Current Paradigms and Future Challenges in Harnessing Nanocellulose for Advanced Applications in Tissue Engineering: A Critical State-of-the-Art Review for Biomedicine
Abstract
1. Introduction
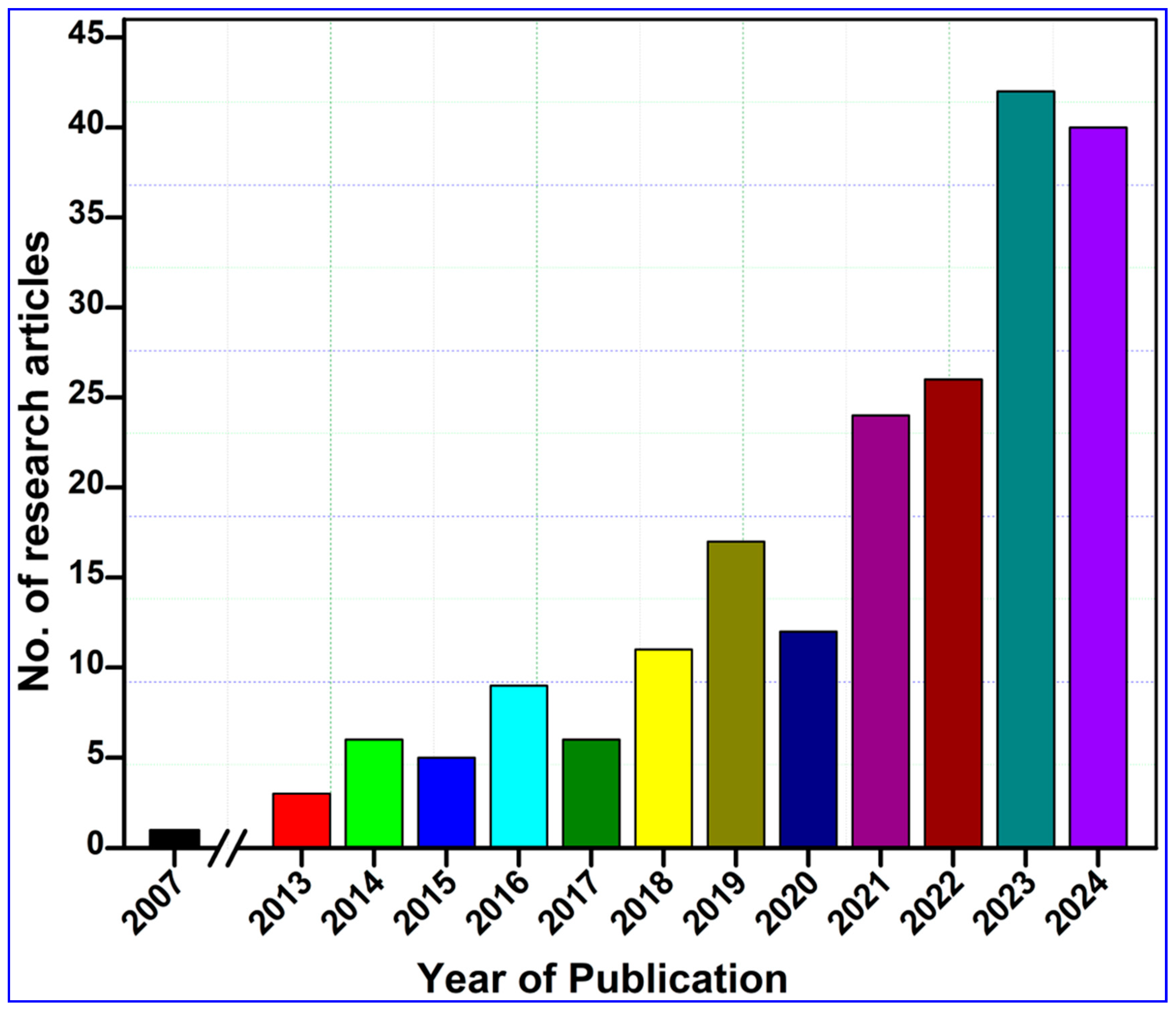
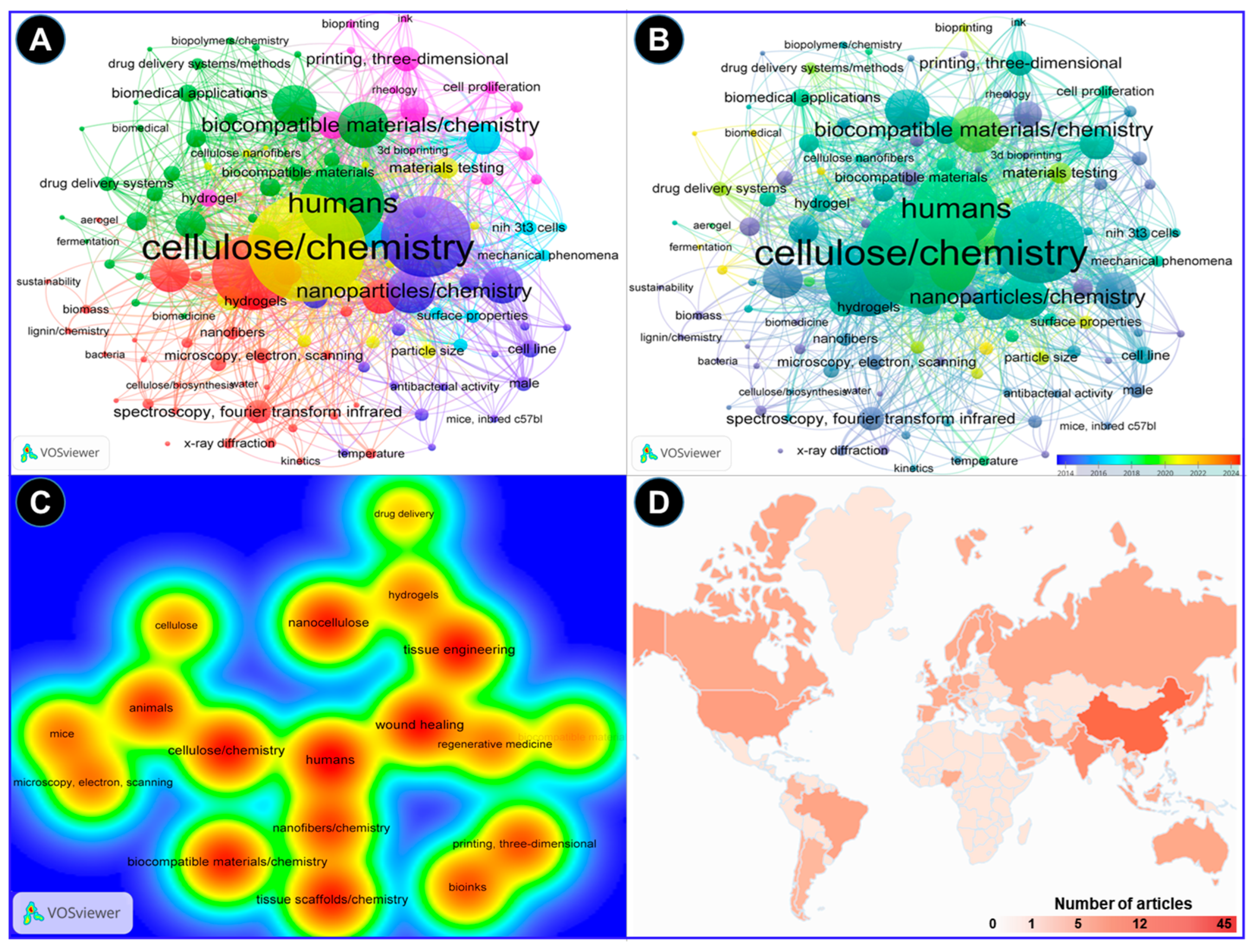
2. Methodology and Bibliometric Analysis
3. Structural and Morphological Characterization of Nanocellulose
| Type of Nanocellulose | Diameter (nm) | Source/Examples | Methods Used for Preparation | Other Characteristics | Reference(s) |
|---|---|---|---|---|---|
| Nanofibrils | 70–140 | Bacteria, plants, algae | Bacterial secretion | High crystallinity and pure cellulose (no hemicellulose, lignin), good mechanical stability, production from low-molecular-weight molecules | [31,32] |
| 3–5 | Softwood-derived cellulose | TEMPO oxidation and enzymatic pretreatment | [33,34] | ||
| Nanofibers | ≤400 | Cellulose acetate, bacterial, lignocellulose, algae, tunicates | Chemical synthesis | Gel-like characteristics in water; long, flexible fibers capable of entanglement; contain both crystalline and amorphous regions | [3,8] |
| ≤6.4 | Pineapple | Electrospinning | [22] | ||
| Nanowhiskers | 5–15 | Pine kraft pulp | Acid hydrolysis | [35] | |
| 10–15 | Kenaf bast | Acid hydrolysis | [36] | ||
| 10–100 | Bacteria, plants, algae | Bacterial secretion | High crystallinity and pure cellulose (no hemicellulose, lignin), good mechanical stability | [37] | |
| Nanocrystals | ≤7.3 | Cotton-derived, plants | Acid hydrolysis | Elongated crystalline, rodlike shapes; rigid rods (elastic modulus ~100 GPa); crystalline; no amorphous regions; form stable hydrogels with up to 99% water | [38,39] |
| Nanorods | 15 ± 3 | Grass-derived | Acid hydrolysis | [40] | |
| Nanoballs | 80–85 | Wood-derived | Acid/alkaline hydrolysis | [41] | |
| Nanoplatelets | ≤80 | Agave-derived | Aqueous dispersion and heat treatment | [42,43] |
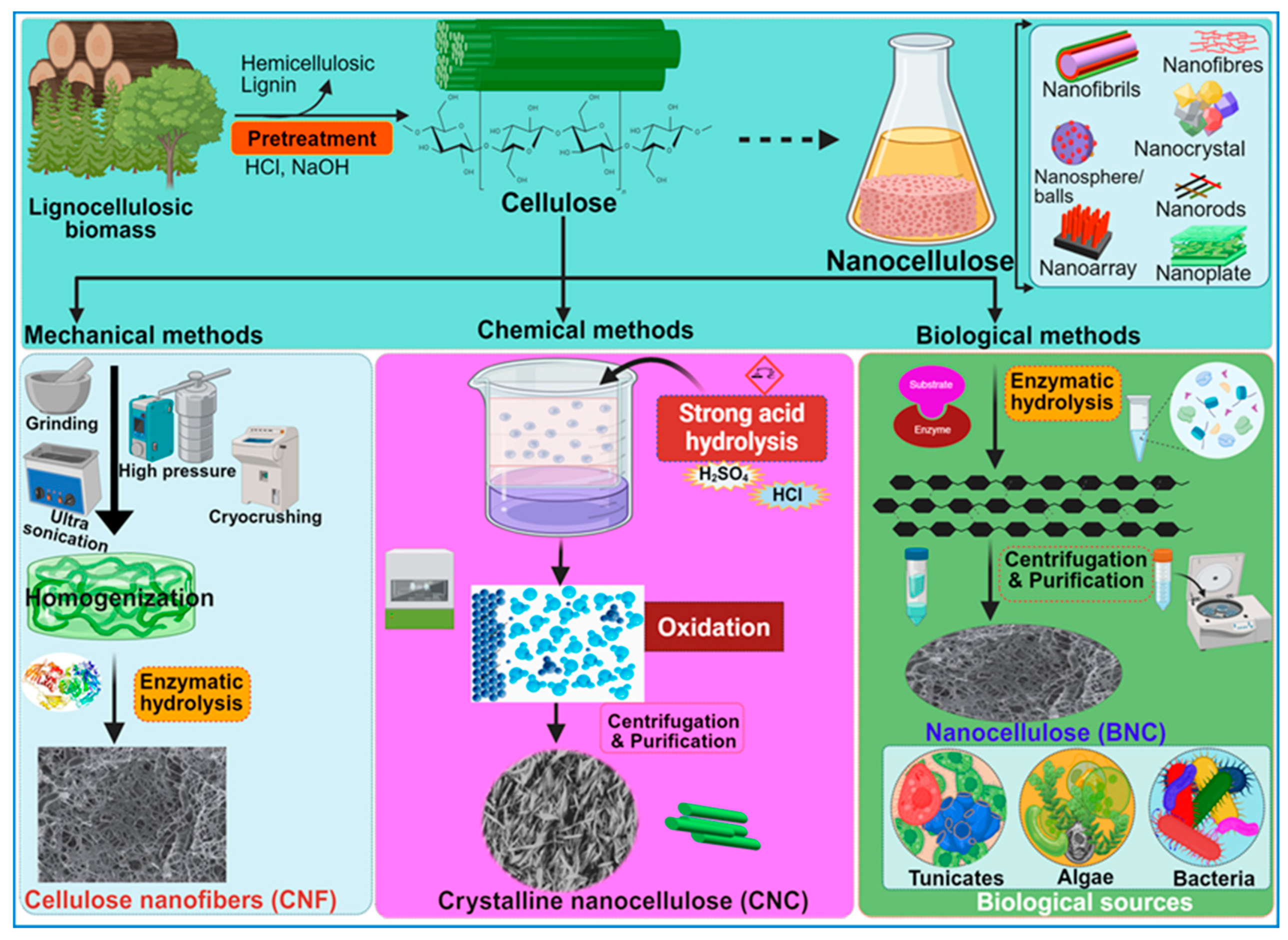
4. Overview of the Nanocellulose Resources
Synthesis of Nanocellulose: Bacterial vs. Chemical Synthesis
5. Physicochemical Properties of Nanocellulose from the Tissue-Engineering Perspective
5.1. Structures and Physical Properties of the Nanocellulose Biomaterials
5.2. Cytocompatibility of Nanocellulose
5.3. Biodegradability of Nanocellulose-Based Biomaterials
5.4. Immunogenicity of Nanocellulose
5.5. Non-Toxic Nature of Nanocellulose
6. Surface Modifications of Nanocellulose
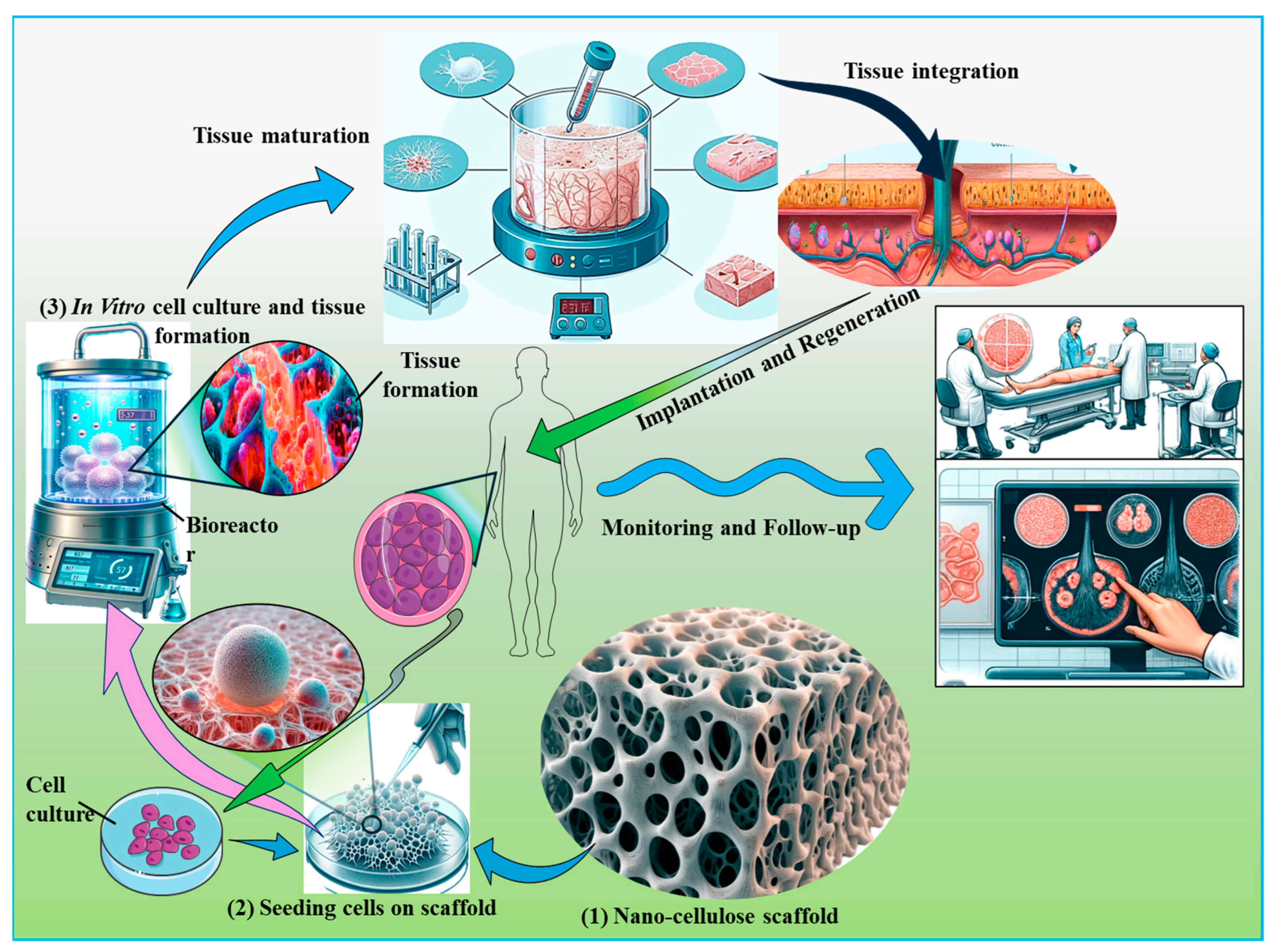
7. Biomedical Applications of Nanocellulose-Based Biomaterials
7.1. Tissue Engineering Applications of Nanocellulose-Based Biomaterials
7.1.1. Nanocellulose-Based Materials for Cartilage Engineering
7.1.2. Nanocellulose and Hepatic Tissue Engineering
7.1.3. Nanocellulose in Adipose Tissue Engineering
7.1.4. Nanocellulose in Vascular Tissue Engineering
7.1.5. Nanocellulose in Bone Engineering
7.1.6. Application of Nanocellulose for Urethra Reconstruction
7.2. Engineering of Nanocellulosic Biomaterials for Other Tissues
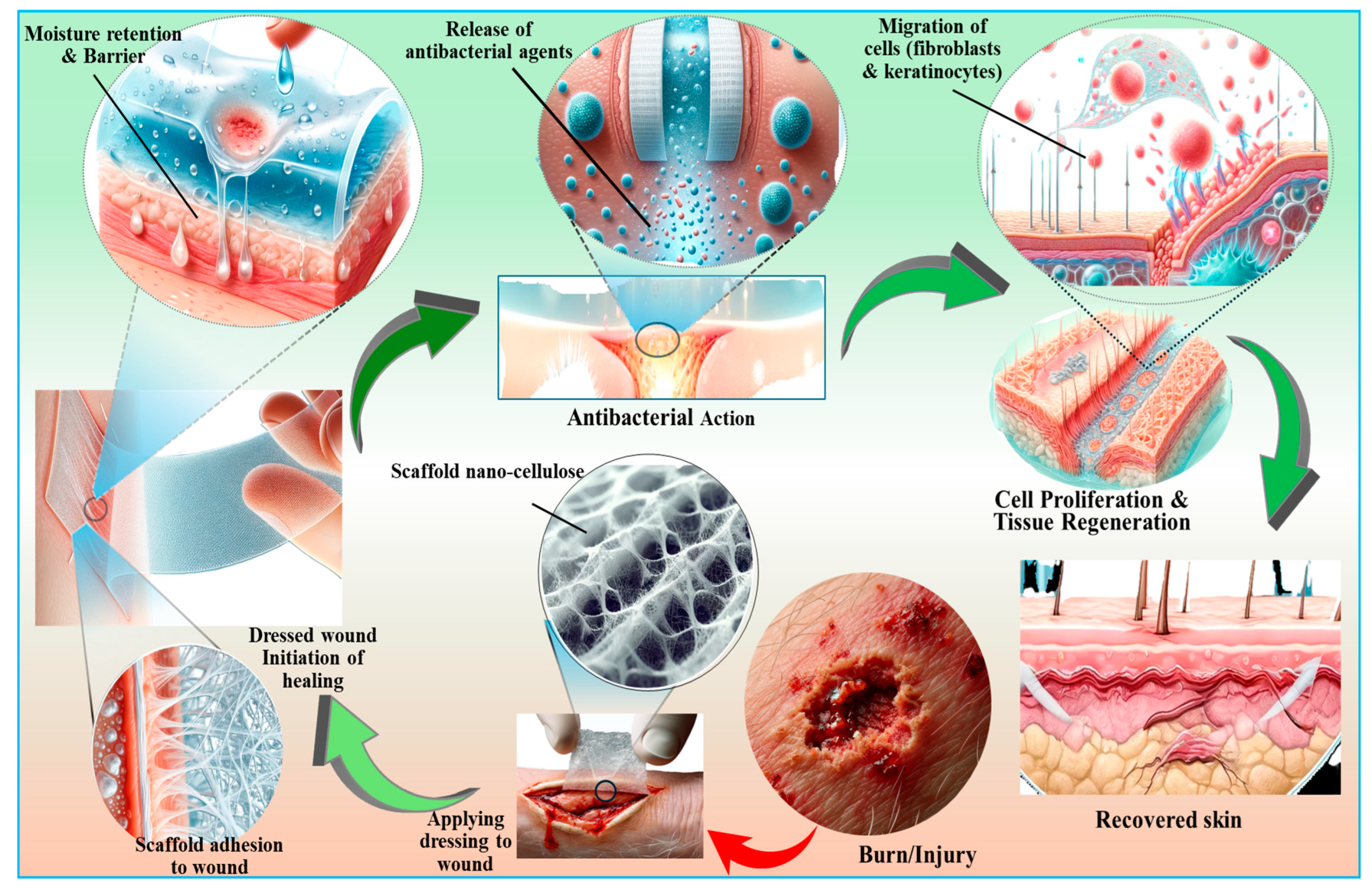
7.3. Wound Healing and Skin Tissue Repair Applications of Nanocellulose
7.4. Biosafety Considerations of Nanocellulose-Based Biomaterials
8. Challenges of Nanocellulose-Based Biomaterials in Tissue Engineering
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, H.; Lu, X.; Li, Y.; Zhang, H.; Fu, Y.; Zhong, C.; Wang, Q.; Ullah, M.W.; Liu, H.; Yong, Y.; et al. In situ biomineralization reinforcing anisotropic nanocellulose scaffolds for guiding the differentiation of bone marrow-derived mesenchymal stem cells. Int. J. Biol. Macromol. 2024, 274, 133515. [Google Scholar] [CrossRef] [PubMed]
- Niknafs, B.; Meskaraf-asadabadi, M.; Hamdi, K.; Ghanbari, E. Incorporating bioactive glass nanoparticles in silk fibroin/bacterial nanocellulose composite scaffolds improves their biological and osteogenic properties for bone tissue engineering applications. Int. J. Biol. Macromol. 2024, 266, 131167. [Google Scholar] [CrossRef]
- Jiao, H.; Shi, Y.; Sun, J.; Lu, X.; Zhang, H.; Li, Y.; Fu, Y.; Guo, J.; Wang, Q.; Liu, H.; et al. Sawdust-derived cellulose nanofibrils with high biosafety for potential bioprinting. Ind. Crops Prod. 2024, 209, 118025. [Google Scholar] [CrossRef]
- Azka, M.A.; Adam, A.; Ridzuan, S.; Sapuan, S.; Habib, A. A review on the enhancement of circular economy aspects focusing on nanocellulose composites. Int. J. Biol. Macromol. 2024, 269, 132052. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Xie, R.; Zabed, H.M.; Ali, S.; Zhu, D.; Sun, J. Termite microbial symbiosis as a model for innovative design of lignocellulosic future biorefinery: Current paradigms and future perspectives. Biomass 2024, 4, 180–201. [Google Scholar] [CrossRef]
- Dar, M.A.; Syed, R.; Pawar, K.D.; Dhole, N.P.; Xie, R.; Pandit, R.S.; Sun, J. Evaluation and characterization of the cellulolytic bacterium Bacillus pumilus SL8 isolated from the gut of oriental leafworm Spodoptera litura: An assessment of its potential value for lignocellulose bioconversion. Environ. Technol. Innov. 2022, 27, 102459. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Fu, Y.; Jiao, H.; Wang, X.; Wang, Q.; Zhou, M.; Yong, Y.; Liu, J. A structure-functionality insight into the bioactivity of microbial polysaccharides toward biomedical applications: A review. Carbohydr. Polym. 2024, 335, 122078. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, C.; Wang, T.; Yang, Y.; Sun, Y.; Zhang, Y.; Cui, L.; Song, Z.; Chen, X.; Cao, X.; et al. Extraction and characterization of nanocellulose from cattail leaves: Morphological microstructural and thermal properties. Int. J. Biol. Macromol. 2024, 255, 128123. [Google Scholar] [CrossRef]
- Heise, K.; Koso, T.; King, A.W.T.; Nypelö, T.; Penttilä, P.; Tardy, B.L.; Beaumont, M. Spatioselective surface chemistry for the production of functional and chemically anisotropic nanocellulose colloids. J. Mater. Chem. A 2022, 10, 23413–23432. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Souza, A.G.; Ajdary, R.; De Souza, L.P.; Lopes, J.H.; Correa, D.S.; Siqueira, G.; Barud, H.S.; Rosa, D.D.S.; Mattoso, L.H.; et al. Nanocellulose-based porous materials: Regulation and pathway to commercialization in regenerative medicine. Bioact. Mater. 2023, 29, 151–176. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, K.; Hazrati, A.; Khosrojerdi, A.; Roshangar, L.; Ahmadi, M. An overview to nanocellulose clinical application: Biocompatibility and opportunities in disease treatment. Regen. Ther. 2023, 24, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Baruah, R.; Hazarika, M.P.; Das, A.M.; Sastry, G.N.; Nath, D.; Talukdar, K. Green synthesis of nanocellulose supported cu-bionanocomposites and their profound applicability in the synthesis of amide derivatives and controlling of food-borne pathogens. Carbohydr. Polym. 2024, 330, 121786. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, B.; Vinoth, S.; Hariharan, M.; Raja, R.K.; Kamaraj, C.; Narayanan, M. Valorization of agro-industry wastes for nanocellulose fabrication and its multifunctional applications. Biocatal. Agric. Biotechnol. 2024, 57, 103124. [Google Scholar] [CrossRef]
- Meng, L.; Xi, J.; Bian, H.; Xiao, H.; Wu, W. Nanocellulose/natural rubber latex composite film with high barrier and preservation properties. Sustain. Chem. Pharm. 2024, 37, 101399. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Syed Najmuddin, S.U.F.; Ilyas, R.A. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Kramer, F.; Klemm, D.; Schumann, D.; Heßler, N.; Wesarg, F.; Fried, W.; Stadermann, D. Nanocellulose polymer composites as innovative pool for (bio)material development. Macromol. Symp. 2006, 244, 136–148. [Google Scholar] [CrossRef]
- Branski, L.; Jeschke, M.; Mittermayr, R.; Redl, H.; Traber, D.; Herndon, D. The development of a porcine model for burns and reconstruction. Inflamm. Res. 2007, 56 (Suppl. 2), S259–S260. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. 2014, 102, 3918–3930. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sabra, A.; Elbery, M.; Cerveira, M.M.; Ghenov, F.; Sunasee, R.; Ckless, K. A mechanistic insight into curcumin modulation of the IL-1β secretion and NLRP3 S-glutathionylation induced by needle-like cationic cellulose nanocrystals in myeloid cells. Chem. Biol. Interact. 2017, 274, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, G.; Liu, D.; Tian, D.; Zhu, Y.; Chang, Y. Vapor sensing with color-tunable multilayered coatings of cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 39–47. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest advances on bacterial cellulose-based antibacterial materials as wound dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Sepúlveda-Guzmán, S.; Silva-Mendoza, J.; Aguilar-Flores, C.; Pérez-Camacho, O. Eco-friendly isolation of cellulose nanoplatelets through oxidation under mild conditions. Carbohydr. Polym. 2018, 181, 642–649. [Google Scholar] [CrossRef]
