No Association Between HIV-1 Subtype and Primary Resistance Mutations with CD4 Reconstitution During Effective Antiretroviral Treatment: An Observational, Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Study Population
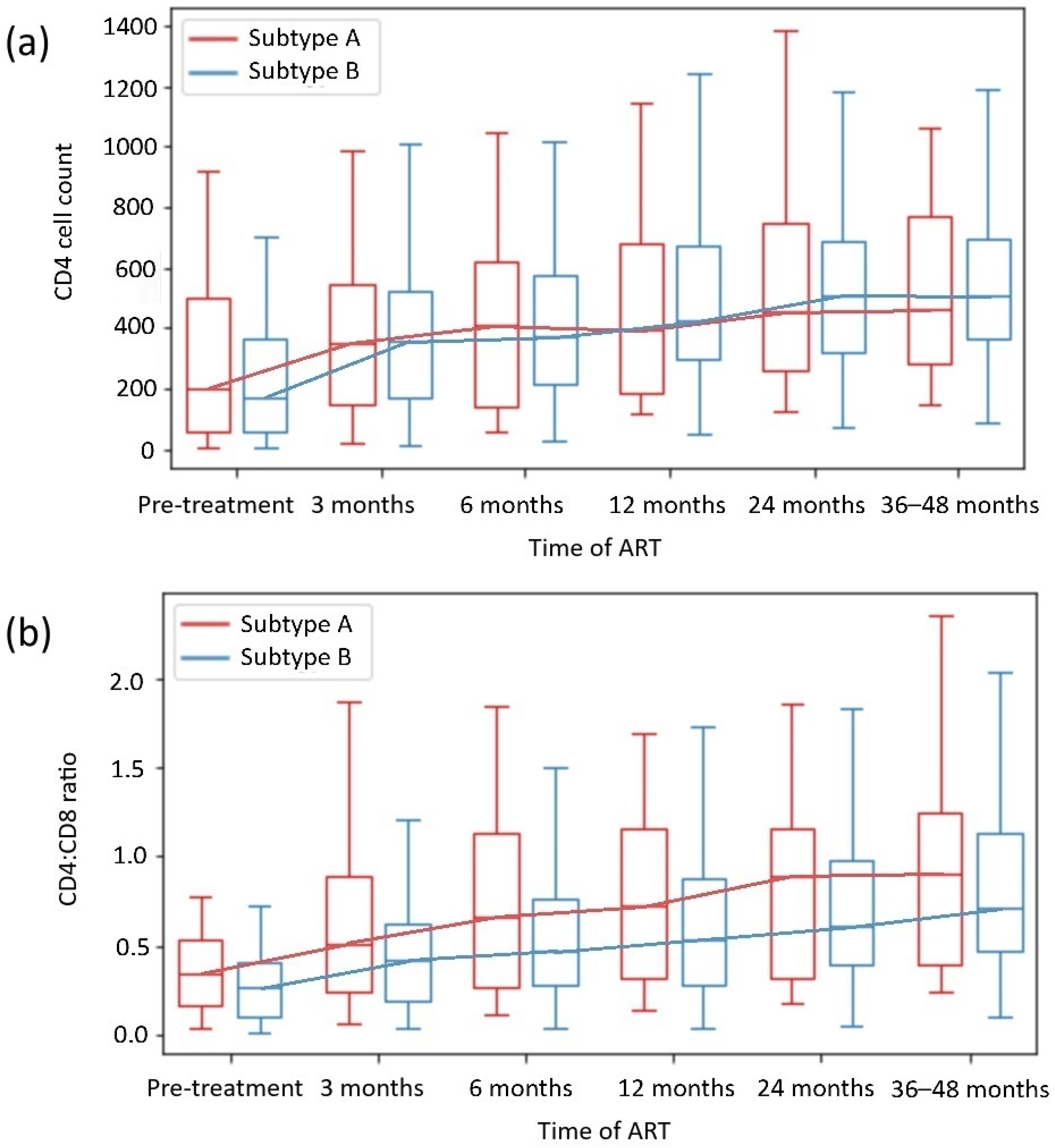
2.2. HIV Subtype-Dependent Restoration
2.3. DRM-Dependent Restoration
3. Discussion
3.1. Time of Observation of CD4 Restoration
3.2. HIV Subtype-Dependent Analysis
3.2.1. HIV Subtype Epidemiology
3.2.2. HIV Subtype Differences
3.2.3. Possible HIV Subtype Influence on Immune Reconstitution
3.3. DRM-Dependent Analysis
3.3.1. DRMs Differences
3.3.2. Possible DRMs-Dependent Mechanisms of Immune Restoration
3.4. Significance and Limitations of This Study
4. Materials and Methods
4.1. Study Design
4.2. Clinical Assessment
4.3. Molecular Investigation
4.4. Statistical Evaluation
4.5. Bioethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HIV | Human Immunodeficiency Virus |
| PWH | People with HIV |
| ART | Antiretroviral therapy |
| INR | Immunological non-responder |
| AIDS | Acquired Immunodeficiency Syndrome |
| IRIS | Immune Reconstitution Inflammatory Syndrome |
| DRM | Drug resistance mutation |
| HIV-1 | HIV type 1 |
| PI | Protease Inhibitor |
| NRTI | Nucleoside Reverse Transcriptase Inhibitor |
| NNRTI | Non-nucleoside Reverse Transcriptase Inhibitor |
| InSTI | Integrase Strand Transfer Inhibitor |
| CDC | Centers for Disease Control and Prevention |
| CRF | Circulating Recombinant Form |
| URF | Unique Recombinant Form |
| hs-CRP | highly sensitive C-Reactive Protein |
| IL-6 | Interleukin 6 |
| EACS | European AIDS Clinical Society |
| ECDC | European Centre for Disease Prevention and Control |
| PR | Protease |
| RT | Reverse Transcriptase |
| RNA | Ribonucleic acid |
| PCR | Polymerase Chain Reaction |
References
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults with and Without HIV Infection, 2000–2016. JAMA 2020, 3, 207954. [Google Scholar] [CrossRef]
- Gazzola, L.; Tincati, C.; Bellistrì, G.M.; Monforte, A.D.; Marchetti, G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: Clinical risk, immunological gaps, and therapeutic options. Clin. Infect. Dis. 2009, 48, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Li, C.X.; Li, Y.Y.; He, L.P.; Kou, J.; Bai, J.S.; Liu, J.; Tian, B.; Cao, L.J.; Wang, K.H.; Kuang, Y.Q. The predictive role of CD4+ cell count and CD4/CD8 ratio in immune reconstitution outcome among HIV/AIDS patients receiving antiretroviral therapy: An eight-year observation in China. BMC Immunol. 