Biological Activities of Constituents from Rosa roxburghii and Their Mechanisms Based on Network Pharmacology and Biological Verification
Abstract
1. Introduction
2. Results and Discussion
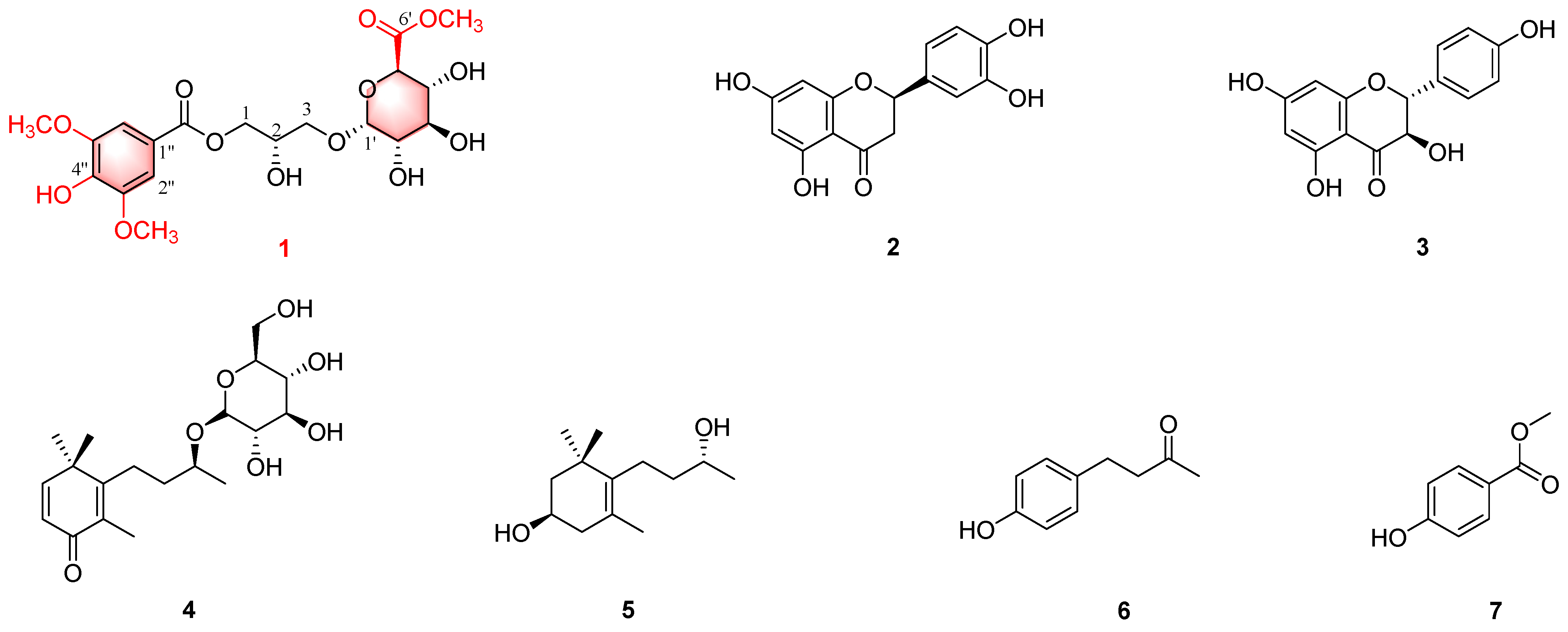
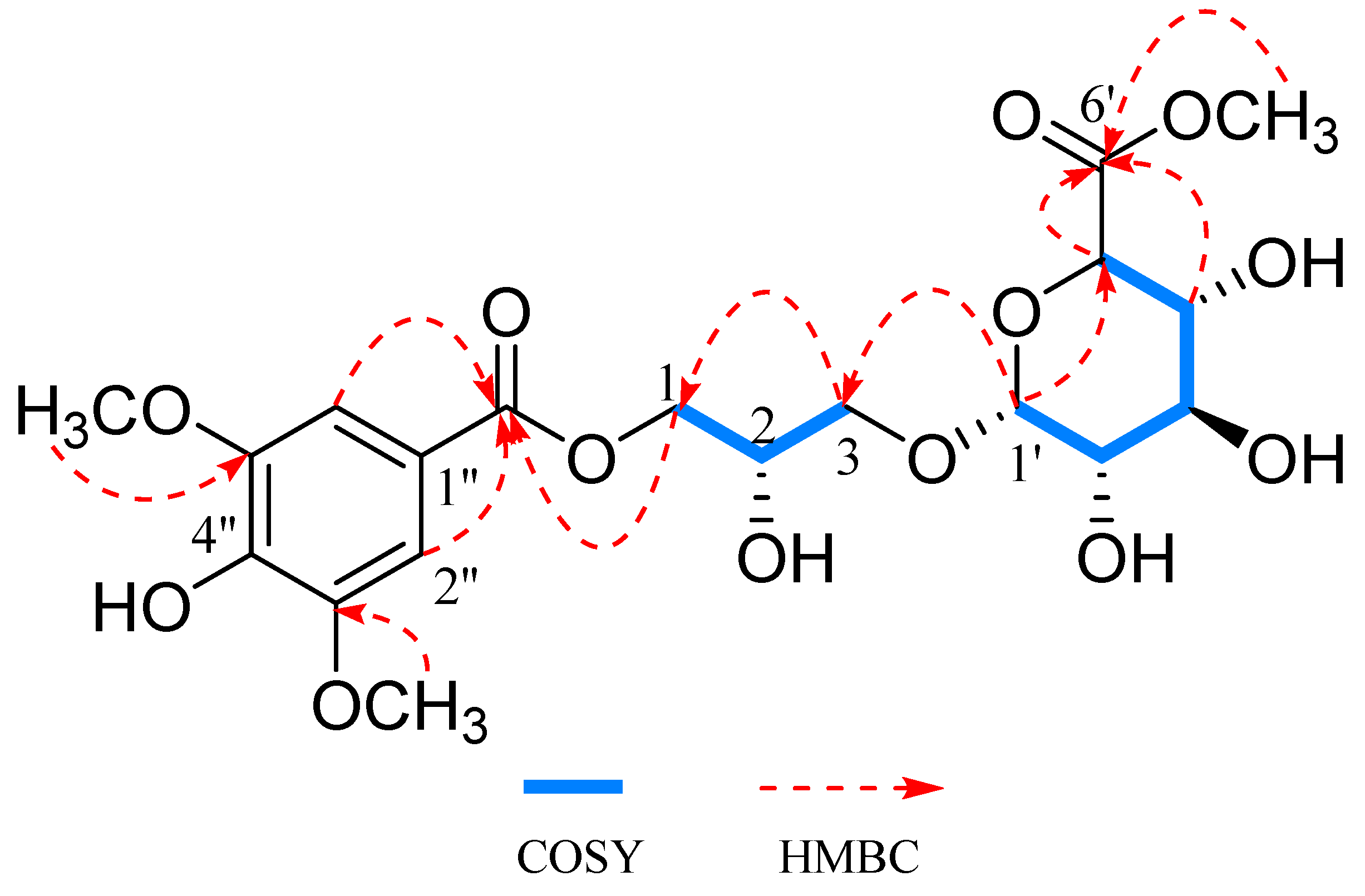
2.1. Structural Elucidation
2.2. Cell Viability of RAW264.7 Cells
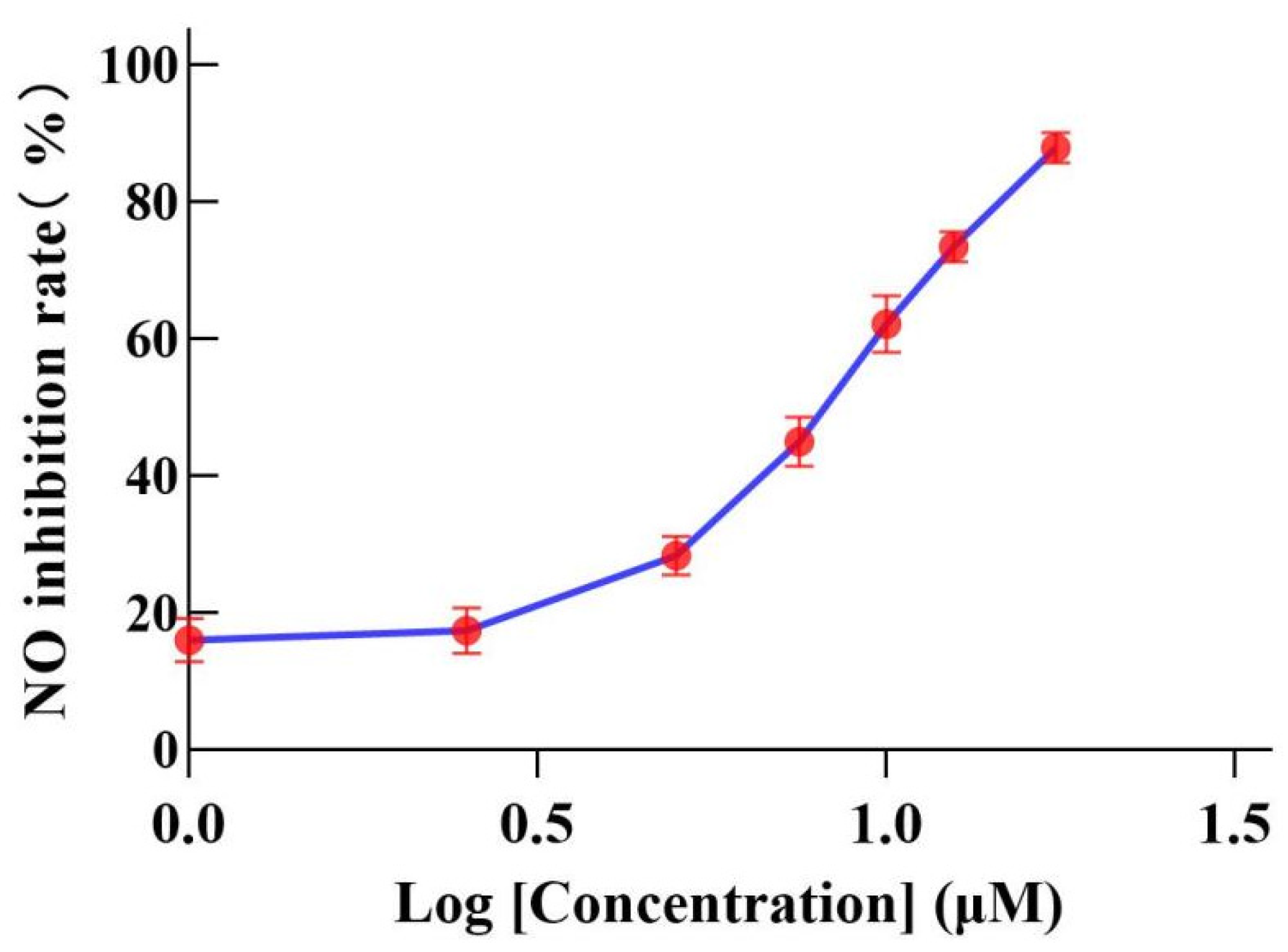
2.3. Inhibition of NO Production Induced by LPS in RAW264.7 Cells
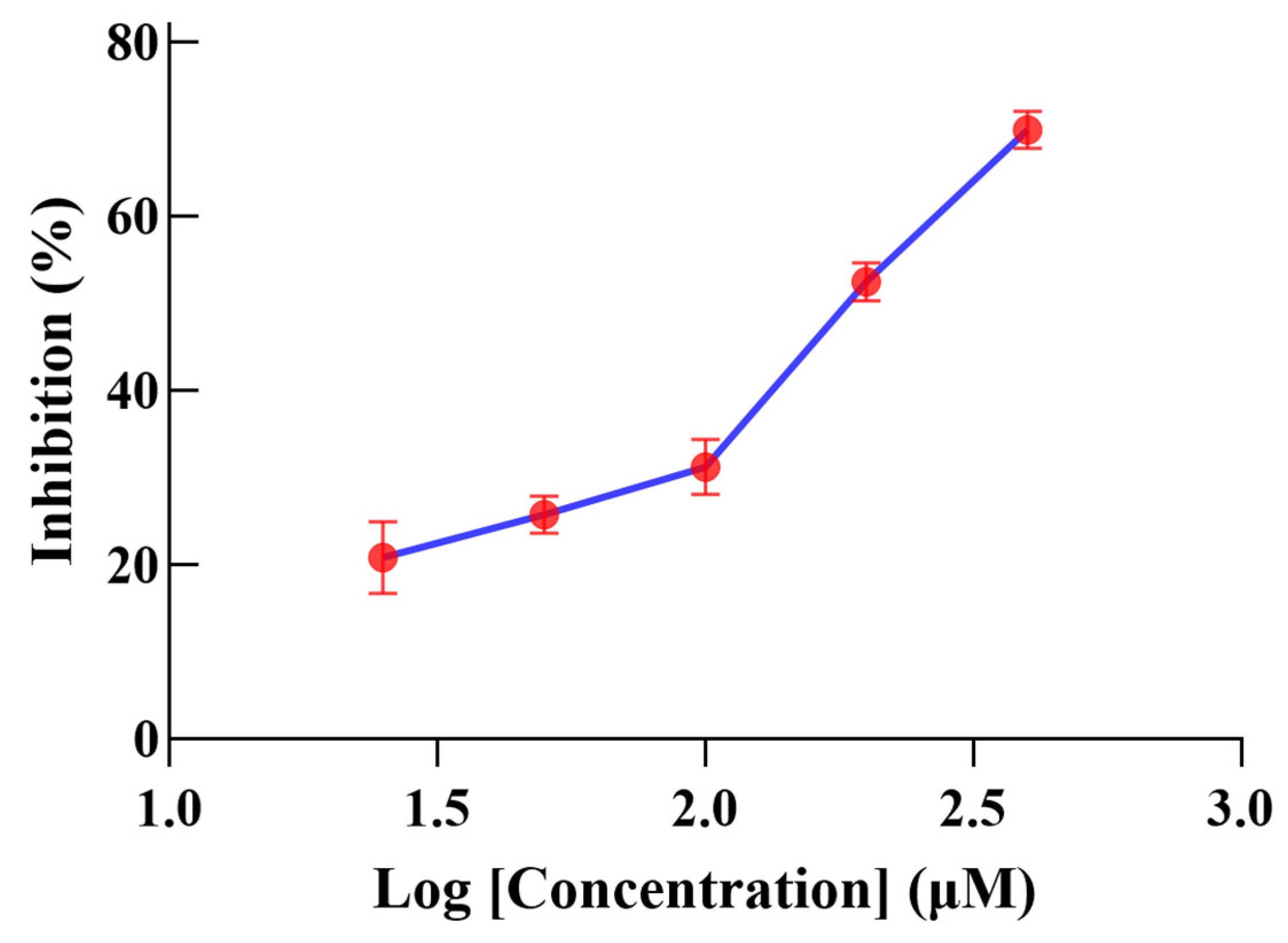
2.4. α-Glucosidase-Inhibitory Activity
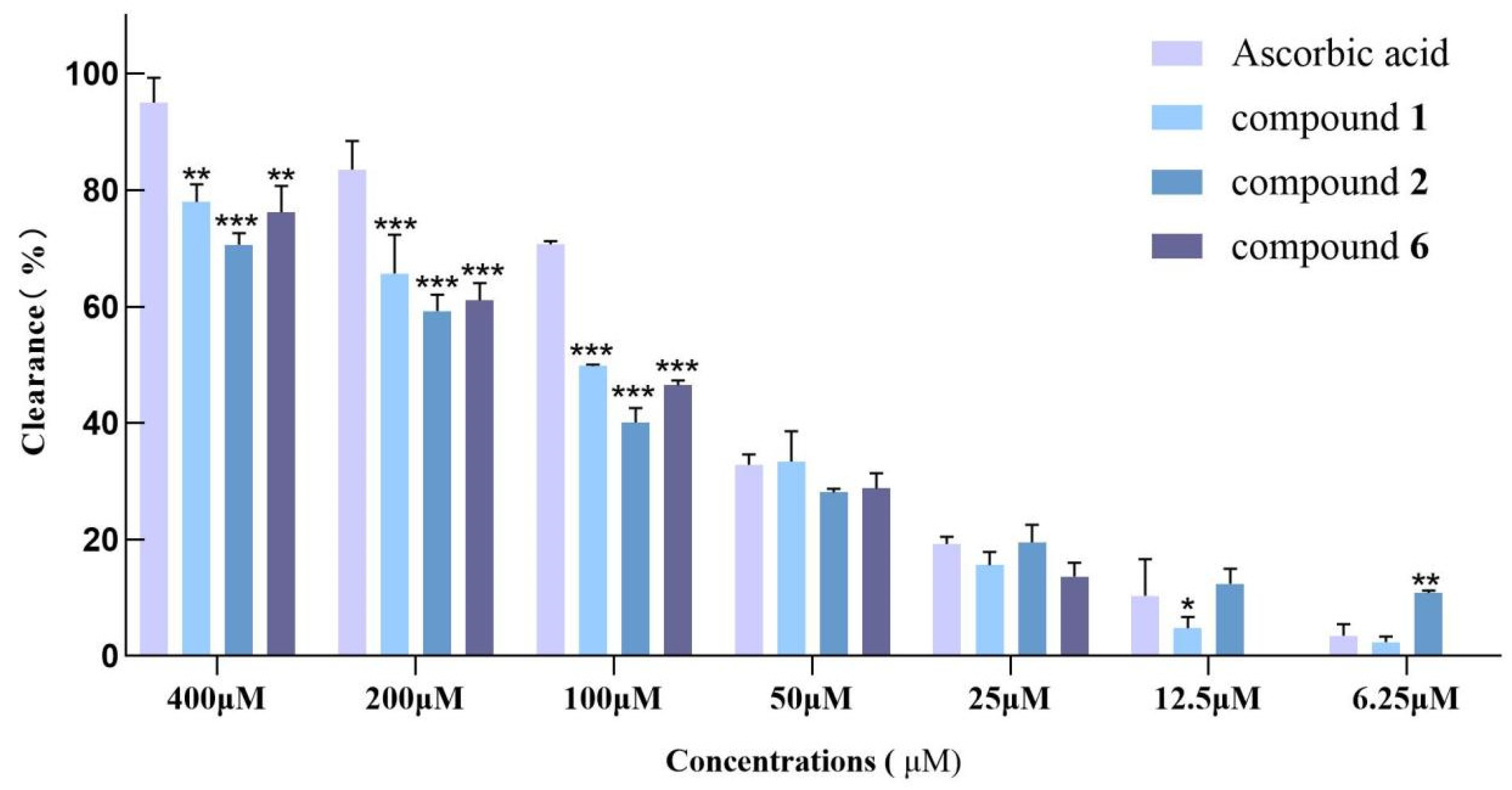
2.5. DPPH and ABTS Free Radical-Scavenging Activity
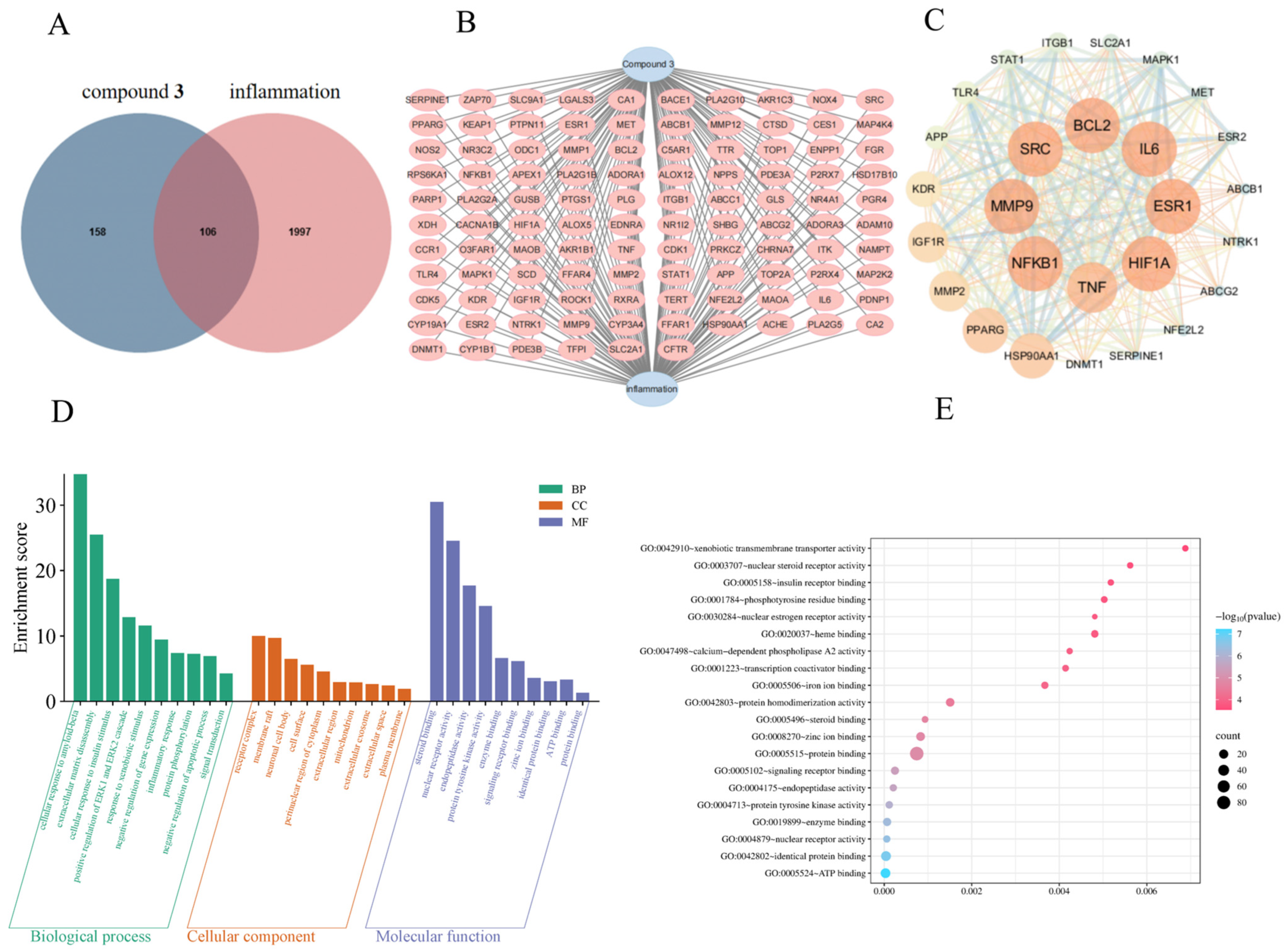
2.6. Network Pharmacology Predicts the Potential Anti-Inflammatory Pathways of Compound 3
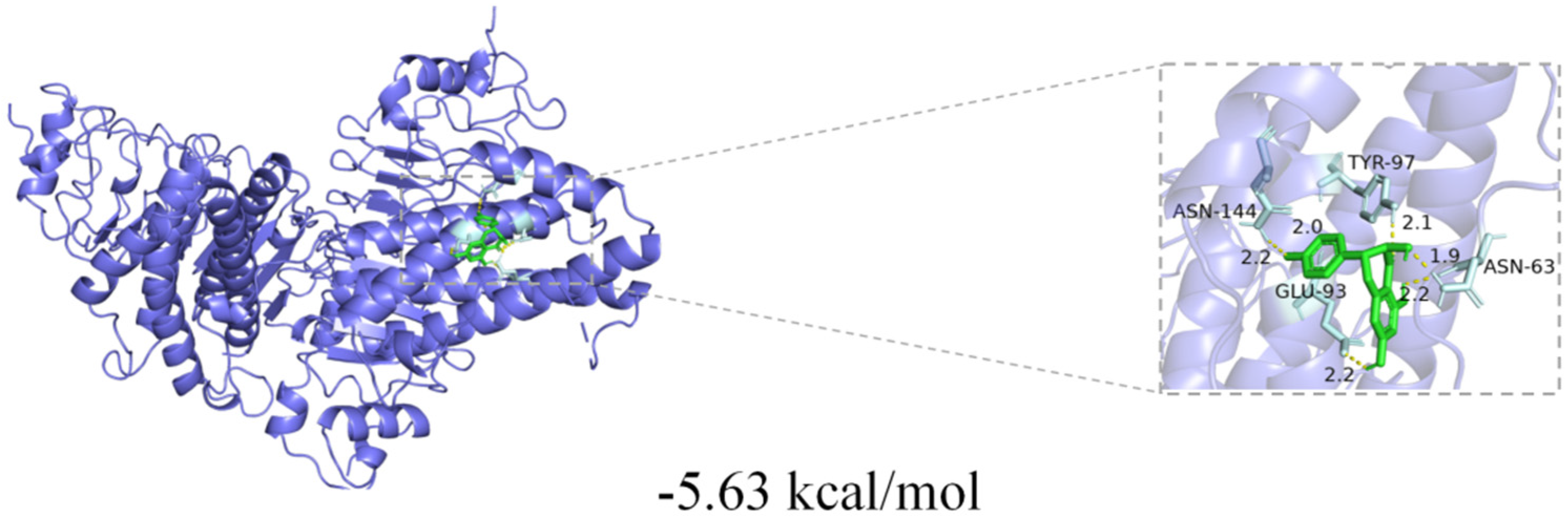
2.7. Molecular Docking Analysis
2.8. The Effect of Compound 3 on the Expression of TNF-α and IL-6
3. Materials and Methods
3.1. Plant Material
3.2. Materials
3.3. Extraction and Isolation
Characterization of Compound 1
3.4. RAW264.7 Cell Culture
3.5. RAW264.7 Cell Viability Experiment
3.6. Assay of Inflammatory Factor Inhibition
3.7. α-Glucosidase Inhibition Assay
