Improvement of Mutant Galactose-1-Phosphate Uridylyltransferase (GALT) Activity by FDA-Approved Pharmacochaperones: A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Results by Computational Studies
2.1.1. Docking Studies with Known PCs Selected from Literature
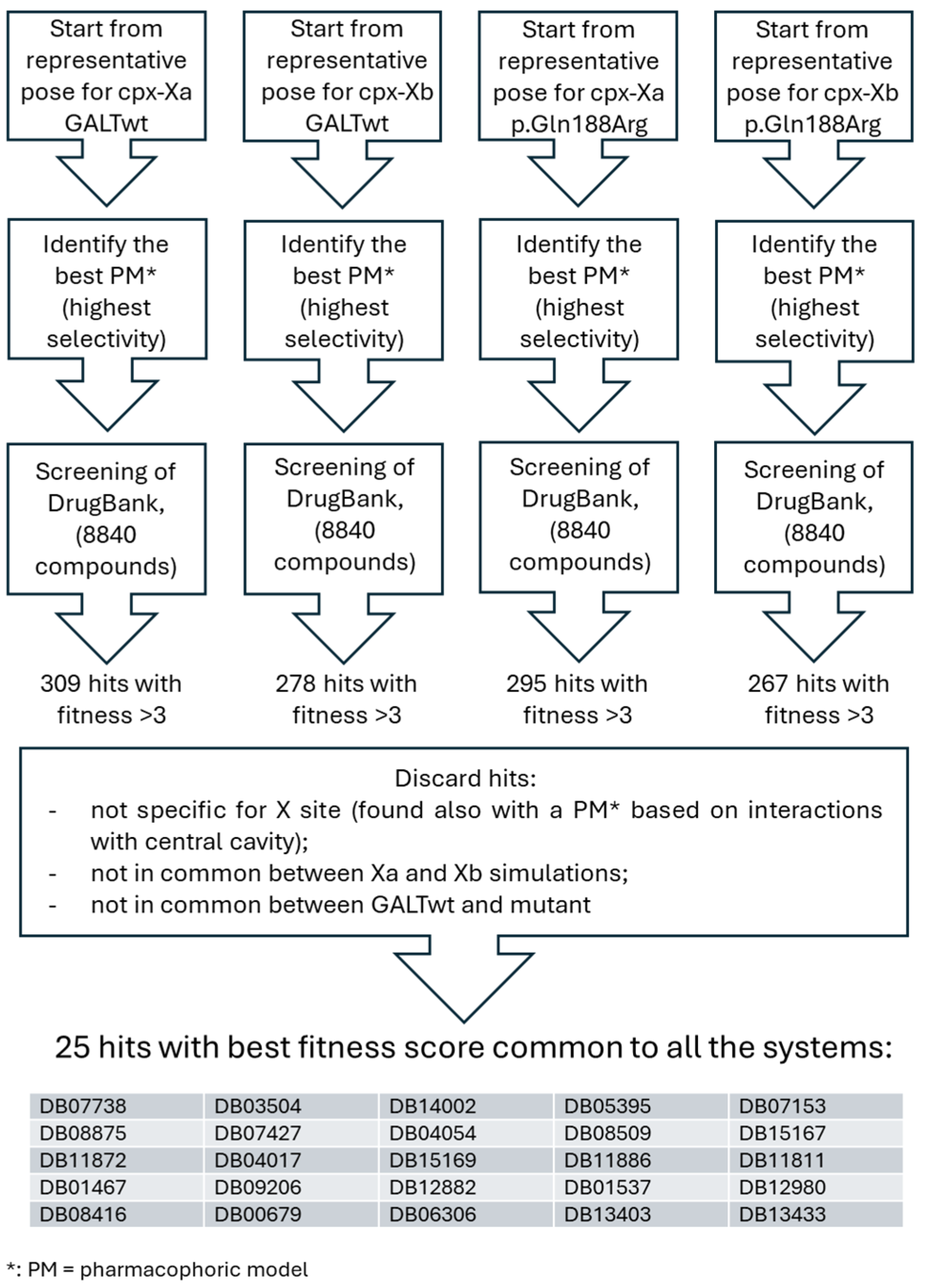
2.1.2. Pharmacophore Screening for Further Selection of Putative Drugs
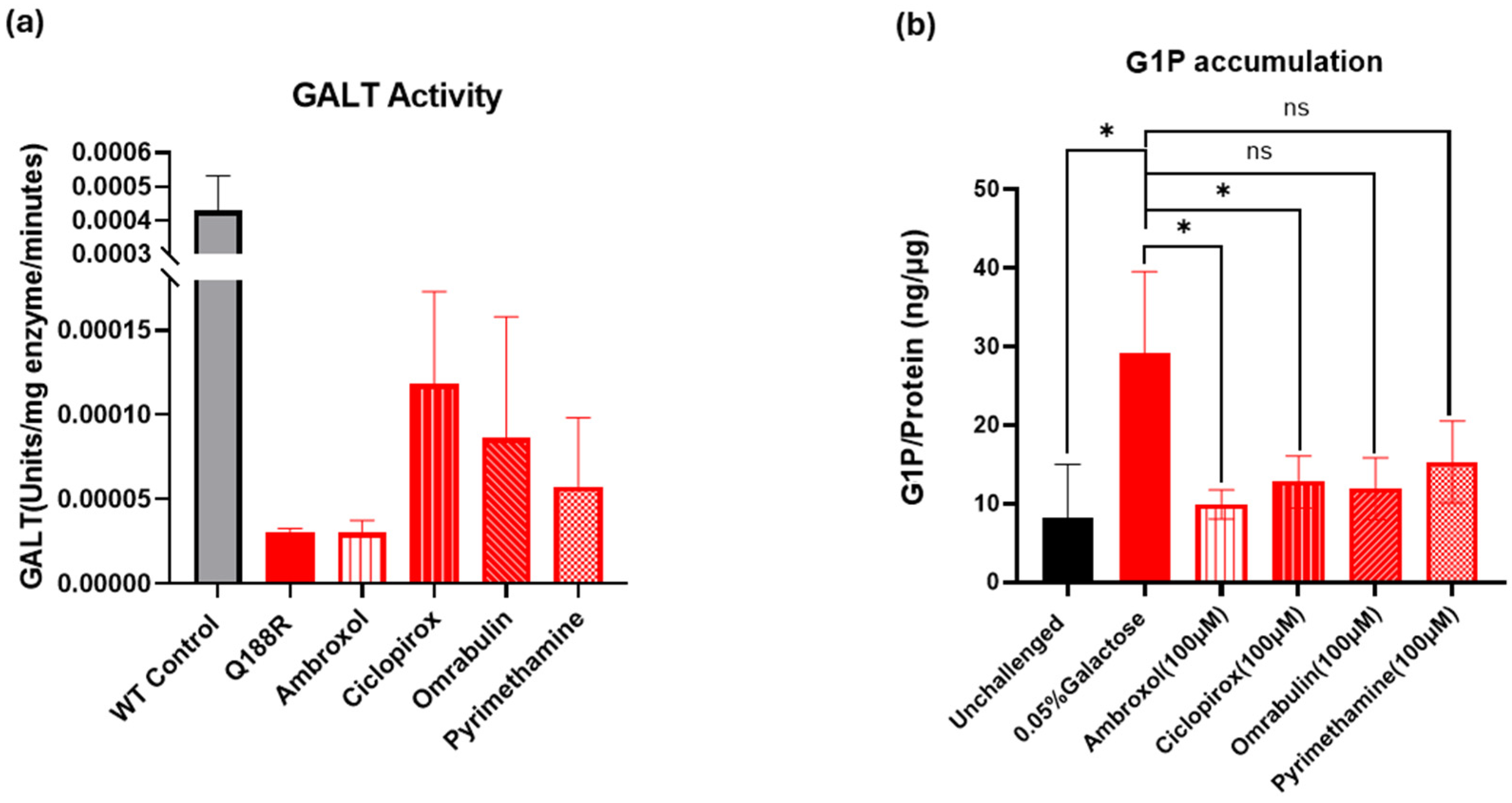
2.2. Results of Preliminary Experimental Assays
2.2.1. In Vitro Evaluation of GALT Enzymatic Activity Augmentation by the Selected Small Molecules
2.2.2. Cell-Based Assessment of GALT Enzymatic Activity Augmentation by the Selected Small Molecules
3. Discussion
4. Materials and Methods
4.1. Computational Approaches
4.2. Preliminary Experimental Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry; Molecular Genetics; Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- d’Acierno, A.; Scafuri, B.; Facchiano, A.; Marabotti, A. The evolution of a Web resource: The Galactosemia Proteins Database 2.0. Hum. Mutat. 2018, 39, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.T. Classic Galactosemia and Clinical Variant Galactosemia. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2024; Updated 11 March 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1518/ (accessed on 12 January 2025).
