Association of Selected STAT Inhibitors with Prolactin-Induced Protein (PIP) in Breast Cancer
Abstract
1. Introduction
2. Results
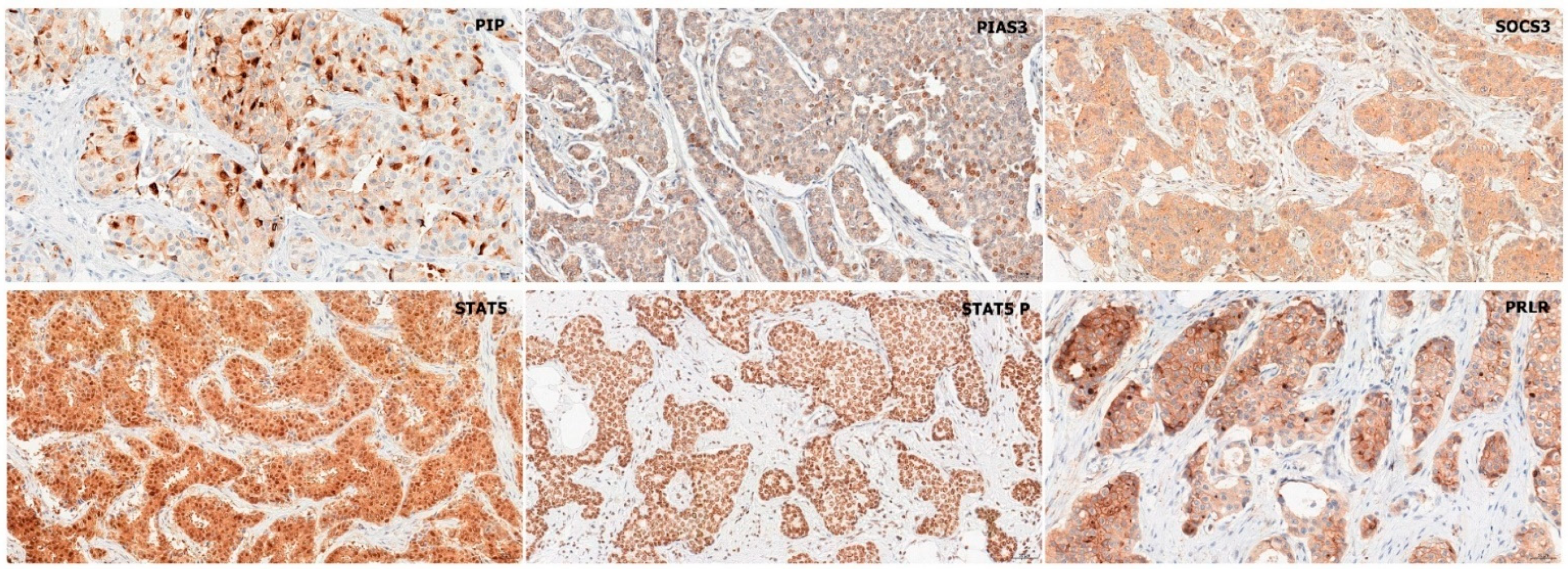
2.1. Immunohistochemistry
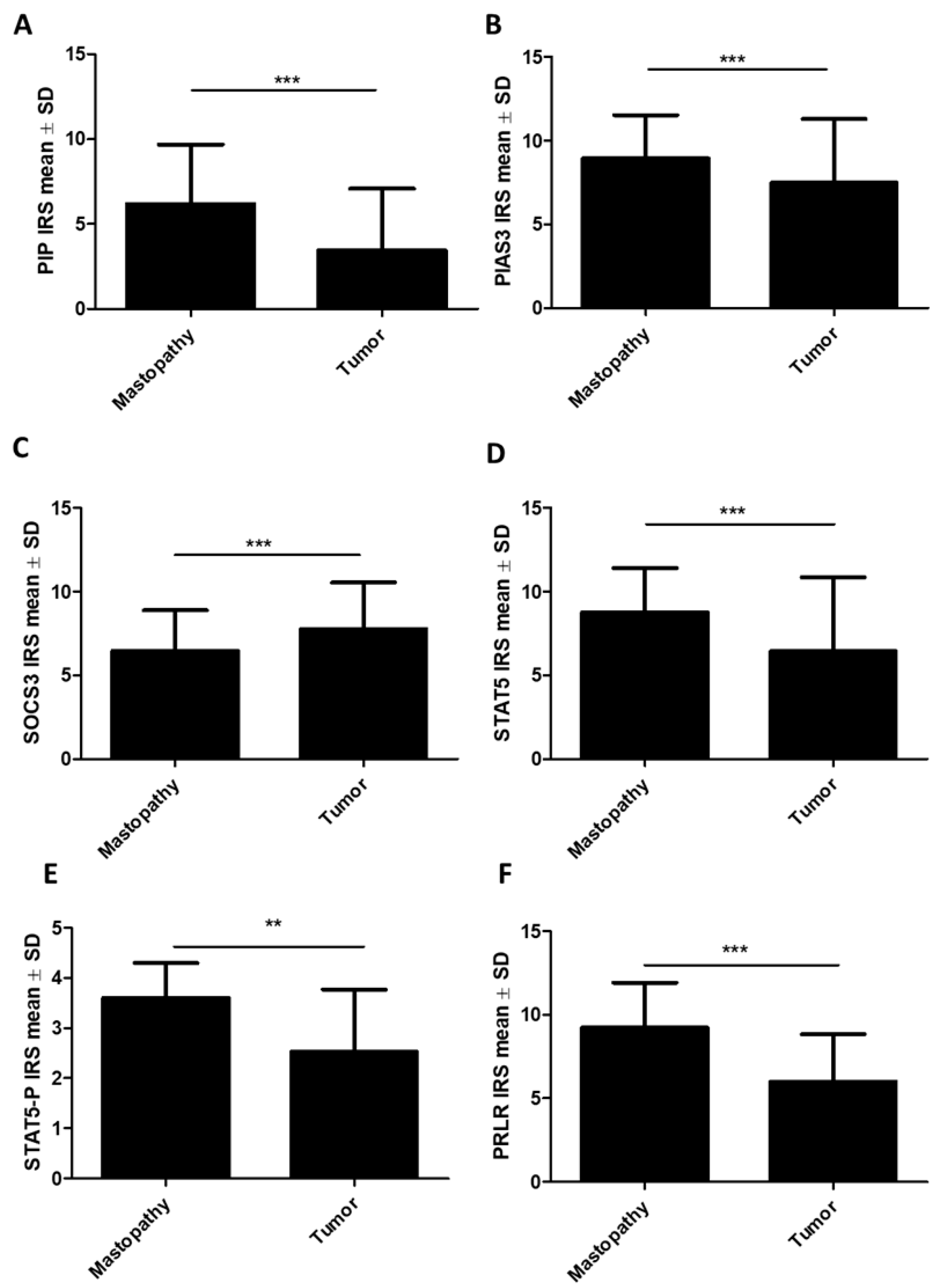
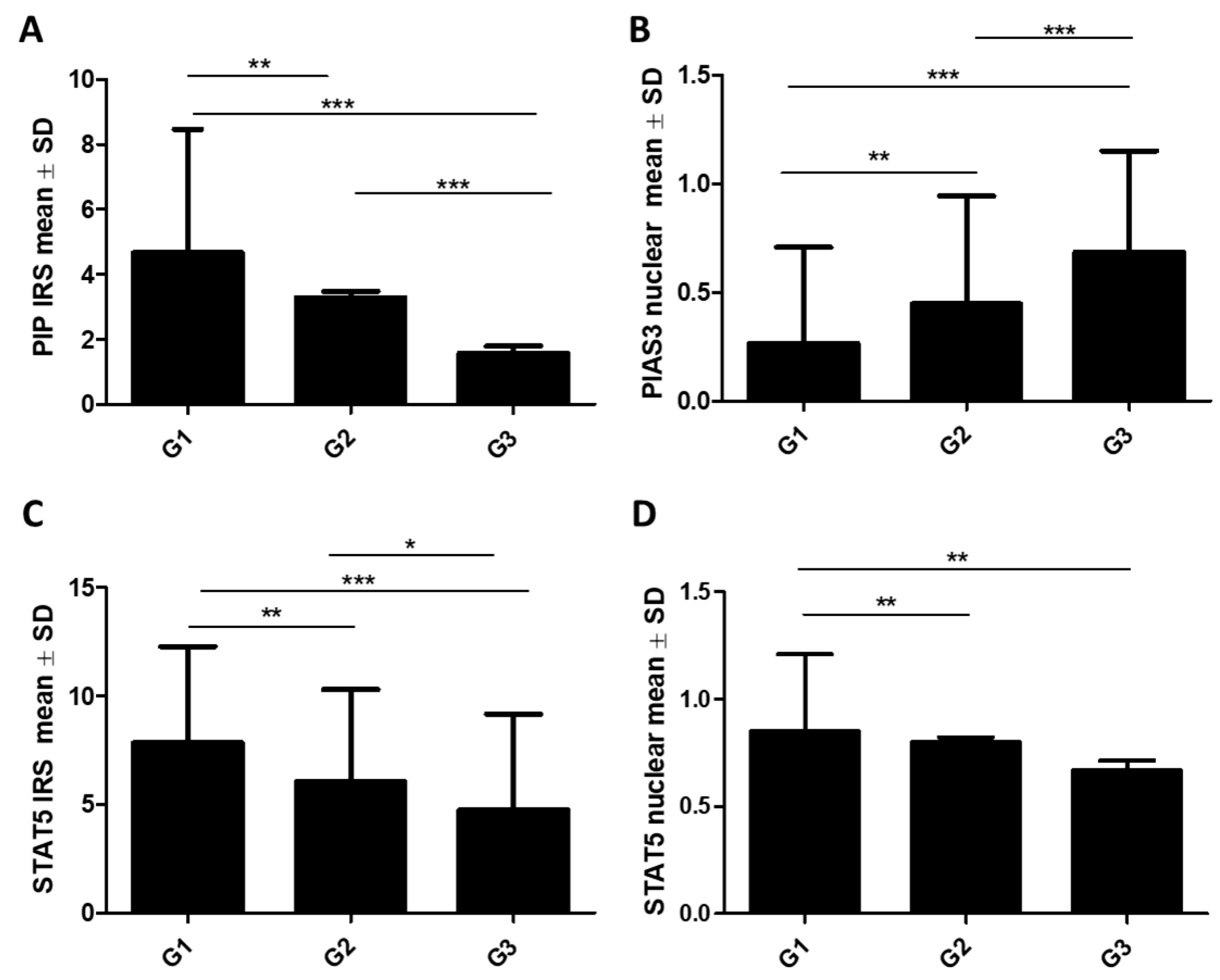
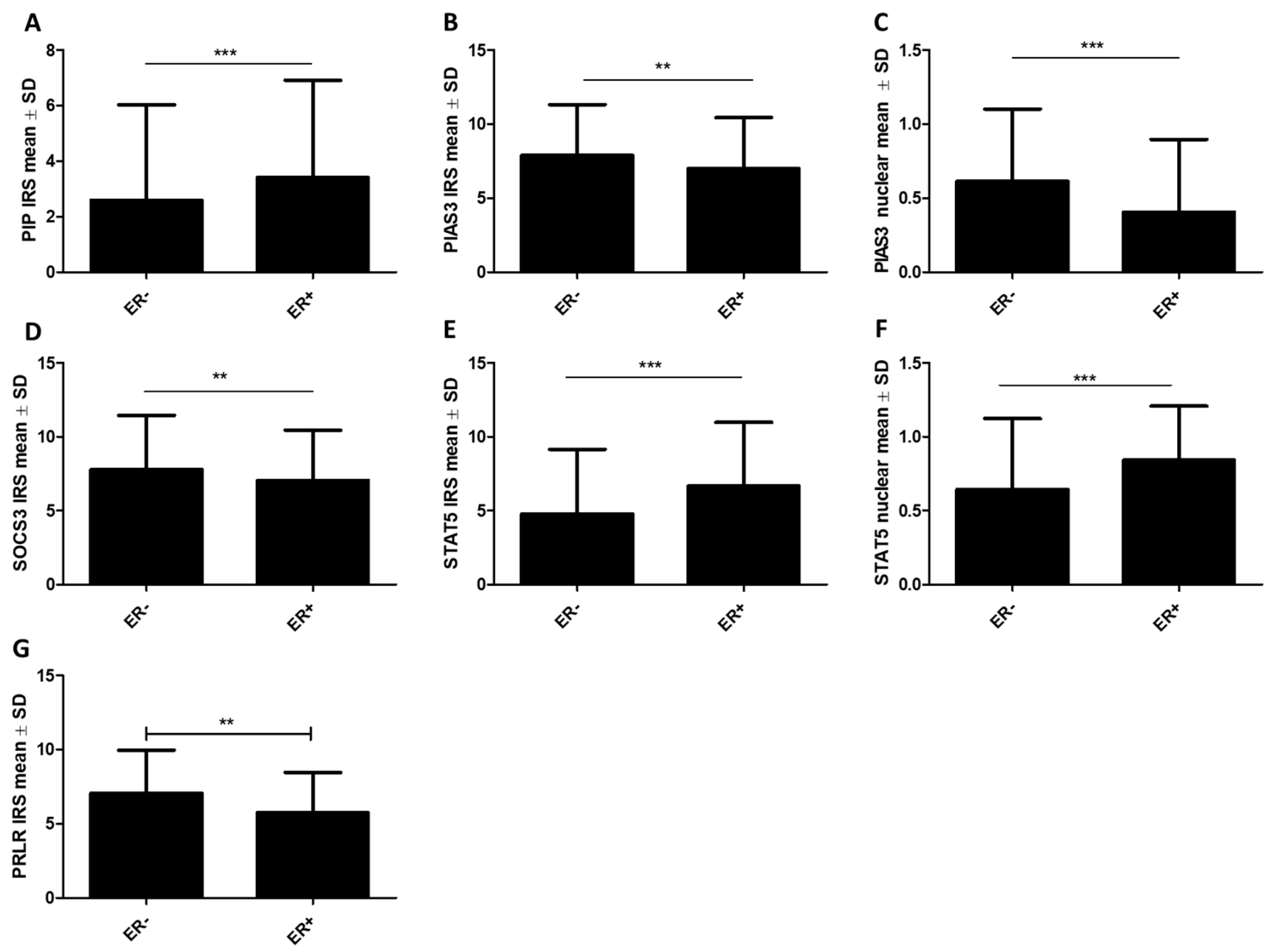
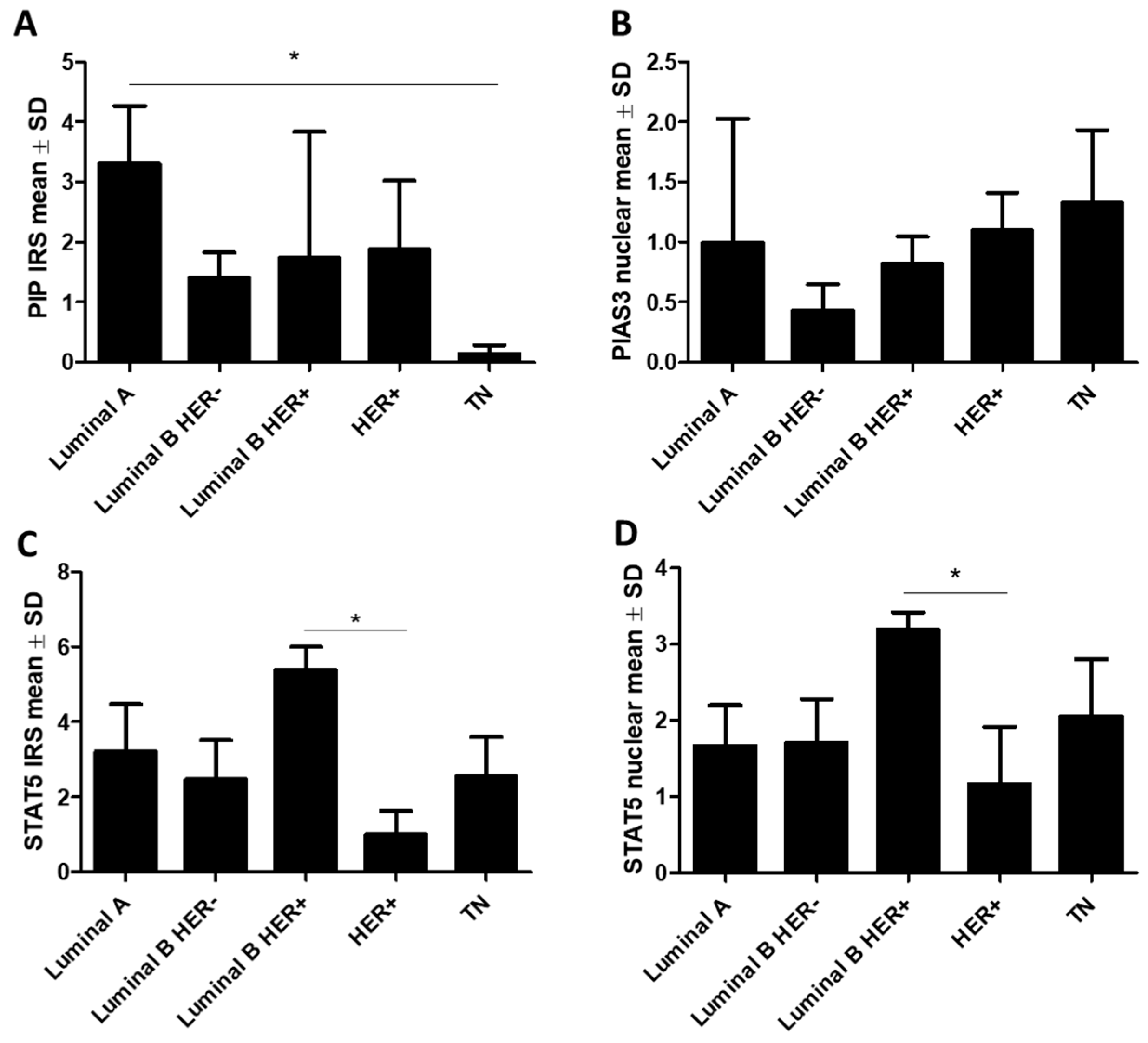
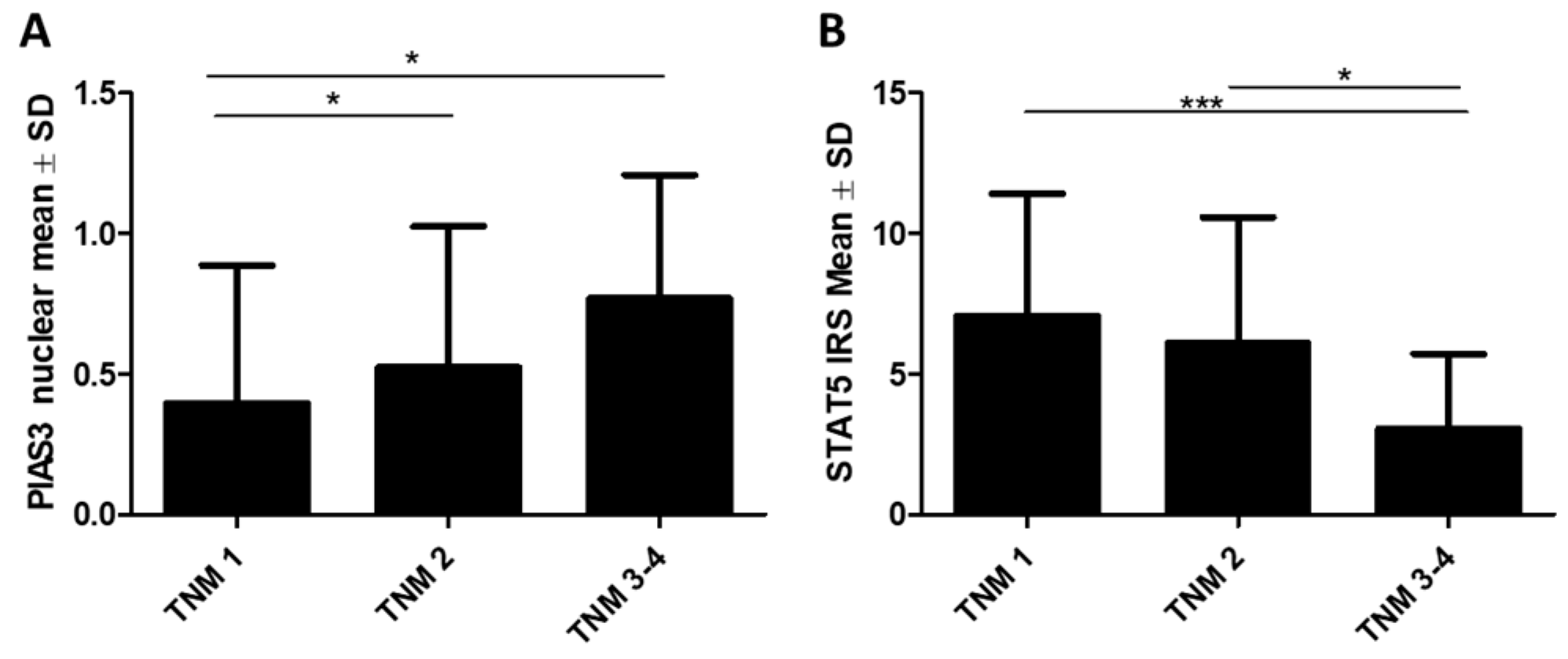