3.8. DPPH and ABTS Free Radical-Scavenging Assays
3.9. Network Pharmacological Studies
3.10. Molecular Docking Verification
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duong, T.H.; Aree, T.; Le, T.K.; Dang, V.S.; Nguyen, N.H.; Sichaem, J. Chemical constituents with their alpha-glucosidase inhibitory activity from the whole plant of Ceratophyllum demersum. Phytochemistry 2024, 229, 114290. [Google Scholar] [CrossRef]
- Li, Q.J.; Zhou, S.; Yang, Y. Ethnobotanical Study on Rosa roxburghii in Guizhou. China Acad. J. Electron. Publ. House 2016, 22, 38–39. [Google Scholar]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food. Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Gong, Q.; Wu, Q.; Shi, J. Chemical diversity, traditional uses, and bioactivities of Rosa roxburghii Tratt: A comprehensive review. Pharmacol. Therapeut. 2024, 259, 108657. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, W.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, L.J.; Long, X.X.; Guo, L.J.; Wei, X.; Zhou, Y.Q.; Feng, T.T.; Zhou, Y.; Yin, X. Anti-Inflammatory and α-Glucosidase Inhibitory Triterpenoid with Diverse Carbon Skeletons from the Fruits of Rosa roxburghii. J. Agric. Food Chem. 2024, 72, 11503–11514. [Google Scholar] [CrossRef]