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary galactosemia. Metabolism 2018, 83, 188–196. [Google Scholar] [CrossRef]
- Lai, K.; Boxer, M.B.; Marabotti, A. GALK inhibitors for classic galactosemia. Future Med. Chem. 2014, 6, 1003–1015. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.Q.; Lee, O.W.; Liu, L.; Tang, M.; Lai, K.; Boxer, M.B.; Hall, M.D.; Shen, M. Discovery of novel inhibitors of human galactokinase by virtual screening. J. Comput. Mol. Des. 2019, 33, 405–417. [Google Scholar] [CrossRef]
- Mackinnon, S.R.; Krojer, T.; Foster, W.R.; Diaz-Saez, L.; Tang, M.; Huber, K.V.M.; von Delft, F.; Lai, K.; Brennan, P.E.; Arruda Bezerra, G.; et al. Fragment screening reveals starting points for rational design of galactokinase 1 inhibitors to treat Classic Galactosemia. ACS Chem. Biol. 2021, 16, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.H.; Karas, B.L.; Barada, F.; Levitsky, A.M. Recent and anticipated novel drug approvals for 2024. Am. J. Health Syst. Pharm. 2024, 81, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, R.; Bailey, E.; Wang, S.; Mills, R.; Mohanlal, R.; Shendelman, S. Safety, Pharmacokinetics; and Pharmacodynamics of the New Aldose Reductase Inhibitor Govorestat (AT-007) After a Single and Multiple Doses in Participants in a Phase 1/2 Study. J. Clin. Pharmacol. 2024, 64, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Timson, D.J. Therapies for galactosemia: A patent landscape. Pharm. Pat. Anal. 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Delnoy, B.; Coelho, A.; Rubio-Gozalbo, M. Current and future treatments for Classic Galactosemia. J. Pers. Med. 2021, 11, 75. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Siddiqi, A.; Mella, J.; Lupo, A.; Hollien, J.; Johnson, J.; Lai, K. Salubrinal enhances eIF2α phosphorylation and improves fertility in a mouse model of Classic Galactosemia. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165516. [Google Scholar] [CrossRef]
- Delnoy, B.; Haskovic, M.; Vanoevelen, J.; Steinbusch, L.K.M.; Vos, E.N.; Knoops, K.; Zimmermann, L.J.I.; Noga, M.; Lefeber, D.J.; Martini, P.G.V.; et al. Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia. J. Inherit. Metab. Dis. 2022, 45, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Yan, X.; McCue, M.D.; Bellagamba, O.; Guo, A.; Winkler, F.; Thall, J.; Crawford, L.; Dimen, R.; Chen, S.; et al. Whole-body galactose oxidation as a robust functional assay to assess the efficacy of gene-based therapies in a mouse model of Galactosemia. Mol. Ther. Methods Clin. Dev. 2024, 32, 101191. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Daenzer, J.M.I.; Fridovich-Keil, J.L. A pilot study of neonatal GALT gene replacement using AAV9 dramatically lowers galactose metabolites in blood; liver; and brain and minimizes cataracts in GALT-null rat pups. J. Inherit. Metab. Dis. 2021, 44, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.L.; Stansfield, J.C.; Ahn, Y.; Cheng, S.H.; Murphy, J.E.; Bell, R.D. AAV-mediated expression of galactose-1-phosphate uridyltransferase corrects defects of galactose metabolism in classic galactosemia patient fibroblasts. J. Inherit. Metab. Dis. 2022, 45, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Facchiano, A.; Rachamadugu, R.; Calderon, F.; Mao, R.; Milanesi, L.; Marabotti, A.; Lai, K. Correlation assessment among clinical phenotypes; expression analysis and molecular modeling of 14 novel variations in the human galactose-1-phosphate uridylyltransferase gene. Hum. Mutat. 2012, 33, 1107–1115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCorvie, T.J.; Gleason, T.J.; Fridovich-Keil, J.L.; Timson, D.J. Misfolding of galactose 1-phosphate uridylyltransferase can result in type I galactosemia. Biochim. Biophys. Acta 2013, 1832, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- McCorvie, T.J.; Kopec, J.; Pey, A.L.; Fitzpatrick, F.; Patel, D.; Chalk, R.; Shrestha, L.; Yue, W.W. Molecular basis of classic galactosemia from the structure of human galactose 1-phosphate uridylyltransferase. Hum. Mol. Genet. 2016, 25, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Verdino, A.; D’Urso, G.; Tammone, C.; Scafuri, B.; Marabotti, A. Analysis of the structure-function-dynamics relationships of GALT enzyme and of its pathogenic mutant p.Q188R: A molecular dynamics simulation study in different experimental conditions. Molecules 2021, 26, 5941. [Google Scholar] [CrossRef] [PubMed]
- Morello, J.P.; Salahpour, A.; Laperriere, A.; Bernier, V.; Arthus, M.F.; Lonergan, M.; Petäjä-Repo, U.; Angers, S.; Morin, D.; Bichet, D.G.; et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded v2 vasopressin receptor mutants. J. Clin. Investig. 2000, 105, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Liguori, L.; Monticelli, M.; Allocca, M.; Hay Mele, B.; Lukas, J.; Cubellis, M.V.; Andreotti, G. Pharmacological chaperones: A therapeutic approach for diseases caused by destabilizing missense mutations. Int. J. Mol. Sci. 2020, 21, 489. [Google Scholar] [CrossRef]
