The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology
Abstract
1. The Endocannabinoid System (ECS)
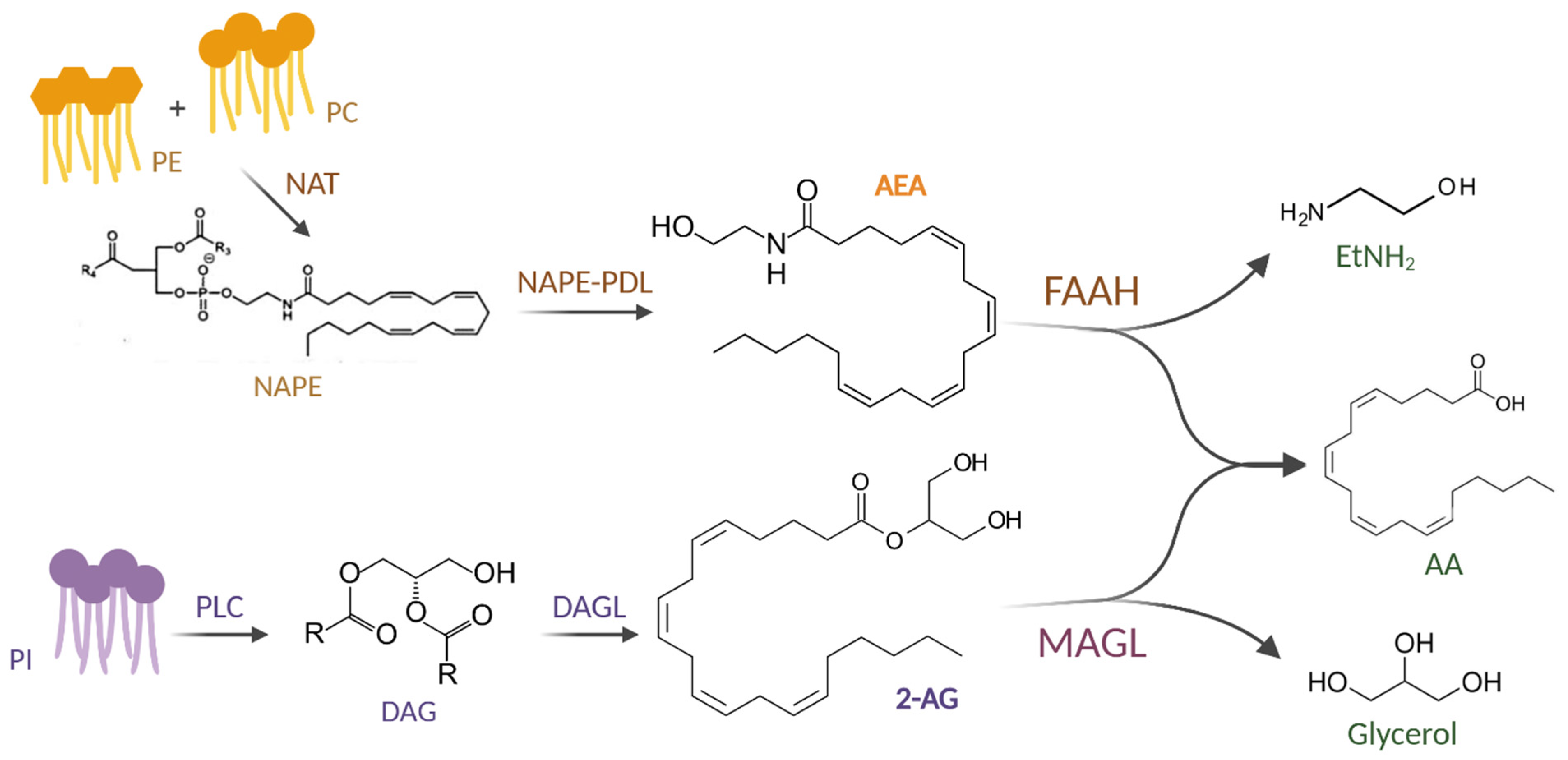
1.1. Endocannabinoids (eCBs)
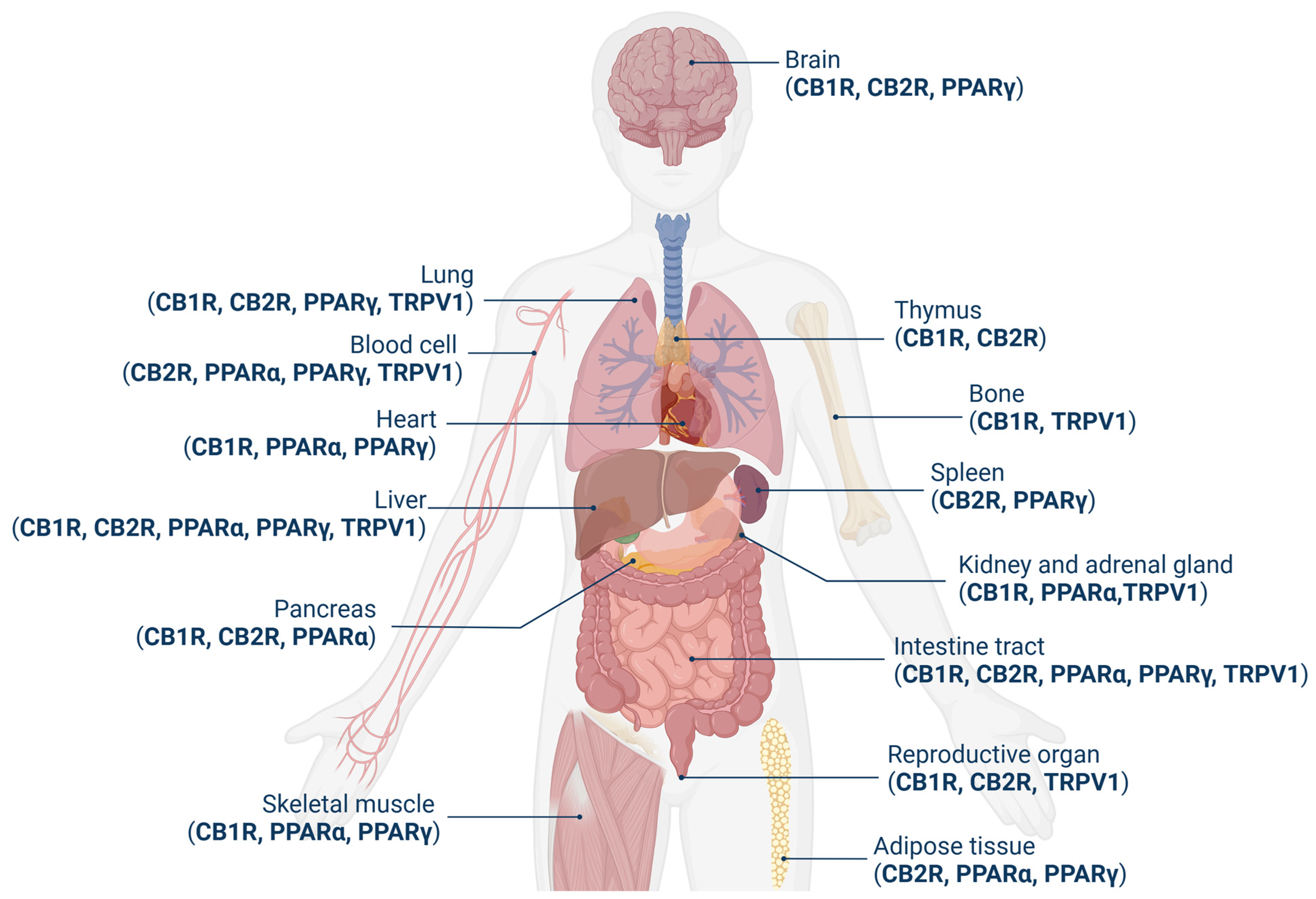
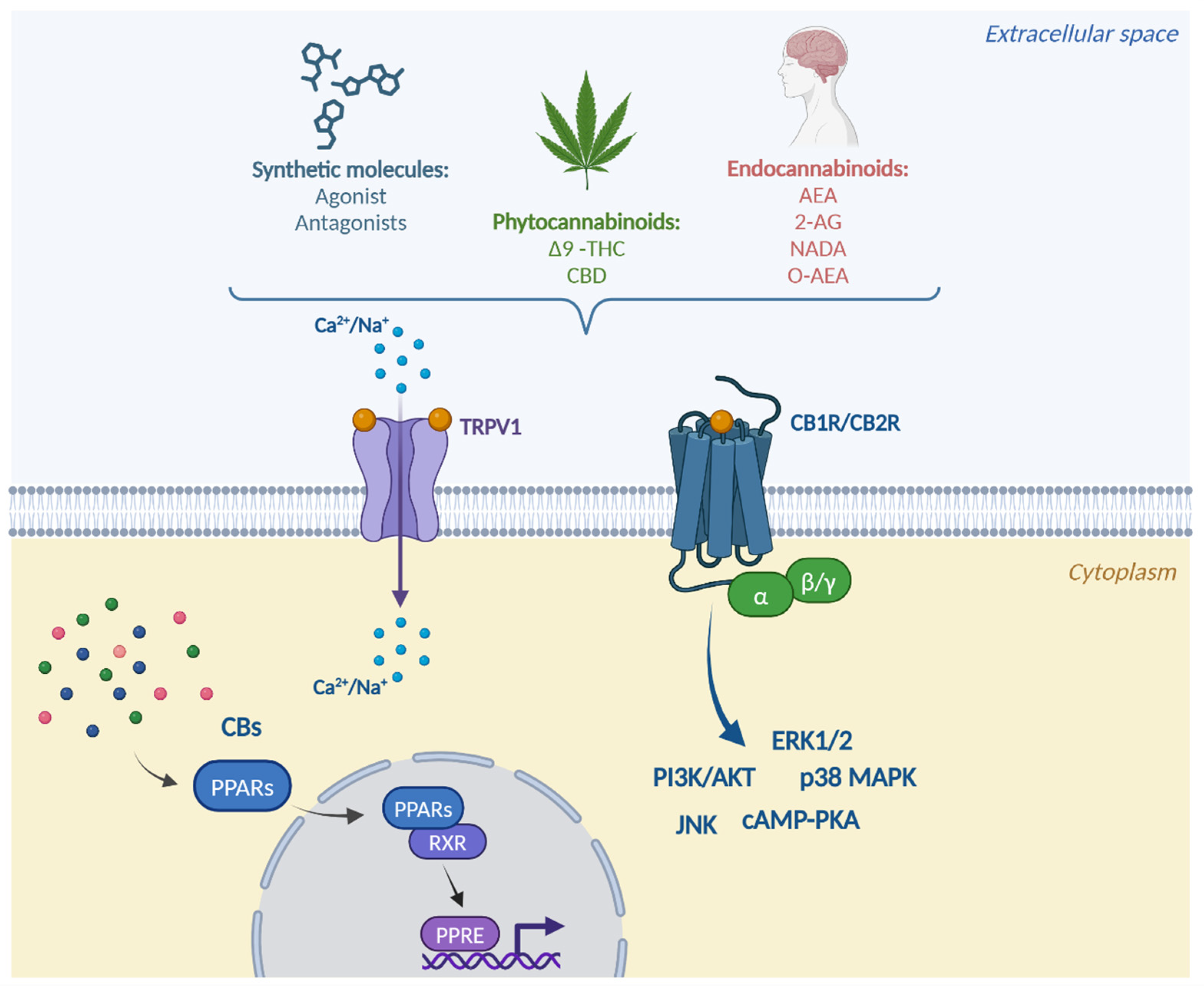
1.2. Receptor System
1.3. Signaling Mechanisms
2. The ECS and Gastrointestinal Physiology
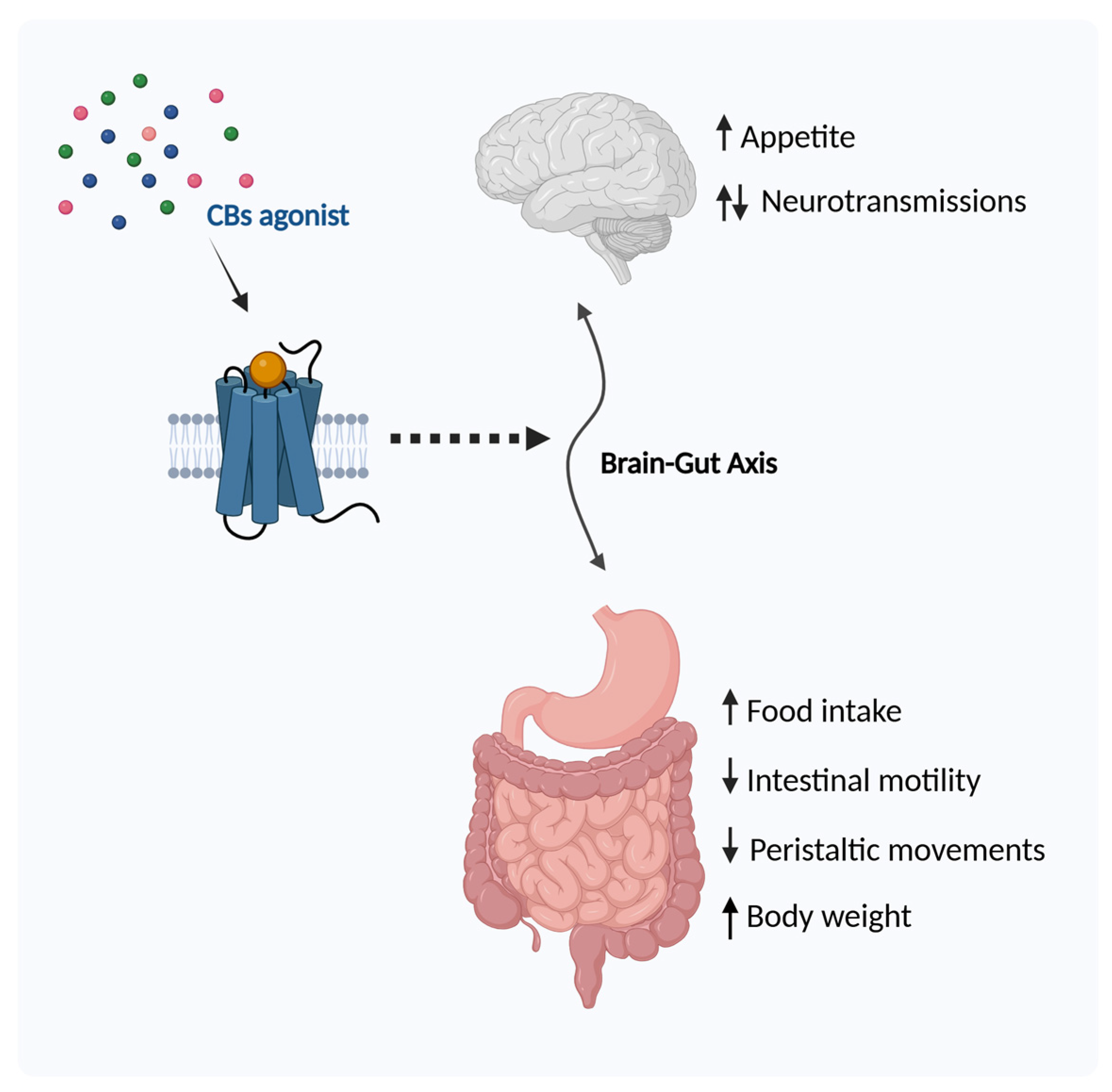
2.1. Regulation of Food Intake
2.2. Obesity
2.3. Intestinal Motility
3. ECS and Gastrointestinal Pathology
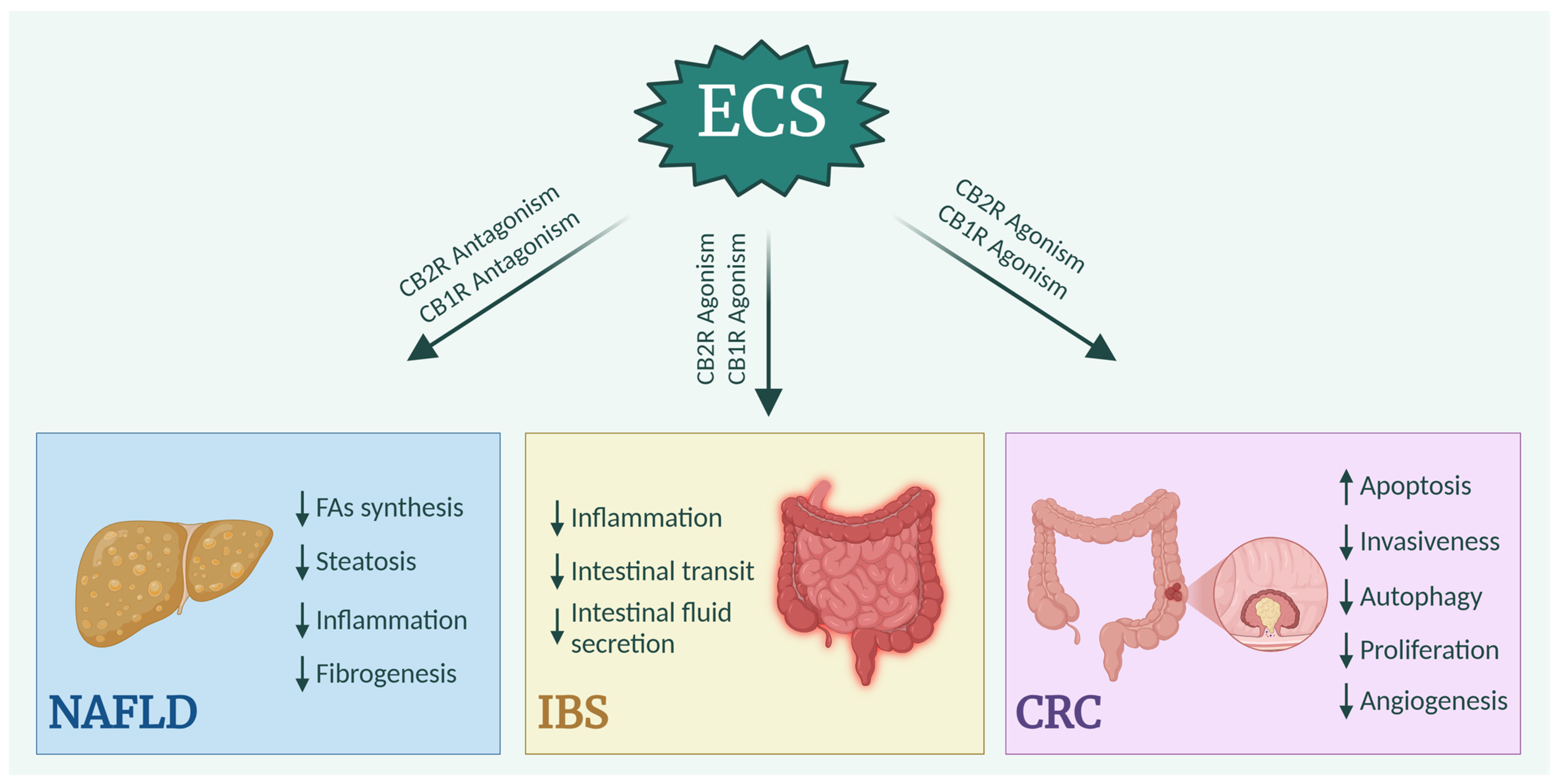
3.1. Non-Alcoholic Fatty Liver Disease
3.2. Irritable Bowel Syndrome
3.3. Colorectal Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Lee, Y.; Jo, J.; Chung, H.Y.; Pothoulakis, C.; Im, E. Endocannabinoids in the Gastrointestinal Tract. Am. J. Physiol. -Gastrointest. Liver Physiol. 2016, 311, G655–G666. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Di Tommaso, M.; Bari, M.; Maccarrone, M. The Endocannabinoid System: An Overview. Front. Behav. Neurosci. 2012, 6, 19633. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef]
- Simard, M.; Archambault, A.-S.; Lavoie, J.-P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and Metabolism of Endocannabinoids and Their Congeners from the Monoacylglycerol and N-Acyl-Ethanolamine Families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB 1 and CB 2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L. De The Endocannabinoid System and Its Therapeutic Exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid Receptor Localization in Brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical Distribution of Cannabinoid CB1 Receptors in the Rat Central Nervous System. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid Activation at Hepatic CB1 Receptors Stimulates Fatty Acid Synthesis and Contributes to Diet-Induced Obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; Lazzari, R.; Macchi, V.; Porzionato, A.; Variola, A.; Colavito, D.; Leon, A.; Guido, M.; Baldo, V.; De Caro, R.; et al. Hepatic Expression of Endocannabinoid Receptors and Their Novel Polymorphisms in Primary Biliary Cirrhosis. J. Gastroenterol. 2010, 45, 68–76. [Google Scholar] [CrossRef]
- Brown, S.M.; Wager-Miller, J.; Mackie, K. Cloning and Molecular Characterization of the Rat CB2 Cannabinoid Receptor. Biochim. Biophys. Acta BBA—Gene Struct. Expr. 2002, 1576, 255–264. [Google Scholar] [CrossRef]
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in Liver Disease. Hepatology 2011, 53, 346–355. [Google Scholar] [CrossRef]
- Klein, T.W. Cannabinoid-Based Drugs as Anti-Inflammatory Therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Zamora-Valdes, D.; Pichardo-Bahena, R.; Barredo-Prieto, B.; Ponciano-Rodriguez, G.; Bermejo-Martínez, L.; Chavez-Tapia, N.C.; Baptista-González, H.A.; Uribe, M. Endocannabinoid Receptor CB2 in Nonalcoholic Fatty Liver Disease. Liver Int. 2007, 27, 215–219. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic Role of the Cannabinoid Receptor CB2 in the Liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of Cannabinoid Receptors CB1 and CB2 Correlates with Improved Prognosis of Patients with Hepatocellular Carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Ahmadiani, A.; Rahimian, R.; Grolla, A.A.; Moradi, F.; Haeri, A. WIN55212-2 Attenuates Amyloid-Beta-Induced Neuroinflammation in Rats through Activation of Cannabinoid Receptors and PPAR-γ Pathway. Neuropharmacology 2012, 63, 653–666. [Google Scholar] [CrossRef]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5Y APP+ Cells Through PPARγ Involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Pistis, M.; Melis, M. From Surface to Nuclear Receptors: The Endocannabinoid Family Extends Its Assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Bouaboula, M.; Hilairet, S.; Marchand, J.; Fajas, L.; Fur, G.L.; Casellas, P. Anandamide Induced PPARγ Transcriptional Activation and 3T3-L1 Preadipocyte Differentiation. Eur. J. Pharmacol. 2005, 517, 174–181. [Google Scholar] [CrossRef]