- Mizuno, T.; Ishikawa-Takano, Y.; Nakane, T.; Devkota, H.P.; Iwashina, T. Flavonoids from the leaves and stems of Sedum japonicum var. senanense and their antioxidant activity. Fitoterapia 2024, 177, 106020. [Google Scholar] [CrossRef]
- Su, J.; Zhang, B.; Fu, X.; Huang, Q.; Li, C.; Liu, G.; Liu, R. Recent advances in polysaccharides from Rose roxburghii Tratt fruits: Isolation, structural characterization, and bioactivities. Food Funct. 2022, 13, 12561–12571. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Fu, X.; Huang, Q.; Li, Y. Purification, Characterization, and Anti-Inflammatory Potential of Free and Bound Polyphenols Extracted from Rosa roxburghii Tratt Pomace. Foods 2024, 13, 2044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Chen, J.H.; Qi, M. The experiment study of chronic gastric ulcer treatment with root of Rose roxburghii Tratt in rats. Guizhou Med. 2001, 25, 1850. [Google Scholar]
- Liang, Y.; Li, L.Q.; Wang, L. Chemical constituents and their anti-inflammatory activities from rhizome of ethnic medicine Rosa roxburghii. Guihaia 2021, 42, 11. [Google Scholar]
- Aravindhan, V.; Bobhate, A.; Sathishkumar, K.; Viswanathan, V. Serum levels of novel anti-inflammatory cytokine Interleukin-38 in diabetes patients infected with latent tuberculosis (DM-LTB-3). J. Diabetes Complic. 2022, 36, 108133. [Google Scholar] [CrossRef]
- Black, H.S. A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Ocampos, F.M.M.; Paetz, C.; Antar, G.M.; Menezes, R.C.; Schneider, B. Phytochemical profile of Schiekia orinocensis (Haemodoraceae). Phytochem. Lett. 2017, 21, 139–145. [Google Scholar] [CrossRef]
- Stark, T.D.; Sch, S.; Balemba, O.B.; Hofmann, T. Two new benzoyl glucuronosyl glycerols from the leaves of Garcinia buchananii baker. Phytochem. Lett. 2017, 19, 187–190. [Google Scholar]
- Gerardo, M.V.; Júnior, C.M.M.; Sousa, A.J.; Cavalheiro, J.H.; Lago, G.; Mariana, H.C. Phenolic Derivatives from Fruits of Dipteryx lacunifera Ducke and Evaluation of Their Antiradical Activities. Helv. Chim. Acta 2008, 91, 2159–2167. [Google Scholar]
