The Synergistic Impact of Glycolysis, Mitochondrial OxPhos, and PEP Cycling on ATP Production in Beta Cells
Abstract
1. Introduction
2. Results
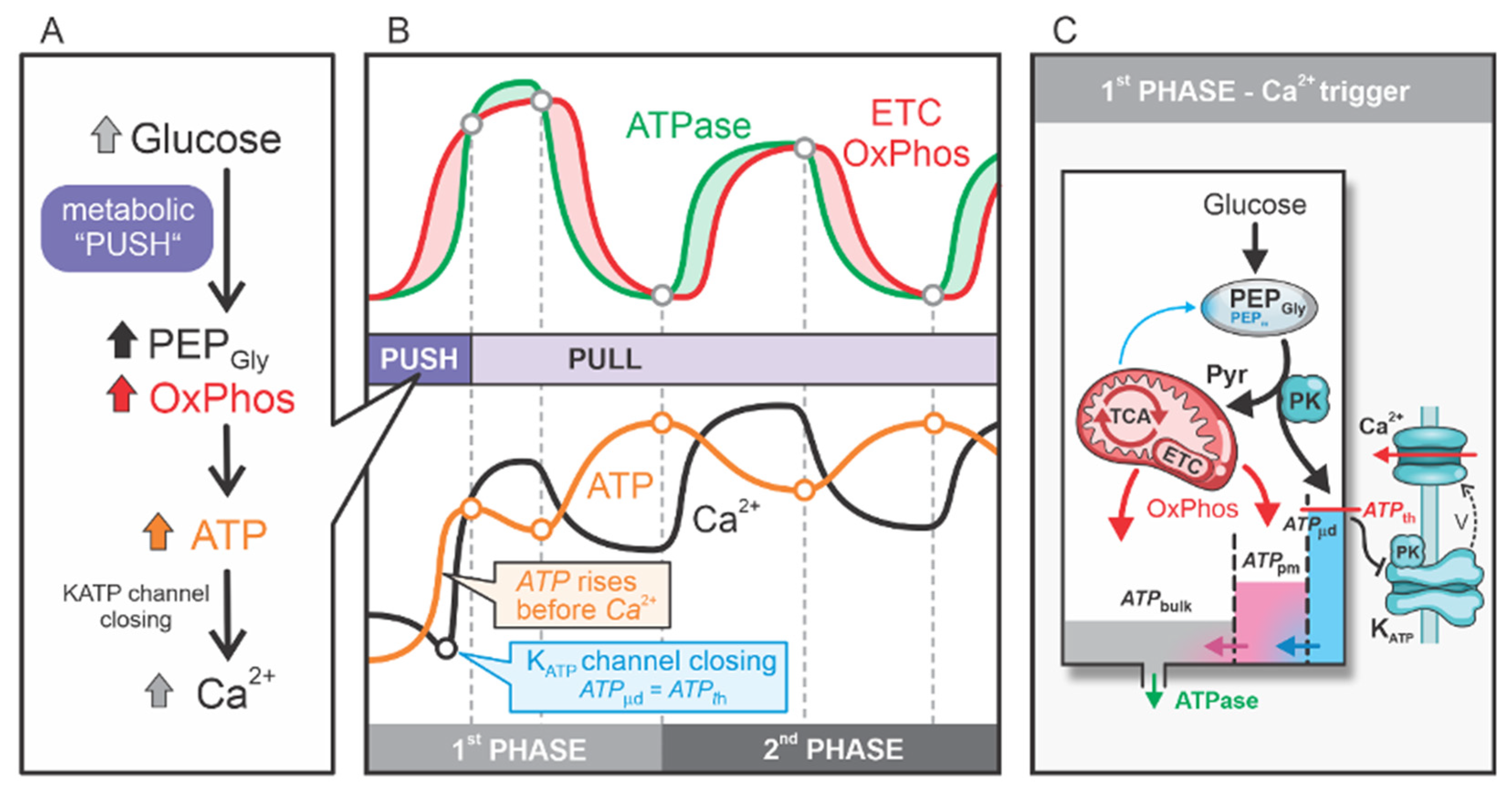
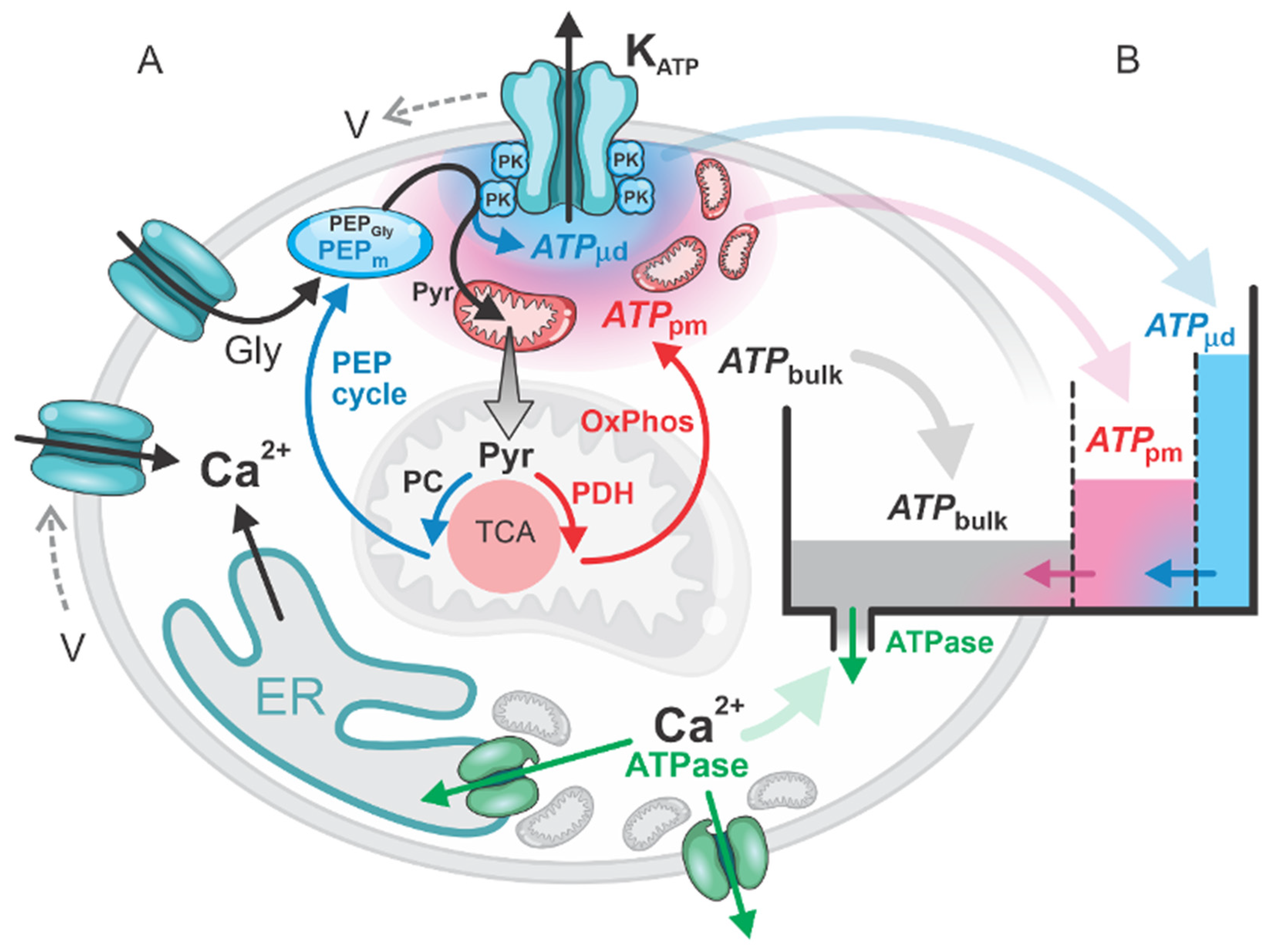
2.1. Glycolytic and Mitochondrial ATP Production in the First Phase
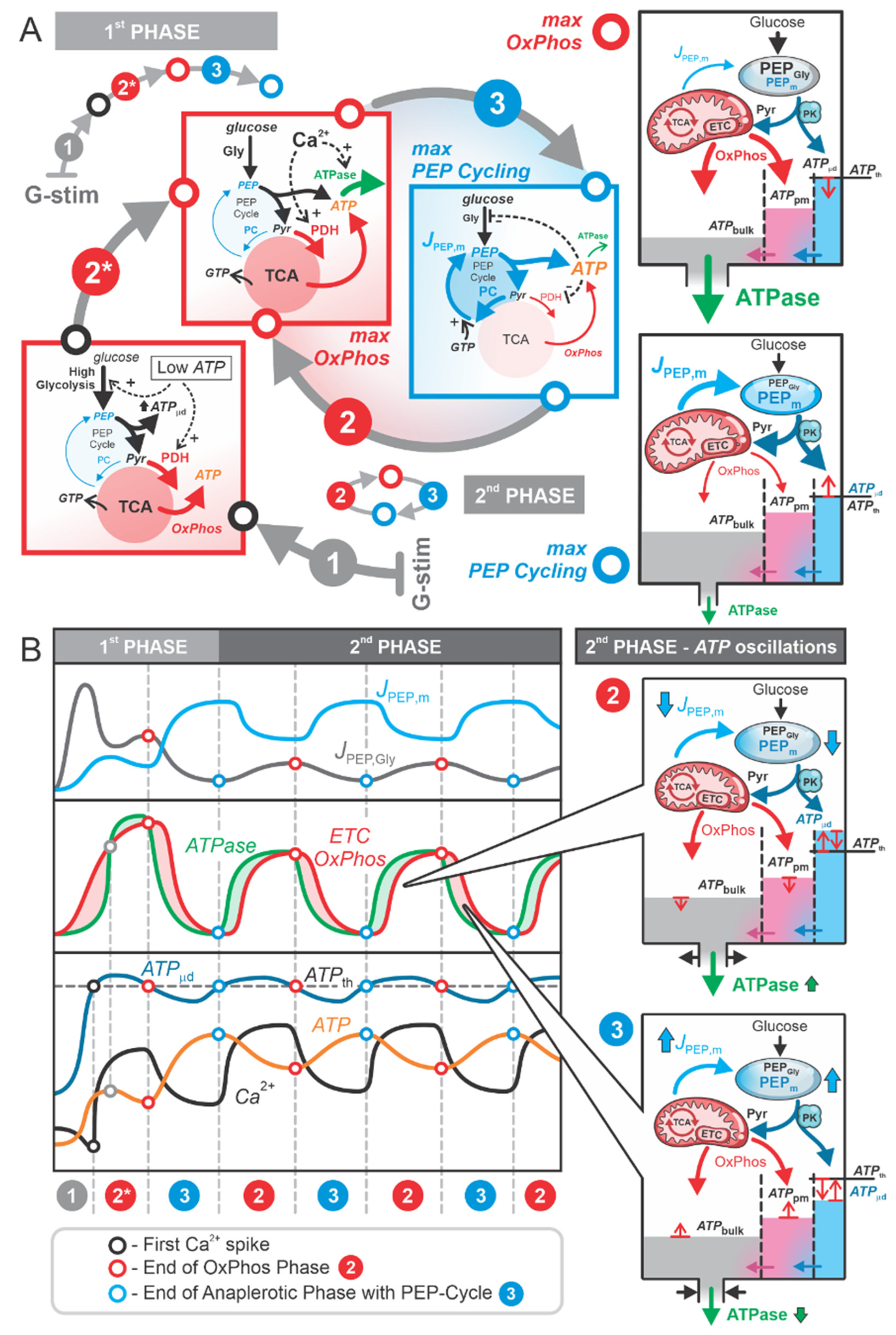
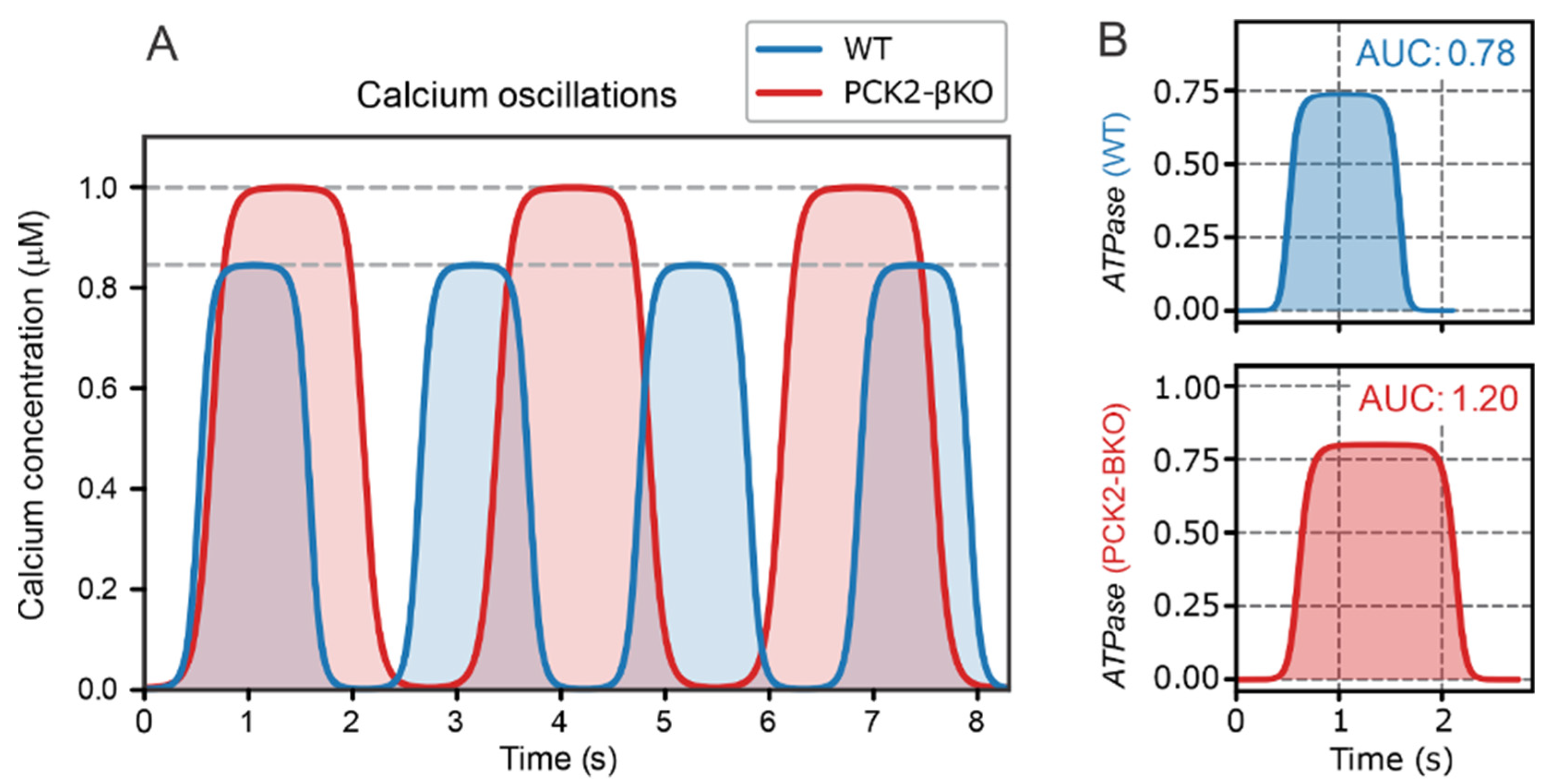
2.2. Role of PEP Cycle and Local ATP Production in the Second Phase
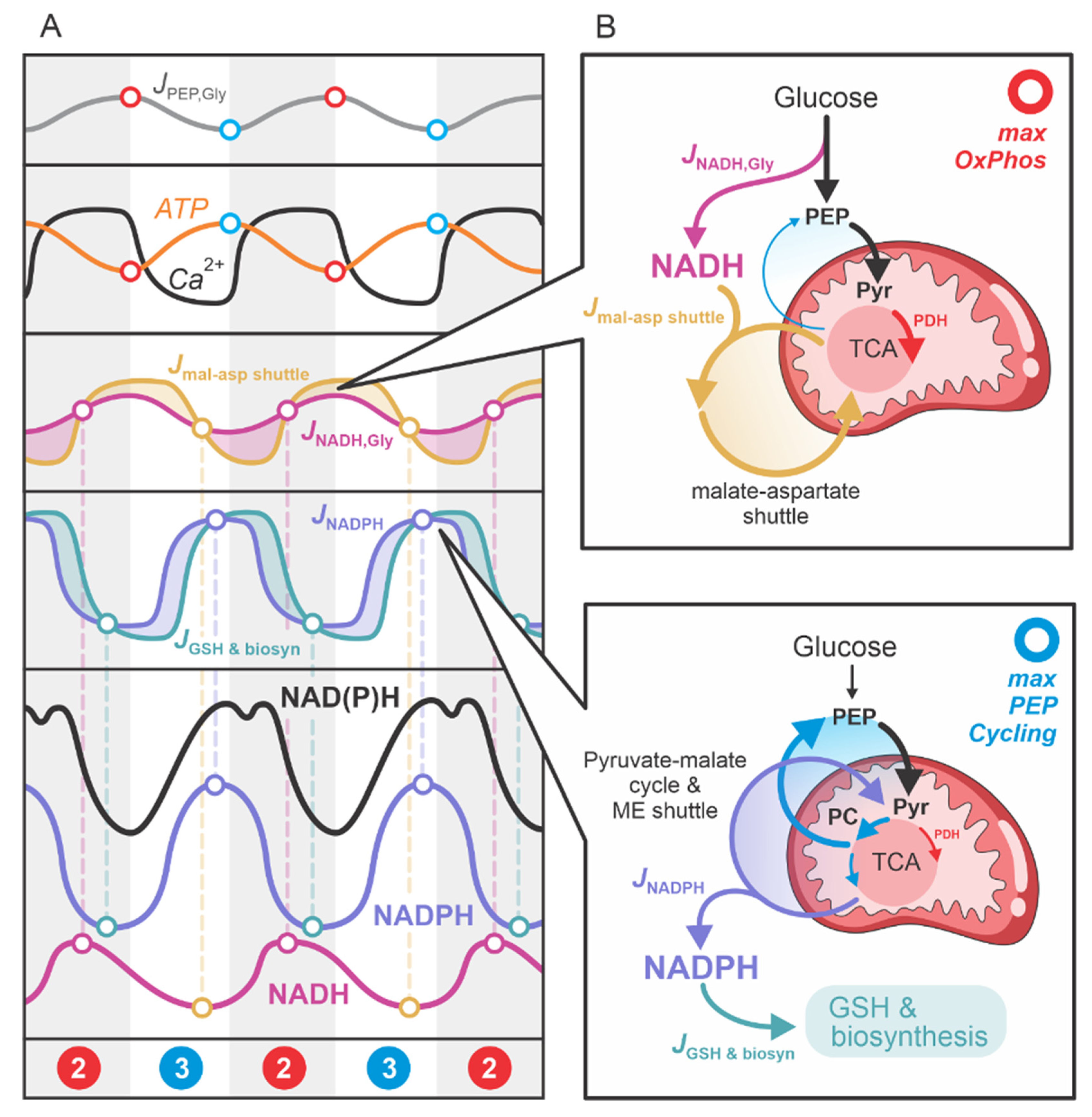
2.3. Cyclic NADH and NADPH Production in the Second Phase
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KATP | ATP-sensitive potassium channels |
| PK | Pyruvate kinase |
| PEP | Phosphoenolpyruvate |
| OAA | Oxaloacetate |
| PC | Pyruvate carboxylase |
| PCK2 | PEP carboxykinase |
| OxPhos | Oxidative phosphorylation |
| PDH | Pyruvate dehydrogenase |
| PEPGly | Glycolytic PEP |
| PEPm | Mitochondrial PEP |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| PCK2-βKO | β-cell deletion of PCK2 |
| ME | Malic enzyme |
| IDH | Isocitrate dehydrogenase |
| GSH | Glutathione |
| TCA | Tricarboxylic acid cycle |
| T2DM | Type 2 diabetes mellitus |
References
- Grubelnik, V.; Zmazek, J.; Gosak, M.; Marhl, M. The Role of Anaplerotic Metabolism of Glucose and Glutamine in Insulin Secretion: A Model Approach. Biophys. Chem. 2024, 311, 107270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shuai, H.Y.; Gylfe, E.; Tengholm, A. Oscillations of Sub-Membrane ATP in Glucose-Stimulated Beta Cells Depend on Negative Feedback from Ca2+. Diabetologia 2013, 56, 1577–1586. