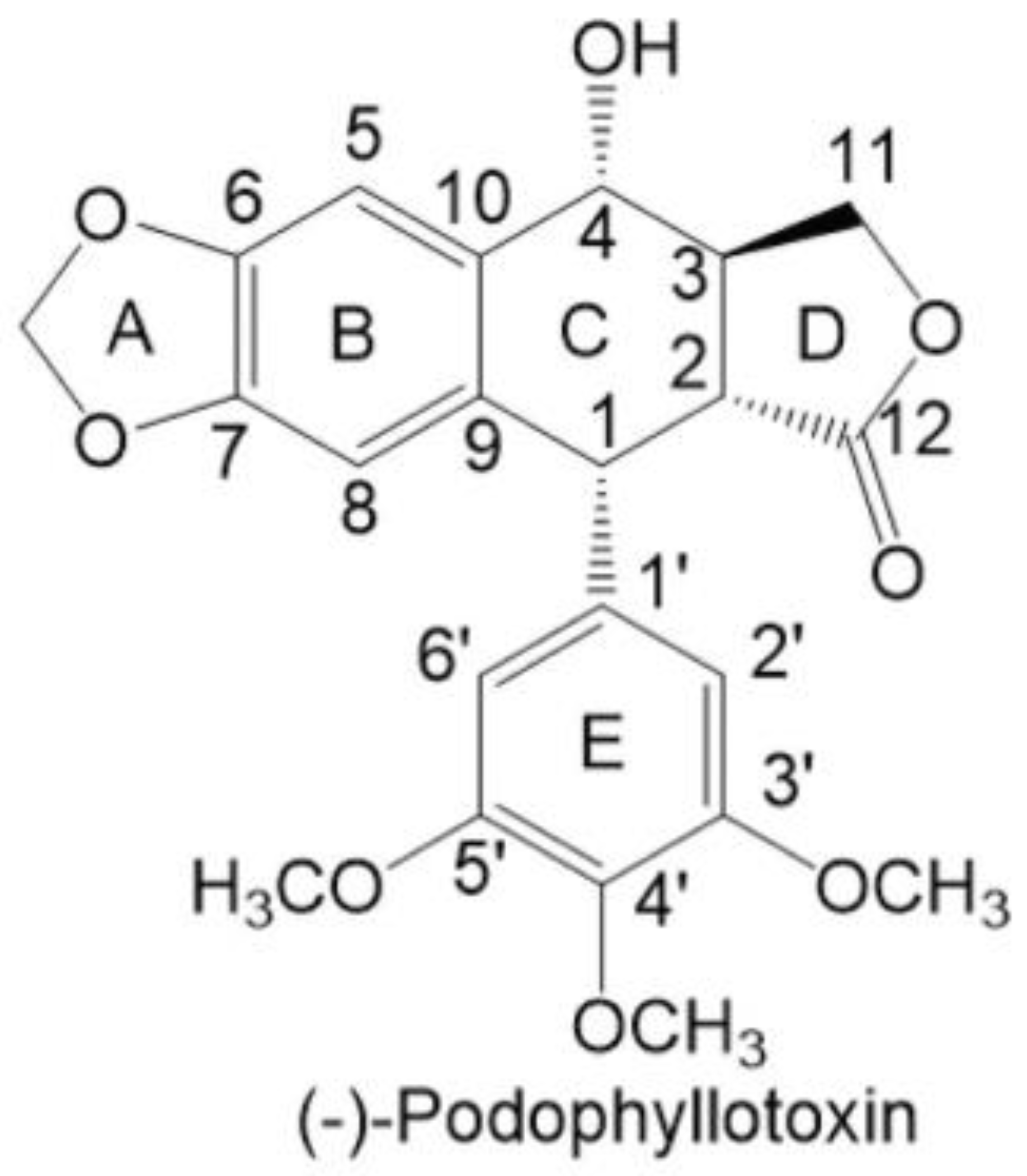
The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review
Abstract
:1. Introduction
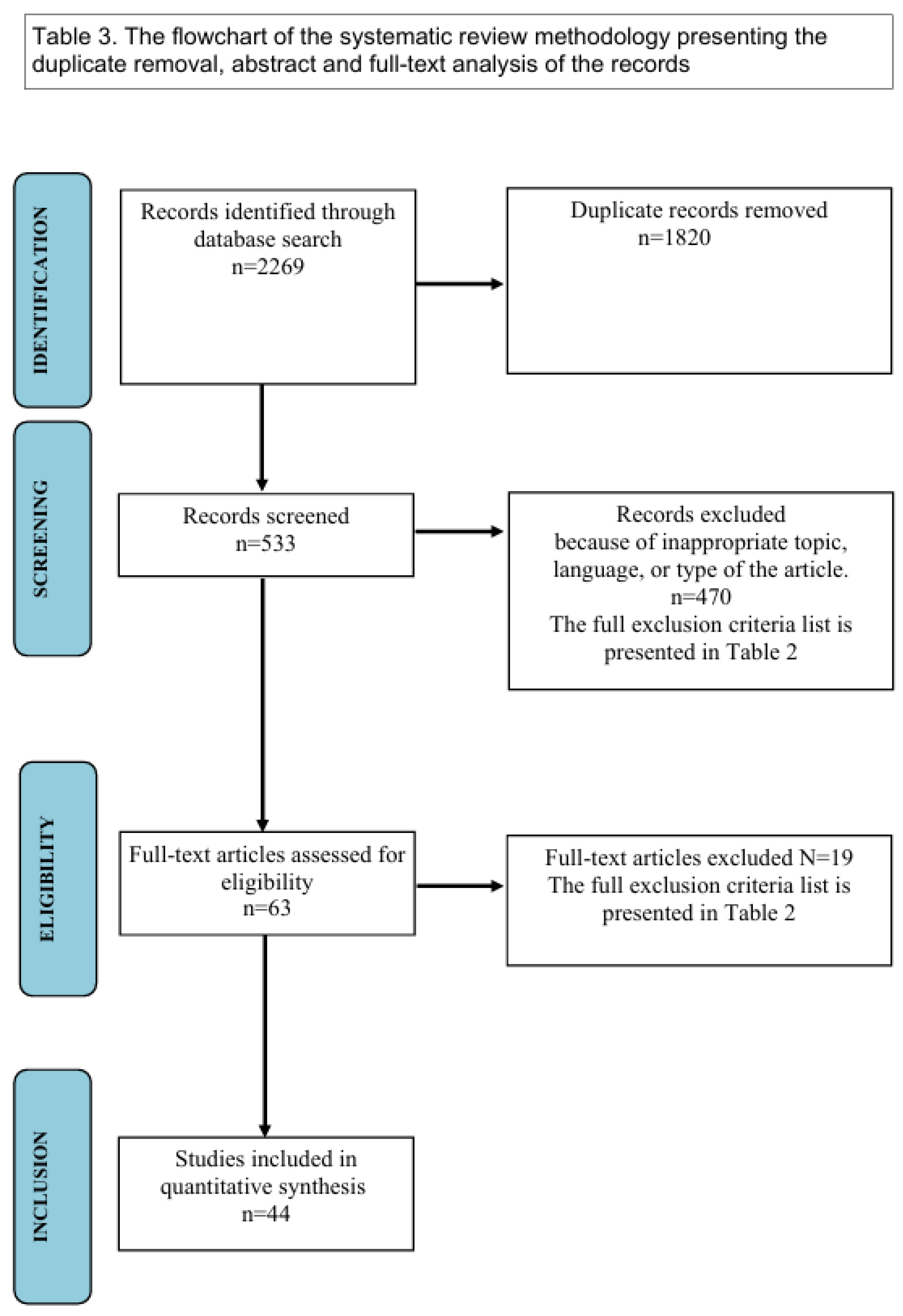
2. Methods
3. Results and Discussion
3.1. Results
3.1.1. Toxic Effects of PPT and Its Derivatives
Toxicity of PPT to Non-Cancerous Cells
Comparative Analysis of PPT Derivatives: KL3
Impact on Organelles
Mitotic Spindle Inhibition
Embryology
3.1.2. Therapeutic Effects of PPT and Its Derivatives
Analgesics and Anti-Inflammatory Properties
Radiation Protection
3.1.3. Graphical Summary
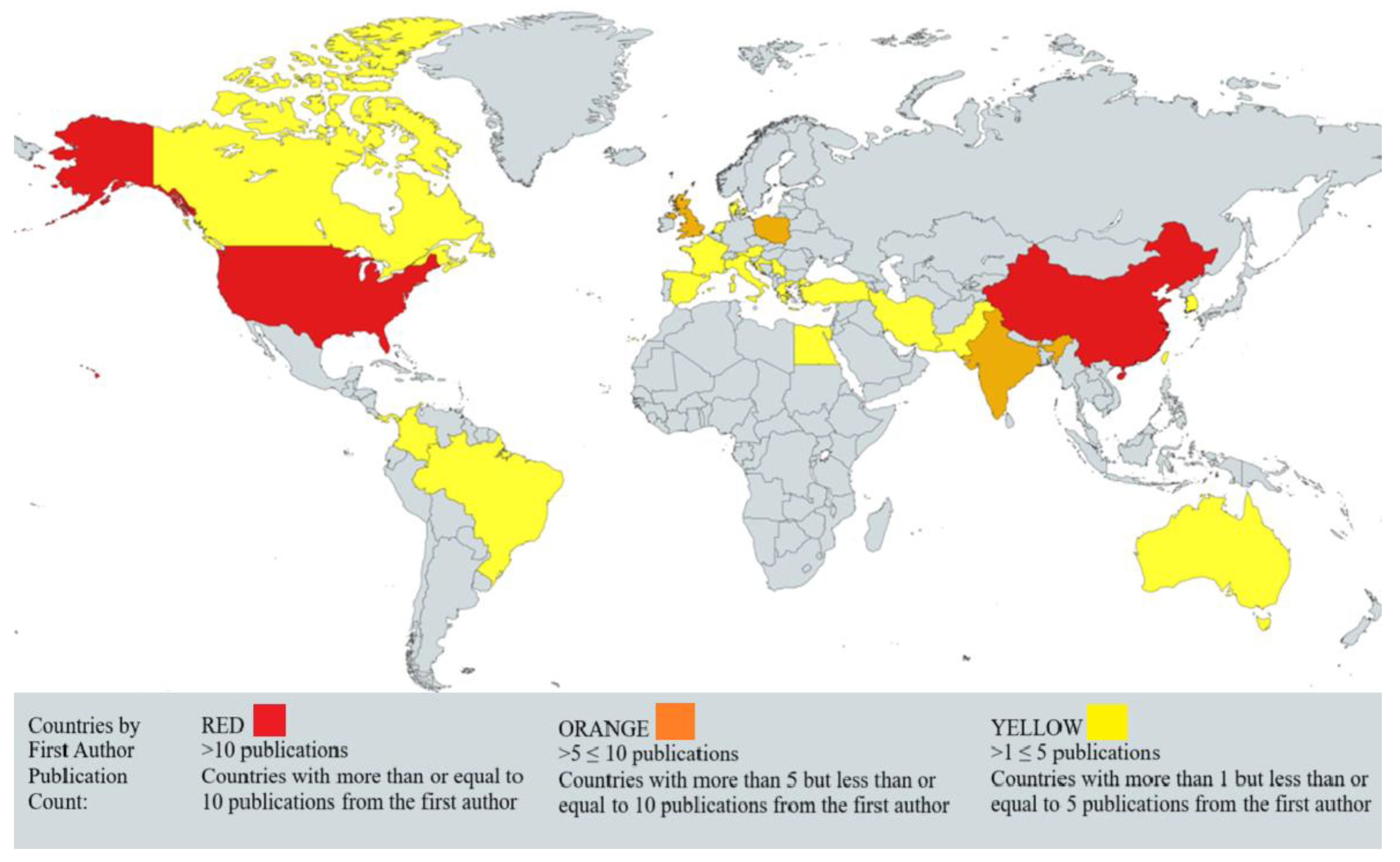
3.1.4. Geographical Distribution of Podophyllotoxin Research
3.2. Discussion
Gaps and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Sofi, F.A.; Tabassum, N. Natural product inspired leads in the discovery of anticancer agents: An update. J. Biomol. Struct. Dyn. 2023, 41, 8605–8628. [Google Scholar] [CrossRef] [PubMed]
- Hejchman, E.; Taciak, P.; Kowalski, S.; Maciejewska, D.; Czajkowska, A.; Borowska, J.; Śladowski, D.; Młynarczuk-Biały, I. Synthesis and anticancer activity of 7-hydroxycoumarinyl gallates. Pharmacol. Rep. 2015, 67, 236–244. [Google Scholar] [CrossRef]
- Singh, D.; Fisher, J.; Shagalov, D.; Varma, A.; Siegel, D.M. Dangerous plants in dermatology: Legal and controlled. Clin. Dermatol. 