Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds
Abstract
:1. Introduction
| Coumarin Name | Molecular Formula | Chemical Structure | Ref. |
|---|---|---|---|
| Simple coumarins | |||
| Coumarin | C9H6O2 | 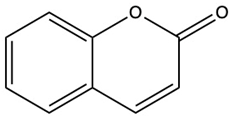 | [5] |
| Umbelliferone (7-hydroxycoumarin) | C9H6O3 |  | [17] |
| Esculetin (6,7-dihydroxycoumarin) | C9H6O4 |  | [18] |
| Scopoletin (6-methoxy-7-hydroxycoumarin) | C10H8O4 |  | [19] |
| Scoparone (6,7-dimethoxycoumarin) | C11H10O4 | 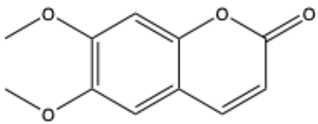 | [20] |
| Osthole (7-methoxy-8-[3-methylpent-2-enyl]coumarin) | C15H16O3 | 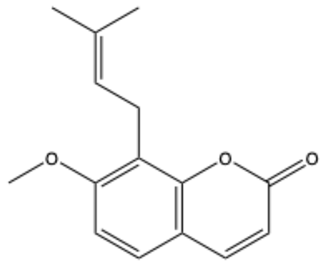 | [21] |
| Warfarin | C19H16O4 | 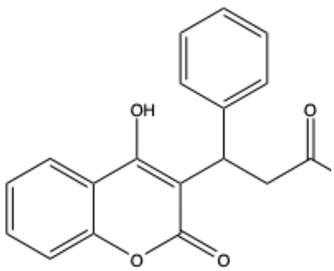 | [22] |
| Isocoumarins | |||
| Isocoumarin (benzopyran-1-one) | C9H6O2 | 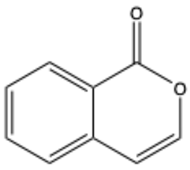 | [23] |
| Mellein (3,4-dihydro-8-hydroxyisocoumarin) | C10H10O3 | 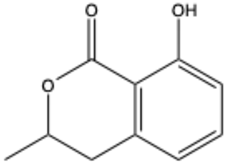 | [24] |
| Monocerin | C16H20O6 | 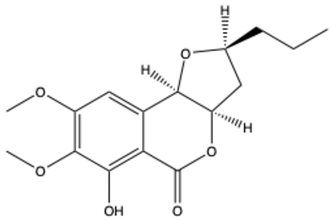 | [25] |
| Furocoumarins | |||
| Psoralen (furocoumarin) | C11H6O3 |  | [26] |
| Angelicin (isopsoralen) | C11H6O3 | 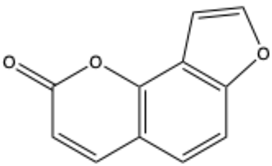 | [27] |
| Xanthotoxin (8-methoxypsoralen) | C12H8O4 | 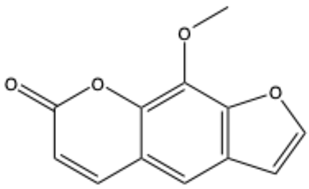 | [28] |
| Pyranocoumarins | |||
| Inophyllum A | C25H24O5 | 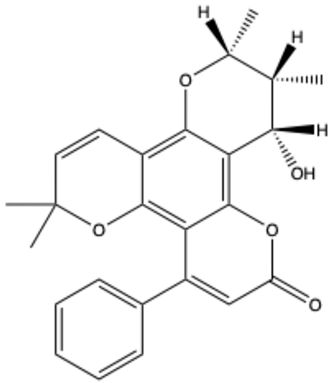 | [29] |
| Biscoumarins | |||
| Dicoumarol | C19H12O6 | 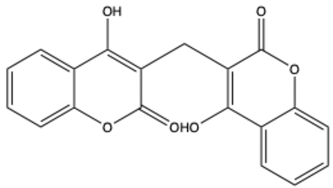 | [30] |
| Thamnosin | C30H28O6 | 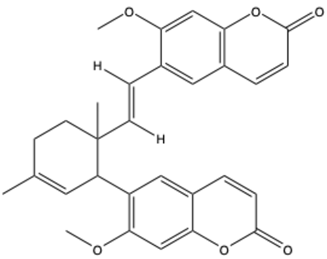 | [31] |
| Phenylocoumarins | |||
| 3-Phenylcoumarin | C15H10O2 | 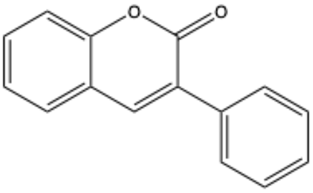 | [32] |
| Isodispar B | C20H18O5 | 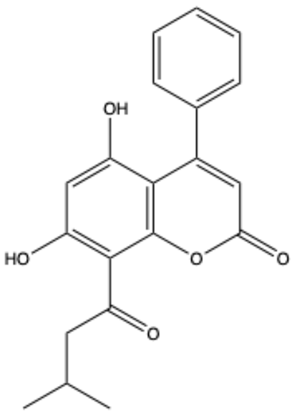 | [33] |
2. Advantages and Disadvantages of Using the Zebrafish Model
- (1)
- Genetic similarity to humans. A substantial proportion of zebrafish genes are identical to those found in humans, representing approximately 70% of the total [47], which makes them an invaluable model for the study of human disease and genetics.
- (2)
- Simplicity of genetic manipulation. The zebrafish is a convenient model organism for genetic modification [48], as it can be modified using a variety of techniques [49], including CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats) [50,51,52], morpholino injection [53], and transgenic approaches [54]. This makes it an ideal subject for the creation of models, which can be used to gain a deeper understanding of human diseases in a number of fields, including oncology, Alzheimer’s disease, immunology, diabetes, regenerative medicine, aging-related research, etc. [46].
- (3)
- High embryo yield and rapid reproduction rate. The zebrafish is a highly prolific species, capable of producing hundreds of embryos per week. This reproductive rate allows for the utilization of large sample sizes in scientific experiments and high-throughput screening in drug discovery and toxicology [46].
- (4)
- Embryo/body transparency. The transparency of zebrafish’s embryos and body throughout adulthood permits direct observation of organ development and cellular processes in real time [55]. This facilitates research into a range of topics, including tissue development, disease progression, and drug effects.
- (5)
- Development outside of the uterus. The external development of zebrafish embryos outside of the uterus provides researchers with convenient access for a range of studies, particularly those requiring post-fertilization observation [46].
- (6)
- Fast development and a short life cycle. The rapid development and short life cycle of zebrafish embryos provide an optimal environment for the evaluation of genetic and environmental interventions across generations. The embryos develop rapidly, with major organs forming within 24 h post-fertilization (hpf) and a fully functional body system within 120 hpf (5 days post-fertilization) [56]. This enables researchers to rapidly evaluate the impact of any intervention.
- (7)
- Cost-effectiveness. In terms of cost-effectiveness, zebrafish offer distinct advantages over mammalian models. Their smaller size and simpler aquatic environment necessitate reduced space requirements and maintenance costs, making them a more cost-effective choice for long-term studies [46,57,58,59].
- (8)
- Ethical guidelines. Zebrafish is categorized as a lower vertebrate, and research involving it is often subject to less stringent ethical guidelines than that conducted on rodents, for example. Additionally, in the case of studies involving larvae of zebrafish up to 120 hpf, the approval of the Ethical Commission is not a prerequisite. This enables the undertaking of studies in this model that might otherwise be challenging or unfeasible in mammals [60].