2.1.1. Association of PIP, PIAS3, SOCS3, STAT5, and PRLR with Clinicopathological Factors
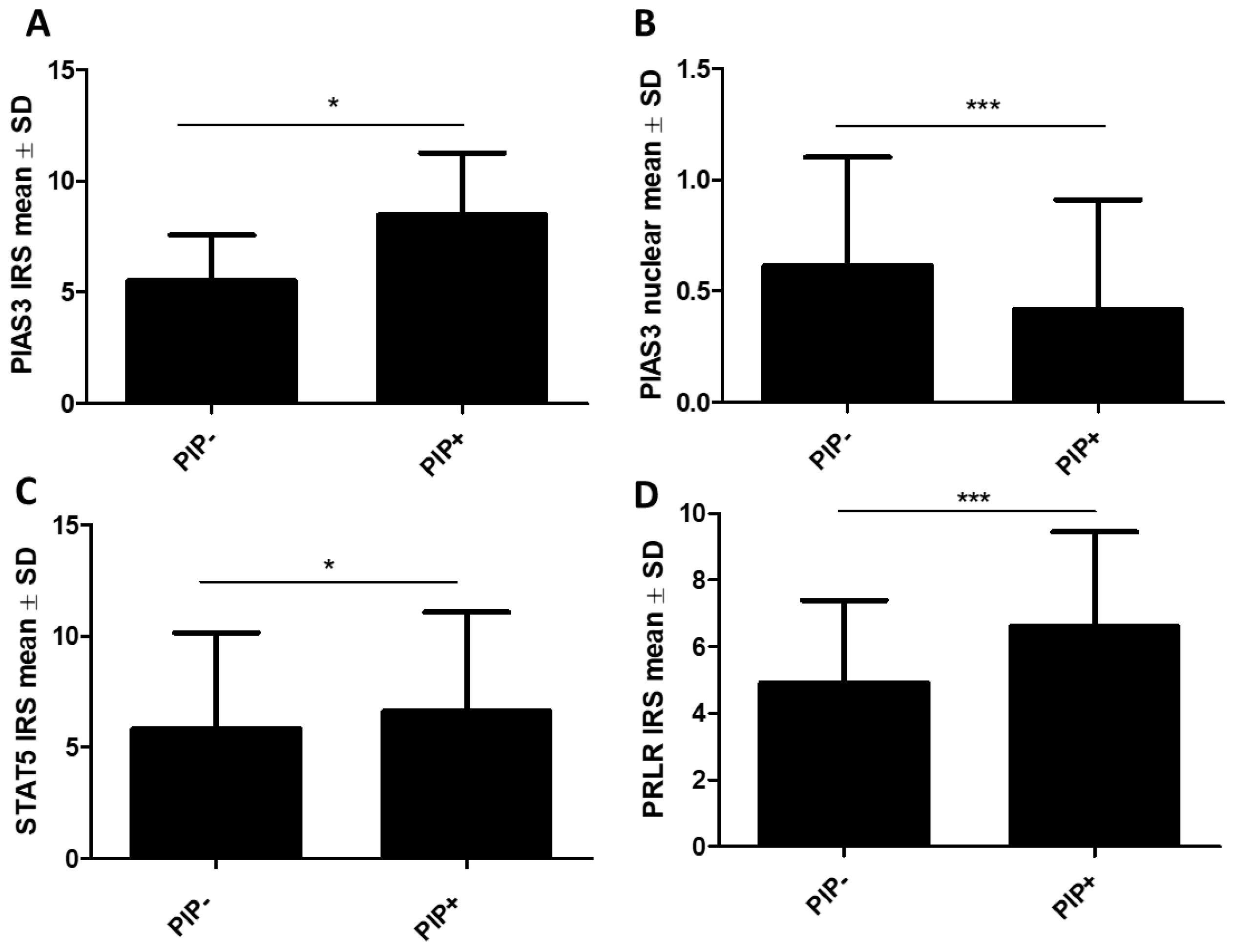
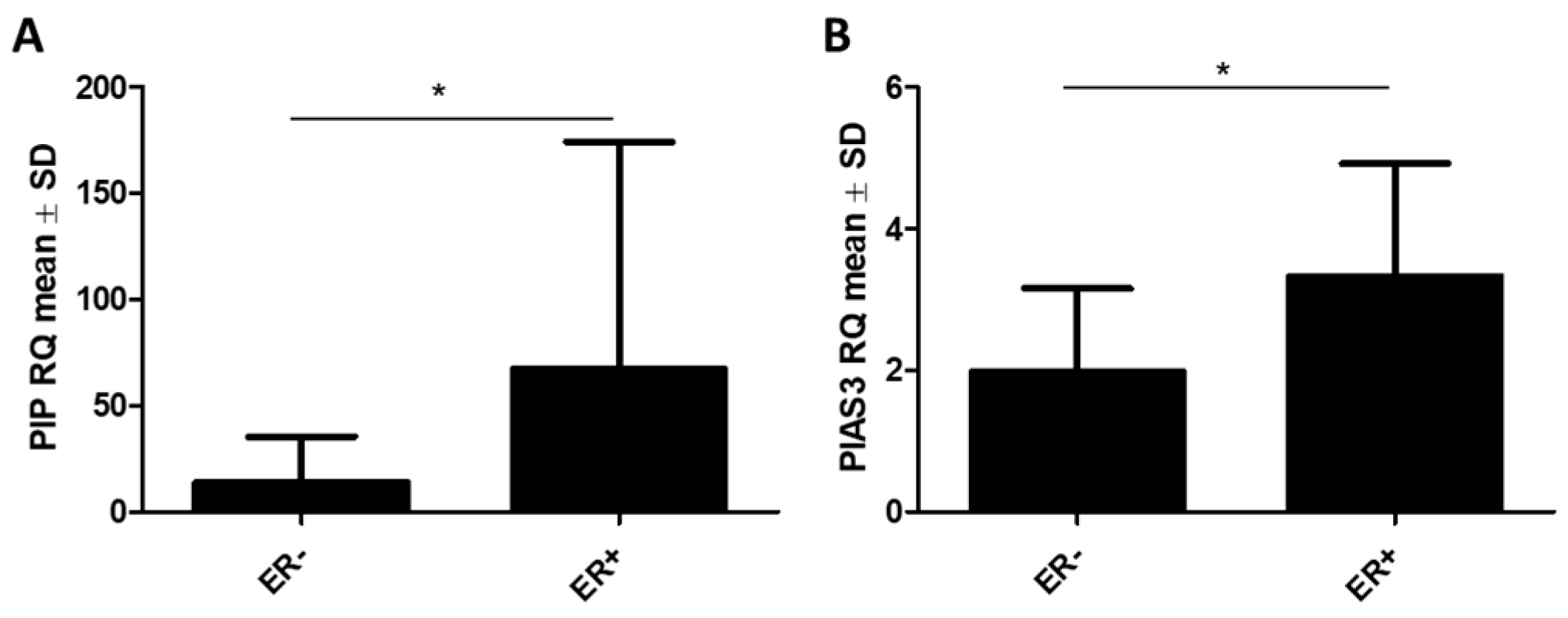
2.1.2. Association of STAT Inhibitors (PIAS3 and SOCS3), STAT5, and PRLR with PIP Expression
2.1.3. Associations with Survival
2.2. Real-Time qPCR
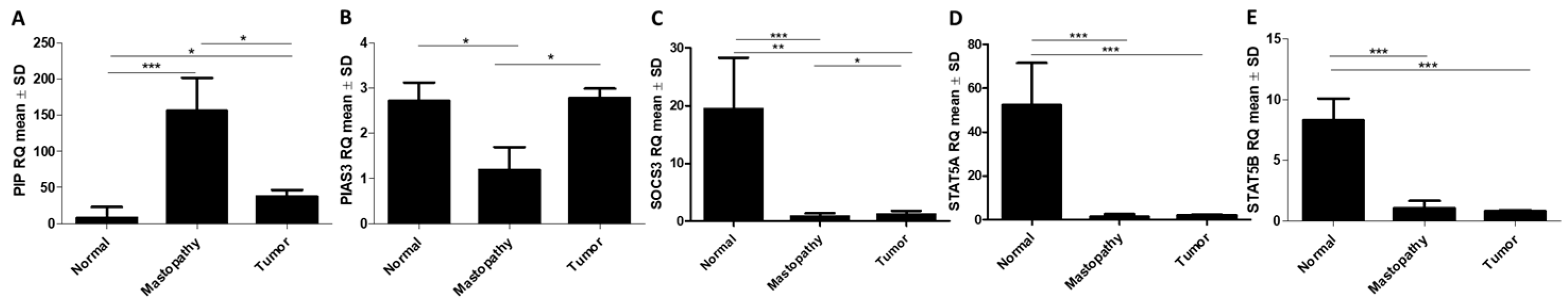
