Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress
Abstract
:1. Introduction
2. Results
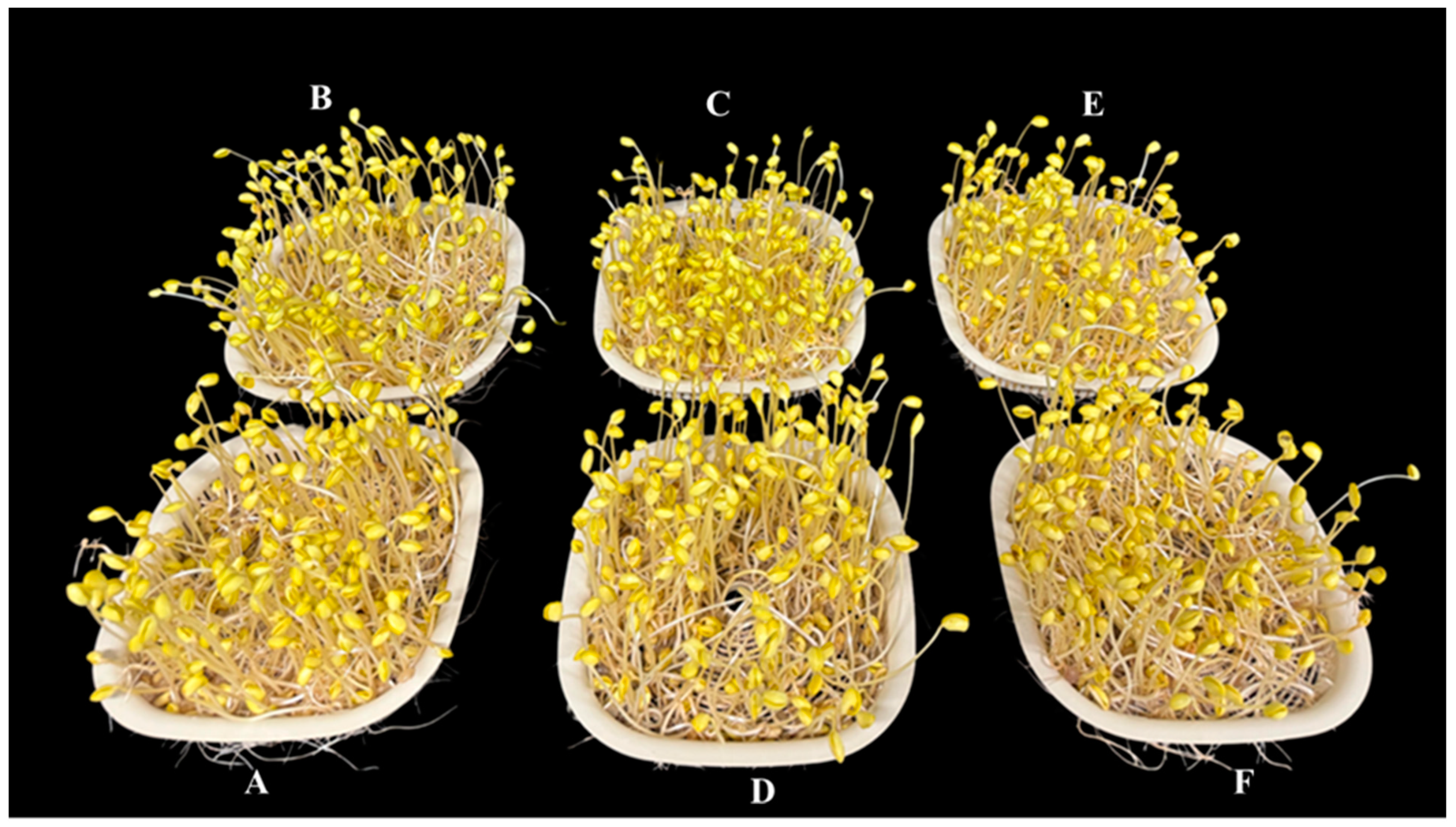
2.1. Growth Parameters of Sprout
- Biomass of sproutThe total biomass of the sprout was considerably increased by 7% in Ca-Mel treated groups and by 5% with Ca 1 mM treated groups. Continuous Mel treatment reduced the fresh weight by 4%, whereas no significant differences were observed in fresh weight with treatment of melatonin alone when compared to the control; Table 1.
- Hypocotyl length of sproutThe hypocotyl length was significantly increased with the treatment of Ca, Mel, and Ca-Mel by 9, 8, and 7%, respectively. The continuous treatment of melatonin (MelCon) reduced the hypocotyl growth by 12%, whereas continuous treatment of calcium (CaCon) increased the hypocotyl length by 5% when compared to the control; Figure 1 and Table 1.
- Radical length of SproutThe radical length was significantly increased by 13%, 7%, 22%, and 19% with the treatment of Ca, Mel, Ca-Mel, and CaCon, respectively, compared to the control. However, the continuous treatment of melatonin significantly reduced the radical length of the sprout by 12%; Table 1.
