Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications
Abstract
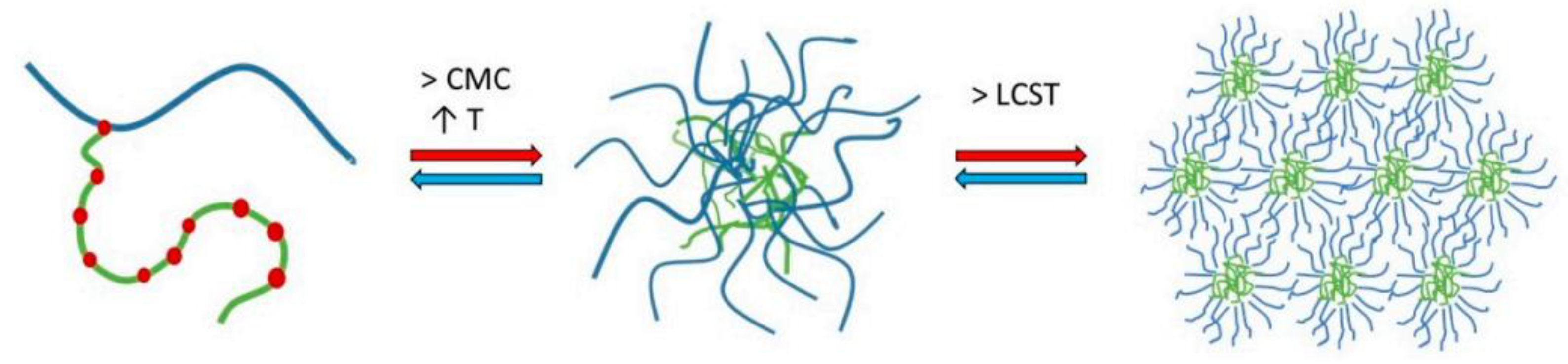
1. Introduction
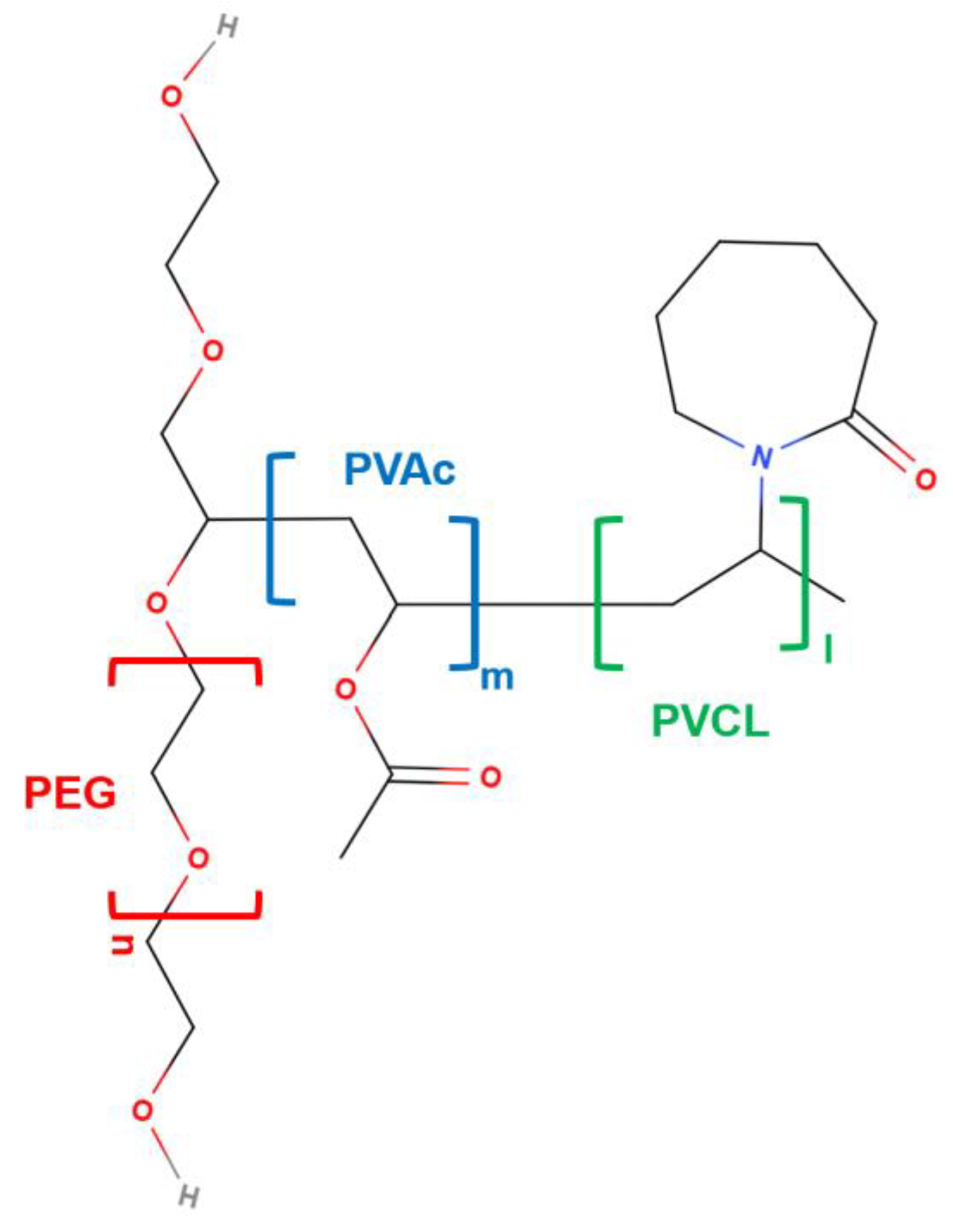
2. Physicochemical Features of SLP
3. SLP Formulation Methods
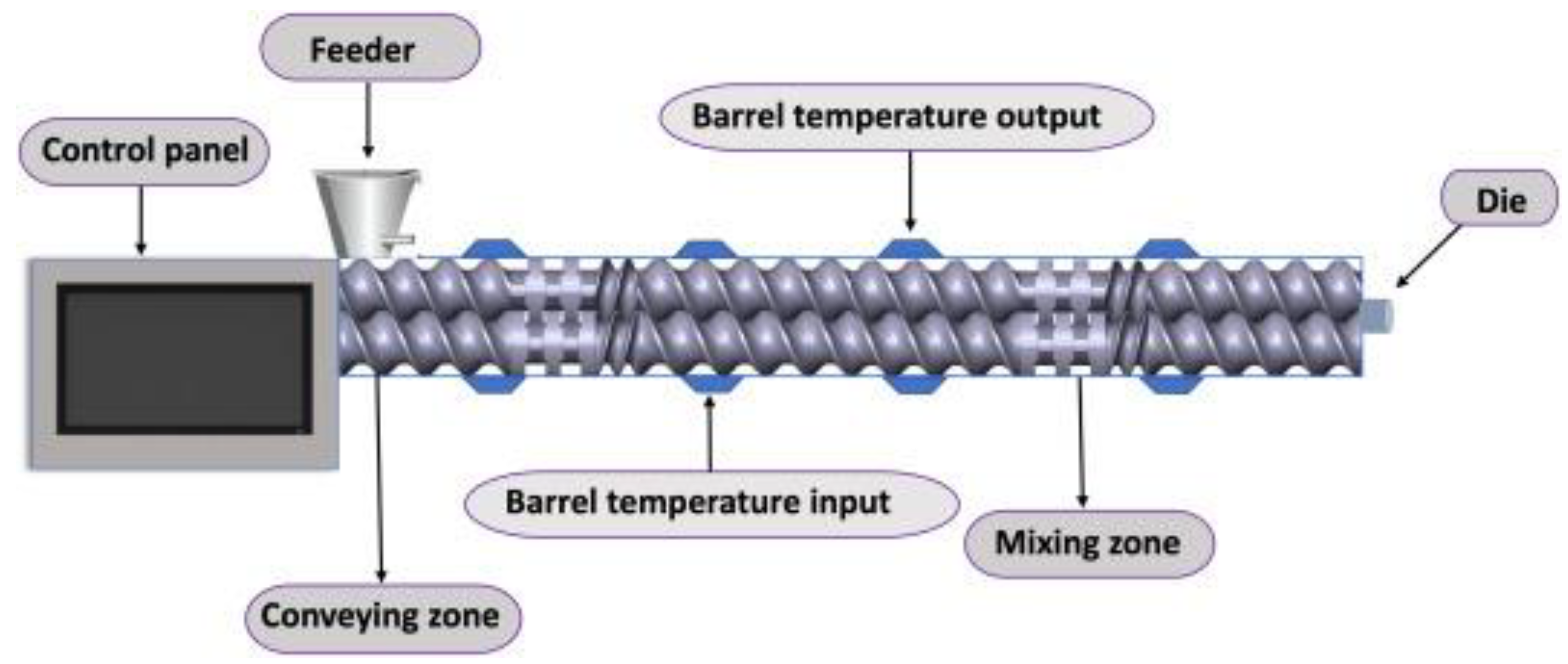
3.1. Hot-Melt Extrusion
3.2. Spray Drying
3.3. Electrospinning
3.4. Drug–Polymer Layering
3.5. Capsule Formulation
3.6. Tablet Formulation
4. Applications of SLP in Biomedicine
4.1. Antitumoral Applications
| Drug | Formulation Type of SLP | Role of SLP in the Formulation | Disease or Application | Reference |
|---|---|---|---|---|
| Albendazole and paclitaxel | SLP, TPGS, and folic acid mixed micelles | Helps in the stability and permits the sustained release of the drugs | Ovarian cancer | [94] |
| β-ionone | SLP matrix | Increases the solubility and bioavailability of the drug | Many forms of cancer | [88] |
| Betulinic acid | SLP micelles | Increases the solubility and bioavailability of the drug | Breast cancer | [95] |
| Brigatinib | SLP and TPGS mixed micelles | Increases the solubility and bioavailability of the drug | Lung cancer (non-small cell lung carcinoma, NSCLC) | [96] |
| Camptothecin analog FLQY2 | SLP micelles produced by solvent evaporation | Increases the solubility and bioavailability of the drug | Solid tumors | [97] |
| Chrysin | SLP and TPGS micelles prepared by solvent evaporation | Increases the solubility and bioavailability of the drug | Hepatocellular carcinoma and anti-inflammatory response | [98] |
| CPD 23 | SLP micelles | Acts as a carrier and enhances the pharmacokinetics of this drug, augmenting its stability in blood | Kidney tumors | [99] |
| Curcumin and piperine | SLP solid dispersion prepared via HME | Increases the solubility and bioavailability of the drugs | Antitumoral and anti-inflammatory properties | [40] |
| DHA-S-CA | SLP and TPGS nanomicelles | Increases the solubility and bioavailability of the drug | Lung cancer cells | [100] |
| Dioscorea bulbifera extracts | SLP + poloxamer F127 films produced by thin-film dispersion | Increases the solubility of the drug and enhances cytotoxicity in tumor cells | Apoptosis of tumors | [83] |
| Docetaxel | SLP and poloxamer F108 micelles produced by spray drying | Helps in the stability and permits the sustained release of the drug | Melanoma | [33] |
| Docetaxel and curcumin | SLP and TPGS mixed micelles | Permits the sustained release of the drugs | Breast cancer | [101] |
| Everolimus | Electrosprayed SLP-PVA | Helps in the stability and improves the targeting of the drug | Cancer therapy | [91] |
| Everolimus | Electrosprayed SLP-PVA | Helps in the stability and improves the targeting of the drug | Brain tumors | [92] |