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Ahooghalandari, P.; Gribble, F.M.; Reimann, F.; Tengholm, A.; Gylfe, E. Submembrane ATP and Ca2+ Kinetics in α-Cells: Unexpected Signaling for Glucagon Secretion. FASEB J. 2015, 29, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.A.; El Azzouny, M.A.; Kennedy, R.T.; Burant, C.F. Metabolome Response to Glucose in the β-Cell Line INS-1 832/13. J. Biol. Chem. 2013, 288, 10923–10935. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, H.J.; Pouli, A.E.; Ainscow, E.K.; Jouaville, L.S.; Rizzuto, R.; Rutter, G.A. Glucose Generates Sub-Plasma Membrane ATP Microdomains in Single Islet β-Cells. J. Biol. Chem. 1999, 274, 13281–13291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gurlo, T.; Matveyenko, A.V.; Elashoff, D.; Wang, P.; Rosenberger, M.; Junge, J.A.; Stevens, R.C.; White, K.L.; Fraser, S.E.; et al. Live-Cell Imaging of Glucose-Induced Metabolic Coupling of β and α Cell Metabolism in Health and Type 2 Diabetes. Commun. Biol. 2021, 4, 594. [Google Scholar] [CrossRef]
- Abulizi, A.; Cardone, R.L.; Stark, R.; Lewandowski, S.L.; Zhao, X.; Hillion, J.; Ma, L.; Sehgal, R.; Alves, T.C.; Thomas, C.; et al. Multi-Tissue Acceleration of the Mitochondrial Phosphoenolpyruvate Cycle Improves Whole-Body Metabolic Health. Cell Metab. 2020, 32, 751–766.e11. [Google Scholar] [CrossRef]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020, 32, 736–750.e5. [Google Scholar] [CrossRef]