2018, 36, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Goldmeier, D. The cost effectiveness of hospital-based 25% podophyllin vs home-based 0.5% podophyllotoxin in the treatment of anogenital warts. Int. J. STD AIDS 1995, 6, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, E.; von Krogh, G. Condyloma eradication: Self-therapy with 0.15–0.5% podophyllotoxin versus 20–25% podophyllin preparations—An integrated safety assessment. Regul. Toxicol. Pharmacol. 2001, 33, 117–137. [Google Scholar] [CrossRef]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-Ui-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Strus, P.; Sadowski, K.; Kostro, J.; Szczepankiewicz, A.A.; Nieznańska, H.; Niedzielska, M.; Zlobin, A.; Nawar Ra’idah, P.; Molęda, Z.; Szawkało, J.; et al. Cellular Distribution and Ultrastructural Changes in HaCaT Cells, Induced by Podophyllotoxin and Its Novel Fluorescent Derivative, Supported by the Molecular Docking Studies. Int. J. Mol. Sci. 2024, 25, 5948. [Google Scholar] [CrossRef]
- Hu, L.L.; Liao, B.Y.; Wei, J.X.; Ling, Y.L.; Wei, Y.X.; Liu, Z.L.; Luo, X.Q.; Wang, J.L. Podophyllotoxin Exposure Causes Spindle Defects and DNA Damage-Induced Apoptosis in Mouse Fertilized Oocytes and Early Embryos. Front. Cell Dev. Biol. 2020, 8, 600521. [Google Scholar] [CrossRef]
- Han, J.; Hu, S.; Hu, Y.; Xu, Y.; Hou, Y.; Yang, Y.; Su, H.; Zhang, Z.; Liu, P.; Sun, X.; et al. Discovery of Podofilox as a Potent cGAMP-STING Signaling Enhancer with Antitumor Activity. Cancer Immunol. Res. 2023, 11, 583–599. [Google Scholar] [CrossRef]
