Abstract
Cancer metastasis is a leading cause of cancer-related deaths and represents one of the most challenging processes to study due to its complexity and dynamic nature. Zebrafish (Danio rerio) have become an invaluable model in metastasis research, offering unique advantages such as optical transparency, rapid development, and the ability to visualize tumor interactions with the microenvironment in real time. This review explores how zebrafish models have elucidated the critical steps of metastasis, including tumor invasion, vascular remodeling, and immune evasion, while also serving as platforms for drug testing and personalized medicine. Advances such as patient-derived xenografts and innovative genetic tools have further established zebrafish as a cornerstone in cancer research, particularly in understanding the molecular drivers of metastasis and identifying therapeutic targets. By bridging the experimental findings with clinical relevance, zebrafish continue transforming our understanding of cancer biology and therapy.
Keywords:
zebrafish; cancer; metastasis; tumor microenvironment; angiogenesis; personalized medicine 1. Introduction
Cancer metastasis, the spread of tumor cells from a primary site to distant organs, is a multistep and highly dynamic process that is responsible for the majority of cancer-related deaths [1,2,3]. The metastatic process occurs through a series of defined stages (Figure 1): cancer cells invade the surrounding tissues, enter the bloodstream or lymphatic system (intravasation), survive in the circulation, exit into distant tissues (extravasation), and establish secondary tumors in new tissues (colonization) [1]. Throughout these stages, tumor cells must overcome mechanical, biochemical, and immune challenges while interacting with their surrounding microenvironment [1]. Gaining a deeper insight into these complex processes is essential for developing strategies to prevent and control metastasis.
The tumor microenvironment (TME) is central to metastatic progression, consisting of a complex network of stromal cells, an extracellular matrix (ECM), immune cells, and soluble signaling molecules [4]. These components can either inhibit or support metastasis, depending on the specific context, which adds to the challenge of fully understanding this process and developing effective treatments [5].
Despite significant progress in understanding metastatic processes, many aspects remain poorly understood, largely due to the challenges of studying each step in vivo [6]. Traditional models like genetically engineered mouse models (GEMMs) and patient-derived xenografts (PDXs) have been valuable in exploring metastasis biology and testing anti-cancer therapies [7]. However, these models have inherent limitations, including high costs, lengthy experimental timelines, and real-time challenges in capturing the dynamic interactions between tumors and the TME [8]. Recent advances have improved the resolution and temporal analysis of these models. Still, these improvements often require invasive surgical procedures and specialized imaging technologies, which can hinder the reproducibility of the studies [9].
Zebrafish (Danio rerio) have emerged as a powerful and versatile model system in cancer research, addressing the numerous limitations associated with traditional models [10]. As a small vertebrate, the zebrafish share highly conserved biological features with humans, including organ systems such as the central nervous system [11], intestines, pancreas, and liver [12]; metabolic pathways like glucose [13] and lipid [14] homeostasis; and physiological functions, including cardiac electrophysiology and heart rate regulation [15]. Additionally, this model presents a high conservation of key human cancer-related genes, such as tumor protein p53 (TP53) [16], Kirsten rat sarcoma viral oncogene homolog (KRAS) [17], and vascular endothelial growth factor-A (VEGFA) [18], and critical signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) (proliferation) [19], RHOA/Rho-associated protein kinase (ROCK) (migration) [20], and B-cell leukemia/lymphoma 2 protein (BCL2) (cell death) [21]. The pathways involved in differentiation (e.g., neurogenic locus notch homolog protein–NOTCH [22]) and immune responses, such as danger-associated molecular pattern (DAMP) signaling [23], are also preserved. These similarities make zebrafish particularly valuable in studying cancer progression and metastasis. One of their most notable advantages is the optical transparency of embryos, which enables the real-time, single-cell high-resolution imaging of critical metastatic events, such as tumor cell invasion, intravasation, extravasation, and migration [10] (Figure 2). Their rapid external embryonic development and high fecundity facilitate large-scale experimental designs and high-throughput approaches, allowing researchers to test hypotheses and identify the mechanisms involved in tumor biology efficiently [10,24]. Zebrafish are also highly amenable to genetic manipulation, with tools like CRISPR/Cas9 enabling the modeling of specific genetic alterations observed in human cancers [25].
Zebrafish models have been widely utilized to study metastatic cancers, offering a valuable platform to investigate tumor dissemination and organotropism [26,27]. In breast cancer, they have elucidated the mechanisms of brain and bone metastasis [28]. Prostate cancer models have revealed conserved genomic alterations in the metastatic site [29], and melanoma studies have explored drivers of tumor invasion [30]. Zebrafish have also provided insights into rarer cancers like glioblastoma and pediatric tumors [31,32,33], highlighting their real-time imaging capabilities to comprehensibly study metastatic behavior.
In addition to their relevance to metastatic cancer, zebrafish support the engraftment of human tumor cells [34,35] and retain the key components of the TME, such as fibroblasts, macrophages, and neutrophils [36]. This allows for the study of tumor–stroma interactions in a dynamic and physiologically relevant setting. Furthermore, zebrafish replicate specific aspects of human cancer biology, including organ-specific metastasis, such as liver and brain tropism [37], making them an invaluable tool for understanding the mechanisms underlying metastatic spread and developing novel therapeutic strategies. Their cost-effectiveness and accessibility [10] further enhance their appeal, enabling resource-limited laboratories to contribute to cutting-edge cancer research without compromising on experimental rigor.
This review examines how zebrafish have transformed metastasis research by offering unique insights into tumor–TME interactions, enabling advanced imaging and genomic studies, and serving as an efficient platform for high-throughput drug testing. It also highlights the model’s strengths and limitations, underscoring its vital role in connecting preclinical research with clinical applications.
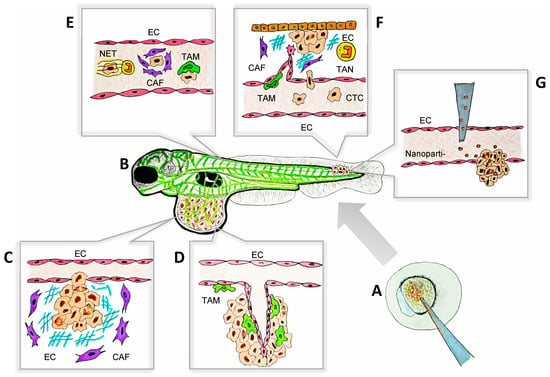
Figure 1.
The zebrafish model as a platform for the visualization of tumor–TME interactions during metastasis. The key aspects of tumor–TME interactions, including ECM remodeling, angiogenesis, immune cell recruitment, and tumor invasion, can be visualized in real time and at a single-cell resolution using zebrafish models. This approach facilitates high-throughput drug testing and provides a dynamic, cost-effective platform for studying metastasis. The processes illustrated in this schematic are examples of observable cell behaviors studied in zebrafish models that have deepened our understanding of metastasis and advanced personalized therapies. (A) Mutant and transgenic zebrafish can be generated with ease due to the accessibility of embryos at the single-cell stage. Techniques such as morpholino oligonucleotides, CRISPR/Cas9, and other gene-editing tools are readily applicable in this model. (B) Schematic representation of a transgenic larval zebrafish xenograft model, where blood vessels are labeled with a fluorescent green protein, enabling clear visualization of the primary tumors and metastatic sites. This set-up facilitates the real-time monitoring of tumor progression and metastasis with a high resolution. (C) CAFs (purple) remodeling the ECM in the primary tumor to facilitate tumor extravasation. (D) TAMs (green) interact closely with ECs, promoting angiogenesis in the primary tumor through direct contact and the release of soluble factors, including VEGF-A. (E) CTCs survive their journey to the metastatic sites with the support of NETs (yellow), CAFs (purple), and TAMs (green). These components create a protective microenvironment, shielding CTCs from immune system attacks and other potential threats, thereby facilitating their successful dissemination and colonization. (F) CTCs extravasate and colonize distant tissues with the support of soluble factors secreted by TAMs (green), TANs (yellow), and CAFs (purple). Furthermore, TAM-induced angiogenesis facilitates tissue invasion and supports the establishment of the metastatic niche, promoting successful tumor colonization. (G) Nanoparticle-based drug delivery in zebrafish models, targeting tumor cells in a metastatic site for therapeutic evaluation. CAF: cancer-associated fibroblast; CTC: circulating tumor cell; EC: endothelial cell; ECM: extracellular matrix; NET: neutrophil extracellular trap; TAM: tumor-associated macrophage; TAN: tumor-associated neutrophil; TME: tumor microenvironment.
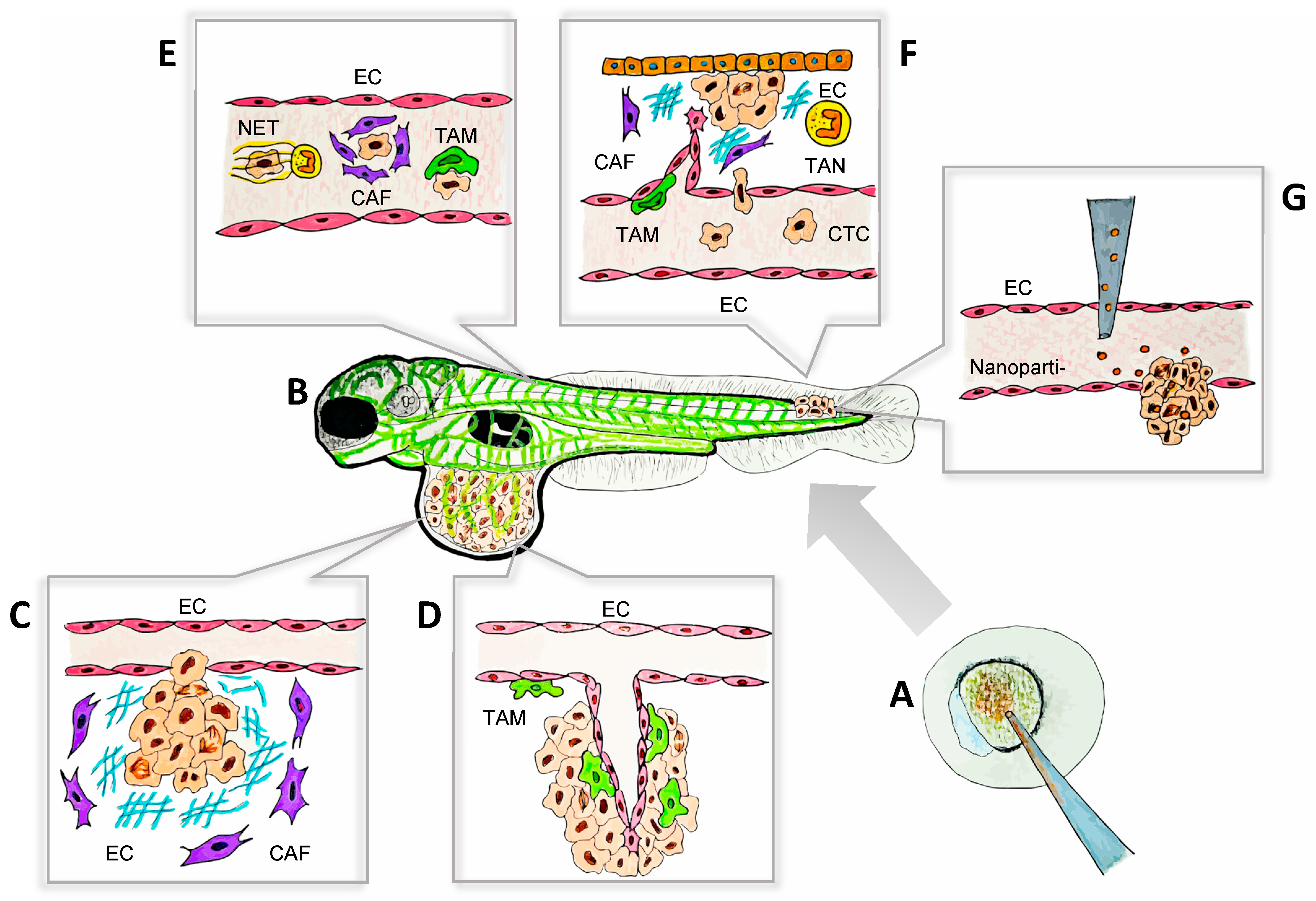
Figure 1.
The zebrafish model as a platform for the visualization of tumor–TME interactions during metastasis. The key aspects of tumor–TME interactions, including ECM remodeling, angiogenesis, immune cell recruitment, and tumor invasion, can be visualized in real time and at a single-cell resolution using zebrafish models. This approach facilitates high-throughput drug testing and provides a dynamic, cost-effective platform for studying metastasis. The processes illustrated in this schematic are examples of observable cell behaviors studied in zebrafish models that have deepened our understanding of metastasis and advanced personalized therapies. (A) Mutant and transgenic zebrafish can be generated with ease due to the accessibility of embryos at the single-cell stage. Techniques such as morpholino oligonucleotides, CRISPR/Cas9, and other gene-editing tools are readily applicable in this model. (B) Schematic representation of a transgenic larval zebrafish xenograft model, where blood vessels are labeled with a fluorescent green protein, enabling clear visualization of the primary tumors and metastatic sites. This set-up facilitates the real-time monitoring of tumor progression and metastasis with a high resolution. (C) CAFs (purple) remodeling the ECM in the primary tumor to facilitate tumor extravasation. (D) TAMs (green) interact closely with ECs, promoting angiogenesis in the primary tumor through direct contact and the release of soluble factors, including VEGF-A. (E) CTCs survive their journey to the metastatic sites with the support of NETs (yellow), CAFs (purple), and TAMs (green). These components create a protective microenvironment, shielding CTCs from immune system attacks and other potential threats, thereby facilitating their successful dissemination and colonization. (F) CTCs extravasate and colonize distant tissues with the support of soluble factors secreted by TAMs (green), TANs (yellow), and CAFs (purple). Furthermore, TAM-induced angiogenesis facilitates tissue invasion and supports the establishment of the metastatic niche, promoting successful tumor colonization. (G) Nanoparticle-based drug delivery in zebrafish models, targeting tumor cells in a metastatic site for therapeutic evaluation. CAF: cancer-associated fibroblast; CTC: circulating tumor cell; EC: endothelial cell; ECM: extracellular matrix; NET: neutrophil extracellular trap; TAM: tumor-associated macrophage; TAN: tumor-associated neutrophil; TME: tumor microenvironment.
2. Advanced Molecular and Cellular Insights of Metastasis: From Mechanisms to Modeling
Metastasis is a highly regulated process influenced not only by the intrinsic properties of tumor cells but also by their interactions with the surrounding cells and molecules, many of them being part of the TME [4]. Importantly, the components of the TME greatly contribute to the success or failure of metastatic dissemination [4,38]. Additionally, tumor–TME interactions are mediated by a range of molecular players, including cytokines, growth factors, proteases, and adhesion molecules, which regulate crucial processes such as ECM remodeling, angiogenesis, immune evasion, and vascular invasion [38,39]. Zebrafish models have proven to be essential tools for studying these mechanisms in vivo, providing key insights into the molecular dynamics of this crosstalk during metastasis [36] (Table 1; Figure 1 and Figure 2). Moreover, the integration of cutting-edge technologies into zebrafish models has significantly expanded their utility in cancer research [40]. Zebrafish now serve as versatile platforms for genomic and proteomic analyses, the real-time imaging of tumor–TME interactions, and high-throughput drug discovery pipelines (Figure 1) [41]. Using tools such as CRISPR screens, RNA sequencing (RNA-seq), and spatial transcriptomics, along with PDX models and advanced drug delivery systems, zebrafish enable groundbreaking insights into metastasis that were previously not easily accessible.