- Merrins, M.J.; Corkey, B.E.; Kibbey, R.G.; Prentki, M. Metabolic Cycles and Signals for Insulin Secretion. Cell Metab. 2022, 34, 947–968. [Google Scholar] [CrossRef]
- Stark, R.; Kibbey, R.G. The Mitochondrial Isoform of Phosphoenolpyruvate Carboxykinase (PEPCK-M) and Glucose Homeostasis: Has It Been Overlooked? Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.R.; Ho, T.; Potapenko, E.; Sdao, S.M.; Huang, S.M.; Lewandowski, S.L.; VanDeusen, H.R.; Davidson, S.M.; Cardone, R.L.; Prentki, M.; et al. β-Cell Deletion of the PKm1 and PKm2 Isoforms of Pyruvate Kinase in Mice Reveals Their Essential Role as Nutrient Sensors for the KATP Channel. Elife 2022, 11, e79422. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Guebre-Egziabher, F.; Zhao, X.; Feriod, C.; Dong, J.; Alves, T.C.; Ioja, S.; Pongratz, R.L.; Bhanot, S.; Roden, M.; et al. A Role for Mitochondrial Phosphoenolpyruvate Carboxykinase (PEPCK-M) in the Regulation of Hepatic Gluconeogenesis. J. Biol. Chem. 2014, 289, 7257–7263. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Pasquel, F.; Turcu, A.; Pongratz, R.L.; Roden, M.; Cline, G.W.; Shulman, G.I.; Kibbey, R.G. Phosphoenolpyruvate Cycling via Mitochondrial Phosphoenolpyruvate Carboxykinase Links Anaplerosis and Mitochondrial GTP with Insulin Secretion. J. Biol. Chem. 2009, 284, 26578–26590. [Google Scholar] [CrossRef] [PubMed]