- Costa, A.G.; Yoshida, N.C.; Garcez, W.S.; Perdomo, R.T.; Matos, M.F.C.; Garcez, F.R. Metabolomics Approach Expands the Classification of Propolis Samples from Midwest Brazil. J. Nat. Prod. 2022, 83, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Ninomiya, K.; Yoshikawa, M. Medicinal foodstuffs. XXVIII. Inhibitors of nitric oxide production and new sesquiterpenes, zedoarofuran, 4-epicurcumenol, neocurcumenol, gajutsulactones A and B, and zedoarolides A and B, from Zedoariae rhizoma. Chem. Pharm. Bull. 2001, 49, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Jie, H.Y.; Wu, D.L.; Qin, S.H.; Tang, X.; Wang, R.Z.; Xu, F.Q. Two new sesquiterpene glycosides from the stems of Dendrobium henanense and their anti-inflammatory activity. Nat. Prod. Res. 2021, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xia, J.; Chen, Z.; Wu, X.; Li, G.; Lai, Q.; Shao, Z.; Wang, W.; Hong, X. Cytotoxic Pentaketide-Sesquiterpenes from the Marine-Derived Fungus Talaromyces variabilis M22734. Mar. Drugs 2024, 22, 274. [Google Scholar] [CrossRef]
- Lewis, M.T.; Koivunen, E.E.; Swett, C.L.; Hamby, K.A. Associations Between Drosophila suzukii (Diptera: Drosophilidae) and Fungi in Raspberries. Environ. Entomol. 2019, 48, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar]