| Gemcitabine and vitamin E succinate | SLP micelles | Helps in the stability and enhances the affinity of the drugs | Pancreatic cancer | [102] |
| Hexyselen (CPD-3B) | SLP micelles | Increases the solubility of the drug | Kidney tumors | [82] |
| Luteolin | SLP amorphous solid dispersions | Increases the solubility and bioavailability of the drug | Antioxidant, antimicrobial, anti-allergic, cardio-protective, and anti-cancer activities | [86] |
| Magestrol acetate | SLP and Cremophor® RH 40 mixed micelles | Acts as a carrier and stabilizes the drug | Endometrial and breast cancers and HIV infections | [85] |
| Naringin | SLP, poloxamer 188, Kollidon® VA30 and Kollidon® VA64 solid dispersions prepared by freeze-drying | Increases the solubility and bioavailability of the drug | Neuroblastoma, antibacterial properties | [103] |
| Olaparib | SLP matrix prepared by antisolvent precipitation | Helps in the stability of the drug and enhances cytotoxicity in tumor cells | Various types of cancer | [104] |
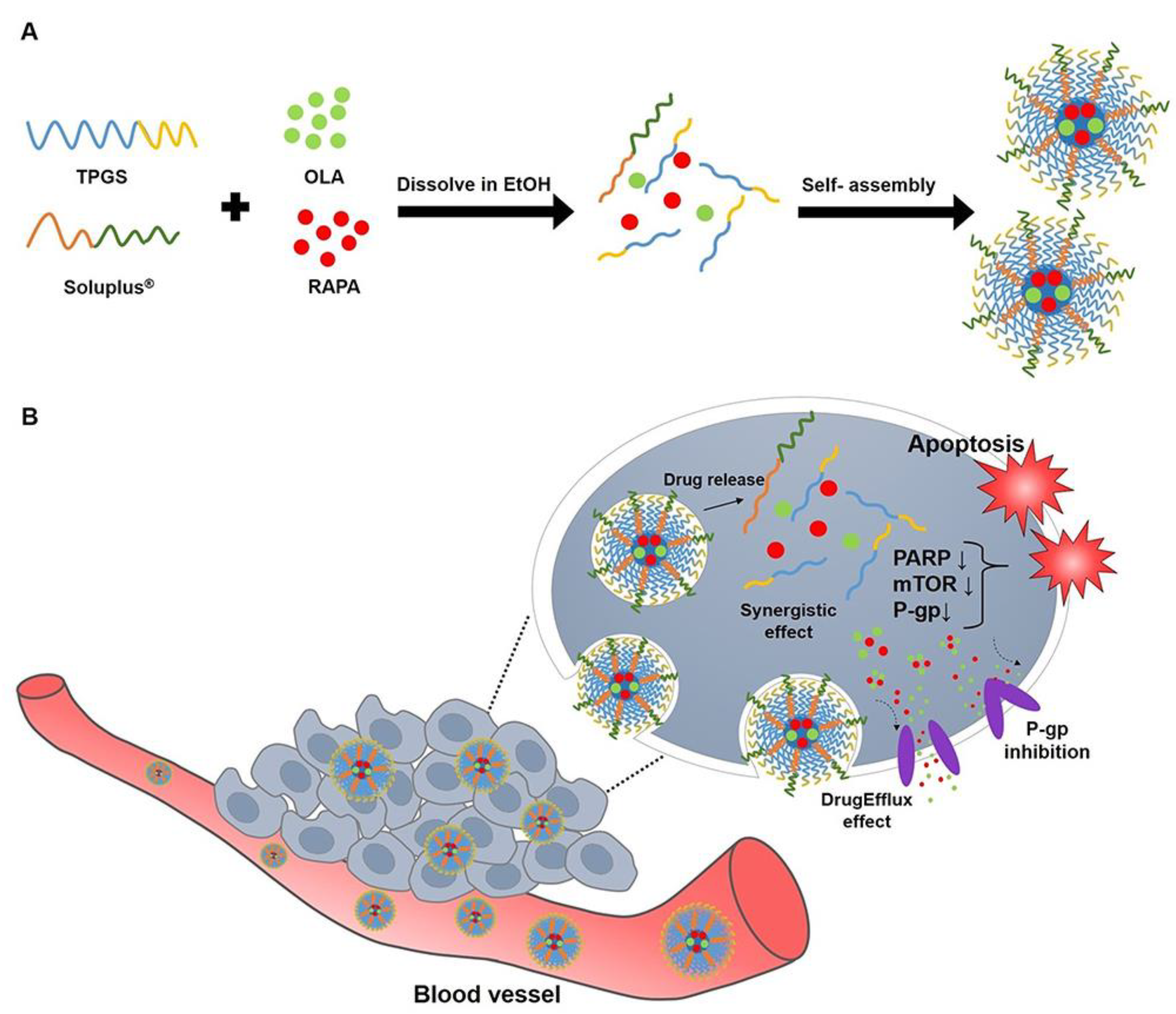
| Olaparib and rapamycin | SLP and TPGS mixed micelles | Helps in the stability of the drugs | Ovarian cancer | [87] |
| Paclitaxel | SLP + poloxamer 407 + Tween 80 stabilizers for lipid nanoparticles | Helps in the stability of the drug | Many types of tumors | [81] |
| Paclitaxel | Glycosylated-SLP and TPGS matrix | Increases the solubility and bioavailability of the drug | Glioblastoma cell lines | [105] |
| Paclitaxel | SLP nanomicelles | Increases the solubility of the drug | Breast cancer (triple negative breast cancer, TNBC) | [106] |
| Paclitaxel and curcumin | SLP and TPGS matrix | Acts as a matrix and carrier for the delivery of these drugs | Breast and ovarian cancers | [107] |
| Paclitaxel and resveratrol | SLP nanoparticles produced by thin-film hydration | Increases the bioavailability of the drugs | Glioma | [108] |
| Quercetin | Polymeric mixed micelles of SLP, vitamin E, TPGS, and poloxamer 407 | Acts as a matrix | Human U87MG glioma cells | [109] |
| Quercetin | SLP micelles produced by thin-film hydration | Increases the solubility of the drug and reduces off-target toxic effects | Tumor angiogenesis | [110] |
| Radiolabeled bevacizumab | SLP micelles and SLP-TPGS mixed micelles | Allows the targeting and imaging | Breast and colon cancers | [93] |
| Silymarin | SLP, Kollidon® VA64, and poloxamer 188 solid dispersions prepared by solvent evaporation, microwave irradiation, and freeze-drying | Increases the solubility of the drug and enhances cytotoxicity in tumor cells | Lung cancer | [111] |
| Simvastatin | SLP nanosuspension encapsulated in Eudragit® and ethyl cellulose | Increases the solubility of the drug | Colorectal cancer | [84] |