- Matalonga, L.; Gort, L.; Ribes, A. Small molecules as therapeutic agents for inborn errors of metabolism. J. Inherit. Metab. Dis. 2017, 40, 177–193. [Google Scholar] [CrossRef]
- Hou, Z.S.; Ulloa-Aguirre, A.; Tao, Y.X. Pharmacoperone drugs: Targeting misfolded proteins causing lysosomal storage- ion channels-; and G protein-coupled receptors-associated conformational disorders. Expert. Rev. Clin. Pharmacol. 2018, 11, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Conn, P.M. Pharmacoperones as novel therapeutics for diverse protein conformational diseases. Physiol. Rev. 2018, 98, 697–725. [Google Scholar] [CrossRef] [PubMed]
- Timson, D.J. The molecular basis of galactosemia—Past; present and future. Gene 2016, 589, 133–141. [Google Scholar] [CrossRef]
- Coelho, A.I.; Trabuco, M.; Silva, M.J.; de Almeida, I.T.; Leandro, P.; Rivera, I.; Vicente, J.B. Arginine functionally improves clinically relevant human galactose-1-phosphate uridylyltransferase (GALT) variants expressed in a prokaryotic model. JIMD Rep. 2015, 23, 1–6. [Google Scholar] [CrossRef]
- Haskovic, M.; Derks, B.; van der Ploeg, L.; Trommelen, J.; Nyakayiru, J.; van Loon, L.J.C.; Mackinnon, S.; Yue, W.W.; Peake, R.W.A.; Zha, L.; et al. Arginine does not rescue p.Q188R mutation deleterious effect in classic galactosemia. Orphanet J. Rare Dis. 2018, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Verdino, A.; D’Urso, G.; Tammone, C.; Scafuri, B.; Catapano, L.; Marabotti, A. Simulation of the interactions of arginine with wild-type GALT enzyme and the Classic Galactosemia-related mutant p.Q188R by a computational approach. Molecules 2021, 26, 6061. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert. Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Hay Mele, B.; Citro, V.; Andreotti, G.; Cubellis, M.V. Drug repositioning can accelerate discovery of pharmacological chaperones. Orphanet J. Rare Dis. 2015, 10, 55. [Google Scholar] [CrossRef]
- Scafuri, B.; Verdino, A.; D’Arminio, N.; Marabotti, A. Computational methods to assist in the discovery of pharmacological chaperones for rare diseases. Brief. Bioinform. 2022, 23, bbac198. [Google Scholar] [CrossRef]
