Look Beyond Plasma Membrane Biophysics: Revealing Considerable Variability of the Dipole Potential Between Plasma and Organelle Membranes of Living Cells
Abstract
1. Introduction
2. Results
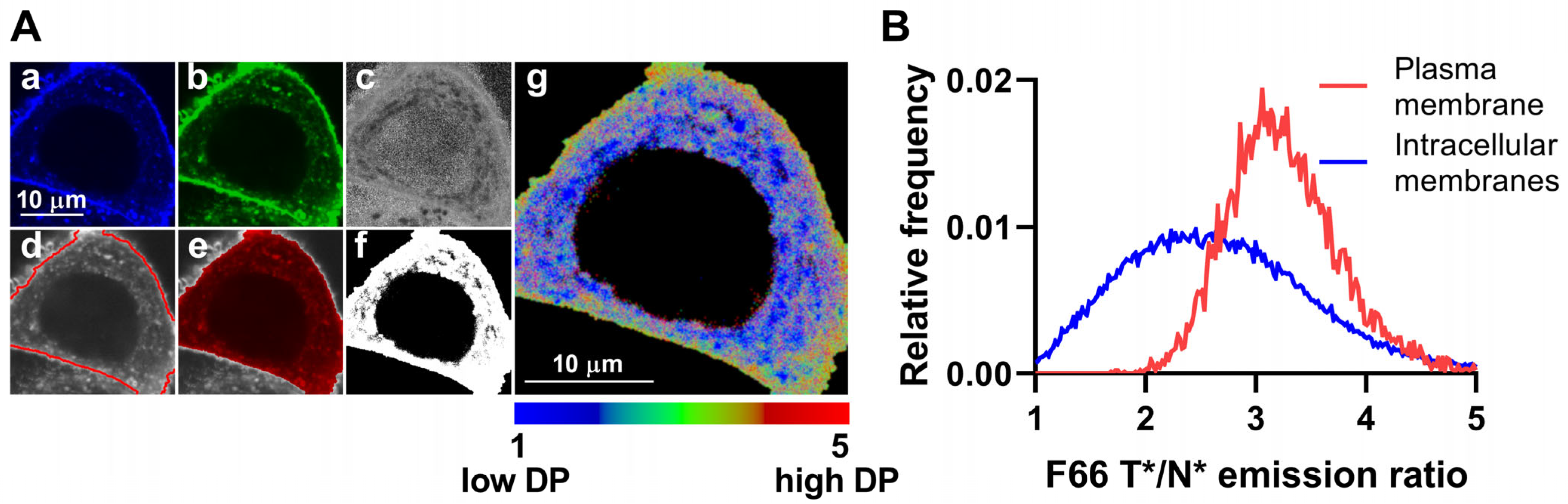
2.1. The Magnitude of Dipole Potential Displays Large Variations in Cellular Membranes
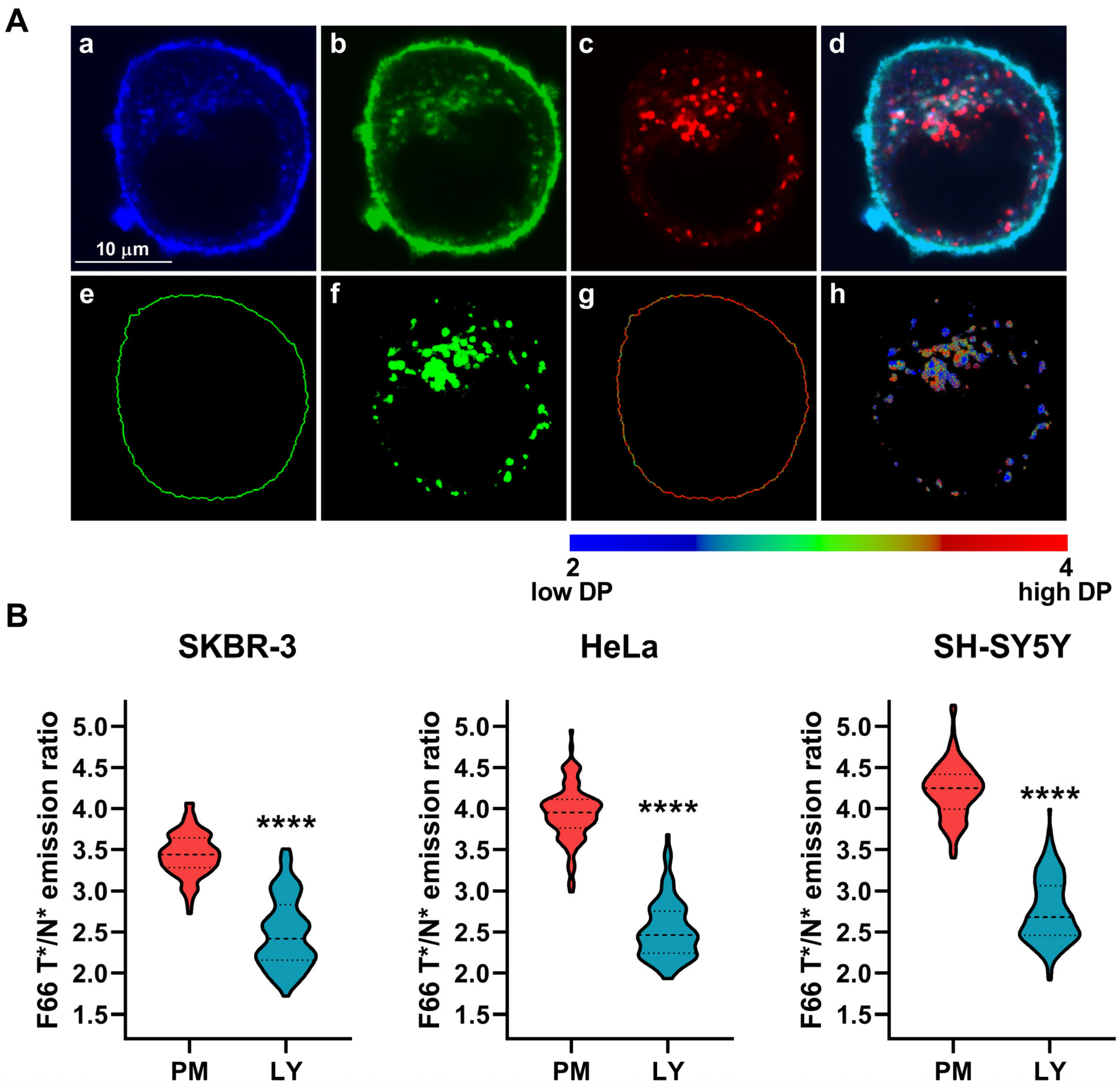
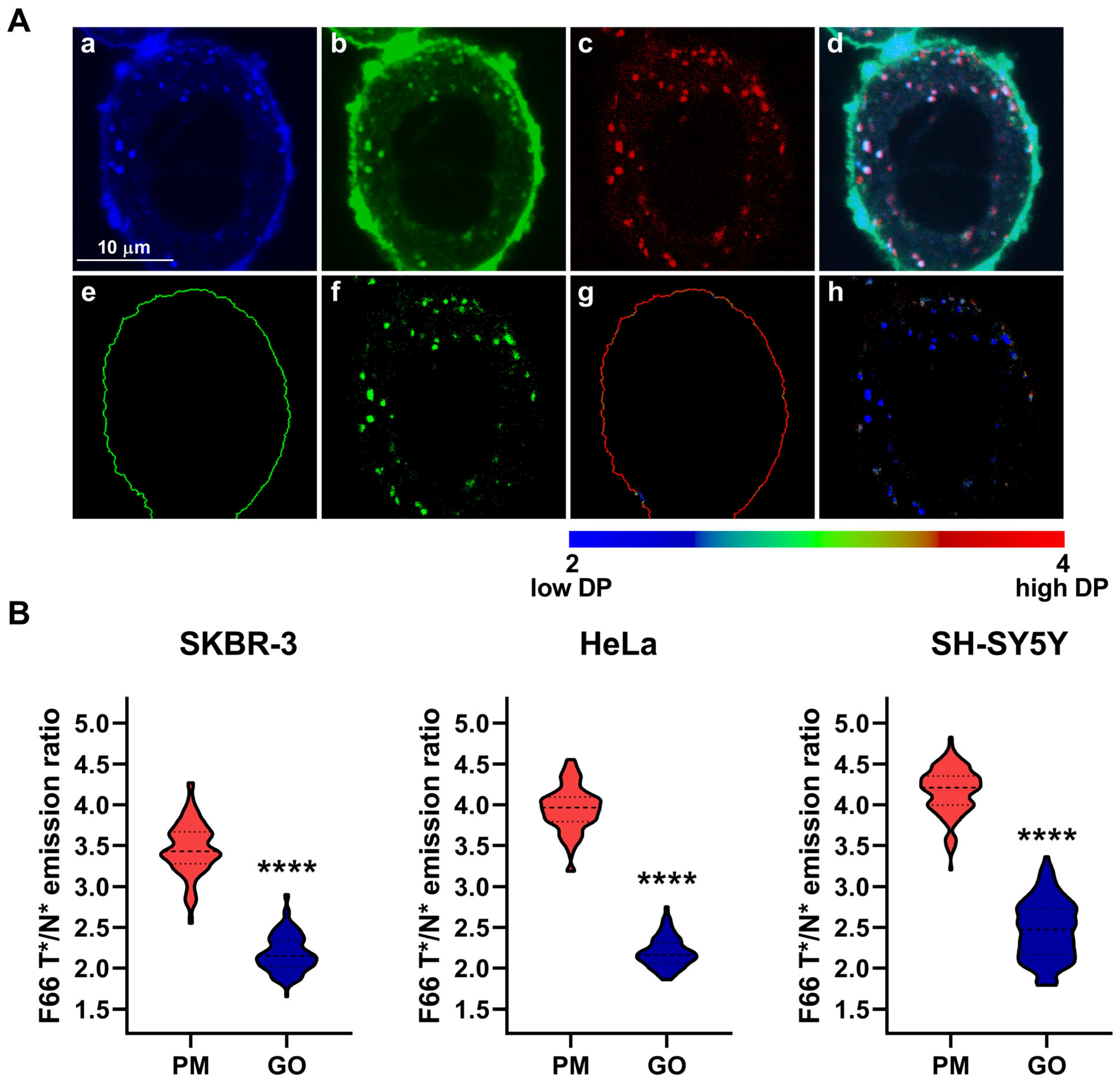
2.2. The Magnitude of Dipole Potential of Intracellular Organelles Compared with That of the Plasma Membrane
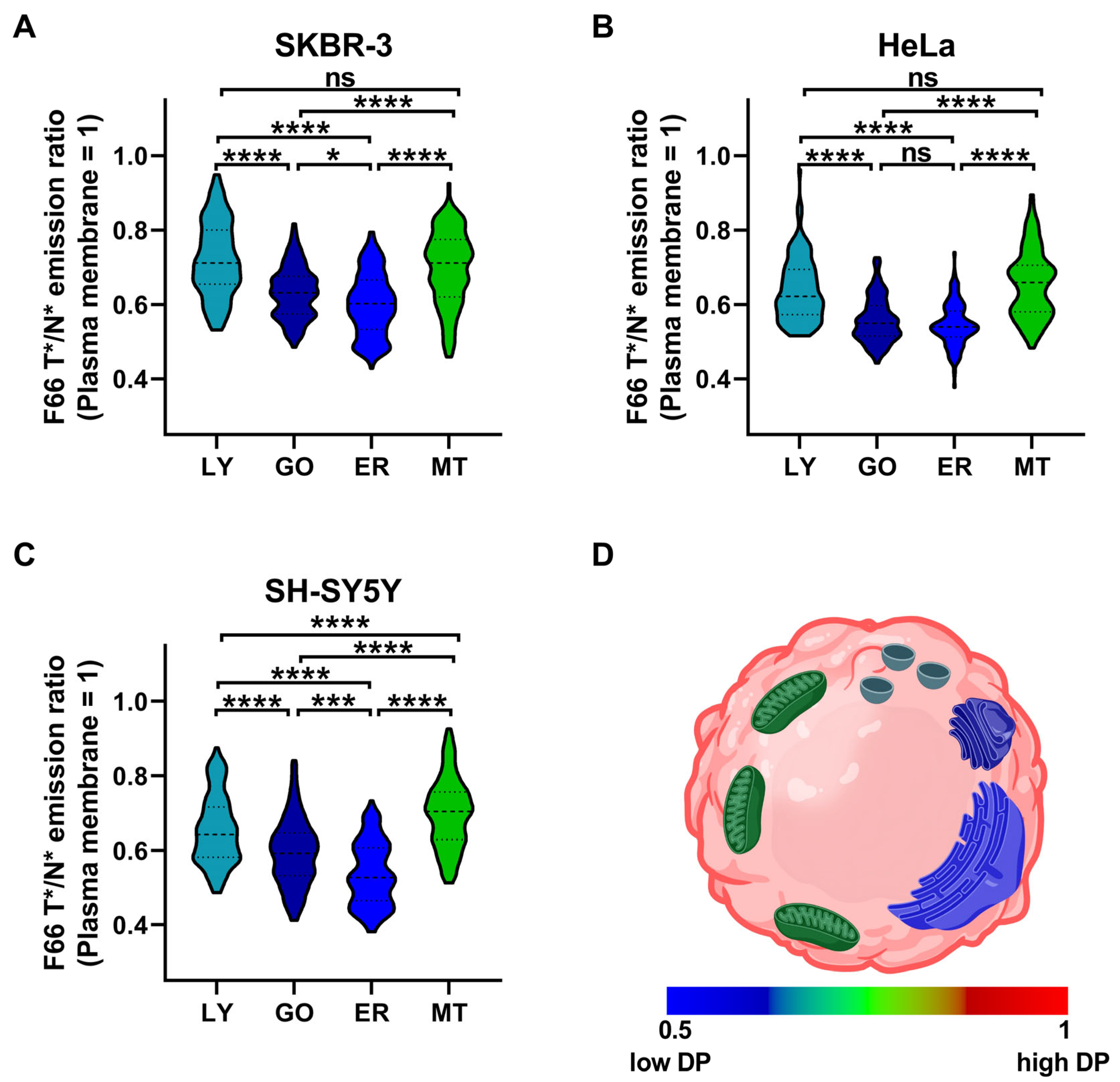
2.3. Comparison of the Magnitude of Dipole Potential in Different Intracellular Organelles
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Labeling Cells with Intracellular Organelle Markers and the Dipole Potential-Sensitive F66 Fluorophore
4.3. Confocal Microscopic Imaging of Cells
4.4. Quantitative Image Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging diversity in lipid-protein interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Zakany, F.; Kovacs, T.; Panyi, G.; Varga, Z. Direct and indirect cholesterol effects on membrane proteins with special focus on potassium channels. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158706. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Doktorova, M.; Molugu, T.R.; Heberle, F.A.; Scott, H.L.; Dzikovski, B.; Nagao, M.; Stingaciu, L.R.; Standaert, R.F.; Barrera, F.N.; et al. How cholesterol stiffens unsaturated lipid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 21896–21905. [Google Scholar] [CrossRef]
- de Santis, A.; Scoppola, E.; Ottaviani, M.F.; Koutsioubas, A.; Barnsley, L.C.; Paduano, L.; D’Errico, G.; Russo Krauss, I. Order vs. Disorder: Cholesterol and omega-3 phospholipids determine biomembrane organization. Int. J. Mol. Sci. 2022, 23, 5322. [Google Scholar] [CrossRef]
- Rajamoorthi, K.; Petrache, H.I.; McIntosh, T.J.; Brown, M.F. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by (2)h nmr and x-ray diffraction. J. Am. Chem. Soc. 2005, 127, 1576–1588. [Google Scholar] [CrossRef]
- Leeb, F.; Maibaum, L. Spatially resolving the condensing effect of cholesterol in lipid bilayers. Biophys. J. 2018, 115, 2179–2188. [Google Scholar] [CrossRef]