- Cabrera-Rivera, G.L.; Madera-Sandoval, R.L.; León-Pedroza, J.I.; Ferat-Osorio, E.; Salazar-Rios, E.; Hernández-Aceves, J.A.; Guadarrama-Aranda, U.; López-Macías, C.; Wong-Baeza, I.; Arriaga-Pizano, L.A.; et al. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin. Exp. Immunol. 2019, 209, 225–235. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Yin, X.; Wang, Z.H.; Yin, Z.; Zhou, Y.Q.; Hu, R.H.; Feng, T.T. Chemical Constituents and Antioxidant Activity of Coicis Semen. Chin. J. Pharm. 2023, 58, 2054–2061. [Google Scholar]
- Shi, P.; Yu, X.; Zhang, M.; Wang, L.; Deng, L.; Yin, J.; Han, N. Biological activities of benzofurans from the fruits of Psoralea corylifolia L. and their mechanism based on network pharmacology and biological verification. Phytochemistry 2024, 230, 114316. [Google Scholar] [CrossRef]
- Du, C.; Li, X.; Chen, J.; Luo, L.; Yuan, C.; Yang, J.; Hao, X.; Gu, W. Discovery of Coumarins from Zanthoxylum dimorphophyllum var. spinifolium as and Their Potential against Rheumatoid Arthritis. Molecules 2024, 29, 4395. [Google Scholar] [CrossRef]