3. Studies of Coumarins and Coumarin-Based Compounds in Zebrafish Model
3.1. Toxicological and Developmental Research
3.2. Evaluation of Pharmacological Properties
3.2.1. Anti-Angiogenic Activity
3.2.2. Central Nervous System Disorders
3.2.3. Other Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Citarella, A.; Vittorio, S.; Dank, C.; Ielo, L. Syntheses, Reactivity, and Biological Applications of Coumarins. Front. Chem. 2024, 12, 1362992. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Norderstedt, Germany, 2015. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 323, Coumarin. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Coumarin (accessed on 10 November 2024).
- Dhara, A.K.; Nayak, A.K. Introduction to Herbal Biomolecules. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–19. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. CMC 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.S.; Payá, M. Pharmacological and Biochemical Actions of Simple Coumarins: Natural Products with Therapeutic Potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Tava, A. Coumarin-Containing Grass: Volatiles from Sweet Vernalgrass (Anthoxanthum odoratum L.). J. Essent. Oil Res. 2001, 13, 367–370. [Google Scholar] [CrossRef]
- Blahová, J.; Svobodová, Z. Assessment of Coumarin Levels in Ground Cinnamon Available in the Czech Retail Market. Sci. World J. 2012, 2012, 263851. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.; Setzer, W.N. Medicinal Plants of Tajikistan. In Vegetation of Central Asia and Environs; Egamberdieva, D., Öztürk, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–209. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural Product Coumarins: Biological and Pharmacological Perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Xu, P. Allelochemicals: A Source for Developing Economically and Environmentally Friendly Plant Growth Regulators. Biochem. Biophys. Res. Commun. 2024, 690, 149248. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; De Jonge, R.; Pieterse, C.M.J. The Age of Coumarins in Plant–Microbe Interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef]
- Zou, Y.; Teng, Y.; Li, J.; Yan, Y. Recent Advances in the Biosynthesis of Coumarin and Its Derivatives. Green Chem. Eng. 2024, 5, 150–154. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 5281426, Umbelliferone. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281426 (accessed on 21 November 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281416, Esculetin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281416 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280460, Scopoletin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280460 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8417, Scoparone. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8417 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10228, Osthol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10228 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 54678486, Warfarin. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Warfarin (accessed on 21 November 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 68108, Isocoumarin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/68108 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 28516, Mellein. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/28516 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 92267, Monocerin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/92267 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6199, Psoralen. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Psoralen (accessed on 21 November 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10658, Angelicin. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Angelicin (accessed on 21 November 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4114, Methoxsalen. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methoxsalen (accessed on 21 November 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 455248, Inophyllum A. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/455248 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 54676038, Dicumarol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/54676038 (accessed on 1 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5377043, Thamnosin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5377043 (accessed on 1 February 2025).
- PubChem Compound Summary for CID 70385, 3-Phenylcoumarin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/70385 (accessed on 1 February 2025).
- PubChem Compound Summary for CID 6483316, Isodispar B. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6483316 (accessed on 1 February 2025).
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent Advances and Therapeutic Journey of Coumarins: Current Status and Perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef]
- Vogel, A. Über Das Coumarin Und Einige Seiner Eigenschaften. Ann. Phys. Chem. 1820, 65, 172–180. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. (Eds.) Coumarins: Biology, Applications and Mode of Action; Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Link, K.P. The Discovery of Dicumarol and Its Sequels. Circulation 1959, 19, 97–107. [Google Scholar] [CrossRef]
- Riveiro, M.; De Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.K.; Khatoon, S.; Khan, M.F.; Akhtar, M.S.; Ahamad, S.; Saquib, M. Coumarins as Versatile Therapeutic Phytomolecules: A Systematic Review. Phytomedicine 2024, 134, 155972. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, Y.H.; Kim, H.; Ha, H.-H. Synthesis of Coumarin Derivatives and Investigation of Their Inhibitory Effects on Lung Cancer Cell Motility. Sci. Rep. 2022, 12, 21635. [Google Scholar] [CrossRef]
- Parichy, D.M. Advancing Biology through a Deeper Understanding of Zebrafish Ecology and Evolution. eLife 2015, 4, e05635. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, M.; Raja, M.; Vijayakumar, C.; Malaiammal, P.; Mayden, R.L. Natural History of Zebrafish (Danio rerio) in India. Zebrafish 2013, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zizioli, D.; Mione, M.; Varinelli, M.; Malagola, M.; Bernardi, S.; Alghisi, E.; Borsani, G.; Finazzi, D.; Monti, E.; Presta, M.; et al. Zebrafish Disease Models in Hematology: Highlights on Biological and Translational Impact. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of Zebrafish Models in Understanding Human Diseases—A Review of Models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, A. Improving Transgenesis in Zebrafish. Lab. Anim. 2024, 53, 192. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Sieliwonczyk, E.; Vandendriessche, B.; Claes, C.; Mayeur, E.; Alaerts, M.; Holmgren, P.; Canter Cremers, T.; Snyders, D.; Loeys, B.; Schepers, D. Improved Selection of Zebrafish CRISPR Editing by Early Next-Generation Sequencing Based Genotyping. Sci. Rep. 2023, 13, 1491. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Salazar, J.M.; Kaya, G.; Sekar, A.; Weyenberg, K.; Ingamells, C.; Dennis, M.Y. Evaluation of CRISPR Gene-Editing Tools in Zebrafish. BMC Genom. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Petree, C.; Requena, T.; Varshney, P.; Varshney, G.K. Expanding the CRISPR Toolbox in Zebrafish for Studying Development and Disease. Front. Cell Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef]
- Stainier, D.Y.R.; Raz, E.; Lawson, N.D.; Ekker, S.C.; Burdine, R.D.; Eisen, J.S.; Ingham, P.W.; Schulte-Merker, S.; Yelon, D.; Weinstein, B.M.; et al. Guidelines for Morpholino Use in Zebrafish. PLoS Genet. 2017, 13, e1007000. [Google Scholar] [CrossRef]