2.1. Exploring Tumor Invasion Through the Modeling of ECM Dynamics
Cancer-associated fibroblasts (CAFs) play a significant role in metastasis by altering the composition of the ECM and secreting signals that enhance tumor cell invasiveness (Figure 1C) [42]. Through the production of matrix metalloproteinases (MMPs), such as MMP2 and MMP9, CAFs break down ECM barriers, facilitating tumor cell migration and invasion [42,43]. In addition to structural changes, CAFs release cytokines, including transforming growth factor beta (TGF-β) and interleukin (IL)-6, which promote epithelial-to-mesenchymal transition (EMT), a critical process in increasing tumor cell motility [44]. The role of MMPs in metastasis was further corroborated by exploring cooperative invasion in melanoma [45]. When co-grafted into zebrafish with highly invasive melanoma cells, poorly invasive melanoma cells acquired invasive properties. This process relied on MMP activity and tumor-derived ECM factors, such as fibronectin and integrin [45]. These findings highlight the critical role of CAFs and MMP activity in remodeling the TME, driving cancer cell invasiveness and facilitating metastatic progression. Furthermore, CAFs enhance circulating tumor cells’ (CTCs) survival and proliferation by producing pro-survival factors such as regulated upon activation, normal T cell expressed and secreted (RANTES), and intercellular adhesion molecule 1 (ICAM1) [46]. Heterotypic CTC–CAF clusters support tumor cell survival (Figure 1C) and proliferation more effectively than homotypic clusters, emphasizing the role of CAF-mediated stromal contributions in metastasis [46].
Zebrafish models have been instrumental in uncovering these processes, offering unique advantages for studying ECM properties, stromal stiffness, and tumor cell motility. Advanced loss-of-function techniques, such as morpholino oligonucleotides (MOs) and short-hairpin RNA (shRNA), have proven particularly valuable in this context [47]. MOs enable the transient suppression of gene expression [48], allowing researchers to investigate the genes involved in ECM remodeling and angiogenesis. Meanwhile, shRNA-mediated knockdown provides a longer-term solution for gene silencing, making it possible to study the effects of specific genes on tumor progression and metastasis [49]. For example, in zebrafish xenograft models of oral squamous cell carcinoma, shRNA-mediated silencing of MMP9 led to reduced ECM degradation, tumor invasion, and metastatic spread. This intervention significantly decreased the number of metastatic lesions, further reinforcing the essential role of MMP9 in metastasis [50]. Together, these approaches demonstrate the power of zebrafish as a model for studying CAF activity, ECM dynamics, and therapeutic interventions targeting the metastatic cascade.
2.2. Real-Time Insights into Angiogenesis and Vascular Dissemination
Angiogenesis, forming new blood vessels from the existing vasculature, is a critical step in cancer progression. It sustains tumor growth by supplying essential nutrients and oxygen while facilitating the entry of tumor cells into the circulation [51]. In zebrafish xenografts, tumors initially develop as avascular masses until they reach a critical size, approximately 100 cells, at which point they recruit endothelial cells to form new blood vessels [52]. This process, known as the angiogenic switch, supports rapid tumor growth. Interestingly, smaller tumors with invasive behavior can bypass angiogenesis altogether, enabling cancer cells to extravasate and form micrometastases in distant tissues [52]. These findings emphasize how the tumor size and cell number influence metastasis, particularly the reliance on angiogenesis for tumor progression.
Zebrafish models offer unparalleled opportunities to observe and manipulate angiogenesis and tumor progression in real time. Among the various genetic tools, the Gal4-UAS system has emerged as a precise approach for controlling gene expression. By utilizing the Gal4 transcription factor to activate UAS-linked genes, this system enables targeted expression in specific tissues or at defined developmental stages [53]. When paired with transgenic reporter lines, the Gal4-UAS system allows for the real-time visualization of signaling pathways, such as TGFβ and Notch, which play critical roles in angiogenesis, tumor progression, and metastatic dissemination [54].
The use of stable transgenic lines facilitates long-term studies of dynamic tumor-microenvironment interactions, including stromal recruitment, immune modulation, and vascular remodeling [55]. Techniques such as the Tol2 transposon system and CRISPR/Cas9-mediated insertion [25,53,56] enhance the generation of zebrafish lines with specific oncogene expression or fluorescently labeled cell populations, such as endothelial cells (Figure 2A–C), further advancing the study of tumor–TME interactions at a single-cell resolution in real time [55]
2.2.1. Key Molecular Drivers of Angiogenesis
The VEGF Pathway
The VEGF family is a major driver of tumor-induced angiogenesis. VEGF-A, the most studied isoform, binds to its primary receptor VEGFR-2 on endothelial cells, activating signaling pathways such as PI3K/AKT and mitogen-activated protein kinase (MAPK). These pathways promote endothelial cell proliferation, migration, and survival [57]. VEGF-A also increases vascular permeability, enabling tumor cells to extravasate into the circulation [58,59]. Hypoxia within tumors upregulates VEGF-A via the stabilization of hypoxia-inducible factor 1-alpha (HIF-1α), creating a feedback loop that sustains angiogenesis [60,61,62]. VEGF overexpression is a hallmark of numerous malignancies. In hematological cancers, such as leukemia, lymphoma, and multiple myeloma, VEGF functions as a key autocrine and paracrine factor driving tumor growth and angiogenesis [63]. Its overexpression correlates with therapeutic resistance and poor prognosis [63,64,65]. Similarly, in solid tumors, including gastrointestinal adenocarcinomas and cancers of the lung, prostate, and ovary, VEGF overexpression is associated with aggressive disease progression and unfavorable outcomes [66,67,68,69].
Functional studies using zebrafish models have reinforced the critical role of VEGF in vascular remodeling, an essential step in the metastatic cascade. For instance, VEGF-A MO silencing in zebrafish xenografts of breast cancer and melanoma revealed the involvement of macrophages in promoting angiogenesis-driven tumor progression, highlighting the interplay between immune cells and tumor-derived signals [70,71]. Moreover, VEGFR-2 knockdown using shRNA in zebrafish models significantly disrupted vessel formation, underscoring the critical role of this signaling pathway in angiogenesis [72].
Interestingly, while VEGF inhibition can suppress angiogenesis, it may paradoxically enhance invasiveness. For example, VEGFR2/3 inhibition in breast cancer xenografts reduced lymph node metastases by decreasing the lymphatic vasculature but simultaneously increased metastases in other organs, such as the diaphragm and pancreas [73]. Similar compensatory upregulation of alternative pro-angiogenic pathways has been observed in mouse models of pancreatic neuroendocrine carcinoma and glioblastoma (GBM) following VEGF inhibition, likely driven by hypoxia-induced stress [74]. VEGF also disrupts the VE–cadherin–β-catenin complex, further facilitating tumor cell extravasation and metastasis [75].
Hypoxia
Due to their inherently aberrant structural characteristics, most solid tumors exhibit areas of significant hypoxia, one of the hallmarks of cancer, particularly in the tumor’s interior regions farthest from the potential blood supply [76]. Hypoxia plays a dual role in driving angiogenesis and metastasis. While hypoxic conditions initially cause vessel regression, they can also stimulate angiogenesis and enhance tumor invasion [77]. In zebrafish, tumors exposed to hypoxic conditions for three days become highly angiogenic, initiating extravasation, invasion, and metastasis, like VEGF-inhibition-induced metastasis [77,78]. Hypoxia-driven changes in the TME, including oxidative stress and VEGF upregulation, can promote vascular regrowth and further metastasis following anti-angiogenic therapy [79]. Additionally, under hypoxia, cancer cells shift from oxidative phosphorylation to glycolysis, a phenomenon known as the Warburg effect, leading to increased glucose uptake and lactate production [80,81]. This metabolic shift not only fuels tumor growth but also creates an acidic extracellular environment that promotes ECM degradation, angiogenesis, and immune evasion [80,81]. Moreover, hypoxia stabilizes HIF-1α, which upregulates the genes involved in glycolysis, glutaminolysis, and lipid biosynthesis [82,83,84]. These changes enhance the ability of tumor cells to adapt to nutrient deprivation and oxidative stress, thereby facilitating their invasion and metastasis [80].
Zebrafish models uniquely enable the study of hypoxia-induced metabolic shifts, and their influence on tumor progression and metastasis. These models facilitate the real-time analysis of glycolysis, oxidative phosphorylation, and lipid metabolism in vivo, revealing how metabolic reprogramming supports angiogenesis, ECM remodeling, and immune evasion. This approach provides critical insights for identifying precise therapeutic targets to inhibit metastasis at its metabolic roots.
Angiopoietin and the TIE Pathway
Angiopoietin-2 (ANGPT2) destabilizes vascular networks by loosening endothelial junctions, preparing vessels for remodeling and sprouting [85]. ANGPT2 interacts with the TEK tyrosine kinase (TIE) 2 receptor, amplifying VEGF-driven angiogenesis. Elevated serum concentrations of ANGPT2 have been observed in patients with multiple myeloma [86], non-small cell lung cancer (NSCLC) [87], metastatic colorectal cancer (CRC) [88], renal cell carcinoma [89], hepatocellular carcinoma [90], and chronic lymphocytic leukemia [91], all of which are associated with a poorer prognosis and rapid disease progression. On the other hand, anti-angiogenic factors like thrombospondin-1 (TSP-1) and endostatin are often downregulated in tumors, tipping the balance toward vascular growth [92,93]. High-resolution imaging of zebrafish with fluorescently labeled endothelial cells showed how ANGPT2 destabilizes vessels and enhances VEGF-mediated sprouting [94]. These findings highlight the critical role of ANGPT2 in vascular destabilization and its contribution to forming abnormal, leaky networks that support tumor growth and metastasis.
ECM Remodeling and MMPs
The MMP-mediated degradation of the basement membrane and ECM is a prerequisite for angiogenesis, clearing pathways for migrating endothelial cells [27]. MMP expression is also directly correlated with a higher metastatic potential in several cancers including CRC, NSCLC, breast cancer, and prostate cancer [95]. Stromal cells, including CAFs and tumor-associated macrophages (TAMs), secrete MMP2 and MMP9, driving ECM degradation [27,96,97,98]. As previously described, the inhibition of MMP activity reduces vascular remodeling and impairs angiogenesis, further confirming the role of ECM dynamics in endothelial migration [55].
Furthermore, zebrafish with fluorescent vasculature reporters (Figure 1B and Figure 2B,C) are essential for studying candidate genes in angiogenesis, showing how tumor and TME signals shape leaky vascular networks that drive metastasis [99]. These findings underscore the complex mechanisms of angiogenesis and its role in metastatic progression.
2.2.2. Endothelial–Tumor Interactions
The interplay between tumor cells and endothelial cells is central to metastasis. Tumor cells adhere to endothelial surfaces through adhesion molecules such as E-selectin, ICAM-1, and vascular cell adhesion molecule 1 (VCAM-1), facilitating both intravasation and extravasation [100]. Tumor-derived extracellular vesicles (EVs) carry pro-angiogenic factors, such as VEGF and ECM remodeling enzymes, that modulate endothelial behavior and promote vascular permeability [101,102].
Zebrafish provide a precise platform for studying endothelial–tumor interactions, with EMT standing out as a critical process in these dynamics [103,104]. This transition, mediated by TGF-β signaling, enhances endothelial plasticity and contributes to the invasive front [105,106]. Knockdown experiments targeting TGF-β in zebrafish revealed reduced metastasis due to less EMT [106]. Additionally, timelapse imaging has captured how VEGF gradients direct vascular sprouting, with tip endothelial cells exhibiting high VEGFR-2 expression and responding to stromal-derived factors like fibroblast growth factors (FGFs) and angiopoietins [107].
The centrality of angiogenesis in tumor progression has made it a prominent target for cancer therapies. Inhibitors of VEGF, such as bevacizumab [108], and small-molecule tyrosine kinase inhibitors targeting VEGFR, such as sunitinib and sorafenib [109], have demonstrated efficacy in reducing tumor vascular density and delaying progression. However, these drugs are often associated with significant side effects and toxicities in clinical settings, including hypertension, proteinuria, bleeding, and impaired wound healing [110,111,112]. Resistance mechanisms further complicate treatment, as tumors can upregulate alternative angiogenic mediators, such as ANGPT2 or basic FGF, to bypass VEGF inhibition [112].
Zebrafish models have been instrumental in advancing research to address these challenges. These models allow the study of compensatory angiogenic pathways and enable the high-throughput screening of drug combinations that could mitigate side effects [34,110,113]. For example, zebrafish studies have shown that dual inhibition of VEGF and ANGPT2 significantly reduces vascular density compared with single-agent therapies, offering potential strategies to overcome resistance while minimizing toxicity [114]. Additionally, zebrafish xenograft models were used to screen combinations of VEGF inhibitors with MMP blockers, providing preclinical evidence for the synergistic anti-angiogenic effects [111,115]. Furthermore, zebrafish xenografts demonstrated high potential as efficient screening platforms for anti-angiogenic therapies like bevacizumab [110]. These models enabled the rapid evaluation of tumor angiogenesis, micrometastasis, and therapeutic responses, while also offering insights into patient-specific outcomes, making them highly valuable for developing targeted therapies with fewer side effects for personalized medicine [110].
Advances in genome editing, particularly CRISPR/Cas9 technology, have further enhanced the utility of zebrafish models in metastasis research [116]. The Cas9 nuclease, guided by a single-guide RNA (sgRNA), creates targeted double-stranded DNA breaks. These breaks are repaired by either non-homologous end joining (NHEJ), which often results in gene knockouts through insertions or deletions, or homology-directed repair (HDR), enabling precise edits like specific mutations or reporter tags [117]. In zebrafish, CRISPR/Cas9 is delivered as either RNA or pre-assembled ribonucleoprotein (RNP) complexes into one-cell-stage embryos, ensuring a high efficiency of mutagenesis in developing tissues [25]. This approach allows for the rapid generation of knockouts for candidate genes involved in metastasis, such as those associated with angiogenesis, ECM remodeling, and chemotaxis [25].
In addition, zebrafish models are particularly valuable for studying metastasis due to their capacity for high-throughput analysis [113]. When combined with CRISPR technology, this feature could enable the rapid, parallel screening of multiple genes and pathways, potentially providing deeper insights into their roles in metastatic progression [118]. Advances in genome editing, such as RNP complexes for precise CRISPR mutagenesis, can enhance this model by ensuring high mutagenesis rates with minimal off-target effects, enabling the efficient generation of functional mutants [116]. This is particularly advantageous in metastasis research, where rapid results are needed to link molecular changes to functional outcomes.
2.3. Immune Cells: Dynamic Regulators of Tumor Progression
Immune cells perform diverse roles that evolve with tumor progression. Initially, many immune cells exhibit anti-tumor activity, but molecular signals from the TME often co-opt these cells to support tumor growth and metastasis [119]. TAMs and neutrophils are particularly notable for their dual roles, toggling between tumor suppression and promotion based on cues from the TME [120,121].
An important breakthrough in studying immune subsets and their interactions within the TME is the advent of single-cell RNA sequencing (scRNA-seq). This technique allows for the precise profiling of tumor, stromal, vascular, and immune cells at a single-cell resolution, uncovering individual transcriptional states and signaling pathways [122,123]. By offering an unprecedented level of detail, scRNA-seq has significantly deepened the understanding of the complex immune-related mechanisms underlying tumor progression and metastasis. For example, scRNA-seq analysis of GBM zebrafish xenografts revealed significant differences in tumor cell transcriptional states across patient samples. These findings highlighted the specific molecular changes linked to tumor progression, such as increased expression of immunosuppressive genes galectin 1 (lgals1), triggering receptor expressed on myeloid cells 2 (trem2), as well as pathways related to extracellular ECM remodeling. Moreover, zebrafish models of CRC have shown how immune interactions within the TME can reshape the genomic profile of immunogenic subclones into a non-immunogenic state, enabling the evasion of immunosurveillance [124].
2.3.1. Macrophages: Orchestrators of Metastatic Spread
Macrophages are among the most versatile immune cells in the TME, responding dynamically to diverse signals [120] (Figure 2D). In metastatic contexts, macrophages are often polarized into a M2-like phenotype, characterized by the secretion of IL-10, TGF-β, and colony stimulating factor 1 (CSF-1), which suppress inflammation and enhance tumor progression [125]. One way that TAMs promote metastasis is by releasing VEGF, which stimulates angiogenesis and, in turn, facilitates tumor cell migration (Figure 1D) [55,70,77]. Additionally, TAMs physically aid tumor cell intravasation, as in vivo imaging of zebrafish cancer models showed macrophages guiding tumor cells to blood vessels and disrupting the endothelial barriers for vascular entry (Figure 1E,F) [70,125]. These interactions were driven by the C-X-C chemokine receptor type 4 (CXCR4)/C-C motif ligand 2 (CCL2) axis and facilitated by ECM degradation through TAM-secreted MMPs, with MMP9 playing a key role [96,126].