- Shen, H.; Deng, M.; Wu, Z.; Zhang, J.; Zhang, Y.; Gao, C.; Cen, C. Effect of cholesterol on membrane dipole potential: Atomistic and coarse-grained molecular dynamics simulations. J. Chem. Theory Comput. 2018, 14, 3780–3795. [Google Scholar] [CrossRef]
- Mills, T.T.; Toombes, G.E.; Tristram-Nagle, S.; Smilgies, D.M.; Feigenson, G.W.; Nagle, J.F. Order parameters and areas in fluid-phase oriented lipid membranes using wide angle x-ray scattering. Biophys. J. 2008, 95, 669–681. [Google Scholar] [CrossRef]
- Wang, L.; Bose, P.S.; Sigworth, F.J. Using cryo-em to measure the dipole potential of a lipid membrane. Proc. Natl. Acad. Sci. USA 2006, 103, 18528–18533. [Google Scholar] [CrossRef]
- Yang, Y.; Mayer, K.M.; Wickremasinghe, N.S.; Hafner, J.H. Probing the lipid membrane dipole potential by atomic force microscopy. Biophys. J. 2008, 95, 5193–5199. [Google Scholar] [CrossRef] [PubMed]
- Batta, G.; Karpati, L.; Henrique, G.F.; Toth, G.; Tarapcsak, S.; Kovacs, T.; Zakany, F.; Mandity, I.M.; Nagy, P. Statin-boosted cellular uptake and endosomal escape of penetratin due to reduced membrane dipole potential. Br. J. Pharmacol. 2021, 178, 3667–3681. [Google Scholar] [CrossRef] [PubMed]
- Batta, G.; Soltesz, L.; Kovacs, T.; Bozo, T.; Meszar, Z.; Kellermayer, M.; Szollosi, J.; Nagy, P. Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of gaucher disease. Sci. Rep. 2018, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Cs. Szabo, B.; Szabo, M.; Nagy, P.; Varga, Z.; Panyi, G.; Kovacs, T.; Zakany, F. Novel insights into the modulation of the voltage-gated potassium channel k(v)1.3 activation gating by membrane ceramides. J. Lipid Res. 2024, 65, 100596. [Google Scholar] [CrossRef]
- Kovacs, T.; Sohajda, T.; Szente, L.; Nagy, P.; Panyi, G.; Varga, Z.; Zakany, F. Cyclodextrins exert a ligand-like current inhibitory effect on the k(v)1.3 ion channel independent of membrane cholesterol extraction. Front. Mol. Biosci. 2021, 8, 735357. [Google Scholar] [CrossRef]
- Pinto, S.N.; Laviad, E.L.; Stiban, J.; Kelly, S.L.; Merrill, A.H., Jr.; Prieto, M.; Futerman, A.H.; Silva, L.C. Changes in membrane biophysical properties induced by sphingomyelinase depend on the sphingolipid n-acyl chain. J. Lipid Res. 2014, 55, 53–61. [Google Scholar] [CrossRef]