- Takeyama, K.; Kodera, Y.; Suzawa, M.; Kato, S. Peroxisome Proliferator-Activated Receptor(PPAR)--Structure, Function, Tissue Distribution, Gene Expression. Nihon Rinsho 2000, 58, 357–363. [Google Scholar]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in Health and Disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Snider, N.T.; Thompson, J.T.; Vanden Heuvel, J.P.; Kaminski, N.E. Interleukin-2 Suppression by 2-Arachidonyl Glycerol Is Mediated through Peroxisome Proliferator-Activated Receptor γ Independently of Cannabinoid Receptors 1 and 2. Mol. Pharmacol. 2006, 70, 101–111. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Fleming, R.M. Cannabinoid Inhibition of Adenylate Cyclase. Pharmacology of the Response in Neuroblastoma Cell Membranes. Mol. Pharmacol. 1984, 26, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Howlett, A.C.; Mukhopadhyay, S. Cellular Signal Transduction by Anandamide and 2-Arachidonoylglycerol. Chem. Phys. Lipids 2000, 108, 53–70. [Google Scholar] [CrossRef]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human Lung-Resident Macrophages Express CB1 and CB2 Receptors Whose Activation Inhibits the Release of Angiogenic and Lymphangiogenic Factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef]
- Ozaita, A.; Puighermanal, E.; Maldonado, R. Regulation of PI3K/Akt/GSK-3 Pathway by Cannabinoids in the Brain. J. Neurochem. 2007, 102, 1105–1114. [Google Scholar] [CrossRef]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of Cell Death Protease Caspase-9 by Phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Chan, L.S.A.; Wells, R.A. Cross-Talk between PPARs and the Partners of RXR: A Molecular Perspective. PPAR Res. 2009, 2009, 925309. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.; Udi, S.; Drori, A.; Hadar, R.; Nemirovski, A.; Vemuri, K.V.; Miller, M.; Sherill-Rofe, D.; Arad, Y.; Gur-Wahnon, D.; et al. Reversal of Diet-Induced Hepatic Steatosis by Peripheral CB1 Receptor Blockade in Mice Is P53/MiRNA-22/SIRT1/PPARα Dependent. Mol. Metab. 2020, 42, 101087. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Pilon, C.; Calcagno, A.; Urbanet, R.; Rossato, M.; Milan, G.; Bianchi, K.; Rizzuto, R.; Bernante, P.; Federspil, G.; et al. The Endogenous Cannabinoid System Stimulates Glucose Uptake in Human Fat Cells via Phosphatidylinositol 3-Kinase and Calcium-Dependent Mechanisms. J. Clin. Endocrinol. Metab. 2007, 92, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Gonthier, M.-P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, T.; Wang, X. Activation of Type 2 Cannabinoid Receptors (CB2R) Promotes Fatty Acid Oxidation through the SIRT1/PGC-1α Pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide Induces Microglia Changes Associated with Increased Migration and Phagocytic Activity: Involvement of the CB2 Receptor. Sci. Rep. 2017, 7, 375. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and Desensitization of TRPV1 Channels in Sensory Neurons by the PPARα Agonist Palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin Induces Browning of White Adipose Tissue and Counters Obesity by Activating TRPV1 Channel-dependent Mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Massa, F.; Monory, K. Endocannabinoids and the Gastrointestinal Tract. J. Endocrinol. Investig. 2006, 29, 47–57. [Google Scholar]
- Fride, E.; Bregman, T.; Kirkham, T.C. Endocannabinoids and Food Intake: Newborn Suckling and Appetite Regulation in Adulthood. Exp. Biol. Med. 2005, 230, 225–234. [Google Scholar] [CrossRef]
- Abel, E.L. Cannabis: Effects on Hunger and Thirst. Behav. Biol. 1975, 15, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Trojniar, W.; Wise, R.A. Facilitory Effect of Δ9-Tetrahydrocannabinol on Hypothalamically Induced Feeding. Psychopharmacology 1991, 103, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Kirkham, T.C. Anandamide Induces Overeating: Mediation by Central Cannabinoid (CB1) Receptors. Psychopharmacology 1999, 143, 315–317. [Google Scholar] [CrossRef]
- Jamshidi, N.; Taylor, D.A. Anandamide Administration into the Ventromedial Hypothalamus Stimulates Appetite in Rats. Br. J. Pharmacol. 2001, 134, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Marzo, V. Di Endocannabinoid Levels in Rat Limbic Forebrain and Hypothalamus in Relation to Fasting, Feeding and Satiation: Stimulation of Eating by 2-arachidonoyl Glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef]
- Hanuš, L.; Avraham, Y.; Ben-Shushan, D.; Zolotarev, O.; Berry, E.M.; Mechoulam, R. Short-Term Fasting and Prolonged Semistarvation Have Opposite Effects on 2-AG Levels in Mouse Brain. Brain Res. 2003, 983, 144–151. [Google Scholar] [CrossRef]
- Oveisi, F. Oleoylethanolamide Inhibits Food Intake in Free-Feeding Rats after Oral Administration. Pharmacol. Res. 2004, 49, 461–466. [Google Scholar] [CrossRef]
- Esfandyari, T.; Camilleri, M.; Ferber, I.; Burton, D.; Baxter, K.; Zinsmeister, A.R. Effect of a Cannabinoid Agonist on Gastrointestinal Transit and Postprandial Satiation in Healthy Human Subjects: A Randomized, Placebo-controlled Study. Neurogastroenterol. Motil. 2006, 18, 831–838. [Google Scholar] [CrossRef]