- Carney, T.J.; Mosimann, C. Switch and Trace: Recombinase Genetics in Zebrafish. Trends Genet. 2018, 34, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The Use of Zebrafish (Danio rerio) as Biomedical Models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish Embryos and Larvae: A New Generation of Disease Models and Drug Screens. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 115–133. [Google Scholar] [CrossRef]
- Detrich, H.W.; Westerfield, M.; Zon, L.I. Chapter 1 Overview of the Zebrafish System. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 59, pp. 3–10. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: An Emerging Real-Time Model System to Study Alzheimer’s Disease and Neurospecific Drug Discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Dooley, K. Zebrafish: A Model System for the Study of Human Disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Konantz, M.; Schürch, C.; Hanns, P.; Müller, J.S.; Sauteur, L.; Lengerke, C. Modeling Hematopoietic Disorders in Zebrafish. Dis. Models Mech. 2019, 12, dmm040360. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Lu, Q.; Wang, Y.; Chen, J.-N. Zebrafish as a Model for Cardiovascular Development and Disease. Drug Discov. Today Dis. Models 2008, 5, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; Van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a Tractable Model of Human Cardiovascular Disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, J.; Pandey, G.; Westhoff, J.H. Zebrafish as a Model for Drug Screening in Genetic Kidney Diseases. Front. Pediatr. 2018, 6, 183. [Google Scholar] [CrossRef]
- Poureetezadi, S.J.; Wingert, R.A. Little Fish, Big Catch: Zebrafish as a Model for Kidney Disease. Kidney Int. 2016, 89, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish Cancer: The State of the Art and the Path Forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Fogley, R.; Zon, L.I. Identifying Novel Cancer Therapies Using Chemical Genetics and Zebrafish. In Cancer and Zebrafish; Langenau, D.M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 916, pp. 103–124. [Google Scholar] [CrossRef]
- Cero, C.; House, J.S.; Verdi, V.; Ferguson, J.L.; Jima, D.D.; Selmek, A.A.; Patania, O.M.; Dwyer, J.E.; Wei, B.-R.; Lloyd, D.T.; et al. Profiling the Cancer-Prone Microenvironment in a Zebrafish Model for MPNST. Oncogene 2024, 44, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, T.M.; Wen, X.; Foster, J.A.; Kennedy, S.H. Zebrafish Models of Major Depressive Disorders. J. Neurosci. Res. 2016, 94, 3–14. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish Models of Autism Spectrum Disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a Fish: Zebra Fish (Danio rerio) as a Behavior Genetic Model to Study Alcohol Effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Bradamante, S.; Maier, J.A.; Alestrom, P.; Van Loon, J.J.; Muller, M. Effects of Microgravity Simulation on Zebrafish Transcriptomes and Bone Physiology—Exposure Starting at 5 Days Post Fertilization. NPJ Microgravity 2016, 2, 16010. [Google Scholar] [CrossRef]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Marée, R.; Dardenne, N.; Jeanray, N.; Wehenkel, L.; Aleström, P.; Van Loon, J.J.W.A.; Muller, M. Zebrafish Bone and General Physiology Are Differently Affected by Hormones or Changes in Gravity. PLoS ONE 2015, 10, e0126928. [Google Scholar] [CrossRef] [PubMed]
- Mrinalini, R.; Tamilanban, T.; Naveen Kumar, V.; Manasa, K. Zebrafish—The Neurobehavioural Model in Trend. Neuroscience 2023, 520, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Mamede, J.P.M.; Araujo-Silva, H.; Galvão-Pereira, M.C.; De Morais Freire, F.A.; Norton, W.J.; Luchiari, A.C. Consistency of Behavioral Profiles in Zebrafish: A Machine Learning Approach to Bold and Shy Individual Differences. Appl. Anim. Behav. Sci. 2024, 276, 106317. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring Behavioral and Endocrine Responses to Novelty Stress in Adult Zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Belo, M.A.A.; Oliveira, M.F.; Oliveira, S.L.; Aracati, M.F.; Rodrigues, L.F.; Costa, C.C.; Conde, G.; Gomes, J.M.M.; Prata, M.N.L.; Barra, A.; et al. Zebrafish as a Model to Study Inflammation: A Tool for Drug Discovery. Biomed. Pharmacother. 2021, 144, 112310. [Google Scholar] [CrossRef] [PubMed]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a Model for Inflammation and Drug Discovery. Drug Discov. Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Sarode, L.P.; Gaddam, R.R.; Kumar, P.; Bhatti, J.S.; Khurana, A.; Navik, U. Zebrafish as an Emerging Tool for Drug Discovery and Development for Thyroid Diseases. Fish Shellfish Immunol. 2022, 130, 53–60. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Uechi, T.; Kenmochi, N. Zebrafish Models of Diamond-Blackfan Anemia: A Tool for Understanding the Disease Pathogenesis and Drug Discovery. Pharmaceuticals 2019, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Smith, C. The Potential of Zebrafish as Drug Discovery Research Tool in Immune-Mediated Inflammatory Disease. Inflammopharmacol 2024, 32, 2219–2233. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In Vivo Drug Discovery in the Zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.N.; Patnaik, L. Flight for Fish in Drug Discovery: A Review of Zebrafish-Based Screening of Molecules. Biol. Lett. 2023, 19, 20220541. [Google Scholar] [CrossRef] [PubMed]
- Rocke, J.; Lees, J.; Packham, I.; Chico, T. The Zebrafish as a Novel Tool for Cardiovascular Drug Discovery. PRC 2009, 4, 1–5. [Google Scholar] [CrossRef]
- Delvecchio, C.; Tiefenbach, J.; Krause, H.M. The Zebrafish: A Powerful Platform for In Vivo, HTS Drug Discovery. ASSAY Drug Dev. Technol. 2011, 9, 354–361. [Google Scholar] [CrossRef]
- Joshi, S. Zebrafish Model for Drug Discovery and Screening. In Zebrafish Model for Biomedical Research; Bhandari, P.R., Bharani, K.K., Khurana, A., Eds.; Springer Nature: Singapore, 2022; pp. 229–258. [Google Scholar] [CrossRef]
- Zhang, T.; Peterson, R.T. Zebrafish as a Platform for Drug Screening. In The Zebrafish in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 659–675. [Google Scholar] [CrossRef]
- Esterberg, R.; Coffin, A.B.; Ou, H.; Simon, J.A.; Raible, D.W.; Rubel, E.W. Fish in a Dish: Drug Discovery for Hearing Habilitation. Drug Discov. Today Dis. Models 2013, 10, e23–e29. [Google Scholar] [CrossRef]
- Fleming, A.; Alderton, W.K. Zebrafish in Pharmaceutical Industry Research: Finding the Best Fit. Drug Discov. Today Dis. Models 2013, 10, e43–e50. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Drug Screening in the Zebrafish: An Overview. Drug Discov. Today Dis. Models 2013, 10, e1–e3. [Google Scholar] [CrossRef]
- Choe, S.-K.; Kim, C.-H. Zebrafish: A Powerful Model for Genetics and Genomics. Int. J. Mol. Sci. 2023, 24, 8169. [Google Scholar] [CrossRef]
- Li, C.; Tan, X.F.; Lim, T.K.; Lin, Q.; Gong, Z. Comprehensive and Quantitative Proteomic Analyses of Zebrafish Plasma Reveals Conserved Protein Profiles between Genders and between Zebrafish and Human. Sci. Rep. 2016, 6, 24329. [Google Scholar] [CrossRef] [PubMed]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A Marvel of High-Throughput Biology for 21st Century Toxicology. Curr. Environ. Health Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M. Beyond the Zebrafish: Diverse Fish Species for Modeling Human Disease. Dis. Models Mech. 2013, 7, 181–192. [Google Scholar] [CrossRef]
- Tobin, D.M.; Ramakrishnan, L. Comparative Pathogenesis of Mycobacterium Marinum and Mycobacterium Tuberculosis. Cell Microbiol. 2008, 10, 1027–1039. [Google Scholar] [CrossRef]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The Use of Zebrafish to Understand Immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef]