- Faradilla, R.F.; Lee, G.; Arns, J.-Y.; Roberts, J.; Martens, P.; Stenzel, M.H.; Arcot, J. Characteristics of a free-standing film from banana pseudostem nanocellulose generated from TEMPO-mediated oxidation. Carbohydr. Polym. 2017, 174, 1156–1163. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Tian, Z.; Zhang, C.; Han, X.; Jiang, S.; Liu, K.; Duan, G. Preparation of nanocellulose and its applications in wound dressing: A review. Int. J. Biol. Macromol. 2023, 254, 127997. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Squinca, P.; Baş, Y.; Zattarin, E.; Aili, D.; Rakar, J.; Junker, J.; Starkenberg, A.; Diamanti, M.; Sivlér, P.; et al. Self-Assembly of Nanocellulose Hydrogels Mimicking Bacterial Cellulose for Wound Dressing Applications. Biomacromolecules 2023, 24, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liu, X.; Liao, P.; Ma, T.; Meng, H.; Xia, T. Nanocellulose Length Determines the Differential Cytotoxic Effects and Inflammatory Responses in Macrophages and Hepatocytes. Small 2021, 17, e2102545. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in-vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef]
- Puglia, D.; Luzi, F.; Tolisano, C.; Rallini, M.; Priolo, D.; Brienza, M.; Costantino, F.; Torre, L.; Del Buono, D. Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass. Polymers 2023, 16, 1395. [Google Scholar] [CrossRef]
- Sun, Q.; Tan, R.; Yan, Y.; Wang, S.; Fu, S.; Zheng, S.; Zhang, Y.; Qu, D.; Zhang, R.; Tan, M.; et al. Production of High-Value-Added Bacterial Nanocellulose with Thinner Fiber Using Novel Yarrowia lipolytica Extract from Erythritol Industry Waste. ACS Sustain. Chem. Eng. 2024, 12, 3450–3460. [Google Scholar] [CrossRef]
- Thipchai, P.; Punyodom, W.; Jantanasakulwong, K.; Thanakkasaranee, S.; Sringarm, K.; Panyathip, R.; Tanadchangsaeng, N.; Worajittiphon, P.; Rachtanapun, P. Property investigation of nanocellulose I and II from different non-wood fibers using chemical acid hydrolysis method. Ind. Crops Prod. 2024, 222, 119534. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Condon, B.D.; Qureshi, H.; Yager, D. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: Implications of material selection for dressing and protease sensor design. J. Biomater. Appl. 2017, 32, 622–637. [Google Scholar] [CrossRef]
- Mahheidari, N.; Nourani, M.R.; Atashi, A.; Alizadeh, M.; Aldaghi, N.; Salehi, M. Biological study of skin wound treated with Alginate/Carboxymethyl cellulose/chorion membrane, diopside nanoparticles, and Botox A. npj Regen. Med. 2024, 9, 9. [Google Scholar] [CrossRef]
- Keplinger, T.; Wang, X.; Burgert, I. Nanofibrillated cellulose composites and wood derived scaffolds for functional materials. J. Mater. Chem. A 2019, 7, 2981–2992. [Google Scholar] [CrossRef]
- Levä, T.; Rissanen, V.; Nikkanen, L.; Siitonen, V.; Heilala, M.; Phiri, J.; Maloney, T.C.; Kosourov, S.; Allahverdiyeva, Y. Mapping Nanocellulose- and Alginate-Based Photosynthetic Cell Factory Scaffolds: Interlinking Porosity, Wet Strength, and Gas Exchange. Biomacromolecules 2023, 24, 3484–3497. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, U.; Ronca, A.; Scialla, S.; Soriente, A.; Manini, P.; Phua, J.W.; Ottenheim, C.; Pezzella, A.; Calabrese, G.; Raucci, M.G.; et al. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials 2023, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Green bionanocomposite based on kefiran and cellulose nanocrystals produced from beer industrial residues. Int. J. Biol. Macromol. 2015, 77, 85–91. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Powell, L.C.; Khan, S.; Chinga-Carrasco, G.; Wright, C.J.; Hill, K.E.; Thomas, D.W. An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohydr. Polym. 2016, 137, 191–197. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Fazita, M.R.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Ramphul, H.; Bhaw-Luximon, A.; Jhurry, D. Sugar-cane bagasse derived cellulose enhances performance of polylactide and polydioxanone electrospun scaffold for tissue engineering. Carbohydr. Polym. 2017, 178, 238–250. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef]
- De Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of Nanocellulose from Imperata brasiliensis Grass Using Taguchi Method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Soni, S.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Patial, V.; Padwad, Y.S.; Yadav, S.K. In Situ Functionalized Nanobiocomposites Dressings of Bamboo Cellulose Nanocrystals and Silver Nanoparticles for Accelerated Wound Healing. Carbohydr. Polym. 2017, 155, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sadalage, P.S.; Pawar, K.D. Production of Microcrystalline Cellulose and Bacterial Nanocellulose through Biological Valorization of Lignocellulosic Biomass Wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial Cellulose and Bacterial Cellulose-Vaccarin Membranes for Wound Healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Dos Reis, E.M.; Berti, F.V.; Colla, G.; Porto, L.M. Bacterial Nanocellulose-IKVAV Hydrogel Matrix Modulates Melanoma Tumor Cell Adhesion and Proliferation and Induces Vasculogenic Mimicry In vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2741–2749. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; Raucci, L.M.S.d.C.; Scarel-Caminaga, R.M.; Franchi, L.P.; dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-Collagen-Apatite Composite Associated with Osteogenic Growth Peptide for Bone Regeneration. Int. J. Biol. Macromol. 2017, 103, 467–476. [Google Scholar] [CrossRef]
- Osorio, M.; Fernández-Morales, P.; Gañán, P.; Zuluaga, R.; Kerguelen, H.; Ortiz, I.; Castro, C. Development of Novel Three-Dimensional Scaffolds Based on Bacterial Nanocellulose for Tissue Engineering and Regenerative Medicine: Effect of Processing Methods, Pore Size, and Surface Area. J. Biomed. Mater. Res. Part A 2018, 107, 348–359. [Google Scholar] [CrossRef]
- Kaminagakura, K.L.N.; Sue Sato, S.; Sugino, P.; Veloso, L.K.d.O.; dos Santos, D.C.; Padovani, C.R.; Basmaji, P.; Olyveira, G.; Schellini, S.A. Nanoskin® to Treat Full Thickness Skin Wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 724–732. [Google Scholar] [CrossRef]
- Zharikov, A.N.; Lubyansky, V.G.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V.; Semyonova, E.N.; Zharikov, A.A.; Sakovich, G.V. Early Morphological Changes in Tissues When Replacing Abdominal Wall Defects by Bacterial Nanocellulose in Experimental Trials. J. Mater. Sci. Mater. Med. 2018, 29, 95. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial Functionalization of Bacterial Nanocellulose by Loading with Polihexanide and Povidone-Iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M.; Raanaei, H. The Removal of Mercury Ion Pollution by Using Fe₃O₄-Nanocellulose: Synthesis, Characterizations, and DFT Studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Rocha, I.; Zhang, P.; Gustafsson, S.; Ning, Y.; Strømme, M.; Mihranyan, A.; Ferraz, N. Transition from Bioinert to Bioactive Material by Tailoring the Biological Cell Response to Carboxylated Nanocellulose. Biomacromolecules 2016, 17, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Lindh, J.; Hong, J.; Strømme, M.; Mihranyan, A.; Ferraz, N. Blood Compatibility of Sulfonated Cladophora Nanocellulose Beads. Molecules 2018, 23, 601. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Mihranyan, A. On Importance of Impurities, Potential Leachables and Extractables in Algal Nanocellulose for Biomedical Use. Carbohydr. Polym. 2017, 172, 11–19. [Google Scholar] [CrossRef]
- Song, S.H.; Seong, K.Y.; Kim, J.E.; Go, J.; Koh, E.K.; Sung, J.E.; Son, H.J.; Jung, Y.J.; Kim, H.S.; Hong, J.T.; et al. Effects of Different Cellulose Membranes Regenerated from Styela clava Tunics on Wound Healing. Int. J. Mol. Med. 2017, 39, 1173–1187. [Google Scholar] [CrossRef]
- Kjesbu, J.S.; Zaytseva-Zotova, D.; Sämfors, S.; Gatenholm, P.; Troedsson, C.; Thompson, E.M.; Strand, B.L. Alginate and Tunicate Nanocellulose Composite Microbeads—Preparation, Characterization, and Cell Encapsulation. Carbohydr. Polym. 2022, 286, 119284. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, M.; Zhang, F.; Liu, C.; Sun, M.; Li, B. All-Tunicate Cellulose Film with Good Light Management Properties for High-Efficiency Organic Solar Cells. Nanomaterials 2023, 13, 1221. [Google Scholar] [CrossRef]
- Lv, X.; Han, J.; Liu, M.; Yu, H.; Liu, K.; Yang, Y.; Sun, Y.; Pan, P.; Liang, Z.; Chang, L.; et al. Overview of preparation modification and application of tunicate-derived nanocellulose. Chem. Eng. J. 2023, 452, 139439. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Clemons, C.; Reiner, R.; Sabo, R.; Agarwal, U.P.; Bissessur, R.; Sojoudiasli, H.; Carreau, P.J.; Acharya, B. Towards the Scalable Isolation of Cellulose Nanocrystals from Tunicates. Sci. Rep. 2020, 10, 19090. [Google Scholar] [CrossRef]