- Coutts, A.A.; Izzo, A.A. The Gastrointestinal Pharmacology of Cannabinoids: An Update. Curr. Opin. Pharmacol. 2004, 4, 572–579. [Google Scholar] [CrossRef]
- Baenas, I.; Miranda-Olivos, R.; Granero, R.; Solé-Morata, N.; Sánchez, I.; Pastor, A.; Del Pino-Gutiérrez, A.; Codina, E.; Tinahones, F.J.; Fernández-Formoso, J.A.; et al. Association of Anandamide and 2-Arachidonoylglycerol Concentrations with Clinical Features and Body Mass Index in Eating Disorders and Obesity. Eur. Psychiatry 2023, 66, e49. [Google Scholar] [CrossRef]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the Peripheral Endocannabinoid System in Human Obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Engeli, S.; Klöting, N.; Berndt, J.; Fasshauer, M.; Bátkai, S.; Pacher, P.; Schön, M.R.; Jordan, J.; Stumvoll, M. Dysregulation of the Peripheral and Adipose Tissue Endocannabinoid System in Human Abdominal Obesity. Diabetes 2006, 55, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Alméras, N.; Després, J.-P.; Di Marzo, V. Circulating Endocannabinoid Levels, Abdominal Adiposity and Related Cardiometabolic Risk Factors in Obese Men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Mancini, G.; Schmidt, H.; Steindel, F.; Mackie, K.; Angioni, C.; Oliet, S.H.R.; Geisslinger, G.; Lutz, B. Alterations in the Hippocampal Endocannabinoid System in Diet-Induced Obese Mice. J. Neurosci. 2010, 30, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S. Effects of the Cannabinoid-1 Receptor Blocker Rimonabant on Weight Reduction and Cardiovascular Risk Factors in Overweight Patients: 1-Year Experience from the RIO-Europe Study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Van Gaal, L.; Pi-Sunyer, X.; Després, J.-P.; McCarthy, C.; Scheen, A. Efficacy and Safety of Rimonabant for Improvement of Multiple Cardiometabolic Risk Factors in Overweight/Obese Patients. Diabetes Care 2008, 31, S229–S240. [Google Scholar] [CrossRef]
- Scheen, A.J. CB1 Receptor Blockade and Its Impact on Cardiometabolic Risk Factors: Overview of the RIO Programme with Rimonabant. J. Neuroendocrinol. 2008, 20, 139–146. [Google Scholar] [CrossRef]
- Cluny, N.; Vemuri, V.; Chambers, A.; Limebeer, C.; Bedard, H.; Wood, J.; Lutz, B.; Zimmer, A.; Parker, L.; Makriyannis, A.; et al. A Novel Peripherally Restricted Cannabinoid Receptor Antagonist, AM6545, Reduces Food Intake and Body Weight, but Does Not Cause Malaise, in Rodents. Br. J. Pharmacol. 2010, 161, 629–642. [Google Scholar] [CrossRef]
- Tam, J.; Cinar, R.; Liu, J.; Godlewski, G.; Wesley, D.; Jourdan, T.; Szanda, G.; Mukhopadhyay, B.; Chedester, L.; Liow, J.-S.; et al. Peripheral Cannabinoid-1 Receptor Inverse Agonism Reduces Obesity by Reversing Leptin Resistance. Cell Metab. 2012, 16, 167–179. [Google Scholar] [CrossRef]
- Ravinet Trillou, C.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrié, P. CB1 Cannabinoid Receptor Knockout in Mice Leads to Leanness, Resistance to Diet-Induced Obesity and Enhanced Leptin Sensitivity. Int. J. Obes. 2004, 28, 640–648. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Ruiz de Azua, I.; Conrad, A.; Purrio, M.; Lutz, B. Cannabinoid CB1 Receptor Deletion from Catecholaminergic Neurons Protects from Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 12635. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte Cannabinoid Receptor CB1 Regulates Energy Homeostasis and Alternatively Activated Macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef] [PubMed]
- Verty, A.N.A.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS ONE 2015, 10, e0140592. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Carpio, O.; Horiuchi, Y.; Shu, A.; Higuchi, S.; Schanz, N.; Benno, R.; Arinami, T.; Onaivi, E.S. A Nonsynonymous Polymorphism in Cannabinoid CB2 Receptor Gene Is Associated with Eating Disorders in Humans and Food Intake Is Modified in Mice by Its Ligands. Synapse 2010, 64, 92–96. [Google Scholar] [CrossRef]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 Cannabinoid Receptor in Mice Improves Insulin Sensitivity but Increases Food Intake and Obesity with Age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef]
- Schmitz, K.; Mangels, N.; Häussler, A.; Ferreirós, N.; Fleming, I.; Tegeder, I. Pro-Inflammatory Obesity in Aged Cannabinoid-2 Receptor-Deficient Mice. Int. J. Obes. 2016, 40, 366–379. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Luongo, L.; Manzo, I.; Tolone, S.; Tortora, C.; Bernardo, M.E.; Grandone, A.; Conforti, A.; Docimo, L.; et al. Cannabinoid Receptor 2 as Antiobesity Target: Inflammation, Fat Storage, and Browning Modulation. J. Clin. Endocrinol. Metab. 2016, 101, 3469–3478. [Google Scholar] [CrossRef]