- Merrins, M.J.; Kibbey, R.G. Glucose Regulation of β-Cell KATP Channels: It Is Time for a New Model! Diabetes 2024, 73, 856–863. [Google Scholar] [CrossRef]
- Rutter, G.A.; Sweet, I.R. Glucose Regulation of β-Cell KATP Channels: Is a New Model Needed? Diabetes 2024, 73, 849–855. [Google Scholar] [CrossRef]
- Satin, L.S.; Corradi, J.; Sherman, A.S. Do We Need a New Hypothesis for KATP Closure in β-Cells? Distinguishing the Baby From the Bathwater. Diabetes 2024, 73, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.L.; Hales, N. Intracellular ATP Directly Blocks K+ Channels in Pancreatic B-Cells. Nature 1984, 311, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Vivaudou, M.; Moreau, C.J.; Terzic, A. ATP-Sensitive K+ Channels. In Ion Channels: From Structure to Function; Kew, J., Davies, C., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 454–473. [Google Scholar]
- Grubelnik, V.; Zahle Larsen, A.; Kummer, U.; Folke Olsen, L.; Marhl, M. Mitochondria Regulate the Amplitude of Simple and Complex Calcium Oscillations. Biophys. Chem. 2001, 94, 59–74. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Ciccarelli, G.; Soldovieri, L.; Capece, U.; Cefalo, C.M.A.; Moffa, S.; Nista, E.C.; Brunetti, M.; Cinti, F.; Gasbarrini, A.; et al. First-Phase Insulin Secretion: Can Its Evaluation Direct Therapeutic Approaches? Trends Endocrinol. Metab. 