In distant organs, TAMs are essential for pre-metastatic niche formation (Figure 1F). Tumor-derived signals, including CCL2 and TGF-β, recruit macrophages to future metastatic sites, remodeling the stroma and suppressing local immune responses [33,127,128]. Live imaging revealed that microglia and macrophages actively contribute to GBM progression by migrating to tumor sites and closely interacting with cancer cells. Through non-phagocytic interactions, these immune cells promote tumor cell survival and enhance their invasiveness [129]. Besides, TAMs deposit ECM proteins, like fibronectin 1, which promote tumor colonization and metastasis [130]. Additionally, melanoma xenograft models demonstrate that tumor-derived EVs deliver metastasis-promoting proteins to macrophages and endothelial cells, further driving the metastatic cycle [101].
Finally, scRNA-seq of TAMs in GBM models showed that they exhibited diverse immunosuppressive gene profiles including the differential expression of lgals1, which supports tumor survival, immune evasion, and cancer progression [122]. These findings emphasize the cellular heterogeneity within the TME and underscore its critical role in determining metastatic behavior and immune escape.
2.3.2. Neutrophils: Partners in Tumor Dissemination
Neutrophils play a crucial role in metastasis, responding to chemokine gradients like CXCL8 (IL-8) and stromal cell-derived factor 1 (CXCL12) to infiltrate the TME [121] (Figure 2E). Although they can exhibit anti-tumor activity in certain conditions, neutrophils are often reprogrammed to support metastasis [121]. A key feature of this pro-tumoral behavior is the formation of neutrophil extracellular traps (NETs), composed of DNA, histones, and proteases such as elastase and MMP9 [131]. These NETs capture CTCs, promote their adhesion to endothelial surfaces, shield them from immune clearance, and, as shown by the real-time tracking of fluorescently labeled neutrophils and tumor cells, guide CTCs to blood vessel walls to prepare them for extravasation (Figure 1E) [132].
Additional observations in zebrafish xenografts revealed that neutrophils are recruited to specific sites, such as the caudal hematopoietic tissue (CHT), the equivalent of the fetal liver in mammals, where they facilitate vascular remodeling and enhance metastatic spread efficiency (Figure 1F) [133]. The CXCL12/CXCR4 axis plays a crucial role in neutrophil-driven metastasis. Tumor cells frequently overexpress CXCL12, which binds to CXCR4 on neutrophils, directing their recruitment and facilitating interactions with tumor cells [134]. Blocking CXCR4 signaling disrupts these interactions, effectively halting early metastatic events [135].
Building on these findings, spatial transcriptomics provides a valuable approach to exploring how these interactions are organized within the TME [136]. This technique has the potential to enable the precise mapping of gene expression and chemokine gradients such as CXCL12 in the vicinity of tumor cells and the surrounding stromal components in zebrafish models [137]. By examining how CXCL12 gradients direct neutrophil and tumor cell migration in vivo, deeper insights can be gained into the spatial dynamics of metastasis [138]. Furthermore, spatial profiling allows for the simultaneous analysis of tumor–stroma interactions, identifying stromal cell populations such as endothelial cells and fibroblasts that contribute to vascular remodeling, immunosuppression, and the establishment of the metastatic niche [139]. This comprehensive approach not only has the potential to reveal the molecular drivers of invasion and dissemination but also to highlight how neutrophil recruitment and chemokine-driven signaling coordinate to facilitate metastatic progression [139].
Moreover, in zebrafish models of HRasV12-transformed epithelial cells, neutrophils promoted EMT by increasing the expression of genes and proteins such as slug, vimentin, and MMP9. This process was triggered by IL-8, which is upregulated during oncogenic transformation and recruits neutrophils to the TME, initiating tumor cell dissemination [140].

Table 1.
Stages of metastasis: mechanisms and therapeutic insights.
Table 1.
Stages of metastasis: mechanisms and therapeutic insights.
| Stage of Metastasis | Description | Key Molecules/Processes | Zebrafish Model Contributions | Therapeutic Implications | Key Differences with Humans | References |
|---|---|---|---|---|---|---|
| Invasion | Tumor cells breach the ECM and invade the surrounding tissues | MMPs (MMP2, MMP9), integrins, fibronectin, TGF-β, IL-6, EMT | CAFs and TAMs promote ECM degradation through MMP2/9. IL-8-mediated recruitment of neutrophils promotes EMT and tumor dissemination. Cooperative melanoma invasion involves ECM factors like fibronectin and integrins. | Development of MMP inhibitors to reduce ECM breakdown. Targeting of integrin interactions to limit adhesion and migration. Potential selective inhibition of neutrophil migration towards the TME. | The genomic diversity of MMPs in humans is higher than in zebrafish. TGF-β is mostly found in scar formation processes in humans whereas in zebrafish it is associated with tissue regeneration. Zebrafish have a high regenerative capacity associated with EMT processes, which has not been described in humans. | [45,50,77,78,98,101,102,105,106,129,135,140,141,142,143] |
| Intravasation | Tumor cells enter the blood or lymphatic vessels | VEGF/VEGFR, ICAM-1, VCAM-1, E-selectin, CXCL12/CXCR4, EVs | Tumor-derived EVs carrying VEGF and remodeling enzymes promote endothelial permeability. TAMs stimulate angiogenesis and guide tumor cells to blood vessels, disrupting endothelial barriers for intravasation. CXCL12/CXCR4 signaling recruits neutrophils to tumor cells, facilitating early metastatic events. | Anti-VEGF therapies to reduce vascular permeability. Blocking CXCL12/CXCR4 to disrupt tumor–endothelial interactions. Inhibition of EV release/trafficking. | Zebrafish have two VEGF paralogs (vegfa and vegfb) and humans have three (VEGF-A, VEGF-B, and VEGF-C). Zebrafish have four genes related to VEGFR (flt1, kdr, flt4 and kdrl) and humans have two (VEGFR-1 and VEGFR-2). | [70,101,102,125,135,144,145] |
| Circulation | Tumor cells survive as CTCs | RANTES, IL-6, IL-8, NETs, TGF-β | TAMs guide tumor cells to blood vessels, disrupting the endothelial barriers for intravasation. TAMs and microglia promote tumor invasiveness through non-phagocytic interactions. NETs capture CTCs and promote adhesion, immune evasion, and extravasation. CAFs enhance CTC survival via RANTES and ICAM1. | Targeting of pro-survival factors to reduce CTC viability. NET inhibitors. Immunomodulators to polarize TAMs towards an M1-like phenotype. | TGF-β is mostly found in scar formation processes in humans whereas in zebrafish it is associated with tissue regeneration. | [46,70,125,129,132,143] |
| Extravasation | Tumor cells exit blood vessels to colonize tissues | VEGF/VEGFR, ANGPT2/TIE2, MMP2, MMP9, EVs. | VEGF-induced vascular leakiness promotes tumor cell extravasation. Hypoxia-triggered angiogenesis drives extravasation. ANGPT2 destabilizes vascular networks by loosening endothelial junctions, facilitating vessel remodeling. CAFs and TAMs induce ECM degradation via MMP2/9 and VEGF, enhancing angiogenesis and tumor cell migration. | Combined VEGF/ANGPT2 inhibitors to stabilize vessels. Inhibition of EV release/trafficking. Development of MMP inhibitors to reduce ECM breakdown. | Zebrafish have two VEGF paralogs (vegfa and vegfb) and humans have three (VEGF-A, VEGF-B, and VEGF-C). Zebrafish have four genes related to VEGFR (flt1, kdr, flt4, and kdrl) and humans have two (VEGFR-1 and VEGFR-2). Ang1, Ang2a, and Ang2b are ligands for Tie1 and 2 in zebrafish. In humans, the ligand for TIE2 is ANG4. The genomic diversity of MMPs in humans is higher than in zebrafish. | [27,70,77,78,85,94,98,141,144,145] |
| Colonization | Tumor cells home distant tissues and grow into secondary tumors | CXCL12/CXCR4, IL-10, TGF-β, CSF-1, VEGF, MMPs | Pre-metastatic niche formation by promoting ECM degradation and angiogenesis. TAMs allow for cancer cell homing of the new tissue by inducing immunosuppression through a M2-like phenotype. | Potential selective inhibition of macrophage and/or neutrophil migration towards the TME. Inhibition of TGF-β. Development of MMP inhibitors to reduce ECM breakdown. | TGF-β is mostly found in scar formation processes in humans whereas in zebrafish it is associated with tissue regeneration. The genomic diversity of MMPs in humans is higher than in zebrafish. | [27,50,73,94,98,106,107,125,141,143] |
| Angiogenesis | New blood vessels form from existing ones to irrigate tumors | VEGF/VEGFR2, HIF-1α, ANGPT2/TIE2, TSP-1 | VEGF-induced vessel formation and hypoxia-driven angiogenesis. The angiogenic switch, triggered at ~100 cells, promotes rapid growth, while smaller tumors bypass angiogenesis, forming micrometastases. ANGPT2 destabilizes vessels, enhancing VEGF sprouting. VEGF gradients direct vascular sprouting via VEGFR-2 on tip endothelial cells. Neutrophils recruited to the caudal hematopoietic tissue facilitate vascular remodeling. | Platform for high-throughput testing of anti-angiogenic therapies (e.g., bevacizumab, VEGFR inhibitors). Dual targeting of VEGF and ANGPT2 pathways to overcome resistance mechanisms. | Zebrafish have four genes related to VEGFR (flt1, kdr, flt4, and kdrl) and humans have two (VEGFR-1 and VEGFR-2). Ang1, Ang2a, and Ang2b are ligands for Tie1 and 2 in zebrafish. In humans, the ligand for TIE2 is ANG4. | [52,70,72,73,77,78,85,94,101,102,107,110,111,115,133,144,145] |
ANGPT2: Angiopoietin-2; CAF: Cancer-associated fibroblast; CSF-1: Colony-stimulating factor 1; CTC: Circulating tumor cell; CXCL12: C-X-C motif chemokine ligand 12; CXCR4: C-X-C chemokine receptor type 4; ECM: Extracellular matrix; EMT: Endothelial-to-mesenchymal transition; EV: Extracellular vesicle; flt: FMS-like tyrosine kinase; HIF-1α: Hypoxia-inducible factor 1-alpha; ICAM-1: Intercellular adhesion molecule 1; IL: Interleukin; kdrl: kinase insert domain receptor; MMP: Matrix metalloproteinase; NET: Neutrophil extracellular trap; RANTES: Regulated on activation, normal T cell expressed and secreted; TAM: Tumor-associated macrophage; TGF-β: Transforming growth factor beta; TIE2: TEK tyrosine kinase; TSP-1: Thrombospondin 1; VCAM-1: Vascular cell adhesion molecule 1; VEGF: vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor.
TAMs and neutrophils are key drivers of metastasis, orchestrating processes like angiogenesis, immune evasion, and tumor cell migration. Their influence on the tumor–TME crosstalk and their extensive study in zebrafish models place them as central topics in this discussion. However, it is also important to acknowledge the contributions of other immune cells, such as NK cells, and B and T lymphocytes, whose complex interactions with the TME, while significant, fall outside the scope of this review.
3. Integration with AI and Computational Models
The increasing complexity of the biological data generated in zebrafish studies has driven the adoption of artificial intelligence (AI) and computational tools to analyze and interpret findings [146]. The ability to integrate advanced imaging techniques with AI-driven analysis has the potential to transform the zebrafish model into a data-rich platform for investigating metastasis and other diseases [146].
GBM zebrafish models have been significantly refined by applying advanced imaging techniques and computational analysis, providing a robust platform to study tumor progression and invasion [147]. Integrating high-throughput imaging with deep convolutional neural networks (CNNs) enables the precise tracking of tumor growth, vascular interactions, and invasive behaviors, including perivascular invasion and single-cell dissemination [147,148]. This combination allows for single-cell resolution imaging, reducing labor-intensive manual monitoring and enabling a more detailed understanding of how GBM cells interact with and adapt to their microenvironment [147]. Importantly, these models have also been used to evaluate patient-specific responses to therapies, such as the proteasome inhibitor marizomib, demonstrating consistent findings with other preclinical models [147].
In another example, the transparent casper zebrafish strain was used to develop a high-resolution imaging system that is capable of tracking tumor progression at a single-cell resolution [149]. In this study, quantitative imaging algorithms and a metastasis scoring framework (m-score) were introduced, allowing for the detailed characterization of tumor spread and metastatic burden in vivo [149]. Building on this foundation, integrating machine learning with imaging tools allows for a more efficient analysis of timelapse data to identify the molecular markers and behavioral patterns linked to aggressive tumor phenotypes [150,151]. For example, computational models could refine the analysis of metastasis-initiating cell (MIC) frequencies, invasion pathways, and TME interactions observed in zebrafish xenografts [149]. This approach could support early cancer diagnosis and the identification of patient-specific therapeutic targets.
Simulating therapeutic outcomes adds another dimension to the utility of zebrafish in cancer research. Integrating zebrafish-derived data with AI-driven simulations allows for the prediction of drug combination effects and the optimization of therapeutic regimens [152,153]. Specifically, computational models based on zebrafish drug screening results could simulate drug delivery dynamics, improving the design and precision of targeted therapies [154].
In conclusion, combining advanced imaging with computational tools enhances the capacity to model complex biological processes in zebrafish models while also supporting the development of early diagnostic tools and personalized therapies.
4. Advanced Drug Screening Pipelines and Translational Applications
Zebrafish models are increasingly essential for studying and testing anti-metastatic therapies, offering unique opportunities to integrate advanced methods such as PDXs and nanoparticle delivery platforms. These innovations enhance drug discovery and serve as a vital bridge between preclinical research and clinical applications.
4.1. Patient-Derived Xenografts in Personalized Medicine
Zebrafish patient-derived xenografts (zPDXs) provide an efficient platform for testing cancer behavior and drug responses in vivo in the context of personalized medicine (Figure 1B and Figure 2A) [35,155,156]. By transplanting tumor cells from individual patients into zebrafish embryos, researchers can rapidly evaluate therapeutic regimes, often delivering actionable results within days of sample collection [157]. zPDXs demonstrated a strong alignment with clinical outcomes, with studies indicating up to ~90% accuracy in predicting responses to chemotherapy and targeted therapies [155]. In this context, brain metastasis (BM) models revealed that microRNA-330-3p (miR-330-3p) enhances the invasive and colonizing capabilities of NSCLC cells. The overexpression of miR-330-3p increases the brain metastatic potential, while its knockdown suppresses metastasis, identifying it as a promising therapeutic target [26]. These models also revealed that although a mature blood–brain barrier (BBB) reduces metastatic efficiency, it does not entirely prevent cancer cell invasion. Live imaging highlighted the influence of the BBB on metastasis and provided a platform for evaluating therapeutic interventions. Notably, osimertinib, with its superior BBB permeability, outperformed gefitinib in treating BM [26].
The incorporation of advanced imaging techniques, such as selective plane illumination microscopy (SPIM) [158], further enhances the utility of zPDXs. In leukemia models, SPIM allowed for the assessment of distinct behaviors among cancer types, such as the rapid migration of leukemic cells within blood vessels [20]. In contrast to leukemia cells, breast cancer cells invaded the avascular tissues through a slower and amoeboid mode of migration [20]. These differences demonstrated the ability of zPDXs to capture the unique characteristics of various malignancies. Beyond investigating the metastatic mechanisms, this zebrafish model also demonstrated effectiveness in drug screening. For example, the rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) inhibitor fasudil significantly reduced leukemic cell migration and overall survival [120], with quantitative metrics, like migration speed and dissemination distance, enhancing the evaluation of therapeutic responses [20].
zPDXs serve as an indispensable resource for advancing metastasis research and precision oncology due to their potential to rapidly model patient-specific tumor biology, predict therapeutic responses with a high accuracy, and uncover novel targets and mechanisms, ultimately contributing to developing more effective and personalized treatment strategies.
4.2. Nanoparticle-Based Drug Delivery
The transparent and vascularized nature of zebrafish embryos provides a unique platform for studying nanoparticle-based drug delivery systems (Figure 1G) [154,159]. This model allows for the real-time tracking of fluorescently labeled nanoparticles, enabling the detailed evaluation of their biodistribution, tumor uptake, and interactions with the metastatic microenvironment [160].