- Yan, X.T.; An, Z.; Huangfu, Y.; Zhang, Y.T.; Li, C.H.; Chen, X.; Liu, P.L.; Gao, J.M. Polycyclic polyprenylated acylphloroglucinol and phenolic metabolites from the aerial parts of Hypericum elatoides and their neuroprotective and anti-neuroinflammatory activities. Phytochemistry 2019, 159, 65–74. [Google Scholar] [CrossRef] [PubMed]


| NO. | δC | δH (J in Hz) |
|---|---|---|
| 1 | 66.6 | 4.39 (1H, dd, J = 11.5, 4.2 Hz) 4.34 (1H, dd, J = 11.5, 4.8 Hz) |
| 2 | 69.5 | 4.17 (H, m) |
| 3 | 70.6 | 3.90 (1H, dd, J = 10.0, 5.2 Hz) 3.54 (1H, dd, J = 10.0, 6.6 Hz) |
| 1′ | 101.2 | 4.89 (1H, d, J = 3.7 Hz) |
| 2′ | 73.3 | 3.47 (1H, dd, J = 9.3, 3.4 Hz) |
| 3′ | 74.4 | 3.68 (1H, t-like, J = 9.3 Hz) |
| 4′ | 73.2 | 3.50 (1H, t-like, J = 9.9 Hz) |
| 5′ | 73.1 | 4.08 (1H, d, J = 9.9 Hz) |
| 6′ | 171.9 | - |
| 1″ | 121.2 | - |
| 2″ | 108.3 | 7.37 (1H, s) |
| 3″ | 148.9 | - |
| 4″ | 142.0 | - |
| 5″ | 148.9 | - |
| 6″ | 108.3 | 7.37 (1H, s) |
| 7″ | 167.9 | - |
| 3″, 5″-OCH3 | 56.9 | 3.90 (6H, s) |
| 6′-OCH3 | 52.7 | 3.66 (3H, s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.-J.; Zhang, S.; Luo, M.-L.; Long, X.