2.2. Antioxidant Properties of Sprout
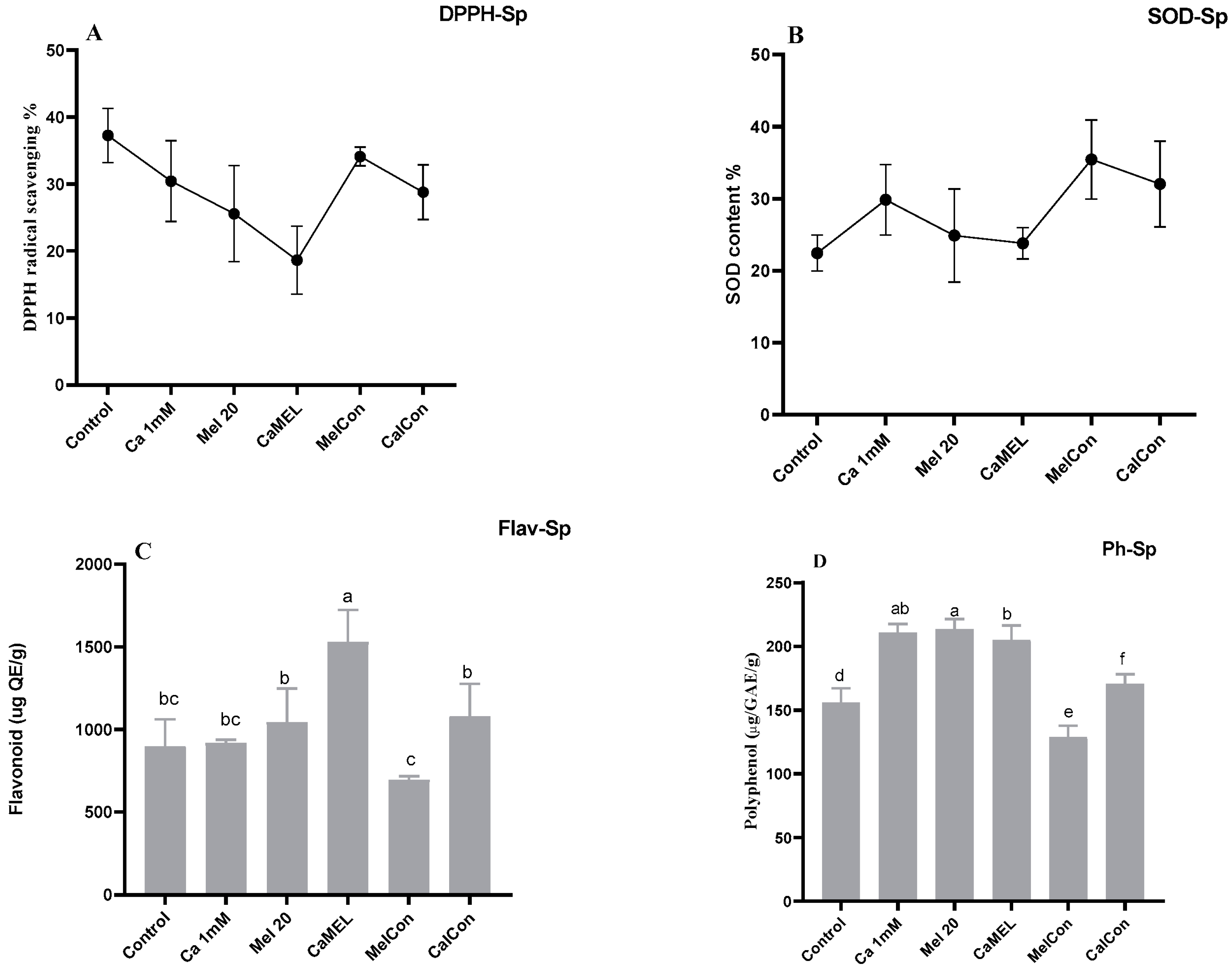
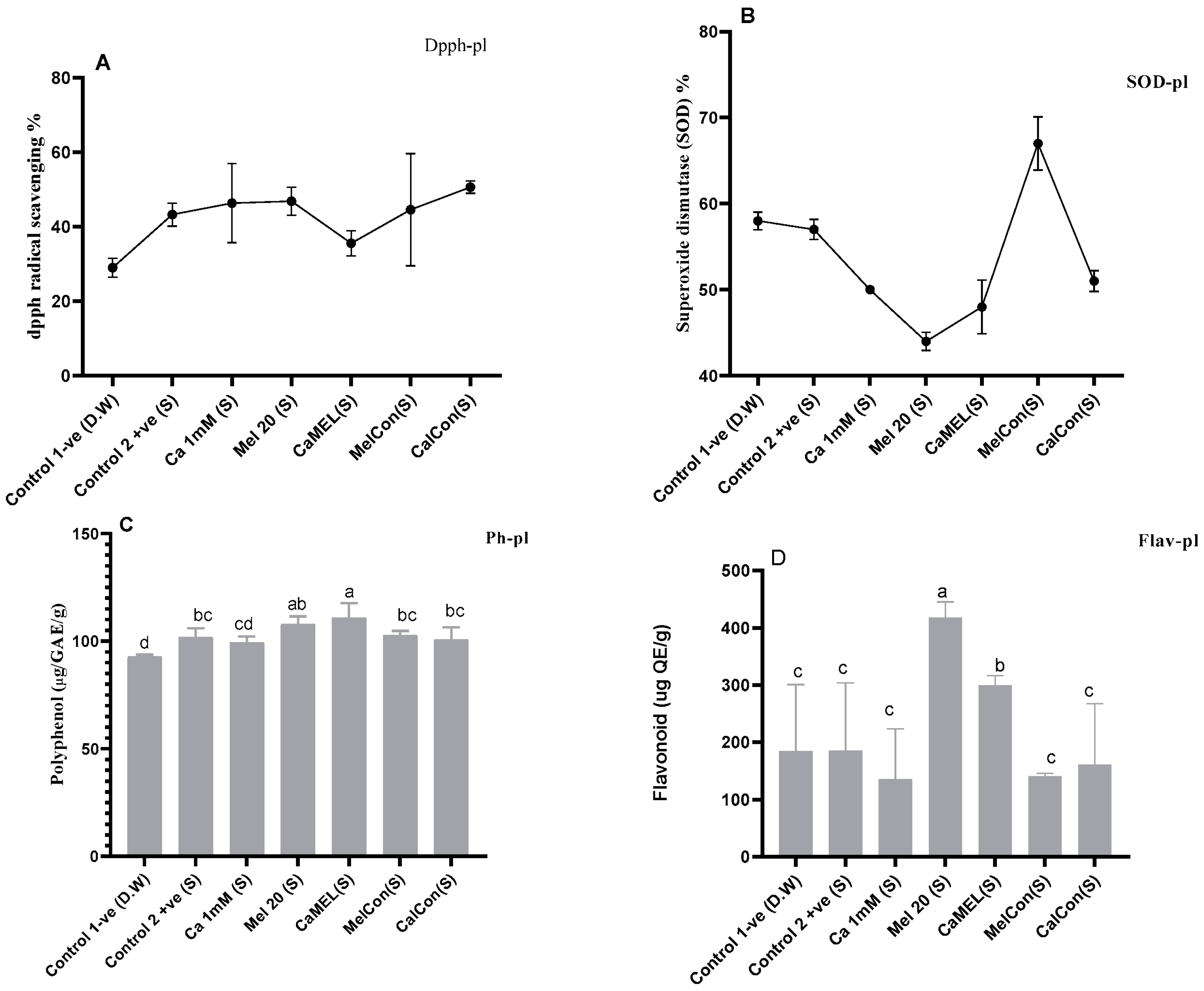
- SODSuperoxide dismutase (SODsp) activity in soybean sprouts increased under all treatments compared to the control (water only). Calcium (Ca 1 mM) enhanced activity by 24.80%, melatonin (Mel 20 µM) by 9.86%, and the combination of calcium and melatonin (CaMel) by 5.77%. Continuous melatonin treatment (MelCon) showed the highest increase of 36.72%, followed by continuous calcium treatment (CaCon) with a 29.98% increase. Continuous treatments proved more effective in enhancing SODsp activity; Figure 2.
- DPPHHere, a contrasting trend was observed in between melatonin alternate treatments vs. continuous treatment. DPPH content was reduced by 15% and increased by 12% with Mel 20 treatment and MelCon, respectively; Figure 2.