- Zakany, F.; Szabo, M.; Batta, G.; Karpati, L.; Mandity, I.M.; Fulop, P.; Varga, Z.; Panyi, G.; Nagy, P.; Kovacs, T. An omega-3, but not an omega-6 polyunsaturated fatty acid decreases membrane dipole potential and stimulates endo-lysosomal escape of penetratin. Front. Cell Dev. Biol. 2021, 9, 647300. [Google Scholar] [CrossRef]
- Ma, Y.; Benda, A.; Kwiatek, J.; Owen, D.M.; Gaus, K. Time-resolved laurdan fluorescence reveals insights into membrane viscosity and hydration levels. Biophys. J. 2018, 115, 1498–1508. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E.; Yu, W.M.; Wilson, P.; Levi, M. Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys. J. 1997, 72, 2413–2429. [Google Scholar] [CrossRef]
- Varela, A.R.; Ventura, A.E.; Carreira, A.C.; Fedorov, A.; Futerman, A.H.; Prieto, M.; Silva, L.C. Pathological levels of glucosylceramide change the biophysical properties of artificial and cell membranes. Phys. Chem. Chem. Phys. 2016, 19, 340–346. [Google Scholar] [CrossRef]
- Kwiatek, J.M.; Owen, D.M.; Abu-Siniyeh, A.; Yan, P.; Loew, L.M.; Gaus, K. Characterization of a new series of fluorescent probes for imaging membrane order. PLoS ONE 2013, 8, e52960. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; Chakraborty, H.; Covey, D.F.; Chattopadhyay, A. Membrane dipole potential is sensitive to cholesterol stereospecificity: Implications for receptor function. Chem. Phys. Lipids 2014, 184, 25–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clarke, R.J.; Kane, D.J. Optical detection of membrane dipole potential: Avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta 1997, 1323, 223–239. [Google Scholar] [CrossRef]
- Davis, S.; Davis, B.M.; Richens, J.L.; Vere, K.A.; Petrov, P.G.; Winlove, C.P.; O’Shea, P. Alpha-tocopherols modify the membrane dipole potential leading to modulation of ligand binding by p-glycoprotein. J. Lipid Res. 2015, 56, 1543–1550. [Google Scholar] [CrossRef]
- Gross, E.; Bedlack, R.S., Jr.; Loew, L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994, 67, 208–216. [Google Scholar] [CrossRef]
- Haldar, S.; Kanaparthi, R.K.; Samanta, A.; Chattopadhyay, A. Differential effect of cholesterol and its biosynthetic precursors on membrane dipole potential. Biophys. J. 2012, 102, 1561–1569. [Google Scholar] [CrossRef]