2.2.1. RT-qPCR Detection of PIAS3, SOCS3, STAT5, and PIP Gene Expression in BC and Control Tissues
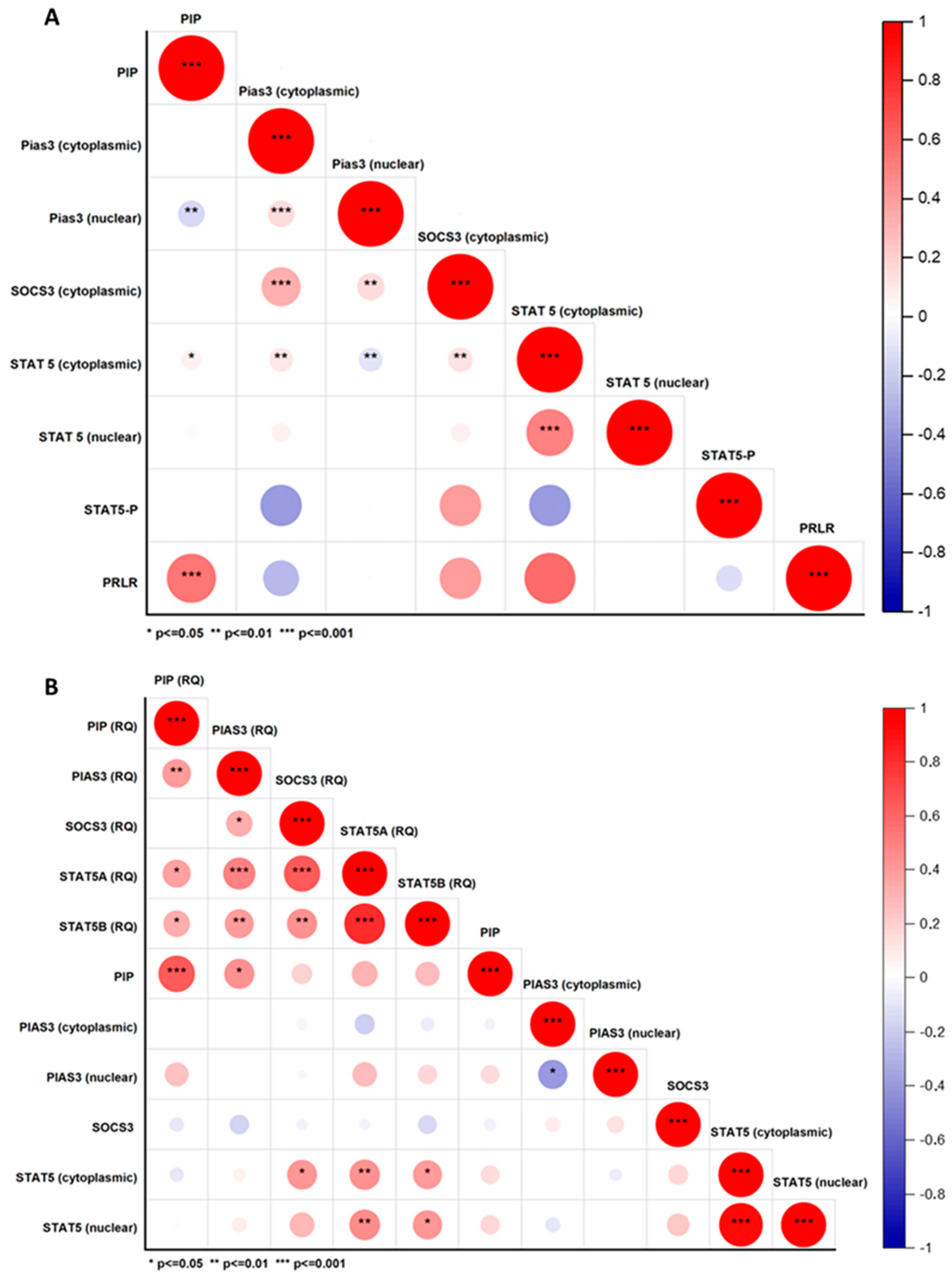
2.2.2. Correlations Between PIP, PIAS3, SOCS3, STAT5, and PRLR at Protein and mRNA Level
2.3. Cell Lines (In Vitro Tests)
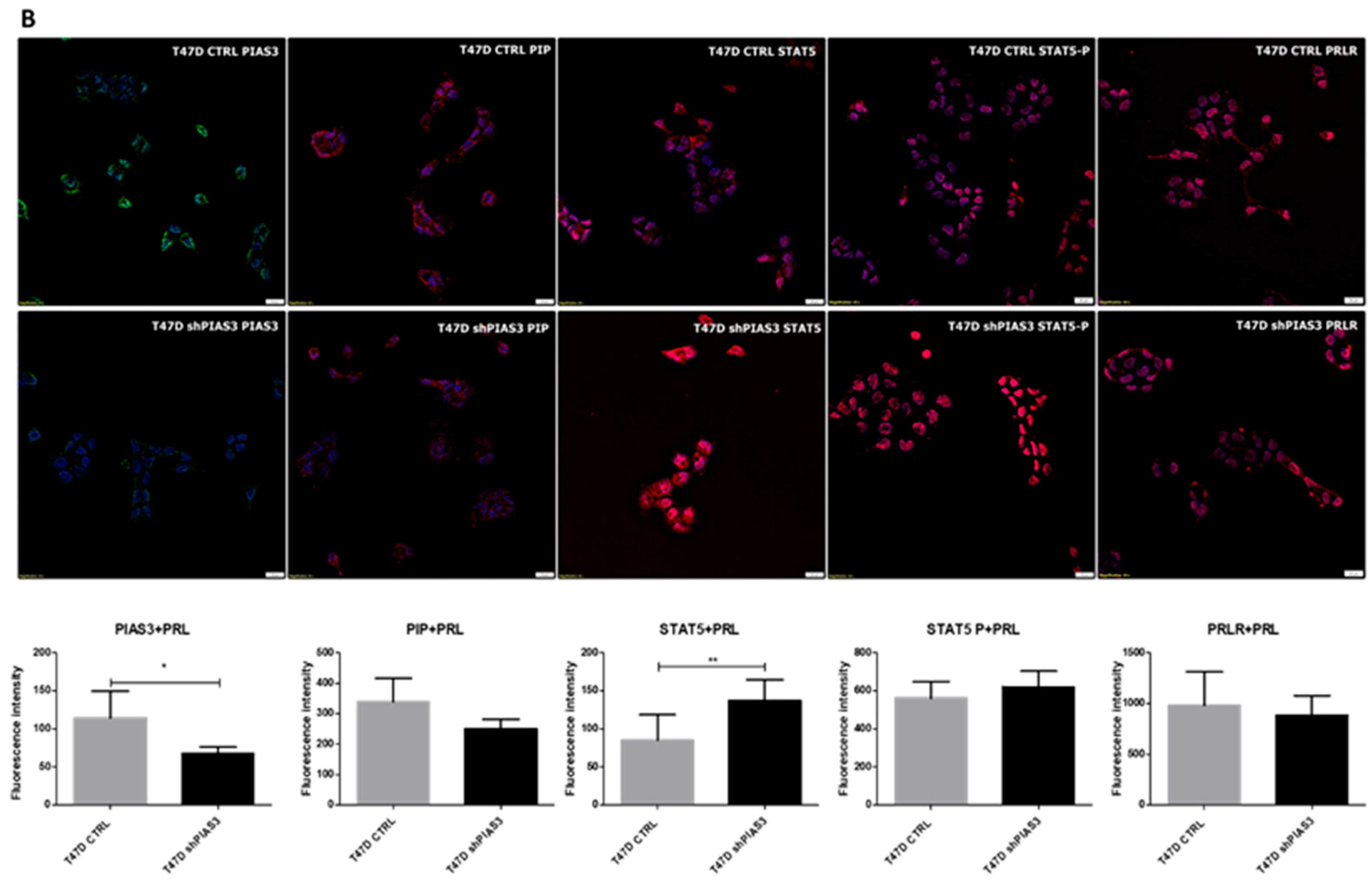
2.3.1. Assessment of Protein Localization and Fluorescence Intensity in the T47D Cell Lines
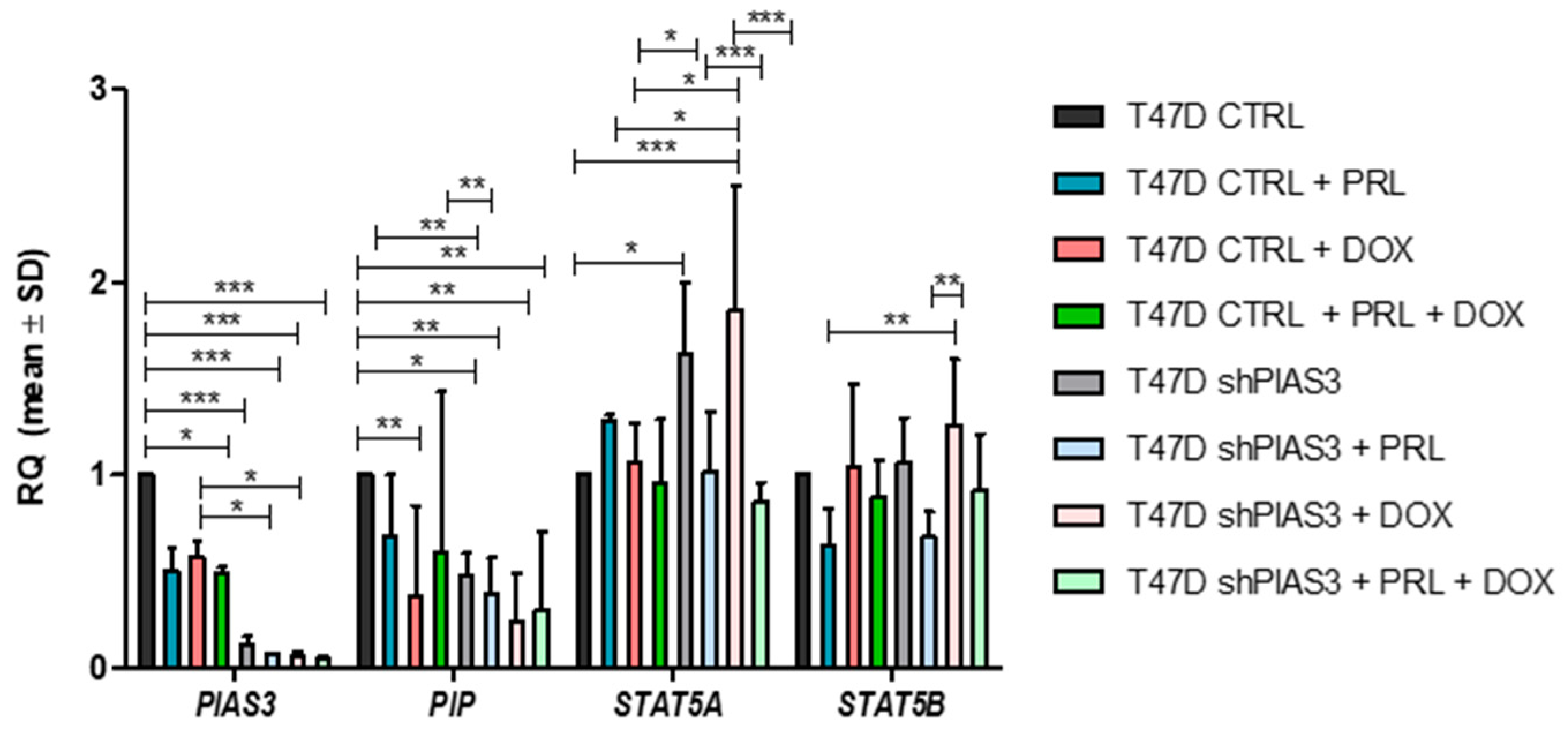