- Jun, S.Y.; Park, J.; Song, H.; Song, H.; Shin, H. Tunicate Cellulose Nanocrystals as Stabilizers for PLGA-Based Polymeric Nanoparticles. Biotechnol. Bioprocess Eng. 2020, 25, 206–214. [Google Scholar] [CrossRef]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J. Excellent chemical and material cellulose from tunicates: Diversity in cellulose production yield and chemical and morphological structures from different tunicate species. Cellulose 2014, 21, 3427–3441. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Mohammed, A.B.A.; El-Malkey, S.E. Bacterial Nanocellulose Production Using Cantaloupe Juice, Statistical Optimization and Characterization. Sci. Rep. 2023, 13, 51. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the Fermentation Process and Properties of Bacterial Cellulose: A Review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial Cellulose Biosynthesis: Diversity of Operons, Subunits, Products, and Functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Sofiah, A.G.N.; Pasupuleti, J.; Samykano, M.; Kadirgama, K.; Koh, S.P.; Tiong, S.K.; Pandey, A.K.; Yaw, C.T.; Natarajan, S.K. Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences. Polymers 2023, 15, 3044. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a Versatile Platform: From the Delivery of Active Molecules to Tissue Engineering Applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, N.; Munagala, M.; Rajkhowa, R.; Aallardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, Physio-Chemical Properties, Current Uses and Future Applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Nuryawan, A.; Abdullah, C.K.; Hazwan, C.M.; Olaiya, N.G.; Yahya, E.B.; Risnasari, I.; Masruchin, N.; Baharudin, M.S.; Khalid, H.; Abdul Khalil, H.P.S. Enhancement of Oil Palm Waste Nanoparticles on the Properties and Characterization of Hybrid Plywood Biocomposites. Polymers 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Simón-Herrero, C.; Caminero-Huertas, S.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Effects of Freeze-Drying Conditions on Aerogel Properties. J. Mater. Sci. 2016, 51, 8977–8985. [Google Scholar] [CrossRef]
- Madyan, O.A.; Fan, M.; Feo, L.; Hui, D. Enhancing Mechanical Properties of Clay Aerogel Composites: An Overview. Compos. Part B Eng. 2016, 98, 314–329. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, Characterization, and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef]
- Apelgren, P.; Sämfors, S.; Säljö, K.; Mölne, J.; Gatenholm, P.; Troedsson, C.; Thompson, E.M.; Kölby, L. Biomaterial and Biocompatibility Evaluation of Tunicate Nanocellulose for Tissue Engineering. Biomater. Adv. 2022, 137, 212828. [Google Scholar] [CrossRef]
- Uddin, K.M.; Orelma, H.; Mohammadi, P.; Borghei, M.; Laine, J.; Linder, M.; Rojas, O.J. Retention of Lysozyme Activity by Physical Immobilization in Nanocellulose Aerogels and Antibacterial Effects. Cellulose 2017, 24, 2837–2848. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite Aerogels Based on Dialdehyde Nanocellulose and Collagen for Potential Applications as Wound Dressing and Tissue Engineering Scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, L.; Zhang, Q.; Hong, F.F. Using In Situ Dynamic Cultures to Rapidly Biofabricate Fabric-Reinforced Composites of Chitosan/Bacterial Nanocellulose for Antibacterial Wound Dressings. Front. Microbiol. 2016, 7, 260. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mahmoudi, N.; Anaran, F.R.; Simchi, A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Mar. Drugs 2016, 14, 128. [Google Scholar] [CrossRef]
- Lamboni, L.; Xu, C.; Clasohm, J.; Yang, J.; Saumer, M.; Schäfer, K.; Yang, G. Silk Sericin-Enhanced Microstructured Bacterial Cellulose as Tissue Engineering Scaffold towards Prospective Gut Repair. Mater. Sci. Eng. C 2019, 102, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk Sericin-Functionalized Bacterial Cellulose as a Potential Wound-Healing Biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Sierakowski, M.R.; Woehl, M.A.; Ono, L.; Cofré, A.R.; Vanin, L.P.; Pontarolo, R.; De Freitas, R.A. Lysozyme-Triggered Epidermal Growth Factor Release from Bacterial Cellulose Membranes Controlled by Smart Nanostructured Films. J. Pharm. Sci. 2014, 103, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, L.; Gong, N.; Tang, Q.; Du, L.; Chen, L. The Effects of Macrophage-Stimulating Protein on the Migration, Proliferation, and Collagen Synthesis of Skin Fibroblasts In vitro and In-vivo. Tissue Eng. Part A 2015, 21, 982–991. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, M.K.; Singh, A. Bacterial Cellulose: A Smart Biomaterial for Biomedical Applications. J. Mater. Res. 2024, 39, 2–18. [Google Scholar] [CrossRef]
- Moraru, A.; Dima, Ș.O.; Tritean, N.; Oprița, E.I.; Prelipcean, A.M.; Trică, B.; Oancea, A.; Moraru, I.; Constantinescu-Aruxandei, D.; Oancea, F. Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota. Pharmaceuticals 2023, 16, 1671. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled Extended Octenidine Release from a Bacterial Nanocellulose/Poloxamer Hybrid System. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The Safety and Efficacy of Bacterial Nanocellulose Wound Dressing Incorporating Sericin and Polyhexamethylene Biguanide: In vitro, In-vivo, and Clinical Studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver Nanoparticle/Bacterial Cellulose Gel Membranes for Antibacterial Wound Dressing: Investigation In vitro and In-vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1700130. [Google Scholar] [CrossRef]
- Kędzierska, M.; Blilid, S.; Miłowska, K.; Katir, N.; Lahcini, M.; El Kadib, A.; Bryszewska, M. Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films. Int. J. Mol. Sci. 2020, 22, 11386. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Uyama, H.; Nguyen, D.H.; Chuah, C.H. Investigations on the Interactions of Proteins with Nanocellulose Produced via Sulphuric Acid Hydrolysis. Int. J. Biol. Macromol. 2021, 193, 1522–1531. [Google Scholar] [CrossRef]
- Štiglic, A.D.; Lackner, F.; Nagaraj, C.; Beaumont, M.; Bračič, M.; Duarte, I.; Kononenko, V.; Drobne, D.; Madhan, B.; Finšgar, M.; et al. 3D-Printed Collagen–Nanocellulose Hybrid Bioscaffolds with Tailored Properties for Tissue Engineering Applications. ACS Appl. Bio Mater. 2023, 6, 5596–5608. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Wu, C.-M.; Mata, J.; Garnier, G. Nano- and Microstructures of Collagen-Nanocellulose Hydrogels as Engineered Extracellular Matrices. ACS Appl. Mater. Interfaces 2024, 16, 1370–1379. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxid. Med. Cell. Longev. 2021, 3687700. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Berry, R.C.; Tam, K.C. Surface Modification of Cellulose Nanocrystals with Chitosan Oligosaccharide for Drug Delivery Applications. Cellulose 2013, 20, 1747–1764. [Google Scholar] [CrossRef]
- Francesco, M.D.; Celia, C.; Cristiano, M.C.; Ruozi, B.; Mircioiu, C.; Cosco, D.; Marzio, L.D.; Fresta, M. Doxorubicin Hydrochloride-Loaded Nonionic Surfactant Vesicles to Treat Metastatic and Non-Metastatic Breast Cancer. ACS Omega 2021, 6, 2973. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of Copper-Containing Mesoporous Bioactive Glass and Nanofibrillated Cellulose: Biocompatibility and Angiogenic Promotion in Chronic Wound Healing Application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Jančič, U.; Trček, J.; Verestiuc, L.; Vukomanović, M.; Gorgieva, S. Bacterial Nanocellulose Loaded with Bromelain and Nisin as a Promising Bioactive Material for Wound Debridement. Int. J. Biol. Macromol. 2024, 266, 131329. [Google Scholar] [CrossRef]
- Li, Q.; Dong, M.; Han, Q.; Zhang, Y.; Yang, D.; Wei, D.; Yang, Y. Enhancing Diabetic Wound Healing with a pH-Responsive Nanozyme Hydrogel Featuring Multi-Enzyme-Like Activities and Oxygen Self-Supply. J. Control. Release 2023, 365, 905–918. [Google Scholar] [CrossRef]
- Stasyuk, N.; Demkiv, O.; Gayda, G.; Zakalska, O.; Zakalskiy, A.; Serkiz, R.; Kavetskyy, T.; Gonchar, M. Reusable Alcohol Oxidase–nPtCu/Alginate Beads for Highly Sensitive Ethanol Assay in Beverages. RSC Adv. 2022, 12, 21309. [Google Scholar] [CrossRef] [PubMed]
- Neubauerova, K.; Carneiro, M.C.; Rodrigues, L.R.; Moreira, F.T.; Sales, M.G.F. Nanocellulose-Based Biosensor for Colorimetric Detection of Glucose. Sens. Bio-Sens. Res. 2020, 29, 100368. [Google Scholar] [CrossRef]
- Macha, P.; Perreault, L.; Hamedani, Y.; Mayes, M.L.; Vasudev, M.C. Molecular Mechanisms of Tryptophan–Tyrosine Nanostructures Formation and Their Influence on PC-12 Cells. ACS Appl. Bio Mater. 2018, 1, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Mackin, R.T.; Fontenot, K.R.; Edwards, J.V.; Prevost, N.T.; Jordan, J.H.; Easson, M.W.; Condon, B.D.; French, A.D. Detection of Human Neutrophil Elastase by Fluorescent Peptide Sensors Conjugated to TEMPO-Oxidized Nanofibrillated Cellulose. Int. J. Mol. Sci. 2022, 23, 3101. [Google Scholar] [CrossRef]
- Ataide, J.A.; de Carvalho, N.M.; de Araújo Rebelo, M.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.G.; Jozala, A.F. Bacterial Nanocellulose Loaded with Bromelain: Assessment of Antimicrobial, Antioxidant, and Physical-Chemical Properties. Sci. Rep. 2017, 7, 18031. [Google Scholar] [CrossRef]