- Kovacs, T.; Batta, G.; Hajdu, T.; Szabo, A.; Varadi, T.; Zakany, F.; Csomos, I.; Szollosi, J.; Nagy, P. The dipole potential modifies the clustering and ligand binding affinity of erbb proteins and their signaling efficiency. Sci. Rep. 2016, 6, 35850. [Google Scholar] [CrossRef]
- Kovacs, T.; Batta, G.; Zakany, F.; Szollosi, J.; Nagy, P. The dipole potential correlates with lipid raft markers in the plasma membrane of living cells. J. Lipid Res. 2017, 58, 1681–1691. [Google Scholar] [CrossRef]
- Sarkar, P.; Rao, B.D.; Chattopadhyay, A. Cell cycle dependent modulation of membrane dipole potential and neurotransmitter receptor activity: Role of membrane cholesterol. ACS Chem. Neurosci. 2020, 11, 2890–2899. [Google Scholar] [CrossRef]
- Singh, P.; Haldar, S.; Chattopadhyay, A. Differential effect of sterols on dipole potential in hippocampal membranes: Implications for receptor function. Biochim. Biophys. Acta 2013, 1828, 917–923. [Google Scholar] [CrossRef]
- Starke-Peterkovic, T.; Clarke, R.J. Effect of headgroup on the dipole potential of phospholipid vesicles. Eur. Biophys. J. 2009, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Nagy, P.; Panyi, G.; Szente, L.; Varga, Z.; Zakany, F. Cyclodextrins: Only pharmaceutical excipients or full-fledged drug candidates? Pharmaceutics 2022, 14, 2559. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Zakany, F.; Nagy, P. It takes more than two to tango: Complex, hierarchal, and membrane-modulated interactions in the regulation of receptor tyrosine kinases. Cancers 2022, 14, 944. [Google Scholar] [CrossRef] [PubMed]
- Zakany, F.; Mandity, I.M.; Varga, Z.; Panyi, G.; Nagy, P.; Kovacs, T. Effect of the lipid landscape on the efficacy of cell-penetrating peptides. Cells 2023, 12, 1700. [Google Scholar] [CrossRef]
- O’Shea, P. Intermolecular interactions with/within cell membranes and the trinity of membrane potentials: Kinetics and imaging. Biochem. Soc. Trans. 2003, 31, 990–996. [Google Scholar] [CrossRef]
- Richens, J.L.; Lane, J.S.; Bramble, J.P.; O’Shea, P. The electrical interplay between proteins and lipids in membranes. Biochim. Biophys. Acta 2015, 1848, 1828–1836. [Google Scholar] [CrossRef]
