HPV Infection and Oral Microbiota: Interactions and Future Implications
Abstract
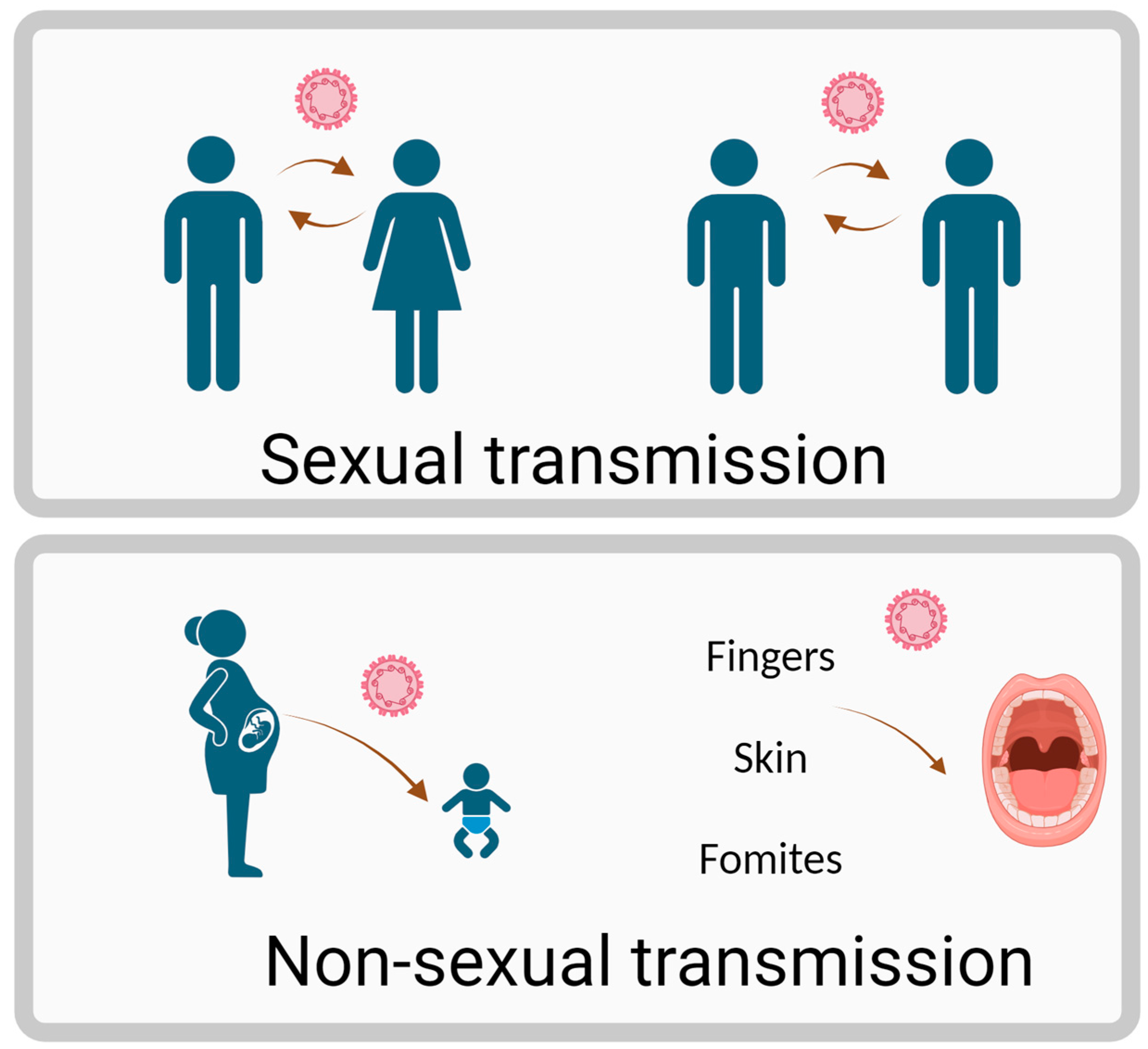
1. Introduction
2. Overview of HPV and Oral Infections
3. The Oral Microbiota
4. HPV and Oral Microbiota Interaction
5. Oral Microbiota as a Biomarker for HPV-Related Cancers
6. Therapeutic Implications and Future Directions
| Bacteria/Probiotics | Cell Line | Outcome | References |
| Milk-isolated L. casei and L. paracasei | HeLa | Upregulating the expression of apoptotic genes | [127] |
| Vagina-isolated L. gasseri | HeLa | Inflammation and proliferation were reduced, and apoptosis was increased | [128] |
| Vagina-isolated L. plantarum | HeLa | Suppression of proliferation and induction of apoptosis | [129] |
| Supernatants of L. rhamnosus and L. crispatus | HeLa | Inhibited cell proliferation and metastasis | [130] |
| Supernatants of L. rhamnosus and L. crispatus | HeLa | Decrease expression of HPV oncogenes | [131] |
| Lacticaseibacillus casei LH23 | HeLa | suppress the proliferation and induced the apoptosis of cervical cancer cells | [132] |
| Bifdobacterium adolescentis SPM1005-A | SiHa | Inhibited E6 and E7 oncogenes | [133] |
| supernatants of L. crispatus, L. jensenii, and L. gasseri | CasKi | inhibitory effects on the viability of cervical cancer cells via regulation of HPV oncogenes and cell cycle-related genes | [134] |
| Twelve standard Lactobacillus | HeLa and SiHa | Reduce tumor invasion and metastasis | [135] |
| Sample Size | Bacterial Strain | Condition | Intervention | Outcome | References |
|---|---|---|---|---|---|
| N = 160 | L. crispatus M247 | ASCUS * or LSIL * HPV+ | Oral administration (no fewer than 20 billion) for 12 months | higher percentage of clearance of PAP-smear abnormalities in patients who took oral Lactobacillus crispatus M247 than in the control group | [136] |
| N = 35 | L. crispatus M247 | ASCUS or LSIL or NILM * HPV+ | Oral administration (no fewer than 20 billion) for 3 months | reduction of approximately 70% in HPV positivity and a significant change in CST status with 94% of women | [137] |
| N = 54 | L. casei Shirota | LSIL HPV+ | Oral administration (no fewer than 20 billion) for 6 months | Probiotic users had a twice as high chance of clearance of cytological abnormalities | [123] |
| N = 121 | L. rhamnosus and L. reuteri | High-risk HPV | Oral administration (no fewer than 5.4 billion) discontinued until negative HPV testing | No significant influence on HR-HPV clearance, but may have decreased the rates of mildly abnormal and unsatisfactory cervical smears | [125] |
| N = 91 | L. crispatus chen01 | High-risk HPV | Intravaginal 1 × 109 CFU per capsule for 5 months | Significantly reduced viral load of HPV, ameliorated HPV clearance rate, and improved vaginal inflammation state | [124] |