2023, 34, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Merrins, M.J.; Poudel, C.; McKenna, J.P.; Ha, J.; Sherman, A.; Bertram, R.; Satin, L.S. Phase Analysis of Metabolic Oscillations and Membrane Potential in Pancreatic Islet β-Cells. Biophys. J. 2016, 110, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-K.; Kauri, L.M.; Qian, W.-J.; Kennedy, R.T. Correlated Oscillations in Glucose Consumption, Oxygen Consumption, and Intracellular Free Ca2+ in Single Islets of Langerhans. J. Biol. Chem. 2000, 275, 6642–6650. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Tiengo, A. The Importance of First-Phase Insulin Secretion: Implications for the Therapy of Type 2 Diabetes Mellitus. Diabetes Metab. Res. Rev. 2001, 17, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Grubelnik, V.; Markovič, R.; Lipovšek, S.; Leitinger, G.; Gosak, M.; Dolenšek, J.; Valladolid-Acebes, I.; Berggren, P.-O.; Stožer, A.; Perc, M.; et al. Modelling of Dysregulated Glucagon Secretion in Type 2 Diabetes by Considering Mitochondrial Alterations in Pancreatic α-Cells. R. Soc. Open Sci. 2020, 7, 191171. [Google Scholar] [CrossRef] [PubMed]
- Grubelnik, V.; Zmazek, J.; Markovič, R.; Gosak, M.; Marhl, M. Mitochondrial Dysfunction in Pancreatic Alpha and Beta Cells Associated with Type 2 Diabetes Mellitus. Life 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, S.; Funcke, J.