Light-triggered drug delivery systems are highly suitable for assessment in zebrafish models due to their embryos’ natural transparency [161]. Folate-modified liposomes, designed to enhance uptake via receptor-mediated endocytosis, have emerged as a promising approach for targeted drug delivery to tumors [162]. In zebrafish models, these liposomes, further stabilized with PEGylation to prolong the circulation time, achieved significantly greater tumor reduction compared with free-circulating drugs [162], demonstrating their potential in precise therapeutic strategies. In another study, zebrafish models were employed to evaluate G23-functionalized, curcumin-loaded zein nanoparticles (CUR-ZpD-G23 NPs), designed to cross the BBB and target GBM cells. This approach showed improved drug delivery with a higher cellular uptake and enhanced therapeutic effects, including increased apoptosis, elevated reactive oxygen species (ROS) production, and reduced cell migration and tumor growth [163].
Based on the previous applications, zebrafish show great potential for testing nanoparticles targeting the molecular pathways involved in metastasis, such as integrins and ECM components [154,160]. Arginine–glycine–aspartate (RGD)-functionalized nanoparticles, targeting integrins that are overexpressed on the tumor vasculature, effectively accumulated in breast cancer models both in vitro and in vivo in mice [164]. By disrupting the integrin-mediated pathways, these nanoparticles inhibited tumor adhesion and invasion, which are crucial steps in metastasis [164]. However, their application in other cancer types remains underexplored, and zebrafish could provide valuable insights into the broader efficacy of these systems and their ability to inhibit metastasis.
Together, these findings highlight the versatility of zebrafish as a model for nanoparticle-based cancer research, providing unique opportunities to investigate drug delivery, therapeutic responses, and molecular mechanisms across a range of tumor types and metastatic pathways.
4.3. Preclinical and Translational Importance
Zebrafish models provide substantial quantitative and statistical advantages in preclinical research. Their rapid development and high fecundity allow for the screening of up to 100 compounds per week, far surpassing the 10–20 compounds typically tested in murine systems [165]. This high throughput, combined with the robust statistical power from large sample sizes, enhances the reliability and reproducibility of the experimental findings [165]. Additionally, meta-analyses highlight the reliability of zebrafish models, demonstrating a predictive accuracy of approximately 80% in correlating drug efficacy with mammalian outcomes [166].
Combining high-throughput screening with mechanistic studies, zebrafish offer a cost-effective, efficient platform for preclinical research, reducing expenses compared with traditional mammalian systems and supporting early-stage drug discovery [24,165,166]. Despite their lower costs, zebrafish models maintain a strong predictive power for downstream mammalian validation, ensuring that results are both reliable and applicable to more complex systems [155,166].
Zebrafish embryonic processes, such as epiboly, share mechanistic parallels with cancer cell migration during metastasis [167,168]. Small-molecule inhibitors delaying epiboly in zebrafish, like the serotonin receptor 2C inhibitor pizotifen, also inhibit metastatic behaviors in human cancer cells [104]. Pizotifen’s anti-metastatic effects were confirmed in zebrafish xenografts and validated in murine models, highlighting zebrafish as a powerful tool for identifying effective therapies [104].
Along the same vein, zebrafish have proven effective in replicating the metastatic potential of various human cancer cells, including those from breast, prostate, colon, and pancreatic cancers [99]. For poorly invasive cell lines, zebrafish consistently show minimal metastasis, mirroring their in vitro behavior and validating their predictive accuracy [99]. Genetic studies in zebrafish, such as the knockdown of metastasis-promoting genes like Wiskott–Aldrich syndrome protein family member 3 (WASF3) [169] or oncogenic kinases like Janus kinase (JAK)1 and JAK2 [46], demonstrated a significant reduction in metastatic spread. These results are consistent with findings from murine models, reinforcing the utility of zebrafish in metastasis research [170,171].
Moreover, zebrafish models are particularly well-suited for testing combination therapies, enabling the efficient evaluation of interactions between multiple agents [172]. Their unique capabilities not only streamline drug development but also refine hypotheses, validate therapeutic targets, and advance personalized treatment strategies. As a key component of preclinical and translational cancer research, zebrafish enhance the relevance and applicability of experimental findings, reinforcing their significance in the field.
4.4. Applications in Rare Cancers
Rare cancers with a high propensity for metastasis pose significant challenges in treatment development due to their low incidence, limited research focus, and insufficient funding [173]. Understanding their metastatic behavior and identifying therapeutic targets is crucial for developing effective, tailored interventions.
4.4.1. Sarcomas
Despite advances in understanding Ewing sarcoma, the role of its hallmark Ewing sarcoma breakpoint region 1-Friend leukemia integration 1 (EWS-FLI1) fusion gene in tumor progression and metastasis remains incompletely understood [32]. A zebrafish model of the disease provided a precise tool for addressing this gap, faithfully recapitulating its small-round-blue-cell morphology and aggressive gene expression profiles [174]. This model enabled the detailed analysis of EWS-FLI1, revealing its disruption of signaling pathways and the activation of oncogenic drivers like NK2 homeobox 2 (NKX2-2) and myelocytomatosis oncogene (MYC), which are critical for neural development, tumor growth, and invasion [32,174].
Building on these insights, zebrafish models have proven valuable for high-throughput drug screening, enabling the identification of compounds that target EWS-FLI1 or its downstream signaling pathways, such as MAPK and PI3K/AKT, which are critical for tumor cell survival and dissemination [175]. This highlights the potential therapeutic strategies to inhibit pathways driving tumor invasiveness and metastatic adaptation, advancing efforts to translate molecular findings into effective treatments.
4.4.2. Pediatric Cancers
Neuroblastoma
Zebrafish models of neuroblastoma uncovered the impact of co-expressing the MYCN oncogene and LIM domain only 1 (LMO1), which drives aggressive tumor growth and metastasis resembling high-risk human neuroblastoma [31,176]. Tumors in these models mirrored their human counterparts morphologically and exhibited increased ECM stiffness and collagen deposition, which are key factors enhancing metastatic potential [176,177].
Medulloblastoma
Medulloblastoma (MB) is another rare cancer that benefits from the use of zebrafish models. Zebrafish enable the orthotopic engraftment of MB cells, with the tumors developing in the hindbrain, closely replicating the localization of MB in humans [178]. Pre-culturing MB cells in a neural stem cell-like medium enhances their ability to home to the hindbrain, mimicking the neurotropism observed in human MB [178]. This process upregulates genes such as Semaphorin 3A (SEMA3A) and Ephrin B1 (EFNB1) and induces a migratory neuronal phenotype associated with poor survival [178]. In another approach, transcription activator-like effector nuclease (TALEN)-mediated inactivation of tumor suppressor genes, such as cyclin-dependent kinase inhibitor 2A (cdkn2a/b) and retinoblastoma 1 (rb1), in zebrafish resulted in the development of tumors that mimic human MB and primitive neuroectodermal tumors (PNETs) [179]. RNA sequencing revealed the activation of pathways involved in cell cycle regulation, DNA replication, and neuronal development, including wingless-related integration site (WNT), sonic hedgehog (SHH), and NOTCH signaling, which are associated with distinct MB subtypes [179]. These pathways not only drive tumor growth but also regulate key processes such as migration and invasion [178,179].
CRISPR-based models have been developed to study SHH MB by knocking out patched 1 (ptch1), a key genetic driver of this cancer [180]. Tumors arising from ptch1 loss closely resemble human SHH MB in both histological and genomic features [180]. Combining the ptch1 knockout with a tp53 mutation resulted in more aggressive tumors, reflecting the poorer outcomes and increased risk of dissemination seen in certain human SHH MB cases [180,181]. Molecular players such as G-protein-coupled receptor (GRK)2/3 kinases were identified as key regulators of both tumor growth and behaviors related to migration and invasion [180]. The inhibition of GRK2/3 significantly slowed tumor progression and decreased markers of invasive capacity, highlighting their role in enabling cancer cells to breach local barriers and potentially metastasize to distant sites [180].
These findings demonstrate how zebrafish models provide critical insights into MB biology, supporting the development of novel therapeutic strategies to target both tumor growth and the mechanisms driving invasion and metastasis.
5. Addressing the Limitations of Zebrafish Models in Metastasis Research
As highlighted in this review, zebrafish have become a crucial model in cancer research, offering unique advantages such as optical transparency for real-time imaging and the potential for high-throughput studies [34,55,113,182]. These attributes make them invaluable for studying tumor progression and metastasis. However, limitations in replicating human disease complexity, such as differences in immune function [183], stromal composition [184], and TME [184], highlight the need for further research. Innovations like humanized zebrafish [185] are essential to overcoming these challenges and unlocking their full preclinical and translational efficacy.
There are several differences between the immune systems of zebrafish and humans [183], which can create challenges when studying immune-related metastatic processes. Larval zebrafish, which are the most suitable for imaging studies, lack a mature adaptive immune system [186]. Although this difference posits an advantage when generating xenografts, it can also hinder studies aimed at the adaptive immune system. Additionally, while adult zebrafish possess an adaptive immune system, it differs from that of humans in several key aspects [183]. For example, zebrafish produce immunoglobulins such as IgZ/T, which are absent in humans [187], and cannot produce IL-18 [188]. To overcome these issues, several zebrafish models that express human immune components, including cytokines like CXCL12 and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been created [185]. These humanized zebrafish have been used to study cancer cell migration, metastasis, and the interaction of cancer cells with immune cells [185]. Additionally, the transplantation of human adaptive immune cells, such as T lymphocytes, has been employed to compensate for the absence of a fully developed adaptive immune system in zebrafish, enabling a more comprehensive exploration of tumor–immune dynamics [189].
The TME plays a critical role in metastasis, but differences between the zebrafish and human components pose challenges for modeling certain interactions. Zebrafish TME differs from its human counterpart, particularly in cellular composition and regenerative capacity [184,190]. Zebrafish stromal cells retain a progenitor-like phenotype that is different to human and mouse fetal stromal cells, which is associated with a robust ability to support tissue repair and regeneration [190,191,192]. While this feature enables dynamic remodeling of the ECM and robust cell–cell signaling, it does not fully mimic the characteristics of adult human stromal cells, which are more differentiated and less regenerative [184]. The zebrafish ECM also differs significantly in stiffness and molecular composition [98], which can influence key processes like tumor migration and invasion. Zebrafish ECM is often less rigid than that of human tissues, a factor that can affect mechanical movement and the behavior of invading cancer cells [98]. Advances in single-cell transcriptomics and cross-species analyses have highlighted these differences, showing that zebrafish stromal cells have a transcriptional profile that is more aligned with regeneration than with the tumor-promoting roles observed in adult human stromal cells [184]. Despite these differences, as previously described in this review, zebrafish models have been crucial in outlining the key molecular drivers of metastasis within the TME (Table 1). These include integrins, which mediate tumor–ECM adhesion and can be blocked to reduce cancer spread [193,194]; VEGF, which is essential for angiogenesis and studied using zebrafish with fluorescently labeled blood vessels [70,110,195]; TGF-β, which influences EMT and immune evasion [105,196]; EVs, which help to establish pre-metastatic niches [101,197]; and MMPs, which enable tumor invasion by degrading the ECM [50,198].
In zPDXs, tumors are engrafted along with the components of their original TME, including stromal, immune, endothelial cells, and microbiome [34]. Introducing foreign TME components into a zebrafish host presents challenges, as interactions between the host and donor TME can alter cell behavior, disrupt signaling pathways, and modulate immune responses [199]. For example, the zebrafish immune system may react to human-derived cells, potentially triggering inflammatory or rejection responses that could confound the experimental results [200]. Despite this, the inclusion of the donor TME can also provide significant benefits. Retaining elements of the original TME can allow for a more accurate replication of the tumor’s native microenvironment [201,202]. This is particularly valuable for studying tumor–TME dynamics, immune evasion mechanisms, and the effects of therapeutic agents in a more human-like context [201]. Moreover, the combination of the donor TME with the zebrafish host environment offers a unique opportunity to explore cross-species interactions. A recent study demonstrated that zebrafish-derived tumor necrosis factor-alpha (TNF-α) can directly induce apoptosis in human cancer cells by engaging their receptors, with macrophages polarized to a pro-inflammatory state driving this effect [203]. This finding highlights that human cells are not only responsive to zebrafish-secreted factors, such as cytokines, but also that they respond through conserved pathways, mimicking the behaviors observed in human physiological settings [203,204]. Additionally, the behavior of the donor TME in a foreign host can help to understand how environmental cues influence tumor progression [36]. By carefully optimizing conditions, such as inducing immune suppression in the host, matching ECM characteristics, or utilizing transgenic zebrafish lines expressing human proteins, it is possible to maximize the advantages of zPDXs. These approaches enable the study of complex tumor–host dynamics while preserving the translational relevance of the original TME.
Zebrafish models have proven to be an invaluable platform for uncovering key aspects of tumor metastasis and progression, despite their inherent limitations in fully replicating human biology. With innovations like humanized zebrafish and methods to incorporate human TME components, these models are evolving to address the current challenges, offering unique opportunities to study tumor–host interactions.
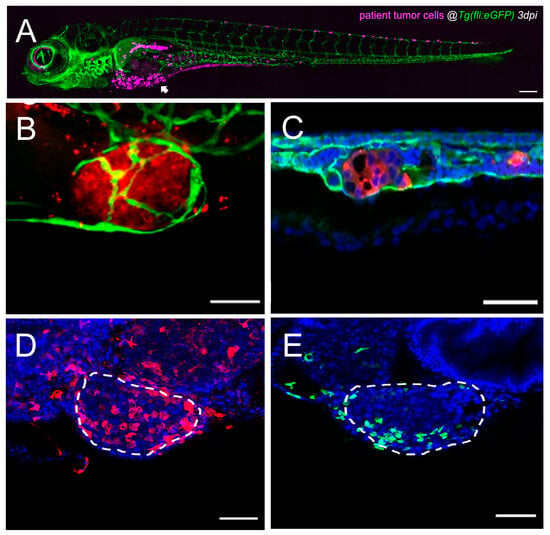
Figure 2.
Single-cell high-resolution visualization of the tumor–TME dynamics in the zebrafish model. (A) Representative confocal image of an overview of a CRC patient-derived zebrafish xenograft 3 dpi, where human cells are labeled in magenta, and blood and lymphatic vessels are labeled in green. The arrowhead points towards the primary tumor, and human cells (magenta) are also observed in metastatic sites. Scale bar 200 µm. Adapted from ref. [155]. (B) Representative confocal image of a primary tumor in a CRC zebrafish xenograft 4 dpi, where human cells are labeled in red, and blood and lymphatic vessels are labeled in green. Scale bar 50 µm. Adapted from ref. [110]. (C) Representative confocal image of a metastatic tumor in the CHT of a CRC zebrafish xenograft 4 dpi, where human cells are labeled in red, blood and lymphatic vessels are labeled in green, and nuclei with DAPI counterstaining in blue. Scale bar 50 µm. Adapted from ref. [110]. (D) Representative confocal image of a primary tumor in a bladder cancer zebrafish xenograft 4 dpi, where macrophages are labeled in red, and nuclei with DAPI counterstaining in blue. White dashes outline the tumor. Scale bar 50 µm. Adapted from ref. [203]. (E) Representative confocal image of a primary tumor in a bladder cancer zebrafish xenograft 4 dpi, where neutrophils are labeled in green, and nuclei with DAPI counterstaining in blue. White dashes outline the tumor. Scale bar 50 µm. Adapted from ref. [203]. CRC: colorectal cancer; CHT: caudal–hematopoietic tissue; dpi: days post injection; TME: tumor microenvironment.
