y. Alterations of Cytokine Profiles in Patients With Recurrent Implantation Failure. Front. Endocrinol. 2022, 13, 949123. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2023, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Kwak-Kim, J.Y.H.; Chung-Bang, H.; Ng, S.; Ntrivalas, E.; Mangubat, C.; Beaman, K.; Beer, A.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sereshki, N.; Gharagozloo, M.; Ostadi, V.; Ghahiri, A.; Roghaei, M.; Mehrabian, F.; Andalib, A.; Hassanzadeh, A.; Hosseini, H.; Rezaei, A.A. Variations in T-helper 17 and Regulatory T Cells during The Menstrual Cycle in Peripheral Blood of Women with Recurrent Spontaneous Abortion. Int. J. Fertil. Steril. 2014, 8, 59–66. [Google Scholar] [PubMed]
- Inagaki, N.; Stern, C.; McBain, J.; Lopata, A.; Kornman, L.; Wilkinson, D. Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum. Reprod. 2003, 18, 608–615. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, H.; Chen, Z.Q.; Zhang, W.; Liu, X.M.; Fang, J.Y.; Liu, F.J.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod. Biol. Endocrinol. 2019, 17, 2. [Google Scholar] [CrossRef]
- Sheikhansari, G.; Soltani-Zangbar, M.S.; Pourmoghadam, Z.; Kamrani, A.; Azizi, R.; Aghebati-Maleki, L.; Danaii, S.; Koushaeian, L.; Hojat-Farsangi, M.; Yousefi, M. Oxidative stress, inflammatory settings, and microRNA regulation in the recurrent implantation failure patients with metabolic syndrome. Am. J. Reprod. Immunol. 2019, 82, e13170. [Google Scholar] [CrossRef]
- O’Hern Perfetto, C.; Fan, X.; Dahl, S.; Krieg, S.A.; Westphal, L.M.; Lathi, R.B.; Nayak, N.R. Expression of interleukin-22 in decidua of patients with early pregnancy and unexplained recurrent pregnancy loss. J. Assist. Reprod. Genet. 2015, 32, 977–984. [Google Scholar] [CrossRef]
- Wang, W.J.; Liu, F.J.; Qu, H.M.; Hao, C.F.; Qu, Q.L.; Bao, H.C.; Wang, X.R. Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J. Reprod. Immunol. 2013, 99, 39–45. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Ye, S.; Liu, Y.; Zhao, X.; Wang, Y. Association of IL-17 and IL-27 polymorphisms with susceptibility to recurrent pregnancy loss and pre-eclampsia: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e1057. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, J.; Ding, F.; Liu, J.; Li, L.; Song, Q.; Fu, Y. IL-33 and Soluble ST2 Are Associated With Recurrent Spontaneous Abortion in Early Pregnancy. Front. Physiol. 2021, 12, 789829. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, B.; Ying, C. Elevated Serum Level of IL-35 Associated with the Maintenance of Maternal-Fetal Immune Tolerance in Normal Pregnancy. PLoS ONE. 2015, 10, e0128219. [Google Scholar] [CrossRef] [PubMed]
- Karaer, A.; Cigremis, Y.; Celik, E.; Urhan Gonullu, R. Prokineticin 1 and leukemia inhibitory factor mRNA expression in the endometrium of women with idiopathic recurrent pregnancy loss. Fertil. Steril 2014, 102, 1091–1095.e1. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Al-Mutawa, E.; Al-Azemi, M.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J. Reprod. Immunol. 2009, 80, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Begum, A.; Ray Das, C.; Datta, R.; Verma, M.K.; Dongre, A.; Husain, S.A.; Ahmad Khan, L.; Deka Bose, P. Aberrations in the progesterone pathway and the Th1/Th2 cytokine dichotomy—An evaluation of RPL predisposition in the northeast Indian population. Am. Reprod. Immunol. 2023, 90, e13745. [Google Scholar] [CrossRef]
- Amjadi, F.; Zandieh, Z.; Mehdizadeh, M.; Aghajanpour, S.; Raoufi, E.; Aghamajidi, A.; Aflatoonian, R. The uterine immunological changes may be responsible for repeated implantation failure. J. Reprod. Immunol. 2020, 138, 103080. [Google Scholar] [CrossRef]
- Laitinen, T. A Set of MHC Haplotypes Found Among Finnish Couples Suffering From Recurrent Spontaneous Abortions. Am. J. Reprod. Immunol. 1993, 29, 148–154. [Google Scholar] [CrossRef]
- Hsiao, T.W.; Chung, M.T.; Wen, J.Y.; Lin, Y.; Lin, L.Y.; Tsai, Y. HLA sharing and maternal HLA expression in couples with recurrent pregnancy loss in Taiwan. Taiwan J. Obstet. Gynecol. 2022, 61, 854–857. [Google Scholar] [CrossRef]
- Gharesi-Fard, B.; Askarinejad-Behbahani, R.; Behdin, S. The effect of HLA-DRB1 sharing between the couples with recurrent pregnancy loss on the pregnancy outcome after leukocyte therapy. Iran. J. Immunol. 2014, 11, 13–20. [Google Scholar]
- Wang, X.P.; Lin, Q.; Peng, L.; Ma, Z.; Zhao, A. Association of HLA-DQB1 coding region with unexplained recurrent spontaneous abortion. Chin. Med. J. 2004, 117, 492–497. [Google Scholar]
- Ho, H.N.; Yang, Y.S.; Hsieh, R.P.; Lin, H.R.; Chen, S.; Huang, S.; Lee, T.Y.; Gill, T.J. Sharing of human leukocyte antigens in couples with unexplained infertility affects the success of in vitro fertilization and tubal embryo transfer. Am. J. Obstet. Gynecol. 1994, 170, 63–71. [Google Scholar] [CrossRef]
- Weckstein, L.N.; Patrizio, P.; Balmaceda, J.P.; Asch, R.H.; Branch, D.W. Human leukocyte antigen compatibility and failure to achieve a viable pregnancy with assisted reproductive technology. Acta Eur. Fertil. 1991, 22, 103–107. [Google Scholar] [PubMed]
- Balasch, J.; Jové, I.; Martorell, J.; Gayà, A.; Vanrell, J.A. Histocompatibility in in vitro fertilization couples. Fertil Steril. 1993, 59, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Apps, R.; Sharkey, A.M.; Farrell, L.E.; Gardner, L.; Mulder, A.; Claas, F.H.; Walker, J.J.; Redman, C.W.; Morgan, L.; et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Investig. 2010, 120, 4102–4110. [Google Scholar] [CrossRef]
- Yang, X.; Meng, T. Killer-cell immunoglobulin-like receptor/human leukocyte antigen-C combination and ‘great obstetrical syndromes’ (Review). Exp Ther Med. 2021, 22, 1178. [Google Scholar] [CrossRef]
- Gil Laborda, R.; de Frías, E.R.; Subhi-Issa, N.; de Albornoz, E.C.; Meliá, E.; Órdenes, M.; Verdú, V.; Vidal, J.; Suárez, E.; Santillán, I.; et al. Centromeric AA motif in KIR as an optimal surrogate marker for precision definition of alloimmune reproductive failure. Sci. Rep. 2024, 14, 3354. [Google Scholar] [CrossRef]
- Dahl, M.; Djurisic, S.; Hviid, T.V. The many faces of human leukocyte antigen-G: Relevance to the fate of pregnancy. J. Immunol. Res. 2014, 2014, 591489. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Z.; Li, S.; Xiao, Z. The HLA-G 14-bp polymorphism and recurrent implantation failure: A meta-analysis. J. Assist. Reprod. Genet. 2017, 34, 1559–1565. [Google Scholar] [CrossRef]
- Hu, L.; He, D.; Zeng, H. Association of parental HLA-G polymorphisms with soluble HLA-G expressions and their roles on recurrent implantation failure: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 988370. [Google Scholar] [CrossRef]
- Nowak, I.; Wilczyńska, K.; Radwan, P.; Wiśniewski, A.; Krasiński, R.; Radwan, M.; Wilczyński, J.R.; Malinowski, A.; Kuśnierczyk, P. Association of Soluble HLA-G Plasma Level and HLA-G Genetic Polymorphism With Pregnancy Outcome of Patients Undergoing in vitro Fertilization Embryo Transfer. Front. Immunol. 2020, 10, 2982. [Google Scholar] [CrossRef]
- Zych, M.; Roszczyk, A.; Kniotek, M.; Dąbrowski, F.; Zagożdżon, R. Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages-Preliminary Studies. J. Clin. Med. 2021, 10, 4182. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Roszczyk, A.; Dąbrowski, F.; Kniotek, M.; Zagożdżon, R. Soluble Forms of Immune Checkpoints and Their Ligands as Potential Biomarkers in the Diagnosis of Recurrent Pregnancy Loss-A Preliminary Study. Int. J. Mol. Sci. 2023, 25, 499. [Google Scholar] [CrossRef] [PubMed]
- Esparvarinha, M.; Madadi, S.; Aslanian-Kalkhoran, L.; Nickho, H.; Dolati, S.; Pia, H.; Danaii, S.; Taghavi, S.; Yousefi, M. Dominant immune cells in pregnancy and pregnancy complications: T helper cells (TH1/TH2, TH17/Treg cells), NK cells, MDSCs, and the immune checkpoints. Cell Biol. Int. 2023, 47, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Pan, C.; Liu, J.; Wu, L.; Pan, J.; Liu, C.; Zhang, H. Differential expression of immune checkpoints (OX40/OX40L and PD-1/PD-L1) in decidua of unexplained recurrent spontaneous abortion women. Hum. Immunol. 2024, 85, 110745. [Google Scholar] [CrossRef]
- Zych, M.; Kniotek, M.; Roszczyk, A.; Dąbrowski, F.; Jędra, R.; Zagożdżon, R. Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 9378. [Google Scholar] [CrossRef]
- Opatrny, L.; David, M.; Kahn, S.R.; Shrier, I.; Rey, E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: A metaanalysis. J. Rheumatol. 2006, 33, 2214–2221. [Google Scholar]
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Metaanalysis of evidence. BMJ 2011, 342, 1–8. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Cavalgante, C.T.; Sarno, M.; Da Silva, A.; Barini, R. Antinuclear antibodies and recurrent miscarriage: Systematic review and meta-analysis. Am. J. Reprod. Immunol. 2020, 83, 13215. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Wu, P.; Sun, Y.; Dai, F.; He, Y.; Qian, H.; Liu, Y.; Shi, G. Antinuclear antibodies positivity is a risk factor of recurrent pregnancy loss: A meta-analysis. Semin. Arthritis Rheum. 2020, 50, 534–543. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurba, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 40–53. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Romero, R.; Feinberg, R.F.; Clyne, L.P.; Coster, B.; Hobbins, J.C. The prevalence and biologic significance of lupus anticoagulant and antic ardiolipin antibodies in a general obstetric population. Am. J. Obstet. Gynecol. 1989, 161, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.M.; Kwak, J.Y.H.; Beer, A.E.; Kim, J.H.; Nelson, L.A.; Beaman, K.D.; Gilman-Sachs, A. Antibodies to phospholipids and nuclear antigens in non-pregnant women with unexplained spontaneous recurrent abortions. J. Reprod. Immunol. 1993, 24, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.H.; Beer, A.E.; Cubillos, J.; Muñoz Sandoval, P.; Mendoza, J.; Espinel, F. Biological Basis of Fetoplacental Antigenic Determinants in the Induction of the Antiphospholipid Antibody Syndrome and Recurrent Pregnancy Loss. Ann. N. Y. Acad. Sci. 1994, 731, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.S.; Regan, L.; Clifford, K.; Pickering, W.; Dave, M.; Mackie, I.; McNally, T.; Cohen, H. Immunology: Antiphospholipid antibodies and β2-glycoprotein-I in 500 women with recurrent miscarriage: Results of a comprehensive screening approach. Hum. Reprod. 1995, 10, 2001–2005. [Google Scholar] [CrossRef]