| Tamoxifen citrate | SLP and chitosan nanoparticles | Increases the targeting and therapeutic effect | Breast cancer | [89] |
| Usnic acid | SLP + TPGS + Solutol® HS15 micelles produced by freeze-drying | Reduces cell migration and enhances the stability and activity of the drug | Human SH-SY5Y neuroblastoma cells | [90] |
4.2. Anti-Inflammatory Applications
| Drug | Formulation Type of SLP | Role of SLP in the Formulation | Disease or Application | Reference |
|---|---|---|---|---|
| 18β-glycyrrhetinic acid | SLP + sodium carboxymethyl cellulose hydrogels | Increases the solubility and permits the sustained release of the drug | Inflammation in wound treatment | [113] |
| Aloe emodin | SLP and glycyrrhizic acid micelles prepared by thin-film hydration | Increases the solubility and bioavailability of the drug | Gouty arthritis (hyperuricemia) | [37] |
| Atorvastatin | SLP solid dispersion prepared by a super critical fluid technology | Increases the solubility and bioavailability and permits the sustained release of the drug | Inflammatory Bowel Disease and Irritable Bowel Syndrome | [118] |
| Budesonide | Microcontainers of polycaprolactone (PCL) coated by SLP films | Permits the amorphous state maintenance and the sustained release of the drug | Inflammatory Bowel Disease | [65] |
| Chrysin | SLP and TPGS micelles prepared by solvent evaporation | Increases the solubility and bioavailability of the drug | Hepatocellular carcinoma and anti-inflammatory response | [98] |
| Colchicine | SLP microarray patches | Acts as a matrix and carrier for the delivery of the drug and permits a sustained release | Gout | [119] |
| Curcumin | Solid dispersion formed with SLP, Syloid®, poloxamer 188 and HPMC E5 | Increases the solubility and bioavailability of the drug and enhances its activity | Anti-inflammatory and antimicrobial responses | [120] |
| Curcumin and piperine | SLP solid dispersion prepared via HME | Increases the solubility and bioavailability of the drugs | Antitumoral and anti-inflammatory properties | [40] |
| Etoricoxib | SLP produced by HME and 3D-printed tablets | Increases the solubility and permits the sustained release of the drug | Anti-inflammatory properties | [117] |
| Flurbiprofen | Pseudopolyrotaxane preparation upon the mixing of SLP micelles and cyclodextrins | Permits the sustained release of the drug | Anterior uveitis (eye inflammation) | [121] |
| Glycyrrhetinic acid and L-arginine | SLP solid dispersion | Increases the solubility and bioavailability of the drugs | Anti-inflammatory activity for gastric ulcers | [112] |
| Ivermectin | SLP microarray patches | Promotes the mechanical robustness and increases the solubility of the drug | Rosacea disease | [122] |