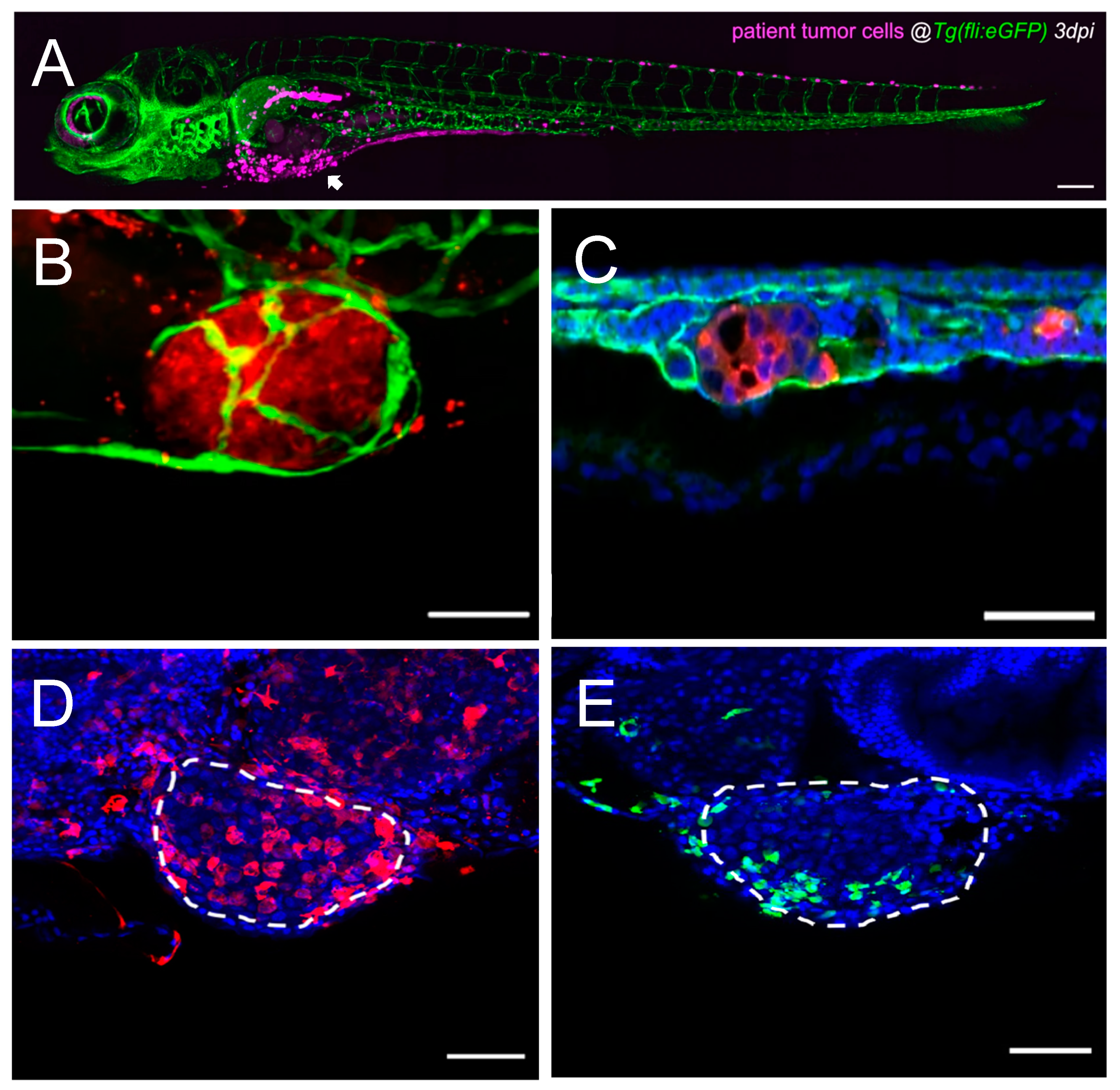
Figure 2.
Single-cell high-resolution visualization of the tumor–TME dynamics in the zebrafish model. (A) Representative confocal image of an overview of a CRC patient-derived zebrafish xenograft 3 dpi, where human cells are labeled in magenta, and blood and lymphatic vessels are labeled in green. The arrowhead points towards the primary tumor, and human cells (magenta) are also observed in metastatic sites. Scale bar 200 µm. Adapted from ref. [155]. (B) Representative confocal image of a primary tumor in a CRC zebrafish xenograft 4 dpi, where human cells are labeled in red, and blood and lymphatic vessels are labeled in green. Scale bar 50 µm. Adapted from ref. [110]. (C) Representative confocal image of a metastatic tumor in the CHT of a CRC zebrafish xenograft 4 dpi, where human cells are labeled in red, blood and lymphatic vessels are labeled in green, and nuclei with DAPI counterstaining in blue. Scale bar 50 µm. Adapted from ref. [110]. (D) Representative confocal image of a primary tumor in a bladder cancer zebrafish xenograft 4 dpi, where macrophages are labeled in red, and nuclei with DAPI counterstaining in blue. White dashes outline the tumor. Scale bar 50 µm. Adapted from ref. [203]. (E) Representative confocal image of a primary tumor in a bladder cancer zebrafish xenograft 4 dpi, where neutrophils are labeled in green, and nuclei with DAPI counterstaining in blue. White dashes outline the tumor. Scale bar 50 µm. Adapted from ref. [203]. CRC: colorectal cancer; CHT: caudal–hematopoietic tissue; dpi: days post injection; TME: tumor microenvironment.
6. Future Directions and the Evolving Role of Zebrafish in Research
This review has summarized the key findings demonstrating how zebrafish models contribute to comprehending the complex mechanisms through which tumors evade their primary sites and home distant tissues. Current work is now focusing on improving the biological accuracy and experimental flexibility of zebrafish models to enhance their value in translational research [205,206,207,208]. One promising approach involves zebrafish models expressing human-specific molecules and components, including stromal elements [185,209,210,211,212]. This emerging strategy has shown potential [185] but requires further development to better replicate the complex TME and produce findings that are more relevant to human biology.
Combining patient-derived tumor organoids (PDTOs) with zebrafish models presents an unexplored opportunity to study tumor–TME interactions in a more dynamic and physiologically relevant context [213]. PDTOs capture the three-dimensional architecture of tumors, offering insights into individual patient biology, but lack the systemic dynamics of a living organism [213]. On the other hand, zPDXs allow for the study of these organism-level processes, though certain tumor characteristics may make direct engraftment challenging [34]. By transplanting PDTOs into zebrafish, it may be possible to integrate the structural fidelity of organoids with the systemic dynamism of zebrafish, overcoming limitations in both models. This approach could provide a versatile platform for investigating cancer progression, including metastasis and immune interactions, while maximizing resources through the high-throughput and cost-effective nature of zebrafish.
Advances in imaging technologies and genetic tools have further expanded the utility of zebrafish models in cancer research [54,55,114,175,182]. Fully optically transparent zebrafish strains now allow for the real-time observation of metastatic behavior over extended periods, even in adult zebrafish, enabling the detailed exploration of the spatial and temporal dynamics of tumor dissemination [214]. Meanwhile, the refinement of gene-editing tools like CRISPR-Cas9 has facilitated the creation of zebrafish models with precise genetic mutations linked to metastasis [25]. Combining these advanced imaging and genetic approaches opens new avenues to directly correlate tumor behaviors with their underlying genetic drivers, enhancing our understanding of cancer progression.
Beyond cancer research, zebrafish models have made significant contributions to broader areas of medicine and biology. Studies of cell migration in zebrafish have elucidated mechanisms of tissue invasion, which are applicable to both cancerous and non-cancerous conditions [215,216,217]. Research on ECM remodeling, a key factor in metastasis, has provided valuable insights into wound healing and tissue regeneration, advancing regenerative medicine [27,98,192]. Zebrafish studies on inflammatory mediators have the potential to contribute to the development of immunotherapies for cancer and autoimmune diseases [124,189,203,218,219]. Together, these findings demonstrate the versatility of zebrafish as a model system, offering unique opportunities to explore complex biological processes that extend beyond oncology.
In addition to their technical advantages, zebrafish offer exceptional accessibility and affordability, making them particularly valuable in resource-limited settings [34]. Their low maintenance costs enable high-throughput screening and advanced research, providing a cost-effective alternative to mammalian models, even in emerging laboratories [165]. This accessibility allows researchers in developing countries to model tumor dynamics relevant to their specific geographic and clinical realities. By focusing on the cancers that are most prevalent in their populations, these studies yield findings that are directly applicable to local healthcare challenges while contributing to global cancer research efforts. This approach fosters inclusivity and collaboration, driving impactful discoveries and advancing cancer diagnosis and treatment worldwide.
7. Conclusions
Zebrafish models are invaluable for studying cancer metastasis, providing key insights into the molecular drivers of tumor progression, angiogenesis, and tumor–TME interactions. Their optical transparency, rapid development, and genetic tractability allow for the real-time analysis of processes like ECM remodeling and vascular invasion. Advances in gene editing, imaging, and PDX technologies have further enhanced their role in exploring metastatic mechanisms and testing therapies. Accessible and cost-effective, zebrafish empower researchers worldwide, including those in resource-limited settings, to advance metastasis research. Despite some limitations, innovations such as humanized zebrafish are addressing these challenges, establishing zebrafish as critical tools for deepening our understanding of metastasis and advancing cancer research.
Author Contributions
Conceptualization, M.F.M.-L.; Investigation, M.F.M.-L.; Visualization, M.F.M.-L.; Writing—original draft, M.F.M.-L.; Writing—review and editing, M.F.M.-L. and J.F.L.-G.; Funding acquisition, J.F.L.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Luis Martínez-López for his assistance with Figure 1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Deng, D.; Lin, C.; Wang, M.; Hsu, M.L.; Zaorsky, N.G. Causes of Death among People Living with Metastatic Cancer. Nat. Commun. 2024, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Bouchalova, P.; Bouchal, P. Current Methods for Studying Metastatic Potential of Tumor Cells. Cancer Cell Int. 2022, 22, 394. [Google Scholar] [CrossRef]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-Derived Xenograft (PDX) Models, Applications and Challenges in Cancer Research. J. Transl. Med. 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef]
- Entenberg, D.; Rodriguez-Tirado, C.; Kato, Y.; Kitamura, T.; Pollard, J.W.; Condeelis, J. In Vivo Subcellular Resolution Optical Imaging in the Lung Reveals Early Metastatic Proliferation and Motility. Intravital 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Yao, T.; Jia, R. Benefits of Zebrafish Xenograft Models in Cancer Research. Front. Cell Dev. Biol. 2021, 9, 616551. [Google Scholar] [CrossRef]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in Zebrafish–from Embryo to Adult. Neural Develop. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.M.; Nguyen, A.T.; Odem, M.A.; Eisenhoffer, G.T.; Krachler, A.M. The Zebrafish as a Model for Gastrointestinal Tract–Microbe Interactions. Cell. Microbiol. 2020, 22, e13152. [Google Scholar] [CrossRef] [PubMed]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval Zebrafish as a Model for Glucose Metabolism: Expression of Phosphoenolpyruvate Carboxykinase as a Marker for Exposure to Anti-Diabetic Compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zeituni, E.M.; Farber, S.A. Studying Lipid Metabolism and Transport During Zebrafish Development. In Zebrafish: Methods and Protocols; Kawakami, K., Patton, E.E., Orger, M., Eds.; Springer: New York, NY, USA, 2016; pp. 237–255. ISBN 978-1-4939-3771-4. [Google Scholar]
- Echeazarra, L.; Hortigón-Vinagre, M.P.; Casis, O.; Gallego, M. Adult and Developing Zebrafish as Suitable Models for Cardiac Electrophysiology and Pathology in Research and Industry. Front. Physiol. 2021, 11, 607860. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.M.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P.; et al. Tp53 Mutant Zebrafish Develop Malignant Peripheral Nerve Sheath Tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407–412. [Google Scholar] [CrossRef]
- Ju, B.; Chen, W.; Orr, B.A.; Spitsbergen, J.M.; Jia, S.; Eden, C.J.; Henson, H.E.; Taylor, M.R. Oncogenic KRAS Promotes Malignant Brain Tumors in Zebrafish. Mol. Cancer 2015, 14, 18. [Google Scholar] [CrossRef]
- Li, D.; Li, X.-P.; Wang, H.-X.; Shen, Q.-Y.; Li, X.-P.; Wen, L.; Qin, X.-J.; Jia, Q.-L.; Kung, H.-F.; Peng, Y. VEGF Induces Angiogenesis in a Zebrafish Embryo Glioma Model Established by Transplantation of Human Glioma Cells. Oncol. Rep. 2012, 28, 937–942. [Google Scholar] [CrossRef]
- Ju, B.; Spitsbergen, J.; Eden, C.J.; Taylor, M.R.; Chen, W. Co-Activation of Hedgehog and AKT Pathways Promote Tumorigenesis in Zebrafish. Mol. Cancer 2009, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Asokan, N.; Daetwyler, S.; Bernas, S.N.; Schmied, C.; Vogler, S.; Lambert, K.; Wobus, M.; Wermke, M.; Kempermann, G.; Huisken, J.; et al. Long-Term in Vivo Imaging Reveals Tumor-Specific Dissemination and Captures Host Tumor Interaction in Zebrafish Xenografts. Sci. Rep. 2020, 10, 13254. [Google Scholar] [CrossRef] [PubMed]
- Langenau, D.M.; Jette, C.; Berghmans, S.; Palomero, T.; Kanki, J.P.; Kutok, J.L.; Look, A.T. Suppression of Apoptosis by Bcl-2 Overexpression in Lymphoid Cells of Transgenic Zebrafish. Blood 2005, 105, 3278–3285. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jette, C.; Kanki, J.P.; Aster, J.C.; Look, A.T.; Griffin, J.D. NOTCH1-Induced T-Cell Leukemia in Transgenic Zebrafish. Leukemia 2007, 21, 462–471. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Cao, X.; Jin, X.; Jin, T. Pattern Recognition Receptors in Zebrafish Provide Functional and Evolutionary Insight into Innate Immune Signaling Pathways. Cell. Mol. Immunol. 2017, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Albadri, S.; De Santis, F.; Di Donato, V.; Del Bene, F. CRISPR/Cas9-Mediated Knockin and Knockout in Zebrafish. In Genome Editing in Neurosciences; Jaenisch, R., Zhang, F., Gage, F., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 41–49. ISBN 978-3-319-60192-2. [Google Scholar]
- Fan, R.-Y.; Wu, J.-Q.; Liu, Y.-Y.; Liu, X.-Y.; Qian, S.-T.; Li, C.-Y.; Wei, P.; Song, Z.; He, M.-F. Zebrafish Xenograft Model for Studying Mechanism and Treatment of Non-Small Cell Lung Cancer Brain Metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 371. [Google Scholar] [CrossRef]
- Allen, T.A.; Cullen, M.M.; Hawkey, N.; Mochizuki, H.; Nguyen, L.; Schechter, E.; Borst, L.; Yoder, J.A.; Freedman, J.A.; Patierno, S.R.; et al. A Zebrafish Model of Metastatic Colonization Pinpoints Cellular Mechanisms of Circulating Tumor Cell Extravasation. Front. Oncol. 2021, 11, 641187. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, Y.; He, S.; Zhang, L.; Snaar-Jagalska, B.E.; ten Dijke, P. Transforming Growth Factor-β Signalling Controls Human Breast Cancer Metastasis in a Zebrafish Xenograft Model. Breast Cancer Res. 2013, 15, R106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Boleslaw Olszewski, M.; Kruithof-de Julio, M.; Snaar-Jagalska, B.E. Zebrafish Microenvironment Elevates EMT and CSC-Like Phenotype of Engrafted Prostate Cancer Cells. Cells 2020, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Rabbie, R.; Garg, M.; Lumaquin, D.; Huang, T.-H.; Montal, E.; Ma, Y.; Cruz, N.M.; Tang, X.; Nsengimana, J.; et al. Identifying the Transcriptional Drivers of Metastasis Embedded within Localized Melanoma. Cancer Discov. 2023, 13, 194–215. [Google Scholar] [CrossRef]
- Her, Z.P.; Yeo, K.S.; Howe, C.; Levee, T.; Zhu, S. Zebrafish Model of Neuroblastoma Metastasis. J. Vis. Exp. JoVE 2021, e62416. [Google Scholar] [CrossRef]
- May, W.A.; Lessnick, S.L.; Braun, B.S.; Klemsz, M.; Lewis, B.C.; Lunsford, L.B.; Hromas, R.; Denny, C.T. The Ewing’s Sarcoma EWS/FLI-1 Fusion Gene Encodes a More Potent Transcriptional Activator and Is a More Powerful Transforming Gene than FLI-1. Mol. Cell. Biol. 1993, 13, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Zhu, H.; Liu, D.; Chen, Z.; Zhang, X.; Guo, Z.; Dong, M.; Wan, L.; Zhang, P.; Liu, G.; et al. TGFBI Secreted by Tumor-Associated Macrophages Promotes Glioblastoma Stem Cell-Driven Tumor Growth via Integrin Avβ5-Src-Stat3 Signaling. Theranostics 2022, 12, 4221–4236. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Estrada, M.F.; Mendes, R.V.; Fior, R. Zebrafish Avatars towards Personalized Medicine—A Comparative Review between Avatar Models. Cells 2020, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Fior, R.; Póvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-Cell Functional and Chemosensitive Profiling of Combinatorial Colorectal Therapy in Zebrafish Xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, C.; Hocking, J.; Kirchberger, S.; Distel, M. Studying the Tumor Microenvironment in Zebrafish. In Tumor Microenvironment: Novel Concepts; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–92. ISBN 978-3-030-73119-9. [Google Scholar]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, G.; Rüegg, C. The Tumor Microenvironment and Its Contribution to Tumor Evolution toward Metastasis. Histochem. Cell Biol. 2008, 130, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Weiss, J.M.; Lumaquin-Yin, D.; Montal, E.; Suresh, S.; Leonhardt, C.S.; White, R.M. Shifting the Focus of Zebrafish toward a Model of the Tumor Microenvironment. eLife 2022, 11, e69703. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Nagaraju, R.; Hurlstone, A. Zebrafish: A New Companion for Translational Research in Oncology. Clin. Cancer Res. 2015, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef]