| N = 117 | L. rhamnosus BMX 54 | C1N1 *, HPV+ | Intravaginal 1 × 104 CFU per tablet for 3 or 6 months | Long term user doubled the chance of resolving HPV-related cytological anomalies compared to short term user | [138] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riva, G.; Albano, C.; Gugliesi, F.; Pasquero, S.; Pacheco, S.F.C.; Pecorari, G.; Landolfo, S.; Biolatti, M.; Dell’Oste, V. HPV Meets APOBEC: New Players in Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upadhyay, R.; Dhakal, A.; Wheeler, C.; Hoyd, R.; Jagjit Singh, M.; Karivedu, V.; Bhateja, P.; Bonomi, M.; Valentin, S.; Gamez, M.E.; et al. Comparative analysis of the tumor microbiome, molecular profiles, and immune cell abundances by HPV status in mucosal head and neck cancers and their impact on survival. Cancer Biol. Ther. 2024, 25, 2350249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; D’Souza, G.; Fakhry, C.; Bigelow, E.O.; Usyk, M.; Burk, R.D.; Zhao, N. Oral Human Papillomavirus Associated With Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J. Infect. Dis. 2022, 226, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannatà, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a potential non-invasive liquid biopsy for early and easy diagnosis/prognosis of head and neck cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell. Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Ma, Q.; Zhang, L.; Ma, L.; Wang, D.; Yang, Y.; Jia, P.; Wu, Y.; Wang, F. Human papillomavirus and cervical cancer in the microbial world: Exploring the vaginal microecology. Front. Cell. Infect. Microbiol. 2024, 14, 1325500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bravo, I.G.; Félez-Sánchez, M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol. Med. Public Health 2015, 2015, 32–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michaud, D.S.; Langevin, S.M.; Eliot, M.; Nelson, H.H.; Pawlita, M.; McClean, M.D.; Kelsey, K.T. High-risk HPV types and head and neck cancer. Int. J. Cancer 2014, 135, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Río-Ospina, L.; Soto-DELeón, S.C.; Camargo, M.; Sánchez, R.; Moreno-Pérez, D.A.; Pérez-Prados, A.; Patarroyo, M.E.; Patarroyo, M.A. Multiple high-risk HPV genotypes are grouped by type and are associated with viral load and risk factors. Epidemiol. Infect. 2017, 145, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Oyouni, A.A.A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Checchi, M.; Burns, L.; Pavitt, C.; Postma, M.J.; Jit, M. Systematic review and evidence synthesis of non-cervical human papillomavirus-related disease health system costs and quality of life estimates. Sex. Transm. Infect. 2019, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shen-Gunther, J.; Xia, Q.; Stacey, W.; Asusta, H.B. Molecular Pap Smear: Validation of HPV Genotype and Host Methylation Profiles of ADCY8, CDH8, and ZNF582 as a Predictor of Cervical Cytopathology. Front. Microbiol. 