| Ketoprofen | SLP tablets prepared by wet granulation | Increases the solubility and bioavailability of the drug | Anti-inflammatory properties | [80] |
| Mefenamic acid | SLP + sorbitol matrix produced via HME | Helps in the stability of the drug | Anti-inflammatory properties | [114] |
| Meloxicam | SLP and poloxamer F127 prepared by fusion and HME | Increases the solubility of the drug | Anti-inflammatory activity on RAW macrophages | [123] |
| Meloxicam | Electrospun nanofibers of SLP and SLS for tablet formulations | Increases the solubility of the drug | Anti-inflammatory activity | [61] |
| Meloxicam | SLP micelles for nasal administration | Promotes the transport to the CNS | Anti-inflammatory activity for brain applications | [115] |
| Narasin | Self-nanomicellizing solid dispersions of SLP in the form of a gel | Permits the skin penetration of the drug and increases its solubility | Anti-inflammatory response for acne and activity against antimicrobial-resistant strains of Cutibacterium acnes | [124] |
| Phloretin | SLP amorphous solid dispersion | Increases the solubility and bioavailability of the drug | NAFLD | [116] |
| Pterostilbene | SLP and poloxamer 188 mixed micelles | Increases the solubility and bioavailability of the drug | Anti-inflammatory properties in acetaminophen-induced acute liver injury | [125] |
4.3. Antimicrobial and Antiparasitic Applications
| Drug | Formulation Type of SLP | Role of SLP in the Formulation | Disease or Application | Reference |
|---|---|---|---|---|
| Albendazole and mebendazole | SLP matrix | Prevents drug precipitation | Helminthiasis | [128] |
| Arteether | SLP capsules | Increases the solubility and bioavailability of the drug | Malaria | [70] |
| Buddleja globosa Hope extracts | Spray-dried SLP or PVP | Increases the solubility of the drug and produces enhanced antimicrobial properties | Skin and gastric ulcers, as it has activity against Pseudomonas aeruginosa | [134] |
| Carbonitrile derivatives (LN002) | SLP and hydroxypropyl-β-cyclodextrin solid dispersions | Increases the solubility and bioavailability of the drug | Activity against Cryptosporidium | [131] |
| Carbothioamide derivatives (LQIT/LT-50) | Solid dispersions made of SLP, PVP K-30, and PEG | Increases the solubility of the drug and enhances its activity | Schistosomiasis | [130] |
| Ciprofloxacin | SLP, PVA, and PEG films prepared by solvent casting | Allows effective drug delivery to the eye and improves corneal and conjunctival permeation | Eye infection | [135] |
| Curcumin | Solid dispersion formed with SLP, Syloid®, poloxamer 188 and HPMC E5 | Increases the solubility and bioavailability of the drug and enhances its activity | Anti-inflammatory and antimicrobial responses | [120] |