2.3.2. RT-PCR Analysis of mRNA Expression Levels of PIAS3, PIP, STAT5A, and STAT5B in T47D Cell Lines
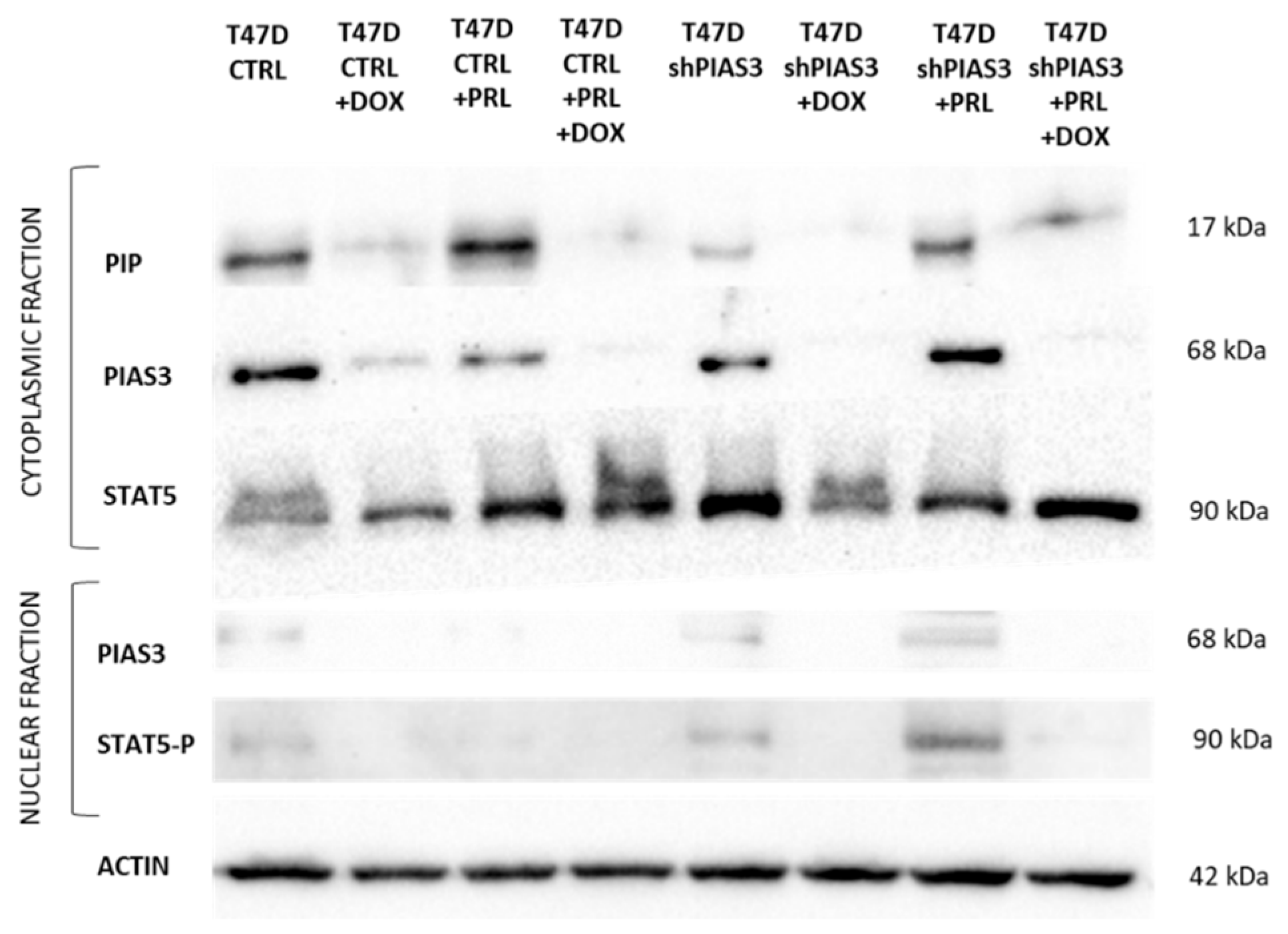
2.3.3. Western Blot Analysis of Cytoplasmic and Nuclear Fractions Isolated from T47D Cell Lines
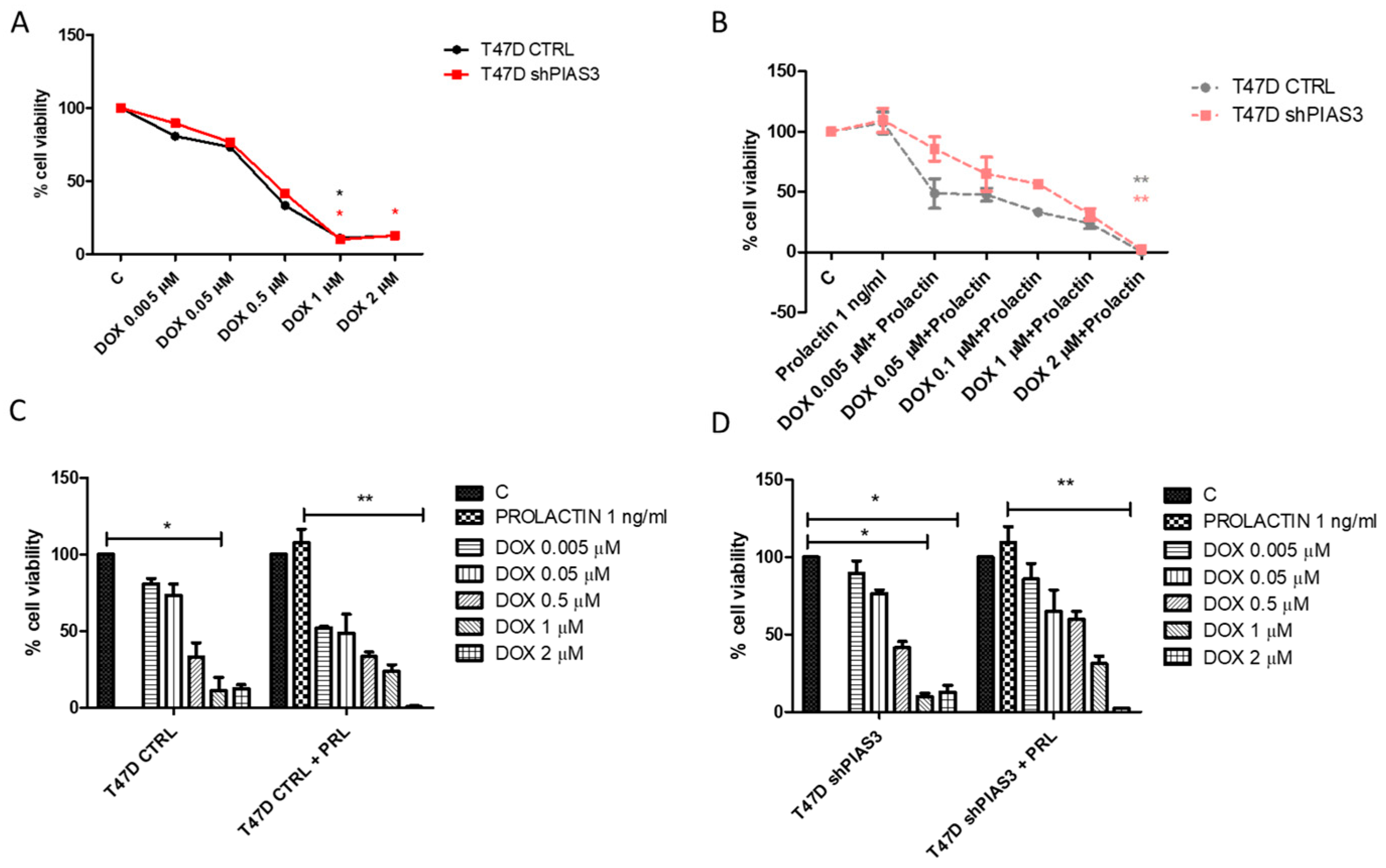
2.3.4. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)—Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. TMAs—Tissue Microarrays
4.3. Immunohistochemistry
4.4. Real-Time PCR
4.5. Cell Line
4.6. Virus Production, Transductions, and Cell Maintenance
4.7. Immunofluorescence
4.8. Western Blot
4.9. Analysis of Cell Viability by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. GLOBOCAN Cancer Fact Sheet 2019. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 4 December 2024).