- Osorio, M.; Ortiz, I.; Gañán, P.; Naranjo, T.; Zuluaga, R.; Van Kooten, T.; Castro, C. Novel Surface Modification of Three-Dimensional Bacterial Nanocellulose with Cell-Derived Adhesion Proteins for Soft Tissue Engineering. Mater. Sci. Eng. C 2019, 100, 697–705. [Google Scholar] [CrossRef]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced Antimicrobial Activity and Structural Transitions of a Nanofibrillated Cellulose–Nisin Biocomposite Suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and Anticancer Properties of Bacterial Cellulose Films Containing Ethanolic Extract of Mangosteen Peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications: A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Lu, Y.; Li, G.; Li, Y.; Yao, Y. Cellulose Nanofibril Matrix Drives the Dynamic Formation of Spheroids. J. Zhejiang Univ. Sci. B 2023, 24, 922. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Jeffrey Basirun, W.; Sanghiran Lee, V.; Rafie Johan, M. A Metal-Free Nanosensor Based on Nanocellulose-Polypyrrole Matrix and Single-Walled Carbon Nanotube: Experimental Study and Electroanalytical Application for Determination of Paracetamol and Ciprofloxacin. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100691. [Google Scholar] [CrossRef]
- Salas-Ambrosio, P.; Vexler, S.; Rajalakshmi, P.S.; Chen, I.A.; Maynard, H.D. Caffeine and Cationic Copolymers with Antimicrobial Properties. ACS Bio Med. Chem Au 2023, 3, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Hong, S.; Hu, G.; Ji, Y.; Hong, F.F. Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers 2020, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Kovalainen, M.; Laaksonen, P.; Häkkinen, M.; Auriola, S.; Peltonen, L.; Linder, M.; Järvinen, K.; Hirvonen, J.; Laaksonen, T. Immobilization of Protein-Coated Drug Nanoparticles in Nanofibrillar Cellulose Matrices—Enhanced Stability and Release. J. Control. Release 2011, 156, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Arola, S.; Tammelin, T.; Setälä, H.; Tullila, A.; Linder, M.B. Immobilization–Stabilization of Proteins on Nanofibrillated Cellulose Derivatives and Their Bioactive Film Formation. Biomacromolecules 2012, 13, 594–603. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface Functionalized Nanofibrillar Cellulose (NFC) Film as a Platform for Immunoassays and Diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Adsorption and Hydrolytic Activity of Trypsin on a Carboxylate-Functionalized Cation Exchanger Prepared from Nanocellulose. J. Colloid Interface Sci. 2012, 381, 125–136. [Google Scholar] [CrossRef]
- Orelma, H.; Johansson, L.; Filpponen, I.; Rojas, O.J.; Laine, J. Generic Method for Attaching Biomolecules via Avidin–Biotin Complexes Immobilized on Films of Regenerated and Nanofibrillar Cellulose. Biomacromolecules 2012, 13, 2802–2810. [Google Scholar] [CrossRef]
- Carvajal-Barriga, E.J.; Fitzgerald, W.; Dimitriadis, E.K.; Margolis, L.; Fields, R.D. Sulfated Endospermic Nanocellulose Crystals Prevent the Transmission of SARS-CoV-2 and HIV-1. Sci. Rep. 2023, 13, 6959. [Google Scholar] [CrossRef]
- Brisola, J.; Andrade, G.J.S.; Oliveira, S.A.; Viana, R.; Tischer, P.C.S.F.; Tischer, C.A. Covalent Immobilization of Lipase on Bacterial Cellulose Membrane and Nanocellulose. Mater. Res. 2022, 25, e20210350. [Google Scholar] [CrossRef]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active Wound Dressings Based on Bacterial Nanocellulose as Drug Delivery System for Octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of Silver Sulfadiazine into Bacterial Cellulose for Antimicrobial and Biocompatible Wound Dressing. Biomed. Mater. 2012, 7, 065006. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Han, G.; Wang, X.; Luo, J.; Sun, R. An Ultra-Light Antibacterial Bagasse-AgNP Aerogel. J. Mater. Chem. B 2017, 5, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Ye, S. Morphological, Release and Antibacterial Performances of Amoxicillin-Loaded Cellulose Aerogels. Molecules 2018, 23, 2082. [Google Scholar] [CrossRef] [PubMed]
- Lazarini, S.C.; Yamada, C.; Barud, H.d.S.; Trovatti, E.; Corbi, P.P.; Lustri, W.R. Influence of Chemical and Physical Conditions in Selection of Gluconacetobacter hansenii ATCC 23769 Strains with High Capacity to Produce Bacterial Cellulose for Application as Sustained Antimicrobial Drug-Release Supports. J. Appl. Microbiol. 2018, 125, 777–791. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojevic, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.; Glamočlija, J.; Dmitrović, S.; Matović, B.; Tasić, N.; Maksimović, V.; et al. Bacterial Cellulose-Lignin Composite Hydrogel as a Promising Agent in Chronic Wound Healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive Bacterial Cellulose Wound Dressings for Burns with Collagen In-Situ and Chitosan Ex-Situ Impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Jankau, J.; Błażyńska-Spychalska, A.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Pankiewicz, T.; Ludwicka, K.; Dettlaff, A.; Pęksa, R. Bacterial Cellulose Properties Fulfilling Requirements for a Biomaterial of Choice in Reconstructive Surgery and Wound Healing. Front. Bioeng. Biotechnol. 2022, 9, 805053. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release Using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef]
- Mohd Amin, M.C.I.; Abadi, A.G.; Ahmad, N.; Katas, H.; Jamal, J.A. Bacterial Cellulose Film Coating as Drug Delivery System: Physicochemical, Thermal and Drug Release Properties. Sains Malays. 2012, 41, 561–568. [Google Scholar]
- Mantas, A.; Petit, M.A.; Mihranyan, A. Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms. Pharmaceutics 2020, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical Caffeine Delivery Using Biocellulose Membranes: A Potential Innovative System for Cellulite Treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef]
- Xiao, L.; Poudel, A.J.; Huang, L.; Wang, Y.; Abdalla, A.M.E.; Yang, G. Nanocellulose Hyperfine Network Achieves Sustained Release of Berberine Hydrochloride Solubilized with β-Cyclodextrin for Potential Anti-Infection Oral Administration. Int. J. Biol. Macromol. 2020, 153, 633–640. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.; Gomes, F.; Silvestre, A.; Freire, C.; Lobo, J.S.; Costa, P. Bacterial Cellulose Membranes as Drug Delivery Systems: An In-vivo Skin Compatibility Study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- Amir, F.; Niazi, M.B.K.; Malik, U.S.; Jahan, Z.; Andleeb, S.; Ahmad, T.; Mustansar, Z. A Multifunctional Vanillin-Infused Chitosan-PVA Hydrogel Reinforced by Nanocellulose and CuO-Ag Nanoparticles as Antibacterial Wound Dressing. Int. J. Biol. Macromol. 2024, 258, 128831. [Google Scholar] [CrossRef]
- Pajorova, J.; Skogberg, A.; Hadraba, D.; Broz, A.; Travnickova, M.; Zikmundova, M.; Honkanen, M.; Hannula, M.; Lahtinen, P.; Tomkova, M.; et al. Cellulose Mesh with Charged Nanocellulose Coatings as a Promising Carrier of Skin and Stem Cells for Regenerative Applications. Biomacromolecules 2020, 21, 4857–4870. [Google Scholar] [CrossRef]
- Pandanaboina, S.C. Functionalized Nanocellulose Drives Neural Stem Cells Toward Neuronal Differentiation. J. Funct. Biomater. 2021, 12, 64. [Google Scholar] [CrossRef]
- Brassolatti, P.; Kido, H.W.; Bossini, P.S.; Gabbai-Armelin, P.R.; Otterço, A.N.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; Avó, L.R.d.S.d.; Forato, L.A.; et al. Bacterial Cellulose Membrane Used as Biological Dressings on Third-Degree Burns in Rats. Biomed. Mater. Eng. 2018, 29, 29–42. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated Chitin Fibers Reinforced with Bacterial Cellulose Nanocrystals as Suture Biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Navarro, J.R.; Rostami, J.; Ahlinder, A.; Mietner, J.B.; Bernin, D.; Saake, B.; Edlund, U.M. Surface-Initiated Controlled Radical Polymerization Approach to In Situ Cross-Link Cellulose Nanofibrils with Inorganic Nanoparticles. Biomacromolecules 2020, 21, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Zywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of Bacterial Cellulose with Quaternary Ammonium Compounds Based on Fatty Acids and Amino Acids and the Effect on Antimicrobial Activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Kung, H.N.; Tsai, Y.-S.; Tseng, T.-N.; Hsu, K.-D.; Cheng, K.-C. Novel Dextran Modified Bacterial Cellulose Hydrogel Accelerating Cutaneous Wound Healing. Cellulose 2017, 24, 4927–4937. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin Entrapped Gelatin/Ionically Modified Bacterial Cellulose Based Self-Healable Hydrogel Film: An Eco-Friendly Sustainable Synthesis Method of Wound Healing Patch. Int. J. Biol. Macromol. 2018, 122, 940–953. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical Properties of pH-Sensitive Hydrogels Based on Hydroxyethyl Cellulose–Hyaluronic Acid for Applications as Transdermal Delivery Systems for Skin Lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; Kim, H.J.; Park, C.H.; Kim, C.S. Three-Dimensional Cellulose Sponge: Fabrication, Characterization, Biomimetic Mineralization, and in vitro Cell Infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose–Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Garg, M.; Apostolopoulou-Kalkavoura, V.; Linares, M.; Kaldéus, T.; Malmström, E.; Bergström, L.; Zozoulenko, I. Moisture Uptake in Nanocellulose: The Effects of Relative Humidity, Temperature, and Degree of Crystallinity. Cellulose 2021, 28, 9007–9021. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Hu, D.; Ren, K.; Yao, F.; Zhu, Y.; Gao, C.; Wan, Y. Characterization of TEMPO-Oxidized Bacterial Cellulose Scaffolds for Tissue Engineering Applications. Mater. Chem. Phys. 2013, 143, 373–379. [Google Scholar] [CrossRef]