- Reichardt, J.K.; Packman, S.; Woo, S.L. Molecular characterization of two galactosemia mutations: Correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase. Am. J. Hum. Genet. 1991, 49, 860–867. [Google Scholar] [PubMed]
- Malerba, M.; Ragnoli, B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, G.H. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 2009, 284, 23502–23516. [Google Scholar] [CrossRef] [PubMed]
- Bendikov-Bar, I.; Maor, G.; Filocamo, M.; Horowitz, M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 2013, 50, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Shirai, K.; Itamura, S.; Matsuda, A.; Ishihara, A.; Matsushita, K.; Fukuda, C.; Kubota, N.; Takayama, R.; Shigematsu, H.; et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: A pilot study. Ann. Clin. Transl. Neurol. 2016, 3, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Sirichaiwat, C.; Intaraudom, C.; Kamchonwongpaisan, S.; Vanichtanankul, J.; Thebtaranonth, Y.; Yuthavong, Y. Target guided synthesis of 5-benzyl-2;4-diamonopyrimidines: Their antimalarial activities and binding affinities to wild type and mutant dihydrofolate reductases from Plasmodium falciparum. J. Med. Chem. 2004, 47, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective; animal models; and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Osher, E.; Fattal-Valevski, A.; Sagie, L.; Urshanski, N.; Amir-Levi, Y.; Katzburg, S.; Peleg, L.; Lerman-Sagie, T.; Zimran, A.; Elstein, D.; et al. Pyrimethamine increases beta-hexosaminidase A activity in patients with Late Onset Tay Sachs. Mol. Genet. Metab. 2011, 102, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Niemir, N.; Aureli, M.; Magini, A.; Loberto, N.; Prinetti, A.; Bassi, R.; Polchi, A.; Emiliani, C.; Caillaud, C.; et al. Chaperone therapy for GM2 gangliosidosis: Effects of pyrimethamine on beta-hexosaminidase activity in Sandhoff fibroblasts. Mol. Neurobiol. 2014, 50, 159–167. [Google Scholar] [CrossRef]
- Clarke, J.T.; Mahuran, D.J.; Sathe, S.; Kolodny, E.H.; Rigat, B.A.; Raiman, J.A.; Tropak, M.B. An open-label Phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants). Mol. Genet. Metab. 2011, 102, 6–12. [Google Scholar] [CrossRef]