- Del Porto, F.; Ferrero, S.; Cifani, N.; Sesti, G.; Proietta, M. Antiphospholipid antibodies and idiopathic infertility. Lupus 2022, 31, 347–353. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Ticconi, C.; Tersigni, C.; Garofalo, S.; Martino, C.; Lanzone, A.; Scambia, G.; Di Simone, N. The pathogenic role of autoantibodies in recurrent pregnancy loss. Am. J. Reprod. Immunol. 2019, 83, e13200. [Google Scholar] [CrossRef]
- Gibbins, K.J.; Mumford, S.L.; Sjaarda, L.A.; Branch, D.W.; Perkins, N.J.; Ye, A.; Schisterman, E.F.; Silver, R.M. Preconception antiphospholipid antibodies and risk of subsequent early pregnancy loss. Lupus 2018, 27, 1437–1445. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Boutzios, G.; Mathioudakis, A.G.; Vlahos, N.F.; Vlachoyiannopoulos, P.; Mastorakos, G. Presence of antiphospholipid antibodies is associated with increased implantation failure following in vitro fertilization technique and embryo transfer: A systematic review and meta-analysis. PLoS ONE. 2022, 17, e0260759. [Google Scholar] [CrossRef]
- Jarne-Borràs, M.; Miró-Mur, F.; Anunciación-Llunell, A.; Alijotas-Reig, J. Antiphospholipid antibodies in women with recurrent embryo implantation failure: A systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 103101. [Google Scholar] [CrossRef]
- Tan, X.F.; Xu, L.; Li, T.T.; Wu, Y.T.; Ma, W.W.; Ding, J.Y.; Dong, H.L. Serum antiphospholipid antibody status may not be associated with the pregnancy outcomes of patients undergoing in vitro fertilization. Medicine 2022, 101, e29146. [Google Scholar] [CrossRef]
- Tan, X.; Ding, J.; Pu, D.; Wu, J. Anti-phospholipid antibody may reduce endometrial receptivity during the window of embryo implantation. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101912. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.T.; Blank, M.B.; Ornoy, A.; Shoenfeld, Y. The Association Between Anti-Thyroid Antibodies and Pregnancy Loss. Am. J. Reprod. Immunol. Microbiol. 2001, 45, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Valeff, N.J.; Ventimiglia, M.S.; Diao, L.; Jensen, F. Lupus and recurrent pregnancy loss: The role of female sex hormones and B cells. Front. Endocrinol. 2023, 14, 1233883. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zeng, X.; Qin, L. Systemic autoimmune diseases and recurrent pregnancy loss: Research progress in diagnosis and treatment. Chin. Med. J. 2021, 134, 2140–2142. [Google Scholar] [CrossRef]
- Mankee, A.; Petri, M.; Magder, L.S. Lupus anticoagulant, disease activity and low complement in the first trimester are predictive of pregnancy loss. Lupus Sci. Med. 2015, 2, e000095. [Google Scholar] [CrossRef]
- Ticconi, C.; Inversetti, A.; Logruosso, E.; Ghio, M.; Casadei, L.; Selmi, C.; Di Simone, N. Antinuclear antibodies positivity in women in reproductive age: From infertility to adverse obstetrical outcomes—A meta-analysis. J. Reprod. Immunol. 2023, 155, 103794. [Google Scholar] [CrossRef]
- Hardy, C.J.; Palmer, B.P.; Morton, S.J.; Muir, K.R.; Powell, R.J. Pregnancy outcome and family size in systemic lupus erythematosus: A case-control study. Rheumatology 1999, 38, 559–563. [Google Scholar] [CrossRef][Green Version]
- Singh, M.; Fayaz, F.F.A.; Wang, J.; Wambua, S.; Subramanian, A.; Reynolds, J.A.; Nirantharakumar, K.; Crowe, F.; MuM-PreDiCT. Pregnancy complications and autoimmune diseases in women: Systematic review and meta-analysis. BMC Med. 2024, 22, 339. [Google Scholar] [CrossRef]
- Motak-Pochrzest, H.; Malinowski, A. Does autoimmunity play a role in the risk of implantation failures? Neuro Endocrinol. Lett. 2018, 38, 575–578. [Google Scholar]
- Salmeri, N.; Gennarelli, G.; Vanni, V.S.; Ferrari, S.; Ruffa, A.; Rovere-Querini, P.; Pagliardini, L.; Candiani, M.; Papaleo, E. Concomitant Autoimmunity in Endometriosis Impairs Endometrium-Embryo Crosstalk at the Implantation Site: A Multicenter Case-Control Study. J. Clin. Med. 2023, 12, 3557. [Google Scholar] [CrossRef]
- Ballester, C.; Grobost, V.; Roblot, P.; Pourrat, O.; Pierre, F.; Laurichesse-Delmas, H.; Gallot, D.; Aubard, Y.; Bezanahary, H.; Fauchais, A.L. Pregnancy and primary Sjögren’s syndrome: Management and outcomes in a multicentre retrospective study of 54 pregnancies. Scand. J. Rheumatol. 2017, 46, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, N. Sjögren Syndrome and Pregnancy: A Literature Review. Perm J. 2017, 21, 16-047. [Google Scholar] [CrossRef] [PubMed]
- Imbroane, M.R.; LeMoine, F.; Gibson, K.S. Autoimmune Condition Diagnosis Following Recurrent Pregnancy Loss. Am. J. Reprod. Immunol. 2024, 92, e70006. [Google Scholar] [CrossRef] [PubMed]
- Masucci, L.; D’Ippolito, S.; De Maio, F.; Quaranta, G.; Mazzarella, R.; Bianco, D.M.; Castellani, R.; Inversetti, A.; Sanguinetti, M.; Gasbarrini, A.; et al. Celiac Disease Predisposition and Genital Tract Microbiota in Women Affected by Recurrent Pregnancy Loss. Nutrients 2023, 15, 221. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Siargkas, A.; Germanidis, G.; Dagklis, T.; Tsakiridis, I. Adverse pregnancy outcomes in women with celiac disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2023, 36, 12–24. [Google Scholar] [CrossRef]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 2014, 20, 582–593. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V.; Sarno, L.; Maruotti, G.M.; Cetin, I.; Greco, L.; Khashan, A.S.; McCarthy, F.; Martinelli, D.; Fortunato, F.; et al. Celiac disease and obstetric complications: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2016, 214, 225–234. [Google Scholar] [CrossRef]
- Di Simone, N.; Silano, M.; Castellani, R.; Di Nicuolo, F.; D’Alessio, M.C.; Franceschi, F.; Tritarelli, A.; Leone, A.M.; Tersigni, C.; Gasbarrini, G.; et al. Anti-tissue transglutaminase antibodies from celiac patients are responsible for trophoblast damage via apoptosis in vitro. Am. J. Gastroenterol. 2010, 105, 2254–2261. [Google Scholar] [CrossRef]
- Di Simone, N.; De Spirito, M.; Di Nicuolo, F.; Tersigni, C.; Castellani, R.; Silano, M.; Maulucci, G.; Papi, M.; Marana, R.; Scambia, G.; et al. Potential new mechanisms of placental damage in celiac disease: Anti-transglutaminase antibodies impair human endometrial angiogenesis. Biol. Reprod. 2013, 89, 88. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Gasbarrini, A.; Castellani, R.; Rocchetti, S.; Sisti, L.G.; Scambia, G.; Di Simone, N. Human leukocyte antigen (HLA) DQ2/DQ8 prevalence in recurrent pregnancy loss women. Autoimmun. Rev. 2016, 15, 638–643. [Google Scholar] [CrossRef]
- Królik, M.; Wrześniak, M.; Jezela-Stanek, A. Possible effect of the HLA-DQ2/DQ8 polymorphism on autoimmune parameters and lymphocyte subpopulation in recurrent pregnancy losses. J. Reprod. Immunol. 2022, 149, 103467. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liang, P.; Diao, L.; Liu, C.; Chen, X.; Li, G.; Chen, C.; Zeng, Y. Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure. Int. J. Environ. Res. Public Health 2015, 12, 10352–10361. [Google Scholar] [CrossRef] [PubMed]
- Huisman, P.; Krogh, J.; Nielsen, C.H.; Nielsen, H.S.; Feldt-Rasmussen, U.; Bliddal, S. Thyroglobulin antibodies in women with recurrent pregnancy loss: A Systematic Review and Meta-Analysis. Thyroid 2023, 33, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ying, Y.; Wu, H.; Zhou, C.; Xu, Y.; Wang, Q.; Li, J.; Shen, X.; Jin, L. Relationship between Antithyroid Antibody and Pregnancy Outcome following in Vitro Fertilization and Embryo Transfer. Int. J. Med. Sci. 2012, 9, 121–125. [Google Scholar] [CrossRef]
- Abdolmohammadi-Vahid, S.; Danaii, S.; Hamdi, K.; Jadidi-Niaragh, F.; Ahmadi, M.; Yousefi, M. Novel immunotherapeutic approaches for treatment of infertility. Biomed. Pharmacother. 2016, 84, 1449–1459. [Google Scholar] [CrossRef]
- Stewart-Akers, A.M.; Krasnow, J.S.; Brekosky, J.; Deloia, J.A. Endometrial Leukocytes Are Altered Numerically and Functionally in Women with Implantation Defects. Am. J. Reprod. Immunol. 1998, 39, 1–11. [Google Scholar] [CrossRef]
- Dhillon-Smith, R.K.; Middleton, L.J.; Sunner, K.K.; Cheed, V.; Baker, K.; Farrell-Carver, S.; Bender-Atik, R.; Agrawal, R.; Bhatia, K.; Edi-Osagie, E.; et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. N. Engl. J. Med. 2019, 380, 1316–1325. [Google Scholar] [CrossRef]
- van Dijk, M.M.; Vissenberg, R.; Fliers, E.; van der Post, J.A.M.; van der Hoorn, M.P.; de Weerd, S.; Kuchenbecker, W.K.; Hoek, A.; Sikkema, J.M.; Verhoeve, H.R.; et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 322–329. [Google Scholar] [CrossRef]
- Leng, T.; Li, X.; Zhang, H. Levothyroxine treatment for subclinical hypothyroidism improves the rate of live births in pregnant women with recurrent pregnancy loss: A randomized clinical trial. Gynecol. Endocrinol. 2022, 38, 488–494. [Google Scholar] [CrossRef]
- Rao, M.; Zeng, Z.; Zhao, S.; Tang, L. Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmunity undergoing in vitro fertilization/intracytoplasmic sperm injection: An updated meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2018, 16, 92. [Google Scholar] [CrossRef]
- Yu, M.; Long, Y.; Wang, Y.; Zhang, R.; Tao, L. Effect of levothyroxine on the pregnancy outcomes in recurrent pregnancy loss women with subclinical hypothyroidism and thyroperoxidase antibody positivity: A systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. 2023, 36, 2233039. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Recurrent-pregnancy-loss (accessed on 19 January 2025).