2019, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, J.; Zhang, L.; Liu, Y.; Chen, N.; Deng, M.; Song, C.; Liu, T.; Zhang, Y.; Zhao, H. Longitudinal analysis of immune reconstitution and metabolic changes in women living with HIV: A real-world observational study. Chin. Med. J. 2023, 136, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Borges, Á.H.; O’Connor, J.L.; Phillips, A.N.; Rönsholt, F.F.; Pett, S.; Vjecha, M.J.; French, M.A.; Lundgren, J.D.; INSIGHT SMART and ESPRIT Study Groups and the SILCAAT Scientific Committee. Factors Associated With Plasma IL-6 Levels During HIV Infection. J. Infect. Dis. 2015, 212, 585–595. [Google Scholar] [CrossRef]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.; Su, L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014, 289, 21716–21726. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Dutta, R.K.; Nair, M.; Chand, H.S.; Rahman, I.; Unwalla, H.J. TGF-β1 increases viral burden and promotes HIV-1 latency in primary differentiated human bronchial epithelial cells. Sci. Rep. 2019, 9, 12552. [Google Scholar] [CrossRef]
- Wang, Y.; Lifshitz, L.; Silverstein, N.J.; Mintzer, E.; Luk, K.; StLouis, P.; Brehm, M.A.; Wolfe, S.A.; Deeks, S.G.; Luban, J. Transcriptional and chromatin profiling of human blood innate lymphoid cell subsets sheds light on HIV-1 pathogenesis. EMBO J. 2023, 42, e114153. [Google Scholar] [CrossRef]
- Grozdeva, R.; Ivanov, D.; Strashimirov, D.; Kapincheva, N.; Yordanova, R.; Mihailova, S.; Georgieva, A.; Alexiev, I.; Grigorova, L.; Partsuneva, A.; et al. Relationship between Modern ART Regimens and Immunosenescence Markers in Patients with Chronic HIV Infection. Viruses 2024, 16, 1205. [Google Scholar] [CrossRef] [PubMed]
- Vandergeeten, C.; Fromentin, R.; Chomont, N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Shrestha, U. Immune Reconstitution Inflammatory Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Meya, D.B.; Manabe, Y.C.; Boulware, D.R.; Janoff, E.N. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: Understanding a conundrum. Curr. Opin. Infect. Dis. 2016, 29, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Martin-Blondel, G.; Mars, L.T.; Liblau, R.S. Pathogenesis of the immune reconstitution inflammatory syndrome in HIV-infected patients. Curr. Opin. Infect. Dis. 2012, 25, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Parczewski, M.; Scheibe, K.; Witak-Jędra, M.; Pynka, M.; Aksak-Wąs, B.; Urbańska, A. Infection with HIV-1 subtype D adversely affects the live expectancy independently of antiretroviral drug use. Infect. Genet. Evol. 2021, 90, 104754. [Google Scholar] [CrossRef] [PubMed]
