Integrins as Key Mediators of Metastasis
Abstract
1. The Metastatic Process: From Pre-Metastatic Niche to Distant Organ Colonization
The Metastatic Cascade
2. ECM in Healthy Tissues and Its Involvement in Metastasis
3. Integrins
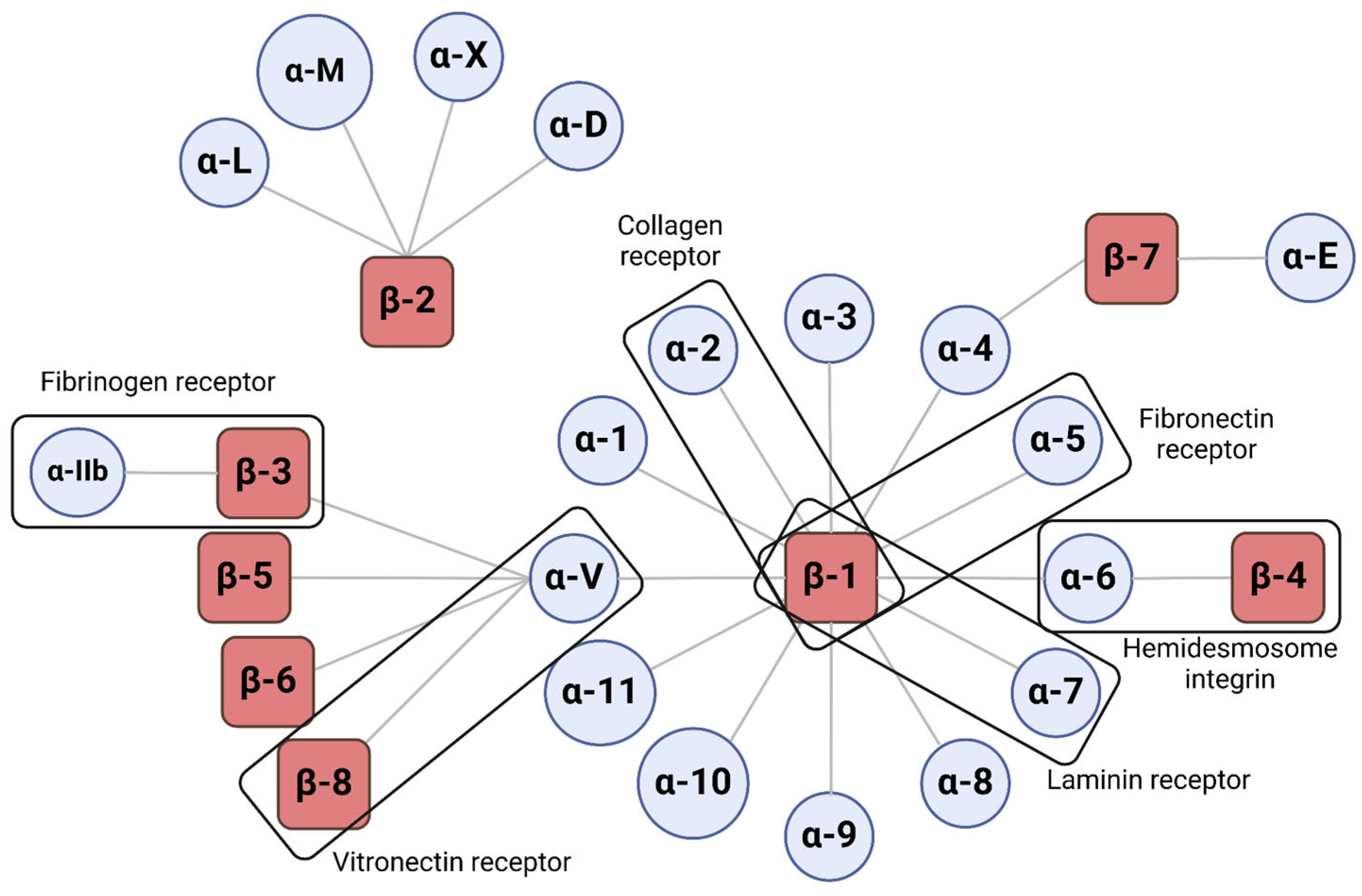
3.1. Structure and Function
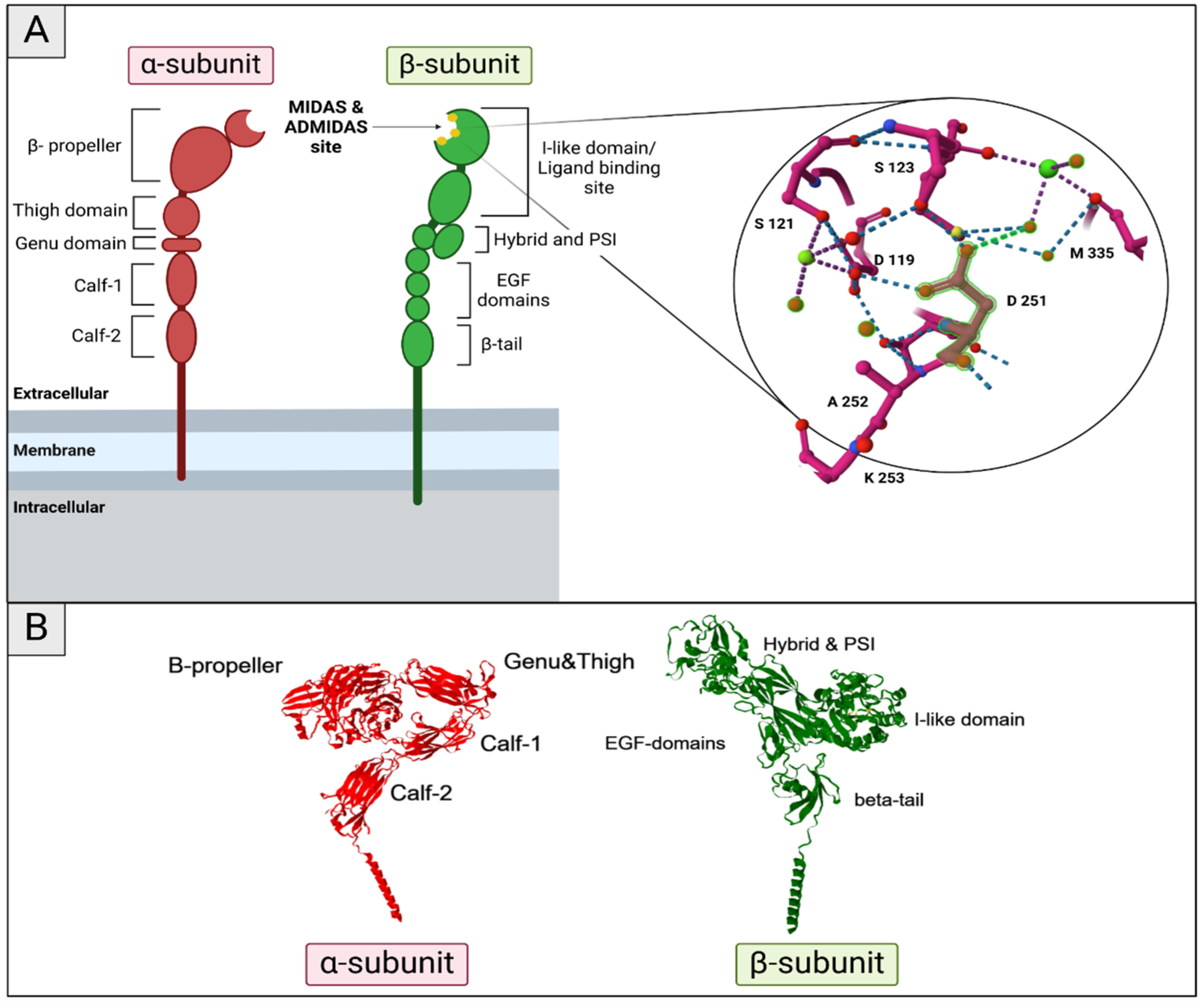
3.2. Integrin Activation
3.3. Intracellular Events Triggered by Integrin Activation
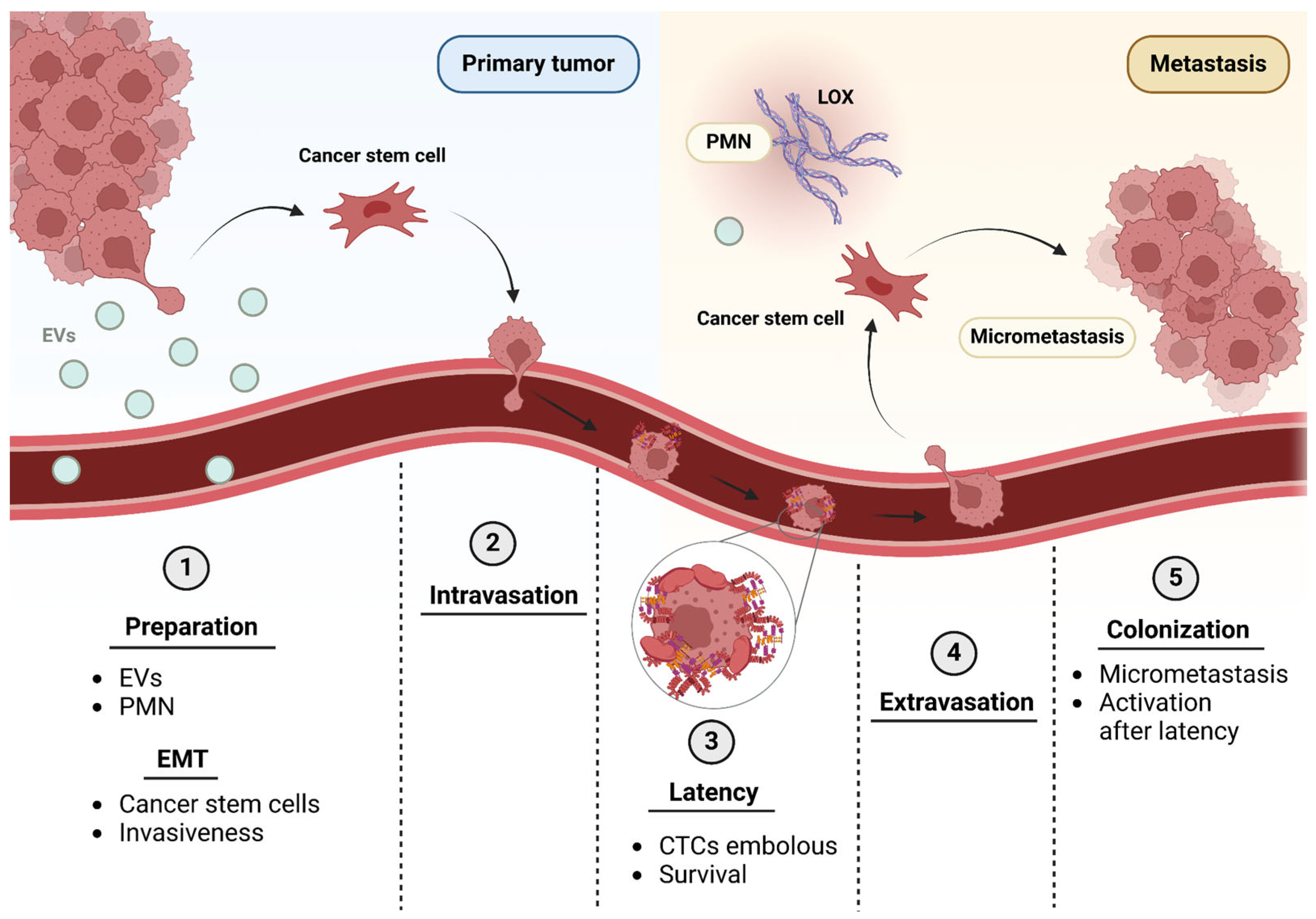
4. Integrins in the Different Steps of the Metastatic Cascade
4.1. Tumor Exosomes and Integrins for Pre-Metastatic Niche Formation
4.2. Matrix Remodeling and Invasive Phenotype Acquisition
4.3. Intravasation, Dissemination and Extravasation
4.4. Awakening from Latency and Colonization of the New Tissue
5. Integrins’ Role in Tumor Vessel Development
6. Therapies Based on Integrin Inhibition and Their Application in Metastasis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Crece la Carga Mundial de Cáncer en Medio de Una Creciente Necesidad de Servicios. Available online: https://www.who.int/es/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 27 November 2024).
- Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 29 November 2024).