- Kwa, M.Q.; Herum, K.M.; Brakebusch, C. Cancer-Associated Fibroblasts: How Do They Contribute to Metastasis? Clin. Exp. Metastasis 2019, 36, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-Associated Fibroblasts: An Emerging Target of Anti-Cancer Immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.; Fernandez del Ama, L.; Ferguson, J.; Kamarashev, J.; Wellbrock, C.; Hurlstone, A. Heterogeneous Tumor Subpopulations Cooperate to Drive Invasion. Cell Rep. 2014, 8, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, P.; Martínez-Pena, I.; Yepes-Rodríguez, S.; Bascoy-Otero, M.; Abuín, C.; Fernández-Santiago, C.; Sánchez, L.; López-López, R.; Piñeiro, R. Modelling Metastasis in Zebrafish Unveils Regulatory Interactions of Cancer-Associated Fibroblasts with Circulating Tumour Cells. Front. Cell Dev. Biol. 2023, 11, 1076432. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Stainier, D.Y.R. Out with the Old, in with the New: Reassessing Morpholino Knockdowns in Light of Genome Editing Technology. Development 2014, 141, 3103–3104. [Google Scholar] [CrossRef]
- Schütte, A.; Hedrich, J.; Stöcker, W.; Becker-Pauly, C. Let It Flow: Morpholino Knockdown in Zebrafish Embryos Reveals a Pro-Angiogenic Effect of the Metalloprotease Meprin A2. PLoS ONE 2010, 5, e8835. [Google Scholar] [CrossRef]
- Dong, M.; Fu, Y.-F.; Du, T.-T.; Jing, C.-B.; Fu, C.-T.; Chen, Y.; Jin, Y.; Deng, M.; Liu, T.X. Heritable and Lineage-Specific Gene Knockdown in Zebrafish Embryo. PLoS ONE 2009, 4, e6125. [Google Scholar] [CrossRef]
- Wen, J.; Yin, P.; Li, L.; Kang, G.; Ning, G.; Cao, Y.; Gao, F.; Su, Y.; Wu, Y.; Zhang, X. Knockdown of Matrix Metallopeptidase 9 Inhibits Metastasis of Oral Squamous Cell Carcinoma Cells in a Zebrafish Xenograft Model. BioMed Res. Int. 2020, 2020, 4350783. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, H.; Shi, H.; Wang, X.; Chen, X.; Yuan, Y.; Lin, S.; Wei, Y. Distinct Contributions of Angiogenesis and Vascular Co-Option during the Initiation of Primary Microtumors and Micrometastases. Carcinogenesis 2011, 32, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, J.; Qie, J.; Zhang, G.; Liu, L.; Yang, P. Optimization of the Gal4/UAS Transgenic Tools in Zebrafish. Appl. Microbiol. Biotechnol. 2019, 103, 1789–1799. [Google Scholar] [CrossRef]
- Schiavone, M.; Rampazzo, E.; Casari, A.; Battilana, G.; Persano, L.; Moro, E.; Liu, S.; Leach, S.D.; Tiso, N.; Argenton, F. Zebrafish Reporter Lines Reveal in Vivo Signaling Pathway Activities Involved in Pancreatic Cancer. Dis. Model. Mech. 2014, 7, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; He, S.; van der Ent, W.; Chen, L.; Groenewoud, A.; Spaink, H.P.; Snaar-Jagalska, B.E. Imaging Cancer Angiogenesis and Metastasis in a Zebrafish Embryo Model. In Cancer and Zebrafish: Mechanisms, Techniques, and Models; Langenau, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 239–263. ISBN 978-3-319-30654-4. [Google Scholar]
- Suster, M.L.; Kikuta, H.; Urasaki, A.; Asakawa, K.; Kawakami, K. Transgenesis in Zebrafish with the Tol2 Transposon System. In Transgenesis Techniques: Principles and Protocols; Cartwright, E.J., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 41–63. ISBN 978-1-60327-019-9. [Google Scholar]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 Signaling Network in Endothelial Cells Relevant to Angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kanada, M.; Zhang, J.; Yan, L.; Sakurai, T.; Terakawa, S. Endothelial Cell-Initiated Extravasation of Cancer Cells Visualized in Zebrafish. PeerJ 2014, 2, e688. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, L.H. Assessing Molecular Regulation of Vascular Permeability Using a VEGF-Inducible Zebrafish Model. In VEGF Signaling: Methods and Protocols; Fiedler, L.R., Pellet-Many, C., Eds.; Springer: New York, NY, USA, 2022; pp. 339–350. ISBN 978-1-07-162217-9. [Google Scholar]
- Wu, Y.-C.; Chang, C.-Y.; Kao, A.; Hsi, B.; Lee, S.-H.; Chen, Y.-H.; Wang, I.-J. Hypoxia-Induced Retinal Neovascularization in Zebrafish Embryos: A Potential Model of Retinopathy of Prematurity. PLoS ONE 2015, 10, e0126750. [Google Scholar] [CrossRef]
- Gerri, C.; Marín-Juez, R.; Marass, M.; Marks, A.; Maischein, H.-M.; Stainier, D.Y.R. Hif-1α Regulates Macrophage-Endothelial Interactions during Blood Vessel Development in Zebrafish. Nat. Commun. 2017, 8, 15492. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.T.; Richter, L.; Frutiger, Y.; Grogan, T.M. Expression of Vascular Endothelial Growth Factor and Its Receptors in Hematopoietic Malignancies. Cancer Res. 1999, 59, 728–733. [Google Scholar] [PubMed]
- Fiedler, W.; Graeven, U.; Ergün, S.; Verago, S.; Kilic, N.; Stockschläder, M.; Hossfeld, D.K. Vascular Endothelial Growth Factor, a Possible Paracrine Growth Factor in Human Acute Myeloid Leukemia. Blood 1997, 89, 1870–1875. [Google Scholar] [CrossRef]
- Bellamy, W.T.; Richter, L.; Sirjani, D.; Roxas, C.; Glinsmann-Gibson, B.; Frutiger, Y.; Grogan, T.M.; List, A.F. Vascular Endothelial Cell Growth Factor Is an Autocrine Promoter of Abnormal Localized Immature Myeloid Precursors and Leukemia Progenitor Formation in Myelodysplastic Syndromes. Blood 2001, 97, 1427–1434. [Google Scholar] [CrossRef]
- Olson, T.A.; Mohanraj, D.; Carson, L.F.; Ramakrishnan, S. Vascular Permeability Factor Gene Expression in Normal and Neoplastic Human Ovaries. Cancer Res. 1994, 54, 276–280. [Google Scholar] [PubMed]
- Ohta, Y.; Endo, Y.; Tanaka, M.; Shimizu, J.; Oda, M.; Hayashi, Y.; Watanabe, Y.; Sasaki, T. Significance of Vascular Endothelial Growth Factor Messenger RNA Expression in Primary Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1411–1416. [Google Scholar]
- Joseph, I.B.; Nelson, J.B.; Denmeade, S.R.; Isaacs, J.T. Androgens Regulate Vascular Endothelial Growth Factor Content in Normal and Malignant Prostatic Tissue. Clin. Cancer Res. 1997, 3, 2507–2511. [Google Scholar]
- Brown, L.F.; Berse, B.; Jackman, R.W.; Tognazzi, K.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of Vascular Permeability Factor (Vascular Endothelial Growth Factor) and Its Receptors in Adenocarcinomas of the Gastrointestinal Tract. Cancer Res. 1993, 53, 4727–4735. [Google Scholar] [PubMed]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages Enhance Vegfa-Driven Angiogenesis in an Embryonic Zebrafish Tumour Xenograft Model. Dis. Model. Mech. 2018, 11, dmm035998. [Google Scholar] [CrossRef] [PubMed]
- Nasevicius, A.; Larson, J.; Ekker, S.C. Distinct Requirements for Zebrafish Angiogenesis Revealed by a VEGF-A Morphant. Yeast Chichester Engl. 2000, 17, 294–301. [Google Scholar] [CrossRef]
- Shin, M.; Beane, T.J.; Quillien, A.; Male, I.; Zhu, L.J.; Lawson, N.D. Vegfa Signals through ERK to Promote Angiogenesis, but Not Artery Differentiation. Development 2016, 143, 3796–3805. [Google Scholar] [CrossRef]
- Astin, J.W.; Jamieson, S.M.F.; Eng, T.C.Y.; Flores, M.V.; Misa, J.P.; Chien, A.; Crosier, K.E.; Crosier, P.S. An In Vivo Antilymphatic Screen in Zebrafish Identifies Novel Inhibitors of Mammalian Lymphangiogenesis and Lymphatic-Mediated Metastasis. Mol. Cancer Ther. 2014, 13, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial Barrier Disruption by VEGF-Mediated Src Activity Potentiates Tumor Cell Extravasation and Metastasis. J. Cell Biol. 2004, 167, 223–229. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.C.; Rouhi, P.; Jensen, L.D.; Zhang, D.; Ji, H.; Hauptmann, G.; Ingham, P.; Cao, Y. Hypoxia-Induced Pathological Angiogenesis Mediates Tumor Cell Dissemination, Invasion, and Metastasis in a Zebrafish Tumor Model. Proc. Natl. Acad. Sci. USA 2009, 106, 19485–19490. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, P.; Jensen, L.D.; Cao, Z.; Hosaka, K.; Länne, T.; Wahlberg, E.; Steffensen, J.F.; Cao, Y. Hypoxia-Induced Metastasis Model in Embryonic Zebrafish. Nat. Protoc. 2010, 5, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The Effect of HIF on Metabolism and Immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Fagundes, R.R.; Malkov, M.I.; Sparkes, R.; Dillon, E.T.; Smolenski, A.; Faber, K.N.; Taylor, C.T. Hypoxia Induces a Glycolytic Complex in Intestinal Epithelial Cells Independent of HIF-1-Driven Glycolytic Gene Expression. Proc. Natl. Acad. Sci. USA 2023, 120, e2208117120. [Google Scholar] [CrossRef]
- Xiang, L.; Mou, J.; Shao, B.; Wei, Y.; Liang, H.; Takano, N.; Semenza, G.L.; Xie, G. Glutaminase 1 Expression in Colorectal Cancer Cells Is Induced by Hypoxia and Required for Tumor Growth, Invasion, and Metastatic Colonization. Cell Death Dis. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Oike, Y.; Satoh, S.; Tabata, Y.; Niikura, Y.; Morisada, T.; Akao, M.; Urano, T.; Ito, Y.; Miyamoto, T.; et al. Cooperative Interaction of Angiopoietin-like Proteins 1 and 2 in Zebrafish Vascular Development. Proc. Natl. Acad. Sci. USA 2005, 102, 13502–13507. [Google Scholar] [CrossRef] [PubMed]
- Pappa, C.A.; Alexandrakis, M.G.; Boula, A.; Thanasia, A.; Konsolas, I.; Alegakis, A.; Tsirakis, G. Prognostic Impact of Angiopoietin-2 in Multiple Myeloma. J. Cancer Res. Clin. Oncol. 2014, 140, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.L.; Araújo, A.; Gomes, M.; Catarino, R.; Marques, A.; Medeiros, R. Circulating Ang-2 mRNA Expression Levels: Looking Ahead to a New Prognostic Factor for NSCLC [Corrected]. PLoS ONE 2014, 9, e90009. [Google Scholar] [CrossRef] [PubMed]
- Jary, M.; Vernerey, D.; Lecomte, T.; Dobi, E.; Ghiringhelli, F.; Monnien, F.; Godet, Y.; Kim, S.; Bouché, O.; Fratte, S.; et al. Prognostic Value of Angiopoietin-2 for Death Risk Stratification in Patients with Metastatic Colorectal Carcinoma. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gayed, B.A.; Gillen, J.; Christie, A.; Peña-Llopis, S.; Xie, X.-J.; Yan, J.; Karam, J.A.; Raj, G.; Sagalowsky, A.I.; Lotan, Y.; et al. Prospective Evaluation of Plasma Levels of ANGPT2, TuM2PK, and VEGF in Patients with Renal Cell Carcinoma. BMC Urol. 2015, 15, 24. [Google Scholar] [CrossRef]
- Miyahara, K.; Nouso, K.; Morimoto, Y.; Takeuchi, Y.; Hagihara, H.; Kuwaki, K.; Onishi, H.; Ikeda, F.; Miyake, Y.; Nakamura, S.; et al. Pro-Angiogenic Cytokines for Prediction of Outcomes in Patients with Advanced Hepatocellular Carcinoma. Br. J. Cancer 2013, 109, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Maffei, R.; Martinelli, S.; Santachiara, R.; Rossi, D.; Guarnotta, C.; Sozzi, E.; Zucchetto, A.; Rigolin, G.M.; Fiorcari, S.; Castelli, I.; et al. Angiopoietin-2 Plasma Dosage Predicts Time to First Treatment and Overall Survival in Chronic Lymphocytic Leukemia. Blood 2010, 116, 584–592. [Google Scholar] [CrossRef]
- Daly, C.; Eichten, A.; Castanaro, C.; Pasnikowski, E.; Adler, A.; Lalani, A.S.; Papadopoulos, N.; Kyle, A.H.; Minchinton, A.I.; Yancopoulos, G.D.; et al. Angiopoietin-2 Functions as a Tie2 Agonist in Tumor Models, Where It Limits the Effects of VEGF Inhibition. Cancer Res. 2013, 73, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, A.; Baek, K.-H.; Lynch, R.C.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.E., Jr.; Ryeom, S. Platelet-Derived Thrombospondin-1 Is a Critical Negative Regulator and Potential Biomarker of Angiogenesis. Blood 2010, 115, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, J.; Zhang, H.; Zi, H.; Liu, M.; An, Q.; Qiu, T.; Li, P.; Song, J.; Liu, P.; et al. Single-Cell Analysis Reveals the Implication of Vascular Endothelial Cell-Intrinsic ANGPT2 in Human Intracranial Aneurysm. Cardiovasc. Res. 2024, cvae186. [Google Scholar] [CrossRef] [PubMed]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.-X. A Pan-Cancer Perspective of Matrix Metalloproteases (MMP) Gene Expression Profile and Their Diagnostic/Prognostic Potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Jessen, J.R. Recent Advances in the Study of Zebrafish Extracellular Matrix Proteins. Dev. Biol. 2015, 401, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Puig, A.; Mosquera, J.L.; Jiménez-Delgado, S.; García-Pastor, C.; Jorba, I.; Navajas, D.; Canals, F.; Raya, A. Proteomics Analysis of Extracellular Matrix Remodeling During Zebrafish Heart Regeneration. Mol. Cell. Proteomics MCP 2019, 18, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating Human Cancer Cell Metastasis in Zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hyenne, V.; Ghoroghi, S.; Collot, M.; Bons, J.; Follain, G.; Harlepp, S.; Mary, B.; Bauer, J.; Mercier, L.; Busnelli, I.; et al. Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev. Cell 2019, 48, 554–572.e7. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; Liu, Y.; Starich, B.; Du, W.; Nair, P.R.; Marar, C.; Faqih, N.; Ciotti, G.E.; Kim, J.H.; Krishnan, S.; et al. Small Extracellular Vesicles Promote Stiffness-Mediated Metastasis. Cancer Res. Commun. 2024, 4, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.-D.; Lerch, M.M.; Bagowski, C.P. Metastatic Behaviour of Primary Human Tumours in a Zebrafish Xenotransplantation Model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef]
- Nakayama, J.; Tan, L.; Li, Y.; Goh, B.C.; Wang, S.; Makinoshima, H.; Gong, Z. A Zebrafish Embryo Screen Utilizing Gastrulation Identifies the HTR2C Inhibitor Pizotifen as a Suppressor of EMT-Mediated Metastasis. eLife 2021, 10, e70151. [Google Scholar] [CrossRef]