- Venner, C.M.; Nankya, I.; Kyeyune, F.; Demers, K.; Kwok, C.; Chen, P.L.; Rwambuya, S.; Munjoma, M.; Chipato, T.; Byamugisha, J.; et al. Infecting HIV-1 Subtype Predicts Disease Progression in Women of Sub-Saharan Africa. EBioMedicine 2016, 13, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Widiyanti, M.; Hadi, M.I. Viral and Host Factors are Related to the Progression of HIV Diseases in Mimika, Papua. Open Access Maced. J. Med. Sci. 2019, 7, 3429–3432. [Google Scholar] [CrossRef] [PubMed]
- Alaeus, A.; Lidman, K.; Björkman, A.; Giesecke, J.; Albert, J. Similar rate of disease progression among individuals infected with HIV-1 genetic subtypes A-D. AIDS 1999, 13, 901–907. [Google Scholar] [CrossRef] [PubMed]
- McPhee, E.; Grabowski, M.K.; Gray, R.H.; Ndyanabo, A.; Ssekasanvu, J.; Kigozi, G.; Makumbi, F.; Serwadda, D.; Quinn, T.C.; Laeyendecker, O. The interaction of HIV set point viral load and subtype on disease progression. AIDS Res. Hum. Retrovir. 2019, 35, 49–51. [Google Scholar] [CrossRef]
- Kiguoya, M.W.; Mann, J.K.; Chopera, D.; Gounder, K.; Lee, G.Q.; Hunt, P.W.; Martin, J.N.; Ball, T.B.; Kimani, J.; Brumme, Z.L.; et al. Subtype-specific differences in Gag-protease-driven replication capacity are consistent with intersubtype differences in HIV-1 disease progression. J. Virol. 2017, 91, e00253-17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Hill, A.; Sawyer, A.W.; Cozzi-Lepri, A.; von Wyl, V.; Yerly, S.; Lima, V.D.; Günthard, H.F.; Gilks, C.; Pillay, D. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: A systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, W.H.; Ziebell, R.A.; Zabina, H.; Pieniazek, D.; Prejean, J.; Bodnar, U.R.; Mahle, K.C.; Heneine, W.; Johnson, J.A.; Hall, H.I.; et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS 2010, 24, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Bokharaei-Salim, F.; Esghaei, M.; Khanaliha, K.; Kalantari, S.; Marjani, A.; Fakhim, A.; Keyvani, H. HIV-1 reverse transcriptase and protease mutations for drug-resistance detection among treatment-experienced and naïve HIV-infected individuals. PLoS ONE 2020, 15, e0229275. [Google Scholar] [CrossRef]
- Stanford University HIV Drug Resistance Database. Available online: http://hivdb.stanford.edu (accessed on 25 January 2025).