- Yu, H.J.; Shin, J.A.; Choi, S.J.; Cho, S.D. Podophyllotoxin reduces the aggressiveness of human oral squamous cell carcinoma through myeloid cell leukemia-1. Int. J. Mol. Med. 2023, 52, 103. [Google Scholar] [CrossRef] [PubMed]
- Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef]
- Ibbeson, B.M.; Laraia, L.; Alza, E.; O’ Connor, C.J.; Tan, Y.S.; Davies, H.M.L.; McKenzie, G.; Venkitaraman, A.R.; Spring, D.R. Diversity-oriented synthesis as a tool for identifying new modulators of mitosis. Nat. Commun. 2014, 5, 3155. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef]
- Screpanti, E.; Santaguida, S.; Nguyen, T.; Silvestri, R.; Gussio, R.; Musacchio, A.; Hamel, E.; De Wulf, P. A screen for kinetochore-microtubule interaction inhibitors identifies novel antitubulin compounds. PLoS ONE 2010, 5, e11603. [Google Scholar] [CrossRef] [PubMed]
- Strus, P.; Borensztejn, K.; Szczepankiewicz, A.A.; Lisiecki, K.; Czarnocki, Z.; Nieznanska, H.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Novel podophyllotoxin and benzothiazole derivative induces transitional morphological and functional changes in HaCaT cells. Toxicol. Vitr. 2021, 73, 105144. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I. Condylomata acuminate. New Orleans Med. Surg. J. 1942, 94, 388–390. [Google Scholar]
- Wartec 1.5 mg/g, Krem Podophyllotoxinum, Ulotka Dołączona do Opakowania: Informacja dla Pacjenta. Available online: https://rejestrymedyczne.ezdrowie.gov.pl/api/rpl/medicinal-products/9913/leaflet (accessed on 23 November 2024).