- Wang, L. Measurements and implications of the membrane dipole potential. Annu. Rev. Biochem. 2012, 81, 615–635. [Google Scholar] [CrossRef]
- Pearlstein, R.A.; Dickson, C.J.; Hornak, V. Contributions of the membrane dipole potential to the function of voltage-gated cation channels and modulation by small molecule potentiators. Biochim. Biophys. Acta Biomembr. 2017, 1859, 177–194. [Google Scholar] [CrossRef]
- Zakany, F.; Pap, P.; Papp, F.; Kovacs, T.; Nagy, P.; Peter, M.; Szente, L.; Panyi, G.; Varga, Z. Determining the target of membrane sterols on voltage-gated potassium channels. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 312–325. [Google Scholar] [CrossRef]
- Clarke, R.J. Dipole-potential-mediated effects on ion pump kinetics. Biophys. J. 2015, 109, 1513–1520. [Google Scholar] [CrossRef]
- Kovacs, T.; Kurtan, K.; Varga, Z.; Nagy, P.; Panyi, G.; Zakany, F. Veklury(r) (remdesivir) formulations inhibit initial membrane-coupled events of SARS-CoV-2 infection due to their sulfobutylether-beta-cyclodextrin content. Br. J. Pharmacol. 2023, 180, 2064–2084. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escriba, P.V.; Rossello, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, M.J.; Llorente, A.; Petan, T.; Khnykin, D.; Popa, I.; Nikolac Perkovic, M.; Konjevod, M.; Jaganjac, M. The expanding organelle lipidomes: Current knowledge and challenges. Cell Mol. Life Sci. 2023, 80, 237. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Shyu, P., Jr.; Ng, B.S.H.; Ho, N.; Chaw, R.; Seah, Y.L.; Marvalim, C.; Thibault, G. Membrane phospholipid alteration causes chronic er stress through early degradation of homeostatic er-resident proteins. Sci. Rep. 2019, 9, 8637. [Google Scholar] [CrossRef]
- Volkmar, N.; Gawden-Bone, C.M.; Williamson, J.C.; Nixon-Abell, J.; West, J.A.; St George-Hyslop, P.H.; Kaser, A.; Lehner, P.J. Regulation of membrane fluidity by rnf145-triggered degradation of the lipid hydrolase adipor2. EMBO J. 2022, 41, e110777. [Google Scholar] [CrossRef]
- Lajevardipour, A.; Chon, J.W.; Chattopadhyay, A.; Clayton, A.H. Imaging cellular dynamics with spectral relaxation imaging microscopy: Distinct spectral dynamics in golgi membranes of living cells. Sci. Rep. 2016, 6, 37038. [Google Scholar] [CrossRef]