- Rhee, S.Y.; Kassaye, S.G.; Barrow, G.; Sundaramurthi, J.C.; Jordan, M.R.; Shafer, R.W. HIV-1 transmitted drug resistance surveillance: Shifting trends in study design and prevalence estimates. J. Int. AIDS Soc. 2020, 23, 25611. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2022 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 2022, 30, 559–574. [Google Scholar]
- Branda, F.; Giovanetti, M.; Sernicola, L.; Farcomeni, S.; Ciccozzi, M.; Borsetti, A. Comprehensive Analysis of HIV-1 Integrase Resistance-Related Mutations in African Countries. Pathogens 2024, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, A.; Dunn, D.; Gascuel, O., on behalf of the UK HIV Drug Resistance Database & the Collaborative HIV, Anti-HIV Drug Resistance Network. Modeling Drug Resistance Emergence and Transmission in HIV-1 in the UK. Viruses 2023, 15, 1244. [Google Scholar] [CrossRef] [PubMed]
- Pingarilho, M.; Pimentel, V.; Diogo, I.; Fernandes, S.; Miranda, M.; Pineda-Pena, A.; Libin, P.; Theys, K.; Martins, M.R.O.; Vandamme, A.M.; et al. Increasing Prevalence of HIV-1 Transmitted Drug Resistance in Portugal: Implications for First Line Treatment Recommendations. Viruses 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, A.; Lapovok, I.; Baryshev, P.; van de Vijver, D.A.M.C.; van Kampen, J.J.A.; Boucher, C.A.B.; Paraskevis, D.; Kireev, D. Genetic Features of HIV-1 Integrase Sub-Subtype A6 Predominant in Russia and Predicted Susceptibility to INSTIs. Viruses 2020, 12, 838. [Google Scholar] [CrossRef]
- Serwin, K.; Urbańska, A.; Scheibe, K.; Witak-Jędra, M.; Jankowska, M.; Hlebowicz, M.; Bociąga-Jasik, M.; Kalinowska-Nowak, A.; Biała, M.; Ciepłucha, H.; et al. Molecular epidemiology and HIV-1 variant evolution in Poland between 2015 and 2019. Sci. Rep. 2021, 11, 16609. [Google Scholar] [CrossRef]
- Beyrer, C.; Wirtz, A.L.; O’Hara, G.; Léon, N.; Kazatchkine, M. The expanding epidemic of HIV-1 in the Russian Federation. PLoS Med. 2017, 14, e1002462. [Google Scholar] [CrossRef]
- CDC. Revised Guidelines for Performing CD4+ T-Cell Determinations in Persons Infected with Human Immunodeficiency Virus (HIV). 1997. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00045580.htm (accessed on 18 November 2024).