- CONDYLINE, 5 mg/mL, Roztwór na Skórę, Charakterystyka Produktu Leczniczego. Available online: https://rejestry.ezdrowie.gov.pl/api/rpl/medicinal-products/1562/characteristic (accessed on 24 November 2024).
- McEvoy, G.K. AHFS Drug Information 2004; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2004. [Google Scholar]
- Loike, J.D.; Horwitz, S.B. Effects of podophyllotoxin and VP-16-213 on microtubule assembly in vitro and nucleoside transport in HeLa cells. Biochemistry 1976, 15, 5435–5443. [Google Scholar] [CrossRef]
- An, J.; Liu, Y.; Duo, S.; Ma, X.; An, L.; Yan, Y.; Ji, D.; Yan, Y.; Cheng, Q.; Su, Z. Podofilox suppresses gastric cancer cell proliferation by regulating cell cycle arrest and the c-Myc/ATG10 axis. Exp. Ther. Med. 2021, 22, 1203. [Google Scholar] [CrossRef]
- Soloway, M.S.; Martino, C. Prophylaxis of bladder tumor implantation. Intravesical and systemic chemotherapy. Urology 1976, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Lu, Y.; Hou, M.; Xu, Z.; Li, B. A thiol-responsive and self-immolative podophyllotoxin prodrug for cancer therapy. Tetrahedron Lett. 2021, 71, 153044. [Google Scholar] [CrossRef]
- Goldschmidt, P.; Glupczynski, Y.; Gueuning, C.; Graff, G.L. Systemic effects of podophyllotoxin on phosphate metabolism in innervated and denervated, slow and fast muscles of the rat. Arch. Int. Physiol. Biochim. 1980, 88, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R.; Ferenczy, A. Therapeutic Approaches to Genital Warts. Am. J. Med. 1997, 102, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Komericki, P.; Akkilic-Materna, M.; Strimitzer, T.; Aberer, W. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of anogenital warts. Sex. Transm. Dis. 2011, 38, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J.; Goodall, R.L.; Tennvall, G.R.; Maw, R.; Kinghorn, G.R.; Fisk, P.G.; Barton, S.; Byren, I. Randomised controlled trial and economic evaluation of podophyllotoxin solution, podophyllotoxin cream, and podophyllin in the treatment of genital warts. Sex. Transm. Infect. 2003, 79, 270–275. [Google Scholar] [CrossRef]
- Chang, L.W.; Yang, C.M.; Chen, C.F.; Deng, J.F. Experimental podophyllotoxin (bajiaolian) poisoning: I. Effects on the nervous system. Biomed. Environ. Sci. 1992, 5, 283–292. [Google Scholar]
- Kao, W.F.; Hung, D.Z.; Tsai, W.J.; Lin, K.P.; Deng, J.F. Podophyllotoxin intoxication: Toxic effect of Bajiaolian in herbal therapeutics. Hum. Exp. Toxicol. 1992, 11, 480–487. [Google Scholar] [CrossRef]
- Slater, G.E.; Rumack, B.H.; Peterson, R.G. Podophyllin poisoning. Systemic toxicity following cutaneous application. Obstet. Gynecol. 1978, 52, 94–96. [Google Scholar]
- Andersson, N.W.; Andersen, J.T. Association Between Fetal Safety Outcomes and Exposure to Local Podophyllotoxin During Pregnancy. JAMA Dermatol. 2020, 156, 303–311. [Google Scholar] [CrossRef]
- Strus, P.; Lisiecki, K.; Czarnocki, Z.; Młynarczuk-Biały, I.; Biały, L. Novel Podophyllotoxin Derivatives as Anticancer Agents: Design, Synthesis, and Biological Screening; Advances in Biomedical Research–Selected Topics; Wydawnictwo Naukowe TYGIEL sp. z oo: Lublin, Poland, 2018; pp. 48–61. [Google Scholar]
- Wantke, F.; Fleischl, G.; Gotz, M.; Jarisch, R. Topical podophyllotoxin in psoriasis vulgaris. Dermatology 1993, 186, 79. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Hon, K.L.E. Molluscum Contagiosum: An Update. Recent. Pat. Inflamm. Allergy Drug Discov. 2017, 11, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.D.; Tackett, A.J.; Shalin, S.C. Proteomics and melanoma: A current perspective. Glob. Dermatol. 2016, 3, 366–370. [Google Scholar] [PubMed]