- Gaál, Z. Role of microRNAs in Immune Regulation with Translational and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 1942. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Warner, L.M.; Lin, L.L.; Chen, M.C.; O’Connell, R.M.; Lu, L.F. miR-155 promotes T reg cell development by safeguarding medullary thymic epithelial cell maturation. J. Exp. Med. 2021, 218, e20192423. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.A.; Motavalli, R.; Soltani-Zangbar, M.S.; Parhizkar, F.; Danaii, S.; Aghebati-Maleki, L.; Noori, M.; Dolati, S.; Ahmadi, M.; Samadi Kafil, H.; et al. A new approach to the preeclampsia puzzle; MicroRNA-326 in CD4+ lymphocytes might be as a potential suspect. J. Reprod. Immunol. 2021, 145, 103317. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ji, X. First-trimester maternal cell microRNA is a superior pregnancy marker to immunological testing for predicting adverse pregnancy outcome. J. Reprod. Immunol. 2015, 110, 22–35. [Google Scholar] [CrossRef]
- Patronia, M.M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Vrachnis, D.; Moustakli, E.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 3361. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, X.; Shi, W.; Ye, M.; Cao, X.; Chen, S.; Xu, C. Integrative analysis of circulating microRNAs and the placental transcriptome in recurrent pregnancy loss. Front. Physiol. 2022, 13, 893744. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Wang, J.; Lei, J.; Liu, C.; Ma, Y.; Zhao, H. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod. Biomed. Online 2012, 25, 415–424. [Google Scholar] [CrossRef]
- Li, L.; Feng, T.; Zhou, W.; Liu, Y.; Li, H. miRNAs in decidual NK cells: Regulators worthy of attention during pregnancy. Reprod. Biol. Endocrinol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Guo, C.; Yin, X.; Yao, S. The effect of MicroRNAs variants on idiopathic recurrent pregnancy loss. J. Assist. Reprod. Genet. 2023, 40, 1589–1595. [Google Scholar] [CrossRef]
- Thapliyal, A.; Tomar, A.K.; Naglot, S.; Dhiman, S.; Datta, S.K.; Sharma, J.B.; Singh, N.; Yadav, S. Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development. Noncoding RNA 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The endometrial microbiota and early pregnancy loss. Hum. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Louwers, Y.V.; Laven, E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef] [PubMed]
- Soyer Caliskan, C.; Yurtcu, N.; Celik, S.; Sezer, O.; Kilic, S.S.; Cetin, A. Derangements of vaginal and cervical canal microbiota determined with real-time PCR in women with recurrent miscarriages. J. Obstet. Gynaecol. 2022, 42, 2105–2114. [Google Scholar] [CrossRef]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case-control study. BJOG 2020, 127, 264–274. [Google Scholar] [CrossRef]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: A nested case-control study. Reprod. BioMed. Online 2022, 45, 1021–1031. [Google Scholar] [CrossRef]
- Vomstein, K.; Reider, S.; Böttcher, B.; Watschinger, C.; Kyvelidou, C.; Tilg, H.; Moschen, A.R.; Toth, B. Uterine microbiota plasticity during the menstrual cycle: Differences between healthy controls and patients with recurrent miscarriage or implantation failure. J. Reprod. Immunol. 2022, 151, 103634. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Shi, Y.; Yamada, H.; Sasagawa, Y.; Tanimura, K.; Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J. Reprod. Immunol. 2022, 152, 103653. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; He, L.; Liu, H.; Liu, Y.; Luan, Z.; Li, H.; Liu, W.; Luo, M. Association between the vaginal and uterine microbiota and the risk of early embryonic arrest. Front. Microbiol. 2023, 14, 1137869. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.asrm.org/practice-guidance/practice-committee-documents/evaluation-and-treatment-of-recurrent-pregnancy-loss-a-committee-opinion-2012 (accessed on 19 January 2025).
- Quenby, S.; Kalumbi, C.; Bates, M.; Farquharson, R.; Vince, G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil. Steril. 2005, 84, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.F.; Elkholy, A.G.; El-Said, M.M.; Abdel-Salam, N.E. Combined oral prednisolone and heparin versus heparin: The effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double-blind placebo randomized controlled trial. Arch. Gynecol. Obstet. 2014, 290, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Rezayat, F.; Esmaeil, N.; Rezaei, A.; Sherkat, R. Contradictory Effect of Lymphocyte Therapy and Prednisolone Therapy on CD3+CD8+CD56+ Natural Killer T Population in Women with Recurrent Spontaneous Abortion. J. Hum. Reprod. Sci. 2023, 16, 246. [Google Scholar] [CrossRef]
- Tang, A.W.; Alfirevic, Z.; Turner, M.A.; Drury, J.A.; Small, R.; Quenby, S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum. Reprod. 2013, 28, 1743–1752. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Kamath, M.S.; Keay, S.D.; Macklon, N.S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2022, 6, CD005996. [Google Scholar] [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Dan, S.; Wei, W.; Yichao, S.; Hongbo, C.; Shenmin, Y.; Jiaxiong, W.; Hong, L. Effect of Prednisolone Administration on Patients with Unexplained Recurrent Miscarriage and in Routine Intracytoplasmic Sperm Injection: A Meta-Analysis. Am. J. Reprod. Immunol. 2015, 74, 89–97. [Google Scholar] [CrossRef]
- He, Y.; Tang, R.; Yu, H.; Mu, H.; Jin, H.; Dong, J.; Wang, W.; Wang, L.; Chen, S.; Wang, X. Comparative effectiveness and safety of 36 therapies or interventions for pregnancy outcomes with recurrent implantation failure: A systematic review and network meta-analysis. J. Assist. Reprod. Genet. 2023, 40, 2343–2356. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, H.; Li, M.; Yang, Y.; Fu, X. Prednisone improves pregnancy outcome in repeated implantation failure by enhance regulatory T cells bias. J. Reprod. Immunol. 2021, 143, 103245. [Google Scholar] [CrossRef]
- Hasegawa, I.; Yamanoto, Y.; Suzuki, M.; Murakawa, H.; Kurabayashi, T.; Takakuwa, K.; Tanaka, K. Prednisolone plus low-dose aspirin improves the implantation rate in women with autoimmune conditions who are undergoing in vitro fertilization. Fertil. Steril. 1998, 70, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhong, Y.; Chen, C. Combined treatment of prednisone and aspirin, starting before ovulation induction, may improve reproductive outcomes in ANA-positive patients. Am. J. Reprod. Immunol. 2016, 76, 391–395. [Google Scholar] [CrossRef]
- Ando, T.; Suganuma, N.; Furuhashi, M.; Asada, Y.; Kondo, I.; Tsutsumi, Y. Successful glucocorticoid treatment for patients with abnormal autoimmunity on in vitro fertilization and embryo transfer. J. Assist. Reprod. Genet. 1996, 13, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cui, L.; Lu, Y.; Tan, J.; Dong, X.; Ni, T.; Yan, J.; Guan, Y.; Hao, G.; Liu, J.Y.; et al. Prednisone vs Placebo and Live Birth in Patients With Recurrent Implantation Failure Undergoing In Vitro Fertilization. JAMA 2023, 329, 1460. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Thomas, M.; Nelson-Piercy, C.; Khamashta, M.; Hunt, B.J. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood 2011, 117, 6948–6951. [Google Scholar] [CrossRef]
- Riancho-Zarrabeitia, L.; Lopez-Marin, L.; Cacho, P.M.; López-Hoyos, M.; Barrio, R.D.; Haya, A.; Martínez-Taboada, V.M. Treatment with low-dose prednisone in refractory obstetric antiphospholipid syndrome: A retrospective cohort study and meta-analysis. Lupus 2022, 31, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Forges, T.; Monnier-Barbarino, P.; Guillet-May, F.; Faure, G.C.; Béné, M.C. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: A prospective pilot study. Eur. J. Clin. Pharmacol. 2006, 62, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Palmsten, K.; Forbess Smith, C.J.; Chambers, C.D. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum. Dis. Clin. N. Am. 2017, 43, 489–502. [Google Scholar] [CrossRef]
- Hooper, A.; Bacal, V.; Bedaiwy, M.A. Does adding hydroxychloroquine to empiric treatment improve the live birth rate in refractory obstetrical antiphospholipid syndrome? A systematic review. Am. J. Reprod. Immunol. 2023, 90, e13761. [Google Scholar] [CrossRef]
- Mekinian, A.; Lazzaroni, M.G.; Kuzenko, A.; Alijotas-Reig, J.; Ruffatti, A.; Levy, P.; Canti, V.; Bremme, K.; Bezanahary, H.; Bertero, T.; et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun. Rev. 2015, 14, 498–502. [Google Scholar] [CrossRef]