- Serrano-Villar, S.; Deeks, S.G. CD4/CD8 ratio: An emerging biomarker for HIV. Lancet HIV 2015, 2, 76–77. [Google Scholar] [CrossRef]
- Lee, S.S.; Wong, N.S.; Wong, B.C.K.; Wong, K.H.; Chan, K.C.W. Combining CD4 recovery and CD4: CD8 ratio restoration as an indicator for evaluating the outcome of continued antiretroviral therapy: An observational cohort study. BMJ Open 2017, 7, 016886. [Google Scholar] [CrossRef] [PubMed]
- Caby, F.; Guihot, A.; Lambert-Niclot, S.; Guiguet, M.; Boutolleau, D.; Agher, R.; Valantin, M.A.; Tubiana, R.; Calvez, V.; Marcelin, A.G.; et al. Determinants of a Low CD4/CD8 Ratio in HIV-1-Infected Individuals Despite Long-term Viral Suppression. Clin. Infect. Dis. 2016, 62, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Mussini, C.; Lorenzini, P.; Cozzi-Lepri, A.; Lapadula, G.; Marchetti, G.; Nicastri, E.; Cingolani, A.; Lichtner, M.; Antinori, A.; Gori, A.; et al. CD4/CD8 ratio normalization and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: An observational cohort study. Lancet HIV 2015, 2, 98–106. [Google Scholar] [CrossRef]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Kirtley, S.; Gouws-Williams, E.; Ghys, P.D.; WHO-UNAIDS Network for HIV Isolation and Characterisation. Global and regional epidemiology of HIV-1 recombinants in 1990–2015: A systematic review and global survey. Lancet HIV 2020, 7, 772–781. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2021-2020 Data. Stockholm: ECDC. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-europe-2021-2020-data (accessed on 6 November 2024).
- Blassel, L.; Tostevin, A.; Villabona-Arenas, C.J.; Peeters, M.; Hué, S.; Gascuel, O.; UK HIV Drug Resistance Database. Using machine learning and big data to explore the drug resistance landscape in HIV. PLoS Comput. Biol. 2021, 17, 1008873. [Google Scholar] [CrossRef]
- Sarabia, I.; Bosque, A. HIV-1 Latency and Latency Reversal: Does Subtype Matter? Viruses 2019, 11, 1104. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Giovanetti, M.; Sagnelli, C.; Ciccozzi, A.; d’Ettorre, G.; Angeletti, S.; Borsetti, A.; Ciccozzi, M. Human Immunodeficiency Virus Type 2: The Neglected Threat. Pathogens 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Sans, B.; Gong, Y.F.; McAuliffe, B.; Dicker, I.; Ho, H.T.; Zhou, N.; Eggers, B.; Lin, P.F.; Ray, N.; Wind-Rotolo, M.; et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob. Agents Chemother. 2021, 56, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, H.; Li, J.; Feng, Y.; Lan, G.; Liang, S.; Liu, M.; Rashid, A.; Xing, H.; Shen, Z.; et al. Immune reconstruction effectiveness of combination antiretroviral therapy for HIV-1 CRF01_AE cluster 1 and 2 infected individuals. Emerg. Microbes Infect. 2022, 11, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Fokam, J.; Santoro, M.M.; Takou, D.; Njom-Nlend, A.E.; Ndombo, P.K.; Kamgaing, N.; Kamta, C.; Essiane, A.; Sosso, S.M.; Ndjolo, A.; et al. Evaluation of treatment response, drug resistance and HIV-1 variability among adolescents on first- and second-line antiretroviral therapy: A study protocol for a prospective observational study in the centre region of Cameroon (EDCTP READY-study). BMC Pediatr. 2019, 19, 226. [Google Scholar] [CrossRef]