| Decoquinate | SLP nanoparticles prepared by HME | Increases the solubility and bioavailability of the drug | Malaria | [136] |
| Dexamethasone and tobramycin | SLP and TPGS micelles embedded in a poloxamer 407 gel for intranasal administration | Enhances the kinetics of dexamethasone and permits a sustained release of tobramycin | Nasal rhinosinusitis | [133] |
| Emamectin benzoate | Spray-dried SLP or sodium alginate prepared by ionic gelation | Increases the solubility of the drug, although the best results were obtained with the sodium alginate solid dispersion | Antiparasitic activity against Caligus rogercresseyi | [127] |
| Lumefantrine | SLP, HPC, and poloxamer F68 matrix combined with piperine | Increases the solubility and bioavailability of the drug | Malaria | [126] |
| Luteolin | SLP amorphous solid dispersions | Increases the solubility and bioavailability of the drug | Antioxidant, antimicrobial, anti-allergic, cardio-protective, and anti-cancer activities | [86] |
| Mebendazole | Spray-dried SLP micelles | Increases the solubility and bioavailability of the drug and enhances its bioabsorption | Infection from roundworms (pinworms and hookworms), trichinosis, capillariasis, and toxocariasis | [48] |
| Metronidazole | Microarray patches of SLP | Augments skin permeation of the drug | Skin and soft tissue infections, as it has activity against Bacteroides fragilus | [137] |
| Narasin | Self-nanomicellizing solid dispersions of SLP in the form of a gel | Permits the skin penetration of the drug and increases its solubility | Anti-inflammatory response for acne and activity against antimicrobial-resistant strains of Cutibacterium acnes | [124] |
| Naringin | SLP, poloxamer 188, Kollidon® VA30 and Kollidon® VA64 solid dispersions prepared by freeze-drying | Increases the solubility and bioavailability of the drug | Neuroblastoma, antibacterial properties | [103] |
| Rifampicin and curcumin | SLP nanomicelles | Allows the drug delivery for an inhalable formulation | Tuberculosis | [138] |
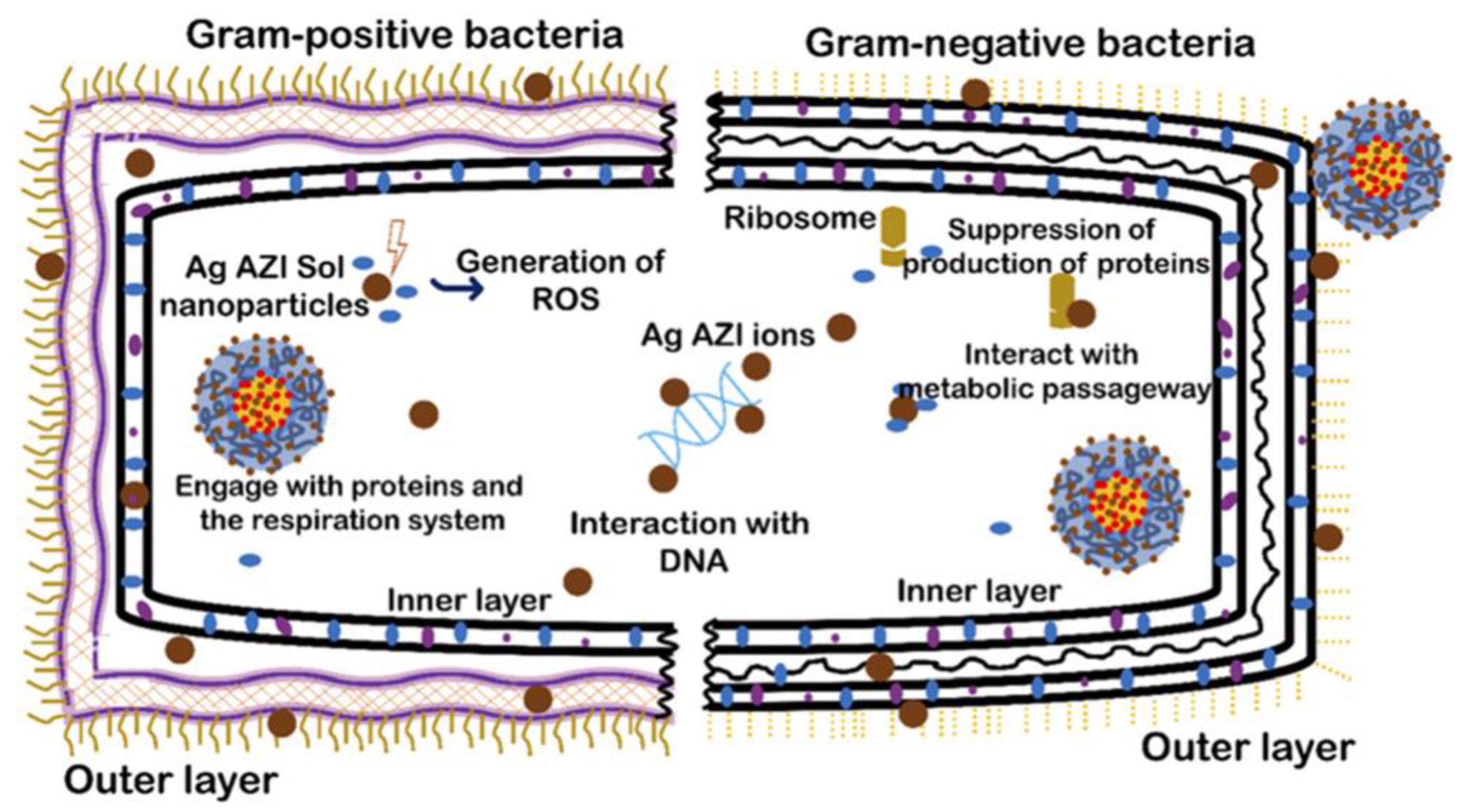
| Silver-decorated azithromycin | SLP nanoparticles developed by controlled emulsion diffusion | Permits the sustained release of the drug for continuous antibacterial efficacy | Antibacterial efficacy against Escherichia coli and Staphylococcus epidermidis | [132] |
| Thiazolidine derivatives (LPSF/GQ-238) | Solid dispersions made of SLP, PVP K-30, and PEG | Increases the solubility of the drug | Schistosomiasis | [129] |
4.4. Other Biomedical Applications
| Drug | Formulation Type of SLP | Role of SLP in the Formulation | Disease or Application | Reference |
|---|---|---|---|---|