- Mekinian, A.; Alijotas-Reig, J.; Carrat, F.; Costedoat-Chalumeau, N.; Ruffatti, A.; Lazzaroni, M.G.; Tabacco, S.; Maina, A.; Masseau, A.; Morel, N.; et al. Refractory obstetrical antiphospholipid syndrome: Features, treatment and outcome in a European multicenter retrospective study. Autoimmun. Rev. 2017, 16, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.L.; Gu, X.K.; Tao, L.Y.; Cong, J.M.; Wang, Y.Q. Efficacy of Different Treatment Regimens for Antiphospholipid Syndrome-related Recurrent Spontaneous Abortion. Chin. Med. J. 2017, 130, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Gerde, M.; Ibarra, E.; Mac Kenzie, R.; Fernandez Suarez, C.; Heer, C.; Alvarez, R.; Iglesias, M.; Balparda, J.; Beruti, E.; Rubinstein, F. The impact of hydroxychloroquine on obstetric outcomes in refractory obstetric antiphospholipid syndrome. Thromb. Res. 2021, 206, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ruffatti, A.; Tonello, M.; Hoxha, A.; Sciascia, S.; Cuadrado, M.J.; Latino, J.O.; Udry, S.; Reshetnyak, T.; Costedoat-Chalumeau, N.; Morel, N.; et al. Effect of Additional Treatments Combined with Conventional Therapies in Pregnant Patients with High-Risk Antiphospholipid Syndrome: A Multicentre Study. Thromb. Haemost. 2018, 47, 639–646. [Google Scholar] [CrossRef]
- Sciascia, S.; Hunt, B.J.; Talavera-Garcia, E.; Lliso, G.; Khamashta, M.A.; Cuadrado, M.J. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am. J. Obstet. Gynecol. 2016, 214, 273.e1–273.e8. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Ghasemnejad Berenji, M.; Nazarian, H.; Ghasemnejad, T.; Nematollahi, M.H.; Abroon, S.; Paktinat, S.; Heidari Khoei, H.; Ghasemnejad Berenji, H.; Ghaffari Novin, M. Effects of treatment with hydroxychloroquine on the modulation of Th17/Treg ratio and pregnancy outcomes in women with recurrent implantation failure: Clinical trial. Immunopharmacol. Immunotoxicol. 2020, 42, 632–642. [Google Scholar] [CrossRef]
- Dernoncourt, A.; Hedhli, K.; Abisror, N.; Cheloufi, M.; Cohen, J.; Kolanska, K.; McAvoy, C.; Selleret, L.; Ballot, E.; Mathieu d’Argent, E.; et al. Hydroxychloroquine in recurrent pregnancy loss: Data from a French prospective multicenter registry. Hum. Reprod. 2024, 39, 1934–1941. [Google Scholar] [CrossRef]
- Halloran, P.F. Molecular mechanisms of new immunosuppressants. Clin. Transplant. 1996, 10, 118–123. [Google Scholar] [CrossRef]
- Saad, A.F.; Pacheco, L.D.; Saade, G.R. Immunosuppressant Medications in Pregnancy. Obstet. Gynecol. 2024, 143, e94–e106. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Tavares, A.C.M.; Rocha, C.A.; de Souza, G.F.; Lima, E.M.; Simões, J.M.L.; de Souza, L.C.; Martins, M.Y.M.; de Araújo, N.O.; Barini, R. Calcineurin inhibitors in the management of recurrent miscarriage and recurrent implantation failure: Systematic review and meta-analysis. J. Reprod. Immunol. 2023, 160, 104157. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sugiyama, R. Tacrolimus treatment in women with repeated implantation failures. Reprod. Med. Biol. 2024, 23, e12558. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Huang, Y.; Chen, C.; Mao, J.; Zhang, H. Low dose Cyclosporin A treatment increases live birth rate of unexplained recurrent abortion—Initial cohort study. Clin. Exp. Obstet. Gynecol. 2017, 44, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Ahmadi, M.; Danaii, S.; Abdollahi-Fard, S.; Mosapour, P.; Eghbal-Fard, S.; Dolati, S.; Kamrani, A.; Rahnama, B.; Mehdizadeh, A.; et al. Cyclosporine A improves pregnancy outcomes in women with recurrent pregnancy loss and elevated Th1/Th2 ratio. J. Cell. Physiol. 2019, 234, 19039–19047. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H. Analysis of the use of cyclosporin A to treat refractory immune recurrent spontaneous abortion. Clin. Exp. Obstet. Gynecol. 2015, 42, 739–742. [Google Scholar] [CrossRef]
- Qu, D.; Tian, X.; Ding, L.; Li, Y.; Zhou, W. Impacts of Cyclosporin A on clinical pregnancy outcomes of patients with a history of unexplained transfer failure: A retrospective cohort study. Reprod. Biol Endocrinol. 2021, 19, 44. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Fu, J.; Yuan, G.; Li, N.; Fu, Y.; Zhao, L. Tacrolimus improved the pregnancy outcomes of patients with refractory recurrent spontaneous abortion and immune bias disorders: A randomized controlled trial. Eur. J. Clin. Pharmacol. 2023, 79, 627–634. [Google Scholar] [CrossRef]
- Kuroda, K.; Ikemoto, Y.; Horikawa, T.; Moriyama, A.; Ojiro, Y.; Takamizawa, S.; Uchida, T.; Nojiri, S.; Nakagawa, K.; Sugiyama, R. Novel approaches to the management of recurrent pregnancy loss: The OPTIMUM (OPtimization of Thyroid function, Thrombophilia, Immunity, and Uterine Milieu) treatment strategy. Reprod. Med. Biol. 2021, 20, 524–536. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. After 12 consecutive miscarriages, a patient received immunosuppressive treatment and delivered an intact baby. Reprod. Med. Biol. 2017, 16, 297–301. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, T.; Han, R.; Xie, H.; Lv, Q. Co-administration of tacrolimus and low molecular weight heparin in patients with a history of implantation failure and elevated peripheral blood natural killer cell proportion. J. Obstet. Gynaecol. Res. 2022, 49, 649–657. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kwak-Kim, J.; Hisano, M.; Kasahara, Y.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. Obstetric and perinatal outcome of the women with repeated implantation failures or recurrent pregnancy losses who received pre- and post-conception tacrolimus treatment. Am. J. Reprod. Immunol. 2019, 82, e13142. [Google Scholar] [CrossRef]
- Nakamura, A.; Tanaka, Y.; Amano, T.; Takebayashi, A.; Takahashi, A.; Hanada, T.; Tsuji, S.; Murakami, T. mTOR inhibitors as potential therapeutics for endometriosis: A narrative review. Mol. Hum. Reprod. 2024, 30, gaae041. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Shen, H.H.; Cao, X.Y.; Gao, X.X.; Xu, F.Y.; Ha, S.Y.; Sun, J.S.; Liu, S.P.; Xie, F.; Li, M.Q. Targeting a mTOR/autophagy axis: A double-edged sword of rapamycin in spontaneous miscarriage. Biomed. Pharmacother. 2024, 177, 116976. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Abdolmohamadi-Vahid, S.; Ghaebi, M.; Dolati, S.; Abbaspour-Aghdam, S.; Danaii, S.; Berjis, K.; Madadi-Javid, R.; Nouri, Z.; Siahmansouri, H.; et al. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: A double-blind, phase II randomized clinical trial. Int. Immunopharmacol. 2019, 74, 105730. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.H.; Kwak, F.M.Y.; Ainbinder, S.W.; Ruiz, A.M.; Beer, A.E. Elevated Peripheral Blood Natural Killer Cells Are Effectively Downregulated by Immunoglobulin G Infusion in Women With Recurrent Spontaneous Abortions. Am. J. Reprod. Immunol. 1996, 35, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Afkham, A.; Danaii, S.; Abdollahi-Fard, S.; Heidari, L.; Jadidi-Niaragh, F.; Younesi, V.; et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed. Pharmacother. 2017, 92, 1095–1102. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; Ghasemzadeh, A.; Hamdi, K.; Younesi, V.; Nouri, M.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017, 63, 350–359. [Google Scholar] [CrossRef]
- Yamada, H.; Deguchi, M.; Saito, S.; Takeshita, T.; Mitsui, M.; Saito, T.; Nagamatsu, T.; Takakuwa, K.; Nakatsuka, M.; Yoneda, S.; et al. High doses of intravenous immunoglobulin stimulate regulatory T cell and suppress natural killer cell in women with recurrent pregnancy loss. J. Reprod. Immunol. 2023, 158, 103977. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, D.; Hao, B.; Zhang, X.; Geng, W.; Wang, Y.; Sun, J.; Zhao, Y. Efficacy of intravenous immunoglobulin in the treatment of recurrent spontaneous abortion: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2022, 88, e13615. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Kolte, A.M.; Krog, M.C.; Nielsen, H.S.; Egerup, P. Treatment with intravenous immunoglobulin in patients with recurrent pregnancy loss: An update. J. Reprod. Immunol. 2019, 133, 37–42. [Google Scholar] [CrossRef]
- Yamada, H.; Deguchi, M.; Saito, S.; Takeshita, T.; Mitsui, M.; Saito, T. Intravenous immunoglobulin treatment in women with four or more recurrent pregnancy losses: A double-blind, randomised, placebo-controlled trial. eClinicalMedicine 2022, 50, 101527. [Google Scholar] [CrossRef]