2020, 11, 595902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litwin, T.R.; Clarke, M.A.; Dean, M.; Wentzensen, N. Somatic Host Cell Alterations in HPV Carcinogenesis. Viruses 2017, 9, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.; Shi, C.; Zhou, R.; Han, Y.; Li, N.; Qu, C.; Xia, R.; Zhang, C.; Hu, Y.; Tian, Z.; et al. Mapping the landscape of HPV integration and characterising virus and host genome interactions in HPV-positive oropharyngeal squamous cell carcinoma. Clin. Transl. Med. 2024, 14, e1556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal microbiota significantly changed for HPV-positive women with high-grade squamous intraepithelial lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osazuwa-Peters, N.; Simpson, M.C.; Massa, S.T.; Adjei Boakye, E.; Antisdel, J.L.; Varvares, M.A. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017, 74, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, K.; Suk, R.; Chiao, E.Y.; Chhatwal, J.; Qiu, P.; Wilkin, T.; Nyitray, A.G.; Sikora, A.G.; Deshmukh, A.A. Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann. Intern. Med. 2017, 167, 714–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghanem, A.S.; Memon, H.A.; Nagy, A.C. Evolving trends in oral cancer burden in Europe: A systematic review. Front. Oncol. 2024, 14, 1444326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Coleman, S.; Fadlullah, M.Z.H.; Spakowicz, D.; Chung, C.H.; Tan, A.C. Deciphering the Tumor-Immune-Microbe Interactions in HPV-Negative Head and Neck Cancer. Genes 2023, 14, 1599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, Z.H.; Butler, S.S.; Mahal, B.A.; Muralidhar, V.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Short-term mortality risks among patients with oropharynx cancer by human papillomavirus status. Cancer 2020, 126, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Christianto, S.; Li, K.Y.; Huang, T.H.; Su, Y.X. The Prognostic Value of Human Papilloma Virus Infection in Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2022, 132, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Hübbers, C.U.; Akgül, B. HPV and cancer of the oral cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Bryant, T.S.; Babrah, J.; Louie, K.; Bryant, J.L.; Spruce, R.J.; Batis, N.; Olaleye, O.; Jones, J.; Struijk, L.; et al. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin. Infect. Dis. 2019, 69, 1296–1302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meites, E.; Winer, R.L.; Newcomb, M.E.; Gorbach, P.M.; Querec, T.D.; Rudd, J.; Collins, T.; Lin, J.; Moore, J.; Remble, T.; et al. Vaccine Effectiveness Against Prevalent Anal and Oral Human Papillomavirus Infection Among Men Who Have Sex With Men-United States, 2016–2018. J. Infect. Dis. 2020, 222, 2052–2060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institute of Health. Human Oral Microbiome Database. 2024. Available online: https://www.homd.org (accessed on 12 December 2024).