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting Metastatic Cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef] [PubMed]
- TRACERx Consortium; Biswas, D.; Birkbak, N.J.; Rosenthal, R.; Hiley, C.T.; Lim, E.L.; Papp, K.; Boeing, S.; Krzystanek, M.; Djureinovic, D.; et al. A Clonal Expression Biomarker Associates with Lung Cancer Mortality. Nat. Med. 2019, 25, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Ganesh, K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021, 11, 971–994. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Ghajar, C.M.; Correia, A.L.; Aguirre-Ghiso, J.A.; Cai, S.; Rescigno, M.; Zhang, P.; Hu, G.; Fendt, S.-M.; Boire, A.; et al. Metastasis. Cancer Cell 2022, 40, 787–791. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic Colonization by Circulating Tumour Cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef]
- Patel, U.; Susman, D.; Allan, A.L. Influence of Extracellular Vesicles on Lung Stromal Cells during Breast Cancer Metastasis. Int. J. Mol. Sci. 2023, 24, 11801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-Mesenchymal Transition Is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Aziz, S.G.-G.; Jaghi, N.Z.Z. EMT, Cancer Stem Cells and Autophagy; The Three Main Axes of Metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar] [CrossRef]
- Zhang, Y.; Donaher, J.L.; Das, S.; Li, X.; Reinhardt, F.; Krall, J.A.; Lambert, A.W.; Thiru, P.; Keys, H.R.; Khan, M.; et al. Genome-Wide CRISPR Screen Identifies PRC2 and KMT2D-COMPASS as Regulators of Distinct EMT Trajectories That Contribute Differentially to Metastasis. Nat. Cell Biol. 2022, 24, 554–564. [Google Scholar] [CrossRef]
- Zeng, H.; Hou, Y.; Zhou, X.; Lang, L.; Luo, H.; Sun, Y.; Wan, X.; Yuan, T.; Wang, R.; Liu, Y.; et al. Cancer-Associated Fibroblasts Facilitate Premetastatic Niche Formation through lncRNA SNHG5-Mediated Angiogenesis and Vascular Permeability in Breast Cancer. Theranostics 2022, 12, 7351–7370. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-Derived Exosomal miR-25-3p Promotes Pre-Metastatic Niche Formation by Inducing Vascular Permeability and Angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Kong, J.; Tian, H.; Zhang, F.; Zhang, Z.; Li, J.; Liu, X.; Li, X.; Liu, J.; Li, X.; Jin, D.; et al. Extracellular Vesicles of Carcinoma-Associated Fibroblasts Creates a Pre-Metastatic Niche in the Lung through Activating Fibroblasts. Mol. Cancer 2019, 18, 175. [Google Scholar] [CrossRef]
- Mo, Y.; Leung, L.L.; Mak, C.S.L.; Wang, X.; Chan, W.-S.; Hui, L.M.N.; Tang, H.W.M.; Siu, M.K.Y.; Sharma, R.; Xu, D.; et al. Tumor-Secreted Exosomal miR-141 Activates Tumor-Stroma Interactions and Controls Premetastatic Niche Formation in Ovarian Cancer Metastasis. Mol. Cancer 2023, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Leite-Gomes, E.; Charatsidou, I.; Zhao, P.; Carrá, G.; Cappellesso, F.; Mourao, L.; De Schepper, M.; Liu, D.; Serneels, J.; et al. Breast Tumors Interfere with Endothelial TRAIL at the Premetastatic Niche to Promote Cancer Cell Seeding. Sci. Adv. 2023, 9, eadd5028. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The Lung Microenvironment: An Important Regulator of Tumour Growth and Metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of Small Cell Lung Cancer Metastasis. EMBO Mol. Med. 2021, 13, e13122. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Jolly, M.K. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a036905. [Google Scholar] [CrossRef]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Kshitiz Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Mak, M.P.; Tong, P.; Diao, L.; Cardnell, R.J.; Gibbons, D.L.; William, W.N.; Skoulidis, F.; Parra, E.R.; Rodriguez-Canales, J.; Wistuba, I.I.; et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin. Cancer Res. 2016, 22, 609–620. [Google Scholar] [CrossRef]
- Lund, A.W. Lymph Node Metastasis: An Immunological Burden. J. Exp. Med. 2023, 220, e20230904. [Google Scholar] [CrossRef]
- Pramanik, D.; Jolly, M.K.; Bhat, R. Matrix Adhesion and Remodeling Diversifies Modes of Cancer Invasion across Spatial Scales. J. Theor. Biol. 2021, 524, 110733. [Google Scholar] [CrossRef]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time Has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.-E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. In The Extracellular Matrix; Vigetti, D., Theocharis, A.D., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1952, pp. 1–20. ISBN 978-1-4939-9132-7. [Google Scholar]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, D.M.; Phillion, A.B.; Kinose, D.; Verleden, S.E.; Vanaudenaerde, B.M.; Verleden, G.M.; Van Raemdonck, D.; Stevenson, C.S.; Hague, C.J.; Han, M.K.; et al. Comprehensive Stereological Assessment of the Human Lung Using Multiresolution Computed Tomography. J. Appl. Physiol. 2020, 128, 1604–1616. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The Extracellular Matrix as a Multitasking Player in Disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen Fibril Assembly and Function. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 130, pp. 107–142. ISBN 978-0-12-809802-8. [Google Scholar]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Paolillo, M.; Schinelli, S. Extracellular Matrix Alterations in Metastatic Processes. Int. J. Mol. Sci. 2019, 20, 4947. [Google Scholar] [CrossRef]