- PolyphenolThe phenolic content showed a significant increase across all treatments, except for MelCon, which exhibited a 17% reduction. In contrast, the phenolic content increased by 26%, 27%, 33%, and 9% under the treatments of Ca, Mel, Ca-Mel, and CaCon, respectively; Figure 2.
- FlavonoidA similar trend was observed in the flavonoid content of the sprouts, with increases of 2%, 13%, 41%, and 16% under the treatments of Ca, Mel, Ca-Mel, and CaCon, respectively, when compared to the control. In contrast, the MelCon group showed a 22% reduction in flavonoid content; Figure 2.
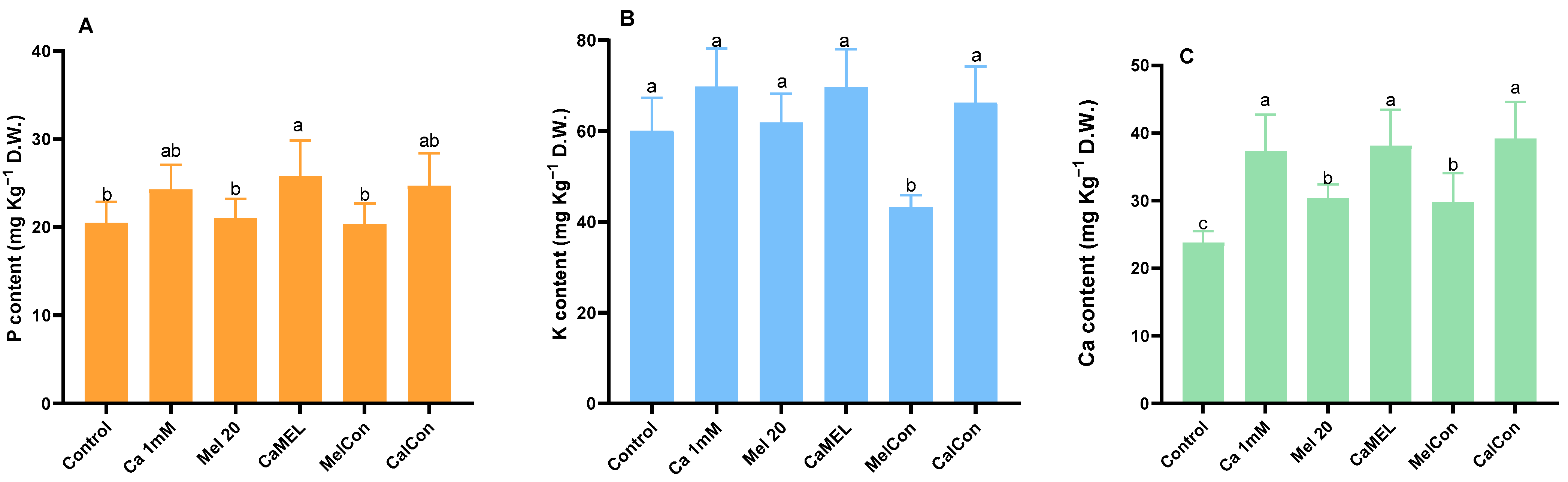
2.3. Ca, P, and K Content of Sprout
2.4. Soybean Seed Germination and Seedlings Resilience Under NaCl+Cd Stress