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Jablonska, K.; Grzegrzolka, J.; Podhorska-Okolow, M.; Stasiolek, M.; Pula, B.; Olbromski, M.; Gomulkiewicz, A.; Piotrowska, A.; Rys, J.; Ambicka, A.; et al. Prolactin-induced protein as a potential therapy response marker of adjuvant chemotherapy in breast cancer patients. Am. J. Cancer Res. 2016, 6, 878–893. [Google Scholar]
- Murphy, L.C.; Tsuyuki, D.; Myal, Y.; Shiu, R.P. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J. Biol. Chem. 1987, 262, 15236–15241. [Google Scholar] [CrossRef]
- Schenkels, L.C.P.M.; Walgreen-Weterings, E.; Oomen, L.C.J.M.; Bolscher, J.G.M.; Veerman, E.C.I.; Amerongen, A.V.N. In Vivo Binding of the Salivary Glycoprotein EP-GP (Identical to GCDFP-15) to Oral and Non-Oral Bacteria Detection and Identification of EP-GP Binding Species. Biol. Chem. 1997, 378, 83–88. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Rathman, W.M.; Veerman, E.C.; Amerongen, A.V.N. Detection of Proteins Related to a Salivary Glycoprotein (EP-GP). Concentrations in Human Secretions (Saliva, Sweat, Tears, Nasal Mucus, Cerumen, Seminal Plasma). Biol. Chem. Hoppe-Seyler 1991, 372, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, A.; Jablonska, K.; Podhorska-Okolow, M.; Ugorski, M.; Dziegiel, P. Prolactin-induced protein (PIP)-characterization and role in breast cancer progression. Am. J. Cancer Res. 2018, 8, 2150–2164. [Google Scholar] [PubMed]
- Blais, Y.; Gingras, S.; Haagensen, D.E.; Labrie, F.; Simard, J. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol. Cell. Endocrinol. 1996, 121, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Haagensen, D.E.; Stewart, P.; Dilley, W.G.; Wells, S.A. Secretion of breast gross cystic disease fluid proteins by T47D breast cancer cells in culture—Modulation by steroid hormones. Breast Cancer Res. Treat. 1992, 23, 77–86. [Google Scholar] [CrossRef]
- Loos, S.; Schulz, K.D.; Hackenberg, R. Regulation of GCDFP-15 expression in human mammary cancer cells. Int. J. Mol. Med. 1999, 4. [Google Scholar] [CrossRef]
- Clark, J.W.; Snell, L.; Shiu, R.P.C.; Orr, F.W.; Maitre, N.; Vary, C.P.H.; Cole, D.J.; Watson, P.H. The potential role for prolactin-inducible protein (PIP) as a marker of human breast cancer micrometastasis. Br. J. Cancer 1999, 81, 1002–1008. [Google Scholar] [CrossRef]
- Ihedioha, O.C.; Shiu, R.P.; Uzonna, J.E.; Myal, Y. Prolactin-Inducible Protein: From Breast Cancer Biomarker to Immune Modulator—Novel Insights from Knockout Mice. DNA Cell Biol. 2016, 35, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, C.; Merino, M.J.; LaPorte, N.; Neumann, R.D. Value of gross cystic disease fluid protein-15 in distinguishing metastatic breast carcinomas among poorly differentiated neoplasms involving the ovary. Hum. Pathol. 1991, 22, 368–372. [Google Scholar] [CrossRef]
- Urbaniak, A.; Jablonska, K.; Suchanski, J.; Partynska, A.; Szymczak-Kulus, K.; Matkowski, R.; Maciejczyk, A.; Ugorski, M.; Dziegiel, P. Prolactin-induced protein (PIP) increases the sensitivity of breast cancer cells to drug-induced apoptosis. Sci. Rep. 2023, 13, 6574. [Google Scholar] [CrossRef]
- Shiu, R.P.; Iwasiow, B.M. Prolactin-inducible proteins in human breast cancer cells. J. Biol. Chem. 1985, 260, 11307–11313. [Google Scholar] [CrossRef]
- McHale, K.; E Tomaszewski, J.; Puthiyaveettil, R.; A LiVolsi, V.; Clevenger, C.V. Altered expression of prolactin receptor-associated signaling proteins in human breast carcinoma. Mod. Pathol. 2008, 21, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Abramicheva, P.A.; Smirnova, O.V. Prolactin Receptor Isoforms as the Basis of Tissue-Specific Action of Prolactin in the Norm and Pathology. Biochemistry 2019, 84, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J. Stat Proteins and Oncogenesis. J. Clin. Investig. 2002, 109, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Chen, X. STAT Proteins: Signal Tranducers and Activators of Transcription. Methods Enzymol. 2001, 333, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Debily, M.-A.; El Marhomy, S.; Boulanger, V.; Eveno, E.; Mariage-Samson, R.; Camarca, A.; Auffray, C.; Piatier-Tonneau, D.; Imbeaud, S. A Functional and Regulatory Network Associated with PIP Expression in Human Breast Cancer. PLoS ONE 2009, 4, e4696. [Google Scholar] [CrossRef]
- Chia, S.Y.; Thike, A.A.; Cheok, P.Y.; Tan, P.H. Utility of mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15) in confirming a breast origin for recurrent tumors. Breast 2010, 19, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Wakao, H.; Harada, N.; Kitamura, T.; Mui, A.L.F.; Miyajima, A. Interleukin 2 and Erythropoietin Activate STAT5/MGF via Distinct Pathways. EMBO J. 1995, 14, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.J.; Sliva, D.; Lobie, P.E.; Pircher, T.J.; Gouilleux, F.; Wakao, H.; Gustafsson, J.; Groner, B.; Norstedt, G.; Haldosén, L.-A. Mediation of Growth Hormone-dependent Transcriptional Activation by Mammary Gland Factor/Stat 5. J. Biol. Chem. 1995, 270, 9448–9453. [Google Scholar] [CrossRef]
- Peck, A.R.; Witkiewicz, A.K.; Liu, C.; Stringer, G.A.; Klimowicz, A.C.; Pequignot, E.; Freydin, B.; Tran, T.H.; Yang, N.; Rosenberg, A.L.; et al. Loss of Nuclear Localized and Tyrosine Phosphorylated Stat5 in Breast Cancer Predicts Poor Clinical Outcome and Increased Risk of Antiestrogen Therapy Failure. J. Clin. Oncol. 2011, 29, 2448–2458. [Google Scholar] [CrossRef]
- Yu, H.; Jove, R. The STATs of cancer — new molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef]
- Krebs, D.L.; Hilton, D.J. SOCS Proteins: Negative Regulators of Cytokine Signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Helman, D.; Sandowski, Y.; Cohen, Y.; Matsumoto, A.; Yoshimura, A.; Merchav, S.; Gertler, A. Cytokine-inducible SH2 protein (CIS3) and JAK2 binding protein (JAB) abolish prolactin receptor-mediated STAT5 signaling. FEBS Lett. 1998, 441, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Pezet, A.; Favre, H.; Kelly, P.A.; Edery, M. Inhibition and Restoration of Prolactin Signal Transduction by Suppressors of Cytokine Signaling. J. Biol. Chem. 1999, 274, 24497–24502. [Google Scholar] [CrossRef]
- Arora, T.; Liu, B.; He, H.; Kim, J.; Murphy, T.L.; Murphy, K.M.; Modlin, R.L.; Shuai, K. PIASx Is a Transcriptional Co-repressor of Signal Transducer and Activator of Transcription 4. J. Biol. Chem. 2003, 278, 21327–21330. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liao, J.; Rao, X.; Kushner, S.A.; Chung, C.D.; Chang, D.D.; Shuai, K. Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. USA 1998, 95, 10626–10631. [Google Scholar] [CrossRef]
- Schmidt, D. PIAS/SUMO: New partners in transcriptional regulation. Cell. Mol. Life Sci. 2003, 60, 2561–2574. [Google Scholar] [CrossRef]
- Prigge, J.R.; Schmidt, E.E. Interaction of Protein Inhibitor of Activated STAT (PIAS) Proteins with the TATA-binding Protein, TBP. J. Biol. Chem. 2006, 281, 12260–12269. [Google Scholar] [CrossRef] [PubMed]
- Dagvadorj, A.; Tan, S.-H.; Liao, Z.; Xie, J.; Nurmi, M.; Alanen, K.; Rui, H.; Mirtti, T.; Nevalainen, M.T. N-terminal truncation of Stat5a/b circumvents PIAS3-mediated transcriptional inhibition of Stat5 in prostate cancer cells. Int. J. Biochem. Cell Biol. 2010, 42, 2037–2046. [Google Scholar] [CrossRef]
- Győrffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation 2024, 5, 100625. [Google Scholar] [CrossRef]