- Haj-Shomaly, J.; Vorontsova, A.; Barenholz-Cohen, T.; Levi-Galibov, O.; Devarasetty, M.; Timaner, M.; Raviv, Z.; Cooper, T.J.; Soker, S.; Hasson, P.; et al. T Cells Promote Metastasis by Regulating Extracellular Matrix Remodeling Following Chemotherapy. Cancer Res. 2022, 82, 278–291. [Google Scholar] [CrossRef]
- Ma, M.; Shi, F.; Zhai, R.; Wang, H.; Li, K.; Xu, C.; Yao, W.; Zhou, F. TGF-β Promote Epithelial-Mesenchymal Transition via NF-κB/NOX4/ROS Signal Pathway in Lung Cancer Cells. Mol. Biol. Rep. 2021, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Li, Q.; Shi, J.; Wei, J.; Li, P.; Chang, C.-H.; Shultz, L.D.; Ren, G. Lung Fibroblasts Facilitate Pre-Metastatic Niche Formation by Remodeling the Local Immune Microenvironment. Immunity 2022, 55, 1483–1500.e9. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.S.; Zhang, H.; Chen, Y.-Y.; Lee, B.; Chew, K.; Moore, D.; Park, C. Increased Β1 Integrin Is Associated with Decreased Survival in Invasive Breast Cancer. Cancer Res. 2007, 67, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Reticker-Flynn, N.E.; Malta, D.F.B.; Winslow, M.M.; Lamar, J.M.; Xu, M.J.; Underhill, G.H.; Hynes, R.O.; Jacks, T.E.; Bhatia, S.N. A Combinatorial Extracellular Matrix Platform Identifies Cell-Extracellular Matrix Interactions That Correlate with Metastasis. Nat. Commun. 2012, 3, 1122. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; Young, J.L.; Van Vliet, K.J.; Kamm, R.D.; Discher, D.; Janmey, P.; Spatz, J.P.; Saif, T. Cell–Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The Roles of Integrins in Cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The Extracellular Matrix in Tumor Progression and Metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Hebert, J.D.; Myers, S.A.; Naba, A.; Abbruzzese, G.; Lamar, J.M.; Carr, S.A.; Hynes, R.O. Proteomic Profiling of the ECM of Xenograft Breast Cancer Metastases in Different Organs Reveals Distinct Metastatic Niches. Cancer Res. 2020, 80, 1475–1485. [Google Scholar] [CrossRef]
- Narciso, M.; Martínez, Á.; Júnior, C.; Díaz-Valdivia, N.; Ulldemolins, A.; Berardi, M.; Neal, K.; Navajas, D.; Farré, R.; Alcaraz, J.; et al. Lung Micrometastases Display ECM Depletion and Softening While Macrometastases Are 30-Fold Stiffer and Enriched in Fibronectin. Cancers 2023, 15, 2404. [Google Scholar] [CrossRef]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal Profiling of the Breast Tumour Microenvironment Reveals Collagen XII as a Driver of Metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef]
- Chang, T.T.; Thakar, D.; Weaver, V.M. Force-Dependent Breaching of the Basement Membrane. Matrix Biol. 2017, 57–58, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chastney, M.R.; Kaivola, J.; Leppänen, V.-M.; Ivaska, J. The Role and Regulation of Integrins in Cell Migration and Invasion. Nat. Rev. Mol. Cell Biol. 2025, 26, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Stanislovas, J.; Kermorgant, S. C-Met-Integrin Cooperation: Mechanisms, Tumorigenic Effects, and Therapeutic Relevance. Front. Cell Dev. Biol. 2022, 10, 994528. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Schumacher, S.; Dedden, D.; Nunez, R.V.; Matoba, K.; Takagi, J.; Biertümpfel, C.; Mizuno, N. Structural Insights into Integrin α5 β1 Opening by Fibronectin Ligand. Sci. Adv. 2021, 7, eabe9716. [Google Scholar] [CrossRef]
- Li, J.; Jo, M.H.; Yan, J.; Hall, T.; Lee, J.; López-Sánchez, U.; Yan, S.; Ha, T.; Springer, T.A. Ligand Binding Initiates Single-Molecule Integrin Conformational Activation. Cell 2024, 187, 2990–3005.e17. [Google Scholar] [CrossRef]
- Anderson, J.M.; Li, J.; Springer, T.A. Regulation of Integrin α5β1 Conformational States and Intrinsic Affinities by Metal Ions and the ADMIDAS. Mol. Biol. Cell 2022, 33, ar56. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin Ligands at a Glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D Cell Migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef]
- Adair, B.D.; Xiong, J.-P.; Yeager, M.; Arnaout, M.A. Cryo-EM Structures of Full-Length Integrin αIIbβ3 in Native Lipids. Nat. Commun. 2023, 14, 4168. [Google Scholar] [CrossRef] [PubMed]
- Cormier, A.; Campbell, M.G.; Ito, S.; Wu, S.; Lou, J.; Marks, J.; Baron, J.L.; Nishimura, S.L.; Cheng, Y. Cryo-EM Structure of the αvβ8 Integrin Reveals a Mechanism for Stabilizing Integrin Extension. Nat. Struct. Mol. Biol. 2018, 25, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, O.; Velyvis, A.; Velyviene, A.; Hu, B.; Haas, T.A.; Plow, E.F.; Qin, J. A Structural Mechanism of Integrin αIIbβ3 “Inside-Out” Activation as Regulated by Its Cytoplasmic Face. Cell 2002, 110, 587–597. [Google Scholar] [CrossRef]
- Haydari, Z.; Shams, H.; Jahed, Z.; Mofrad, M.R.K. Kindlin Assists Talin to Promote Integrin Activation. Biophys. J. 2020, 118, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, M.J.; Van De Water, B. Focal Adhesion Kinase: A Potential Target in Cancer Therapy. Biochem. Pharmacol. 2007, 73, 597–609. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.-L. Focal Adhesion Kinase and Its Signaling Pathways in Cell Migration and Angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J. The Regulation of Integrin Function by Divalent Cations. Cell Adhes. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef]
- Tiwari, S.; Askari, J.A.; Humphries, M.J.; Bulleid, N.J. Divalent Cations Regulate the Folding and Activation Status of Integrins during Their Intracellular Trafficking. J. Cell Sci. 2011, 124, 1672–1680. [Google Scholar] [CrossRef]