-B.; Straub, L.G.; Pirro, V.; Emont, M.P.; Droz, B.A.; Collins, K.A.I.; Joung, C.; Pearson, M.J.; et al. The GIP Receptor Activates Futile Calcium Cycling in White Adipose Tissue to Increase Energy Expenditure and Drive Weight Loss in Mice. Cell Metab. 2025, 37, 187–204.e7. [Google Scholar] [CrossRef] [PubMed]
- Polino, A.J.; Sviben, S.; Melena, I.; Piston, D.W.; Hughes, J.W. Scanning Electron Microscopy of Human Islet Cilia. Proc. Natl. Acad. Sci. USA 2023, 120, e2302624120. [Google Scholar] [CrossRef] [PubMed]
- Gregg, T.; Sdao, S.M.; Dhillon, R.S.; Rensvold, J.W.; Lewandowski, S.L.; Pagliarini, D.J.; Denu, J.M.; Merrins, M.J. Obesity-Dependent CDK1 Signaling Stimulates Mitochondrial Respiration at Complex I in Pancreatic β-Cells. J. Biol. Chem. 2019, 294, 4656–4666. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Jabůrek, M.; Holendová, B.; Tauber, J.; Pavluch, V.; Berková, Z.; Cahová, M.; Schröder, K.; Brandes, R.P.; Siemen, D.; et al. Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes 2020, 69, 1341–1354. [Google Scholar] [CrossRef]
- Roma, L.P.; Jonas, J.-C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and β-Cells. J. Mol. Biol. 2020, 432, 1461–1493. [Google Scholar] [CrossRef]
- Bensellam, M.; Duvillié, B.; Rybachuk, G.; Laybutt, D.R.; Magnan, C.; Guiot, Y.; Pouysségur, J.; Jonas, J.-C. Glucose-Induced O2 Consumption Activates Hypoxia Inducible Factors 1 and 2 in Rat Insulin-Secreting Pancreatic Beta-Cells. PLoS ONE 2012, 7, e29807. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.; Van Vleck, A.; Mawla, A.M.; Li, A.M.; Ye, J.; Huising, M.O.; Annes, J.P. β-Cell Succinate Dehydrogenase Deficiency Triggers Metabolic Dysfunction and Insulinopenic Diabetes. Diabetes 2022, 71, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cai, X.; Liu, T.; Zou, J.; Wang, L.; Wang, G.; Liu, Y.; Ding, X.; Zhang, B.; Sun, P.; et al. Hypoxia-Inducible Factor-1α Mediates the Expression of Mature β Cell-Disallowed Genes in Hypoxia-Induced β Cell Dedifferentiation. Biochem. Biophys. Res. Commun. 2020, 523, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Marhl, M. What Do Stimulated Beta Cells Have in Common with Cancer Cells? BioSystems 2024, 242, 105257. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry, 5th ed.; W. H. Freeman Publishing: New York, NY, USA, 2002. [Google Scholar]
- Marinelli, I.; Thompson, B.M.; Parekh, V.S.; Fletcher, P.A.; Gerardo-Giorda, L.; Sherman, A.S.; Satin, L.S.; Bertram, R. Oscillations in K(ATP) Conductance Drive Slow Calcium Oscillations in Pancreatic β-Cells. Biophys. J. 2022, 121, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.T.; Kauri, L.M.; Dahlgren, G.M.; Jung, S.-K. Metabolic Oscillations in β-Cells. Diabetes 2002, 51, S152–S161. [Google Scholar] [CrossRef]
- Jesinkey, S.R.; Madiraju, A.K.; Alves, T.C.; Yarborough, O.H.; Cardone, R.L.; Zhao, X.; Parsaei, Y.; Nasiri, A.R.; Butrico, G.; Liu, X.; et al. Mitochondrial GTP Links Nutrient Sensing to β Cell Health, Mitochondrial Morphology, and Insulin Secretion Independent of OxPhos. Cell Rep. 2019, 28, 759–772.e10. [Google Scholar] [CrossRef] [PubMed]