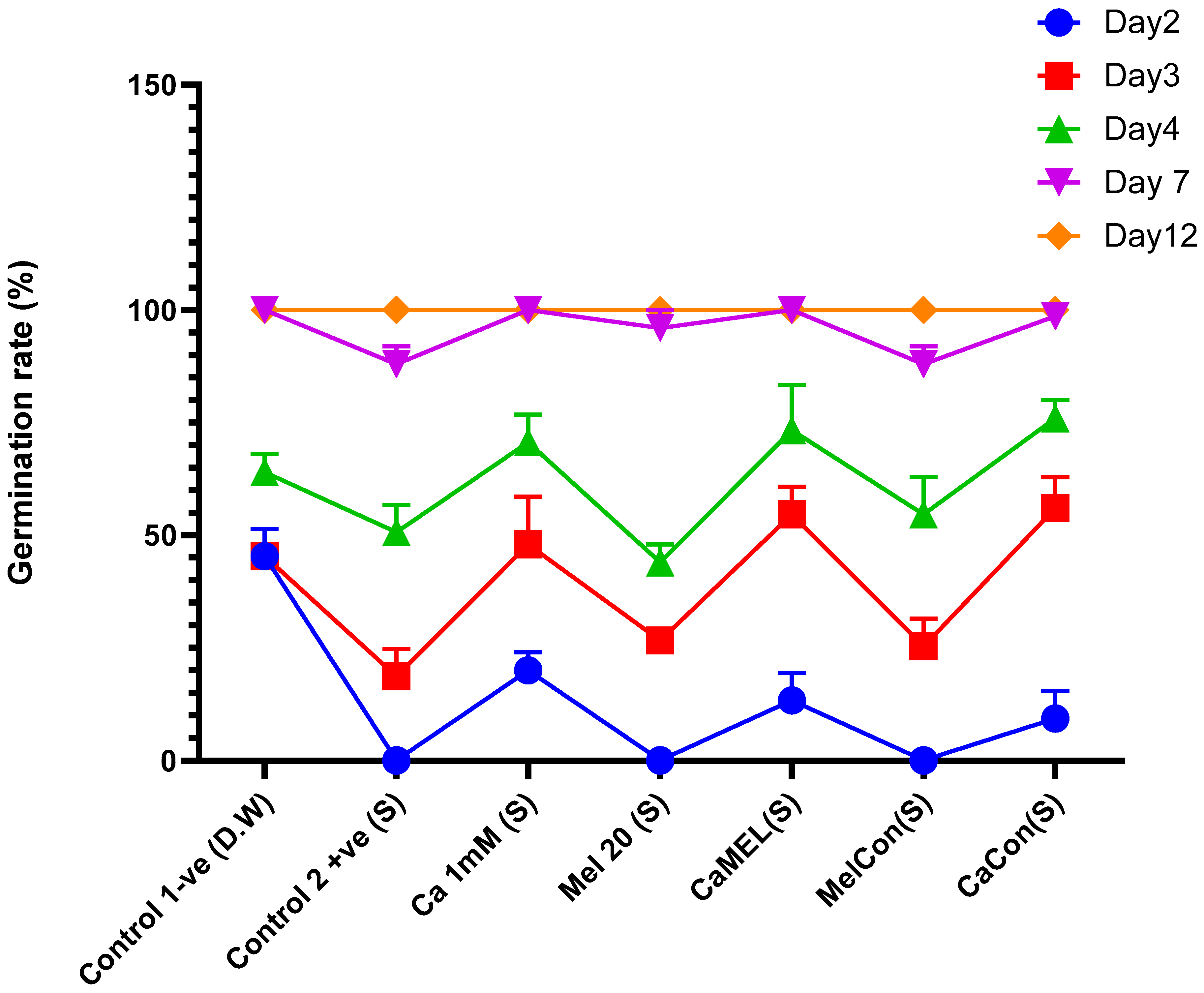
2.4.1. Germination Rate
2.4.2. Morphological Attributes
2.4.3. Chlorophyll Content and Soil pH
2.4.4. Antioxidant Like Activities of Soybean Seedlings Under Stressed Condition
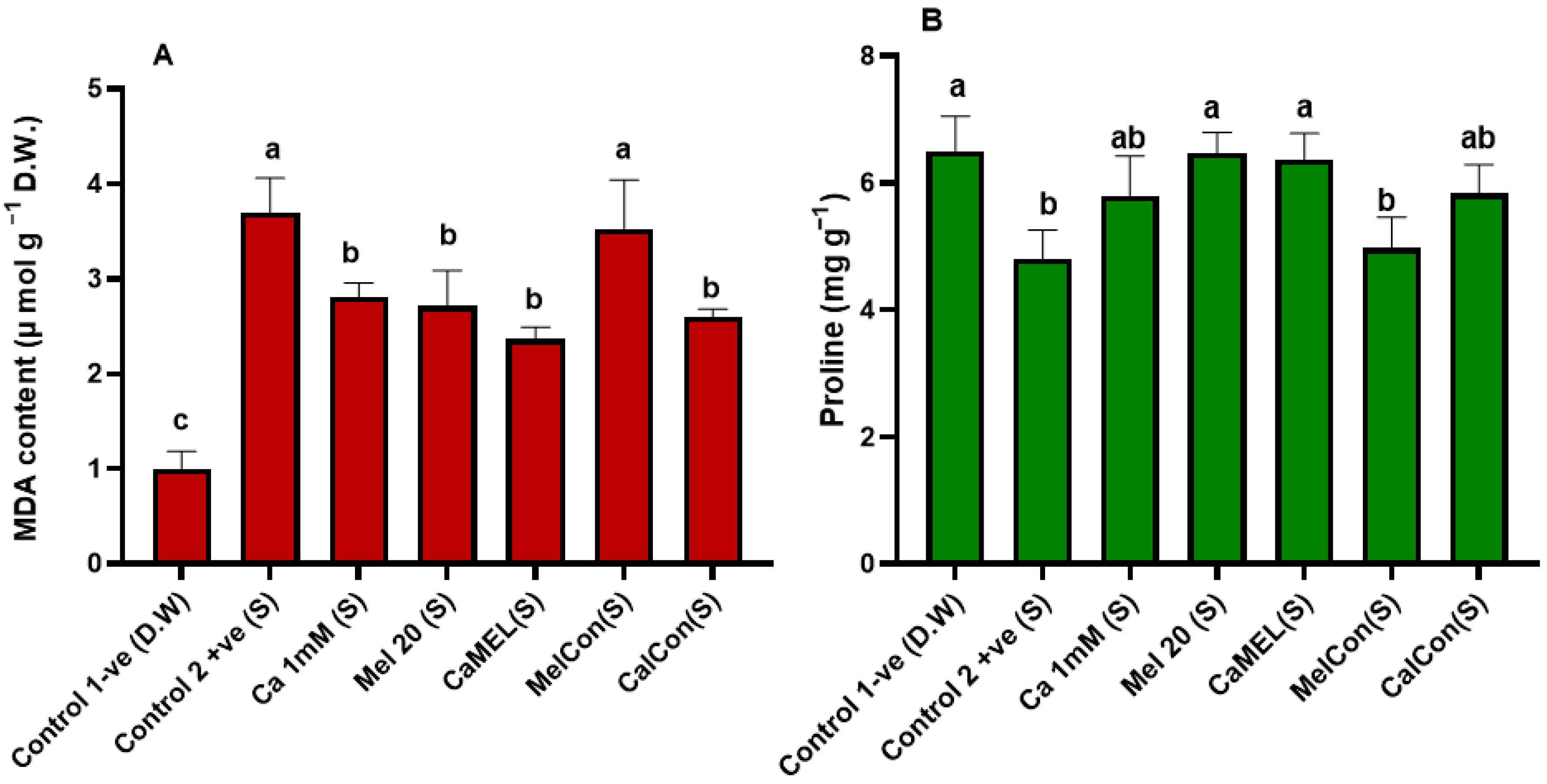
2.4.5. MDA and Proline Content of Soybean Seedlings Treated with Ca/Mel/Ca-Mel Under NaCl+Cd Stress
3. Discussion
3.1. Soybean Seed Priming and Sprout Development
3.2. Sprout Quantity and Quality
3.3. Seed Germination and Seedlings Resilience
3.4. Impact on Nutrient Content and Antioxidant Properties in Sprout
3.5. Reduced Oxidative Damage in Seedlings Subjected to NaCl+Cd
3.6. Precaution for Melatonin and Calcium Application for Enhancing Sprout Quality and Seedling Resilience Under Salt+Cd Stress
3.7. Notable Insights on Melatonin in Diet, Animal, and Plant Interactions Highlighting the Current Research Significance
4. Materials and Methods
4.1. Experiment to Improve the Quality and Sprouting of Soybean
4.1.1. Screening of Optimum Doses of Ca and Melatonin for Sprouting of Soybean
4.1.2. Plant Material and Sprouting Procedure
- Sprout Cultivation Treatments:
- ○
- Control (water only): Seeds were treated with distilled water (D.W.) only.
- ○
- Ca 1 mM: Seeds were treated with 1 mM calcium.
- ○
- Mel 20: Seeds were treated with 20 µM melatonin.
- ○
- CaMel: Seeds were treated with a combination of 1 mM calcium and 20 µM melatonin.
- ○
- MelCon: Continuous treatment with 20 µM melatonin.
- ○
- CaCon: Continuous treatment with 1 mM calcium.