-X.; Zhou, Y.; Yin, X. Biological Activities of Constituents from Rosa roxburghii and Their Mechanisms Based on Network Pharmacology and Biological Verification. Int. J. Mol. Sci. 2025, 26, 1353. https://doi.org/10.3390/ijms26031353
Xiang L-J, Zhang S, Luo M-L, Long X-X, Zhou Y, Yin X. Biological Activities of Constituents from Rosa roxburghii and Their Mechanisms Based on Network Pharmacology and Biological Verification. International Journal of Molecular Sciences. 2025; 26(3):1353. https://doi.org/10.3390/ijms26031353
Chicago/Turabian StyleXiang, Li-Juan, Shuang Zhang, Ming-Liang Luo, Xing-Xiang Long, Ying Zhou, and Xin Yin. 2025. "Biological Activities of Constituents from Rosa roxburghii and Their Mechanisms Based on Network Pharmacology and Biological Verification" International Journal of Molecular Sciences 26, no. 3: 1353. https://doi.org/10.3390/ijms26031353
APA StyleXiang, L.-J., Zhang, S., Luo, M.-L., Long, X.-X., Zhou, Y., & Yin, X. (2025). Biological Activities of Constituents from Rosa roxburghii and Their Mechanisms Based on Network Pharmacology and Biological Verification. International Journal of Molecular Sciences, 26(3), 1353. https://doi.org/10.3390/ijms26031353