- Wang, L.; Banerjee, S. Differential PIAS3 expression in human malignancy. Oncol. Rep. 2004, 11, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, Z.; Tao, Y.; Liang, W.; Hu, W.; Zhou, S.; Fu, X.; Wang, X. Emerging roles of suppressor of cytokine signaling 3 in human cancers. Biomed. Pharmacother. 2021, 144, 112262. [Google Scholar] [CrossRef] [PubMed]
- Schieler, J.M.; Henderson, J.O. Treating a Dysregulated JAK/STAT Pathway in Cancer Cells. J. Stud. Res. 2016, 5, 11–17. [Google Scholar] [CrossRef]
- Sauer, N.; Matkowski, I.; Bodalska, G.; Murawski, M.; Dzięgiel, P.; Calik, J. Prognostic Role of Prolactin-Induced Protein (PIP) in Breast Cancer. Cells 2023, 12, 2252. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; Duggan, M.A.; Sherman, M.E.; Garcia-Closas, M.; Yang, X.R.; Lissowska, J.; Brinton, L.A.; Peplonska, B.; Vonderhaar, B.K.; Figueroa, J.D. Prolactin Receptor Expression and Breast Cancer: Relationships with Tumor Characteristics among Pre- and Post-menopausal Women in a Population-Based Case–Control Study from Poland. Discov. Oncol. 2013, 5, 42–50. [Google Scholar] [CrossRef]
- Barclay, J.L.; Anderson, S.T.; Waters, M.J.; Curlewis, J.D. SOCS3 as a tumor suppressor in breast cancer cells, and its regulation by PRL. Int. J. Cancer 2009, 124, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Sharrocks, A.D. PIAS Proteins and Transcriptional Regulation-More than Just SUMO E3 Ligases? Genes Dev. 2006, 20, 754–758. [Google Scholar] [CrossRef]
- Nishida, T.; Yasuda, H. PIAS1 and PIASxα Function as SUMO-E3 Ligases toward Androgen Receptor and Repress Androgen Receptor-dependent Transcription. J. Biol. Chem. 2002, 277, 41311–41317. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Liu, B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat. Rev. Immunol. 2005, 5, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Xie, J.; LeBaron, M.J.; Ealley, E.L.; Nevalainen, M.T.; Rui, H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 2004, 24, 746–760. [Google Scholar] [CrossRef]
- Alvarez, J.V.; Frank, D.A. Genome-wide analysis of STAT target genes: Elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol. Ther. 2004, 3, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, W.; Zhang, Y.; Hong, J.; Su, W.; Tang, J.; Wang, Y.; Lu, R.; Fang, J. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol. Carcinog. 2011, 51, 174–184. [Google Scholar] [CrossRef]
- Watson, C.; Miller, W. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. Br. J. Cancer 1995, 71, 840–844. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.-W.; Liu, P.; Yu, W.; Liu, T.; Yu, J. Dysregulation of SOCS-Mediated Negative Feedback of Cytokine Signaling in Carcinogenesis and Its Significance in Cancer Treatment. Front. Immunol. 2017, 8, 70. [Google Scholar] [CrossRef]
- Raccurt, M.; Tam, S.P.; Lau, P.; Mertani, H.C.; Lambert, A.; Garcia-Caballero, T.; Li, H.; Brown, R.J.; A McGuckin, M.; Morel, G.; et al. Suppressor of cytokine signalling gene expression is elevated in breast carcinoma. Br. J. Cancer 2003, 89, 524–532. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, X. Decreased prolactin-inducible protein expression exhibits inhibitory effects on the metastatic potency of breast cancer cells. Chin. -Ger. J. Clin. Oncol. 2013, 12, 101–105. [Google Scholar] [CrossRef]
- Barclay, J.L.; Anderson, S.T.; Waters, M.J.; Curlewis, J.D. Characterization of the SOCS3 Promoter Response to Prostaglandin E2 in T47D Cells. Mol. Endocrinol. 2007, 21, 2516–2528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, X.; Shao, J.; Wei, S.; Gu, Y.; Qian, J. Prognostic Significance of SOCS3 in Patients With Solid Tumors: A Meta-Analysis. Front. Surg. 2022, 8, 802143. [Google Scholar] [CrossRef] [PubMed]
- Hähnel, E.; Harvey, J.; Robbins, P.; Sterrett, G.; Hähnel, R. Hormone-Regulated Genes (pS2, PIP, FAS) in Breast Cancer and Nontumoral Mammary Tissue. Pathobiology 1994, 62, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hähnel, R.; Hähnel, E. Expression of the PIP/GCDFP-15 gene and survival in breast cancer. Virchows Arch. 1996, 429, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nishio, M.; Ando, Y.; Zhang, Z.; Hamaguchi, M.; Mita, K.; Kobayashi, S.; Fujii, Y.; Iwase, H. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr. -Relat. Cancer 2006, 13, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, M.; Gupta, S. Jak2/Stat5a/b Pathway in Prostate Cancer. In Cancer Therapeutic Targets; Springer: New York, NY, USA, 2017; Volume 2, pp. 1–8. [Google Scholar] [CrossRef]
- Nevalainen, M.; Gupta, S. Jak2/Stat5a/b Pathway in Prostate Cancer. In Cancer Therapeutic Targets; Springer: New York, NY, USA, 2013; Volume 2, pp. 745–752. [Google Scholar] [CrossRef]
- Walker, S.R.; Xiang, M.; Frank, D.A. Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer. Mol. Cell. Endocrinol. 2013, 382, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, C.A.; Rice, M.S.; Collins, L.C.; Chen, D.; Frank, D.A.; Walker, S.; Clevenger, C.V.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Prolactin levels and breast cancer risk by tumor expression of prolactin-related markers. Breast Cancer Res. 2023, 25, 1–9. [Google Scholar] [CrossRef]
- Taheri, M.; Oskooei, V.K.; Ghafouri-Fard, S. Protein Inhibitor of Activated STAT Genes are Differentially Expressed in Breast Tumor Tissues. Pers. Med. 2019, 16, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.; Fu, Y.; Guo, F.; Zhang, X.; Xiao, S.; Zhu, W.; Huang, Z.; Zhang, J.; Chen, J. PIAS3-mediated feedback loops promote chronic colitis-associated malignant transformation. Theranostics 2018, 8, 3022–3037. [Google Scholar] [CrossRef]
- Wang, L.H.; Yang, X.Y.; Mihalic, K.; Xiao, W.; Li, D.; Farrar, W.L. Activation of Estrogen Receptor Blocks Interleukin-6-inducible Cell Growth of Human Multiple Myeloma Involving Molecular Cross-talk between Estrogen Receptor and STAT3 Mediated by Co-regulator PIAS3. J. Biol. Chem. 2001, 276, 31839–31844. [Google Scholar] [CrossRef]
- Yamashita, H.; Iwase, H. The role of stat5 in estrogen receptor-positive breast cancer. Breast Cancer 2002, 9, 312–318. [Google Scholar] [CrossRef]
- Kavarthapu, R.; Dufau, M.L. Prolactin receptor gene transcriptional control, regulatory modalities relevant to breast cancer resistance and invasiveness. Front. Endocrinol. 2022, 13, 949396. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, R.; Morris, C.-H.T.; Dufau, M.L. Prolactin induces up-regulation of its cognate receptor in breast cancer cells via transcriptional activation of its generic promoter by cross-talk between ERα and STAT5. Oncotarget 2014, 5, 9079–9091. [Google Scholar] [CrossRef] [PubMed]
- Bjornstrom, L.; Kilic, E.; Norman, M.; Parker, M.; Sjoberg, M. Cross-talk between Stat5b and estrogen receptor-alpha and -beta in mammary epithelial cells. J. Mol. Endocrinol. 2001, 27, 93–106. [Google Scholar] [CrossRef][Green Version]
- Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in Oncogenesis. Biomedicines 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Able, A.A.; Burrell, J.A.; Stephens, J.M. STAT5-Interacting Proteins: A Synopsis of Proteins that Regulate STAT5 Activity. Biology 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Yu, M.; Clark, B.D.; Snyderwine, E.G. Possible role of Stat5a in rat mammary gland carcinogenesis. Breast Cancer Res. Treat. 2004, 88, 263–272. [Google Scholar] [CrossRef]
- Yang, S.-F.; Hou, M.-F.; Chen, F.-M.; Ou-Yang, F.; Wu, Y.-C.; Chai, C.-Y.; Yeh, Y.-T. Prognostic value of protein inhibitor of activated STAT3 in breast cancer patients receiving hormone therapy. BMC Cancer 2016, 16, 20. [Google Scholar] [CrossRef][Green Version]