- Bhargava, M.; Cajas, J.M.; Wainberg, M.A.; Klein, M.B.; Pant Pai, N. Do HIV-1 non-B subtypes differentially impact resistance mutations and clinical disease progression in treated populations? Evidence from a systematic review. J. Int. AIDS Soc. 2014, 17, 18944. [Google Scholar] [CrossRef]
- Tamalet, C.; Tissot-Dupont, H.; Motte, A.; Tourrès, C.; Dhiver, C.; Ravaux, I.; Poizot-Martin, I.; Dieng, T.; Tomei, C.; Bregigeon, S.; et al. Emergence of uncommon HIV-1 non-B subtypes and circulating recombinant forms and trends in transmission of antiretroviral drug resistance in patients with primary infection during the 2013-2015 period in Marseille, Southeastern France. J. Med. Virol. 2018, 90, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Oomen, P.G.A.; Dijkstra, S.; Hofstra, L.M.; Nijhuis, M.M.; Verbon, A.; Mudrikova, T.; Wensing, A.M.J.; Hoepelman, A.I.M.; Van Welzen, B.J. Integrated analysis of viral blips, residual viremia, and associated factors in people with HIV: Results from a retrospective cohort study. J. Med. Virol. 2023, 95, 29178. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Broom, J.; Bopage, R.; Templeton, D.J.; Edmiston, N.; Petoumenos, K.; Australian HIV Observational Database. Investigating rates and predictors of viral blips, low-level viraemia and virological failure in the Australian HIV observational database. Trop. Med. Int. Health 2024, 29, 42–56. [Google Scholar] [CrossRef]
- Suzuki, K.; Levert, A.; Yeung, J.; Starr, M.; Cameron, J.; Williams, R.; Rismanto, N.; Stark, T.; Druery, D.; Prasad, S.; et al. HIV-1 viral blips are associated with repeated and increasingly high levels of cell-associated HIV-1 RNA transcriptional activity. AIDS 2021, 35, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, W.A.; van Deuren, R.C.; van de Wijer, L.; van den Munckhof, I.C.L.; Steehouwer, M.; Riksen, N.P.; Netea, M.G.; de Mast, Q.; Vandekerckhove, L.; de Over, R.M.; et al. Clonal Hematopoiesis Is Associated With Low CD4 Nadir and Increased Residual HIV Transcriptional Activity in Virally Suppressed Individuals With HIV. J. Infect. Dis. 2022, 225, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; SMART study group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Szubert, A.J.; Pimundu, G.; Berejena, C.; Pala, P.; Shonhai, A.; Hunter, P.; Arrigoni, F.I.F.; Musiime, V.; Bwakura-Dangarembizi, M.; et al. The impact of viraemia on inflammatory biomarkers and CD4+ cell subpopulations in HIV-infected children in sub-Saharan Africa. AIDS 2021, 35, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Jiamsakul, A.; Khusuwan, S.; Khol, V.; Pham, T.T.; Chaiwarith, R.; Avihingsanon, A.; Kumarasamy, N.; Wong, W.W.; Kiertiburanakul, S.; et al. IeDEA Asia-Pacific. The influence of age-associated comorbidities on responses to combination antiretroviral therapy in older people living with HIV. J. Int. AIDS Soc. 2019, 22, 25228. [Google Scholar] [CrossRef] [PubMed]
- EACS 2024 Guidelines for the Management of People Living with HIV in Europe. Available online: https://eacs.sanfordguide.com/ (accessed on 25 January 2025).
- European Centre for Disease Prevention and Control. HIV Infection and AIDS. Available online: https://www.ecdc.europa.eu/en/hiv-infection-and-aids (accessed on 25 January 2025).
- MMWR. Appendix A—AIDS-Defining Conditions. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a2.htm (accessed on 20 January 2025).