- Wang, H.; Shi, C.; Dong, B.; Tian, M. Dual-emissive near-infrared fluorescent probe revealing fluidity changes in lysosomal membranes during lysosomal fission and fusion. Sens. Actuators B Chem. 2025, 422, 136550. [Google Scholar] [CrossRef]
- Singh, G.; George, G.; Raja, S.O.; Kandaswamy, P.; Kumar, M.; Thutupalli, S.; Laxman, S.; Gulyani, A. A molecular rotor flim probe reveals dynamic coupling between mitochondrial inner membrane fluidity and cellular respiration. Proc. Natl. Acad. Sci. USA 2023, 120, e2213241120. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, H.; Zheng, L.; Liu, A.; Zhuan, Q.; Luo, Y.; Zhou, G.; Meng, L.; Hou, Y.; Wu, G.; et al. Metformin alleviates cryoinjuries in porcine oocytes by reducing membrane fluidity through the suppression of mitochondrial activity. Commun. Biol. 2024, 7, 925. [Google Scholar] [CrossRef]
- Darwich, Z.; Kucherak, O.A.; Kreder, R.; Richert, L.; Vauchelles, R.; Mely, Y.; Klymchenko, A.S. Rational design of fluorescent membrane probes for apoptosis based on 3-hydroxyflavone. Methods Appl. Fluoresc. 2013, 1, 025002. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Fluorescent probes for lipid membranes: From the cell surface to organelles. Acc. Chem. Res. 2023, 56, 1–12. [Google Scholar] [CrossRef]
- Gunther, G.; Malacrida, L.; Jameson, D.M.; Gratton, E.; Sanchez, S.A. Laurdan since weber: The quest for visualizing membrane heterogeneity. Acc. Chem. Res. 2021, 54, 976–987. [Google Scholar] [CrossRef]
- Rui, X.; Okamoto, Y.; Fukushima, S.; Morishita Watanabe, N.; Umakoshi, H. Investigating the impact of 2-ohoa-embedded liposomes on biophysical properties of cancer cell membranes via laurdan two-photon microscopy imaging. Sci. Rep. 2024, 14, 15831. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef]
- Panconi, L.; Euchner, J.; Tashev, S.A.; Makarova, M.; Herten, D.P.; Owen, D.M.; Nieves, D.J. Mapping membrane biophysical nano-environments. Nat. Commun. 2024, 15, 9641. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Duportail, G.; Mely, Y.; Demchenko, A.P. Ultrasensitive two-color fluorescence probes for dipole potential in phospholipid membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 11219–11224. [Google Scholar] [CrossRef]