- Corradi, J.; Thompson, B.; Fletcher, P.A.; Bertram, R.; Sherman, A.S.; Satin, L.S. KATP Channel Activity and Slow Oscillations in Pancreatic Beta Cells Are Regulated by Mitochondrial ATP Production. J. Physiol. 2023, 601, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubelnik, V.; Zmazek, J.; Marhl, M. The Synergistic Impact of Glycolysis, Mitochondrial OxPhos, and PEP Cycling on ATP Production in Beta Cells. Int. J. Mol. Sci. 2025, 26, 1454. https://doi.org/10.3390/ijms26041454
Grubelnik V, Zmazek J, Marhl M. The Synergistic Impact of Glycolysis, Mitochondrial OxPhos, and PEP Cycling on ATP Production in Beta Cells. International Journal of Molecular Sciences. 2025; 26(4):1454. https://doi.org/10.3390/ijms26041454
Chicago/Turabian StyleGrubelnik, Vladimir, Jan Zmazek, and Marko Marhl. 2025. "The Synergistic Impact of Glycolysis, Mitochondrial OxPhos, and PEP Cycling on ATP Production in Beta Cells" International Journal of Molecular Sciences 26, no. 4: 1454. https://doi.org/10.3390/ijms26041454
APA StyleGrubelnik, V., Zmazek, J., & Marhl, M. (2025). The Synergistic Impact of Glycolysis, Mitochondrial OxPhos, and PEP Cycling on ATP Production in Beta Cells. International Journal of Molecular Sciences, 26(4), 1454. https://doi.org/10.3390/ijms26041454