| Agomelatine | Intranasal gel containing SLP, hydroxypropyl-β-cyclodextrin and poloxamer 188 | Enhances the drug efficacy | Depression | [143] |
| Bortezomib and lenalidomide | SLP solutions | Enhances HSCs growth | Modification of the culture medium for transplantations | [144] |
| Carvedilol and curcumin | SLP micelles | Increases the solubility and optimizes the therapeutic potential of the drug | Hypertension | [145] |
| Curcumin | SLP micelles produced by thin-film hydration | Increases the solubility and bioavailability of the drug | Alcohol-use disorders | [146] |
| Ketamine hydrochloride | SLP and Eudragit® prepared via HME and then formulated in tablets | Permits the sustained release of the drug | Refractory depression and chronic pain | [42] |
| Polydatin (Polygoni cuspidati extracts) | SLP solid dispersion prepared by HME and then formulated into tablets with HPMC | Increases the muco-adhesivity and enhances the kinetics of the drug | Buccal applications | [141] |
| Quercetin | SLP microarray patches | Increases the solubility and bioavailability of the drug | Fibrosis lowering, scar formation limitation, and fibroblast proliferation | [147] |
| Sildenafil | SLP, keratin, and merwinite scaffolds formed via electrospinning | Enhances the osteogenic and angiogenic capacities and allows a robust structure | Bone tissue regeneration | [142] |
| Tacrolimus | Eye drop formulation formed by Zein-SLP nanoparticles and hydroxypropyl-β-cyclodextrin | Increases the solubility and bioavailability of the drug | Retinal diseases | [148] |
| No drug was used in this study | SLP nanomicelles | Allows the encapsulation of proteins. Some model proteins employed were bovine serum albumin (BSA), lysozyme, and bovine hemoglobin (BHb) | Allows the encapsulation of drugs for their delivery | [139] |
| No drug was used in this study | SLP solutions | Permits the long-term ex vivo expansion of HSCs | Hematological diseases | [140] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ammar, H.; Salama, H.; Ghorab, M.; Mahmoud, A. Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems. Int. J. Pharm. 2006, 309, 129–138. [Google Scholar] [CrossRef]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Ehmad-Eldin, M.; Ghazy, F.E.S.; Chopra, H.; Ibrahum, T.M. Soluplus® as a Solubilizing Excipient for Poorly Water-Soluble Drugs: Recent Advances in Formulation Strategies and Pharmaceutical Product Features. J. Drug Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Prajapati, B.G.; Patel, M.M. Conventional and alternative pharmaceutical methods to improve oral bioavailability of lipophilic drugs. Asian J. Pharm. 2007, 1, 1–8. [Google Scholar]