- Van Der Vaart, M.; Spaink, H.P.; Meijer, A.H. Pathogen Recognition and Activation of the Innate Immune Response in Zebrafish. Adv. Hematol. 2012, 2012, 159807. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think Small: Zebrafish as a Model System of Human Pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef]
- Loerracher, A.-K.; Braunbeck, T. Cytochrome P450-Dependent Biotransformation Capacities in Embryonic, Juvenile and Adult Stages of Zebrafish (Danio rerio)—A State-of-the-Art Review. Arch. Toxicol. 2021, 95, 2299–2334. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Grech, A.; Tebby, C.; Brochot, C.; Bois, F.Y.; Bado-Nilles, A.; Dorne, J.-L.; Quignot, N.; Beaudouin, R. Generic Physiologically-Based Toxicokinetic Modelling for Fish: Integration of Environmental Factors and Species Variability. Sci. Total Environ. 2019, 651, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Villacrez, M.; Hellman, K.; Ono, T.; Sugihara, Y.; Rezeli, M.; Ek, F.; Marko-Varga, G.; Olsson, R. Evaluation of Drug Exposure and Metabolism in Locust and Zebrafish Brains Using Mass Spectrometry Imaging. ACS Chem. Neurosci. 2018, 9, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, R.C.; Krekels, E.H.J.; Kantae, V.; Ordas, A.; Kreling, T.; Harms, A.C.; Hankemeier, T.; Spaink, H.P.; Van Der Graaf, P.H. Mechanistic and Quantitative Understanding of Pharmacokinetics in Zebrafish Larvae through Nanoscale Blood Sampling and Metabolite Modeling of Paracetamol. J. Pharmacol. Exp. Ther. 2019, 371, 15–24. [Google Scholar] [CrossRef]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish Embryos as Models for Embryotoxic and Teratological Effects of Chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Corum, D.; Padnos, B.; Hunter, D.L.; Beam, A.; Houck, K.A.; Sipes, N.; Kleinstreuer, N.; Knudsen, T.; Dix, D.J.; et al. Zebrafish Developmental Screening of the ToxCastTM Phase I Chemical Library. Reprod. Toxicol. 2012, 33, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jia, Y.; Chen, N.; Bian, W.; Li, Q.; Ma, Y.; Chen, Y.; Pei, D. Zebrafish as a Model System to Study Toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef]
- Brannen, K.C.; Panzica-Kelly, J.M.; Danberry, T.L.; Augustine-Rauch, K.A. Development of a Zebrafish Embryo Teratogenicity Assay and Quantitative Prediction Model. Birth Defects Res. Pt.B 2010, 89, 66–77. [Google Scholar] [CrossRef]
- Chakraborty, C.; Hsu, C.; Wen, Z.; Lin, C.; Agoramoorthy, G. Zebrafish: A Complete Animal Model for In Vivo Drug Discovery and Development. CDM 2009, 10, 116–124. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.T.; Van Rompay, A.R.; De Coen, W.; Witters, H.E. Development of a Screening Assay to Identify Teratogenic and Embryotoxic Chemicals Using the Zebrafish Embryo. Reprod. Toxicol. 2009, 28, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Ton, C.; Lin, Y.; Willett, C. Zebrafish as a Model for Developmental Neurotoxicity Testing. Birth Defects Res. 2006, 76, 553–567. [Google Scholar] [CrossRef]
- Modarresi Chahardehi, A.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.-A.; Bondesson, M. Comparison of Toxicity Values across Zebrafish Early Life Stages and Mammalian Studies: Implications for Chemical Testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aspatwar, A.; Berrino, E.; Bua, S.; Carta, F.; Capasso, C.; Parkkila, S.; Supuran, C.T. Toxicity Evaluation of Sulfamides and Coumarins That Efficiently Inhibit Human Carbonic Anhydrases. J. Enzym. Inhib. Med. Chem. 2020, 35, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Developmental Effects of Coumarin and the Anticoagulant Coumarin Derivative Warfarin on Zebrafish (Danio rerio) Embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef]
- Veselinović, J.B.; Kocić, G.M.; Pavic, A.; Nikodinovic-Runic, J.; Senerovic, L.; Nikolić, G.M.; Veselinović, A.M. Selected 4-Phenyl Hydroxycoumarins: In Vitro Cytotoxicity, Teratogenic Effect on Zebrafish (Danio rerio) Embryos and Molecular Docking Study. Chem.-Biol. Interact. 2015, 231, 10–17. [Google Scholar] [CrossRef]
- Verstraelen, S.; Peers, B.; Maho, W.; Hollanders, K.; Remy, S.; Berckmans, P.; Covaci, A.; Witters, H. Phenotypic and Biomarker Evaluation of Zebrafish Larvae as an Alternative Model to Predict Mammalian Hepatotoxicity. J. Appl. Toxicol. 2016, 36, 1194–1206. [Google Scholar] [CrossRef]
- Haque, M.N.; Nam, S.-E.; Han, Y.-S.; Park, H.S.; Rhee, J.-S. Chronic Exposure to Sublethal Concentrations of Saxitoxin Reduces Antioxidant Activity and Immunity in Zebrafish but Does Not Affect Reproductive Parameters. Aquat. Toxicol. 2022, 243, 106070. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 204: Fish, Prolonged Toxicity Test: 14-Day Study; OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 1984. [Google Scholar] [CrossRef]
- Nagel, R.; Isberner, K. Testing of Chemicals with Fish—A Critical Evaluation of Tests with Special Regard to Zebrafish. In Fish Ecotoxicology; Braunbeck, T., Hinton, D.E., Streit, B., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 337–352. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Blanc, M.; Cormier, B.; Hyötyläinen, T.; Krauss, M.; Scherbak, N.; Cousin, X.; Keiter, S.H. Multi- and Transgenerational Effects Following Early-Life Exposure of Zebrafish to Permethrin and Coumarin 47: Impact on Growth, Fertility, Behavior and Lipid Metabolism. Ecotoxicol. Environ. Saf. 2020, 205, 111348. [Google Scholar] [CrossRef] [PubMed]
- Anegundi, N.; Pancharatna, K. Evidence for Antiangiogenic Potentials of Herniarin (7-Methoxycoumarin). Pharm. Lett. 2017, 9, 96–104. [Google Scholar]
- Veselinović, J.B.; Veselinović, A.M.; Ilic-Tomic, T.; Davis, R.; O’Connor, K.; Pavic, A.; Nikodinovic-Runic, J. Potent Anti-Melanogenic Activity and Favorable Toxicity Profile of Selected 4-Phenyl Hydroxycoumarins in the Zebrafish Model and the Computational Molecular Modeling Studies. Bioorganic Med. Chem. 2017, 25, 6286–6296. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nishimura, Y.; Oka, T.; Nomoto, T.; Kon, T.; Shintou, T.; Hirano, M.; Shimada, Y.; Umemoto, N.; Kuroyanagi, J.; et al. In Vivo Imaging of Zebrafish Retinal Cells Using Fluorescent Coumarin Derivatives. BMC Neurosci. 2010, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- K, I.B.; K, V.B. Synthesis, Evaluation of Antiangiogenic Activity of Novel Coumarinyl 4-Thiazolidinone Derivatives. Int. J. Drug Deliv. Technol. 2024, 14, 1547–1552. [Google Scholar] [CrossRef]
- Huang, H.-T.; Huang, C.-Y.; Lee, C.-J.; Sun, B.-J.; Jhang, Z.-W.; Wen, C.-C.; Wang, Y.-H.; Li, T.-S.; Chern, C.-Y.; Chen, Y.-H. The Angiogenesis-Modulating Effects of Coumarin-Derivatives. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 278, 109862. [Google Scholar] [CrossRef]
- Kim, D.-C.; Kim, S.; Hwang, K.-S.; Kim, C.-H. p-Coumaric Acid Potently Down-Regulates Zebrafish Embryo Pigmentation: Comparison of in Vivo Assay and Computational Molecular Modeling with Phenylthiourea. Biomed. Sci. Lett. 2017, 23, 8–16. [Google Scholar] [CrossRef]
- Feng, C.; Chen, G.; Zhao, Y.; Xin, S.; Li, S.; Tang, J.; Li, X.; Hu, D.; Liu, X.; Gao, H. New Isocoumarins from a Cold-Adapted Fungal Strain Mucor sp. and Their Developmental Toxicity to Zebrafish Embryos. Chem. Biodivers. 2014, 11, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egaña, J.T. Zebrafish as an Emerging Model Organism to Study Angiogenesis in Development and Regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef]
