Abstract
Human papillomavirus (HPV) is a leading cause of mucosal cancers, including the increasing incidence of HPV-related head and neck cancers. The oral microbiota—a diverse community of bacteria, fungi, and viruses—play a critical role in oral and systemic health. Oral microbiota dysbiosis is increasingly linked to inflammation, immune suppression, and cancer progression. Recent studies have highlighted a complex interaction between HPV and oral microbiota, suggesting this interplay influences viral persistence, immune response and the tumor microenvironment. These interactions hold significant implications for disease progression, clinical outcomes, and therapeutic approaches. Furthermore, the oral microbiota has emerged as a promising biomarker for HPV detection and disease progress assessment. In addition, probiotic-based treatments are gaining attention as an innovative approach for preventing or treating HPV-related cancers by modulating the microbial environment. In this review, current research on the interaction between HPV and oral microbiota is provided, their clinical implications are explored, and the future potential for utilizing microbiota for diagnostic and therapeutic innovations in HPV-associated cancers is discussed.
1. Introduction
Human papillomavirus (HPV) is one of the most prevalent viruses associated with the development of various mucosal cancers, particularly cervical and oropharyngeal cancers. While much attention has been paid to its role in genital cancers, the increasing incidence of HPV-related head and neck cancer highlights the importance of HPV in the oral cavity [1,2]. The oral microbial flora, which includes bacteria, fungi, and virus, plays a crucial role in maintaining both oral and systemic health [3]. Dysbiosis, or imbalance, in this microbial community has been associated with excessive inflammatory reaction, immunosuppression of the host, promotion of malignant transformation, antiapoptotic activity, and secretion of carcinogens [4,5,6].
Emerging research has suggested that the interaction between HPV and the oral microbiota may play a critical role in the pathogenesis of HPV-related oral cancers, which could influence viral persistence, immune response, and the local tumor microenvironment, potentially altering disease outcomes and responses to treatment [5,7,8]. Recent studies have highlighted the complex relationship between HPV and dynamic oral microbiota, emphasizing that its balance is essential not only for oral health but also for disease progression and cancer development [9,10]. However, the role of microbial-viral interactions in the initiation, development, and progression of HPV-related cancers remains unclear.
The oral microbiota has been increasingly recognized as a biomarker for HPV-related cancers [11,12]. Understanding the interaction between HPV and oral microbiota is essential for identifying novel biomarkers for early detection, risk assessment, and therapeutic interventions. The composition of the oral microbiota has been shown to influence HPV infection persistence, and disease progression, suggesting that microbiota-based approaches could offer novel therapeutic strategies for HPV-related cancers [9]. Furthermore, manipulating the microbiota to prevent or treat HPV-related cancers is an area of growing interest [13,14,15].
In this narrative review, the aim is to provide a comprehensive overview of HPV infection and its interaction with the oral microbiota. We will explore the intricate relationship between HPV and oral microbiota, highlighting current research findings, clinical implications, and future directions in this emerging field. By advancing our understanding of these connections, we can better assess the role of oral health in HPV-related disease processes and develop targeted strategies for prevention, diagnosis, and treatment.
Approach: We conducted a narrative review by searching on PubMed, ScienceDirect, and Scopus using key words such as “HPV AND oral microbiota (microbiome), HPV AN D head-and -neck cancer (Oropharyngeal cancer, Oral cancer), oral microbiota AND treatment, oral cancer AND biomarker”. Articles published in English from 2000 to 2024 were considered. Studies were included if they discussed the role of oral microbiota in HPV-related carcinogenesis. The papers unrelated to HPV or the oral microbiome were excluded.
2. Overview of HPV and Oral Infections
Human papillomaviruses (HPVs) are non-enveloped, circular double-stranded DNA viruses (~8 kb) that primarily infect epithelial cells [16]. Over 200 HPV genotypes have been identified, broadly classified into low-risk and high-risk categories based on their association with benign or malignant lesions [17]. Low-risk types, such as HPV-6 and HPV-11, cause benign conditions like warts, while high-risk types, including HPV-16 and HPV-18, are linked to cancers of the cervix, anus, and the head and neck. Other high-risk types, such as HPV-31, 33, 35, and 52, show a variable frequency and geographical distribution [17,18,19,20].
HPV can be transmitted through both sexual and non-sexual routes, as illustrated in Figure 1. Oral HPV is primarily transmitted through oral–genital contact, deep kissing, and exposure to infected saliva. HPV infects the squamous cells that line the inner surface of organs, gaining access to the basal layer through micro abrasions or wounds. This infection can lead to malignancies, such as head and neck cancers in both sexes [21,22,23]. While approximately 90% of HPV infections are cleared by the innate and adaptive immune systems within 1–2 years, some infections persist due to the virus’s immune evasion strategies. These include inhibiting antigen presentation, modulating host cell apoptosis, disrupting DNA methylation, and silencing tumor suppressor genes [24,25,26]. Persistent HPV infections can integrate viral DNA into the host genome, hijacking cellular machinery for replication and altering gene expression [27,28].
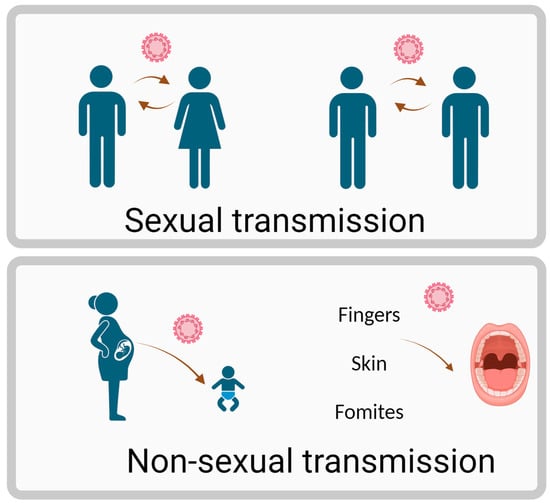
Figure 1.
Transmission route of HPV. Figure created using Biorender.com.
The prevalence of oral HPV infections is lower than that of genital HPV infections but remains a significant concern due to its association with oropharyngeal cancers. Despite declining rates of head and neck cancers overall, the incidence of HPV-positive oropharyngeal cancers has risen, particularly among younger patients with minimal tobacco and alcohol use [29,30,31]. HPV-16 is detected in 60–80% of head and neck cancers, while HPV-18 has been identified in 34% of oral cavity squamous cell cancers and 17% of laryngeal squamous cell cancers [32]. Men have a higher prevalence of oral HPV infections (11.5% vs. 3.2%) and HPV-16 infections (1.8% vs. 0.3%) compared to women [33]. A systematic review highlighted geographic variability in HPV-positive oropharyngeal cancers, with higher rates in North America, Northern Europe, and Oceania [34]. In Europe, HPV has emerged as a growing risk factor for oropharyngeal cancer, especially among younger populations [34].