- Danen, E.H.J.; Sonneveld, P.; Brakebusch, C.; Fässler, R.; Sonnenberg, A. The Fibronectin-Binding Integrins α5β1 and αvβ3 Differentially Modulate RhoA–GTP Loading, Organization of Cell Matrix Adhesions, and Fibronectin Fibrillogenesis. J. Cell Biol. 2002, 159, 1071–1086. [Google Scholar] [CrossRef]
- Rottner, K.; Hall, A.; Small, J.V. Interplay between Rac and Rho in the Control of Substrate Contact Dynamics. Curr. Biol. 1999, 9, 640–648. [Google Scholar] [CrossRef]
- Bouvard, D.; Pouwels, J.; De Franceschi, N.; Ivaska, J. Integrin Inactivators: Balancing Cellular Functions In Vitro and In Vivo. Nat. Rev. Mol. Cell Biol. 2013, 14, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylänne, J.; Calderwood, D.A. The Molecular Basis of Filamin Binding to Integrins and Competition with Talin. Mol. Cell 2006, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, D.; Vignoud, L.; Dupé-Manet, S.; Abed, N.; Fournier, H.-N.; Vincent-Monegat, C.; Retta, S.F.; Fässler, R.; Block, M.R. Disruption of Focal Adhesions by Integrin Cytoplasmic Domain-Associated Protein-1α. J. Biol. Chem. 2003, 278, 6567–6574. [Google Scholar] [CrossRef]
- Rantala, J.K.; Pouwels, J.; Pellinen, T.; Veltel, S.; Laasola, P.; Mattila, E.; Potter, C.S.; Duffy, T.; Sundberg, J.P.; Kallioniemi, O.; et al. SHARPIN Is an Endogenous Inhibitor of Β1-Integrin Activation. Nat. Cell Biol. 2011, 13, 1315–1324. [Google Scholar] [CrossRef]
- Gao, J.; Bao, Y.; Ge, S.; Sun, P.; Sun, J.; Liu, J.; Chen, F.; Han, L.; Cao, Z.; Qin, J.; et al. Sharpin Suppresses Β1-Integrin Activation by Complexing with the Β1 Tail and Kindlin-1. Cell Commun. Signal. 2019, 17, 101. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins, Oncogenes, and Anchorage Independence. J. Cell Biol. 1997, 139, 575–578. [Google Scholar] [CrossRef]
- McNamee, H.P.; Ingber, D.E.; Schwartz, M.A. Adhesion to Fibronectin Stimulates Inositol Lipid Synthesis and Enhances PDGF-Induced Inositol Lipid Breakdown. J. Cell Biol. 1993, 121, 673–678. [Google Scholar] [CrossRef]
- Shin, S.; Wolgamott, L.; Yoon, S.-O. Integrin Trafficking and Tumor Progression. Int. J. Cell Biol. 2012, 2012, 516789. [Google Scholar] [CrossRef]
- Caswell, P.T.; Vadrevu, S.; Norman, J.C. Integrins: Masters and Slaves of Endocytic Transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 843–853. [Google Scholar] [CrossRef]
- Chia, W.J.; Tang, B.L. Emerging Roles for Rab Family GTPases in Human Cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2009, 1795, 110–116. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.D.; Donaldson, J.G. Pathways and Mechanisms of Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lobert, V.H.; Brech, A.; Pedersen, N.M.; Wesche, J.; Oppelt, A.; Malerød, L.; Stenmark, H. Ubiquitination of α5β1 Integrin Controls Fibroblast Migration through Lysosomal Degradation of Fibronectin-Integrin Complexes. Dev. Cell 2010, 19, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Lamar, J.M.; Li, R.; Hynes, R.O.; Kamm, R.D. Elucidation of the Roles of Tumor Integrin Β1 in the Extravasation Stage of the Metastasis Cascade. Cancer Res. 2016, 76, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Aguado, B.A.; Bushnell, G.G.; Rao, S.S.; Jeruss, J.S.; Shea, L.D. Engineering the Pre-Metastatic Niche. Nat. Biomed. Eng. 2017, 1, 0077. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Sun, L.; Wang, S.-M.; Zheng, X.-Y.; Xu, R. Exosomal Integrins in Tumor Progression, Treatment and Clinical Prediction (Review). Int. J. Oncol. 2024, 65, 118. [Google Scholar] [CrossRef]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-Derived Exosomal miR-1247-3p Induces Cancer-Associated Fibroblast Activation to Foster Lung Metastasis of Liver Cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-Mediated Mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Schmidt, S.; Friedl, P. Interstitial Cell Migration: Integrin-Dependent and Alternative Adhesion Mechanisms. Cell Tissue Res. 2010, 339, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual Determinants of TGFβ Action in Development, Immunity and Cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Arthur, H.M. Extracellular Control of TGFβ Signalling in Vascular Development and Disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef]
- Cuypers, A.; Truong, A.-C.K.; Becker, L.M.; Saavedra-García, P.; Carmeliet, P. Tumor Vessel Co-Option: The Past & the Future. Front. Oncol. 2022, 12, 965277. [Google Scholar] [CrossRef]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Β1 Integrin Inhibitory Antibody Induces Apoptosis of Breast Cancer Cells, Inhibits Growth, and Distinguishes Malignant from Normal Phenotype in Three Dimensional Cultures and In Vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef]
- Nam, J.-M.; Onodera, Y.; Bissell, M.J.; Park, C.C. Breast Cancer Cells in Three-Dimensional Culture Display an Enhanced Radioresponse after Coordinate Targeting of Integrin α5β1 and Fibronectin. Cancer Res. 2010, 70, 5238–5248. [Google Scholar] [CrossRef]
- Luo, J.; Yao, J.-F.; Deng, X.-F.; Zheng, X.-D.; Jia, M.; Wang, Y.-Q.; Huang, Y.; Zhu, J.-H. 14, 15-EET Induces Breast Cancer Cell EMT and Cisplatin Resistance by Up-Regulating Integrin αvβ3 and Activating FAK/PI3K/AKT Signaling. J. Exp. Clin. Cancer Res. 2018, 37, 23. [Google Scholar] [CrossRef]
- Mamuya, F.A.; Duncan, M.K. aV Integrins and TGF-β-induced EMT: A Circle of Regulation. J. Cell. Mol. Med. 2012, 16, 445–455. [Google Scholar] [CrossRef]