- Ramos-Medina, R.; García-Segovia, A.; Gil, J.; Carbone, J.; Aguarón de la Cruz, A.; Seyfferth, A.; Alonso, B.; Alonso, J.; León, J.A.; Alecsandru, D.; et al. Experience in IVIg Therapy for Selected Women with Recurrent Reproductive Failure and NK Cell Expansion. Am. J. Reprod. Immunol. 2014, 71, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Han, A.R.; Hur, S.E.; Kim, C.J.; Kim, T.H.; Cho, B.R.; Han, J.W.; Han, S.G.; Na, B.J.; et al. Intravenous Immunoglobulin G Improves Pregnancy Outcome in Women with Recurrent Pregnancy Losses with Cellular Immune Abnormalities. Am J Reprod Immunol. 2016, 75, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Banjar, S.; Kadour, E.; Khoudja, R.; Ton-Leclerc, S.; Beauchamp, C.; Beltempo, M.; Dahan, M.H.; Gold, P.; Jacques Kadoch, I.; Jamal, W.; et al. Intravenous immunoglobulin use in patients with unexplained recurrent pregnancy loss. Am. J. Reprod. Immunol. 2023, 90, e13737. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.H.; Yang, N.; Ko, Y.; Lee, S.R.; Chae, H.D. Outcomes of Empirical Treatment With Intravenous Immunoglobulin G Combined With Low-Dose Aspirin in Women With Unexplained Recurrent Pregnancy Loss. J. Korean Med. Sci. 2022, 37, e200. [Google Scholar] [CrossRef] [PubMed]
- Habets, D.H.J.; Pelzner, K.; Wieten, L.; Spaanderman, M.E.A.; Villamor, E.; Al-Nasiry, S. Intravenous immunoglobulins improve live birth rate among women with underlying immune conditions and recurrent pregnancy loss: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2022, 18, 23. [Google Scholar] [CrossRef]
- Clark, D.A.; Coulam, C.B.; Stricker, R.B. Is intravenous immunoglobulins (IVIG) efficacious in early pregnancy failure? A critical review and meta-analysis for patients who fail in vitro fertilization and embryo transfer (IVF). J. Assist. Reprod. Genet. 2006, 23, 1–13. [Google Scholar] [CrossRef]
- Kumar, P.; Philip, C.E.; Eskandar, K.; Marron, K.; Harrity, C. Effect of intravenous immunoglobulin therapy in recurrent implantation failure: A Systematic review and meta-analysis. J. Reprod. Immunol. 2024, 166, 104323. [Google Scholar] [CrossRef]
- Park, J.S.; Song, A.Y.; Bae, J.Y.; Han, J.W.; Kim, T.H.; Kim, C.J.; Lee, S.K. IL-17 Producing T to Foxp3+CD4+ Regulatory T Cell Ratio as a Diagnostic and Prognostic Marker in Women With Recurrent Pregnancy Loss and Its Implications for Intravenous Immunoglobulin Therapy. Am. J. Reprod. Immunol. 2024, 92, e70020. [Google Scholar] [CrossRef]
- Velikova, T.; Sekulovski, M.; Bogdanova, S.; Vasilev, G.; Peshevska-Sekulovska, M.; Miteva, D.; Georgiev, T. Intravenous Immunoglobulins as Immunomodulators in Autoimmune Diseases and Reproductive Medicine. Antibodies 2023, 12, 20. [Google Scholar] [CrossRef]
- Perricone, R.; De Carolis, K.B.; Greco, E.; Giacomelli, R.; Cipriani, P.; Fontana, L.; Perricone, C. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology 2008, 47, 646–651. [Google Scholar] [CrossRef]
- Wang, S.W.; Zhong, S.Y.; Lou, L.J.; Hu, Z.F.; Sun, H.Y.; Zhu, H.Y. The effect of intravenous immunoglobulin passive immunotherapy on unexplained recurrent spontaneous abortion: A meta-analysis. Reprod. BioMed. Online 2016, 33, 720–736. [Google Scholar] [CrossRef] [PubMed]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Elevated Preconception CD56+16+ and/or Th1:Th2 Levels Predict Benefit from IVIG Therapy in Subfertile Women Undergoing IVF. Am. J. Reprod. Immunol. 2011, 66, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.; Han, A.R.; Park, C.W.; Park, D.W.; Park, J.C.; Kim, N.Y.; Lim, K.S.; Shin, J.E.; Joo, C.W.; Lee, S.E.; et al. Intravenous immunoglobulin G in women with reproductive failure: The Korean Society for Reproductive Immunology practice guidelines. Clin. Exp. Reprod. Med. 2017, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Woon, E.V.; Day, A.; Bracewell-Milnes, T.; Male, V.; Johnson, M. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 142, 103189. [Google Scholar] [CrossRef]
- Porter, T.A.; Lacoursiere, Y.; Scott, J. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2006, CD000112. [Google Scholar] [CrossRef]
- Urban, M.L.; Bettiol, A.; Serena, C.; Comito, C.; Turrini, I.; Fruttuoso, S.; Silvestri, E.; Vannacci, A.; Ravaldi, C.; Petraglia, F.; et al. Intravenous immunoglobulin for the secondary prevention of stillbirth in obstetric antiphospholipid syndrome: A case series and systematic review of literature. Autoimmun. Rev. 2020, 19, 102620. [Google Scholar] [CrossRef]
- Perricone, R.; Di Muzio, G.; Perricone, C.; Giacomelli, R.; De Nardo, D.; Fontana, L.; De Carolis, C. High Levels of Peripheral Blood NK Cells in Women Suffering from Recurrent Spontaneous Abortion are Reverted from High-Dose Intravenous Immunoglobulins. Am. J. Reprod. Immunol. 2006, 55, 232–239. [Google Scholar] [CrossRef]
- Elram, T.; Simon, A.; Israel, S.; Revel, A.; Shveiky, D.; Laufer, N. Treatment of recurrent IVF failure and human leukocyte antigen similarity by intravenous immunoglobulin. Reprod. BioMed. Online 2005, 11, 745–749. [Google Scholar] [CrossRef]
- Rutella, S. Granulocyte Colony-Stimulating Factor for the Induction of T-Cell Tolerance. Transplantation 2007, 84 (Supplement), S26–S30. [Google Scholar] [CrossRef]
- Perobelli, S.M.; Mercadante, A.C.; Galvani, R.G.; Gonçalves-Silva, T.; Alves, A.P.; Pereira-Neves, A.; Benchimol, M.; Nóbrega, A.; Bonomo, A. G-CSF-Induced Suppressor IL-10+ Neutrophils Promote Regulatory T Cells That Inhibit Graft-Versus-Host Disease in a Long-Lasting and Specific Way. J. Immunol 2016, 197, 3725–3734. [Google Scholar] [CrossRef]
- Scarpellini, F.; Sbracia, M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: A randomised controlled trial. Hum. Reprod. 2009, 24, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; Joing, M.; Kwon, P.; Tong, J.; Maneta, E.; Santo, C.D.; Mussai, F.; Lissauer, D.; Carter, D.; RESPONSE study group; et al. Recombinant human granulocyte- colony stimulating factor in women with unexplained recurrent pregnancy losses: A randomized clinical trial. Hum. Reprod. 2019, 34, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Chittawar, P.B.; Kirubakaran, R.; Mascarenhas, M. Use of granulocyte-colony stimulating factor in assisted reproductive technology: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Arefi, S.; Fazeli, E.; Esfahani, M.; Borhani, N.; Yamini, N.; Hosseini, A.; Farifteh, F. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: An RCT. Int. J. Reprod. Biomed. 2018, 16, 299–304. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, Y.; Qiao, Y.; Tang, Y.; Sui, X.; Yin, P.; Yang, D. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 18434. [Google Scholar] [CrossRef]
- Li, J.; Mo, S.; Chen, Y. The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst. Biol. Reprod. Med. 2017, 63, 239–247. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Qi, L.; Zhao, L. A randomized controlled trial of etanercept in the treatment of refractory recurrent spontaneous abortion with innate immune disorders. Taiwan J. Obstet. Gynecol. 2019, 58, 621–625. [Google Scholar] [CrossRef]
- Santiago, K.Y.; Porchia, L.M.; López-Bayghen, E. Endometrial preparation with etanercept increased embryo implantation and live birth rates in women suffering from recurrent implantation failure during IVF. Reprod. Biol. 2021, 21, 100480. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L. Treatment with Tumor Necrosis Factor Inhibitors and Intravenous Immunoglobulin Improves Live Birth Rates in Women with Recurrent Spontaneous Abortion. Am. J. Reprod. Immunol. 2008, 60, 8–16. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; Ahuja, S.; El-Toukhy, T.; Taranissi, M. Treatment with Adalimumab (Humira®) and Intravenous Immunoglobulin Improves Pregnancy Rates in Women Undergoing IVF. Am. J. Reprod. Immunol. 2008, 61, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, H.; Nejabati, H.R.; Latifi, Z.; Hamdi, K.; Bahrami-Asl, Z.; Fattahi, A.; Nouri, M. Lymphocytes immunotherapy for preserving pregnancy: Mechanisms and Challenges. Am. J. Reprod. Immunol. 2018, 80, e12853. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qiu, L.; Di, W.; Zhao, A.; Chen, G.; Hu, K.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells after lymphocyte therapy in unexplained recurrent spontaneous abortion patients. Fertil. Steril. 2009, 92, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Sarkesh, A.; Sorkhabi, A.D.; Parhizkar, F.; Soltani-Zangbar, M.S.; Yousefi, M.; Aghebati-Maleki, L. The immunomodulatory effect of intradermal allogeneic PBMC therapy in patients with recurrent spontaneous abortion. J. Reprod. Immunol. 2023, 156, 103818. [Google Scholar] [CrossRef]
- Liu, S.; Gu, X.; Weng, R. Clinical effect of lymphocyte immunotherapy on patients with unexplained recurrent spontaneous abortion. Immun. Inflamm. Dis. 2021, 9, 1272–1278. [Google Scholar] [CrossRef]