- Pascual, A.S.; Rapanan, J.L.; Uppalapati, C.K.; Cooper, K.E.; Leyva, K.J.; Hull, E.E. Dual Inhibition of TGFβR and ROCK Reverses the Epithelial to Mesenchymal Transition in Collectively Migrating Zebrafish Keratocytes. Cell Biol. Int. 2021, 45, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Noonan, H.R.; Thornock, A.M.; Barbano, J.; Xifaras, M.E.; Baron, C.S.; Yang, S.; Koczirka, K.; McConnell, A.M.; Zon, L.I. A Chronic Signaling TGFb Zebrafish Reporter Identifies Immune Response in Melanoma. eLife 2024, 13, e83527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Deng, M.; Jing, Q. The Zebrafish Tie2 Signaling Controls Tip Cell Behaviors and Acts Synergistically with Vegf Pathway in Developmental Angiogenesis. Acta Biochim. Biophys. Sin. 2014, 46, 641–646. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Maione, P.; Del Gaizo, F.; Colantuoni, G.; Guerriero, C.; Ferrara, C.; Nicolella, D.; Comunale, D.; De Vita, A.; Rossi, A. Sorafenib and Sunitinib in the Treatment of Advanced Non-Small Cell Lung Cancer. Oncologist 2007, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Rebelo de Almeida, C.; Mendes, R.V.; Pezzarossa, A.; Gago, J.; Carvalho, C.; Alves, A.; Nunes, V.; Brito, M.J.; Cardoso, M.J.; Ribeiro, J.; et al. Zebrafish Xenografts as a Fast Screening Platform for Bevacizumab Cancer Therapy. Commun. Biol. 2020, 3, 299. [Google Scholar] [CrossRef] [PubMed]
- Chimote, G.; Sreenivasan, J.; Pawar, N.; Subramanian, J.; Sivaramakrishnan, H.; Sharma, S. Comparison of Effects of Anti-Angiogenic Agents in the Zebrafish Efficacy–Toxicity Model for Translational Anti-Angiogenic Drug Discovery. Drug Des. Devel. Ther. 2014, 8, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-Angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.; Otterstrom, J.; Hoade, Y.; Bjedov, I.; Stead, E.; Whelan, M.; Gestri, G.; Paran, Y.; Payne, E. A Versatile, Automated and High-Throughput Drug Screening Platform for Zebrafish Embryos. Biol. Open 2021, 10, bio058513. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.N.; Turgeon, P.J.; Gupta, S.; Brand-Arzamendi, K.; Chen, H.; Malone, J.H.; Ng, R.; Ho, K.; Dubinsky, M.; Di Ciano-Oliveira, C.; et al. Automated in Vivo Compound Screening with Zebrafish and the Discovery and Validation of PD 81,723 as a Novel Angiogenesis Inhibitor. Sci. Rep. 2022, 12, 14537. [Google Scholar] [CrossRef]
- Wanting, H.; Jian, Z.; Chaoxin, X.; Cheng, Y.; Chengjian, Z.; Lin, Z.; Dan, C. Using a Zebrafish Xenograft Tumor Model to Compare the Efficacy and Safety of VEGFR-TKIs. J. Cancer Res. Clin. Oncol. 2023, 149, 5975–5987. [Google Scholar] [CrossRef]
- Uribe-Salazar, J.M.; Kaya, G.; Sekar, A.; Weyenberg, K.; Ingamells, C.; Dennis, M.Y. Evaluation of CRISPR Gene-Editing Tools in Zebrafish. BMC Genom. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-Throughput Functional Genomics Using CRISPR–Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Di Mitri, D.; Garlanda, C. Macrophage Diversity in Cancer Dissemination and Metastasis. Cell. Mol. Immunol. 2024, 21, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Finotto, L.; Cole, B.; Giese, W.; Baumann, E.; Claeys, A.; Vanmechelen, M.; Decraene, B.; Derweduwe, M.; Dubroja Lakic, N.; Shankar, G.; et al. Single-cell Profiling and Zebrafish Avatars Reveal LGALS1 as Immunomodulating Target in Glioblastoma. EMBO Mol. Med. 2023, 15, e18144. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, N.; Hong, Y.; Tie, R.; Fan, D.; Lin, A.; Chen, Y.; Xiang, L.; Shao, J. Single-Cell RNA Sequencing Unveils the Hidden Powers of Zebrafish Kidney for Generating Both Hematopoiesis and Adaptive Antiviral Immunity. eLife 2024, 13, RP92424. [Google Scholar] [CrossRef]
- Póvoa, V.; Rebelo de Almeida, C.; Maia-Gil, M.; Sobral, D.; Domingues, M.; Martinez-Lopez, M.; de Almeida Fuzeta, M.; Silva, C.; Grosso, A.R.; Fior, R. Innate Immune Evasion Revealed in a Colorectal Zebrafish Xenograft Model. Nat. Commun. 2021, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Z.; Zhang, X.-M.; Nakamura, M.; Sun, M.; Hartman, J.; Harris, R.A.; Sun, Y.; Cao, Y. Novel Mechanism of Macrophage-Mediated Metastasis Revealed in a Zebrafish Model of Tumor Development. Cancer Res. 2015, 75, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Midavaine, É.; Côté, J.; Sarret, P. The Multifaceted Roles of the Chemokines CCL2 and CXCL12 in Osteophilic Metastatic Cancers. Cancer Metastasis Rev. 2021, 40, 427–445. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-Induced Chemokine Cascade Promotes Breast Cancer Metastasis by Enhancing Retention of Metastasis-Associated Macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.A.G.; Nascimento, C.R.; Rodrigues Fernandes, N.A.; Silva, A.L.P.; D’Silva, N.J.; Rossa, C., Jr. Influence of Tumor Cell-Derived TGF-β on Macrophage Phenotype and Macrophage-Mediated Tumor Cell Invasion. Int. J. Biochem. Cell Biol. 2022, 153, 106330. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.; Astell, K.R.; Velikova, G.; Sieger, D. A Zebrafish Live Imaging Model Reveals Differential Responses of Microglia Toward Glioblastoma Cells In Vivo. Zebrafish 2016, 13, 523–534. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Xing, Z.; Lou, C.; Fang, J.; Wang, Z.; Li, M.; He, H.; Bai, H. Fibronectin 1 Derived from Tumor-Associated Macrophages and Fibroblasts Promotes Metastasis through the JUN Pathway in Hepatocellular Carcinoma. Int. Immunopharmacol. 2022, 113, 109420. [Google Scholar] [CrossRef]
- Isles, H.M.; Loynes, C.A.; Alasmari, S.; Kon, F.C.; Henry, K.M.; Kadochnikova, A.; Hales, J.; Muir, C.F.; Keightley, M.-C.; Kadirkamanathan, V.; et al. Pioneer Neutrophils Release Chromatin within in Vivo Swarms. eLife 2021, 10, e68755. [Google Scholar] [CrossRef]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed Neutrophils Sequestered at Entrapped Tumor Cells via Chemotactic Confinement Promote Tumor Cell Extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef]
- He, S.; Lamers, G.E.; Beenakker, J.-W.M.; Cui, C.; Ghotra, V.P.; Danen, E.H.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Neutrophil-Mediated Experimental Metastasis Is Enhanced by VEGFR Inhibition in a Zebrafish Xenograft Model. J. Pathol. 2012, 227, 431–445. [Google Scholar] [CrossRef]
- Mir, M.A.; Naik, A.Q.; Shah, M.Z.U.H.; Zafar, T. CXCL12–CXCR4 Axis in Cancer Metastasis. In Cytokine and Chemokine Networks in Cancer; Mir, M.A., Ed.; Springer Nature: Singapore, 2023; pp. 191–217. ISBN 978-981-9946-57-0. [Google Scholar]
- Tulotta, C.; Stefanescu, C.; Chen, Q.; Torraca, V.; Meijer, A.H.; Snaar-Jagalska, B.E. CXCR4 Signaling Regulates Metastatic Onset by Controlling Neutrophil Motility and Response to Malignant Cells. Sci. Rep. 2019, 9, 2399. [Google Scholar] [CrossRef]
- Cilento, M.A.; Sweeney, C.J.; Butler, L.M. Spatial Transcriptomics in Cancer Research and Potential Clinical Impact: A Narrative Review. J. Cancer Res. Clin. Oncol. 2024, 150, 296. [Google Scholar] [CrossRef]
- Hunter, M.V.; Moncada, R.; Weiss, J.M.; Yanai, I.; White, R.M. Spatially Resolved Transcriptomics Reveals the Architecture of the Tumor-Microenvironment Interface. Nat. Commun. 2021, 12, 6278. [Google Scholar] [CrossRef]
- Zheng, B.; Fang, L. Spatially Resolved Transcriptomics Provide a New Method for Cancer Research. J. Exp. Clin. Cancer Res. 2022, 41, 179. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, C.; Peng, A.; Sun, T.; Ji, X.; Mi, J.; Wei, L.; Shen, S.; Feng, Q. Pan-Cancer Spatially Resolved Single-Cell Analysis Reveals the Crosstalk between Cancer-Associated Fibroblasts and Tumor Microenvironment. Mol. Cancer 2023, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, C.M.; Huttenlocher, A. Live Imaging and Gene Expression Analysis in Zebrafish Identifies a Link between Neutrophils and Epithelial to Mesenchymal Transition. PLoS ONE 2014, 9, e112183. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.E.; Vuong, T.T.; Rønning, S.B.; Kolset, S.O. Matrix Metalloproteinases in Fish Biology and Matrix Turnover. Matrix Biol. 2015, 44–46, 86–93. [Google Scholar] [CrossRef]
- Tang, W.J.; Watson, C.J.; Olmstead, T.; Allan, C.H.; Kwon, R.Y. Single-Cell Resolution of MET- and EMT-like Programs in Osteoblasts during Zebrafish Fin Regeneration. iScience 2022, 25, 103784. [Google Scholar] [CrossRef]
- Conedera, F.M.; Quintela Pousa, A.M.; Presby, D.M.; Mercader, N.; Enzmann, V.; Tschopp, M. Diverse Signaling by TGFβ Isoforms in Response to Focal Injury Is Associated with Either Retinal Regeneration or Reactive Gliosis. Cell. Mol. Neurobiol. 2021, 41, 43–62. [Google Scholar] [CrossRef]
- Nakajima, H.; Chiba, A.; Fukumoto, M.; Morooka, N.; Mochizuki, N. Zebrafish Vascular Development: General and Tissue-Specific Regulation. J. Lipid Atheroscler. 2021, 10, 145–159. [Google Scholar] [CrossRef]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Fan, Y.-L.; Hsu, F.-R.; Wang, Y.; Liao, L.-D. Unlocking the Potential of Zebrafish Research with Artificial Intelligence: Advancements in Tracking, Processing, and Visualization. Med. Biol. Eng. Comput. 2023, 61, 2797–2814. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, R.; Carro, M.S. Neural Networks Help Zebrafish to Step up as a Model for Efficient Drug Screening in Glioblastoma. Neuro-Oncology 2022, 24, 739–740. [Google Scholar] [CrossRef]
- Pudelko, L.; Edwards, S.; Balan, M.; Nyqvist, D.; Al-Saadi, J.; Dittmer, J.; Almlöf, I.; Helleday, T.; Bräutigam, L. An Orthotopic Glioblastoma Animal Model Suitable for High-Throughput Screenings. Neuro-Oncology 2018, 20, 1475–1484. [Google Scholar] [CrossRef]
- Heilmann, S.; Ratnakumar, K.; Langdon, E.M.; Kansler, E.R.; Kim, I.S.; Campbell, N.R.; Perry, E.B.; McMahon, A.J.; Kaufman, C.K.; van Rooijen, E.; et al. A Quantitative System for Studying Metastasis Using Transparent Zebrafish. Cancer Res. 2015, 75, 4272–4282. [Google Scholar] [CrossRef]
- Liu, K.; Qiao, H.; Wu, J.; Wang, H.; Fang, L.; Dai, Q. Fast 3D Cell Tracking with Wide-Field Fluorescence Microscopy through Deep Learning. arXiv 2018, arXiv:1805.05139. [Google Scholar]
- Forsgren, E.; Edlund, C.; Oliver, M.; Barnes, K.; Sjögren, R.; Jackson, T.R. High-Throughput Widefield Fluorescence Imaging of 3D Samples Using Deep Learning for 2D Projection Image Restoration. PLoS ONE 2022, 17, e0264241. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in Artificial Intelligence for Drug Delivery and Development: A Comprehensive Review. Comput. Biol. Med. 2024, 178, 108702. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Dal, N.-J.K.; Kocere, A.; Wohlmann, J.; Van Herck, S.; Bauer, T.A.; Resseguier, J.; Bagherifam, S.; Hyldmo, H.; Barz, M.; De Geest, B.G.; et al. Zebrafish Embryos Allow Prediction of Nanoparticle Circulation Times in Mice and Facilitate Quantification of Nanoparticle–Cell Interactions. Small 2020, 16, 1906719. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Estrada, M.F.; Gomes, A.; Fernandez, L.M.; Azevedo, J.M.; Póvoa, V.; Fontes, M.; Alves, A.; Galzerano, A.; Castillo-Martin, M.; et al. Zebrafish Avatar-Test Forecasts Clinical Response to Chemotherapy in Patients with Colorectal Cancer. Nat. Commun. 2024, 15, 4771. [Google Scholar] [CrossRef]
- Costa, B.; Estrada, M.F.; Barroso, M.T.; Fior, R. Zebrafish Patient-Derived Avatars from Digestive Cancers for Anti-Cancer Therapy Screening. Curr. Protoc. 2022, 2, e415. [Google Scholar] [CrossRef]
- Martinez-Lopez, M.; Póvoa, V.; Fior, R. Generation of Zebrafish Larval Xenografts and Tumor Behavior Analysis. JoVE J. Vis. Exp. 2021, 172, e62373. [Google Scholar] [CrossRef]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical Sectioning Deep Inside Live Embryos by Selective Plane Illumination Microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, H.F.; Shurbaji, S.; Yalcin, H.C. Zebrafish as a Model for Anticancer Nanomedicine Studies. Pharmaceuticals 2021, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Kannan, R.R. Zebrafish: A Promising Real-Time Model System for Nanotechnology-Mediated Neurospecific Drug Delivery. Nanoscale Res. Lett. 2021, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Rapp, T.L.; DeForest, C.A. Targeting Drug Delivery with Light: A Highly Focused Approach. Adv. Drug Deliv. Rev. 2021, 171, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Chen, Q.; Campbell, F.; Snaar-Jagalska, E.; Kros, A. Light-Triggered Cancer Cell Specific Targeting and Liposomal Drug Delivery in a Zebrafish Xenograft Model. Adv. Healthc. Mater. 2020, 9, 1901489. [Google Scholar] [CrossRef]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of Curcumin-Loaded Zein Nanoparticles for Transport across the Blood–Brain Barrier and Inhibition of Glioblastoma Cell Growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Shan, D.; Li, J.; Cai, P.; Prasad, P.; Liu, F.; Rauth, A.M.; Wu, X.Y. RGD-Conjugated Solid Lipid Nanoparticles Inhibit Adhesion and Invasion of Avβ3 Integrin-Overexpressing Breast Cancer Cells. Drug Deliv. Transl. Res. 2015, 5, 15–26. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Rand-Weaver, M.; Winter, M.J. Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs. Front. Pharmacol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Kane, D.A.; Hammerschmidt, M.; Mullins, M.C.; Maischein, H.-M.; Brand, M.; van Eeden, F.J.M.; Furutani-Seiki, M.; Granato, M.; Haffter, P.; Heisenberg, C.-P.; et al. The Zebrafish Epiboly Mutants. Development 1996, 123, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Makinoshima, H.; Gong, Z. Gastrulation Screening to Identify Anti-Metastasis Drugs in Zebrafish Embryos. Bio-protocol 2022, 12, e4525. [Google Scholar] [CrossRef]
- Silva, J.; Omar, N.; Sittaramane, V.; Cowell, J.K. Identification of Small Molecules That Suppress Cell Invasion and Metastasis Promoted by WASF3 Activation. Heliyon 2023, 9, e20662. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Herrmann, A.; Reckamp, K.; Zhang, W.; Pal, S.; Hedvat, M.; Zhang, C.; Liang, W.; Scuto, A.; Weng, S.; et al. Anti-Angiogenic and Anti-Metastatic Activity of JAK Inhibitor AZD1480. Cancer Res. 2011, 71, 6601–6610. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Lu, S.; Thangaraju, M.; Cowell, J.K. Wasf3 Deficiency Reveals Involvement in Metastasis in a Mouse Model of Breast Cancer. Am. J. Pathol. 2019, 189, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.e14. [Google Scholar] [CrossRef] [PubMed]
- Rare Cancers, Cancer Subtypes, and Pre-Cancers. Available online: https://www.cancer.org/cancer/types/rare-cancers.html (accessed on 10 December 2024).