HPV status is a strong prognostic factor for oropharyngeal cancer. HPV-positive oropharyngeal squamous cell carcinomas (OPSCCs) are biologically distinct, showing increased treatment responsiveness and improved survival compared to HPV-negative tumors [35,36,37]. Some studies have shown that the presence of HPV in oropharyngeal SCC is associated with a significantly lower mortality risk and better prognosis compared to HPV-negative cases [38,39]. However, a 2022 systematic review indicated that HPV-positive oral squamous cell carcinoma (OSCC) was associated with worse overall survival (OS) and distant control (DC) compared to HPV-negative OSCC, warranting further investigation [40]. Notably, OPSCC and OSCC are two distinct subtypes of head and neck cancers that differ significantly in their anatomical origin, etiology, and clinical characteristics. OPSCC is strongly associated with high-risk HPV infection, while the correlation between OSCC and HPV remains controversial [35,41,42]. It is important to distinguish between OPSCC and OSCC when discussing HPV-related carcinogenesis and its therapeutic approaches.
Vaccination against HPV has shown substantial promise in reducing oral HPV infections. A cross-sectional study in the UK reported significantly lower oropharyngeal HPV-16 prevalence in vaccinated versus unvaccinated females (0.5% vs. 5.6%) [43]. Similarly, a U.S. study found a reduction in oral HPV-16/18/6/11 prevalence among vaccinated individuals compared to unvaccinated ones (0.11% vs. 1.61%) [44]. Vaccine efficacy appears higher among younger individuals (≤18 years: 59% effectiveness) compared to those vaccinated at older ages (≥18 years: 18% effectiveness) [45]. These findings highlight the critical role of vaccination in preventing HPV-related oral diseases and cancers.
3. The Oral Microbiota
The human oral microbial ecosystem includes over 700 bacterial species, of which 58% are officially named, 16% are unnamed but cultivated, and 26% are uncultivated phylotypes [46]. Beyond bacteria, the oral cavity hosts archaea, fungi, and viruses [47]. As the body’s second most diverse microbiota [48], the oral microbiome inhabits various surfaces, including the tongue, teeth, gums, and cheeks, playing a crucial role in filtering, processing, and defending against foreign elements before they reach internal systems [47,49].
Different oral niches are dominated by specific microbial species. For example, Streptococcus species are abundant across habitats, while Haemophilus dominates the buccal mucosa, Actinomyces the supragingival plaque, and Prevotella the subgingival plaque and mucosal surfaces [50,51].
The oral microbiota is vital for maintaining oral and systemic health through several key functions, as follows: (1) Pathogen Protection: commensal bacteria prevent pathogenic overgrowth by competing for nutrients and attachment sites on mucosal surfaces; (2) Immune Modulation: microbes engage with the host’s immune system to prime defenses and maintain immune tolerance; (3) Metabolic Support: oral bacteria help break down dietary components, aiding carbohydrate digestion and producing metabolic byproducts [52].
The composition of the oral microbiome is influenced by intrinsic factors (e.g., age, genetics) and extrinsic factors (e.g., diet, hygiene, tobacco, and alcohol use). Unlike other microbiota, such as the gut or skin microbiomes, the oral microbiota is distinct due to its diverse niches, constant exposure to external factors, interaction with saliva, and role in biofilm formation [52,53]. This dynamic interaction among bacteria, fungi, and viruses highlights the importance of maintaining microbial balance to prevent disease.
An imbalance in the oral microbiota, or dysbiosis, contributes to both oral and systemic diseases. In the oral cavity, bacterial dysbiosis is linked to conditions such as dental caries, periodontal disease, aphthous stomatitis, and endodontic abscesses. Furthermore, oral bacteria have been implicated in systemic diseases, including bacterial endocarditis, aspiration pneumonia, osteomyelitis, rheumatoid arthritis, cardiovascular disease, and Alzheimer’s disease [54,55,56,57]. Notably, periodontitis and HPV infection have been associated with an increased risk of OPSCCs [58]. Shin et al. highlighted that several publications have suggested the oral microbiome contribute to the etiology of different types of cancers due to their ability to alter the community composition and induce inflammatory reactions, DNA damage, apoptosis, and altered metabolism [59]. The study showed that HPV infection and cervical cancer impact not only the vaginal but also the oral microenvironments, and the oral microbial diversity exhibited an inverse pattern to that of the vaginal microbiome, indicating a unique relationship [60].
Microorganisms can colonize biotic and abiotic surfaces, including teeth, soft tissues, dental implants, and restorative materials. As microorganisms accumulate, they form structures known as biofilms, complex microbial communities enmeshed in a matrix containing extracellular polymeric substances [61], which protect the microbial community from environmental stressors, antimicrobial agents, and host immune responses [62,63]. These biofilms are primarily composed of bacteria, but can also include fungi, viruses, and archaea. In a healthy oral microbiome, biofilms play a protective role, helping to maintain the microbial balance and defend against pathogenic species. However, when dysbiosis occurs, pathogenic bacteria dominate, leading to chronic inflammation and a disruption in the biofilm’s protective functions. Pathogenic biofilms are implicated in several oral diseases, including periodontal disease, dental caries, and infections [64].
4. HPV and Oral Microbiota Interaction
The interaction between the oral microbiota and HPV infection is an emerging area of research, given the significant role oral microorganisms play in both maintaining oral and systemic health. HPV infection, particularly with high-risk types like HPV16, is a well-established risk factor for OPSCCs, with 40–80% of OPSCC cases linked to this virus [41,65,66,67]. However, the presence of HPV alone is insufficient for cancer development. Additional factors, such as epithelial surface integrity, mucosal secretions, immune regulation, and the surrounding microbial environment, influence how HPV infection progresses and how the immune system responds [68,69].
Recent studies have suggested that the oral microbiota may play a crucial role in HPV persistence and its associated cancer risks. Chronic oral infections, such as periodontal disease caused by pathogens such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Treponema denticola, create a pro-inflammatory environment in the oral cavity [70]. This chronic inflammation can impair local immune responses, allowing HPV to evade detection and persist longer than in a healthy environment. Additionally, pathogens like P. gingivalis and F. nucleatum further exacerbate the issue by interfering with host immune cells, such as macrophages and dendritic cells, further facilitating HPV persistence, replication, and malignant transformation [71,72]. A prospective nested case-control study has demonstrated that a greater abundance of oral commensal bacteria in the genera Corynebacterium and Kingella were associated with a decreased risk for HNSCC, with potential implications for cancer prevention [73].