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.R., III; Soomer-James, J.T.A.; Frenette, M.; Pombert, J.F. Complete Genome Sequences of Two Human Oral Microbiome Commensals, Streptococcus salivarius ATCC 25975 and S. salivarius ATCC 27945. Genome Announc. 2017, 5, e00536-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, J.; Yang, L.; Paster, B.J.; Ganly, I.; Morris, L.; Pei, Z.; Hayes, R.B. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS ONE 2011, 6, e22788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damgaard, C.; Reinholdt, J.; Enevold, C.; Fiehn, N.E.; Nielsen, C.H.; Holmstrup, P. Immunoglobulin G antibodies against Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans in cardiovascular disease and Periodontitis. J. Oral. Microbiol. 2017, 9, 1374154, Erratum in J. Oral Microbiol. 2018, 10, 1433122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koziel, J.; Mydel, P.; Potempa, J. The link between periodontal disease and rheumatoid arthritis: An updated review. Curr. Rheumatol. Rep. 2014, 16, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dibello, V.; Lozupone, M.; Manfredini, D.; Dibello, A.; Zupo, R.; Sardone, R.; Daniele, A.; Lobbezoo, F.; Panza, F. Oral frailty and neurodegeneration in Alzheimer’s disease. Neural Regen. Res. 2021, 16, 2149–2153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Metabolomics of Head and Neck Cancer: A Mini-Review. Front. Physiol. 2016, 7, 526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Yin, Y.; Jiang, Y.; Yang, Y.; Wang, W.; Wang, X.; Ge, Y.; Liu, B.; Yao, L. Relationship between vaginal and oral microbiome in patients of human papillomavirus (HPV) infection and cervical cancer. J. Transl. Med. 2024, 22, 396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Cunha Villar, C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillison, M.L. Human papillomavirus-related diseases: Oropharynx cancers and potential implications for adolescent HPV vaccination. J. Adolesc. Health 2008, 43 (Suppl. S4), S52–S60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayaprakash, V.; Reid, M.; Hatton, E.; Merzianu, M.; Rigual, N.; Marshall, J.; Gill, S.; Frustino, J.; Wilding, G.; Loree, T.; et al. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: A meta-analysis, 1985–2010. Oral Oncol. 2011, 47, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Chifiriuc, M.C.; Mihaescu, G.; Vrancianu, C.O.; Dobre, E.G.; Cristian, R.E.; Bleotu, C.; Bertesteanu, S.V.; Grigore, R.; Serban, B.; et al. Implications of oral dysbiosis and HPV infection in head and neck cancer: From molecular and cellular mechanisms to early diagnosis and therapy. Front. Oncol. 2023, 13, 1273516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajishengallis, G. Immune evasion strategies of Porphyromonas gingivalis. J. Oral Biosci. 2011, 53, 233–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.S.; Kim, C.G.; Kim, W.K.; Kim, K.A.; Yoo, J.; Min, B.S.; Paik, S.; Shin, S.J.; Lee, H.; Lee, K.; et al. Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front. Cell. Infect. Microbiol. 2023, 13, 1101291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmerini, C.A.; Palombari, R.; Perito, S.; Arienti, G. NO synthesis in human saliva. Free Radic. Res. 2003, 37, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Burleigh, M.C.; Easton, C. The oral microbiome, nitric oxide and exercise performance. Nitric Oxide 2022, 125–126, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.C.; Falcinelli, S.; Ciabatti, I.; Cutruzzolà, F.; Brunori, M. Pseudomonas aeruginosa nitrite reductase (or cytochrome oxidase): An overview. Biochimie 1994, 76, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, M.Z.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral microbiota and oral cancer: Review. Oncol. Rev. 2020, 14, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hinten, F.; Hilbrands, L.B.; Meeuwis, K.A.P.; IntHout, J.; Quint, W.G.V.; Hoitsma, A.J.; Massuger, L.F.A.G.; Melchers, W.J.G.; de Hullu, J.A. Reactivation of Latent HPV Infections After Renal Transplantation. Am. J. Transplant. 2017, 17, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, X.; Lei, Z.; Feng, H.; Wang, S.; Cen, X.; Gao, S.; Jiang, Y.; Jiang, J.; Chen, Q.; et al. Chronic Inflammation-Related HPV: A Driving Force Speeds Oropharyngeal Carcinogenesis. PLoS ONE 2015, 10, e0133681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA integration and carcinogenesis: Putative roles for inflammation and oxidative stress. Future Virol. 