- Rane, Y.; Mashru, R.; Sankalia, M.; Sankalia, J. Effect of hydrophilic swellable polymers on dissolution enhancement of carbamazepine solid dispersions studied using response surface methodology. AAPS PharmSciTech 2007, 8, E1–E11. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Murthy, P.N.; Biswal, S.; Mahapatra, A.P.K.; Pradhan, S.P. Dissolution enhancement and physicochemical characterization of valsartan in solid dispersions with β-CD, HP β-CD, and PVP K-30. Dissolution Technol. 2011, 18, 39–45. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Kollidon® VA 64 and Soluplus® as modern polymeric carriers for amorphous solid dispersions. Polim. Med. 2022, 52, 19–29. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Srivastava, B.; Rai, A.K. Solid dispersions: An overview to modify bioavailability of poorly water soluble drugs. Int. J. PharmTech Res. 2009, 1, 1338–1349. [Google Scholar]
- Attia, M.S.; Yahya, A.; Monaem, N.A.; Sabry, S.A. Mesoporous silica nanoparticles: Their potential as drug delivery carriers and nanoscavengers in Alzheimer’s and Parkinson’s diseases. Saudi Pharm. J. 2023, 31, 417–432. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. B 2018, 8, 733–755. [Google Scholar] [CrossRef]
- Leonida, M.; Ispas-Szabo, P.; Mateescu, M.A. Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery. Bioact. Mater. 2018, 3, 34–340. [Google Scholar] [CrossRef]
- López-Rios de Castro, R.; Ziolek, R.M.; Ulmschneider, M.B.; Lorenz, C.D. Therapeutic Peptides Are Preferentially Solubilized in Specific Microenvironments within PEG-PLGA Polymer Nanoparticles. Nano Lett. 2024, 24, 2011–2017. [Google Scholar] [CrossRef]
- Dirany, Z.; El-Dirany, R.; Smith, G.N.; Nguewa, P.; González-Gaitano, G. Mixed micelles and gels of a hydrophilic poloxamine (Tetronic 1307) and miltefosine: Structural characterization by small-angle neutron scattering and in vitro evaluation for the treatment of leishmaniasis. J. Mol. Liq. 2023, 379, 121654. [Google Scholar] [CrossRef]
- Dirany, Z.; Smith, G.N.; Aydillo, C.; Nguewa, P.; González-Gaitano, G. Structure and activity of amphiphilic PEO-PPO-based polymeric micelles and gels incorporating host -guest complexes of miltefosine as novel formulations for the treatment of leishmaniasis. J. Mol. Liq. 2024, 400, 124455. [Google Scholar] [CrossRef]
- Dirany, Z.; González-Benito, J.; Ginatta, P.; Nguewa, P.; González-Gaitano, G. Solution blow spun polymeric nanofibres embedding cyclodextrin complexes of miltefosine: An approach to the production of sprayable dressings for the treatment of cutaneous leishmaniasis. Carbohydr. Polym. 2025, 353, 123173. [Google Scholar] [CrossRef]
- BASF. Soluplus—The First Polymeric Solubilizer and Matrix-Forming Polymer. Available online: https://pharma.basf.com/products/soluplus (accessed on 10 December 2024).