- Czaja, W.; Kyryliouk, D.; DePaula, C.A.; Buechter, D.D. Oxidation of Irradiated Microbial Cellulose Results in Bioresorbable, Highly Conformable Biomaterial. J. Appl. Polym. Sci. 2014, 131, 39995. [Google Scholar] [CrossRef]
- Shen, T.; Dong, H.; Wang, P. Research Progress on Nanocellulose and Its Composite Materials as Orthopedic Implant Biomaterials. Alex. Eng. J. 2024, 87, 575–590. [Google Scholar] [CrossRef]
- Catalán, J.; Ilves, M.; Järventaus, H.; Hannukainen, K.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and Immunotoxic Effects of Cellulose Nanocrystals in vitro. Environ. Mol. Mutagen. 2017, 56, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Kiliç, G.; Costa, P.M.; Fadeel, B. Cytotoxicity Screening and Cytokine Profiling of Nineteen Nanomaterials Enables Hazard Ranking and Grouping Based on Inflammogenic Potential. Nanotoxicology 2017, 11, 809–826. [Google Scholar]
- Yanamala, N.; Farcas, M.T.; Hatfield, M.K.; Kisin, E.R.; Kagan, V.E.; Geraci, C.L.; Shvedova, A.A. In-vivo Evaluation of the Pulmonary Toxicity of Cellulose Nanocrystals: A Renewable and Sustainable Nanomaterial of the Future. ACS Sustain. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Yanamala, N.; Farcas, M.T.; Menas, A.L.; Williams, A.; Fournier, P.M.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Gender Differences in Murine Pulmonary Responses Elicited by Cellulose Nanocrystals. Part. Fibre Toxicol. 2016, 13, 28. [Google Scholar] [CrossRef]
- Adewuyi, A.; Otuechere, C.A.; Adebayo, O.L.; Anazodo, C.; Pereira, F.V. Renal Toxicological Evaluations of Sulphonated Nanocellulose from Khaya sengalensis Seed in Wistar Rats. Chem. Biol. Interact. 2018, 284, 56–68. [Google Scholar] [CrossRef]
- Tullio, S.; McCoy, K.; Chalcraft, D. Chronic Toxicity and Liver Histopathology of Mosquito Fish (Gambusia holbrooki) Exposed to Natural and Modified Nanoclays. Sci. Total Environ. 2024, 908, 168060. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Zeng, S.; Wu, J.; Cheng, X.; Luo, T.; Wang, W.; Zeng, W.; Chen, Y. Cellulose Nanowhiskers: Preparation, Characterization and Cytotoxicity Evaluation. Biomed. Mater. Eng. 2012, 22, 121–127. [Google Scholar] [CrossRef]
- Pinto, F.; Lourenço, A.F.; Pedrosa, J.F.S.; Gonçalves, L.; Ventura, C.; Vital, N.; Bettencourt, A.; Fernandes, S.N.; da Rosa, R.R.; Godinho, M.H.; et al. Analysis of the In vitro Toxicity of Nanocelluloses in Human Lung Cells as Compared to Multi-Walled Carbon Nanotubes. Nanomaterials 2022, 12, 1432. [Google Scholar] [CrossRef]
- Patel, D.K.; Ganguly, K.; Dutta, S.D.; Patil, T.V.; Lim, K. Cellulose Nanocrystals vs. Cellulose Nanospheres: A Comparative Study of Cytotoxicity and Macrophage Polarization Potential. Carbohydr. Polym. 2023, 303, 120464. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native Cellulose Nanofibrils Induce Immune Tolerance In vitro by Acting on Dendritic Cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Raposo, N.R.B.; Brayner, R.; Teixeira, E.M.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.S.A.; Mattoso, L.H.C.; Brandão, H.M. Cytotoxicity and Expression of Genes Involved in the Cellular Stress Response and Apoptosis in Mammalian Fibroblasts Exposed to Cotton Cellulose Nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Carlsson, D.O.; Ålander, E.; Lindström, T.; Strømme, M.; Mihranyan, A.; Ferraz, N. Translational Study Between Structure and Biological Response of Nanocellulose from Wood and Green Algae. RSC Adv. 2014, 4, 2892–2903. [Google Scholar] [CrossRef]
- Staňková, L.; Kutová, A.; Doubková, M.; Kvítek, O.; Vokatá, B.; Sedlář, A.; Idriss, H.; Slepička, P.; Švorčík, V.; Bačáková, L. Argon Plasma-Modified Bacterial Nanocellulose: Cell-Specific Differences in the Interaction with Fibroblasts and Endothelial Cells. Carbohydr. Polym. Technol. Appl. 2024, 7, 100470. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro Biological Responses to Nanofibrillated Cellulose by Human Dermal, Lung, and Immune Cells: Surface Chemistry Aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Menas, A.L.; Yanamala, N.; Farcas, M.T.; Russo, M.; Friend, S.; Fournier, P.M.; Star, A.; Iavicoli, I.; Shurin, G.V.; Vogel, U.B.; et al. Fibrillar vs. Crystalline Nanocellulose Pulmonary Epithelial Cell Responses: Cytotoxicity or Inflammation? Chemosphere 2017, 171, 671–680. [Google Scholar] [CrossRef]
- Park, E.J.; Khaliullin, T.O.; Shurin, M.R.; Kisin, E.R.; Yanamala, N.; Fadeel, B.; Chang, J.; Shvedova, A.A. Fibrous Nanocellulose, Crystalline Nanocellulose, Carbon Nanotubes, and Crocidolite Asbestos Elicit Disparate Immune Responses upon Pharyngeal Aspiration in Mice. J. Immunotoxicol. 2018, 15, 12–23. [Google Scholar] [CrossRef]
- Liu, J.; Bacher, M.; Rosenau, T.; Willför, S.; Mihranyan, A. Potentially Immunogenic Contaminants in Wood-Based and Bacterial Nanocellulose: Assessment of Endotoxin and (1,3)-β-D-Glucan Levels. Biomacromolecules 2018, 19, 150–157. [Google Scholar] [CrossRef]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An Ecotoxicological Characterization of Nanocrystalline Cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and Cellular Uptake of Cellulose Nanocrystals. Nano LIFE 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Foster, E.J.; Vanhecke, D.; Studer, D.; Wick, P.; Gehr, P.; Rothen-Rutishauser, B.; Weder, C. Investigating the Interaction of Cellulose Nanofibers Derived from Cotton with a Sophisticated 3D Human Lung Cell Coculture. Biomacromolecules 2011, 12, 3666–3673. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and Environmental Safety Aspects of Friction Grinding and Spray Drying of Microfibrillated Cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose Reinforced Gellan-Gum Hydrogels as Potential Biological Substitutes for Annulus Fibrosus Tissue Regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 897–908. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity Tests of Cellulose Nanofibril-Based Structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Hua, K.; Ålander, E.; Lindström, T.; Mihranyan, A.; Strømme, M.; Ferraz, N. Surface Chemistry of Nanocellulose Fibers Directs Monocyte/Macrophage Response. Biomacromolecules 2015, 16, 2787–2795. [Google Scholar] [CrossRef]
- Song, L.Y.; Wu, Y.Z.; Pei, X.X.; Li, R.; Chen, H.T.; Sun, X.Z. Pulmonary Toxicity and RNA Sequencing Analyses of Mouse in Response to Exposure to Cellulose Nanofibrils. Inhal. Toxicol. 2020, 32, 388–401. [Google Scholar] [CrossRef]
- Jeon, M.J.; Randhawa, A.; Kim, H.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Lee, J.; Acharya, R.; Park, H.; Seol, Y.; et al. Electroconductive Nanocellulose: A Versatile Hydrogel Platform for Biomedical Engineering Applications. Adv. Healthc. Mater. 2025, 14, 2403983. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, P.; Sarkar, S.; Chowdhury, A.A. Applications of Nanocellulose in Tissue Engineering and Tissue Grafting. In Nanocellulose; Mukhopadhyay, M.; Bhattacharya, D., Eds.; 2024, pp. 159–191. [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Sreedharan, M.; Vijayamma, R.; Liyaskina, E.; Revin, V.V.; Ullah, M.W.; Shi, Z.; Yang, G.; Grohens, Y.; Kalarikkal, N.; Khan, K.A.; et al. Nanocellulose-Based Hybrid Scaffolds for Skin and Bone Tissue Engineering: A 10-Year Overview. Biomacromolecules 2024, 25, 2136–2155. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Kassie, B.B.; Getahun, M.J.; Azanaw, A.; Ferede, B.T.; Tassew, D.F. Surface modification of cellulose nanocrystals for biomedical and personal hygiene applications. Int. J. Biol. Macromol. 2024, 282, 136949. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Patoary, M.K.; Islam, S.R.; Farooq, A.; Rashid, M.A.; Sarker, S.; Hossain, M.Y.; Rakib, M.A.N.; Al-Amin, M.; Liu, L. Phosphorylation of Nanocellulose: State of the Art and Prospects. Ind. Crops Prod. 2023, 201, 116965. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Bai, X.; Xiao, Y.; Che, J. Bio-inspired composite by hydroxyapatite mineralization on (bis)phosphonate-modified cellulose-alginate scaffold for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 127958. [Google Scholar] [CrossRef]
- Wang, Q.; Karadas, Ö.; Rosenholm, J.M.; Xu, C.; Näreoja, T.; Wang, X. Bioprinting Macroporous Hydrogel with Aqueous Two-Phase Emulsion-Based Bioink: In Vitro Mineralization and Differentiation Empowered by Phosphorylated Cellulose Nanofibrils. Adv. Funct. Mater. 2024, 34, 2400431. [Google Scholar] [CrossRef]
- Ugrin, M.; Dinic, J.; Jeremic, S.; Dragicevic, S.; Djeri, B.B.; Nikolic, A. Bacterial Nanocellulose as a Scaffold for In vitro Cell Migration Assay. Nanomaterials 2021, 11, 2322. [Google Scholar] [CrossRef]
- Liu, D.; Meng, Q.; Hu, J. Bacterial Nanocellulose Hydrogel: A Promising Alternative Material for the Fabrication of Engineered Vascular Grafts. Polymers 2023, 15, 183812. [Google Scholar] [CrossRef]