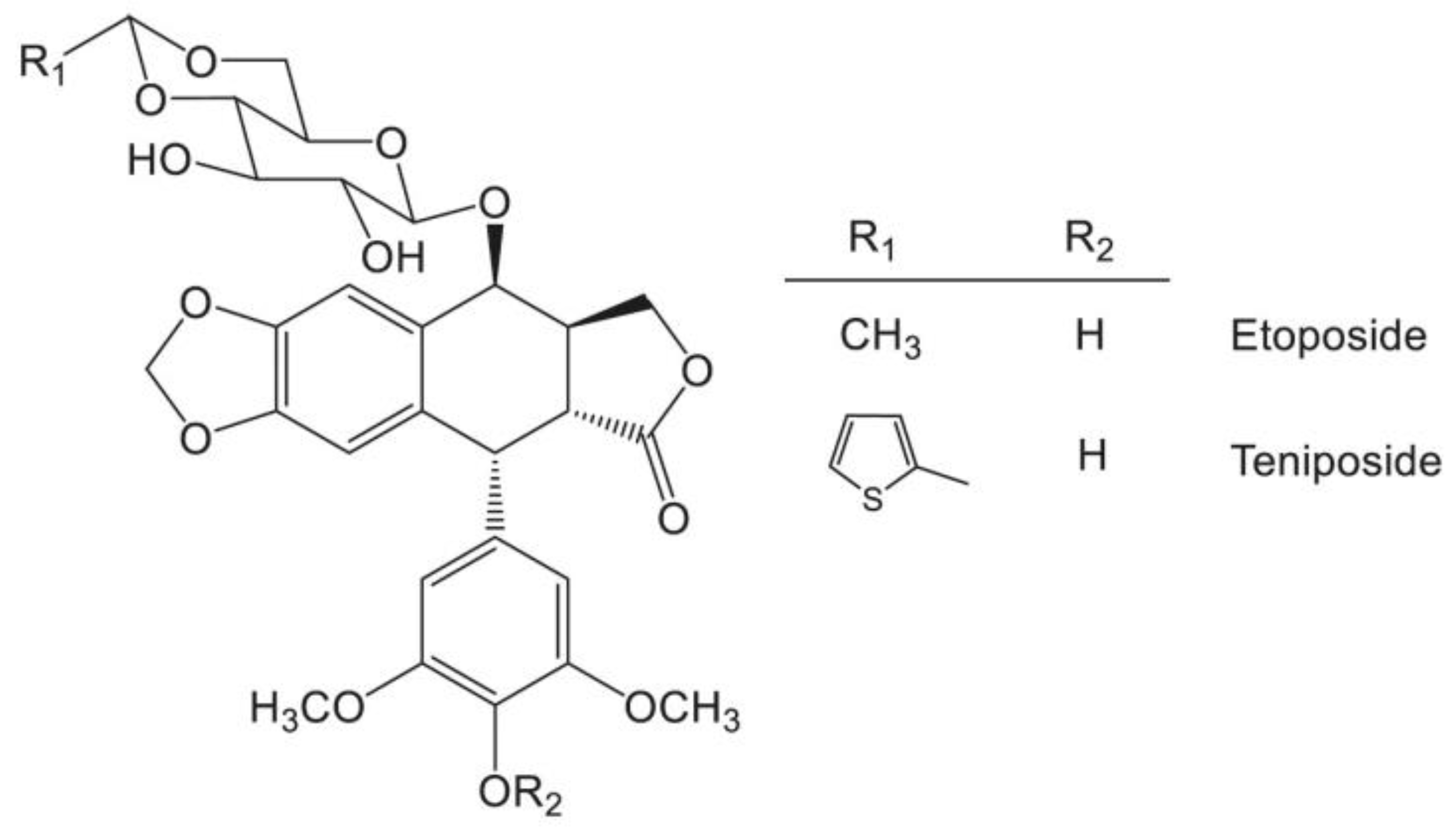
- Clark, P.I.; Slevin, M.L. The clinical pharmacology of etoposide and teniposide. Clin. Pharmacokinet. 1987, 12, 223–252. [Google Scholar] [CrossRef]
- Holthuis, J.J. Etoposide and teniposide. Bioanalysis, metabolism and clinical pharmacokinetics. Pharm. Weekbl. Sci. 1988, 10, 101–116. [Google Scholar] [CrossRef]
- Noronha, V.; Sekhar, A.; Patil, V.M.; Menon, N.; Joshi, A.; Kapoor, A.; Prabhash, K. Systemic therapy for limited stage small cell lung carcinoma. J. Thorac. Dis. 2020, 12, 6275–6290. [Google Scholar] [CrossRef]
- Economides, M.P.; McCue, D.; Borthakur, G.; Pemmaraju, N. Topoisomerase II inhibitors in AML: Past, present, and future. Expert. Opin. Pharmacother. 2019, 20, 1637–1644. [Google Scholar] [CrossRef]
- Najar, I.A.; Johri, R.K. Pharmaceutical and pharmacological approaches for bioavailability enhancement of etoposide. J. Biosci. 2014, 39, 139–144. [Google Scholar] [CrossRef]
- Zhang, W.; Gou, P.; Dupret, J.M.; Chomienne, C.; Rodrigues-Lima, F. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Transl. Oncol. 2021, 14, 101169. [Google Scholar] [CrossRef]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Bishop, J.F.; Lowethal, R.; Joshua, D.; Matthews, J.P.; Wolf, M.M.; Cooper, I.A. Etoposide in leukemia. Cancer 1991, 67 (Suppl. S1), 285–291. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Delle Monache, G.; Misiti, D.; Vitali, A.; Zappia, G. Aryltetralin lignans: Chemistry, pharmacology and biotransformations. Curr. Med. Chem. 2001, 8, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Wozniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar] [PubMed]
- Xie, S.; Li, G.; Qu, L.; Zhong, R.; Chen, P.; Lu, Z.; Zhou, J.; Guo, X.; Li, Z.; Ma, A.; et al. Podophyllotoxin Extracted from Juniperus sabina Fruit Inhibits Rat Sperm Maturation and Fertility by Promoting Epididymal Epithelial Cell Apoptosis. Evid. Based Complement. Alternat Med. 2017, 2017, 6958982. [Google Scholar] [CrossRef]
- Lu, P.S.; Xie, L.P.; Kong, X.H.; Xu, Y.; Sun, S.C. Podophyllotoxin Exposure Affects Organelle Distribution and Functions in Mouse Oocyte Meiosis. Front. Cell Dev. Biol. 2021, 9, 672590. [Google Scholar] [CrossRef]
- Jiang, W.J.; Hu, L.L.; Ren, Y.P.; Lu, X.; Luo, X.Q.; Li, Y.H.; Xu, Y.N. Podophyllotoxin affects porcine oocyte maturation by inducing oxidative stress-mediated early apoptosis. Toxicon 2020, 176, 15–20. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; He, T.; Sun, L.; Wang, Q.; Han, S.; Wang, W.; Kong, J.; Yuan, F.; Huang, J. Study on potential toxic material base and mechanisms of hepatotoxicity induced by Dysosma versipellis based on toxicological evidence chain (TEC) concept. Ecotoxicol. Environ. Saf. 2020, 190, 110073. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Kong, J.; Li, X.; Wang, Y.; Zhang, F.; Duan, J. Podophyllotoxin mediates hepatic toxicity via the C5a/C5aR/ROS/NLRP3 and cGMP/PKG/mTOR axis in rats based on toxicological evidence chain (TEC) concept by phosphoproteomic analysis. Ecotoxicol. Environ. Saf. 2025, 289, 117441. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, M.L.; Kalita, B. The combination of the active principles of Podophyllum hexandrum supports early recovery of the gastrointestinal system via activation of Nrf2-HO-1 signaling and the hematopoietic system, leading to effective whole-body survival in lethally irradiated mice. Free Radic. Res. 2015, 49, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Tang, Y.A.; Li, W.S.; Chiou, Y.C.; Shieh, J.M.; Wang, Y.C. A synthetic podophyllotoxin derivative exerts anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER stress in lung cancer preclinical models. PLoS ONE 2013, 8, e62082. [Google Scholar] [CrossRef]