- Gore, A.V.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular Development in the Zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A New Target for Future Therapy. Vasc. Pharmacol. 2006, 44, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hariprabu, K.N.G.; Yuvashree, R.; Vimalraj, S. Zebrafish: A Model Organism to Understand Tumor Angiogenesis Mechanism. In Zebrafish Model for Biomedical Research; Bhandari, P.R., Bharani, K.K., Khurana, A., Eds.; Springer Nature: Singapore, 2022; pp. 17–42. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Cheng, Y.-B.; Chuang, Y.-T.; Yang, K.-H.; Chang, F.-R.; Liu, W.; Chang, H.-W. Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides. Cells 2022, 11, 961. [Google Scholar] [CrossRef]
- Chimote, G.; Sreenivasan, J.; Pawar, N.; Subramanian, J.; Sharma, S.; Sivaramakrishnan, H. Comparison of Effects of Anti-Angiogenic Agents in the Zebrafish Efficacy–Toxicity Model for Translational Anti-Angiogenic Drug Discovery. Drug Des. Dev. Ther. 2014, 8, 1107. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [PubMed]
- Anegundi, N.; Pancharatna, K. 7-Hydroxycoumarin Elicit Anti-Angiogenic Effects Through Cellular Apoptosis in Developing Embryos of Zebrafish (Danio rerio). Eur. Sci. J. 2017, 13, 53. [Google Scholar] [CrossRef]
- Plein, A.; Fantin, A.; Ruhrberg, C. Neuropilin Regulation of Angiogenesis, Arteriogenesis, and Vascular Permeability. Microcirculation 2014, 21, 315–323. [Google Scholar] [CrossRef]
- Jung, M.H.; Lee, S.H.; Ahn, E.-M.; Lee, Y.M. Decursin and Decursinol Angelate Inhibit VEGF-Induced Angiogenesis via Suppression of the VEGFR-2-Signaling Pathway. Carcinogenesis 2009, 30, 655–661. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish Models in Neuropsychopharmacology and CNS Drug Discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Ochenkowska, K.; Herold, A.; Samarut, É. Zebrafish Is a Powerful Tool for Precision Medicine Approaches to Neurological Disorders. Front. Mol. Neurosci. 2022, 15, 944693. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The Developing Utility of Zebrafish Models of Neurological and Neuropsychiatric Disorders: A Critical Review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Gerlai, R.; Kalueff, A.V. Developing highER-Throughput Zebrafish Screens for in-Vivo CNS Drug Discovery. Front. Behav. Neurosci. 2015, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Rihel, J.; Ortiz, J.G.; Gerlai, R. The Zebrafish as a Promising Tool for Modeling Human Brain Disorders: A Review Based upon an IBNS Symposium. Neurosci. Biobehav. Rev. 2018, 85, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish Models for Translational Neuroscience Research: From Tank to Bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Plavan, G.; Ureche, D.; Jijie, R.; Nicoara, M. Zebrafish as a Potential Model for Neurodegenerative Diseases: A Focus on Toxic Metals Implications. Int. J. Mol. Sci. 2023, 24, 3428. [Google Scholar] [CrossRef]
- d’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef]
- Newman, M.; Verdile, G.; Martins, R.N.; Lardelli, M. Zebrafish as a Tool in Alzheimer’s Disease Research. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Banerjee, M.; Upadhya, A.; Bagwe-Parab, S.; Kaur, G. The Brilliance of the Zebrafish Model: Perception on Behavior and Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 861155. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of Coumarins towards Central Nervous System Disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.B.; Ferreira, R.S.; Dos Santos, C.C.; Castro E Silva, J.H.; Pereira, E.P.; De Almeida, M.M.A.; Do Nascimento, R.P.; De Sampaio Schitine, C.; De Oliveira, J.V.R.; Dos Santos, B.L.; et al. Neuroprotection Induced by Coumarins in Central Nervous System Disease Models. In Natural Molecules in Neuroprotection and Neurotoxicity; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1411–1440. [Google Scholar] [CrossRef]
- Burgess, H.A.; Burton, E.A. A Critical Review of Zebrafish Neurological Disease Models−1. The Premise: Neuroanatomical, Cellular and Genetic Homology and Experimental Tractability. Oxf. Open Neurosci. 2023, 2, kvac018. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.-C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The Comparative Neuroanatomy and Neurochemistry of Zebrafish CNS Systems of Relevance to Human Neuropsychiatric Diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Selderslaghs, I.W.T.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor Activity in Zebrafish Embryos: A New Method to Assess Developmental Neurotoxicity. Neurotoxicol. Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Selderslaghs, I.W.T.; Hooyberghs, J.; Blust, R.; Witters, H.E. Assessment of the Developmental Neurotoxicity of Compounds by Measuring Locomotor Activity in Zebrafish Embryos and Larvae. Neurotoxicol. Teratol. 2013, 37, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lakstygal, A.M.; Demin, K.A.; Kalueff, A.V. Motor Patterns and Swim Path Characteristics: The Ethogram of Zebrafish. In Behavioral and Neural Genetics of Zebrafish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–140. [Google Scholar] [CrossRef]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling Anxiety Using Adult Zebrafish: A Conceptual Review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Speedie, N.; Gerlai, R. Alarm Substance Induced Behavioral Responses in Zebrafish (Danio rerio). Behav. Brain Res. 2008, 188, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The Habenula Is Crucial for Experience-Dependent Modification of Fear Responses in Zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Mathuru, A.S.; Kibat, C.; Cheong, W.F.; Shui, G.; Wenk, M.R.; Friedrich, R.W.; Jesuthasan, S. Chondroitin Fragments Are Odorants That Trigger Fear Behavior in Fish. Curr. Biol. 2012, 22, 538–544. [Google Scholar] [CrossRef]
- Lee, A.; Mathuru, A.S.; Teh, C.; Kibat, C.; Korzh, V.; Penney, T.B.; Jesuthasan, S. The Habenula Prevents Helpless Behavior in Larval Zebrafish. Curr. Biol. 2010, 20, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Chatterjee, D.; Buske, C.; Gerlai, R. Maturation of Shoaling in Two Zebrafish Strains: A Behavioral and Neurochemical Analysis. Behav. Brain Res. 2013, 247, 1–8. [Google Scholar] [CrossRef]
- Arganda, S.; Pérez-Escudero, A.; De Polavieja, G.G. A Common Rule for Decision Making in Animal Collectives across Species. Proc. Natl. Acad. Sci. USA 2012, 109, 20508–20513. [Google Scholar] [CrossRef]
- Valente, A.; Huang, K.-H.; Portugues, R.; Engert, F. Ontogeny of Classical and Operant Learning Behaviors in Zebrafish. Learn. Mem. 2012, 19, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.S.; Kumar, A.; Kaur, K.; Jaitak, V. Recent Developments in Coumarin Derivatives as Neuroprotective Agents. Curr. Med. Chem. 2024, 31, 5702–5738. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, in press. [Google Scholar] [CrossRef]
- Choe, C.P.; Choi, S.-Y.; Kee, Y.; Kim, M.J.; Kim, S.-H.; Lee, Y.; Park, H.-C.; Ro, H. Transgenic Fluorescent Zebrafish Lines That Have Revolutionized Biomedical Research. Lab. Anim. Res. 2021, 37, 26. [Google Scholar] [CrossRef]
- Briñez-Gallego, P.; Da Costa Silva, D.G.; Cordeiro, M.F.; Horn, A.P.; Hort, M.A. Experimental Models of Chemically Induced Parkinson’s Disease in Zebrafish at the Embryonic Larval Stage: A Systematic Review. J. Toxicol. Environ. Health Part B 2023, 26, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.M.; Croll, R.P. A Critical Review of Zebrafish Models of Parkinson’s Disease. Front. Pharmacol. 2022, 13, 835827. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Wadan, A.-H.S.; Mohamed, W. Various Zebrafish Models of Parkinson’s Disease: What Gives Us Hope. In Translational Models of Parkinson’s Disease and Related Movement Disorders; Elsevier: Amsterdam, The Netherlands, 2025; pp. 219–230. [Google Scholar] [CrossRef]