Notably, pathogenic oral bacteria may secrete certain carcinogenic metabolites that contribute to the carcinogenesis process under dysbiosis. For example, the oral bacterial species S. salivarius, S. mitis, and S. bovis, contain nitrate and nitrite reductases, capable of reducing the nitrate secreted in the oral cavity as a salivary component to nitrite and NO [74], carcinogenic metabolites which are associated with oral carcinogenesis [74,75]. Since P. aeruginosa can catalyze the reduction of nitrite to NO by cd1 nitrite reductase, a 2022 study posited that P. aeruginosa increases the concentration of NO in oral cavity by converting salivary nitrite to NO [76,77]. HPV infections can also exist in a latent state, where the virus is present but is not actively replicating. Under conditions of immunosuppression or microbiota dysbiosis, HPV may reactivate, leading to viral replication, shedding, and increased risk of cancer [78,79]. Chronic inflammation, driven by the presence of pro-inflammatory anaerobic bacteria, such as Prevotella spp., Porphyromonas spp., and Fusobacerium spp., leads to the release of inflammatory cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ). These cytokines activate protein kinase-mediated signaling pathways, resulting in the formation of reactive oxygen species (ROS) that are associated with invasive and aggressive tumor phenotypes and tumor migration [5,6,80,81]. In one study using a 4NQO-induced mouse model of oral cancer, the inoculation of P. gingivalis promoted tumor progression by invading precancerous lesions and recruiting the myeloid-derived suppressor cells via the expression of chemokines such as C-C motif ligand 2 (CCL2) and chemokine (C-X-C motif) ligand 2 (CXCL2), and cytokines such as IL-6 and IL-8 [82]. Another study showed that Rothia mucilaginosa can generate acetaldehyde (ACH) from ethanol in vitro and induce oxidative stress in oral keratinocytes [83]. Another study suggested that dysbiosis can be dangerous due to its potential to generate ACH not only from the consumption of alcohol but also from food that does not contain alcohol [84]. Lastly, persistent inflammation depletes antioxidants, increasing oxidative damage and genetic mutation, thereby disrupting cell cycle regulation and promoting malignancy. Studies have confirmed elevated cytokine levels and reduced antioxidant defenses in patients with persistent HPV infection [80,85].
Furthermore, certain microbial profiles have been linked to HPV infection across different sites of the body. For instance, an increased abundance of Fusobacterium species has been associated with HPV-related cancers in the oral cavity, vagina, and anus [29,86,87]. These microbial species may not only influence HPV infection but also patient survival. As an example, Pseudomonas viridiflava has been associated with improved survival, while Yersinia pseudotuberculosis has been linked to worsened survival in head and neck cancer patients [8]. Moreover, a study has shown that the response of tumors to radiation can be regulated by the microbiome and that both the bacterial and fungal constituents of the gut microbiota impact antitumor immunity. Certain bacterial species can affect treatment response [88], which may help inform therapeutic strategies aimed at modifying the microbiome to enhance cancer treatment efficiency.
Another critical factor in the HPV–microbiota interaction is the formation of biofilms. Pathogenic biofilms, particularly those associated with periodontal disease, create a protective niche that shields HPV from immune surveillance, potentially allowing the virus to evade detection and integrate into the host genome—a key step in HPV-driven carcinogenesis [89]. Although direct evidence of HPV integration within biofilm structures is limited, biofilms likely play a role in HPV persistence by fostering immune evasion.
HPV infections can also exist in a latent state, where the virus is present but is not actively replicating. Under conditions of immunosuppression or microbiota dysbiosis, HPV may reactivate, leading to viral replication, shedding, and increased risk of cancer [78,79]. Chronic inflammation, driven by the presence of pro-inflammatory bacteria, leads to the release of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), which activate protein kinase-mediated signaling pathways, resulting in the formation of reactive oxygen species (ROS) [80,81]. In addition, persistent inflammation depletes antioxidants, increasing oxidative damage and genetic mutations, thereby disrupting cell cycle regulation and promoting malignancy. Studies have confirmed elevated cytokine levels and reduced antioxidant defenses in patients with persistent HPV infection [80,85].
The role of fungi and other viruses in HPV infections in the oral cavity also warrants attention. Candida albicans, the predominant fungal species in the oral cavity, are often elevated in individuals with compromised immunity, such as HIV patients, diabetes patients, infants, and elderly populations [90]. It produces the cytolytic toxin candida lysin, leading to epithelial barrier lesions and making it easier for HPV to both infect and persist in basal epithelial cells [91]. While direct evidence of fungal involvement in HPV-driven malignancies is limited, Candida infections are known to produce carcinogens such as nitrosamine and promote the development of carcinoma [92]. Co-infection with other viruses, such as the Epstein–Barr virus (EBV) and herpes simplex virus (HSV), may further exacerbate HPV-related diseases. EBV is a similar virus that causes cellular transformation and chronic inflammation and may support HPV persistence [93]. In a study using an in vitro model that mimics the setting of co-infection (overexpression of E6 and E7 simulating HPV integration followed by EBV infection), the cancerous cell line FaDu and the normal cell line NOK demonstrated a significant increase in invasion in the absence of any effect on proliferation [94].
The synergistic effects of chronic inflammation and co-infections with bacteria, fungi, and viruses in the oral cavity create a persistent inflammatory environment that supports HPV persistence, reactivation, and oncogenesis. The evidence suggests that individuals with periodontal disease or other chronic oral infections may be at a higher risk for developing HPV-related cancers, emphasizing the need to maintain a balanced oral microbiota to reduce cancer risk [95,96].
5. Oral Microbiota as a Biomarker for HPV-Related Cancers
Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer, accounting for approximately 90% of cases [97]. The five-year survival rate for OSCC is around 30–41% in advanced stages, but it exceeds 85% when detected early [98,99]. Unfortunately, nearly half of OSCC cases are diagnosed at later stages, making early detection critical for improving patient outcomes [100]. Reliable biomarkers for detecting and predicting the progression of HPV-related oral cancers, including OSCC, are urgently needed.
Traditionally, biomarkers such as HPV DNA, p16 expression, and antibodies against HPV oncogenes (e.g., E6 and E7) have been used to assess the risk of HPV-driven malignancies [101,102]. However, the recent evidence has suggested that changes in the oral microbial composition—particularly patterns of dysbiosis—may provide valuable insights into the risk and progression of HPV-related oral cancers, offering a non-invasive alternative to traditional biomarkers. Saliva has emerged as a promising medium for detecting both HPV DNA and microbial signatures. Salivary samples can provide a comprehensive overview of the oral microbial ecosystem and its interactions with HPV, offering a snapshot of the dynamic changes occurring within the oral cavity [10,11]. Advances in sequencing technologies and metagenomic analyses have made it possible to identify even subtle shifts in microbial composition, allowing for the detection of early dysbiosis associated with HPV-related malignancies [102,103].