- Zhao, W.; Bai, J.-K.; Li, H.-M.; Chen, T.; Tang, Y.-J. Tubulin structure-based drug design for the development of novel 4β-sulfur-substituted podophyllum tubulin inhibitors with anti-tumor activity. Sci. Rep. 2015, 5, 10172. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Ghayour, A.h.; Delavari, M.; Arbabi, M. Antileishmanial effect of podophyllotoxin and podophyllin on Leishmania major in vitro and in vivo. J. Parasit. Dis. 2024, 48, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, J.; Ju, P.; Ma, L.; Chen, L.; Ma, W.; Zheng, T.; Yang, G.; Wang, Y.X. Synthesis and Biological Evaluation of 4beta-N-Acetylamino Substituted Podophyllotoxin Derivatives as Novel Anticancer Agents. Front. Chem. 2019, 7, 253. [Google Scholar] [CrossRef]
- Cobo, F. Human Papillomavirus Infections: From Laboratory to Clinical Practice; Woodhead Pub.: Cambridge, UK, 2012; 149p. [Google Scholar]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Zhu, P.; Qi, R.Q.; Yang, Y.; Huo, W.; Zhang, Y.; He, L.; Wang, G.; Xu, J.; Zhang, F.; Yang, R.; et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J. Evid. Based Med. 2022, 15, 284–301. [Google Scholar] [CrossRef]
- Gilson, R.; Nugent, D.; Bennett, K.; Dore, C.J.; Murray, M.L.; Meadows, J.; Haddow, L.J.; Lacey, C.; Sandmann, F.; Jit, M.; et al. Imiquimod versus podophyllotoxin, with and without human papillomavirus vaccine, for anogenital warts: The HIPvac factorial RCT. Health Technol. Assess. 2020, 24, 1–86. [Google Scholar] [CrossRef]
- Murray, M.L.; Meadows, J.; Dore, C.J.; Copas, A.J.; Haddow, L.J.; Lacey, C.; Jit, M.; Soldan, K.; Bennett, K.; Tetlow, M.; et al. Human papillomavirus infection: Protocol for a randomised controlled trial of imiquimod cream (5%) versus podophyllotoxin cream (0.15%), in combination with quadrivalent human papillomavirus or control vaccination in the treatment and prevention of recurrence of anogenital warts (HIPvac trial). BMC Med. Res. Methodol. 2018, 18, 125. [Google Scholar] [CrossRef]
- Lee, C.N.; Hsu, C.K.; Lee, J.Y. Recalcitrant extragenital giant condyloma acuminatum: A need for combination therapy. Dermatol. Ther. 2019, 32, e12867. [Google Scholar] [CrossRef] [PubMed]
- Golusin, Z.; Jovanovic, M.; Matic, M.; Ros, T.; Vujanovic, L.; Nikolic, O. Clinical Efficacy of Combination Therapy with Podophyllotoxin and Liquid Nitrogen Cryotherapy in the Treatment of Genital Warts in Men. Acta Dermatovenerol. Croat. 2019, 27, 250–259. [Google Scholar]
- Ghonemy, S. Treatment of recalcitrant plantar warts with long-pulsed Nd:YAG laser versus cantharidin-podophylline resin-salicylic acid. J. Cosmet. Laser Ther. 2017, 19, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, E.; Kanelleas, A.; Nikolakopoulos, S.; Bezrodnii, G.; Nearchou, E.; Gerodimou, M.; Papadopoulou-Skordou, E.; Paparizos, V.; Rigopoulos, D. A short, 8-week course of imiquimod 5% cream versus podophyllotoxin in the treatment of anogenital warts: A retrospective comparative cohort study. Indian. J. Dermatol. Venereol. Leprol. 2021, 87, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Kacar, N.; Tasli, L.; Korkmaz, S.; Ergin, S.; Erdogan, B.S. Cantharidin-podophylotoxin-salicylic acid versus cryotherapy in the treatment of plantar warts: A randomized prospective study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Calik, J.; Zawada, T.; Bove, T. Treatment of Condylomata Acuminata Using a New Non-Vapor-Generating Focused Ultrasound Method following Imiquimod 5% Cream. Case Rep. Dermatol. 2022, 14, 275–282. [Google Scholar] [CrossRef]