- Tan, J.K.; Nazar, F.H.; Makpol, S.; Teoh, S.L. Zebrafish: A Pharmacological Model for Learning and Memory Research. Molecules 2022, 27, 7374. [Google Scholar] [CrossRef]
- Capatina, L.; Napoli, E.M.; Ruberto, G.; Hritcu, L. Origanum Vulgare Ssp. Hirtum (Lamiaceae) Essential Oil Prevents Behavioral and Oxidative Stress Changes in the Scopolamine Zebrafish Model. Molecules 2021, 26, 7085. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef]
- Rajesh, V.; Mridhulmohan, M.; Jayaseelan, S.; Sivakumar, P.; Ganesan, V. Mefenamic Acid Attenuates Chronic Alcohol Induced Cognitive Impairment in Zebrafish: Possible Role of Cholinergic Pathway. Neurochem. Res. 2018, 43, 1392–1404. [Google Scholar] [CrossRef]
- Maeda, H.; Fukushima, N.; Hasumi, A. Standardized Method for the Assessment of Behavioral Responses of Zebrafish Larvae. Biomedicines 2021, 9, 884. [Google Scholar] [CrossRef]
- Bertoncello, K.T.; Bonan, C.D. Zebrafish as a Tool for the Discovery of Anticonvulsant Compounds from Botanical Constituents. Eur. J. Pharmacol. 2021, 908, 174342. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.J.; Bai, Q.; Burton, E.A. Transgenic Zebrafish Models of Neurodegenerative Diseases. Brain Struct. Funct. 2010, 214, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish Neurotransmitter Systems as Potential Pharmacological and Toxicological Targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef]
- Verma, R.; Raj Choudhary, P.; Kumar Nirmal, N.; Syed, F.; Verma, R. Neurotransmitter Systems in Zebrafish Model as a Target for Neurobehavioural Studies. Mater. Today Proc. 2022, 69, 1565–1580. [Google Scholar] [CrossRef]
- Montalbano, G.; Levanti, M.; Mhalhel, K.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Germanà, A. Acid-Sensing Ion Channels in Zebrafish. Animals 2021, 11, 2471. [Google Scholar] [CrossRef]
- Doszyn, O.; Dulski, T.; Zmorzynska, J. Diving into the Zebrafish Brain: Exploring Neuroscience Frontiers with Genetic Tools, Imaging Techniques, and Behavioral Insights. Front. Mol. Neurosci. 2024, 17, 1358844. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Langlois, M.; Martins, T.; Van Der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the Moment: Zebrafish Epilepsy Models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mussulini, B.H.M.; Leite, C.E.; Zenki, K.C.; Moro, L.; Baggio, S.; Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Souza, T.M.; Calcagnotto, M.E.; et al. Seizures Induced by Pentylenetetrazole in the Adult Zebrafish: A Detailed Behavioral Characterization. PLoS ONE 2013, 8, e54515. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, D.; Kim, Y.-H.; Lee, H.; Lee, C.-J. Improvement of Pentylenetetrazol-Induced Learning Deficits by Valproic Acid in the Adult Zebrafish. Eur. J. Pharmacol. 2010, 643, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, E.; Jóźwiak, K.; Budzyńska, B.; De Witte, P.A.M.; Copmans, D.; Skalicka-Woźniak, K. Comparative Antiseizure Analysis of Diverse Natural Coumarin Derivatives in Zebrafish. Int. J. Mol. Sci. 2021, 22, 11420. [Google Scholar] [CrossRef]
- Skiba, A.; Kozioł, E.; Luca, S.V.; Budzyńska, B.; Podlasz, P.; Van Der Ent, W.; Shojaeinia, E.; Esguerra, C.V.; Nour, M.; Marcourt, L.; et al. Evaluation of the Antiseizure Activity of Endemic Plant Halfordia Kendack Guillaumin and Its Main Constituent, Halfordin, on a Zebrafish Pentylenetetrazole (PTZ)-Induced Seizure Model. Int. J. Mol. Sci. 2023, 24, 2598. [Google Scholar] [CrossRef]
- Maximino, C.; De Brito, T.M.; Da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring Anxiety in Zebrafish: A Critical Review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.D.; Kalueff, A.V.; Echevarria, D.J. Zebrafish Models of Anxiety-Like Behaviors. In The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Kalueff, A.V., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 45–72. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Fang, Y.; Ma, J.; Wang, J.; Qu, L.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Advances in Zebrafish as a Comprehensive Model of Mental Disorders. Depress. Anxiety 2023, 2023, 6663141. [Google Scholar] [CrossRef]
- Demin, K.A.; Kolesnikova, T.O.; Galstyan, D.S.; Krotova, N.A.; Ilyin, N.P.; Derzhavina, K.A.; Levchenko, N.A.; Strekalova, T.; De Abreu, M.S.; Petersen, E.V.; et al. Modulation of Behavioral and Neurochemical Responses of Adult Zebrafish by Fluoxetine, Eicosapentaenoic Acid and Lipopolysaccharide in the Prolonged Chronic Unpredictable Stress Model. Sci. Rep. 2021, 11, 14289. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Saszik, S.M.; Smith, C.M. The Impact of Stress on Social Behavior in Adult Zebrafish (Danio rerio). Behav. Pharmacol. 2018, 29, 53–59. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Wang, C.; Gao, D.; Zhou, Y.; Zuo, Z. Maternal Exposure to the Water Soluble Fraction of Crude Oil, Lead and Their Mixture Induces Autism-like Behavioral Deficits in Zebrafish (Danio rerio) Larvae. Ecotoxicol. Environ. Saf. 2016, 134, 23–30. [Google Scholar] [CrossRef]
- Gawel, K.; Banono, N.S.; Michalak, A.; Esguerra, C.V. A Critical Review of Zebrafish Schizophrenia Models: Time for Validation? Neurosci. Biobehav. Rev. 2019, 107, 6–22. [Google Scholar] [CrossRef]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring Thigmotaxis in Larval Zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, M.S.; Friend, A.J.; Demin, K.A.; Amstislavskaya, T.G.; Bao, W.; Kalueff, A.V. Zebrafish Models: Do We Have Valid Paradigms for Depression? J. Pharmacol. Toxicol. Methods 2018, 94, 16–22. [Google Scholar] [CrossRef]
- Mezzomo, N.J.; Silveira, A.; Giuliani, G.S.; Quadros, V.A.; Rosemberg, D.B. The Role of Taurine on Anxiety-like Behaviors in Zebrafish: A Comparative Study Using the Novel Tank and the Light–Dark Tasks. Neurosci. Lett. 2016, 613, 19–24. [Google Scholar] [CrossRef]
- Fulcher, N.; Tran, S.; Shams, S.; Chatterjee, D.; Gerlai, R. Neurochemical and Behavioral Responses to Unpredictable Chronic Mild Stress Following Developmental Isolation: The Zebrafish as a Model for Major Depression. Zebrafish 2017, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus Plantarum ST-III Alleviates the Toxic Effects of Triclosan on Zebrafish (Danio rerio) via Gut Microbiota Modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Ngoc Anh, N.T.; Ngoc Hieu, B.T.; Malhotra, N.; Siregar, P.; Villalobos, O.; Villaflores, O.B.; Ger, T.-R.; Huang, J.-C.; Chen, K.H.-C.; et al. Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments. Biomolecules 2020, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social Preference Tests in Zebrafish: A Systematic Review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, M.; Michalak, A.; Skalicka-Woźniak, K.; Zykubek, M.; Ciszewski, A.; Budzyńska, B. Zebrafish and Mouse Models for Anxiety Evaluation—A Comparative Study with Xanthotoxin as a Model Compound. Brain Res. Bull. 2020, 165, 139–145. [Google Scholar] [CrossRef]
- Widelski, J.; Luca, S.V.; Skiba, A.; Maciąg, M.; Budzyńska, B.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Woźniak, K. Coumarins from Seseli Devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model. Int. J. Mol. Sci. 2021, 22, 1829. [Google Scholar] [CrossRef]
- Widelski, J.; Kasica, N.; Maciąg, M.; Luca, S.V.; Budzyńska, B.; Fondai, D.; Podlasz, P.; Skalicka-Woźniak, K. Simple Coumarins from Peucedanum Luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 8693. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Figueras, A. Zebrafish: Model for the Study of Inflammation and the Innate Immune Response to Infectious Diseases. In Current Topics in Innate Immunity II; Lambris, J.D., Hajishengallis, G., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 946, pp. 253–275. [Google Scholar] [CrossRef]