- Tod, J.; Hanley, C.J.; Morgan, M.R.; Rucka, M.; Mellows, T.; Lopez, M.; Kiely, P.; Moutasim, K.A.; Frampton, S.J.; Sabnis, D.; et al. Pro-migratory and TGF-β-activating Functions of αvβ6 Integrin in Pancreatic Cancer Are Differentially Regulated via an Eps8-dependent GTPase Switch. J. Pathol. 2017, 243, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pylayeva, Y.; Pepe, A.; Yoshioka, T.; Muller, W.J.; Inghirami, G.; Giancotti, F.G. Β4 Integrin Amplifies ErbB2 Signaling to Promote Mammary Tumorigenesis. Cell 2006, 126, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Wadhwa, H.; Sudhir, S.; Chang, A.C.-C.; Jain, S.; Chandra, A.; Nguyen, A.T.; Spatz, J.M.; Pappu, A.; Shah, S.S.; et al. Role of C-Met/Β1 Integrin Complex in the Metastatic Cascade in Breast Cancer. JCI Insight 2021, 6, e138928. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Aguado, I.; Marcos-Zazo, L.; Carrancio-Salán, P.; Guerra-Paes, E.; Sánchez-Juanes, F.; Muñoz-Félix, J.M. The Inhibition of Vessel Co-Option as an Emerging Strategy for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 921. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Decaris, M.L.; Schaub, J.R.; Chen, C.; Cha, J.; Lee, G.G.; Rexhepaj, M.; Ho, S.S.; Rao, V.; Marlow, M.M.; Kotak, P.; et al. Dual Inhibition of αvβ6 and αvβ1 Reduces Fibrogenesis in Lung Tissue Explants from Patients with IPF. Respir. Res. 2021, 22, 265. [Google Scholar] [CrossRef]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the Unexplored Extracellular Matrix in Cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef]
- Hu, P.; Miller, A.E.; Yeh, C.-R.; Bingham, G.C.; Civelek, M.; Barker, T.H. SEMA7a Primes Integrin A5β1 Engagement Instructing Fibroblast Mechanotransduction, Phenotype and Transcriptional Programming. Matrix Biol. 2023, 121, 179–193. [Google Scholar] [CrossRef]
- Legan, S.K.; Lee, D.D.; Schwarz, M.A. A5β1 Integrin Mediates Pulmonary Epithelial Cyst Formation. Dev. Dyn. 2017, 246, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Cherwinski, H.; Venetsanakos, E.; Bhat, A.; Gysin, S.; Humbert, M.; Bray, P.F.; Saylor, V.L.; McMahon, M. Induction of Β3-Integrin Gene Expression by Sustained Activation of the Ras-Regulated Raf–MEK–Extracellular Signal-Regulated Kinase Signaling Pathway. Mol. Cell. Biol. 2001, 21, 3192–3205. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Caswell, P.T.; Doyle, B.; Iwanicki, M.P.; Tan, E.H.; Karim, S.; Lukashchuk, N.; Gillespie, D.A.; Ludwig, R.L.; Gosselin, P.; et al. Mutant P53 Drives Invasion by Promoting Integrin Recycling. Cell 2009, 139, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.-H.; Clifford, J.L.; Chung, J. Crosstalk between Integrin and Receptor Tyrosine Kinase Signaling in Breast Carcinoma Progression. BMB Rep. 2010, 43, 311–318. [Google Scholar] [CrossRef]
- Garmy-Susini, B.; Avraamides, C.J.; Desgrosellier, J.S.; Schmid, M.C.; Foubert, P.; Ellies, L.G.; Lowy, A.M.; Blair, S.L.; Vandenberg, S.R.; Datnow, B.; et al. PI3Kα Activates Integrin A4β1 to Establish a Metastatic Niche in Lymph Nodes. Proc. Natl. Acad. Sci. USA 2013, 110, 9042–9047. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin Secreted by Glioblastoma Stem Cells Recruits M2 Tumour-Associated Macrophages and Promotes Malignant Growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef]
- Yuan, Y.; Engler, A.J.; Raredon, M.S.; Le, A.; Baevova, P.; Yoder, M.C.; Niklason, L.E. Epac Agonist Improves Barrier Function in iPSC-Derived Endothelial Colony Forming Cells for Whole Organ Tissue Engineering. Biomaterials 2019, 200, 25–34. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Rohlenova, K.; Goveia, J.; García-Caballero, M.; Subramanian, A.; Kalucka, J.; Treps, L.; Falkenberg, K.D.; De Rooij, L.P.M.H.; Zheng, Y.; Lin, L.; et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab. 2020, 31, 862–877.e14. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Rüdiger, D.; Zahler, S. Mechanical Aspects of Angiogenesis. Cancers 2021, 13, 4987. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C.; Griffioen, A.W. Pathological Angiogenesis: Mechanisms and Therapeutic Strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Hood, J.L. Melanoma Exosome Induction of Endothelial Cell GM-CSF in Pre-Metastatic Lymph Nodes May Result in Different M1 and M2 Macrophage Mediated Angiogenic Processes. Med. Hypotheses 2016, 94, 118–122. [Google Scholar] [CrossRef]
- Hodivala-Dilke, K. αvβ3 Integrin and Angiogenesis: A Moody Integrin in a Changing Environment. Curr. Opin. Cell Biol. 2008, 20, 514–519. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Drake, C.J.; Cheresh, D.A.; Little, C.D. An Antagonist of Integrin αvβ3 Prevents Maturation of Blood Vessels during Embryonic Neovascularization. J. Cell Sci. 1995, 108 Pt 7, 2655–2661. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Cheresh, D.A. The Role of Alphav Integrins during Angiogenesis: Insights into Potential Mechanisms of Action and Clinical Development. J. Clin. Investig. 1999, 103, 1227–1230. [Google Scholar] [CrossRef]
- Demircioglu, F.; Hodivala-Dilke, K. αvβ3 Integrin and Tumour Blood Vessels-Learning from the Past to Shape the Future. Curr. Opin. Cell Biol. 2016, 42, 121–127. [Google Scholar] [CrossRef]