- Shynkar, V.V.; Klymchenko, A.S.; Duportail, G.; Demchenko, A.P.; Mely, Y. Two-color fluorescent probes for imaging the dipole potential of cell plasma membranes. Biochim. Biophys. Acta 2005, 1712, 128–136. [Google Scholar] [CrossRef]
- Sassano, M.L.; Felipe-Abrio, B.; Agostinis, P. Er-mitochondria contact sites; a multifaceted factory for ca(2+) signaling and lipid transport. Front. Cell Dev. Biol. 2022, 10, 988014. [Google Scholar] [CrossRef]
- Wong, A.M.; Budin, I. Organelle-targeted laurdans measure heterogeneity in subcellular membranes and their responses to saturated lipid stress. ACS Chem. Biol. 2024, 19, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Danylchuk, D.I.; Jouard, P.H.; Klymchenko, A.S. Targeted solvatochromic fluorescent probes for imaging lipid order in organelles under oxidative and mechanical stress. J. Am. Chem. Soc. 2021, 143, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Danylchuk, D.I.; Benaissa, H.; Broch, F.; Vauchelles, R.; Gautier, A.; Klymchenko, A.S. Genetic targeting of solvatochromic dyes for probing nanoscale environments of proteins in organelles. Anal. Chem. 2023, 95, 8512–8521. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.; Martin, L.A.; Karten, B. Mitochondrial cholesterol import. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 90–101. [Google Scholar] [CrossRef]
- Goicoechea, L.; Conde de la Rosa, L.; Torres, S.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023, 61, 102643. [Google Scholar] [CrossRef]
- Brown, M.F. Soft matter in lipid-protein interactions. Annu. Rev. Biophys. 2017, 46, 379–410. [Google Scholar] [CrossRef]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 2004, 1666, 62–87. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Bruning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Pilon, M. Revisiting the membrane-centric view of diabetes. Lipids Health Dis. 2016, 15, 167. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of lipids in alzheimer’s disease pathology and potential therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Sheng, Y.; He, B.; Liu, Z.; Li, W.; Yu, S.; Wang, J.; Zhang, Y.; Chen, J.; et al. The function of sphingolipids in different pathogenesis of alzheimer’s disease: A comprehensive review. Biomed. Pharmacother. 