- Stream, A.; Madigan, C.A. Zebrafish: An Underutilized Tool for Discovery in Host–Microbe Interactions. Trends Immunol. 2022, 43, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rostom, B.; Karaky, R.; Kassab, I.; Sylla-Iyarreta Veitía, M. Coumarins Derivatives and Inflammation: Review of Their Effects on the Inflammatory Signaling Pathways. Eur. J. Pharmacol. 2022, 922, 174867. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An Emerging Antiviral Agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, D.-W.; Liu, G.-L.; Shan, L.-P.; Qiu, T.-X.; Chen, J.; State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-Products, Ningbo University; Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Meishan Campus, Ningbo University; Key Laboratory of Applied Marine Biotechnology of Ministry of Education, Meishan Campus, Ningbo University; School of Chemistry & Chemical Engineering, Zhoukou Normal University. Hydroxycoumarin Efficiently Inhibits Spring Viraemia of Carp Virus Infection in Vitro and in Vivo. Zool. Res. 2020, 41, 395–409. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.; Lu, J.; Wang, G. An Imidazole Coumarin Derivative Enhances the Antiviral Response to Spring Viremia of Carp Virus Infection in Zebrafish. Virus Res. 2019, 263, 112–118. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Mei, W.; Wang, Y.; Yu, C.; Yu, B.; Deng, S.; Hu, J. Anti-Inflammatory and Proresolution Activities of Bergapten Isolated from the Roots of Ficus Hirta in an in Vivo Zebrafish Model. Biochem. Biophys. Res. Commun. 2018, 496, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Y.; Li, L. Coumarins as Potential Antidiabetic Agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, J.; Hu, Y.; Li, L.; Yu, M.; Huang, Y.; Zhang, T.; Shang, H.; Zou, Z. (+)/(−)-Gerbeloid A, a Pair of Unprecedented Coumarin-Based Polycyclic Meroterpenoid Enantiomers from Gerbera Piloselloides: Structural Elucidation, Semi-Synthesis, and Lipid-Lowering Activity. Acta Pharm. Sin. B 2024, 14, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
| Tested Agents | Tested Period | Methods | Main Outcomes | Limitations | Ref. |
|---|---|---|---|---|---|
| Coumarin and warfarin (31.25 μM to 1500 μM) | 0–72 hpf | Determination of LC50, EC20, EC50 and TI; evaluation (scoring) of lethality and teratogenic effects | The LC50 and EC50 values for coumarin are 855 μM and 314 μM, respectively. The LC50 and EC50 values for warfarin are 988 μM and 194 μM, respectively. Both coumarin and warfarin produce teratogenic and lethal effects in zebrafish embryos. In the case of coumarin, three endpoints are identified, namely malformation of the head and tail and growth delay. In contrast, malformation of the tail is the only one endpoint observed in the case of warfarin. | The study was limited to warfarin and coumarin, not exploring a wider range of substances. It also compared the effects of human therapeutic concentrations, rather than examining other potential exposure scenarios. | [124] |
| Herniarin (7-methoxycoumarin, 7MC) (1, 2, 3, 4, and 5 mM) | 6–72 hpf | Evaluation of cardiac anomalies/heart rates, cellular apoptosis, morphological deformities, and mortality rates; determination of LC50 | The exposure of embryos to concentrations of 7MC in excess of 4 mM resulted in a reduction in heart rate, distortion of the tail, an increase in cellular apoptosis, and an elevated mortality rate. | Long-term effects of herniarin were not examined, and the study was conducted using a limited concentration range, overlooking the activity at sub-lethal doses. | [132] |
| 4-phenyl hydroxycoumarins: 7C, 5,7C, and 7,8C (1, 10, and 100 μg/mL) | 0–96 hpf | Evaluation of developmental effects and determination of TI; determination of LC50 and EC50 | LC50 are lower for 7,8C and 5,7C compared to coumarin. 5,7C and 7,8C are more embryotoxic while 7C is less toxic in comparison to coumarin. Observed teratogenic and lethal effects of 4-phenyl hydroxycoumarins are concentration dependent. | There is further need to explore pharmacokinetic properties of 4-phenyl hydroxycoumarins, especially the mechanism of cAMP-dependent protein kinase inhibition, perhaps on other model organisms. | [125] |
| 4-phenyl hydroxycoumarins: 7C and 5,7C (1, 5, 10, 25, and 50 μg/mL) | 24–72 hpf | Determination of anti-melanogenic effect | The compounds 7C and 5,7C effectively reduce pigmentation in zebrafish embryos at a low dose of 5 µg/mL, and no toxicity is observed at higher doses. The 7C has a more favorable toxicity profile in comparison to the commonly used depigmenting agents, namely hydroquinone and kojic acid. This suggests that they may be more suitable candidates for use as skin-whitening agents. The anti-melanogenic effect of the 7C is likely attributable to the inhibition of the enzyme tyrosinase, as evidenced by computational molecular modeling studies. | The study does not report toxicity. The authors have exclusively concentrated on the anti-melanogenic effect of tested agents. | [133] |
| Fluorescent coumarin derivatives (1 μg/mL) | 0–120 hpf | Imaging of retinal development | Four coumarin derivatives were identified as potential stains and imaging agents for zebrafish retinal cells in vivo. The coumarin derivatives may be employed to evaluate retinal development and identify morphological irregularities, in addition to serving as a counterstain for transgenic zebrafish expressing fluorescent proteins in particular retinal cell types. | While the study does not report toxicity, the long-term effects of coumarin derivatives on zebrafish development and retinal function remain unknown. The study also does not extensively compare the performance of coumarin derivatives with other established fluorescent dyes for retinal imaging. | [134] |
| Hybrid compounds incorporating 6- and 7-substituted coumarins (12.5 μM to 3 mM) | 0–120 hpf | Determination of LC50; phenotypic analysis (hatching, oedema, movement pattern, yolk sack utilization, heartbeat, body shape, swim bladder development, and otolith sac development); swim pattern analysis; histological studies | The compounds were observed to exhibit minimal toxicity. The analysis revealed that none of the compounds induced discernible alterations in the developing zebrafish larvae, nor did they exhibit any indications of damage to internal tissues. The larvae exposed to the tested compounds did not display any abnormal or ataxic movement patterns. | The study was conducted over a short exposure period, without acknowledging long-term effects. It also lacked the comprehensive histopathological analysis and other toxicological parameters, such as organ damage or immune system effects. | [122] |
| Coumarinyl 4-thiazolidinone derivatives (TZL1-TZL7) (1, 3, 5, and 10 ppm) | 0–120 hpf | Assessment of developmental toxicity, cardiotoxicity, neurotoxicity, and hepatotoxicity | Notable abnormalities or alterations in the embryos’ development were observed, indicating the potential for toxicity of coumarinyl 4-thiazolidinone derivatives at varying concentrations. | The study noted that higher concentrations caused significant adverse effects. It also lacks the information about the long-term efficacy of the compounds. | [135] |