- Stupp, R.; Ruegg, C. Integrin Inhibitors Reaching the Clinic. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Hodivala-Dilke, K.M.; McHugh, K.P.; Tsakiris, D.A.; Rayburn, H.; Crowley, D.; Ullman-Culleré, M.; Ross, F.P.; Coller, B.S.; Teitelbaum, S.; Hynes, R.O. β3-Integrin-Deficient Mice Are a Model for Glanzmann Thrombasthenia Showing Placental Defects and Reduced Survival. J. Clin. Investig. 1999, 103, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Griffiths, M.; Wu, J.; Farese, R.V.; Sheppard, D. Normal Development, Wound Healing, and Adenovirus Susceptibility in Β5-Deficient Mice. Mol. Cell. Biol. 2000, 20, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.E.; Wyder, L.; Lively, J.C.; Taverna, D.; Robinson, S.D.; Huang, X.; Sheppard, D.; Hynes, R.O.; Hodivala-Dilke, K.M. Enhanced Pathological Angiogenesis in Mice Lacking β3 Integrin or β3 and β5 Integrins. Nat. Med. 2002, 8, 27–34. [Google Scholar] [CrossRef]
- Kim, S.; Bell, K.; Mousa, S.A.; Varner, J.A. Regulation of Angiogenesis in Vivo by Ligation of Integrin α5β1 with the Central Cell-Binding Domain of Fibronectin. Am. J. Pathol. 2000, 156, 1345–1362. [Google Scholar] [CrossRef]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular Endothelial Growth Factor (VEGF) and Its Receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Plate, K.H.; Breier, G.; Millauer, B.; Ullrich, A.; Risau, W. Up-Regulation of Vascular Endothelial Growth Factor and Its Cognate Receptors in a Rat Glioma Model of Tumor Angiogenesis. Cancer Res. 1993, 53, 5822–5827. [Google Scholar]
- Kerbel, R.; Folkman, J. Clinical Translation of Angiogenesis Inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Reynolds, L.E.; Nagel, T.E.; Lively, J.C.; Robinson, S.D.; Hicklin, D.J.; Bodary, S.C.; Hodivala-Dilke, K.M. Elevated Flk1 (Vascular Endothelial Growth Factor Receptor 2) Signaling Mediates Enhanced Angiogenesis in β3-Integrin-Deficient Mice. Cancer Res. 2004, 64, 8643–8650. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Mammoto, T.; Jiang, A.; Jiang, E.; Panigrahy, D.; Kieran, M.W.; Mammoto, A. Role of Collagen Matrix in Tumor Angiogenesis and Glioblastoma Multiforme Progression. Am. J. Pathol. 2013, 183, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Chen, H.; Lin, X.; Yang, L.; Ge, Z. Collagen Triple Helix Repeat Containing 1 Promotes Tumor Angiogenesis in Gastrointestinal Stromal Tumors. Oncol. Lett. 2017, 14, 7499–7505. [Google Scholar] [CrossRef][Green Version]
- Zhou, F.; Sun, J.; Ye, L.; Jiang, T.; Li, W.; Su, C.; Ren, S.; Wu, F.; Zhou, C.; Gao, G. Fibronectin Promotes Tumor Angiogenesis and Progression of Non-Small-Cell Lung Cancer by Elevating WISP3 Expression via FAK/MAPK/HIF-1α Axis and Activating Wnt Signaling Pathway. Exp. Hematol. Oncol. 2023, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, W.S.; DeLay, M.; Jahangiri, A.; Park, C.C.; Aghi, M.K. Β1 Integrin Targeting Potentiates Antiangiogenic Therapy and Inhibits the Growth of Bevacizumab-Resistant Glioblastoma. Cancer Res. 2013, 73, 3145–3154. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Yin, M.; Bar-Zion, A.; Lee, C.R.; Butz, H.; Man, S.; Daley, F.; Vermeulen, P.B.; Yousef, G.M.; Foster, F.S.; et al. Co-Option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2016, 108, djw030. [Google Scholar] [CrossRef]
- Yao, H.; Price, T.T.; Cantelli, G.; Ngo, B.; Warner, M.J.; Olivere, L.; Ridge, S.M.; Jablonski, E.M.; Therrien, J.; Tannheimer, S.; et al. Leukaemia Hijacks a Neural Mechanism to Invade the Central Nervous System. Nature 2018, 560, 55–60. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.-F.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins Promote Cancer Cell Survival and Vascular Co-Option in Brain Metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef]
- Lin, T.-C.; Yang, C.-H.; Cheng, L.-H.; Chang, W.-T.; Lin, Y.-R.; Cheng, H.-C. Fibronectin in Cancer: Friend or Foe. Cells 2019, 9, 27. [Google Scholar] [CrossRef]
- Oudart, J.-B.; Villemin, M.; Brassart, B.; Sellier, C.; Terryn, C.; Dupont-Deshorgue, A.; Monboisse, J.C.; Maquart, F.-X.; Ramont, L.; Brassart-Pasco, S. F4, a Collagen XIX-Derived Peptide, Inhibits Tumor Angiogenesis through αvβ3 and α5β1 Integrin Interaction. Cell Adhes. Migr. 2021, 15, 215–223. [Google Scholar] [CrossRef]
- Veschini, L.; Crippa, L.; Dondossola, E.; Doglioni, C.; Corti, A.; Ferrero, E. The Vasostatin-1 Fragment of Chromogranin A Preserves a Quiescent Phenotype in Hypoxia-driven Endothelial Cells and Regulates Tumor Neovascularization. FASEB J. 2011, 25, 3906–3914. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumor Microenvironment Enriches the Stemness Features: The Architectural Event of Therapy Resistance and Metastasis. Mol. Cancer 2022, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.S.; Gökmen-Polar, Y. Targeting the Tumor-Tumor Microenvironment Crosstalk. Expert Opin. Ther. Targets 2023, 27, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Barkan, D.; Chambers, A.F. Β1-Integrin: A Potential Therapeutic Target in the Battle against Cancer Recurrence. Clin. Cancer Res. 2011, 17, 7219–7223. [Google Scholar] [CrossRef] [PubMed]
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parry, G.C.; Parikh, A.A.; McCarty, M.F.; Bucana, C.D.; Mazar, A.P.; Ellis, L.M. Inhibition of Integrin α5 β1 Function with a Small Peptide (ATN-161) plus Continuous 5-FU Infusion Reduces Colorectal Liver Metastases and Improves Survival in Mice. Int. J. Cancer 2003, 104, 496–503. [Google Scholar] [CrossRef]
- Park, C.C.; Zhang, H.J.; Yao, E.S.; Park, C.J.; Bissell, M.J. Β1 Integrin Inhibition Dramatically Enhances Radiotherapy Efficacy in Human Breast Cancer Xenografts. Cancer Res. 2008, 68, 4398–4405. [Google Scholar] [CrossRef]