- Mendoza, L.; Gunawardhana, T.; Batchelor, W.; Garnier, G. Effects of Fibre Dimension and Charge Density on Nanocellulose Gels. J. Colloid Interface Sci. 2018, 525, 119–125. [Google Scholar] [CrossRef]
- Stoudmann, N.; Schmutz, M.; Hirsch, C.; Nowack, B.; Som, C. Human Hazard Potential of Nanocellulose: Quantitative Insights from the Literature. Nanotoxicology 2020, 14, 1241–1257. [Google Scholar] [CrossRef]
- Skogberg, A.; Mäki, A.J.; Mettänen, M.; Lahtinen, P.; Kallio, P. Cellulose Nanofiber Alignment Using Evaporation-Induced Droplet-Casting, and Cell Alignment on Aligned Nanocellulose Surfaces. Biomacromolecules 2017, 18, 3936–3953. [Google Scholar] [CrossRef]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of Nanocellulose with a Xyloglucan–RGD Conjugate Enhances Adhesion and Proliferation of Endothelial Cells: Implications for Tissue Engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Anton-Sales, I.; D’Antin, J.C.; Engroba, J.F.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial Nanocellulose as a Corneal Bandage Material: A Comparison with Amniotic Membrane. Biomater. Sci. 2020, 8, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Svanström, A.; Berglin, M.; Petronis, S.; Bogestål, Y.; Stenlund, P.; Standoft, S.; Ståhlberg, A.; Landberg, G.; Chinga-Carrasco, G.; et al. 3D Printed Nanocellulose Scaffolds as a Cancer Cell Culture Model System. Bioengineering 2021, 8, 97. [Google Scholar] [CrossRef]
- Szustak, M.; Gendaszewska-Darmach, E. Nanocellulose-Based Scaffolds for Chondrogenic Differentiation and Expansion. Front. Bioeng. Biotechnol. 2021, 9, 736213. [Google Scholar] [CrossRef]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and Differentiation of SH-SY5Y Neuroblastoma Cells on Bacterial Nanocellulose Scaffolds. Artif. Cells Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Jonsson, M.; Brackmann, C.; Puchades, M.; Brattås, K.; Ewing, A.; Gatenholm, P.; Enejder, A. Neuronal Networks on Nanocellulose Scaffolds. Tissue Eng. Part C Methods 2015, 21, 1162–1170. [Google Scholar] [CrossRef]
- Abbasi-Moayed, S.; Golmohammadi, H.; Hormozi-Nezhad, M.R. A Nanopaper-Based Artificial Tongue: A Ratiometric Fluorescent Sensor Array on Bacterial Nanocellulose for Chemical Discrimination Applications. Nanoscale 2018, 10, 2492–2502. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel Bilayer Bacterial Nanocellulose Scaffold Supports Neocartilage Formation In vitro and In-vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-Based Interpenetrating Polymer Network (IPN) Hydrogels for Cartilage Applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical Evaluation of Bacterial Nanocellulose as an Implant Material for Ear Cartilage Replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef]
- Pretzel, D.; Linss, S.; Ahrem, H.; Endres, M.; Kaps, C.; Klemm, D.; Kinne, R.W. A Novel In vitro Bovine Cartilage Punch Model for Assessing the Regeneration of Focal Cartilage Defects with Biocompatible Bacterial Nanocellulose. Arthritis Res. Ther. 2013, 15, R59. [Google Scholar] [CrossRef]
- Kasturi, M.; Mathur, V.; Gadre, M.; Srinivasan, V.; Vasanthan, K.S. Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In vitro Models to Clinical Applications. Tissue Eng. Regen. Med. 2024, 21, 21–52. [Google Scholar] [CrossRef]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of Liver Progenitor Cell Line to Functional Organotypic Cultures in 3D Nanofibrillar Cellulose and Hyaluronan-Gelatin Hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef]
- Krontiras, P.; Gatenholm, P.; Hägg, D.A. Adipogenic Differentiation of Stem Cells in Three-Dimensional Porous Bacterial Nanocellulose Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 195–203. [Google Scholar] [CrossRef]
- Henriksson, I.; Gatenholm, P.; Hägg, D.A. Increased Lipid Accumulation and Adipogenic Gene Expression of Adipocytes in 3D Bioprinted Nanocellulose Scaffolds. Biofabrication 2017, 9, 015022. [Google Scholar] [CrossRef]
- Sun, C.; Xie, Y.; Zhu, H.; Zheng, X.; Hou, R.; Shi, Z.; Li, J.; Yang, Q. Highly Electroactive Tissue Engineering Scaffolds Based on Nanocellulose/Sulfonated Carbon Nanotube Composite Hydrogels for Myocardial Tissue Repair. Biomacromolecules 2023, 24, 5989–5997. [Google Scholar] [CrossRef]
- Weber, C.; Reinhardt, S.; Eghbalzadeh, K.; Wacker, M.; Guschlbauer, M.; Maul, A.; Sterner-Kock, A.; Wahlers, T.; Wippermann, J.; Scherner, M. Patency and In-vivo Compatibility of Bacterial Nanocellulose Grafts as Small-Diameter Vascular Substitute. J. Vasc. Surg. 2018, 68, 177S–187S.e1. [Google Scholar] [CrossRef]
- Hu, G.; Bao, L.; Li, G.; Chen, L.; Hong, F.F. Vascular Cells Responses to Controlled Surface Structure and Properties of Bacterial Nanocellulose Artificial Blood Vessel After Mercerization. Carbohydr. Polym. 2023, 306, 120572. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Reece, L.M.; Pastrana, F.; Arias, S.L.; Shetty, A.R.; Pavón, J.J.; Allain, J.P. Bacterial Nanocellulose Magnetically Functionalized for Neuro-Endovascular Treatment. Macromol. Biosci. 2017, 17, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Pavón, J.J.; Allain, J.P.; Verma, D.; Echeverry-Rendón, M.; Cooper, C.L.; Reece, L.M.; Shetty, A.R.; Tomar, V. In Situ Study Unravels Bio-Nanomechanical Behavior in a Magnetic Bacterial Nano-Cellulose (MBNC) Hydrogel for Neuro-Endovascular Reconstruction. Macromol. Biosci. 2018, 19, 1800225. [Google Scholar] [CrossRef]
- Mariño, M.A.; Oyarce, K.; Tobar, C.; Del Rio, R.S.; Paredes, M.G.; Pavez, P.; Sarabia, M.; Amroroso, A.; Concha, J.L.; Norambuena-Contreras, J.; et al. Crosslinked Oxidized-Nanocellulose/Chitosan Hydrogels as a Scaffold Matrix for Mesenchymal Stem Cell Growth. Cellulose 2024, 31, 363–379. [Google Scholar] [CrossRef]
- Rashad, A.; Grøndahl, M.; Heggset, E.B.; Mustafa, K.; Syverud, K. Responses of Rat Mesenchymal Stromal Cells to Nanocellulose with Different Functional Groups. ACS Appl. Bio Mater. 2023, 6, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Vielreicher, M.; Kralisch, D.; Völkl, S.; Sternal, F.; Arkudas, A.; Friedrich, O. Bacterial Nanocellulose Stimulates Mesenchymal Stem Cell Expansion and Formation of Stable Collagen-I Networks as a Novel Biomaterial in Tissue Engineering. Sci. Rep. 2018, 8, 9401. [Google Scholar] [CrossRef]
- Sundberg, J.; Götherström, C.; Gatenholm, P. Biosynthesis and In vitro Evaluation of Microporous Mineralized Bacterial Nanocellulose Scaffolds for Bone Tissue Engineering. Biomed. Mater. Eng. 2015, 25, 39–52. [Google Scholar]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.; Liu, C. Biomimetic Composite Scaffolds Based on Mineralization of Hydroxyapatite on Electrospun Poly (Caprolactone)/Nanocellulose Fibers. Carbohydr. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef]
- Chen, Q.; Garcia, R.P.; Munoz, J.; de Larraya, U.P.; Garmendia, N.; Yao, Q.; Boccaccini, A.R. Cellulose Nanocrystals—Bioactive Glass Hybrid Coating as Bone Substitutes by Electrophoretic Co-Deposition: In Situ Control of Mineralization of Bioactive Glass and Enhancement of Osteoblastic Performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef]
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.A.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef]
- Ying, Y.; Cai, K.; Cai, X.; Zhang, K.; Qiu, R.; Jiang, G.; Luo, K. Recent Advances in the Repair of Degenerative Intervertebral Disc for Preclinical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1259731. [Google Scholar] [CrossRef]
- Farzamfar, S.; Richer, M.; Rahmani, M.; Naji, M.; Aleahmad, M.; Chabaud, S.; Bolduc, S. Biological Macromolecule-Based Scaffolds for Urethra Reconstruction. Biomolecules 2023, 13, 1167. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Feng, C.; Liu, Y.; Peng, X.; Chen, S.; Xiao, D.; Wang, H.; Li, Z.; Xu, Y.; Lu, M. A Smart Bilayered Scaffold Supporting Keratinocytes and Muscle Cells in Micro/Nano-Scale for Urethral Reconstruction. Theranostics 2018, 8, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Lv, X.G.; Li, Z.; Song, L.J.; Feng, C.; Xie, M.K.; Li, C.; Li, H.B.; Wang, J.H.; Zhu, W.D. Urethral Reconstruction with a 3D Porous Bacterial Cellulose Scaffold Seeded with Lingual Keratinocytes in a Rabbit Model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Nicu, R.; Ciolacu, D.E.; Ciolacu, F. Insight into the Latest Medical Applications of Nanocellulose. Materials 2023, 16, 4447. [Google Scholar] [CrossRef]
- Doench, I.W.; Montembault, A.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef]
- Empson, Y.M.; Ekwueme, E.C.; Hong, J.K.; Paynter, D.M.; Kwansa, A.L.; Brown, C.; Pekkanen, A.M.; Roman, M.; Rylander, N.M.; Brolinson, G.P.; et al. High Elastic Modulus Nanoparticles: A Novel Tool for Subfailure Connective Tissue Matrix Damage. Transl. Res. 2014, 164, 244–257. [Google Scholar] [CrossRef]
- Coelho, F.; Do Vale Braido, G.V.; Cavicchioli, M.; Mendes, L.S.; Specian, S.S.; Franchi, L.P.; Lima Ribeiro, S.J.; Messaddeq, Y.; Scarel-Caminaga, R.M.; Capote, T.S.O. Toxicity of Therapeutic Contact Lenses Based on Bacterial Cellulose with Coatings to Provide Transparency. Contact Lens Anterior Eye 2019, 42, 512–519. [Google Scholar] [CrossRef]