Although many studies have examined the microbiome in the oral cavity and oropharyngeal cancers, most do not specify HPV positivity. In a study of oral cavity cancers with unknown HPV status, the bacterial composition of tumor sites was significantly different from that of contralateral normal mucosa samples [104]. Furthermore, patients with detectable oral HPV or HPV-positive OPSCC exhibit significant shifts in their oral microbiomes compared to patients with HPV-negative counterparts [105].
Healthy individuals typically maintain a diverse and balanced oral microbiota dominated by commensal species that promote oral health. In contrast, individuals with HPV-related oropharyngeal cancers often experience oral dysbiosis, characterized by an overrepresentation of pathogenic species and a reduction in beneficial commensals. Patients with oral HPV exhibit similar alpha diversity in their oral microbiomes to healthy individuals but show distinct differences in beta-diversity [9,106]. Specifically, HPV-positive patients demonstrate an increased abundance of Actinomycetaceae, Prevotellaceae, Veillonellaceae, Campylobacteraceae, and Bacteroidetes, while species such as Gemellaceae, Neisseria, and Lactobacillus are less abundant [9,106].
In HPV-positive cancer patients, members of the genera Actinomyces, Granulicatella, Oribacterium, and Campylobacter, as well as the species Veillonella dispar, Rothia mucilaginosa, and Haemophilus parainfluenzae, are significantly increased, while Streptococcus anginosus, Peptoniphilus, and Mycoplasma are significantly decreased [107]. Furthermore, Mougeot et al. identified higher relative abundances of Alloprevotella tannerae, Fusobacterium periodonticum, Haemophilus pittaniae, and Leptotrichia spp. in HPV-positive head and neck cancer patients compared to HPV-negative patients [108]. Additionally, studies have found that patients with HPV-positive oropharyngeal cancers often show increased abundance of bacterial taxa such as Fusobacterium nucleatum, Porphyromonas, and Treponema denticola, which are also associated with periodontal disease [109]. The recent research has demonstrated that significant shifts in the composition of the oral microbiota occur by disease stage and after chemoradiotherapy in HPV-positive oropharyngeal squamous cell carcinoma patients [86], indicating oral microbiota could play a role in disease progression, response to treatment, or recovery, highlighting its potential as a therapeutic target or biomarker.
The shift in oral microbial communities in HPV-related cancers may contribute to carcinogenesis through several mechanisms. Pathogenic bacteria associated with oral dysbiosis can promote chronic inflammation, a well-established factor in cancer development. Certain bacterial species, such as Fusobacterium nucleatum, have been shown to modulate the immune response, potentially facilitating HPV persistence and promoting epithelial cell transformation. In a mouse model of oral tumorigenesis, co-infection with Porphyromonas gingivalis and Fusobacterium nucleatum significantly enhanced the severity of tongue tumors and increased IL-6 levels in the tongue epithelium [110]. Furthermore, bacterial metabolites may influence the host’s immune surveillance or directly interact with HPV to enhance viral replication. These microbial-induced changes in the local immune environment could create a favorable niche for HPV infection and persistence, critical steps in the development of oral cancers.
The identification of specific microbial signatures linked to cancer progression could aid in risk stratification, allowing clinicians to monitor individuals with high-risk HPV infection more closely. Longitudinal studies that track microbiota changes in HPV-positive individuals could help define microbiota profiles associated with either viral clearance or progression to malignancy. These microbial signatures could be incorporated into predictive models to improve early detection and intervention.
Despite the potential of the oral microbiota as a biomarker for HPV-related cancer, several challenges remain. One major hurdle is the variability in the oral microbiota among individuals, which can be influenced by factors such as diet, smoking, hygiene, and genetics [111]. These confounding variables make it difficult to establish universal microbial signatures that can be applied across diverse populations.
In the future, oral microbiota-based diagnostic tests could be integrated into clinical practice to enhance early detection strategies for HPV-related cancers. By combining traditional HPV biomarkers with microbial profiling, clinicians may be able to develop more comprehensive screening protocols that improve patient outcomes. Such testing could also be used to monitor treatment responses, as changes in the oral microbiota may reflect shifts in the immune response or cancer progression following therapy.
Additionally, more research is needed to establish causality between microbial shifts and HPV-related cancer progression. While associations between specific bacterial species and cancer have been observed, it is not yet clear whether these microbial changes are a cause or consequence of disease progression. Longitudinal studies that track microbial changes from the onset of HPV infection to cancer development are essential to clarify these relationships.
Finally, standardization of sampling techniques and analytical methods is necessary to ensure consistent and reproducible results across studies. As the field advances, the development of standardized protocols for microbiota sampling, sequencing, and analysis will be crucial for translating research findings into clinical applications.
6. Therapeutic Implications and Future Directions
In most oral carcinoma cases, the main treatment method is surgical removal. In advanced cases, postoperative radiotherapy, chemotherapy, oncogene-targeted therapy, and immunotherapy may be administered [112]. However, these treatments can cause alteration in oral microflora and lead to oral mucositis, oral candidiasis, and hyposalivation [113,114]. Another important role of the oral microbiome is modulating the immune system and influencing the effectiveness of treatments for oral cancers. Overgrowth of opportunistic pathogens like Staphylococci, enteric rods, and Candida sp. tend to increase in prevalence after radiotherapy with or without chemotherapy [115]. In a study from China, mutant streptococci were not isolated in radiotherapy patients while lactobacilli, S. mitis, and S. salivarius mutation increased significantly following radiotherapy [116]. Some studies have shown that modulating or manipulating the oral microbiome can help alleviate the side effects caused by cancer therapies. A randomized, double-blind, placebo-controlled trial showed that probiotics can significantly enhance the immune response of patients and reduce the severity of oral mucositis induced by chemoradiotherapy through modification of gut microbiota [117]. Lactobacillus brevis CD2 lozenges have been shown to reduce the severity and incidence of radio/chemotherapy-induced mucositis in head and neck cancer patients, thereby increasing the likelihood of anticancer treatment completion [118]. Studies have pointed out microbial species which can be categorized as pro-tumorigenic and anti-tumorigenic [107,119,120]. One study indicated that the presence of Luteibacter, Flammeovirgo, and Lachnoclostridium was correlated with total T-cell receptor reads, number of clones, leukocytes, and CD8+ T-cell infiltration suggesting their potential influence on the tumor microenvironment and immunotherapy response regulation [37]. It was proposed by Sivan et al. that oral administration of Bifidobacterium, a particular taxon of microbial commensals, improved melanoma control to the same degree as programmed cell death protein 1 ligand 1 (PD-L1) specific antibody therapy (checkpoint blockade), and combination treatment nearly abolished tumor outgrowth in a mouse model [121].