- Tomic, L.; Skerlev, M.; Ljubojevic Hadzavdic, S. Meatal Intraurethral Warts Successfully Treated with 5-fluorouracil Cream. Acta Dermatovenerol. Croat. 2022, 30, 188–191. [Google Scholar]
- Brand, Y.M.; Roa-Linares, V.; Santiago-Dugarte, C.; Del Olmo, E.; Lopez-Perez, J.L.; Betancur-Galvis, L.; Gallego-Gomez, J.C.; Feliciano, A.S. A new host-targeted antiviral cyclolignan (SAU-22.107) for Dengue Virus infection in cell cultures. Potential action mechanisms based on cell imaging. Virus Res. 2023, 323, 198995. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Purohit, P.; Roy, P.K. Neuroprotective Drug Discovery From Phytochemicals and Metabolites for CNS Viral Infection: A Systems Biology Approach With Clinical and Imaging Validation. Front. Neurosci. 2022, 16, 917867. [Google Scholar] [CrossRef]
- Guerrero, E.; Abad, A.; Montenegro, G.; Del Olmo, E.; Lopez-Perez, J.L.; San Feliciano, A. Analgesic and anti-inflammatory activity of podophyllotoxin derivatives. Pharm. Biol. 2013, 51, 566–572. [Google Scholar] [CrossRef]
- Kalita, B.; Ranjan, R.; Singh, A.; Yashavarddhan, M.H.; Bajaj, S.; Gupta, M.L. A Combination of Podophyllotoxin and Rutin Attenuates Radiation Induced Gastrointestinal Injury by Negatively Regulating NF-kappaB/p53 Signaling in Lethally Irradiated Mice. PLoS ONE 2016, 11, e0168525. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kalita, B.; Bajaj, S.; Prakash, H.; Singh, A.K.; Gupta, M.L. A Combination of Podophyllotoxin and Rutin Alleviates Radiation-Induced Pneumonitis and Fibrosis through Modulation of Lung Inflammation in Mice. Front. Immunol. 2017, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Kalita, B.; Singh, A.; Yashavarddhan, M.H.; Prakash, H.; Gupta, M.L. Prophylactic administration of podophyllotoxin and rutin combination assists the revival of radiation-induced hematopoietic suppression in lethally irradiated mice. Biochem. Biophys. Res. Commun. 2021, 549, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Gupta, M.L.; Kumar, K. A combined prophylactic modality of podophyllotoxin and rutin alleviates radiation induced injuries to the lymphohematopoietic system of mice by modulating cytokines, cell cycle progression, and apoptosis. Free Radic. Res. 2020, 54, 497–516. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Candido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, M.L. Radiation-induced hematopoietic myelosuppression and genotoxicity get significantly countered by active principles of Podophyllum hexandrum: A study in strain ‘A’ mice. Int. J. Radiat. Biol. 2015, 91, 757–770. [Google Scholar] [CrossRef] [PubMed]

| Database | Number of Records | Search Strategy |
|---|---|---|
| PubMed/MEDLINE | 408 | ((podophyllotoxin[Title/Abstract]) OR (ppt[Title/Abstract])) AND ((non-cancerous[Title/Abstract]) OR (antiviral[Title/Abstract]) OR (disease[Title/Abstract])) AND ((“2013/01/01”[Date—Publication]: “3000”[Date—Publication])) |
| Embase | 1104 | (‘podophyllotoxin’ OR ‘ppt’) AND (‘non-cancerous’ OR ‘antiviral’ OR ‘disease’) AND [2013–2025]/py AND ‘article’/it |
| Web of Science | 757 | TS = (podophyllotoxin OR ppt) AND TS = (non-cancerous OR antiviral OR disease) AND PY = 2013–2025 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Original studies about Podophyllotoxin and its derivatives | Meta-analysis, systematic review, books, guidelines |
| Language of the article: English | Other unoriginal articles |
| Describing non-cancerous application and activity | Language other than English |
| Human, animal, cellular, and molecular studies | Not finished work |