2011, 6, 45–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Whelan, A.; Al-Hebshi, N.N.; Healy, C.M.; Moran, G.P. Acetaldehyde production by Rothia mucilaginosa isolates from patients with oral leukoplakia. J. Oral Microbiol. 2020, 12, 1743066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smędra, A.; Berent, J. The Influence of the Oral Microbiome on Oral Cancer: A Literature Review and a New Approach. Biomolecules 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, G.J.; Chung, H.W.; Lee, K.H.; Ahn, H.S. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J. Korean Med. Sci. 2005, 20, 267–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliva, M.; Schneeberger, P.H.H.; Rey, V.; Cho, M.; Taylor, R.; Hansen, A.R.; Taylor, K.; Hosni, A.; Bayley, A.; Hope, A.J.; et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br. J. Cancer 2021, 124, 1543–1551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elnaggar, J.H.; Huynh, V.O.; Lin, D.; Hillman, R.T.; Abana, C.O.; El Alam, M.B.; Tomasic, K.C.; Karpinets, T.V.; Kouzy, R.; Phan, J.L.; et al. HPV-related anal cancer is associated with changes in the anorectal microbiome during cancer development. Front. Immunol. 2023, 14, 1051431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rieth, K.K.S.; Gill, S.R.; Lott-Limbach, A.A.; Merkley, M.A.; Botero, N.; Allen, P.D.; Miller, M.C. Prevalence of High-Risk Human Papillomavirus in Tonsil Tissue in Healthy Adults and Colocalization in Biofilm of Tonsillar Crypts. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 231–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L.; et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021, 15, 894–908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. Biomed. Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKeon, M.G.; Gallant, J.N.; Kim, Y.J.; Das, S.R. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers 2022, 14, 3120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortiz, A.P.; González, D.; Vivaldi-Oliver, J.; Castañeda, M.; Rivera, V.; Díaz, E.; Centeno, H.; Muñoz, C.; Palefsky, J.; Joshipura, K.; et al. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus Res. 2018, 5, 128–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazul, A.L.; Taylor, J.M.; Divaris, K.; Weissler, M.C.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Olshan, A.F.; Zevallos, J.P. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2017, 123, 71–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NHS Inform Mouth Cancer Mouth Cancer | NHS Inform. Available online: https://www.nhsinform.scot/illnesses-and-conditions/cancer/cancer-types-in-adults/mouth-cancer/ (accessed on 18 November 2024).
- Yang, J.; Guo, K.; Zhang, A.; Zhu, Y.; Li, W.; Yu, J.; Wang, P. Survival analysis of age-related oral squamous cell carcinoma: A population study based on SEER. Eur. J. Med. Res. 2023, 28, 413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tajmirriahi, N.; Razavi, S.M.; Shirani, S.; Homayooni, S.; Gasemzadeh, G. Evaluation of metastasis and 5-year survival in oral squamous cell carcinoma patients in Isfahan (2001–2015). Dent Res. J. 2019, 16, 117–121. [Google Scholar] [PubMed] [PubMed Central]
- Bramati, C.; Abati, S.; Bondi, S.; Lissoni, A.; Arrigoni, G.; Filipello, F.; Trimarchi, M. Early diagnosis of oral squamous cell carcinoma may ensure better prognosis: A case series. Clin. Case Rep. 2021, 9, e05004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colevas, A.D. HPV DNA as a Biomarker in Oropharyngeal Cancer: A Step in the Right Direction. Clin. Cancer Res. 2022, 28, 4171–4172. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake Weeramange, C.; Liu, Z.; Hartel, G.; Li, Y.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Morris, L.; Frazer, I.; Tang, K.D.; et al. Salivary High-Risk Human Papillomavirus (HPV) DNA as a Biomarker for HPV-Driven Head and Neck Cancers. J. Mol. Diagn. 2021, 23, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, A.; Moissl-Eichinger, C.; Perras, A.; Koskinen, K.; Tomazic, P.V.; Thurnher, D. The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: A pilot study. Sci. Rep. 2017, 7, 5867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuominen, H.; Rautava, S.; Syrjänen, S.; Collado, M.C.; Rautava, J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci. Rep. 2018, 8, 9787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahlstrom, K.R.; Sikora, A.G.; Liu, Y.; Chang, C.C.; Wei, P.; Sturgis, E.M.; Li, G. Characterization of the oral microbiota among middle-aged men with and without human papillomavirus infection. Oral Oncol. 