The relationship between the oral microbiota and HPV infection offers promising therapeutic opportunities. A study analyzing vaginal flora in 26 HPV patients found significantly lower bacterial diversity in those who cleared HPV within a year compared to those with persistent infection [122], suggesting that a healthy microbiota aids HPV clearance. Although oral and vaginal microbiota differ, it is reasonable to hypothesize that a healthy oral microbiota could similarly support oral HPV clearance. Beyond influencing infection dynamics, the oral microbiota plays a critical role in immunity, making microbial balance restoration a potential approach for reducing HPV-related cancer risk. Thus, restoring an imbalanced oral microbiota may be the fundamental key for preventing HPV persistence and progression to malignancy.
Current studies on considering probiotics as an HPV treatment have primarily focused on cervical rather than oral cancer. While data on oral HPV are limited, some studies have shown promising probiotic effects on cervical cancer cell lines (Table 1). Clinical trials have also explored probiotics for HPV-related infections. A prospective study of 54 HPV-positive patients with low-grade squamous intraepithelial lesions showed that daily consumption of Lactobacillus casei Shirota (Yakult) doubled the chance of clearing cytological abnormalities (p = 0.05). HPV clearance occurred in 19% of controls versus 29% of probiotic users (p = 0.41), indicating potential benefits [123]. Another study with 100 women found that vaginal L. crispatus chen-01 transplantation significantly reduced HPV viral load, improved clearance rates, and ameliorated vaginal inflammation without notable side effects [124]. Conversely, a randomized, double-blinded trial found no significant difference in high-risk HPV clearance between a probiotic group (Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14) and a placebo group [125]. However, the bacterial concentration used in this study was lower than other studies, the strains that were used in this study were also different from the other study, indicating dosing, strain, and duration may all play important roles in viral clearance. A summary of the probiotics used for HPV-related infections is presented in Table 2.
While most current studies focus on cervical HPV infections, research on the role of probiotics in oral HPV remains underexplored. Preliminary studies in other areas of the body, such as the vaginal microbiota, have shown that probiotics can be effective in reducing viral infections and improve immune function. These findings suggested that a similar approach could be applied to the oral cavity. Given the differences between oral and vaginal microbiota, understanding how probiotics influence oral HPV clearance is crucial for developing future, targeted therapies. Future research should prioritize clinical trials investigating the effects of probiotics on oral HPV infections, focusing on identifying specific strains and treatment durations that optimize clearance rates. Additionally, studies should aim to determine the ideal composition of a “healthy” oral microbiota in the context of HPV. Despite promising results, current findings are limited by variability in probiotic strains, dosages, and study designs, underscoring the need for standardized approaches and larger sample sizes.
Since microbial dysbiosis may serve as a bridge between periodontal disease and oral cancer, another potential therapeutic avenue to restore oral dysbiosis is the direct targeting of pathogenic bacteria associated with HPV persistence. Antimicrobial therapies, such as antibiotics or antimicrobial peptides, could be used to eliminate or reduce the abundance of specific bacterial species that promote chronic inflammation and create a conducive environment for HPV. However, this approach requires careful consideration, as broad-spectrum antibiotics could disrupt the overall balance of the oral microbiota, leading to unintended consequences. A more targeted approach could involve the use of narrow-spectrum antimicrobial peptides or bacteriophages. Notably, the functional peptides derived from bacteriophages offer the benefits of targeting cancer cells with high specificity while causing minimal immune response in humans. A study revealed that oral vaccination with bacteriophage MS2-L2 virus-like particles (VLPs) could provide protection against oral infections caused by multiple HPV types linked to head and neck cancers [126], highlighting the potential of phage-based vaccines in combating oral cancers. By selectively targeting pathogenic bacteria while preserving beneficial commensals, these therapies could reduce inflammation and improve immune responses without causing widespread disruption of the microbiota. Integrating HPV vaccination and phage vaccination into preventive strategies for oral dysbiosis and HPV infections could significantly enhance control measures. HPV vaccination would reduce the incidence of HPV-related oral cancers, while phage vaccination could specifically target oral pathogens associated with HPV persistence.
As the research on HPV and the oral microbiota continues to evolve, the future of therapeutic interventions is likely to focus on personalized medicine. Personalized medicine approaches could involve using the oral microbiota as both a diagnostic tool and a therapeutic target. By identifying specific microbial profiles associated with increased cancer risk, clinicians could tailor treatment strategies to reduce pathogenic bacterial populations, restore microbial balance, and modulate immune responses. Advances in microbiome research, combined with genetic and immune profiling, may allow for the development of individualized treatment plans that take into account each patient’s unique microbial and immune landscape. Together with personalized microbiota interventions, these tools could provide a multi-layered, individualized approach to prevent and treat oral dysbiosis and HPV infections, improving overall oral health outcomes. This combined strategy holds great promise for the future of preventive healthcare in oral diseases.

Table 1.
Summary of probiotic effects on HPV-related cancer cells.
Table 1.
Summary of probiotic effects on HPV-related cancer cells.
| Bacteria/Probiotics | Cell Line | Outcome | References |
| Milk-isolated L. casei and L. paracasei | HeLa | Upregulating the expression of apoptotic genes | [127] |
| Vagina-isolated L. gasseri | HeLa | Inflammation and proliferation were reduced, and apoptosis was increased | [128] |
| Vagina-isolated L. plantarum | HeLa | Suppression of proliferation and induction of apoptosis | [129] |
| Supernatants of L. rhamnosus and L. crispatus | HeLa | Inhibited cell proliferation and metastasis | [130] |
| Supernatants of L. rhamnosus and L. crispatus | HeLa | Decrease expression of HPV oncogenes | [131] |
| Lacticaseibacillus casei LH23 | HeLa | suppress the proliferation and induced the apoptosis of cervical cancer cells | [132] |
| Bifdobacterium adolescentis SPM1005-A | SiHa | Inhibited E6 and E7 oncogenes | [133] |
| supernatants of L. crispatus, L. jensenii, and L. gasseri | CasKi | inhibitory effects on the viability of cervical cancer cells via regulation of HPV oncogenes and cell cycle-related genes | [134] |
| Twelve standard Lactobacillus | HeLa and SiHa | Reduce tumor invasion and metastasis | [135] |

Table 2.
A summary of studies exploring the use of probiotics for HPV-related infections.
Table 2.