- Gazzerro, P.; Caruso, M.G.; Notarnicola, M.; Misciagna, G.; Guerra, V.; Laezza, C.; Bifulco, M. Association between Cannabinoid Type-1 Receptor Polymorphism and Body Mass Index in a Southern Italian Population. Int. J. Obes. 2007, 31, 908–912. [Google Scholar] [CrossRef]
- Crater, G.D.; Lalonde, K.; Ravenelle, F.; Harvey, M.; Després, J. Effects of CB1R Inverse Agonist, INV-202, in Patients with Features of Metabolic Syndrome. A Randomized, Placebo-controlled, Double-blind Phase 1b Study. Diabetes Obes. Metab. 2024, 26, 642–649. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Fernando, S.R.; Griffin, G.; Abadji, V.; Makriyannis, A. Effect of Phenylmethylsulphonyl Fluoride on the Potency of Anandamide as an Inhibitor of Electrically Evoked Contractions in Two Isolated Tissue Preparations. Eur. J. Pharmacol. 1995, 272, 73–78. [Google Scholar] [CrossRef]
- Coutts, A.A.; Pertwee, R.G. Evidence That Cannabinoid-Induced Inhibition of Electrically Evoked Contractions of the Myenteric Plexus--Longitudinal Muscle Preparation of Guinea-Pig Small Intestine Can Be Modulated by Ca2+ and CAMP. Can. J. Physiol. Pharmacol. 1998, 76, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Mang, C.F.; Erbelding, D.; Kilbinger, H. Differential Effects of Anandamide on Acetylcholine Release in the Guinea-pig Ileum Mediated via Vanilloid and Non-CB 1 Cannabinoid Receptors. Br. J. Pharmacol. 2001, 134, 161–167. [Google Scholar] [CrossRef]
- Pinto, L.; Izzo, A.A.; Mascolo, N.; Capasso, F.; Cascio, M.G.; Bisogno, T.; Di Marzo, V.; Hospodar–Scott, K.; Brown, D.R. Endocannabinoids as Physiological Regulators of Colonic Propulsion in Mice. Gastroenterology 2002, 123, 227–234. [Google Scholar] [CrossRef]
- Capasso, R.; Matias, I.; Lutz, B.; Borrelli, F.; Capasso, F.; Marsicano, G.; Mascolo, N.; Petrosino, S.; Monory, K.; Valenti, M.; et al. Fatty Acid Amide Hydrolase Controls Mouse Intestinal Motility In Vivo. Gastroenterology 2005, 129, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Sałaga, M.; Stuart, J.; Saur, D.; Sobczak, M.; Zatorski, H.; Timmermans, J.-P.; Bradshaw, H.B.; Ahn, K.; Storr, M.A. Selective Inhibition of FAAH Produces Antidiarrheal and Antinociceptive Effect Mediated by Endocannabinoids and Cannabinoid-like Fatty Acid Amides. Neurogastroenterol. Motil. 2014, 26, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Grider, J.R.; Mahavadi, S.; Li, Y.; Qiao, L.-Y.; Kuemmerle, J.F.; Murthy, K.S.; Martin, B.R. Modulation of Motor and Sensory Pathways of the Peristaltic Reflex by Cannabinoids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G539–G549. [Google Scholar] [CrossRef]
- Mathison, R.; Ho, W.; Pittman, Q.J.; Davison, J.S.; Sharkey, K.A. Effects of Cannabinoid Receptor-2 Activation on Accelerated Gastrointestinal Transit in Lipopolysaccharide-treated Rats. Br. J. Pharmacol. 2004, 142, 1247–1254. [Google Scholar] [CrossRef]
- Hinds, N.M.; Ullrich, K.; Smid, S.D. Cannabinoid 1 (CB1) Receptors Coupled to Cholinergic Motorneurones Inhibit Neurogenic Circular Muscle Contractility in the Human Colon. Br. J. Pharmacol. 2006, 148, 191–199. [Google Scholar] [CrossRef]
- Zheng, T.; BouSaba, J.; Taylor, A.; Dilmaghani, S.; Busciglio, I.; Carlson, P.; Torres, M.; Ryks, M.; Burton, D.; Harmsen, W.S.; et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin. Gastroenterol. Hepatol. 2023, 21, 3405–3414.e4. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Elachouri, G.; Gallas, J.F.; Janiak, P.; Marini, P.; Ravinet-Trillou, C.; Chabbert, M.; Cruccioli, N.; Pfersdorff, C.; Roque, C.; et al. Rimonabant Reduces Obesity-Associated Hepatic Steatosis and Features of Metabolic Syndrome in Obese Zucker Fa/Fa Rats. Hepatology 2007, 46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Vemuri, V.K.; Liu, J.; Bátkai, S.; Mukhopadhyay, B.; Godlewski, G.; Osei-Hyiaman, D.; Ohnuma, S.; Ambudkar, S.V.; Pickel, J.; et al. Peripheral CB1 Cannabinoid Receptor Blockade Improves Cardiometabolic Risk in Mouse Models of Obesity. J. Clin. Investig. 2010, 120, 2953–2966. [Google Scholar] [CrossRef]
- De Gottardi, A.; Spahr, L.; Ravier-Dall’Antonia, F.; Hadengue, A. Cannabinoid Receptor 1 and 2 Agonists Increase Lipid Accumulation in Hepatocytes. Liver Int. 2010, 30, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Nhieu, J.T.-V.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 Receptor Potentiates Obesity-Associated Inflammation, Insulin Resistance and Hepatic Steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Azar, S.; Nemirovski, A.; Webb, M.; Halpern, Z.; Shibolet, O.; Tam, J. Serum Levels of Endocannabinoids Are Independently Associated with Nonalcoholic Fatty Liver Disease. Obesity 2017, 25, 94–101. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kotronen, A.; Fielding, B.A.; Wahren, J.; Hodson, L.; Perttilä, J.; Seppänen–Laakso, T.; Suortti, T.; Arola, J.; Hultcrantz, R.; et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects with Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 1961–1971.e1. [Google Scholar] [CrossRef]