2023, 142, 106401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Börnigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mougeot, J.C.; Beckman, M.F.; Langdon, H.C.; Lalla, R.V.; Brennan, M.T.; Bahrani Mougeot, F.K. Haemophilus pittmaniae and Leptotrichia spp. Constitute a Multi-Marker Signature in a Cohort of Human Papillomavirus-Positive Head and Neck Cancer Patients. Front. Microbiol. 2022, 12, 794546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shigeishi, H.; Sugiyama, M.; Ohta, K. Relationship between the prevalence of oral human papillomavirus DNA and periodontal disease (Review). Biomed. Rep. 2021, 14, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuchit, S.; Lam-Ubol, A.; Paemuang, W.; Talungchit, S.; Chokchaitam, O.; Mungkung, O.O.; Pongcharoen, T.; Trachootham, D. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: A randomized controlled trial. Support. Care Cancer 2020, 28, 2817–2828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfouzan, A.F. Radiation therapy in head and neck cancer. Saudi Med. J. 2021, 42, 247–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.; Langendijk, J.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016, 58, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.C.; Gao, X.J.; Dong, X.Z. Non-mutans streptococci in patients receiving radiotherapy in the head and neck area. Caries Res. 2003, 37, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, G.; Su, H.; Johnson, C.H.; Khan, S.A.; Kluger, H.; Lu, L. Intratumour microbiome associated with the infiltration of cytotoxic CD8+ T cells and patient survival in cutaneous melanoma. Eur. J. Cancer 2021, 151, 25–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, M.; Li, X.; Jiao, X.; Cai, X.; Yao, F.; Xu, S.; Huang, X.; Zhang, Q.; Chen, J. Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance. Front. Cell. Infect. Microbiol. 2023, 12, 1036869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: A prospective controlled pilot study. Eur. J. Cancer Prev. 2013, 22, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ou, Y.C.; Fu, H.C.; Tseng, C.W.; Wu, C.H.; Tsai, C.C.; Lin, H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: A randomized placebo-controlled trial. BMC Womens Health 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antivir. Res. 2019, 166, 56–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Karami, F.; Neyazi, N.; Modarressi, M.H.; Karimi, R.; Khorramizadeh, M.R.; Motevaseli, E. Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J. 2016, 18, 127. [Google Scholar]
- Motevaseli, E.; Azam, R.; Akrami, S.M.; Mazlomy, M.; Saffari, M.; Modarressi, M.H.; Daneshvar, M.; Ghafouri-Fard, S. The Effect of Lactobacillus crispatus and Lactobacillus rhamnosusCulture Supernatants on Expression of Autophagy Genes and HPV E6 and E7 Oncogenes in The HeLa Cell Line. Cell J. 2016, 17, 601–607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, S.; Hao, Y.; Zhang, X.; Yang, Y.; Liu, M.; Wang, N.; Zhang, T.C.; He, H. Lacticaseibacillus casei LH23 Suppressed HPV Gene Expression and Inhibited Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2023, 15, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.K.; Lee, D.K.; An, H.M.; Lee, S.W.; Shin, S.H.; Kwon, J.H.; Ha, N.J. Antiviral activity of Bifdobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med. 2012, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Aranha, C. Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr. Res. Toxicol. 2022, 3, 100088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect. Agent Cancer 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DIPierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: Results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Pierson, S. HPV Infection and Oral Microbiota: Interactions and Future Implications. Int. J. Mol. Sci. 2025, 26, 1424. https://doi.org/10.3390/ijms26041424
Xia Q, Pierson S. HPV Infection and Oral Microbiota: Interactions and Future Implications. International Journal of Molecular Sciences. 2025; 26(4):1424. https://doi.org/10.3390/ijms26041424
Chicago/Turabian StyleXia, Qingqing, and Sarah Pierson. 2025. "HPV Infection and Oral Microbiota: Interactions and Future Implications" International Journal of Molecular Sciences 26, no. 4: 1424. https://doi.org/10.3390/ijms26041424
APA StyleXia, Q., & Pierson, S. (2025). HPV Infection and Oral Microbiota: Interactions and Future Implications. International Journal of Molecular Sciences, 26(4), 1424. https://doi.org/10.3390/ijms26041424