2024, 171, 116071. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.A.; Antommaria, A.H.M.; Kolodny, E.H.; Mistry, P.K. Gaucher disease: Basic and translational science needs for more complete therapy and management. Mol. Genet. Metab. 2021, 132, 59–75. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Kovacs, T.; Cs Szabo, B.; Kothalawala, R.C.; Szekelyhidi, V.; Nagy, P.; Varga, Z.; Panyi, G.; Zakany, F. Inhibition of the Hv1 voltage-gated proton channel compromises the viability of human polarized macrophages in a polarization- and ceramide-dependent manner. Front. Immunol. 2024, 15, 1487578. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, M.; Cs. Szabo, B.; Kurtan, K.; Varga, Z.; Panyi, G.; Nagy, P.; Zakany, F.; Kovacs, T. Look Beyond Plasma Membrane Biophysics: Revealing Considerable Variability of the Dipole Potential Between Plasma and Organelle Membranes of Living Cells. Int. J. Mol. Sci. 2025, 26, 889. https://doi.org/10.3390/ijms26030889
Szabo M, Cs. Szabo B, Kurtan K, Varga Z, Panyi G, Nagy P, Zakany F, Kovacs T. Look Beyond Plasma Membrane Biophysics: Revealing Considerable Variability of the Dipole Potential Between Plasma and Organelle Membranes of Living Cells. International Journal of Molecular Sciences. 2025; 26(3):889. https://doi.org/10.3390/ijms26030889
Chicago/Turabian StyleSzabo, Mate, Bence Cs. Szabo, Kitti Kurtan, Zoltan Varga, Gyorgy Panyi, Peter Nagy, Florina Zakany, and Tamas Kovacs. 2025. "Look Beyond Plasma Membrane Biophysics: Revealing Considerable Variability of the Dipole Potential Between Plasma and Organelle Membranes of Living Cells" International Journal of Molecular Sciences 26, no. 3: 889. https://doi.org/10.3390/ijms26030889
APA StyleSzabo, M., Cs. Szabo, B., Kurtan, K., Varga, Z., Panyi, G., Nagy, P., Zakany, F., & Kovacs, T. (2025). Look Beyond Plasma Membrane Biophysics: Revealing Considerable Variability of the Dipole Potential Between Plasma and Organelle Membranes of Living Cells. International Journal of Molecular Sciences, 26(3), 889. https://doi.org/10.3390/ijms26030889