| Variable | Total (n = 109) | Patients with Drug Resistance Mutations (n = 62) | Patients with No Drug Resistance Mutations (n = 47) | p | Patients with HIV Subtype A (n = 24) | Patients with HIV Subtype B (n = 82) | p |
|---|---|---|---|---|---|---|---|
| Age mean (SD) | 38.05 (10.61) | 35.65 (10.16) | 41.21 (10.47) | 0.001 | 40.42 (10.21) | 36.88 (10.28) | 0.050 |
| Male gender n (%) | 92 (84.40) | 58 (93.55) | 34 (72.34) | 0.003 | 16 (66.67) | 76 (92.68) | 0.004 |
| Recent HIV infection n (%) | 12 (11.01) | 7 (11.29) | 5 (10.64) | 0.187 | 4 (16.67) | 8 (0.10) | 0.462 |
| AIDS-defining disease n (%) | 38 (34.86) | 20 (32.26) | 18 (38.30) | 0.123 | 8 (33.33) | 28 (34.15) | 1.000 |
| Baseline CD4 mean (SD) | 247.64 (244.50) | 270.02 (282.19) | 218.13 (182.18) | 0.325 | 277.17 (246.79) | 244.79 (246.85) | 0.306 |
| Baseline CD4:CD8 mean (SD) | 0.31 (0.28) | 0.33 (0.31) | 0.30 (0.24) | 0.352 | 0.41 (0.38) | 0.29 (0.24) | 0.078 |
| Variable | Subtype A with Mutations (n = 9) | Subtype A Without Mutations (n = 15) | p | Subtype B with Mutations (n = 50) | Subtype B Without Mutations (n = 32) | p |
|---|---|---|---|---|---|---|
| Baseline CD4 | 290.67 (329.32) | 269.07 (194.68) | 0.5 | 276.28 (280.87) | 194.25 (174.03) | 0.170 |
| CD4 after 36–48 months | 527.11 (302.85) | 524.47 (281.22) | 0.983 | 591.34 (283.46) | 450 (165.84) | 0.013 |
| ΔCD4 | 236.44 (186.37) | 255.40 (165.80) | 0.798 | 315.06 (259.88) | 256.69 (140.41) | 0.065 |
| CD4 normalization | 1 (16.67) | 2 (18.18) | 1.000 | 20 (50.00) | 11 (36.67) | 0.106 |
| No CD4 normalization | 5 (83.33) | 9 (81.82) | 1.000 | 20 (50.00) | 19 (63.33) | 0.106 |
| Baseline CD4:CD8 | 0.46 (0.54) | 0.38 (0.25) | 0.406 | 0.32 (0.25) | 0.25 (0.22) | 0.104 |
| CD4:CD8 after 36–48 months | 0.94 (0.74) | 0.96 (0.62) | 0.441 | 0.96 (0.74) | 0.75 (0.52) | 0.062 |
| ΔCD4:CD8 | 0.48 (0.69) | 0.58 (0.46) | 0.085 | 0.64 (0.64) | 0.49 (0.44) | 0.067 |
| CD4:CD8 normalization | 0 (0.00) | 4 (36.36) | 0.237 | 13 (32.50) | 6 (20.00) | 0.108 |
| No CD4:CD8 normalization | 6 (100.00) | 7 (63.64) | 0.237 | 27 (67.50) | 24 (80.00) | 0.108 |
| Variable | Subtype A with Mutations (n = 9) | Subtype B with Mutations (n = 50) | p | Subtype A Without Mutations (n = 15) | Subtype B Without Mutations (n = 32) | p |
|---|---|---|---|---|---|---|
| Baseline CD4 | 290.67 (329.32) | 276.28 (280.87) | 0.479 | 269.07 (194.68) | 194.25 (174.03) | 0.120 |
| CD4 after 36–48 months | 527.11 (302.85) | 591.34 (283.46) | 0.538 | 524.47 (281.22) | 450 (165.84) | 0.266 |
| ΔCD4 | 236.44 (186.37) | 315.06 (259.88) | 0.057 | 255.40 (165.80) | 256.69 (140.41) | 0.432 |
| CD4 normalization | 1 (16.67) | 20 (50.00) | 0.198 | 2 (18.18) | 11 (36.67) | 0.165 |
| No CD4 normalization | 5 (83.33) | 20 (50.00) | 0.198 | 9 (81.82) | 19 (63.33) | 0.165 |
| Baseline CD4:CD8 | 0.46 (0.54) | 0.32 (0.25) | 0.336 | 0.38 (0.25) | 0.25 (0.22) | 0.058 |