4.1.3. Measurement of Growth Parameters
4.1.4. Preparation of Seeds for Seedlings Experiment
4.2. Effect of Mel/Ca Treated Seeds Sown Under NaCl+Cd Contaminated Soil
4.2.1. Study Site and Experimental Setup
4.2.2. Experimental Treatments
4.2.3. Observation and Sample Collection
4.3. Analysis of Antioxidant Activities
- DPPH Radical Scavenging Activity DeterminationDPPH radical scavenging activity was assessed using a freshly prepared 0.05% DPPH solution in absolute methanol as described by Wang et al. [52]. Equal volumes (100 µL each) of DPPH solution and sample extract were mixed in microplates. The reaction mixture was incubated in the dark for 30 min at room temperature (22–25 °C). For the control, 100 µL of DPPH solution was combined with 100 µL of methanol. After incubation, the absorbance of the reaction mixtures was measured at 517 nm using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland). The DPPH radical scavenging activity was calculated using the following formula:DPPHradical scavenging activity (%) = [1 − ((A − Ao)/(B − Bo))] × 100
- Quantification of Total polyphenol and Flavonoid ContentTotal polyphenol and flavonoid content was quantified using a modified colorimetric method [53]. For flavonoids, 30 µL of 5% NaNO2 was added to 30 µL of sample extract, followed by 60 µL of 10% AlCl3 after 5 min of incubation. After vortexing, the mixture was incubated for another 5 min, and then 200 µL of 1 M NaOH was added. Absorbance at 500 nm was measured using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland). Results were expressed as quercetin equivalents (QE) in mg/g extract.For polyphenols, 50 µL of extract was mixed with 1 mL of 2% sodium carbonate solution, followed by 50 µL of 1N Folin–Ciocalteu reagent. After incubating for 30 min in the dark, absorbance at 750 nm was measured using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland). Results were expressed as gallic acid equivalents (GAE) per gram of sample.
- Superoxide Dismutase (SOD)-like ActivityThe SOD-like activity was analyzed using a method described by Ha et al. [54]. Frozen plant shoot samples were ground using a grinder, and a reaction mixture consisting of 300 µL of 50 mM Tris-HCl buffer (pH 8.5) + 10 mM EDTA, 200 µL of 7.2 mM pyrogallol, and 200 µL of sample extract was incubated at 25 °C for 10 min. After completion of the reaction, 50 µL of 1N HCl was added to stop the reaction. The absorbance of the oxidized pyrogallol was measured at 420 nm using a microplate spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland).
4.4. Analysis of the Extent of the Lipid Peroxidation and Proline Content
4.5. Quantification of Mineral Elements (K, Ca, P)
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Experimental Parameters and Treatments for Soybean Research
- Parameters AssessedThe experiment focuses on assessing various biochemical, physiological, and morphological parameters in soybean sprouts and seedlings. These are categorized as follows:
- Sprout-Specific Parameters:
- ○
- DPPHsp: Antioxidant activity (2,2-diphenyl-1-picrylhydrazyl assay).
- ○
- SODsp: Superoxide dismutase activity.
- ○
- Phenolsp: Total phenolic content.
- ○
- Flavsp: Flavonoid content in sprouts.
- ○
- Nutritional Elements: Phosphorus (P), potassium (K), calcium (Ca).
- ○
- Growth Parameters: Radical length, hypocotyl length, and fresh weight (Frwt).
- Seedling and Soil-Specific Parameters:
- ○
- Growth Parameters: Shoot length (Sl) and root length (Rl).
- ○
- Biomass: Total biomass accumulation.
- ○
- Stress Markers: Malondialdehyde (MDA) and proline (Prol) content.
- ○
- Antioxidant and Polyphenol Assays: DPPHpl, SODpl, polyphenol content (Phpl), and flavonoid content (Flavpl).
- ○
- Soil pH: Changes in soil pH.
Appendix B
References
- Mariyam, S.; Upadhyay, S.K.; Chakraborty, K.; Verma, K.K.; Duhan, J.S.; Muneer, S.; Meena, M.; Sharma, R.K.; Ghodake, G.; Seth, C.S. Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine—A review. Sci. Total Environ. 2024, 912, 169097. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Z.; Luo, Y. Advances and emerging trends in cultivation substrates for growing sprouts and microgreens toward safe and sustainable agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bose, B. An Impact of Seed Priming on Disease Resistance: A Review. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 193–203. [Google Scholar]
- Tamindžić, G.; Miljaković, D.; Ignjatov, M.; Miladinović, J.; Đorđević, V.; Milošević, D.; Jovičić, D.; Vlajić, S.; Budakov, D.; Grahovac, M. Impact of Simultaneous Nutrient Priming and Biopriming on Soybean Seed Quality and Health. Plants 2024, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Shamsi, I.H.; Zhang, G.-p. Synergistic interaction of NaCl and Cd on growth and photosynthetic parameters in soybean genotypes differing in salinity tolerance. J. Zhejiang Univ. Sci. B 2007, 8, 266–271. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdel Latef, A.A.; Abd_Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef]