A summary of studies exploring the use of probiotics for HPV-related infections.
| Sample Size | Bacterial Strain | Condition | Intervention | Outcome | References |
|---|---|---|---|---|---|
| N = 160 | L. crispatus M247 | ASCUS * or LSIL * HPV+ | Oral administration (no fewer than 20 billion) for 12 months | higher percentage of clearance of PAP-smear abnormalities in patients who took oral Lactobacillus crispatus M247 than in the control group | [136] |
| N = 35 | L. crispatus M247 | ASCUS or LSIL or NILM * HPV+ | Oral administration (no fewer than 20 billion) for 3 months | reduction of approximately 70% in HPV positivity and a significant change in CST status with 94% of women | [137] |
| N = 54 | L. casei Shirota | LSIL HPV+ | Oral administration (no fewer than 20 billion) for 6 months | Probiotic users had a twice as high chance of clearance of cytological abnormalities | [123] |
| N = 121 | L. rhamnosus and L. reuteri | High-risk HPV | Oral administration (no fewer than 5.4 billion) discontinued until negative HPV testing | No significant influence on HR-HPV clearance, but may have decreased the rates of mildly abnormal and unsatisfactory cervical smears | [125] |
| N = 91 | L. crispatus chen01 | High-risk HPV | Intravaginal 1 × 109 CFU per capsule for 5 months | Significantly reduced viral load of HPV, ameliorated HPV clearance rate, and improved vaginal inflammation state | [124] |
| N = 117 | L. rhamnosus BMX 54 | C1N1 *, HPV+ | Intravaginal 1 × 104 CFU per tablet for 3 or 6 months | Long term user doubled the chance of resolving HPV-related cytological anomalies compared to short term user | [138] |
* ASCUS: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; NILM: negative for intraepithelial lesion or malignancy; C1N1: cervical intraepithelial neoplasia grade 1.
7. Conclusions
By synthesizing the current findings, in this review, the importance of understanding how oral microbiota interaction develops in cancer pathogenesis has been underscored and the need for further studies to establish new strategies for prevention, diagnosis, and treatment of HPV-associated oral diseases has been highlighted. While much progress has been made, longitudinal studies, large-scale clinical trials, and in-depth microbiome analysis will be needed to identify potential biomarkers, therapeutic targets, and strategies for intervention. In conclusion, the interaction between HPV and oral microbiota represents a promising area of research that could revolutionize the prevention, diagnosis, and treatment of HPV-related oral cancers.
Author Contributions
Conceptualization, Q.X. writing—original draft preparation, Q.X. writing—review and editing, Q.X. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Department of Clinical Investigation at Brooke Army Medical Center, Fort Sam Houston, Texas.
Conflicts of Interest
The views expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, the Department of Defense, nor any agencies under the U.S. Government.
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riva, G.; Albano, C.; Gugliesi, F.; Pasquero, S.; Pacheco, S.F.C.; Pecorari, G.; Landolfo, S.; Biolatti, M.; Dell’Oste, V. HPV Meets APOBEC: New Players in Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upadhyay, R.; Dhakal, A.; Wheeler, C.; Hoyd, R.; Jagjit Singh, M.; Karivedu, V.; Bhateja, P.; Bonomi, M.; Valentin, S.; Gamez, M.E.; et al. Comparative analysis of the tumor microbiome, molecular profiles, and immune cell abundances by HPV status in mucosal head and neck cancers and their impact on survival. Cancer Biol. Ther. 2024, 25, 2350249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; D’Souza, G.; Fakhry, C.; Bigelow, E.O.; Usyk, M.; Burk, R.D.; Zhao, N. Oral Human Papillomavirus Associated With Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J. Infect. Dis. 2022, 226, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannatà, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a potential non-invasive liquid biopsy for early and easy diagnosis/prognosis of head and neck cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell. Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Ma, Q.; Zhang, L.; Ma, L.; Wang, D.; Yang, Y.; Jia, P.; Wu, Y.; Wang, F. Human papillomavirus and cervical cancer in the microbial world: Exploring the vaginal microecology. Front. Cell. Infect. Microbiol. 2024, 14, 1325500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bravo, I.G.; Félez-Sánchez, M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol. Med. Public Health 2015, 2015, 32–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michaud, D.S.; Langevin, S.M.; Eliot, M.; Nelson, H.H.; Pawlita, M.; McClean, M.D.; Kelsey, K.T. High-risk HPV types and head and neck cancer. Int. J. Cancer 2014, 135, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Río-Ospina, L.; Soto-DELeón, S.C.; Camargo, M.; Sánchez, R.; Moreno-Pérez, D.A.; Pérez-Prados, A.; Patarroyo, M.E.; Patarroyo, M.A. Multiple high-risk HPV genotypes are grouped by type and are associated with viral load and risk factors. Epidemiol. Infect. 2017, 145, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Oyouni, A.A.A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Checchi, M.; Burns, L.; Pavitt, C.; Postma, M.J.; Jit, M. Systematic review and evidence synthesis of non-cervical human papillomavirus-related disease health system costs and quality of life estimates. Sex. Transm. Infect. 2019, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shen-Gunther, J.; Xia, Q.; Stacey, W.; Asusta, H.B. Molecular Pap Smear: Validation of HPV Genotype and Host Methylation Profiles of ADCY8, CDH8, and ZNF582 as a Predictor of Cervical Cytopathology. Front. Microbiol. 2020, 11, 595902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litwin, T.R.; Clarke, M.A.; Dean, M.; Wentzensen, N. Somatic Host Cell Alterations in HPV Carcinogenesis. Viruses 2017, 9, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.; Shi, C.; Zhou, R.; Han, Y.; Li, N.; Qu, C.; Xia, R.; Zhang, C.; Hu, Y.; Tian, Z.; et al. Mapping the landscape of HPV integration and characterising virus and host genome interactions in HPV-positive oropharyngeal squamous cell carcinoma. Clin. Transl. Med. 2024, 14, e1556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal microbiota significantly changed for HPV-positive women with high-grade squamous intraepithelial lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osazuwa-Peters, N.; Simpson, M.C.; Massa, S.T.; Adjei Boakye, E.; Antisdel, J.L.; Varvares, M.A. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017, 74, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, K.; Suk, R.; Chiao, E.Y.; Chhatwal, J.; Qiu, P.; Wilkin, T.; Nyitray, A.G.; Sikora, A.G.; Deshmukh, A.A. Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann. Intern. Med. 2017, 167, 714–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghanem, A.S.; Memon, H.A.; Nagy, A.C. Evolving trends in oral cancer burden in Europe: A systematic review. Front. Oncol. 2024, 14, 1444326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Coleman, S.; Fadlullah, M.Z.H.; Spakowicz, D.; Chung, C.H.; Tan, A.C. Deciphering the Tumor-Immune-Microbe Interactions in HPV-Negative Head and Neck Cancer. Genes 2023, 14, 1599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, Z.H.; Butler, S.S.; Mahal, B.A.; Muralidhar, V.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Short-term mortality risks among patients with oropharynx cancer by human papillomavirus status. Cancer 2020, 126, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Christianto, S.; Li, K.Y.; Huang, T.H.; Su, Y.X. The Prognostic Value of Human Papilloma Virus Infection in Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2022, 132, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Hübbers, C.U.; Akgül, B. HPV and cancer of the oral cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Bryant, T.S.; Babrah, J.; Louie, K.; Bryant, J.L.; Spruce, R.J.; Batis, N.; Olaleye, O.; Jones, J.; Struijk, L.; et al. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin. Infect. Dis. 2019, 69, 1296–1302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meites, E.; Winer, R.L.; Newcomb, M.E.; Gorbach, P.M.; Querec, T.D.; Rudd, J.; Collins, T.; Lin, J.; Moore, J.; Remble, T.; et al. Vaccine Effectiveness Against Prevalent Anal and Oral Human Papillomavirus Infection Among Men Who Have Sex With Men-United States, 2016–2018. J. Infect. Dis. 2020, 222, 2052–2060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institute of Health. Human Oral Microbiome Database. 2024. Available online: https://www.homd.org (accessed on 12 December 2024).