- Teixeira-Clerc, F.; Julien, B.; Grenard, P.; Van Nhieu, J.T.; Deveaux, V.; Li, L.; Serriere-Lanneau, V.; Ledent, C.; Mallat, A.; Lotersztajn, S. CB1 Cannabinoid Receptor Antagonism: A New Strategy for the Treatment of Liver Fibrosis. Nat. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, L.; Fan, X.; Zhao, X.; Chang, N.; Yang, L.; Li, L. Neutrophil Chemotaxis and NETosis in Murine Chronic Liver Injury via Cannabinoid Receptor 1/Gαi/o/ROS/P38 MAPK Signaling Pathway. Cells 2020, 9, 373. [Google Scholar] [CrossRef]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable Bowel Syndrome. JAMA 2015, 313, 949. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Coutts, A.A.; Irving, A.J.; Mackie, K.; Pertwee, R.G.; Anavi-Goffer, S. Localisation of Cannabinoid CB1 Receptor Immunoreactivity in the Guinea Pig and Rat Myenteric Plexus. J. Comp. Neurol. 2002, 448, 410–422. [Google Scholar] [CrossRef]
- Duncan, M.; Mouihate, A.; Mackie, K.; Keenan, C.M.; Buckley, N.E.; Davison, J.S.; Patel, K.D.; Pittman, Q.J.; Sharkey, K.A. Cannabinoid CB2 Receptors in the Enteric Nervous System Modulate Gastrointestinal Contractility in Lipopolysaccharide-Treated Rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G78–G87. [Google Scholar] [CrossRef]
- McKee, D.P.; Quigley, E.M.M. Intestinal Motility in Irritable Bowel Syndrome: Is IBS a Motility Disorder? Dig. Dis. Sci. 1993, 38, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.Y.; Jin, H.O.; Lee, M.H.; Sun, S.W.; Lee, K.Y. Colonic Motility Abnormality in Patients with Irritable Bowel Syndrome Exhibiting Abdominal Pain and Diarrhea. Am. J. Gastroenterol. 2001, 96, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Cann, P.A.; Read, N.W.; Brown, C.; Hobson, N.; Holdsworth, C.D. Irritable Bowel Syndrome: Relationship of Disorders in the Transit of a Single Solid Meal to Symptom Patterns. Gut 1983, 24, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Chistolini, F.; Marinozzi, G.; Morelli, A. Abnormal Colonic Propagated Activity in Patients with Slow Transit Constipation and Constipation-Predominant Irritable Bowel Syndrome. Digestion 2003, 68, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Engel, B.T.; Schuster, M.M. Irritable Bowel Syndrome. Dig. Dis. Sci. 1980, 25, 404–413. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The Endogenous Cannabinoid System Protects against Colonic Inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-regulation of Anandamide Levels as an Endogenous Mechanism and a Pharmacological Strategy to Limit Colon Inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef]
- Troy-Fioramonti, S.; Demizieux, L.; Gresti, J.; Muller, T.; Vergès, B.; Degrace, P. Acute Activation of Cannabinoid Receptors by Anandamide Reduces Gastrointestinal Motility and Improves Postprandial Glycemia in Mice. Diabetes 2015, 64, 808–818. [Google Scholar] [CrossRef]
- Esfandyari, T.; Camilleri, M.; Busciglio, I.; Burton, D.; Baxter, K.; Zinsmeister, A.R. Effects of a Cannabinoid Receptor Agonist on Colonic Motor and Sensory Functions in Humans: A Randomized, Placebo-Controlled Study. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G137–G145. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Camilleri, M.; Eckert, D.; Carlson, P.; Ryks, M.; Burton, D.; Zinsmeister, A.R. Randomized Pharmacodynamic and Pharmacogenetic Trial of Dronabinol Effects on Colon Transit in Irritable Bowel Syndrome-diarrhea. Neurogastroenterol. Motil. 2012, 24, 358. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Camilleri, M.; Busciglio, I.; Carlson, P.; Szarka, L.A.; Burton, D.; Zinsmeister, A.R. Pharmacogenetic Trial of a Cannabinoid Agonist Shows Reduced Fasting Colonic Motility in Patients with Nonconstipated Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1638–1647.e7. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Mascolo, N.; Borrelli, F.; Capasso, F. Defaecation, Intestinal Fluid Accumulation and Motility in Rodents: Implications of Cannabinoid CB1 Receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 359, 65–70. [Google Scholar] [CrossRef]
- Fichna, J.; Sibaev, A.; Sałaga, M.; Sobczak, M.; Storr, M. The Cannabinoid-1 Receptor Inverse Agonist Taranabant Reduces Abdominal Pain and Increases Intestinal Transit in Mice. Neurogastroenterol. Motil. 2013, 25, e550–e559. [Google Scholar] [CrossRef]