- Tummala, G.K.; Joffre, T.; Rojas, R.; Persson, C.; Mihranyan, A. Strain-Induced Stiffening of Nanocellulose-Reinforced Poly (Vinyl Alcohol) Hydrogels Mimicking Collagenous Soft Tissues. Soft Matter 2017, 13, 3936–3945. [Google Scholar] [CrossRef]
- Goldschmidt, E.; Cacicedo, M.; Kornfeld, S.; Valinoti, M.; Ielpi, M.; Ajler, P.M.; Yampolsky, C.; Rasmussen, J.; Castro, G.R.; Argibay, P. Construction and In vitro Testing of a Cellulose Dura Mater Graft. Neurol. Res. 2016, 38, 25–31. [Google Scholar] [CrossRef]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. New methods Modified Bacterial Cellulose Tubes for Regeneration of Damaged Peripheral Nerves. Arch. Med. Sci. 2013, 3, 527–534. [Google Scholar] [CrossRef]
- Elert, A.M.; Chen, C.; Smales, G.J.; Topolniak, I.; Sturm, H.; Schönhals, A.; Szymoniak, P. Effects of the Charge Density of Nanopapers Based on Carboxymethylated Cellulose Nanofibrils Investigated by Complementary Techniques. ACS Omega 2024, 9, 20152–20166. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of Surface Charge Density of Bacterial Cellulose Nanofibrils on the Rheology Property of O/W Pickering Emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Sämfors, S.; Hägg, D.; Gatenholm, P. Universal Method for Protein Bioconjugation with Nanocellulose Scaffolds for Increased Cell Adhesion. Mater. Sci. Eng. C 2013, 33, 4599–4607. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial Cellulose Scaffolds and Cellulose Nanowhiskers for Tissue Engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Amin, M.C.I.M. Development of a Bacterial Cellulose-Based Hydrogel Cell Carrier Containing Keratinocytes and Fibroblasts for Full-Thickness Wound Healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the Use of Ion-Crosslinked Nanocellulose Hydrogels for Wound Healing Solutions: Physicochemical Properties and Application-Oriented Biocompatibility Studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of Bacterial Nanocellulose-Based Uniform Wound Dressing for Large Area Skin Transplantation. Mater. Sci. Eng. C 2013, 33, 2995–3000. [Google Scholar] [CrossRef]
- Bencurova, E.; Shityakov, S.; Schaack, D.; Kaltdorf, M.; Sarukhanyan, E.; Hilgarth, A.; Rath, C.; Montenegro, S.; Roth, G.; Lopez, D.; et al. Nanocellulose Composites as Smart Devices with Chassis, Light-Directed DNA Storage, Engineered Electronic Properties, and Chip Integration. Front. Bioeng. Biotechnol. 2022, 10, 869111. [Google Scholar] [CrossRef]
- Khodayari, A.; Vats, S.; Mertz, G.; Schnell, C.N.; Rojas, C.F.; Seveno, D. Electrospinning of Cellulose Nanocrystals; Procedure and Optimization. Carbohydr. Polym. 2025, 347, 122698. [Google Scholar] [CrossRef]
- Li, M.; Mu, Y.; Xu, Q.; Jin, L.; Fu, Y. Injectable, Rapid Self-Healing, Antioxidant, and Antibacterial Nanocellulose-Tannin Hydrogels Formed via Metal-Ligand Coordination for Drug Delivery and Wound Dressing. Ind. Crops Prod. 2024, 208, 117876. [Google Scholar] [CrossRef]
- Le Gars, M.; Dhuiège, B.; Delvart, A.; Belgacem, M.N.; Missoum, K.; Bras, J. High-Barrier and Antioxidant Poly(Lactic Acid)/Nanocellulose Multilayered Materials for Packaging. ACS Omega 2020, 5, 22816–22826. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Antunes, J.C.; Seabra, C.L.; Fertuzinhos, A.; Tohidi, S.D.; Reis, S.; Amorim, M.T.P.; Ferreira, D.P.; Felgueiras, H.P. Antibacterial and Hemostatic Capacities of Cellulose Nanocrystalline-Reinforced Poly(Vinyl Alcohol) Electrospun Mats Doped with Tiger 17 and Pexiganan Peptides for Prospective Wound Healing Applications. Biomater. Adv. 2022, 137, 212830. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Lee, K.M.; Liu, Z.X.; Lai, R.Y.; Chen, C.K.; Chen, W.C.; Hsu, J.F. Antimicrobial Activity of Electrospun Polyvinyl Alcohol Nanofibers Filled with Poly [2-(tert-Butylaminoethyl) Methacrylate]-Grafted Graphene Oxide Nanosheets. Polymers 2020, 12, 1449. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Makharita, R.R.; Qarabai, F.A.K.; Shahabuddin, F.S.; Saddiq, A.A.; Bahammam, L.A.; El-Far, S.W.; Bukhari, M.A.; Elaidarous, M.A.; Abdella, A. Antibacterial Activities of Ag/Cellulose Nanocomposites Derived from Marine Environment Algae Against Bacterial Tooth Decay. Microorganisms 2023, 12, 1. [Google Scholar] [CrossRef]
- Matei, E.; Gaidau, C.; Râpă, M.; Stefan, L.M.; Ditu, L.-M.; Predescu, A.M.; Stanca, M.; Pantilimon, M.C.; Berechet, M.D.; Predescu, C.; et al. Sustainable Coated Nanostructures Based on Alginate and Electrospun Collagen Loaded with Antimicrobial Agents. Coatings 2021, 11, 121. [Google Scholar] [CrossRef]
- Abdullah, T.; Saeed, U.; Memic, A.; Gauthaman, K.; Hussain, M.A.; Al-Turaif, H.; Abudula, T. Electrospun Cellulose Nano Fibril Reinforced PLA/PBS Composite Scaffold for Vascular Tissue Engineering. J. Polym. Res. 2019, 26, 110. [Google Scholar] [CrossRef]
- Sultana, T.; Hossain, M.; Rahaman, S.; Kim, Y.S.; Gwon, J.; Lee, B. Multi-Functional Nanocellulose-Chitosan Dressing Loaded with Antibacterial Lawsone for Rapid Hemostasis and Cutaneous Wound Healing. Carbohydr. Polym. 2021, 272, 118482. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Chen, X.; Han, H.; Yang, G. Double Network Bacterial Cellulose Hydrogel to Build a biology–Device Interface. Nanoscale 2014, 6, 970–977. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Sanchavanakit, N.; Sangrungraungroj, W.; Kaomongkolgit, R.; Banaprasert, T.; Pavasant, P.; Phisalaphong, M. Growth of Human Keratinocytes and Fibroblasts on Bacterial Cellulose Film. Biotechnol. Prog. 2006, 22, 1194–1199. [Google Scholar] [CrossRef]
- Kingkaew, J.; Jatupaiboon, N.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Biocompatibility and Growth of Human Keratinocytes and Fibroblasts on Biosynthesized Cellulose–Chitosan Film. J. Biomater. Sci. Polym. Ed. 2010, 21, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Bottan, S.; Robotti, F.; Jayathissa, P.; Hegglin, A.; Bahamonde, N.; Heredia-Guerrero, J.A.; Bayer, I.S.; Scarpellini, A.; Merker, H.; Lindenblatt, N.; et al. Surface-Structured Bacterial Cellulose with Guided Assembly-Based Biolithography (GAB). ACS Nano 2015, 9, 206–219. [Google Scholar] [CrossRef]
- Keskin, Z.; Sendemir Urkmez, A.; Hames, E.E. Novel Keratin Modified Bacterial Cellulose Nanocomposite Production and Characterization for Skin Tissue Engineering. Mater. Sci. Eng. C 2017, 75, 1144–1153. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and Structural Characterization of Surface Modified Microporous Bacterial Cellulose Scaffolds: A Potential Material for Skin Regeneration Applications In vitro and In-vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef]
- Brião, G.V.; Rosa, D.S.; Frollini, E. Hydrogels from non-woody lignocellulosic biomass for toxic metal uptake from wastewater: A brief overview. Cellulose 2025, 32, 691–712. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, H.; Zhang, H.; Wang, X.; Fu, Y.; Wang, Q.; Liu, H.; Yong, Y.C.; Guo, J.; Liu, J. Biosafety consideration of nanocellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2024, 265, 130900. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Q.; He, H.; Guo, X.; Yang, S.; Zhang, L.; Wang, L. Metal removal from heavy metal-enriched plants by deep eutectic solvents and its mechanism investigation. Sep. Purif. Technol. 2025, 357, 130189. [Google Scholar] [CrossRef]
- Zuber, J.; Cascabulho, P.L.; Piperni, S.G.; do Amaral, R.J.; Vogt, C.; Carre, V.; Hertzog, J.; Kontturi, E.; Trubetskaya, A. Fast Easy and Reproducible Fingerprint Methods for Endotoxin Characterization in Nanocellulose and Alginate-Based Hydrogel Scaffolds. Biomacromolecules 2024, 25, 6762–6772. [Google Scholar] [CrossRef]
- Negi, A. Cationized Cellulose Materials: Enhancing Surface Adsorption Properties Towards Synthetic and Natural Dyes. Polymers 2025, 17, 36. [Google Scholar] [CrossRef]
- Madani, M.; Borandeh, S.; Teotia, A.K.; Seppälä, J.V. Direct and Indirect Cationization of Cellulose Nanocrystals: Structure–Properties Relationship and Virus Capture Activity. Biomacromolecules 2023, 24, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose Composite Wound Dressings for Real-Time pH Wound Monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef]
- Yadav, V.; Sun, L.; Panilaitis, B.; Kaplan, D.L. In vitro Chondrogenesis with Lysozyme Susceptible Bacterial Cellulose as a Scaffold. J. Tissue Eng. Regen. Med. 2015, 9, E276–E288. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; R, R.; Antony, S.; Madhavan, A.; Sindhu, R.; Kumar Awasthi, M.; Kuddus, M.; Pillai, S.; Varjani, S.; Pandey, A.; et al. Nanocellulose in Tissue Engineering and Bioremediation: Mechanism of Action. Bioengineered 2022, 13, 12823–12833. [Google Scholar] [CrossRef] [PubMed]



| Nanocellulose Type | Biomedical Application | Biocompatible Molecule | Nature of the Compound | Reference(s) |
|---|---|---|---|---|
| CNCs | Wound dressing | Elastase tripeptide | Biosensor | [31] |
| Tissue engineering | Propranolol hydrochloride | Drug | [12] | |
| Theophylline | Stimulant | [101] | ||
| Diagnostics | Bovine serum albumin | Protein | [102] | |