- Élez, E.; Kocáková, I.; Höhler, T.; Martens, U.M.; Bokemeyer, C.; Van Cutsem, E.; Melichar, B.; Smakal, M.; Csőszi, T.; Topuzov, E.; et al. Abituzumab Combined with Cetuximab plus Irinotecan versus Cetuximab plus Irinotecan Alone for Patients with KRAS Wild-Type Metastatic Colorectal Cancer: The Randomised Phase I/II POSEIDON Trial. Ann. Oncol. 2015, 26, 132–140. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Hart, I.R.; Watson, A.R.; Welti, J.C.; Silva, R.G.; Robinson, S.D.; Da Violante, G.; Gourlaouen, M.; Salih, M.; Jones, M.C.; et al. Stimulation of Tumor Growth and Angiogenesis by Low Concentrations of RGD-Mimetic Integrin Inhibitors. Nat. Med. 2009, 15, 392–400. [Google Scholar] [CrossRef]



| Clinical Trial Code | Molecule | Target | Pathology | Status |
|---|---|---|---|---|
| NCT06389123 | [68Ga] Ga DOTA-5G [177Lu] Lu DOTA-ABM-5G | αvβ6 integrin | Metastatic Cancer | Recruiting |
| NCT00066196 | MEDI-522 | αvβ3 integrin | Metastatic Melanoma | Completed |
| NCT06228482 | [68Ga] Ga DOTA-5G | αvβ6 integrin | Metastatic Non-Small-Cell Lung Cancer (NSCLC) | Recruiting |
| NCT00100685 | Volociximab | α5β1 integrin | Metastatic Renal Cell Carcinoma (RCC) | Terminated |
| NCT00099970 | Volociximab | α5β1 integrin | Metastatic Melanoma Not Previously Treated With Chemotherapy | Completed |
| NCT01664273 | Plasmid AMEP | α5β1 and αvβ3 integrins | Disseminated Cancer | Terminated |
| NCT03164486 | 18F-αvβ6-Binding-Peptide | αvβ6 integrin | Breast, Colorectal, Lung or Pancreatic | Active, not recruiting |
| NCT03688230 | Abituzumab | ανβ6 integrin | Metastatic Colorectal Cancer | Withdrawn |
| NCT06435741 | 99mTc-3PRGD2 | αvβ3 integrin | Advanced Gastric Cancer | Not yet recruiting |
| NCT00915278 | PF-04605412 | α5β1 integrin | Solid tumors | Terminated |
| NCT01008475 | EMD 525797 | α integrins | Subjects With K-ras Wild-Type Metastatic Colorectal | Completed |
| NCT05101655 | NA | NA | Lung Metastasis of Osteosarcoma | Completed |
| NCT04712721 | 68Ga-FF58 | αvβ3 and αvβ5 integrin | Solid tumors | Terminated |
| NCT01806675 | 18F-FPPRGD2 | Imaging of αvβ3 integrins | Glioblastoma Multiforme (GBM), Gynecological Cancers and Renal Cell Carcinoma (RCC) | Completed |
| NCT06460298 | ProAgio (Anti-vβ3 Integrin Cytotoxin) | αvβ3 integrin | Metastatic Triple-Negative Breast Cancer | Recruiting |
| NCT00705016 | Cilengitide | αvβ3 integrin | Squamous Cell Carcinoma of the Head and Neck (SCCHN) | Terminated |
| NCT04665947 | [68Ga] Ga DOTA-5G and [177Lu] Lu DOTA-ABM-5G | αvβ6 integrin | Pancreatic cancer | Recruiting |
| NCT00401570 | Volociximab | α5β1 integrin | Metastatic Pancreatic Cancer | Completed |
| NCT00537381 | CNTO 95 | αv integrins | Metastatic Hormone Refractory Prostate Cancer | Completed |
| NCT01961583 | [18F] Fluciclatide | αvβ3 and αvβ5 integrins | Metastatic Renal Cell Carcinoma | Terminated |
| NCT00072930 | MEDI-522 | αvβ3 integrin | Metastatic Androgen- Independent Prostate Cancer | Completed |
| NCT04152018 | PF-06940434 | αvβ8 integrin antagonist | Advanced or Metastatic Solid Tumors | Active, not recruiting |
| NCT00684996 | MEDI-522 | αvβ3 integrin | Unresectable or Metastatic Kidney Cancer | Terminated |
| NCT01327313 | EMD525797 | αv integrins | Solid tumors | Completed |
| NCT01849744 | VS-4718 | FAK | Metastatic Non-Hematologic Malignancies | Terminated |
| NCT00563290 | Dasatinib | Src | Metastatic Squamous Cell Skin | Completed |
| NCT00835679 | Dasatinib | Src | Colorectal Cancer Patients With Resectable Liver Metastases | Terminated |
| NCT00546104 | Dasatinib | Src | Advanced Breast Cancer | Completed |
| NCT01335269 | BI 853520 | FAK | Metastatic Non-hematologic Malignancies | Completed |
| NCT00597038 | Dasatinib | Src | Melanoma | Completed |
| NCT01306942 | Dasatinib | Src | Metastatic breast cancer | Completed |
| NCT04161391 | TPX-0046 | Src | Solid tumors | Completed |
| NCT00528645 | AZD0530 | Src | Small-cell lung cancer | Completed |
| NCT03993873 | TPX-0022 | Src | Advanced NSCLC, Gastric Cancer or Solid Tumors | Active, not recruiting |
| NCT01015222 | Dasatinib | Src | Advanced cancers | Completed |
| NCT00504153 | Dasatinib | Src | Metastatic Colorectal Cancer | Completed |
| NCT00277303 | XL999 | Src | Metastatic Colorectal Cancer | Terminated |
| NCT00277316 | XL999 | Src | Metastatic Renal Cell Carcinoma | Terminated |
| NCT01505413 | Erlotinib | Src | Advanced Pancreatic Cancer | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Calle, D.; Torre-Cea, I.; Marcos-Zazo, L.; Carrera-Aguado, I.; Guerra-Paes, E.; Berlana-Galán, P.; Muñoz-Félix, J.M.; Sánchez-Juanes, F. Integrins as Key Mediators of Metastasis. Int. J. Mol. Sci. 2025, 26, 904. https://doi.org/10.3390/ijms26030904
Cáceres-Calle D, Torre-Cea I, Marcos-Zazo L, Carrera-Aguado I, Guerra-Paes E, Berlana-Galán P, Muñoz-Félix JM, Sánchez-Juanes F. Integrins as Key Mediators of Metastasis. International Journal of Molecular Sciences. 2025; 26(3):904. https://doi.org/10.3390/ijms26030904
Chicago/Turabian StyleCáceres-Calle, Daniel, Irene Torre-Cea, Laura Marcos-Zazo, Iván Carrera-Aguado, Elena Guerra-Paes, Patricia Berlana-Galán, José M. Muñoz-Félix, and Fernando Sánchez-Juanes. 2025. "Integrins as Key Mediators of Metastasis" International Journal of Molecular Sciences 26, no. 3: 904. https://doi.org/10.3390/ijms26030904
APA StyleCáceres-Calle, D., Torre-Cea, I., Marcos-Zazo, L., Carrera-Aguado, I., Guerra-Paes, E., Berlana-Galán, P., Muñoz-Félix, J. M., & Sánchez-Juanes, F. (2025). Integrins as Key Mediators of Metastasis. International Journal of Molecular Sciences, 26(3), 904. https://doi.org/10.3390/ijms26030904