- Camilleri, M.; Carlson, P.; McKinzie, S.; Grudell, A.; Busciglio, I.; Burton, D.; Baxter, K.; Ryks, M.; Zinsmeister, A.R. Genetic Variation in Endocannabinoid Metabolism, Gastrointestinal Motility, and Sensation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G13–G19. [Google Scholar] [CrossRef]
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; et al. Endocannabinoid and Cannabinoid-like Fatty Acid Amide Levels Correlate with Pain-Related Symptoms in Patients with IBS-D and IBS-C: A Pilot Study. PLoS ONE 2013, 8, e85073. [Google Scholar] [CrossRef]
- Capasso, R.; Izzo, A.A.; Fezza, F.; Pinto, A.; Capasso, F.; Mascolo, N.; Di Marzo, V. Inhibitory Effect of Palmitoylethanolamide on Gastrointestinal Motility in Mice. Br. J. Pharmacol. 2001, 134, 945–950. [Google Scholar] [CrossRef]
- Cluny, N.L.; Keenan, C.M.; Lutz, B.; Piomelli, D.; Sharkey, K.A. The Identification of Peroxisome Proliferator-activated Receptor Alpha-independent Effects of Oleoylethanolamide on Intestinal Transit in Mice. Neurogastroenterol. Motil. 2009, 21, 420–429. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.F.; Anand, P.; Ghosh, S. Increased Capsaicin Receptor TRPV1-Expressing Sensory Fibres in Irritable Bowel Syndrome and Their Correlation with Abdominal Pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1–Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [PubMed]
- Karwad, M.A.; Macpherson, T.; Wang, B.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. Oleoylethanolamine and Palmitoylethanolamine Modulate Intestinal Permeability in Vitro via TRPV1 and PPARα. FASEB J. 2017, 31, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The Role of Transient Receptor Potential Vanilloid 1 in Mechanical and Chemical Visceral Hyperalgesia Following Experimental Colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Gigante, I.; Tutino, V.; Russo, F.; De Nunzio, V.; Coletta, S.; Armentano, R.; Crovace, A.; Caruso, M.G.; Orlando, A.; Notarnicola, M. Cannabinoid Receptors Overexpression in a Rat Model of Irritable Bowel Syndrome (IBS) after Treatment with a Ketogenic Diet. Int. J. Mol. Sci. 2021, 22, 2880. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Editorial: Diet, Inflammation and Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2017, 45, 1278–1279. [Google Scholar] [CrossRef]
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2023, 50, 411–427. [Google Scholar] [CrossRef]
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis Induces a Clinical Response in Patients With Crohn’s Disease: A Prospective Placebo-Controlled Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Battista, N.; Biancheri, P.; Rapino, C.; Rovedatti, L.; Astarita, G.; Vanoli, A.; Dainese, E.; Guerci, M.; Piomelli, D.; et al. The Endogenous Cannabinoid System in the Gut of Patients with Inflammatory Bowel Disease. Mucosal Immunol. 2011, 4, 574–583. [Google Scholar] [CrossRef]
- Naftali, T.; Bar-Lev Schleider, L.; Sklerovsky Benjaminov, F.; Lish, I.; Konikoff, F.M.; Ringel, Y. Medical Cannabis for Inflammatory Bowel Disease: Real-Life Experience of Mode of Consumption and Assessment of Side-Effects. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1376–1381. [Google Scholar] [CrossRef]
- Tartakover Matalon, S.; Azar, S.; Meiri, D.; Hadar, R.; Nemirovski, A.; Abu Jabal, N.; Konikoff, F.M.; Drucker, L.; Tam, J.; Naftali, T. Endocannabinoid Levels in Ulcerative Colitis Patients Correlate with Clinical Parameters and Are Affected by Cannabis Consumption. Front. Endocrinol. 2021, 12, 685289. [Google Scholar] [CrossRef]
- Weiss, A.; Friedenberg, F. Patterns of Cannabis Use in Patients with Inflammatory Bowel Disease: A Population Based Analysis. Drug Alcohol Depend. 2015, 156, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Prasad, N.; Ryan, M.; Tangri, S.; Silverberg, M.S.; Gordon, A.; Steinhart, H. Cannabis Use amongst Patients with Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, R.; Omara, E.A.; Salama, A. Cannabis Sativa Alleviates Experimentally Acetic Acid- Induced Ulcerative Colitis in Rats: Targeting CB1/SIRT/MAPK Signaling Pathways. Immunopharmacol. Immunotoxicol. 2025, 47, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Burge, M.; Price, T.; Karapetis, C.S. First-line Therapy for Metastatic Colorectal Cancer: Current Perspectives and Future Directions. Asia-Pac. J. Clin. Oncol. 2019, 15, 3–14. [Google Scholar] [CrossRef]