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.R., III; Soomer-James, J.T.A.; Frenette, M.; Pombert, J.F. Complete Genome Sequences of Two Human Oral Microbiome Commensals, Streptococcus salivarius ATCC 25975 and S. salivarius ATCC 27945. Genome Announc. 2017, 5, e00536-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, J.; Yang, L.; Paster, B.J.; Ganly, I.; Morris, L.; Pei, Z.; Hayes, R.B. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS ONE 2011, 6, e22788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damgaard, C.; Reinholdt, J.; Enevold, C.; Fiehn, N.E.; Nielsen, C.H.; Holmstrup, P. Immunoglobulin G antibodies against Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans in cardiovascular disease and Periodontitis. J. Oral. Microbiol. 2017, 9, 1374154, Erratum in J. Oral Microbiol. 2018, 10, 1433122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koziel, J.; Mydel, P.; Potempa, J. The link between periodontal disease and rheumatoid arthritis: An updated review. Curr. Rheumatol. Rep. 2014, 16, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dibello, V.; Lozupone, M.; Manfredini, D.; Dibello, A.; Zupo, R.; Sardone, R.; Daniele, A.; Lobbezoo, F.; Panza, F. Oral frailty and neurodegeneration in Alzheimer’s disease. Neural Regen. Res. 2021, 16, 2149–2153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Metabolomics of Head and Neck Cancer: A Mini-Review. Front. Physiol. 2016, 7, 526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Yin, Y.; Jiang, Y.; Yang, Y.; Wang, W.; Wang, X.; Ge, Y.; Liu, B.; Yao, L. Relationship between vaginal and oral microbiome in patients of human papillomavirus (HPV) infection and cervical cancer. J. Transl. Med. 2024, 22, 396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Cunha Villar, C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillison, M.L. Human papillomavirus-related diseases: Oropharynx cancers and potential implications for adolescent HPV vaccination. J. Adolesc. Health 2008, 43 (Suppl. S4), S52–S60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayaprakash, V.; Reid, M.; Hatton, E.; Merzianu, M.; Rigual, N.; Marshall, J.; Gill, S.; Frustino, J.; Wilding, G.; Loree, T.; et al. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: A meta-analysis, 1985–2010. Oral Oncol. 2011, 47, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Chifiriuc, M.C.; Mihaescu, G.; Vrancianu, C.O.; Dobre, E.G.; Cristian, R.E.; Bleotu, C.; Bertesteanu, S.V.; Grigore, R.; Serban, B.; et al. Implications of oral dysbiosis and HPV infection in head and neck cancer: From molecular and cellular mechanisms to early diagnosis and therapy. Front. Oncol. 2023, 13, 1273516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajishengallis, G. Immune evasion strategies of Porphyromonas gingivalis. J. Oral Biosci. 2011, 53, 233–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.S.; Kim, C.G.; Kim, W.K.; Kim, K.A.; Yoo, J.; Min, B.S.; Paik, S.; Shin, S.J.; Lee, H.; Lee, K.; et al. Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front. Cell. Infect. Microbiol. 2023, 13, 1101291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmerini, C.A.; Palombari, R.; Perito, S.; Arienti, G. NO synthesis in human saliva. Free Radic. Res. 2003, 37, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Burleigh, M.C.; Easton, C. The oral microbiome, nitric oxide and exercise performance. Nitric Oxide 2022, 125–126, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.C.; Falcinelli, S.; Ciabatti, I.; Cutruzzolà, F.; Brunori, M. Pseudomonas aeruginosa nitrite reductase (or cytochrome oxidase): An overview. Biochimie 1994, 76, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, M.Z.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral microbiota and oral cancer: Review. Oncol. Rev. 2020, 14, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hinten, F.; Hilbrands, L.B.; Meeuwis, K.A.P.; IntHout, J.; Quint, W.G.V.; Hoitsma, A.J.; Massuger, L.F.A.G.; Melchers, W.J.G.; de Hullu, J.A. Reactivation of Latent HPV Infections After Renal Transplantation. Am. J. Transplant. 2017, 17, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, X.; Lei, Z.; Feng, H.; Wang, S.; Cen, X.; Gao, S.; Jiang, Y.; Jiang, J.; Chen, Q.; et al. Chronic Inflammation-Related HPV: A Driving Force Speeds Oropharyngeal Carcinogenesis. PLoS ONE 2015, 10, e0133681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA integration and carcinogenesis: Putative roles for inflammation and oxidative stress. Future Virol. 2011, 6, 45–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Whelan, A.; Al-Hebshi, N.N.; Healy, C.M.; Moran, G.P. Acetaldehyde production by Rothia mucilaginosa isolates from patients with oral leukoplakia. J. Oral Microbiol. 2020, 12, 1743066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smędra, A.; Berent, J. The Influence of the Oral Microbiome on Oral Cancer: A Literature Review and a New Approach. Biomolecules 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, G.J.; Chung, H.W.; Lee, K.H.; Ahn, H.S. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J. Korean Med. Sci. 2005, 20, 267–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliva, M.; Schneeberger, P.H.H.; Rey, V.; Cho, M.; Taylor, R.; Hansen, A.R.; Taylor, K.; Hosni, A.; Bayley, A.; Hope, A.J.; et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br. J. Cancer 2021, 124, 1543–1551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elnaggar, J.H.; Huynh, V.O.; Lin, D.; Hillman, R.T.; Abana, C.O.; El Alam, M.B.; Tomasic, K.C.; Karpinets, T.V.; Kouzy, R.; Phan, J.L.; et al. HPV-related anal cancer is associated with changes in the anorectal microbiome during cancer development. Front. Immunol. 2023, 14, 1051431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rieth, K.K.S.; Gill, S.R.; Lott-Limbach, A.A.; Merkley, M.A.; Botero, N.; Allen, P.D.; Miller, M.C. Prevalence of High-Risk Human Papillomavirus in Tonsil Tissue in Healthy Adults and Colocalization in Biofilm of Tonsillar Crypts. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 231–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L.; et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021, 15, 894–908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. Biomed. Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKeon, M.G.; Gallant, J.N.; Kim, Y.J.; Das, S.R. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers 2022, 14, 3120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortiz, A.P.; González, D.; Vivaldi-Oliver, J.; Castañeda, M.; Rivera, V.; Díaz, E.; Centeno, H.; Muñoz, C.; Palefsky, J.; Joshipura, K.; et al. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus Res. 2018, 5, 128–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazul, A.L.; Taylor, J.M.; Divaris, K.; Weissler, M.C.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Olshan, A.F.; Zevallos, J.P. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2017, 123, 71–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NHS Inform Mouth Cancer Mouth Cancer | NHS Inform. Available online: https://www.nhsinform.scot/illnesses-and-conditions/cancer/cancer-types-in-adults/mouth-cancer/ (accessed on 18 November 2024).