- Issam, N.; Kawther, M.; Haythem, M.; Moez, J. Effects of CaCl2 pretreatment on antioxidant enzyme and leaf lipid content of faba bean (Vicia faba L.) seedlings under cadmium stress. Plant Growth Regul. 2012, 68, 37–47. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.A.; Gomes Domingos, A.L.; Aguiar, A.S.d. Relationship between food consumption and improvements in circulating melatonin in humans: An integrative review. Crit. Rev. Food Sci. Nutr. 2022, 62, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Calvo, J.R.; Maldonado-Aibar, M.D.; Millan-Linares, M.D.; Montserrat-de la Paz, S. Mediterranean Diet and Melatonin: A Systematic Review. Antioxidants 2023, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodríguez-Rodríguez, P.; de Pablo, Á.L.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of Melatonin from Lentil Sprouts and Its Role in the Plasmatic Antioxidant Status in Rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.-x. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Sulkowska-Ziaja, K.; Muszynska, B.; Zagrodzki, P. Serotonin, melatonin, and certain indole derivatives profiles in rutabaga and kohlrabi seeds, sprouts, bulbs, and roots. LWT-Food Sci. Technol. 2014, 59, 740–745. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Adetunji, T.L.; Varghese, B.; Sershen; Pammenter, N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy 2021, 11, 2369. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, pH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Sakouhi, L.; Hussaan, M.; Murata, Y.; Chaoui, A. Role of calcium signaling in cadmium stress mitigation by indol-3-acetic acid and gibberellin in chickpea seedlings. Environ. Sci. Pollut. Res. 2024, 31, 16972–16985. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol. Biochem. 2022, 185, 123–131. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Wang, Y.-X.; Beta, T.; Wang, S.-Y.; Mendez-Zamora, G.; Laborda, P.; Herrera-Balandrano, D.D. Effect of exogenous melatonin on the isoflavone content and antioxidant properties of soybean sprouts. LWT 2023, 175, 114498. [Google Scholar] [CrossRef]
- Xue, J.; Quan, X.; Yang, J.; Fang, W.; Yin, Y. Study on the Mechanism of Flavonoid Enrichment in Black Soybean Sprouts by Abscisic Acid/Melatonin Under Slight Acid Treatment. Foods 2024, 13, 3567. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Munir, R.; Yasin, M.U.; Afzal, M.; Jan, M.; Muhammad, S.; Jan, N.; Nana, C.; Munir, F.; Iqbal, H.; Tawab, F.; et al. Melatonin alleviated cadmium accumulation and toxicity by modulating phytohormonal balance and antioxidant metabolism in rice. Chemosphere 2024, 346, 140590. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Basalah, M.O.; Ali, H.M. Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef] [PubMed]
- Menhas, S.; Yang, X.; Hayat, K.; Ali, A.; Ali, E.F.; Shahid, M.; Shaheen, S.M.; Rinklebe, J.; Hayat, S.; Zhou, P. Melatonin enhanced oilseed rape growth and mitigated Cd stress risk: A novel trial for reducing Cd accumulation by bioenergy crops. Environ. Pollut. 2022, 308, 119642. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Yao, T.; Wang, H.; Wang, Z.; Zhang, H.; Sun, G.; Zhang, H. Potassium ion regulates hormone, Ca2+ and H2O2 signal transduction and antioxidant activities to improve salt stress resistance in tobacco. Plant Physiol. Biochem. 2022, 186, 40–51. [Google Scholar] [CrossRef]
- Vafadar, F.; Ehsanzadeh, P. Synergistic effects of calcium and melatonin on physiological and phytochemical attributes of Dracocephalum kotschyi genotypes under salinity stress. Physiol. Plant. 2023, 175, e13912. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761. [Google Scholar] [CrossRef] [PubMed]
- Colak, N. Melatonin Priming Regulates Mineral Uptake, Osmolite Accumulation, and Cell Wall Structure in Buckwheat Under Cadmium Stress. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y.-C. Pre treatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2023, 445, 130530. [Google Scholar] [CrossRef] [PubMed]
- Parwez, R.; Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Melatonin supplementation combats nickel-induced phytotoxicity in Trigonella foenum-graecum L. plants through metal accumulation reduction, upregulation of NO generation, antioxidant defence machinery and secondary metabolites. Plant Physiol. Biochem. 2023, 202, 107981. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, L.; Tanveer, M.; Song, J. Seed Heteromorphism: An Important Adaptation of Halophytes for Habitat Heterogeneity. Front. Plant Sci. 2018, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Qu, H.; Tian, S.; Xu, Z.; Zhao, L.; Li, X.; Liu, B. A new method based on melatonin-mediated seed germination to quickly remove pesticide residues and improve the nutritional quality of contaminated grains. PLoS ONE 2024, 19, e0303040. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, X.; Yang, H.; Yu, L.; Zhang, Y. A simple new method for aged seed utilization based on melatonin-mediated germination and antioxidant nutrient production. Sci. Rep. 2021, 11, 5937. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.J.; Wang, Q.; Eneji, A.E.; Li, Z.H.; Duan, L.S. Coronatine enhances chilling tolerance in cucumber (Cucumis sativus L.) seedlings by improving the antioxidative defence system. J. Agron. Crop Sci. 2009, 195, 377–383. [Google Scholar] [CrossRef]