- Pal, D.; Tyagi, A.; Chandrasekaran, B.; Alattasi, H.; Ankem, M.K.; Sharma, A.K.; Damodaran, C. Suppression of Notch1 and AKT Mediated Epithelial to Mesenchymal Transition by Verrucarin J in Metastatic Colon Cancer. Cell Death Dis. 2018, 9, 798. [Google Scholar] [CrossRef]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids. JNCI J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The Endogenous Cannabinoid Anandamide Inhibits Human Breast Cancer Cell Proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-Tumoral Action of Cannabinoids: Involvement of Sustained Ceramide Accumulation and Extracellular Signal-Regulated Kinase Activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A Pilot Clinical Study of Δ9-Tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Sánchez, C.; Galve-Roperh, I. Control of the Cell Survival/Death Decision by Cannabinoids. J. Mol. Med. 2001, 78, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential Anticancer Agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; González-Feria, L.; Alvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef]
- Pulgar, T.G.D.; Velasco, G.; Sánchez, C.; Haro, A.; Guzmán, M. De Novo-Synthesized Ceramide Is Involved in Cannabinoid-Induced Apoptosis. Biochem. J. 2002, 363, 183–188. [Google Scholar] [CrossRef]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide Accumulation Mediates Cytotoxic Autophagy of Cancer Cells via Autolysosome Destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-Tumoral Action of Cannabinoids on Hepatocellular Carcinoma: Role of AMPK-Dependent Activation of Autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; José Real, P.; Luis Fernández-Luna, J.; Velasco, G.; Malumbres, M.; Guzmán, M.; Blázquez, C.; Carracedo, A.; et al. Cannabinoid Receptors as Novel Targets for the Treatment of Melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef]
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The Cannabinoid Δ9-tetrahydrocannabinol Inhibits RAS-MAPK and PI3K-AKT Survival Signalling and Induces BAD-mediated Apoptosis in Colorectal Cancer Cells. Int. J. Cancer 2007, 121, 2172–2180. [Google Scholar] [CrossRef]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible Endocannabinoid Control of Colorectal Cancer Growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid Receptor Activation Induces Apoptosis through Tumor Necrosis Factor α–Mediated Ceramide De Novo Synthesis in Colon Cancer Cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef] [PubMed]
- Patsos, H.A. The Endogenous Cannabinoid, Anandamide, Induces Cell Death in Colorectal Carcinoma Cells: A Possible Role for Cyclooxygenase 2. Gut 2005, 54, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Patsos, H.A.; Greenhough, A.; Hicks, D.J.; Al Kharusi, M.; Collard, T.J.; Lane, J.D.; Paraskeva, C.; Williams, A.C. The Endogenous Cannabinoid, Anandamide, Induces COX-2-Dependent Cell Death in Apoptosis-Resistant Colon Cancer Cells. Int. J. Oncol. 2010, 37, 187–193. [Google Scholar] [CrossRef]
- Benedicto, A.; Arteta, B.; Duranti, A.; Alonso-Alconada, D. The Synthetic Cannabinoid URB447 Exerts Antitumor and Antimetastatic Effect in Melanoma and Colon Cancer. Pharmaceuticals 2022, 15, 1166. [Google Scholar] [CrossRef]
- Lee, H.-S.; Tamia, G.; Song, H.-J.; Amarakoon, D.; Wei, C.-I.; Lee, S.-H. Cannabidiol Exerts Anti-Proliferative Activity via a Cannabinoid Receptor 2-Dependent Mechanism in Human Colorectal Cancer Cells. Int. Immunopharmacol. 2022, 108, 108865. [Google Scholar] [CrossRef]
- Alenabi, A.; Malekinejad, H. Cannabinoids Pharmacological Effects Are beyond the Palliative Effects: CB2 Cannabinoid Receptor Agonist Induced Cytotoxicity and Apoptosis in Human Colorectal Cancer Cells (HT-29). Mol. Cell. Biochem. 2021, 476, 3285–3301. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive Effect of the Non-Psychotropic Phytocannabinoid Cannabidiol on Experimental Colon Cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Izzo, A.A.; Aviello, G.; Petrosino, S.; Orlando, P.; Marsicano, G.; Lutz, B.; Borrelli, F.; Capasso, R.; Nigam, S.; Capasso, F.; et al. Increased Endocannabinoid Levels Reduce the Development of Precancerous Lesions in the Mouse Colon. J. Mol. Med. 2008, 86, 89–98. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon Carcinogenesis Is Inhibited by the TRPM8 Antagonist Cannabigerol, a Cannabis-Derived Non-Psychotropic Cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Iden, J.A.; Raphael-Mizrahi, B.; Awida, Z.; Naim, A.; Zyc, D.; Liron, T.; Kasher, M.; Livshits, G.; Vered, M.; Gabet, Y. The Anti-Tumorigenic Role of Cannabinoid Receptor 2 in Colon Cancer: A Study in Mice and Humans. Int. J. Mol. Sci. 2023, 24, 4060. [Google Scholar] [CrossRef]