- Fainboim, L.; Belén, S.; González, V.; Fernández, P. Evaluation of paternal lymphocyte immunotherapy and potential biomarker mixed lymphocyte reaction-blocking factor in an Argentinian cohort of women with unexplained recurrent spontaneous abortion and unexplained infertility. Am. J. Reprod. Immunol. 2021, 86, e13422. [Google Scholar] [CrossRef]
- Sarno, M.; Cavalcante, M.B.; Niag, M.; Pimentel, K.; Luz, I.; Figueiredo, B.; Michelon, T.; Neumann, J.; Lima, S.; Machado, I.N.; et al. Gestational and perinatal outcomes in recurrent miscarriages couples treated with lymphocyte immunotherapy. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100036. [Google Scholar] [CrossRef]
- Chen, J.L.; Yang, J.M.; Huang, Y.Z.; Li, Y. Clinical observation of lymphocyte active immunotherapy in 380 patients with unexplained recurrent spontaneous abortion. Int. Immunopharmacol. 2016, 40, 347–350. [Google Scholar] [CrossRef]
- Gharesi-Fard, B.; Zolghadri, J.; Foroughinia, L.; Tavazoo, F.; Samsami Dehaghani, A. Effectiveness of leukocyte immunotherapy in primary recurrent spontaneous abortion (RPL). Iran. J. Immunol. 2007, 4, 173–178. [Google Scholar]
- Pandey, M.K.; Agrawal, S. Induction of MLR-Bf and protection of fetal loss: A current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int. Immunopharmacol. 2004, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.; Karrison, T.; Odem, R.R.; Barnes, R.B.; Branch, D.W.; Stephenson, M.D.; Baron, B.; Walker, M.A.; Scott, J.R.; Schreiber, J.R. Mononuclear-cell immunisation in prevention of recurrent miscarriages: A randomised trial. Lancet 1999, 354, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.F.; Porter, T.F.; Scott, J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2014, 2014, CD000112. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Alkatout, I.; Meyerholz, L.; Maass, N.; Görg, S.; von Otte, S.; Ziemann, M. Live Birth Rates after Active Immunization with Partner Lymphocytes. Biomedicines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Kang, X.; Wang, T.; He, L.; Zhao, A. Allogenic Lymphocyte Immunotherapy for Unexplained Recurrent Spontaneous Abortion: A Meta-Analysis. Am. J. Reprod. Immunol. 2016, 76, 443–453. [Google Scholar] [CrossRef]
- Rasmark Roepke, E.; Hellgren, M.; Hjertberg, R.; Blomqvist, L.; Matthiesen, L.; Henic, E.; Lalitkumar, S.; Strandell, A. Treatment efficacy for idiopathic recurrent pregnancy loss—A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2018, 97, 921–941. [Google Scholar] [CrossRef]
- Melo, P.; Thornton, T.; Coomarasamy, A.; Granne, I. Evidence for the effectiveness of immunologic therapies in women with subfertility and/or undergoing assisted reproduction. Fertil. Steril. 2022, 117, 1144–1159. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, B.; Xu, M.; Wang, S.; Liu, R.; Wu, J.; Yang, J.; Feng, L. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: A prospective randomized study. Am. J. Reprod. Immunol. 2016, 76, 212–216. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Cheng, Y.; Zhou, D.; Yin, T.; Xu, W.; Yu, N.; Yang, J. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 2017, 119, 15–22. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rezaeinejad, M.; Rouholamin, S.; Almasi-Hashiani, A.; Pirjani, R.; Sepidarkish, M. Intrauterine administration of autologous peripheral blood mononuclear cells in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2019, 131, 50–56. [Google Scholar] [CrossRef]
- Pourmoghadam, Z.; Abdolmohammadi-Vahid, S.; Pashazadeh, F.; Aghebati-Maleki, L.; Ansari, F.; Yousefi, M. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103077. [Google Scholar] [CrossRef] [PubMed]
- Yakin, K.; Oktem, O. Urman B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 3897. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Dai, S.; Lin, R.; Huang, C.; Zeng, Y.; Diao, L.; Lian, R.; Tu, W. The effectiveness and safety of intrauterine infusion of autologous regulatory T cells (Tregs) in patients with recurrent pregnancy loss and low levels of endometrial FoxP3+ cells: A retrospective cohort study. Am. J. Reprod. Immunol. 2023, 90, e13735. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Yang, X.; Xing, Y.; Que, W.; Yu, Z.; Gui, W.; Chen, Y.; Liu, X. Intrauterine Infusion of Leukocyte-Poor Platelet-Rich Plasma Is an Effective Therapeutic Protocol for Patients with Recurrent Implantation Failure: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 2823. [Google Scholar] [CrossRef]
- Kong, X.; Tang, G.; Liu, Y.; Zheng, Z.; Li, Y.; Yan, F. Efficacy of intrauterine infusion therapy before embryo transfer in recurrent implantation failure: A systematic review and network meta-analysis. J. Reprod. Immunol. 2023, 156, 103819. [Google Scholar] [CrossRef]
- Deng, H.; Wang, S.; Li, Z.; Xiao, L.; Mao, Y. Effect of intrauterine infusion of platelet-rich plasma for women with recurrent implantation failure: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2023, 43, 2144177. [Google Scholar] [CrossRef]
- Mehrafza, M.; Pourseify, G.; Zare Yousefi, T.; Azadeh, R.; Saghati Jalali, S.; Hosseinzadeh, E.; Samadnia, S.; Habibdoost, M.; Tamimi, A.; Hosseini, A. The Efficiency of Introducing Intrauterine Infusion of Autologous Platelet-Rich Plasma versus Granulocyte Colony-Stimulating Factor in Repeated Implantation Failure Patients: An Unblinded Randomised Clinical Trial. Int. J. Fertil. Steril. 2024, 18 (Suppl. 1), 30–34. [Google Scholar] [CrossRef]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: Is there any evidence? Reprod. Fertil. 2021, 2, 173–186. [Google Scholar] [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of Intralipid’s Suppressive Effect on NK Cell’s Functional Activity. Am. J. Reprod. Immunol. 2008, 60, 258–263. [Google Scholar] [CrossRef]
- Singh, N.; Davis, A.A.; Kumar, S.; Kriplani, A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: A randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 45–51. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Bayoumi, Y.A.; Sharkawy, M.; Gad Allah, S.H.; Hassan, M.A.; Gouda, H.M.; Hashem, A.T.; Hatem, D.L.; Ahmed, M.F.; El-Khayat, W. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Int. J. Gynecol. Obstet. 2016, 135, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin. Exp. Reprod. Med. 2021, 48, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, M.P.; Black, N.; Keay, S.; Quenby, S.; Al Wattar, B.H. Intralipid infusion at time of embryo transfer in women with history of recurrent implantation failure: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.J.; Masoud, A.T.; Ulibarri, H.; Arroyo, A.; Coriell, C.; Goetz, S.; Moir, C.; Moberly, A.; Gonzalez, D.; Blanco, M.; et al. Effect of a 20% intravenous fat emulsion therapy on pregnancy outcomes in women with RPL or RIF undergoing IVF/ICSI: A systematic review and meta-analysis. J. Clin. Transl. Res. 2023, 9, 236–245. [Google Scholar] [PubMed]
- Ndukwe, G. Recurrent embryo implantation failure after in vitro fertilisation: Improved outcome following intralipid infusion in women with elevated T Helper 1 response. Hum. Fertil. 2011, 14, 1–8. [Google Scholar]
- Coulam, C.B. Intralipid treatment for women with reproductive failures. Am. J. Reprod. Immunol. 2021, 85, e13290. [Google Scholar] [CrossRef]
- Martini, A.; Jasulaitis, S.; Fogg, L.; Uhler, M.; Hirshfeld-Cytron, J. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J. Hum. Reprod. Sci. 2018, 11, 261. [Google Scholar] [CrossRef]
- Carta, G.; Iovenitti, P.; Falciglia, K. Recurrent miscarriage associated with antiphospholipid antibodies: Prophylactic treatment with low-dose aspirin and fish oil derivates. Clin. Exp. Obstet. Gynecol. 2005, 32, 49–51. [Google Scholar]
- Mu, F.; Huo, H.; Wang, M.; Wang, F. Omega-3 fatty acid supplements and recurrent miscarriage: A perspective on potential mechanisms and clinical evidence. Food Sci. Nutr. 2023, 11, 4460–4471. [Google Scholar] [CrossRef]
- Canella, P.R.B.C.; Vinces, S.S.; Silva, Á.A.R.; Sanches, P.H.G.; Barini, R.; Porcari, A.M.; Razolli, D.S.; Carvalho, P.O. Altered profile of plasma phospholipids in woman with recurrent pregnancy loss and recurrent implantation failure treated with lipid emulsion therapy. Am. J. Reprod. Immunol. 2023, 89, e13673. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Mcheik, S.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss: An update in 2022. Hum. Reprod. Open 2023, 2023, hoad002. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. The Investigation and Treatment of Couples with Recurrent First- trimester and Second-trimester Miscarriage Green-top Guideline No. 17. 2022. Available online: https://www.rcog.org.uk/media/3cbgonl0/gtg_17.pdf (accessed on 8 December 2024).