- Niewerth, M.; Kunze, D.; Seibold, M.; Schaller, M.; Korting, H.C.; Hube, B. Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors; iron metabolism proteins; and drug resistance factors. Antimicrob. Agents Chemother. 2003, 47, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Sigle, H.C.; Thewes, S.; Niewerth, M.; Korting, H.C.; Schafer-Korting, M.; Hube, B. Oxygen accessibility and iron levels are critical factors for the antifungal action of ciclopirox against Candida albicans. J. Antimicrob. Chemother. 2005, 55, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Urquiza, P.; Laín, A.; Sanz-Parra, A.; Moreno, J.; Bernardo-Seisdedos, G.; Dubus, P.; González, E.; Gutiérrez-de-Juan, V.; García, S.; Eraña, H.; et al. Repurposing ciclopirox as a pharmacological chaperone in a model of congenital erythropoietic porphyria. Sci. Transl. Med. 2018, 19, eaat7467. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Grove, L.E.; Hall, D.R.; Bohnuud, T.; Mottarella, S.E.; Luo, L.; Xia, B.; Beglov, D.; Vajda, S. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 2015, 10, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Lemieux, M.J.; Mark, B.L.; Cherney, M.M.; Withers, S.G.; Mahuran, D.J.; James, M.N. Crystallographic structure of human beta-hexosaminidase A: Interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis. J. Mol. Biol. 2006, 359, 913–929. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mayes, J.S.; Hanson, R.G. Galactose 1-phosphate uridyl transferase. Methods Enzymol. 1966, 9, 708–713. [Google Scholar] [CrossRef]
- Lai, K.; Willis, A.C.; Elsas, L.J. The biochemical role of glutamine 188 in human galactose-1-phosphate uridyltransferase. J. Biol. Chem. 1999, 274, 6559–6566. [Google Scholar] [CrossRef] [PubMed]


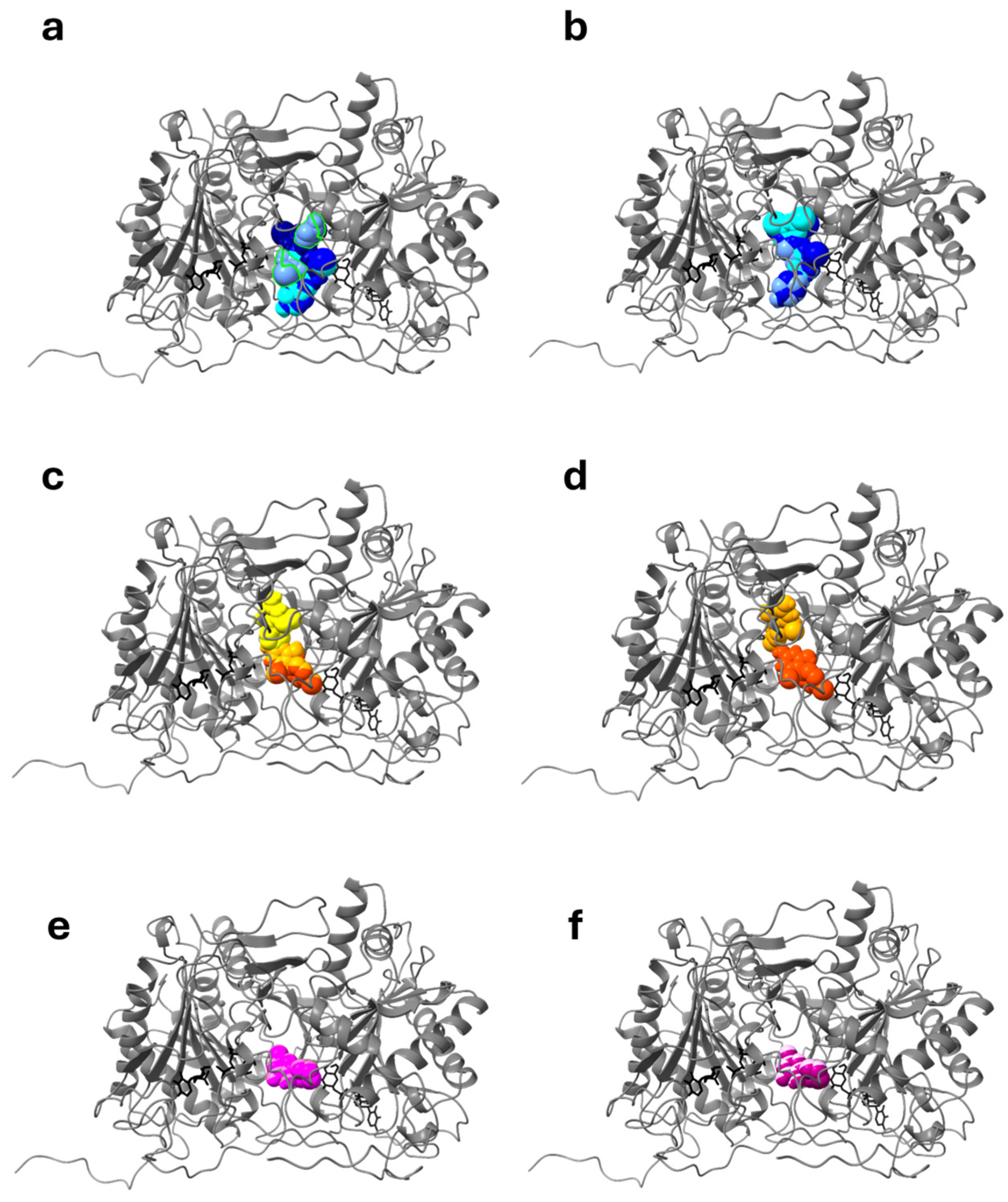
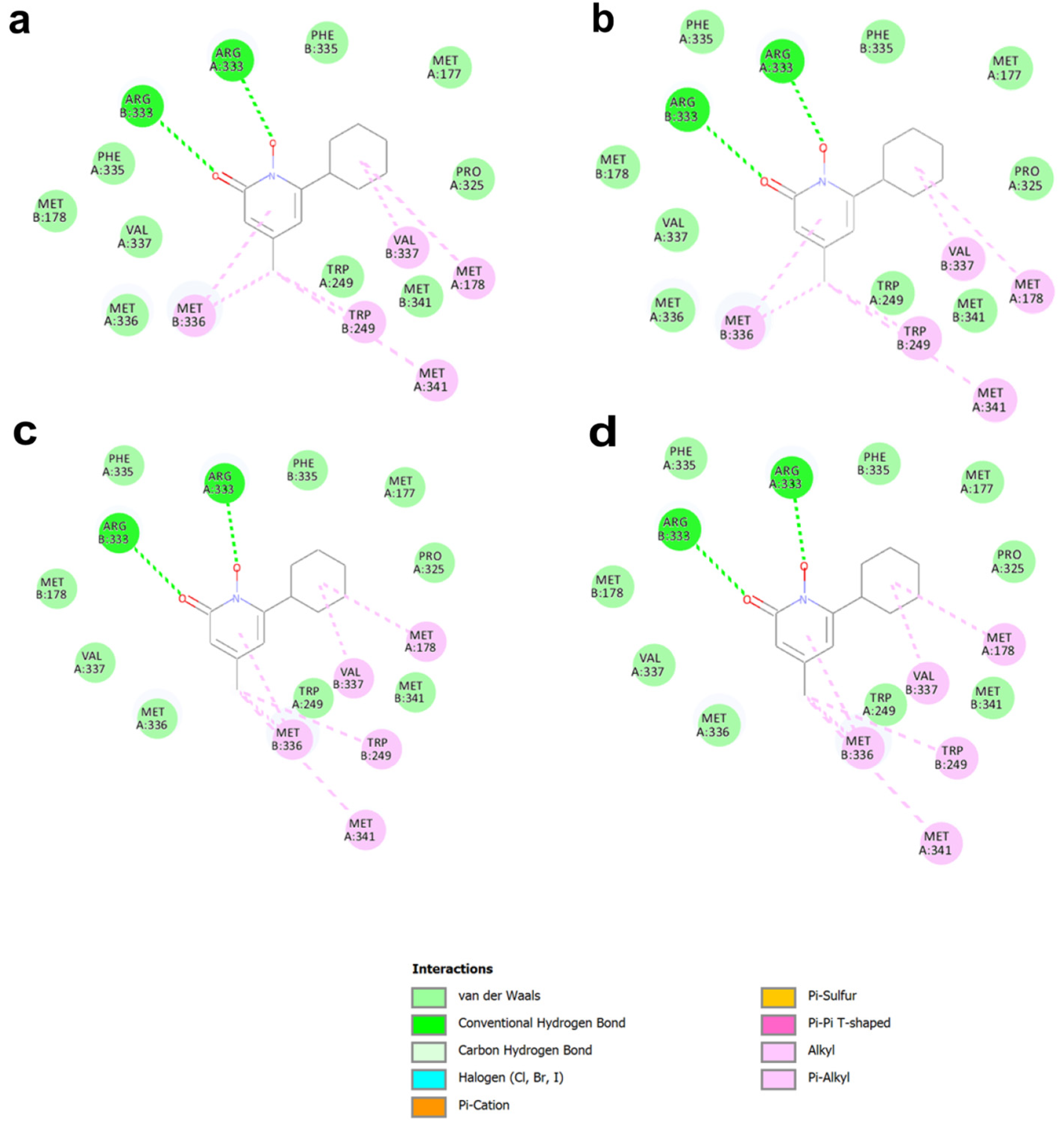

| wtGALT Xa 1 | wtGALT Xb 1 | p.Gln188Arg Xa 1 | p.Gln188Arg Xb 1 | |
|---|---|---|---|---|
| Ambroxol | ||||
| Total number of clusters | 10 | 8 | 13 | 8 |
| Representative result for cluster at lowest energy (predicted binding energy of the best pose—number of poses) | −8.29 kcal/mol—17 poses | −8.30 kcal/mol—19 poses | −8.14 kcal/mol —12 poses | −8.15 kcal/mol—21 poses |
| Representative result for cluster with higher population (predicted binding energy of the best pose—number of poses) | −7.01 kcal/mol—33 poses | −7.26 kcal/mol—35 poses | −6.90 kcal/mol—27 poses | −7.12 kcal/mol—40 poses |
| Pyrimethamine | ||||
| Total number of clusters | 4 | 2 | 1 | 3 |