| CD4:CD8 after 36–48 months | 0.94 (0.74) | 0.96 (0.74) | 0.384 | 0.96 (0.62) | 0.75 (0.52) | 0.142 |
| ΔCD4:CD8 | 0.48 (0.69) | 0.64 (0.64) | 0.047 | 0.58 (0.46) | 0.49 (0.44) | 0.219 |
| CD4:CD8 normalization | 0 (0.00) | 13 (32.50) | 0.163 | 4 (36.36) | 6 (20.00) | 0.709 |
| No CD4:CD8 normalization | 6 (100.00) | 27 (67.50) | 0.163 | 7 (63.64) | 24 (80.00) | 0.709 |
| Variable | NRTI Mutations (n = 11) | NNRTI Mutations (n = 10) | PI Mutations (n = 50) | p |
|---|---|---|---|---|
| Baseline CD4 | 265.27 (309.33) | 261.20 (430.21) | 291.86 (295.41) | 0.942 |
| CD4 after 36–48 months | 602.64 (238.39) | 392.70 (209.36) | 580.24 (291.39) | 0.128 |
| ΔCD4 | 337.36 (341.06) | 131.5 (247.41) | 288.38 (251.77) | 0.170 |
| CD4 normalization | 6 (60.00) | 1 (12.50) | 17 (44.74) | 0.117 |
| No CD4 normalization | 4 (40.00) | 7 (87.50) | 21 (55.26) | 0.117 |
| Baseline CD4:CD8 | 0.31 (0.17) | 0.23 (0.18) | 0.35 (0.33) | 0.514 |
| CD4:CD8 after 36–48 months | 0.98 (0.39) | 0.65 (0.33) | 1.00 (0.79) | 0.356 |
| ΔCD4:CD8 | 0.67 (0.28) | 0.42 (0.26) | 0.66 (0.70) | 0.534 |
| CD4:CD8 normalization | 4 (40.00) | 1 (12.50) | 12 (31.58) | 0.503 |
| No CD4:CD8 normalization | 6 (60.00) | 7 (87.50) | 26 (68.42) | 0.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Załęski, A.; Lembas, A.; Dyda, T.; Osińska, J.; Jabłońska, J.; Stempkowska-Rejek, J.; Orzechowska, J.; Wiercińska-Drapało, A. No Association Between HIV-1 Subtype and Primary Resistance Mutations with CD4 Reconstitution During Effective Antiretroviral Treatment: An Observational, Cohort Study. Int. J. Mol. Sci. 2025, 26, 1410. https://doi.org/10.3390/ijms26041410
Załęski A, Lembas A, Dyda T, Osińska J, Jabłońska J, Stempkowska-Rejek J, Orzechowska J, Wiercińska-Drapało A. No Association Between HIV-1 Subtype and Primary Resistance Mutations with CD4 Reconstitution During Effective Antiretroviral Treatment: An Observational, Cohort Study. International Journal of Molecular Sciences. 2025; 26(4):1410. https://doi.org/10.3390/ijms26041410
Chicago/Turabian StyleZałęski, Andrzej, Agnieszka Lembas, Tomasz Dyda, Joanna Osińska, Joanna Jabłońska, Justyna Stempkowska-Rejek, Justyna Orzechowska, and Alicja Wiercińska-Drapało. 2025. "No Association Between HIV-1 Subtype and Primary Resistance Mutations with CD4 Reconstitution During Effective Antiretroviral Treatment: An Observational, Cohort Study" International Journal of Molecular Sciences 26, no. 4: 1410. https://doi.org/10.3390/ijms26041410
APA StyleZałęski, A., Lembas, A., Dyda, T., Osińska, J., Jabłońska, J., Stempkowska-Rejek, J., Orzechowska, J., & Wiercińska-Drapało, A. (2025). No Association Between HIV-1 Subtype and Primary Resistance Mutations with CD4 Reconstitution During Effective Antiretroviral Treatment: An Observational, Cohort Study. International Journal of Molecular Sciences, 26(4), 1410. https://doi.org/10.3390/ijms26041410