- Hamulyák, E.N.; Scheres, L.J.; Marijnen, M.C.; Goddijn, M.; Middeldorp, S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst. Rev. 2020, 5, CD012852. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, Y.; Yu, E.D.; Xiang, S.; Meng, R.; Niu, K.F.; Zhu, H. Comparison of therapeutic interventions for recurrent pregnancy loss in association with antiphospholipid syndrome: A systematic review and network meta-analysis. Am. J. Reprod. Immunol. 2020, 83, e13219. [Google Scholar] [CrossRef] [PubMed]
- Grandone, E.; Tiscia, G.L.; Mastroianno, M.; Larciprete, G.; Kovač, M.; Tamborini Permunian, E.; Lojacono, A.; Barcellona, D.; Bitsadze, V.; Khizroeva, J.; et al. Findings from a multicentre, observational study on reproductive outcomes in women with unexplained recurrent pregnancy loss: The OTTILIA registry. Hum. Reprod. 2021, 36, 2083–2090. [Google Scholar] [CrossRef]
- Aynıoglu, O.; Isik, H.; Sahbaz, A.; Alptekın, H.; Bayar, U. Does anticoagulant therapy improve adverse pregnancy outcomes in patients with history of recurrent pregnancy loss? Ginekol. Pol. 2016, 87, 585–591. [Google Scholar] [CrossRef]
- Shaaban, O.M.; Abbas, A.M.; Zahran, K.M.; Fathalla, M.M.; Anan, M.A.; Salman, S.A. Low-Molecular-Weight Heparin for the Treatment of Unexplained Recurrent Miscarriage With Negative Antiphospholipid Antibodies: A Randomized Controlled Trial. Clin. Appl. Thromb. Hemost. 2016, 23, 567–572. [Google Scholar] [CrossRef]
- Jiang, F.; Hu, X.; Jiang, K.; Pi, H.; He, Q.; Chen, X. The role of low molecular weight heparin on recurrent pregnancy loss: A systematic review and meta-analysis. Taiwan J. Obstet. Gynecol. 2021, 60, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.H.; Xu, L.; Li, Z.Y. Meta-analysis of heparin combined with aspirin versus aspirin alone for unexplained recurrent spontaneous abortion. Int. J. Gynaecol. Obstet. 2020, 151, 23–32. [Google Scholar] [CrossRef]
- Skeith, L.; Carrier, M.; Kaaja, R.; Martinelli, I.; Petroff, D.; Schleußner, E.; Laskin, C.A.; Rodger, M.A. A meta-analysis of low-molecular-weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood 2016, 127, 1650–1655. [Google Scholar] [CrossRef]
- de Jong, P.; Kaandorp, S.; Di Nisio, M.; Goddijn, M.; Middeldorp, S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst. Rev. 2014, 2014, CD004734. [Google Scholar] [CrossRef]
- Schleussner, E.; Kamin, G.; Seliger, G.; Rogenhofer, N.; Ebner, S.; Toth, B.; Schenk, M.; Henes, M.; Bohlmann, M.K.; Fischer, T.; et al. Low-Molecular-Weight Heparin for Women With Unexplained Recurrent Pregnancy Loss. Ann. Intern. Med. 2015, 162, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, C.; Akar, B.; Gönenç, G.; Aslancan, R.; Yılmaz, N.; Çalışkan, E. Aspirin, low molecular weight heparin, or both in preventing pregnancy complications in women with recurrent pregnancy loss and factor V Leiden mutation. J. Matern.-Fetal Neonatal Med. 2020, 33, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, Y.; Cheng, X.; Li, N.; Sheng, X. Enoxaparin (or plus aspirin) for the prevention of recurrent miscarriage: A meta-analysis of randomized controlled studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, R.; Li, C.; Chen, A. Evaluation of the effect of low molecular weight heparin in unexplained recurrent pregnancy loss: A meta-analysis of randomized controlled trials. J. Matern.-Fetal Neonatal Med. 2021, 35, 7601–7608. [Google Scholar] [CrossRef] [PubMed]
- Scarrone, M.; Salmeri, N.; Buzzaccarini, G.; Canti, V.; Pasi, F.; Papaleo, E.; Rovere-Querini, P.; Candiani, M.; Alteri, A.; Busnelli, A.; et al. Low-molecular-weight heparin in the prevention of unexplained recurrent miscarriage: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 14168. [Google Scholar] [CrossRef]
- Scarrone, M.; Canti, V.; Vanni, V.S.; Bordoli, S.; Pasi, F.; Quaranta, L.; Erra, R.; De Lorenzo, R.; Rosa, S.; Castiglioni, M.T.; et al. Treating unexplained recurrent pregnancy loss based on lessons learned from obstetric antiphospholipid syndrome and inherited thrombophilia: A propensity-score adjusted retrospective study. J. Reprod. Immunol. 2022, 154, 103760. [Google Scholar] [CrossRef]
- Quenby, S.; Booth, K.; Hiller, L.; Coomarasamy, A.; de Jong, P.G.; Hamulyák, E.N.; Scheres, L.J.; van Haaps, T.F.; Ewington, L.; Tewary, S.; et al. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): An international open-label, randomised controlled trial. Lancet 2023, 402, 54–61. [Google Scholar] [CrossRef]
- Giouleka, S.; Tsakiridis, I.; Arsenaki, E.; Kalogiannidis, I.; Mamopoulos, A.; Papanikolaou, E.; Athanasiadis, A.; Dagklis, T. Investigation and Management of Recurrent Pregnancy Loss: A Comprehensive Review of Guidelines. Obstet. Gynecol. Surv. 2023, 78, 287–301. [Google Scholar] [CrossRef]
- Kuroda, K.; Matsumura, Y.; Ikemoto, Y.; Segawa, T.; Hashimoto, T.; Fukuda, J.; Nakagawa, K.; Uchida, T.; Ochiai, A.; Horimoto, Y.; et al. Analysis of the risk factors and treatment for repeated implantation failure: OPtimization of Thyroid function, IMmunity, and Uterine Milieu (OPTIMUM) treatment strategy. Am. J. Reprod. Immunol. 2021, 85, e13376. [Google Scholar] [CrossRef]
- Kuroda, K.; Horikawa, T.; Moriyama, A.; Ojiro, Y.; Takamizawa, S.; Watanabe, H.; Maruyama, T.; Nojiri, S.; Nakagawa, K.; Sugiyama, R. Therapeutic efficacy of the optimization of thyroid function, thrombophilia, immunity and uterine milieu (OPTIMUM) treatment strategy on pregnancy outcomes after single euploid blastocyst transfer in advanced age women with recurrent reproductive failure. Reprod. Med. Biol. 2023, 22, e12554. [Google Scholar] [CrossRef]
- Mohammad-Akbari, A.; Mohazzab, A.; Tavakoli, M.; Karimi, A.; Zafardoust, S.; Zolghadri, Z.; Shahali, S.; Tokhmechi, R.; Ansaripour, S. The effect of low-molecular-weight heparin on live birth rate of patients with unexplained early recurrent pregnancy loss: A two-arm randomized clinical trial. J. Res. Med. Sci. 2022, 27, 78. [Google Scholar] [CrossRef] [PubMed]
- Dolitzky, M.; Inbal, A.; Segal, Y.; Weiss, A.; Brenner, B.; Carp, H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil. Steril. 2006, 86, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.I.; Perkins, N.J.; Sjaarda, L.A.; Mumford, S.L.; Platt, R.W.; Silver, R.M.; Schisterman, E.F. The Effect of Preconception-Initiated Low-Dose Aspirin on Human Chorionic Gonadotropin-Detected Pregnancy, Pregnancy Loss, and Live Birth: Per Protocol Analysis of a Randomized Trial. Ann. Intern. Med. 2021, 174, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Silver, R.M.; Sjaarda, L.A.; Wactawski-Wende, J.; Townsend, J.M.; Lynch, A.M.; Galai, N.; Lesher, L.L.; Faraggi, D.; Perkins, N.J.; et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum. Reprod. 2016, 31, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.; Hellgren, M.; Strandell, A. Acetylsalicylic acid does not prevent first-trimester unexplained recurrent pregnancy loss: A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2018, 97, 1365–1372. [Google Scholar] [CrossRef]