- Leacock, S.W.; Basse, A.N.; Chandler, G.L.; Kirk, A.M.; Rakheja, D.; Amatruda, J.F. A Zebrafish Transgenic Model of Ewing’s Sarcoma Reveals Conserved Mediators of EWS-FLI1 Tumorigenesis. Dis. Model. Mech. 2012, 5, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, C.; Grissenberger, S.; Bozatzi, P.; Scheuringer, E.; Wenninger-Weinzierl, A.; Zajec, Z.; Dernovšek, J.; Pascoal, S.; Gehl, V.; Kutsch, A.; et al. Refined High-Content Imaging-Based Phenotypic Drug Screening in Zebrafish Xenografts. NPJ Precis. Oncol. 2023, 7, 44. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, X.; Weichert-Leahey, N.; Dong, Z.; Zhang, C.; Lopez, G.; Tao, T.; He, S.; Wood, A.C.; Oldridge, D.; et al. LMO1 Synergizes with MYCN to Promote Neuroblastoma Initiation and Metastasis. Cancer Cell 2017, 32, 310–323.e5. [Google Scholar] [CrossRef]
- Bagatell, R.; Park, J.R.; Acharya, S.; Aldrink, J.; Allison, J.; Alva, E.; Arndt, C.; Benedetti, D.; Brown, E.; Cho, S.; et al. Neuroblastoma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, 413–433. [Google Scholar] [CrossRef] [PubMed]
- van Bree, N.; Oppelt, A.-S.; Lindström, S.; Zhou, L.; Boutin, L.; Coyle, B.; Swartling, F.J.; Johnsen, J.I.; Bräutigam, L.; Wilhelm, M. Development of an Orthotopic Medulloblastoma Zebrafish Model for Rapid Drug Testing. Neuro-Oncology 2024, noae210. [Google Scholar] [CrossRef]
- Shim, J.; Choi, J.-H.; Park, M.-H.; Kim, H.; Kim, J.H.; Kim, S.-Y.; Hong, D.; Kim, S.; Lee, J.E.; Kim, C.-H.; et al. Development of Zebrafish Medulloblastoma-like PNET Model by TALEN-Mediated Somatic Gene Inactivation. Oncotarget 2017, 8, 55280–55297. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.J.; Chan, P.P.; Li, Q.; Zu, J.-F.; Jette, C.A.; Kohler, M.; Myers, B.R.; Stewart, R.A. A Simple and Scalable Zebrafish Model of Sonic Hedgehog Medulloblastoma. Cell Rep. 2024, 43, 114559. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.C.; Kalra, R.R.; Dubuc, A.; Sivakumar, W.; Pedone, C.A.; Wu, X.; Taylor, M.D.; Fults, D.W. Genetic Drivers of Metastatic Dissemination in Sonic Hedgehog Medulloblastoma. Acta Neuropathol. Commun. 2014, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.P.; Choi, S.-Y.; Kee, Y.; Kim, M.J.; Kim, S.-H.; Lee, Y.; Park, H.-C.; Ro, H. Transgenic Fluorescent Zebrafish Lines That Have Revolutionized Biomedical Research. Lab. Anim. Res. 2021, 37, 26. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune System and Immune Responses in Fish and Their Role in Comparative Immunity Study: A Model for Higher Organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xiao, Y.; E, W.; Ma, L.; Wang, J.; Chen, H.; Gao, C.; Liao, Y.; Guo, Q.; Peng, J.; et al. Characterization of the Zebrafish Cell Landscape at Single-Cell Resolution. Front. Cell Dev. Biol. 2021, 9, 743421. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.; Melong, N.; Wong, W.H.; King, B.; Tong, R.S.; Mahajan, N.; Gaston, D.; Lund, T.; Rittenberg, D.; Dellaire, G.; et al. Humanized Zebrafish Enhance Human Hematopoietic Stem Cell Survival and Promote Acute Myeloid Leukemia Clonal Diversity. Haematologica 2019, 105, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, A.; Zon, L. The Zebrafish as a Model System for the Study of Hematopoiesis: Zebrafish Mutants Point the Way to Novel Genes Involved in the Generation of Vertebrate Blood Cells. BioScience 1999, 49, 382–392. [Google Scholar] [CrossRef][Green Version]
- Zimmerman, A.M.; Moustafa, F.M.; Romanowski, K.E.; Steiner, L.A. Zebrafish Immunoglobulin IgD: Unusual Exon Usage and Quantitative Expression Profiles with IgM and IgZ/T Heavy Chain Isotypes. Mol. Immunol. 2011, 48, 2220–2223. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, Y.-Y.; Shao, T.; Fan, D.-D.; Lin, A.-F.; Xiang, L.-X.; Shao, J.-Z. The Zebrafish NLRP3 Inflammasome Has Functional Roles in ASC-Dependent Interleukin-1β Maturation and Gasdermin E–Mediated Pyroptosis. J. Biol. Chem. 2020, 295, 1120–1141. [Google Scholar] [CrossRef]
- He, X.; Yin, X.; Wu, J.; Wickström, S.L.; Duo, Y.; Du, Q.; Qin, S.; Yao, S.; Jing, X.; Hosaka, K.; et al. Visualization of Human T Lymphocyte-Mediated Eradication of Cancer Cells in Vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 22910–22919. [Google Scholar] [CrossRef]
- Lund, T.C.; Glass, T.J.; Somani, A.; Nair, S.; Tolar, J.; Nyquist, M.; Patrinostro, X.; Blazar, B.R. Zebrafish Stromal Cells Have Endothelial Properties and Support Hematopoietic Cells. Exp. Hematol. 2012, 40, 61–70.e1. [Google Scholar] [CrossRef][Green Version]
- Wolf, A.; Aggio, J.; Campbell, C.; Wright, F.; Marquez, G.; Traver, D.; Stachura, D.L. Zebrafish Caudal Haematopoietic Embryonic Stromal Tissue (CHEST) Cells Support Haematopoiesis. Sci. Rep. 2017, 7, 44644. [Google Scholar] [CrossRef]
- Anderson, T.; Mo, J.; Gagarin, E.; Sherwood, D.; Blumenkrantz, M.; Mao, E.; Leon, G.; Levitz, H.; Chen, H.-J.; Tseng, K.-C.; et al. Ligament Injury in Adult Zebrafish Triggers ECM Remodeling and Cell Dedifferentiation for Scar-Free Regeneration. NPJ Regen. Med. 2023, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, D.; Orth, M.; Meissler, M.; Geuer, S.; Knaup, S.; Köblitz, I.; Klopocki, E. ECM Alterations in Fndc3a (Fibronectin Domain Containing Protein 3A) Deficient Zebrafish Cause Temporal Fin Development and Regeneration Defects. Sci. Rep. 2019, 9, 13383. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, R.J.; Ho, K.Y.L.; Venkatesh, B.; Tanentzapf, G. Integrins Modulate Extracellular Matrix Organization to Control Cell Signaling during Hematopoiesis. Curr. Biol. 2020, 30, 3316–3329.e5. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Chang, J.R.; Chin, A.J.; Smith, A.; Kelly, C.; Weinberg, E.S.; Ge, R. The Role of Vascular Endothelial Growth Factor (VEGF) in Vasculogenesis, Angiogenesis, and Hematopoiesis in Zebrafish Development. Mech. Dev. 2001, 108, 29–43. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Q.; Gong, Z. Transgenic Expression of Tgfb1a Induces Hepatic Inflammation, Fibrosis and Metastasis in Zebrafish. Biochem. Biophys. Res. Commun. 2019, 509, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shtam, T.; Naryzhny, S.; Samsonov, R.; Karasik, D.; Mizgirev, I.; Kopylov, A.; Petrenko, E.; Zabrodskaya, Y.; Kamyshinsky, R.; Nikitin, D.; et al. Plasma Exosomes Stimulate Breast Cancer Metastasis through Surface Interactions and Activation of FAK Signaling. Breast Cancer Res. Treat. 2019, 174, 129–141. [Google Scholar] [CrossRef]
- Shan, Y.; You, B.; Shi, S.; Shi, W.; Zhang, Z.; Zhang, Q.; Gu, M.; Chen, J.; Bao, L.; Liu, D.; et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018, 9, 382. [Google Scholar] [CrossRef]
- Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Challenges and Prospects of Patient-Derived Xenografts for Cancer Research. Cancers 2023, 15, 4352. [Google Scholar] [CrossRef] [PubMed]
- El Omar, R.; Abdellaoui, N.; Coulibaly, S.T.; Fontenille, L.; Lanza, F.; Gachet, C.; Freund, J.-N.; Negroni, M.; Kissa, K.; Tavian, M. Macrophage Depletion Overcomes Human Hematopoietic Cell Engraftment Failure in Zebrafish Embryo. Cell Death Dis. 2024, 15, 305. [Google Scholar] [CrossRef]
- Delitto, D.; Pham, K.; Vlada, A.C.; Sarosi, G.A.; Thomas, R.M.; Behrns, K.E.; Liu, C.; Hughes, S.J.; Wallet, S.M.; Trevino, J.G. Patient-Derived Xenograft Models for Pancreatic Adenocarcinoma Demonstrate Retention of Tumor Morphology through Incorporation of Murine Stromal Elements. Am. J. Pathol. 2015, 185, 1297–1303. [Google Scholar] [CrossRef]
- Sunil, H.S.; O’Donnell, K.A. Capturing Heterogeneity in PDX Models: Representation Matters. Nat. Commun. 2024, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, M.F.; de Almeida, C.R.; Fontes, M.; Mendes, R.V.; Kaufmann, S.H.E.; Fior, R. Macrophages Directly Kill Bladder Cancer Cells through TNF Signaling as an Early Response to BCG Therapy. Dis. Model. Mech. 2024, 17, dmm050693. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A Road to Inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Cavalcante, L.; Lopera Higuita, M.; González-Rosa, J.M.; Marques, B.; To, S.; Pendexter, C.A.; Cronin, S.E.J.; Gopinathan, K.A.; de Vries, R.J.; Ellett, F.; et al. Zebrafish as a High Throughput Model for Organ Preservation and Transplantation Research. FASEB J. 2023, 37, e23187. [Google Scholar] [CrossRef]
- Hua, X.; Wu, X.; Xu, K.; Zhan, P.; Liu, H.; Zhang, F.; Lv, T.; Song, Y. Zebrafish Patient-Derived Xenografts Accurately and Quickly Reproduce Treatment Outcomes in Non-Small Cell Lung Cancer Patients. Exp. Biol. Med. Maywood NJ 2023, 248, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Nardi, J.; Freddo, N.; Biazus, I.C.; Oliveira, A.P.; Soares, S.M.; Fortuna, M.; Varela, A.C.C.; Siqueira, L.; Pompermaier, A.; Tamagno, W.A.; et al. Methylphenidate Exposure in Juvenile Period Elicits Locomotion Changes and Anxiolytic-like Behavior in Adulthood: Evidence Using Zebrafish as a Translational Model. Behav. Brain Res. 2024, 457, 114709. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yin, H.-M.; Shih, Y.-H.; Nozaki, T.; Portman, D.; Toles, B.; Kolb, A.; Luk, K.; Isogai, S.; Ishida, K.; et al. Generation and Application of Endogenously Floxed Alleles for Cell-Specific Knockout in Zebrafish. Dev. Cell 2023, 58, 2614–2626.e7. [Google Scholar] [CrossRef]
- Häberlein, F.; Mingardo, E.; Merten, N.; Schulze Köhling, N.-K.; Reinoß, P.; Simon, K.; Japp, A.; Nagarajan, B.; Schrage, R.; Pegurier, C.; et al. Humanized Zebrafish as a Tractable Tool for in Vivo Evaluation of Pro-Myelinating Drugs. Cell Chem. Biol. 2022, 29, 1541–1555.e7. [Google Scholar] [CrossRef] [PubMed]
- Schellens, R.; de Vrieze, E.; Slijkerman, R.; Kremer, H.; van Wijk, E. Generation of Humanized Zebrafish Models for the In Vivo Assessment of Antisense Oligonucleotide-Based Splice Modulation Therapies. Methods Mol. Biol. Clifton NJ 2022, 2434, 281–299. [Google Scholar] [CrossRef]
- Uribe-Salazar, J.M.; Kaya, G.; Weyenberg, K.; Radke, B.; Hino, K.; Soto, D.C.; Shiu, J.-L.; Zhang, W.; Ingamells, C.; Haghani, N.K.; et al. Zebrafish Models of Human-Duplicated SRGAP2 Reveal Novel Functions in Microglia and Visual System Development. bioRxiv 2024. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania Somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. Drug Des. Dev. Ther. 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.-B.; et al. Patient-Derived Tumor Organoids: A New Avenue for Preclinical Research and Precision Medicine in Oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Antinucci, P.; Hindges, R. A Crystal-Clear Zebrafish for in Vivo Imaging. Sci. Rep. 2016, 6, 29490. [Google Scholar] [CrossRef] [PubMed]
- Demy, D.L.; Ranta, Z.; Giorgi, J.-M.; Gonzalez, M.; Herbomel, P.; Kissa, K. Generating Parabiotic Zebrafish Embryos for Cell Migration and Homing Studies. Nat. Methods 2013, 10, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.D.; Bishop, K.; Devine, A.; Paine, E.L.; Staunton, J.R.; Thomas, S.M.; Thomas, J.R.; Doyle, A.D.; Miller Jenkins, L.M.; Morgan, N.Y.; et al. Tissue Architectural Cues Drive Organ Targeting of Tumor Cells in Zebrafish. Cell Syst. 2019, 9, 187–206.e16. [Google Scholar] [CrossRef] [PubMed]
- Dries, R.; Lange, A.; Heiny, S.; Berghaus, K.I.; Bastmeyer, M.; Bentrop, J. Cell Proliferation and Collective Cell Migration During Zebrafish Lateral Line System Development Are Regulated by Ncam/Fgf-Receptor Interactions. Front. Cell Dev. Biol. 2021, 8, 591011. [Google Scholar] [CrossRef]
- Quintana, F.J.; Iglesias, A.H.; Farez, M.F.; Caccamo, M.; Burns, E.J.; Kassam, N.; Oukka, M.; Weiner, H.L. Adaptive Autoimmunity and Foxp3-Based Immunoregulation in Zebrafish. PLoS ONE 2010, 5, e9478. [Google Scholar] [CrossRef]
- Cero, C.; House, J.S.; Verdi, V.; Ferguson, J.L.; Jima, D.D.; Selmek, A.A.; Patania, O.M.; Dwyer, J.E.; Wei, B.-R.; Lloyd, D.T.; et al. Profiling the Cancer-Prone Microenvironment in a Zebrafish Model for MPNST. Oncogene 2025, 44, 179–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