- Yang, J.; Guo, K.; Zhang, A.; Zhu, Y.; Li, W.; Yu, J.; Wang, P. Survival analysis of age-related oral squamous cell carcinoma: A population study based on SEER. Eur. J. Med. Res. 2023, 28, 413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tajmirriahi, N.; Razavi, S.M.; Shirani, S.; Homayooni, S.; Gasemzadeh, G. Evaluation of metastasis and 5-year survival in oral squamous cell carcinoma patients in Isfahan (2001–2015). Dent Res. J. 2019, 16, 117–121. [Google Scholar] [PubMed] [PubMed Central]
- Bramati, C.; Abati, S.; Bondi, S.; Lissoni, A.; Arrigoni, G.; Filipello, F.; Trimarchi, M. Early diagnosis of oral squamous cell carcinoma may ensure better prognosis: A case series. Clin. Case Rep. 2021, 9, e05004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colevas, A.D. HPV DNA as a Biomarker in Oropharyngeal Cancer: A Step in the Right Direction. Clin. Cancer Res. 2022, 28, 4171–4172. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake Weeramange, C.; Liu, Z.; Hartel, G.; Li, Y.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Morris, L.; Frazer, I.; Tang, K.D.; et al. Salivary High-Risk Human Papillomavirus (HPV) DNA as a Biomarker for HPV-Driven Head and Neck Cancers. J. Mol. Diagn. 2021, 23, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, A.; Moissl-Eichinger, C.; Perras, A.; Koskinen, K.; Tomazic, P.V.; Thurnher, D. The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: A pilot study. Sci. Rep. 2017, 7, 5867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuominen, H.; Rautava, S.; Syrjänen, S.; Collado, M.C.; Rautava, J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci. Rep. 2018, 8, 9787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahlstrom, K.R.; Sikora, A.G.; Liu, Y.; Chang, C.C.; Wei, P.; Sturgis, E.M.; Li, G. Characterization of the oral microbiota among middle-aged men with and without human papillomavirus infection. Oral Oncol. 2023, 142, 106401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Börnigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mougeot, J.C.; Beckman, M.F.; Langdon, H.C.; Lalla, R.V.; Brennan, M.T.; Bahrani Mougeot, F.K. Haemophilus pittmaniae and Leptotrichia spp. Constitute a Multi-Marker Signature in a Cohort of Human Papillomavirus-Positive Head and Neck Cancer Patients. Front. Microbiol. 2022, 12, 794546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shigeishi, H.; Sugiyama, M.; Ohta, K. Relationship between the prevalence of oral human papillomavirus DNA and periodontal disease (Review). Biomed. Rep. 2021, 14, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuchit, S.; Lam-Ubol, A.; Paemuang, W.; Talungchit, S.; Chokchaitam, O.; Mungkung, O.O.; Pongcharoen, T.; Trachootham, D. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: A randomized controlled trial. Support. Care Cancer 2020, 28, 2817–2828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfouzan, A.F. Radiation therapy in head and neck cancer. Saudi Med. J. 2021, 42, 247–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.; Langendijk, J.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016, 58, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.C.; Gao, X.J.; Dong, X.Z. Non-mutans streptococci in patients receiving radiotherapy in the head and neck area. Caries Res. 2003, 37, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, G.; Su, H.; Johnson, C.H.; Khan, S.A.; Kluger, H.; Lu, L. Intratumour microbiome associated with the infiltration of cytotoxic CD8+ T cells and patient survival in cutaneous melanoma. Eur. J. Cancer 2021, 151, 25–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, M.; Li, X.; Jiao, X.; Cai, X.; Yao, F.; Xu, S.; Huang, X.; Zhang, Q.; Chen, J. Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance. Front. Cell. Infect. Microbiol. 2023, 12, 1036869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: A prospective controlled pilot study. Eur. J. Cancer Prev. 2013, 22, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ou, Y.C.; Fu, H.C.; Tseng, C.W.; Wu, C.H.; Tsai, C.C.; Lin, H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: A randomized placebo-controlled trial. BMC Womens Health 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antivir. Res. 2019, 166, 56–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Karami, F.; Neyazi, N.; Modarressi, M.H.; Karimi, R.; Khorramizadeh, M.R.; Motevaseli, E. Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J. 2016, 18, 127. [Google Scholar]
- Motevaseli, E.; Azam, R.; Akrami, S.M.; Mazlomy, M.; Saffari, M.; Modarressi, M.H.; Daneshvar, M.; Ghafouri-Fard, S. The Effect of Lactobacillus crispatus and Lactobacillus rhamnosusCulture Supernatants on Expression of Autophagy Genes and HPV E6 and E7 Oncogenes in The HeLa Cell Line. Cell J. 2016, 17, 601–607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, S.; Hao, Y.; Zhang, X.; Yang, Y.; Liu, M.; Wang, N.; Zhang, T.C.; He, H. Lacticaseibacillus casei LH23 Suppressed HPV Gene Expression and Inhibited Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2023, 15, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.K.; Lee, D.K.; An, H.M.; Lee, S.W.; Shin, S.H.; Kwon, J.H.; Ha, N.J. Antiviral activity of Bifdobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med. 2012, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Aranha, C. Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr. Res. Toxicol. 2022, 3, 100088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect. Agent Cancer 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DIPierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: Results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).