| Representative result for cluster at lowest energy (predicted binding energy of the best pose—number of poses) | −6.39 kcal/mol—11 poses | −5.88 kcal/mol—62 poses | −6.69 kcal/mol—100 poses | −5.71 kcal/mol—65 poses |
| Representative result for cluster with higher population (predicted binding energy of the best pose—number of poses) | −5.56 kcal/mol—55 poses | Same as above | Same as above | Same as above |
| Ciclopirox | ||||
| Total number of clusters | 2 | 2 | 1 | 1 |
| Representative result for cluster at lowest energy (predicted binding energy of the best pose—number of poses) | −7.10 kcal/mol—99 poses | −7.09 kcal/mol—99 poses | −7.12 kcal/mol—100 poses | −7.09 kcal/mol—100 poses |
| Representative result for cluster with higher population (predicted binding energy of the best pose—number of poses) | Same as above | Same as above | Same as above | Same as above |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scafuri, B.; Piscosquito, S.; Giliberti, G.; Facchiano, A.; Miner, J.; Balakrishnan, B.; Lai, K.; Marabotti, A. Improvement of Mutant Galactose-1-Phosphate Uridylyltransferase (GALT) Activity by FDA-Approved Pharmacochaperones: A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 888. https://doi.org/10.3390/ijms26030888
Scafuri B, Piscosquito S, Giliberti G, Facchiano A, Miner J, Balakrishnan B, Lai K, Marabotti A. Improvement of Mutant Galactose-1-Phosphate Uridylyltransferase (GALT) Activity by FDA-Approved Pharmacochaperones: A Preliminary Study. International Journal of Molecular Sciences. 2025; 26(3):888. https://doi.org/10.3390/ijms26030888
Chicago/Turabian StyleScafuri, Bernardina, Stefania Piscosquito, Giulia Giliberti, Angelo Facchiano, Jaden Miner, Bijina Balakrishnan, Kent Lai, and Anna Marabotti. 2025. "Improvement of Mutant Galactose-1-Phosphate Uridylyltransferase (GALT) Activity by FDA-Approved Pharmacochaperones: A Preliminary Study" International Journal of Molecular Sciences 26, no. 3: 888. https://doi.org/10.3390/ijms26030888
APA StyleScafuri, B., Piscosquito, S., Giliberti, G., Facchiano, A., Miner, J., Balakrishnan, B., Lai, K., & Marabotti, A. (2025). Improvement of Mutant Galactose-1-Phosphate Uridylyltransferase (GALT) Activity by FDA-Approved Pharmacochaperones: A Preliminary Study. International Journal of Molecular Sciences, 26(3), 888. https://doi.org/10.3390/ijms26030888