- Ikemoto, Y.; Kuroda, K.; Nakagawa, K.; Ochiai, A.; Ozaki, R.; Murakami, K.; Jinushi, M.; Matsumoto, A.; Sugiyama, R.; Takeda, S. Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients 2018, 10, 902. [Google Scholar] [CrossRef]
- Ota, K.; Dambaeva, S.; Kim, M.W.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015, 45, 3188–3199. [Google Scholar] [CrossRef]
- Ichikawa, T.; Toyoshima, M.; Watanabe, T.; Negishi, Y.; Kuwabara, Y.; Takeshita, T.; Suzuki, S. Associations of Nutrients and Dietary Preferences with Recurrent Pregnancy Loss and Infertility. J. Nippon Med. Sch. 2024, 91, 254–260. [Google Scholar] [CrossRef]
- Chen, X.; Yin, B.; Lian, R.C.; Zhang, T.; Zhang, H.Z.; Diao, L.H.; Li, Y.Y.; Huang, C.Y.; Liang, D.S.; Zeng, Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016, 76, 432–438. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Pilarski, N.S.P.; Markland, A.D.; Marson, E.J.; Devall, A.; Hewison, M.; Morris, R.K.; Coomarasamy, A. Vitamin D and miscarriage: A systematic review and meta-analysis. Fertil. Steril. 2022, 118, 111–122. [Google Scholar] [CrossRef]
- Raghupathy, R.; Szekeres-Bartho, J. Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. Int. J. Mol. Sci. 2022, 23, 1333. [Google Scholar] [CrossRef]
- Lee, J.H.; Lydon, J.P.; Kim, C.H. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur. J. Immunol. 2012, 42, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Moldenhauer, L.M.; Groome, H.M.; Sharkey, D.J.; Chin, P.Y.; Care, A.S.; Robker, R.L.; McColl, S.R.; Robertson, S.A. Regulatory T cells are paramount effectors in progesterone regulation of embryo implantation and fetal growth. JCI Insight 2023, 8, e162995. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.M.; Hathaway, T.J.; Ramsey, P.S. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst. Rev. 2019, 2019, CD003511. [Google Scholar] [CrossRef] [PubMed]
- Devall, A.J.; Papadopoulou, A.; Haas, D.M.; Price, M.J.; Coomarasamy, A.; Gallos, I.D. Progestogens for preventing miscarriage: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 4, CD013792. [Google Scholar] [CrossRef]
- Zhao, Y.; D’Souza, R.; Gao, Y.; Hao, Q.; Kallas-Silva, L.; Steen, J.P.; Guyatt, G. Progestogens in women with threatened miscarriage or recurrent miscarriage: A meta-analysis. Acta Obstet. Gynecol. Scand. 2024, 103, 1689–1701. [Google Scholar] [CrossRef]
- Xie, H.; Zeng, H.; He, D.; Liu, N. Effect of intrauterine perfusion of human chorionic gonadotropin before embryo transfer after two or more implantation failures: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 133–138. [Google Scholar] [CrossRef]
- Bakry, M.S.; Eldesouky, E.; Alghazaly, M.M.; Farag, E.; Sultan, E.E.K.; Elazzazy, H.; Mohamed, A.; Ali, S.M.S.; Anwar, A.; Elrashedy, A.A.; et al. Granulocyte colony stimulating factor versus human chorionic gonadotropin for recurrent implantation failure in intra cytoplasmic sperm injection: A randomized clinical trial. BMC Pregnancy Childbirth 2022, 22, 881. [Google Scholar] [CrossRef]
- Amooee, S.; Shomali, Z.; Namazi, N.; Jannati, F. Is There any Role for Granulocyte Colony Stimulating Factor in Improvement of Implantation in Intrauterine Insemination? A Prospective Double-Blind Randomized Control Trial. Int. J. Fertil. Steril. 2022, 16, 281–285. [Google Scholar] [CrossRef]
- Bellver, J.; Marín, C.; Lathi, R.B.; Murugappan, G.; Labarta, E.; Vidal, C.; Giles, J.; Cabanillas, S.; Marzal, A.; Galliano, D.; et al. Obesity Affects Endometrial Receptivity by Displacing the Window of Implantation. Reprod. Sci. 2021, 28, 3171–3180. [Google Scholar] [CrossRef]
- Gonnella, F.; Konstantinidou, F.; Donato, M.; Gatta, D.M.P.; Peserico, A.; Barboni, B.; Stuppia, L.; Nothnick, W.B.; Gatta, V. The Molecular Link between Obesity and the Endometrial Environment: A Starting Point for Female Infertility. Int. J. Mol. Sci. 2024, 25, 6855. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.C.R.A.; Feitosa, B.M.; Cavalcante, B.V.; Lima, A.L.G.S.B.; de Souza, C.M.; Joventino, L.B.; Cavalcante, M.B. Obesity and recurrent miscarriage: The interconnections between adipose tissue and the immune system. Am. J. Reprod. Immunol. 2023, 90, e13757. [Google Scholar] [CrossRef] [PubMed]
- Ramidi, G.; Khan, N.; Glueck, C.J.; Wang, P.; Goldenberg, N. Enoxaparin-metformin and enoxaparin alone may safely reduce pregnancy loss. Trans. Res. J. Lab. Clin. Med. 2009, 153, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Silverii, G.A. Optimizing metformin therapy in practice: Tailoring therapy in specific patient groups to improve tolerability, efficacy and outcomes. Diabetes Obes. Metab. 2024, 26 (Suppl. 3), 42–54. [Google Scholar] [CrossRef]
- Rajeev, D.; MacIver, N.J. Metformin as a Therapeutic Agent for Obesity-Associated Immune Dysfunction. J. Nutr. 2024, 154, 2534–2542. [Google Scholar] [CrossRef]
- Sola-Leyva, A.; Pathare, A.D.S.; Apostolov, A.; Aleksejeva, E.; Kask, K.; Tammiste, T.; Ruiz-Durán, S.; Risal, S.; Acharya, G.; Salumets, A. The hidden impact of GLP-1 receptor agonists on endometrial receptivity and implantation. Acta Obstet. Gynecol. Scand. 2024. [Google Scholar] [CrossRef]
- Maslin, K.; Alkutbe, R.; Gilbert, J.; Pinkney, J.; Shawe, J. What is known about the use of weight loss medication in women with overweight/obesity on fertility and reproductive health outcomes? A scoping review. Clin. Obes. 2024, 14, e12690. [Google Scholar] [CrossRef]
- Dang, D.; Dearholt, S.; Bissett, K.; Ascenzi, J.; Whalen, M. Johns Hopkins Evidence-Based Practice for Nurses and Healthcare Professionals: Model and Guidelines, 4th ed.; Sigma Theta Tau International: Indianapolis, IN, USA, 2022. [Google Scholar]
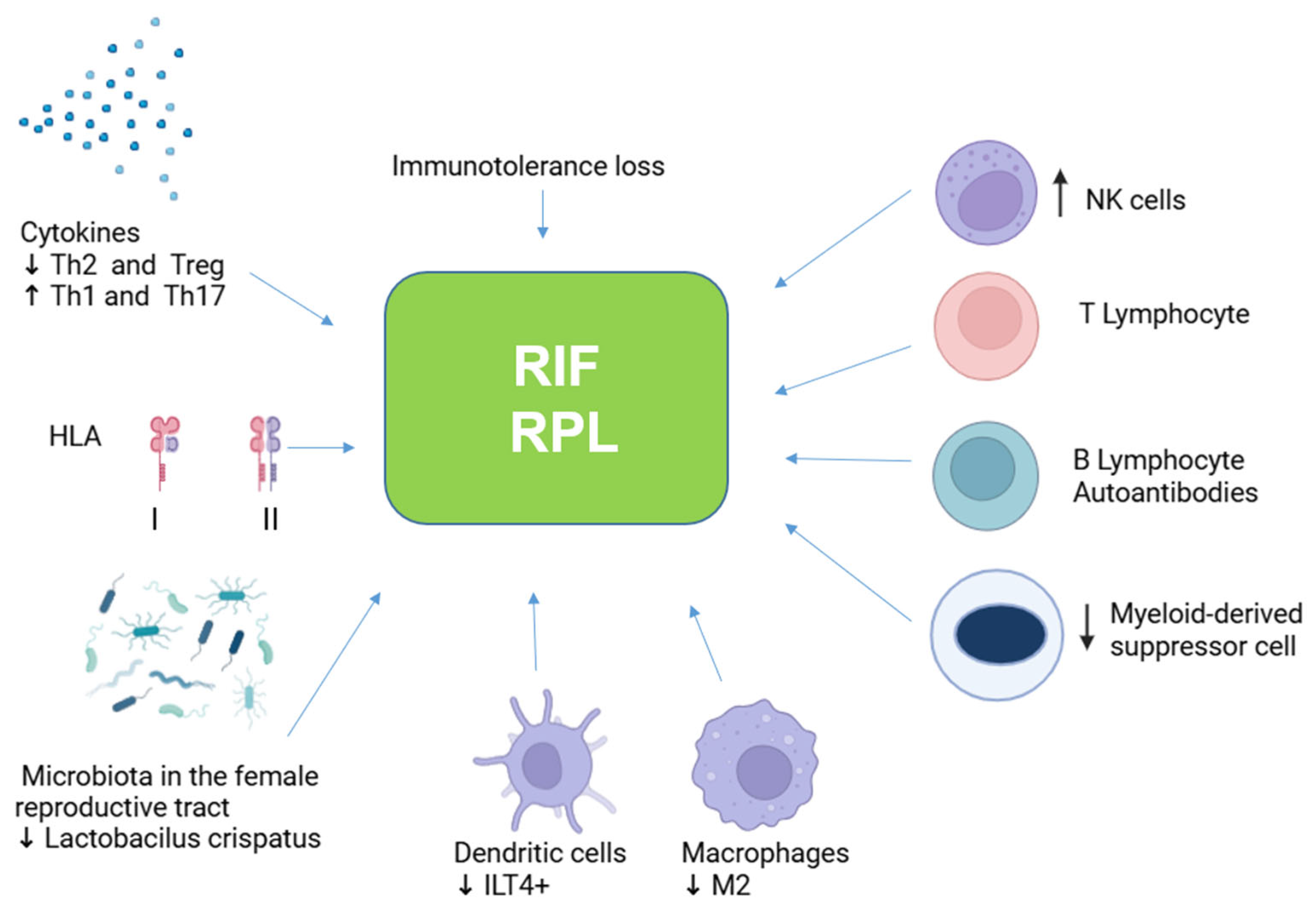
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure. Int. J. Mol. Sci. 2025, 26, 1295. https://doi.org/10.3390/ijms26031295
Garmendia JV, De Sanctis CV, Hajdúch M, De Sanctis JB. Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure. International Journal of Molecular Sciences. 2025; 26(3):1295. https://doi.org/10.3390/ijms26031295
Chicago/Turabian StyleGarmendia, Jenny Valentina, Claudia Valentina De Sanctis, Marián Hajdúch, and Juan Bautista De Sanctis. 2025. "Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure" International Journal of Molecular Sciences 26, no. 3: 1295. https://doi.org/10.3390/ijms26031295
APA StyleGarmendia, J. V., De Sanctis, C. V., Hajdúch, M., & De Sanctis, J. B. (2025). Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure. International Journal of Molecular Sciences, 26(3), 1295. https://doi.org/10.3390/ijms26031295