| Skin repair/tissue engineering | Collagen, hyaluronan | Growth factor | [103,104] | |
| Tissue engineering | Paclitaxel, docetaxel, etoposide | Anticancer drug | [105] | |
| Skin repair/wound healing | Procaine hydrochloride | Local anesthetics | [106] | |
| Tissue engineering | Doxorubicin hydrochloride | Anticancer drug | [107] | |
| Riboflavin | Vitamin | [108] | ||
| Wound healing | Lysozyme | Enzyme | [109] | |
| Tissue engineering | Peroxidase | Enzyme | [110] | |
| Alcohol oxidase | Enzyme | [111] | ||
| Glucose oxidase | Enzyme | [112] | ||
| Tryptophan-based peptides | Protein | [113] | ||
| Human neutrophil elastase | Enzyme | [114] | ||
| Diagnostics | Papain enzyme | Enzyme | [1,115] | |
| Tissue engineering | ||||
| Heptapeptide | Protein | [116] | ||
| Diblock protein (Elastin-co-Cartilage oligomeric matrix) | Drug | [116] | ||
| Wound healing | Nisin | Antibacterial peptide | [117] | |
| Tissue engineering | Anticancer drugs | Biochemical | [118] | |
| CNFs | Skin repair | Alkonnin and Shikonin | Antibacterial agent | [119,120] |
| Tissue engineering | Indomethacin, nadolol, atenolol, metoprolol tartrate, verapamil, ibuprofen | Drug | [31] | |
| Paracetamol | Drug | [121] | ||
| Caffeine | Stimulant | [122] | ||
| Wound healing | Itraconazole | Drug | [123] | |
| Lysozyme | Enzyme | |||
| Indomethacin | Drug | [124] | ||
| Itraconazole | Antifungal agent | |||
| Tissue engineering | Beclomethasone dipropionate | Drug | ||
| Alkaline phosphatase; anti-hydrocortisone antibody | Enzyme | [125] | ||
| Wound healing | Avidins | Protein | [126] | |
| Tissue engineering | Pancreatic serine protease trypsin | Enzyme | [127] | |
| Antihuman IgG antibody | Biomolecule | [128] | ||
| Human immunoglobulin G | Biomolecule | [129] | ||
| Lipase | Enzyme | [130] | ||
| Wound healing | AgNPs | Metal and antibiotic | [87] | |
| Tissue engineering | Collagen | Protein | [88] | |
| Wound healing/tissue engineering | Chitosan, diamond nanoparticle | Polysaccharide | [89] | |
| Skin repair | Sericin | Protein | [90,92] | |
| Wound dressing | Povidone-iodine, Polyhexamethylene biguanide (PHMB), Octenidine | Antiseptics | [60,98,131] | |
| Silver sulfadiazine | Antibacterial agent | [132] | ||
| AuNPs | Nanoparticle | [100] | ||
| AgNPs and antibiotics | Antibiotic | [133] | ||
| Amoxicillin | Antibiotic | [134] | ||
| Ceftriaxone | Antibiotic | [135] | ||
| Curcumin and Lignin | Antibacterial agent | [136] | ||
| BNC | Skin repair | Vaccarin | Glycoside | [32,137,138] |
| Diagnostics | Bovine serum albumin | Protein | [102,139] | |
| Tissue engineering | Theophylline | Drug/stimulant | [140] | |
| Wound healing | Paracetamol | Drug | [141] | |
| Tissue engineering | Lidocaine, ibuprofen | Local anesthetic | [142] | |
| Caffeine | Stimulant | [143] | ||
| Berberine hydrochloride, Berberine sulfate | Drug | [144] | ||
| Skin repair | Glycerin | Moisturizer | [145] | |
| Vanillin | Phenol | [146] | ||
| Skin repair/wound healing | Hemoglobin, myoglobin, albumin, lysozyme | Proteins | [147] | |
| Tissue engineering | Glutamate decarboxylase | Enzyme | [148] | |
| Wound healing | Macrophage-stimulating protein | Protein | [94] | |
| Chitosan | Antibacterial | [89] | ||
| Lidocaine | Anesthesia | [149] | ||
| Skin repair | Glycerin | Moisturizer | [145] | |
| Chitin | Polysaccharide | [150] |
| Nanocellulose | Test | Cell Type/Organism | Toxicity Evaluation | Reference(s) |
|---|---|---|---|---|
| CNCs | Acute lethal test | Hepatocytes of fishes, in vitro rainbow trout hepatocyte assay | Low toxicity potential | [167] |
| Multi-trophic assays | Low environmental risk | |||
| Cytotoxicity to human epithelial airways barrier | Monocyte-derived macrophages, dendritic cells, and bronchial epithelial cells | Low cytotoxicity, pro-inflammatory, cytokine production | [124] | |
| Skin irritation and sensitization tests | L929 cells | Low cytotoxicity | [168] | |
| Cytotoxicity | No cytotoxicity up to 48 h | |||
| MTT assayLDH assay | HBMEC, bEnd.3, RAW 264.7, MCF-10A, MDA-MB-231, MDA-MB-468, KB, PC-3 and C6 | No cytotoxicity or inflammation | [169] | |
| CNFs | Acute environmental toxicity, cytotoxicity | Human monocyte and mouse macrophages, Kinetic luminescent bacteria | No evidence of inflammatory effects or cytotoxicity | [162,170] |
| Neurotoxicity and systemic effects | Nematode model | Low cytotoxicity | [33] | |
| In vitro pharyngeal aspiration study for pulmonary immunotoxicity and genotoxicity | Mice | Pulmonary inflammation, no DNA or chromosome damage | [171] | |
| Cytotoxicity, test of cell membrane, cell mitochondrial activity, DNA proliferation | 3T3 fibroblast cells | Pure CNF: non-toxic, low cytotoxicity for CNF modified via PEI or CTAB surface modification | [123,172] | |
| Cytotoxicity | Bovine fibroblasts cells | Low cytotoxicity at low CNF concentration (0.02–100 µg/mL), no evidence of cytotoxicity for pure CNF, improved cytocompatibility of EPTMAC-modified CNF | [173] | |
| BNC | Cytotoxicity | Osteoblast cells, L929 fibroblast cells | No evidence of cytotoxicity | [109,137] |
| Cytotoxicity | Human umbilical vein endothelial cells | No evidence of toxicity in vitro and in vivo | [174] | |
| Cytotoxicity | C57/Bl6 male mouse | Non-toxic, non-immunogenic | [32] | |
| In vitro immunoreactivity, Cytotoxicity | Human umbilical vein endothelial cells |
| Nanocellulose Type | Reinforcement Material | Synthesis Method | Enhanced Features | Application | Reference(s) |
|---|---|---|---|---|---|
| CNCs | Polyvinyl alcohol (PVA)/NCC scaffolds | Freeze-drying | Mechanical, thermal, and swelling features, uniform pore size | Cell adherence, growth, and metabolism | [120,159,249] |
| Polylactide-polyglycolide (PLGA) | Electrospinning | Stretching and formation of fibrous types of scaffolds | Cell adhesion and fibroblast proliferation | ||
| Cellulose acetate propionate (CAP | Electrospinning and magnetic field | Small diameter | Development of blood vessels | [250] | |
| Coumarin and curcumin | Emulsion | Antioxidant, anti-inflammatory, antimicrobial, and anticancer activities | Wound dressings | [251,252] | |
| Polyvinyl alcohol/polyethylene oxide/CMC cellulose matrix | Electrospun | Antimicrobial/therapeutic | Wound dressings | [253,254] | |
| CNFs | Brown algae nanofibrillar cellulose (BANFC)/ quaternized chitin/organic rectorite | Freeze-drying | Antibacterial activity and mechanical strength | Collagen formation and neovascularization | [255,256] |
| Butylene succinate (PBS) and poly lactic acid (PLA) | Electron spun | Betterment of fiber structure, tensile strength, elastic modulus, and biocompatibility | Blood vessels | [257] | |
| Charged cellulose nanofibrils (cCNFs) | Polymer coating | Cell survival, adhesion, and proliferation | Artificial skin constructs | [147] | |
| Ca2+-crosslinked | Topical drug delivery | Chronic wound healing | [247] | ||
| Copper-containing mesoporous bioactive glass | Antibacterial activity, angiogenic activity | Wound healing | [108] | ||
| Gelatin and aminated silver nanoparticles | Mechanical andself-recovery properties, antibacterial activity | Skin wounds | [178] | ||
| BNC | Chitosan | Cell adhesion | Adhesion of human keratinocytes | [170,258] | |
| Keratin | Cell adhesion, proliferation, and morphology | Human skin keratinocytes, human skin fibroblasts | [95] | ||
| Gelatin | Cell adhesion and proliferation | HaCaT line keratinocytes, wound closure efficacy | [226] | ||
| Polypyrrole and polyaniline | Cell proliferation | Skin tissue engineering | [259] | ||
| Paraffin microspheres | Support and cell proliferation | Mouse embryonic NIH 3T3 fibroblasts/tissue engineering | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar, M.A.; Xie, R.; Liu, J.; Ali, S.; Pawar, K.D.; Sudiana, I.M.; Sun, J. Current Paradigms and Future Challenges in Harnessing Nanocellulose for Advanced Applications in Tissue Engineering: A Critical State-of-the-Art Review for Biomedicine. Int. J. Mol. Sci. 2025, 26, 1449. https://doi.org/10.3390/ijms26041449
Dar MA, Xie R, Liu J, Ali S, Pawar KD, Sudiana IM, Sun J. Current Paradigms and Future Challenges in Harnessing Nanocellulose for Advanced Applications in Tissue Engineering: A Critical State-of-the-Art Review for Biomedicine. International Journal of Molecular Sciences. 2025; 26(4):1449. https://doi.org/10.3390/ijms26041449
Chicago/Turabian StyleDar, Mudasir A., Rongrong Xie, Jun Liu, Shehbaz Ali, Kiran D. Pawar, I Made Sudiana, and Jianzhong Sun. 2025. "Current Paradigms and Future Challenges in Harnessing Nanocellulose for Advanced Applications in Tissue Engineering: A Critical State-of-the-Art Review for Biomedicine" International Journal of Molecular Sciences 26, no. 4: 1449. https://doi.org/10.3390/ijms26041449
APA StyleDar, M. A., Xie, R., Liu, J., Ali, S., Pawar, K. D., Sudiana, I. M., & Sun, J. (2025). Current Paradigms and Future Challenges in Harnessing Nanocellulose for Advanced Applications in Tissue Engineering: A Critical State-of-the-Art Review for Biomedicine. International Journal of Molecular Sciences, 26(4), 1449. https://doi.org/10.3390/ijms26041449