- Gutzman, J.H.; E Rugowski, D.; E Nikolai, S.; A Schuler, L. Stat5 activation inhibits prolactin-induced AP-1 activity: Distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene 2007, 26, 6341–6348. [Google Scholar] [CrossRef]
- Wysokinski, D.; Blasiak, J.; Pawlowska, E. Role of RUNX2 in Breast Carcinogenesis. Int. J. Mol. Sci. 2015, 16, 20969–20993. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Anderson, E.; Hunter, T.; Farnie, G.; Clarke, R.B. Prolactin receptor antagonism reduces the clonogenic capacity of breast cancer cells and potentiates doxorubicin and paclitaxel cytotoxicity. Breast Cancer Res. 2008, 10, R68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Lan, T.; He, J.; Lei, B.; Wang, H.; Mei, Z.; Lv, C. Integrative analysis revealed a correlation of PIAS family genes expression with prognosis, immunomodulation and chemotherapy. Eur. J. Med Res. 2024, 29, 1–21. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Kocab, A.J.; Duckett, C.S. Inhibitor of apoptosis proteins as intracellular signaling intermediates. FEBS J. 2015, 283, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Denkert, C. Updated WHO Classification of Tumors of the Breast: The Most Important Changes. Pathologe 2021, 42, 270–280. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for Uniform Definition of an Immunoreactive Score (IRS) for Immunohisto-chemical Estrogen Receptor Detection (ER-ICA) in Breast Cancer Tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]




| (A) | Overall Survival (OS) | |||||
| Clinicopathological Parameters | Univariate | Multivariate | ||||
| p-Value | HR | 95% HR CI | p-Value | HR | 95% HR CI | |
| Age (<60 vs. ≥60) | 0.069 | 1.229 | 0.196–7.707 | - | - | - |
| Tumor size (pT1 vs. pT2–T4) | 0.239 | 1.789 | 0.679–4.714 | - | - | - |
| Lymph nodes (pN− vs. pN+) | 0.999 | 0.99 | 0.196–5.108 | - | - | - |
| Clinical stage TNM (I–II vs. III–IV) | 0.042 | 7.726 | 0.13–62.345 | 0.981 | 1.005 | 0.641–1.575 |
| Grade of malignancy (G1, G2 vs. G3) | 0.563 | 1.774 | 0.392–1.829 | - | - | - |
| Estrogen receptor (ER− vs. ER+) | 0.448 | 1.063 | 0.511–2.212 | - | - | - |
| Progesterone receptor (PR− vs. PR+) | 0.338 | 1.767 | 0.922–3.387 | - | - | - |
| HER2 (negative vs. positive) | 0.110 | 3.025 | 0.778–11.766 | - | - | - |
| Ki-67 (<25% vs. ≥25%) | 0.037 | 8.426 | 1.127–62.976 | 0.889 | 1.052 | 0.516–2.145 |
| PIP (‘low’ vs. ‘high’) | 0.29 | 0.523 | 0.154–1.777 | - | - | - |
| PIAS3 cytoplasmic (‘low’ vs. ‘high’) | 0.98 | 1.008 | 0.296–3.866 | - | - | - |
| PIAS3 nuclear (PIAS3− vs. PIAS3+) | 0.08 | 3.414 | 0.856- 13.603 | - | - | - |
| SOCS3 (SOCS3− vs. SOCS3+) | 0.812 | 0.812 | 0.147–4.482 | - | - | - |
| STAT5 cytoplasmic (‘low’ vs. ‘high’) | 0.032 | 0.502 | 0.107–2.347 | 0.171 | 0.686 | 0.400–1.177 |
| STAT5 nuclear(STAT5 nuclear− vs. STAT5 nuclear+) | 0.011 | 0.355 | 0.065–1.947 | 0.27 | 1.409 | 0.759–2.615 |
| STAT5-P (‘low’ vs. ‘high’) | 0.859 | 1.091 | 0.416–2.859 | - | - | - |
| PRLR (‘low’ vs. ‘high’) | 0.524 | 1.464 | 0.452—4.748 | - | - | - |
| (B) | Disease-Free Survival (DFS) | |||||
| Clinicopathological Parameters | Univariate | Multivariate | ||||
| p-Value | HR | 95% HR CI | p-Value | HR | 95% HR CI | |
| Age (<60 vs. ≥60) | 0.107 | 1.191 | 0.192–7.403 | - | - | - |
| Tumor size (pT1 vs. pT2–T4) | 0.271 | 1.679 | 0.666–4.233 | - | - | - |
| Lymph nodes (pN− vs. pN+) | 0.966 | 1.03 | 0.216–4.946 | - | - | - |
| Clinical stage TNM (I–II vs. III–IV) | 0.055 | 1.726 | 0.957–2.345 | - | - | - |
| Grade of malignancy (G1, G2 vs. G3) | 0.272 | 1.823 | 0.396–2.710 | - | - | - |
| Estrogen receptor (ER− vs. ER+) | 0.121 | 1.605 | 0.133–2.777 | - | - | - |
| Progesterone receptor (PR− vs. PR+) | 0.529 | 1.546 | 0.083–3.584 | - | - | - |
| HER2 (negative vs. positive) | 0.047 | 3.669 | 1.017–13.233 | 0.011 | 2.008 | 1.176–3.429 |
| Ki-67 (<25% vs. ≥25%) | 0.034 | 8.108 | 1.172–56.102 | 0.801 | 0.943 | 0.600–1.483 |
| PIP (‘low’ vs. ‘high’) | 0.25 | 0.503 | 0.153–1.656 | - | - | - |
| PIAS3 cytoplasmic (‘low’ vs. ‘high’) | 0.81 | 0.868 | 0.263–2.866 | - | - | - |
| PIAS3 nuclear (PIAS3− vs. PIAS3+) | 0.13 | 2.764 | 0.736–10.377 | - | - | - |
| SOCS3 (SOCS3− vs. SOCS3+) | 0.895 | 0.898 | 0.185–4.376 | - | - | - |
| STAT5 cytoplasmic (‘low’ vs. ‘high’) | 0.041 | 0.539 | 0.105–2.752 | 0.157 | 0.684 | 0.405–1.157 |
| STAT5 nuclear(STAT5 nuclear− vs. STAT5 nuclear+) | 0.002 | 0.253 | 0.041–1.564 | 0.360 | 1.355 | 0.707–2.599 |
| STAT5-P (‘low’ vs. ‘high’) | 0.954 | 0.951 | 0.318–2.843 | - | - | - |
| PRLR (‘low’ vs. ‘high’) | 0.696 | 1.601 | 0.746–3.436 | - | - | - |
| Clinicopathological Parameters | IHC Patients (n = 554) | RT-PCR Patients (n = 42) |
|---|---|---|
| Age | ||
| ≤50 | 99 (18%) | 9 (19%) |
| >50 | 455 (82%) | 33 (81%) |
| Tumor size | ||
| pT1 | 255 (46%) | 23 (55%) |
| pT2 | 272 (49%) | 16 (38%) |
| pT3–T4 | 27 (5%) | 3 (7%) |
| Lymph nodes (N) | ||
| pN0 | 316 (57%) | 25 (60%) |
| pN1–N3 | 227 (41%) | 13 (32%) |
| pNx | 11 (2%) | 4 (8%) |
| TNM clinical stage | ||
| I | 161 (29%) | 15 (35%) |
| II | 238 (43%) | 18 (44%) |
| III | 67 (12%) | 6 (15%) |
| IV | 88 (16%) | 3 (6%) |
| Grade of malignancy | ||
| G1 | 89 (16%) | 7 (17%) |
| G2 | 299 (54%) | 24 (57%) |
| G3 | 166 (30%) | 11 (26%) |
| Estrogen receptor | ||
| Negative | 144 (26%) | 10 (23%) |
| Positive | 410 (74%) | 32 (77%) |
| Progesterone receptor | ||
| Negative | 194 (35%) | 15 (35%) |
| Positive | 360 (65%) | 27 (65%) |
| HER2 | ||
| Negative | 343 (62%) | 28 (67%) |
| Positive | 211 (38%) | 14 (33%) |
| Ki-67 | ||
| ≤25 | 327 (59%) | 27 (64%) |
| >25 | 227 (41%) | 15 (36%) |
| Molecular tumor types | ||
| Triple-negative | 55 (10%) | 9 (14%) |
| Other types | 499 (90%) | 33 (86%) |
| Hormonal therapy | ||
| Negative | 95 (17%) | 7 (17%) |
| Positive | 262 (47%) | 35 (83%) |
| No data | 197 (36%) | |
| Chemotherapy | ||
| Negative | 197 (36%) | 20 (48%) |
| Positive | 161 (29%) | 22 (52%) |
| No data | 194 (35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, K.; Kmiecik, A.; Nowińska, K.; Piotrowska, A.; Suchański, J.; Ratajczak-Wielgomas, K.; Partyńska, A.; Romanowicz, H.; Smolarz, B.; Matkowski, R.; et al. Association of Selected STAT Inhibitors with Prolactin-Induced Protein (PIP) in Breast Cancer. Int. J. Mol. Sci. 2025, 26, 1416. https://doi.org/10.3390/ijms26041416
Jabłońska K, Kmiecik A, Nowińska K, Piotrowska A, Suchański J, Ratajczak-Wielgomas K, Partyńska A, Romanowicz H, Smolarz B, Matkowski R, et al. Association of Selected STAT Inhibitors with Prolactin-Induced Protein (PIP) in Breast Cancer. International Journal of Molecular Sciences. 2025; 26(4):1416. https://doi.org/10.3390/ijms26041416
Chicago/Turabian StyleJabłońska, Karolina, Alicja Kmiecik, Katarzyna Nowińska, Aleksandra Piotrowska, Jarosław Suchański, Katarzyna Ratajczak-Wielgomas, Aleksandra Partyńska, Hanna Romanowicz, Beata Smolarz, Rafał Matkowski, and et al. 2025. "Association of Selected STAT Inhibitors with Prolactin-Induced Protein (PIP) in Breast Cancer" International Journal of Molecular Sciences 26, no. 4: 1416. https://doi.org/10.3390/ijms26041416
APA StyleJabłońska, K., Kmiecik, A., Nowińska, K., Piotrowska, A., Suchański, J., Ratajczak-Wielgomas, K., Partyńska, A., Romanowicz, H., Smolarz, B., Matkowski, R., & Dzięgiel, P. (2025). Association of Selected STAT Inhibitors with Prolactin-Induced Protein (PIP) in Breast Cancer. International Journal of Molecular Sciences, 26(4), 1416. https://doi.org/10.3390/ijms26041416