- Dhungana, S.K.; Seo, J.-H.; Kang, B.-K.; Park, J.-H.; Kim, J.-H.; Sung, J.-S.; Baek, I.-Y.; Shin, S.-O.; Jung, C.-S. Protein, Amino Acid, Oil, Fatty Acid, Sugar, Anthocyanin, Isoflavone, Lutein, and Antioxidant Variations in Colored Seed-Coated Soybeans. Plants 2021, 10, 1765. [Google Scholar] [CrossRef]
- Ha, M.-C.; Im, D.-Y.; Park, H.-S.; Dhungana, S.K.; Kim, I.-D.; Shin, D.-H. Seed Treatment with Illite Enhanced Yield and Nutritional Value of Soybean Sprouts. Molecules 2022, 27, 1152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rani, R.; Chandra, A.; Kumar, V. Potential applications of Pseudomonas sp.(strain CPSB21) to ameliorate Cr6+ stress and phytoremediation of tannery effluent contaminated agricultural soils. Sci. Rep. 2018, 8, 4860. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ 2017, 5, e3107. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Kwon, E.-H.; Khan, M.A.; Shaffique, S.; Kang, S.-M.; Lee, I.-J. Enhanced use of chemical fertilizers and mitigation of heavy metal toxicity using biochar and the soil fungus Bipolaris maydis AF7 in rice: Genomic and metabolomic perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115938. [Google Scholar] [CrossRef] [PubMed]

| Trt | Radical (Cm) | Hypocotyl (Cm) | Total Biomass (g) |
|---|---|---|---|
| Control | 12.71 ± 0.90 b | 11.89 ± 0.32 b | 198.64 ± 6.88 b |
| Ca 1 mM | 11.81 ± 0.66 a | 13.02 ± 0.51 a | 209.33 ± 9.01 a |
| Mel 20 | 8.83 ± 0.81 a | 12.94 ± 0.35 a | 198.64 ± 6.88 b |
| Ca-Mel | 5.72 ± 0.28 a | 12.77 ± 0.64 a | 209.33 ± 9.01 a |
| MelCon | 5.65 ± 0.34 c | 10.4 ± 0.29 c | 188.72 ± 7.15 c |
| CaCon | 10.62 ± 0.36 a | 12.5 ± 0.70 a | 198.64 ± 6.88 b |
| Trt | Shoot Length (cm) | Root Length (cm) | Biomass (g) | Soil pH | Chlorophyll (SPAD) |
|---|---|---|---|---|---|
| Control 1−ve (D.W) | 8.13 ± 0.70 a | 11.30 ± 0.78 ab | 1.32 ± 0.28 ab | 6.83 ± 0.01 a | 303.87 ± 57.22 |
| Control 2 +ve (S) | 4.43 ± 1.05 c | 7.11 ± 1.53 bc | 1.10 ± 0.23 bc | 6.61 ± 0.01 b | 160.84 ± 24.61 |
| Ca 1 mM (S) | 5.43 ± 0.92 c | 4.20 ± 6.14 c | 1.54 ± 0.33 c | 6.63 ± 0.01 b | 276.98 ± 75.04 |
| Mel 20 (S) | 4.66 ± 0.75 c | 9.81 ± 0.99 ab | 1.42 ± 0.17 ab | 6.61 ± 0.09 b | 227.58 ± 104.74 |
| CaMEL (S) | 6.66 ± 1.52 c | 12.52 ± 1.33 a | 1.91 ± 0.23 a | 6.50 ± 0.01 b | 222.72 ± 89.88 |
| MelCon (S) | 4.26 ± 1.04 b | 9.29 ± 2.00 ab | 0.60 ± 0.10 ab | 6.70 ± 0.01 b | 162.27 ± 20.27 |
| CaCon (S) | 4.66 ± 2.02 c | 13.15 ± 0.86 a | 1.34 ± 0.28 a | 6.63 ± 0.01 b | 293.07 ± 147.97 |
| Day | Control (D.W) | Ca 1 mM | Mel20 | CaMel | MelCon | CaCon |
|---|---|---|---|---|---|---|
| 1 | D.W. | D.W. | D.W. | D.W. | Treatment | Treatment |
| 2 | Treatment | Treatment | Treatment | Treatment | Treatment | Treatment |
| 3 | D.W. | D.W. | D.W. | D.W. | Treatment | Treatment |
| 4 | Treatment | Treatment | Treatment | Treatment | Treatment | Treatment |
| 5 | D.W. | D.W. | D.W. | D.W. | Treatment | Treatment |
| 6 | Treatment | Treatment | Treatment | Treatment | Treatment | Treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, A.; Sapkota, M.; Savidya, R.N.; Tosin, A.T.; Adam, M.; Alam, M.N.; Kwon, E.-H.; Kang, S.-M.; Shaffique, S.; Lee, I.-J. Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress. Int. J. Mol. Sci. 2025, 26, 878. https://doi.org/10.3390/ijms26030878
Adhikari A, Sapkota M, Savidya RN, Tosin AT, Adam M, Alam MN, Kwon E-H, Kang S-M, Shaffique S, Lee I-J. Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress. International Journal of Molecular Sciences. 2025; 26(3):878. https://doi.org/10.3390/ijms26030878
Chicago/Turabian StyleAdhikari, Arjun, Mahesh Sapkota, Raddella Nishani Savidya, Ajayi Tolulope Tosin, Muchanji Adam, Mohammad Naushad Alam, Eun-Hae Kwon, Sang-Mo Kang, Shifa Shaffique, and In-Jung Lee. 2025. "Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress" International Journal of Molecular Sciences 26, no. 3: 878. https://doi.org/10.3390/ijms26030878
APA StyleAdhikari, A., Sapkota, M., Savidya, R. N., Tosin, A. T., Adam, M., Alam, M. N., Kwon, E.-H., Kang, S.-M., Shaffique, S., & Lee, I.-J. (2025). Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress. International Journal of Molecular Sciences, 26(3), 878. https://doi.org/10.3390/ijms26030878