- Linsalata, M.; Notarnicola, M.; Tutino, V.; Bifulco, M.; Santoro, A.; Laezza, C.; Messa, C.; Orlando, A.; Caruso, M.G. Effects of Anandamide on Polyamine Levels and Cell Growth in Human Colon Cancer Cells. Anticancer Res. 2010, 30, 2583–2589. [Google Scholar] [PubMed]
- Tutino, V.; Caruso, M.G.; De Nunzio, V.; Lorusso, D.; Veronese, N.; Gigante, I.; Notarnicola, M.; Giannelli, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and Its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers 2019, 11, 708. [Google Scholar] [CrossRef]
- Notarnicola, M.; Messa, C.; Orlando, A.; Bifulco, M.; Laezza, C.; Gazzerro, P.; Gabriella Caruso, M. Estrogenic Induction of Cannabinoid CB1 Receptor in Human Colon Cancer Cell Lines. Scand. J. Gastroenterol. 2008, 43, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; De Nunzio, V.; Tafaro, A.; Bianco, G.; Gigante, I.; Scavo, M.P.; D’alessandro, R.; Refolo, M.G.; Messa, C.; Caruso, M.G.; et al. Cannabinoid Receptor-1 Up-Regulation in Azoxymethane (AOM)-Treated Mice After Dietary Treatment with Quercetin. Anticancer Res. 2018, 38, 4485–4491. [Google Scholar] [CrossRef]
- Bar-Lev Schleider, L.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective Analysis of Safety and Efficacy of Medical Cannabis in Large Unselected Population of Patients with Cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients with Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal THC Spray in Patients with Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer Mechanisms of Cannabinoids. Curr. Oncol. 2016, 23, 23–32. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Bifulco, M.; Laezza, C. Molecular Mechanism of Cannabinoids in Cancer Progression. Int. J. Mol. Sci. 2021, 22, 3680. [Google Scholar] [CrossRef]
- Thapa, D.; Kang, Y.; Park, P.-H.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Ku, S.K.; Jung, Y.; Kim, J.-A. Anti-Tumor Activity of the Novel Hexahydrocannabinol Analog LYR-8 in Human Colorectal Tumor Xenograft Is Mediated through the Inhibition of Akt and Hypoxia-Inducible Factor-1α Activation. Biol. Pharm. Bull. 2012, 35, 924–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus Acidophilus Modulates Intestinal Pain and Induces Opioid and Cannabinoid Receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-Induced Microbiota Perturbation Causes Gut Endocannabinoidome Changes, Hippocampal Neuroglial Reorganization and Depression in Mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Castonguay-Paradis, S.; Lacroix, S.; Rochefort, G.; Parent, L.; Perron, J.; Martin, C.; Lamarche, B.; Raymond, F.; Flamand, N.; Di Marzo, V.; et al. Dietary Fatty Acid Intake and Gut Microbiota Determine Circulating Endocannabinoidome Signaling beyond the Effect of Body Fat. Sci. Rep. 2020, 10, 15975. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Dione, N.; Lacroix, S.; Taschler, U.; Deschênes, T.; Abolghasemi, A.; Leblanc, N.; Di Marzo, V.; Silvestri, C. Mgll Knockout Mouse Resistance to Diet-Induced Dysmetabolism Is Associated with Altered Gut Microbiota. Cells 2020, 9, 2705. [Google Scholar] [CrossRef]
- Ellermann, M.; Pacheco, A.R.; Jimenez, A.G.; Russell, R.M.; Cuesta, S.; Kumar, A.; Zhu, W.; Vale, G.; Martin, S.A.; Raj, P.; et al. Endocannabinoids Inhibit the Induction of Virulence in Enteric Pathogens. Cell 2020, 183, 650–665.e15. [Google Scholar] [CrossRef]
- Manca, C.; Boubertakh, B.; Leblanc, N.; Deschênes, T.; Lacroix, S.; Martin, C.; Houde, A.; Veilleux, A.; Flamand, N.; Muccioli, G.G.; et al. Germ-Free Mice Exhibit Profound Gut Microbiota-Dependent Alterations of Intestinal Endocannabinoidome Signaling. J. Lipid Res. 2020, 61, 70–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisio Caruso, E.; De Nunzio, V.; Tutino, V.; Notarnicola, M. The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. Int. J. Mol. Sci. 2025, 26, 1306. https://doi.org/10.3390/ijms26031306
Aloisio Caruso E, De Nunzio V, Tutino V, Notarnicola M. The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. International Journal of Molecular Sciences. 2025; 26(3):1306. https://doi.org/10.3390/ijms26031306
Chicago/Turabian StyleAloisio Caruso, Emanuela, Valentina De Nunzio, Valeria Tutino, and Maria Notarnicola. 2025. "The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology" International Journal of Molecular Sciences 26, no. 3: 1306. https://doi.org/10.3390/ijms26031306
APA StyleAloisio Caruso, E., De Nunzio, V., Tutino, V., & Notarnicola, M. (2025). The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. International Journal of Molecular Sciences, 26(3), 1306. https://doi.org/10.3390/ijms26031306