| Good quality of the research | Articles about only anti-cancerous activity |
| Compound | Anticancer Applications | Non-Anticancer Applications | Special Features | Citation |
|---|---|---|---|---|
| Compounds Tested in Clinical Settings in Humans | ||||
| Podophyllotoxin (PPT) | Treatment of skin cancers (topical) | Treatment of genital warts, psoriasis, molluscum contagiosum, keratoacanthoma; antiviral properties (e.g., HPV, SARS-CoV-2) | Topical use only due to high systemic toxicity; interacts with microtubule spindle formation | [27,28,29,30,31,32,33] |
| Etoposide | Ovarian, testicular, and lung cancer; leukemia; Hodgkin’s lymphoma; non-Hodgkin’s lymphoma | Not currently used in non-cancer treatment. | Systemic application; a first-line treatment for testicular and small-cell lung cancers | [40,41,42,43,44,45] |
| Teniposide | Hodgkin’s lymphoma, bladder cancer, acute lymphocytic leukemia, immature neuroblastoma | Not currently used in non-cancer treatment. | Systemic application, similar to etoposide but distinguished by its thienyl group | [46] |
| Compounds tested in preclinical settings in animals | ||||
| G-003M (PPT + rutin) | Not currently used in cancer treatment. | Radioprotection against gamma radiation-induced lung and tissue damage; reduces oxidative stress; enhances survival rates in preclinical models | Administered prophylactically; strong antioxidant properties; preserves pulmonary vascular integrity during radiation exposure | [77,78,79,80,81] |
| G-002M (PPT, rutin, derivatives) | Not currently used in cancer treatment. | Radioprotection against hematopoietic suppression and chromosomal aberrations; protects bone marrow and reduces DNA damage post-radiation | Single-dose preventative administration; protective effects for radiosensitive organs, including the gastrointestinal tract and bone marrow | [55,76,77,78,79,80,81,82] |
| Compounds tested in preclinical settings on cells | ||||
| KL3 | Used in pre-clinical study as anticancer agent for Hela, MDA-MB, MCF7, PC3, DU-145, CFPAC cell lines | Reduces cytotoxicity in keratinocyte models compared to PPT; potential for less toxic therapeutic use | No necrotic effects; activates caspase-9 in keratinocytes | [17,34] |
| SAU-22.107 | Not currently used in cancer treatment. | Decreases replication of Dengue virus; neuroprotective potential against SARS-CoV-2; modulates interferon-regulatory factors to prevent viral replication | Experimental compound; antiviral activity via modulation of viral binding to host cells | [73,74,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strus, P.; Sadowski, K.; Ploch, W.; Jazdzewska, A.; Oknianska, P.; Raniszewska, O.; Mlynarczuk-Bialy, I. The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 958. https://doi.org/10.3390/ijms26030958
Strus P, Sadowski K, Ploch W, Jazdzewska A, Oknianska P, Raniszewska O, Mlynarczuk-Bialy I. The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(3):958. https://doi.org/10.3390/ijms26030958
Chicago/Turabian StyleStrus, Piotr, Karol Sadowski, Weronika Ploch, Adrianna Jazdzewska, Paulina Oknianska, Oliwia Raniszewska, and Izabela Mlynarczuk-Bialy. 2025. "The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review" International Journal of Molecular Sciences 26, no. 3: 958. https://doi.org/10.3390/ijms26030958
APA StyleStrus, P., Sadowski, K., Ploch, W., Jazdzewska, A., Oknianska, P., Raniszewska, O., & Mlynarczuk-Bialy, I. (2025). The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review. International Journal of Molecular Sciences, 26(3), 958. https://doi.org/10.3390/ijms26030958