| Coumarin derivates (1, 5, 10, and 15 ppm) | 12–72 hpf | Survival rate analysis | Compounds 1, 2 (naturally occur in some plants, e.g., Plumbago zeylanica, Citrus grandis, Naucleopsis caloneura) and 4 (survival rates: 100%, 82.5–100%, and 100%, respectively) are less toxic than compounds 3, 5, and 6. | The study was limited to zebrafish and chick models, without further investigation in a mammal model. It also only covered gene expression changes, not directly measuring angiogenesis. | [136] |
| p-Coumaric acid (50 μM and 100 μM) | 10–48 hpf | Determination of anti-melanogenic effect | The exposure of developing zebrafish embryos to p-coumaric acid results in a significant reduction in pigmentation. In comparison to phenylthiourea (200 μM), a known melanogenic inhibitor, p-coumaric acid has been demonstrated to be more effective at inhibiting pigmentation. p-Coumaric acid has been shown to exhibit a stronger binding affinity to the tyrosinase enzyme than phenylthiourea. | There is no information about the long-term toxicity of p-coumaric acid. The study also did not explore in depth the effects on organ development and reproductive health. | [137] |
| Mucorisocoumarins A, B, and C (10, 20, 30, and 50 μM) | 0–48 hpf | Developmental toxicity test | Mucorisocoumarin C at a dose 20 μM causes defects in embryos (i.e., abnormal brain shape and a short tail). Higher concentrations led to more severe defects and embryo death. None of the other compounds showed any toxicity. | The study has limited structural data—some of the structures were not confirmed with X-ray crystallography. The long-term effects were also not explored. | [138] |
| Behavioral Test | Age of Zebrafish | Setup | Behavioral Endpoints | Interpretation | Ref. |
|---|---|---|---|---|---|
| Video-tracking analysis | Larvae of zebrafish | Larval zebrafish are observed in 6-well plate (1 zebrafish/well). | The angular velocity and turn angle, thigmotaxis (preference for the tank walls), total distance traveled during the test, time spent in predefined zones, duration of immobility. | An increase in the time spent in the bottom zone or dark areas, a higher freezing response, or a reduction in exploration and a decrease in angular velocity and turn angle are indicative of anxiety-like behavior. Anxiolytic compounds reduce these anxiety markers, thereby promoting exploration and reducing immobility. | [200,204] |
| Acoustic startle response test | Larvae of zebrafish | Larval zebrafish are observed in 96-well plate (1 zebrafish/well). Fish are presented with a flat spectrum “white noise” (1–10,000 Hz) stimulus at 20 dB re·1 ms−2. | The interval between the onset of the stimulus and the initiation of the motor response, the intensity of the C-movement, a reduction in the magnitude over the course of repeated trials. | An increased startle response indicates a heightened state of anxiety or stress. Anxiolytic drugs (e.g., benzodiazepines) can reduce acoustic startle response magnitude. | [200,205] |
| Thigmotaxis measure | Larvae of zebrafish | Larval zebrafish are observed in 24-well plate (1 zebrafish/well). The experimental procedure is performed in two steps: acclimatization (0–6 min, light on) and visual motor challenge (7–10 min, light off). | Swimming activity: general locomotor activity, thigmotaxis (distance moved and time spent in outer and inner zone). | Fish that are experiencing anxiety tend to exhibit a preference for swimming in close proximity to the tank walls (thigmotaxis), as opposed to exploring the central area. The anxiolytic effects are indicated by an increased exploration of the central area. | [200,206] |
| Light-dark preference test | Adult zebrafish | The tank is subdivided into two distinct zones, characterized by varying levels of illumination. One area of the tank is opaque black, with a partition situated in the center and a 2 cm height leak at the base, allowing the fish to pass through. | Time spent in the dark and light area. | Anxiolytic compounds enhance the time spent in the light area. | [200,207,208] |
| Novel tank diving test | Adult zebrafish | The zebrafish are placed into an entirely novel environment—in a new tank. The novel tank is comprised of two distinct arenas, delineated by a baseline situated at the center of the tank. This baseline serves to partition the tank into upper and lower arenas. | Time spent in the upper/lower zone, erratic movements, time duration of freezing and swimming toward the bottom. | The typical effect of anxiolytic compounds is a reduction in the time spent at the bottom of the tank and an increase in exploration of the upper zones. | [200,202,209] |
| Predator exposure test | Adult zebrafish | The tank incorporates a vertical plane, the function of which is to separate the predator from the test fish. | Freezing time, erratic swimming, time spent in the greatest distance from the predator, thigmotaxis. | Fish exhibiting a normal anxiety response demonstrate increased freezing, erratic swimming, and avoidance of the predator zone upon exposure to predator cues. The administration of anxiolytics was associated with a reduction in freezing and avoidance behaviors, an increase in exploration, and a decrease in thigmotaxis. | [204,210] |
| Shoaling test | Adult zebrafish | The observation tank is constructed with plexiglass walls and has dimensions of 17 cm × 17 cm × 5.75 cm. The tank holds 250 mL of water per fish (10 fish/tank). | Reduction in shoaling behavior, characterized by fish swimming in closer proximity to one another. | The reduction in shoaling distances indicates an elevated level of anxiety. | [200,203] |
| Social interaction test | Adult zebrafish | The observation tank is divided into three zones: nonsocial, social zone, and zebrafish room. | Frequency and duration of zebrafish activity in social zone. | The fish with higher level of anxiety exhibits reduced social interaction, avoidance of conspecifics, and displays freezing or erratic movements. | [200,208,211] |
| Social preference test | Adult zebrafish | The tank is divided into three chambers (five zones): a, e—social stimulus zone; b, d—area of social preference; c—area of no social preference. | Time spent near stimulus zone, number of entries into stimulus zone, distance maintained from the stimulus fish, freezing or avoidance. | Fish with a low level of anxiety tend to spend more time in the stimulus zone and frequently interact with conspecifics. A reduction in the time spent in the stimulus zone, along with evidence of avoidance or freezing, may indicate the presence heightened anxiety, or stress. | [200,212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz-Radulska, J.; Widelski, J.; Nowaczyński, F.; Serefko, A.; Sobczyński, J.; Ludwiczuk, A.; Kasica, N.; Szopa, A. Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds. Int. J. Mol. Sci. 2025, 26, 1444. https://doi.org/10.3390/ijms26041444
Lachowicz-Radulska J, Widelski J, Nowaczyński F, Serefko A, Sobczyński J, Ludwiczuk A, Kasica N, Szopa A. Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds. International Journal of Molecular Sciences. 2025; 26(4):1444. https://doi.org/10.3390/ijms26041444
Chicago/Turabian StyleLachowicz-Radulska, Joanna, Jarosław Widelski, Filip Nowaczyński, Anna Serefko, Jan Sobczyński, Agnieszka Ludwiczuk, Natalia Kasica, and Aleksandra Szopa. 2025. "Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds" International Journal of Molecular Sciences 26, no. 4: 1444. https://doi.org/10.3390/ijms26041444
APA StyleLachowicz-Radulska, J., Widelski, J., Nowaczyński, F., Serefko, A., Sobczyński, J., Ludwiczuk, A., Kasica, N., & Szopa, A. (2025). Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds. International Journal of Molecular Sciences, 26(4), 1444. https://doi.org/10.3390/ijms26041444