- Mateos, H.; Gentile, L.; Murgia, S.; Colafemmina, G.; Collu, M.; Smets, J.; Palazzo, G. Understanding the self-assembly of the polymeric drug solubilizer Soluplus®. J. Colloid Interface Sci. 2022, 611, 224–234. [Google Scholar] [CrossRef]
- Sofroniou, C.; Baglioni, M.; Mamusa, M.; Resta, C.; Doutch, J.; Smets, J.; Baglioni, P. Self-Assembly of Soluplus in Aqueous Solutions: Characterization and Prospectives on Perfume Encapsulation. ACS Appl. Mater. Interfaces 2022, 14, 14791–14804. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. [Google Scholar] [CrossRef]
- Wu, H.; Wang, K.; Wang, H.; Chen, F.; Huang, W.; Chen, Y.; Chen, J.; Tao, J.; Wen, X.; Xiong, S. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy. Colloids Surf. B 2017, 149, 97–104. [Google Scholar] [CrossRef]
- Soluplus®: Information on Toxicological Data. BASF 2021. Available online, upon registration, at: http://www.virtualpharmaassistants.basf.com (accessed on 4 February 2025).
- Soluplus®: Safety Summary File, PRD-No.: 30446233, RegXcellence®. BASF 2021. Available online: https://virtualpharmaassistants.basf.com/s/product?recordId=01t2p00000AjDlJAAV (accessed on 4 February 2025).
- Schmitt, G. Safety of Soluplus® in Pediatrics. Appl. Clin. Res. Clin. Trials Regul. Aff. 2022, 9, e161122210943. [Google Scholar] [CrossRef]
- Suresh, G.A.; Anil, V.M.; Dillip, J.S.; Kishor, M.R.; Sonawane, R.O. Soluplus is a polymeric carrier which increased the solubility bioavailability, dissolution of various dosage form: An overview. Int. J. Pharm. Res. Appl. 2024, 9, 1435–1442. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.; Tao, J.; Zeng, J.; Zhu, Q.; Zhu, C.; Gan, Y. Supersaturated polymeric micelles for oral cyclosporine A delivery: The role of Soluplus–sodium dodecyl sulfate complex. Colloids Surf. B 2016, 141, 301–310. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.W.; Wang, C.Y.; Ding, Y.F.; Chen, M.Y.; Wang, Y.F.; Peng, J.Y.; Li, L.; Lv, L. Soluplus/TPGS mixed micelles for dioscin delivery in cancer therapy. Drug Dev. Ind. Pharm. 2017, 43, 1197–1204. [Google Scholar] [CrossRef]
- Ding, Y.F.; Ding, Y.Y.; Wang, Y.T.; Wang, C.Y.; Gao, M.; Xu, Y.W.; Ma, X.D.; Wu, J.P.; Li, L. Soluplus®/TPGS mixed micelles for co-delivery of docetaxel and piperine for combination cancer therapy. Pharm. Dev. Technol. 2020, 25, 107–115. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B 2016, 140, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Cui, C.C.; Wei, F.; Lv, H.X. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Li, G.Y.; Lu, Y.T.; Fan, Y.C.; Ning, Q.; Li, W.G. Enhanced oral bioavailability of magnolol via mixed micelles and nanosuspensions based on Soluplus(R)-Poloxamer 188. Drug Deliv. 2020, 27, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.C.; Zhang, Z.H.; Wu, H.; Kia, X.B.; Wang, Y.J.E. Optimization and evaluation of Oridonin-loaded Soluplus®-Pluronic P105 mixed micelles for oral administration. Int. J. Pharm. 2017, 518, 193–202. [Google Scholar] [CrossRef]
- Chougale, R.; Patil, K.; Disouza, J.; Hajare, A.; Jadhav, N.; Kumbhar, P. Development of docetaxel-loaded (Soluplus®-PF108) mixed micelles vacuum foam-dried product for improved stability and melanoma treatment by QbD approach. Future J. Pharm. Sci. 2024, 10, 54. [Google Scholar] [CrossRef]
- Hou, J.; Sun, E.; Sun, C.Y.; Wang, J.; Yang, L.; Jia, X.B.; Zhang, Z.H. Improved oral bioavailability and anticancer efficacy on breast cancer of paclitaxel via Novel Soluplus®-Solutol® HS15 binary mixed micelles system. Int. J. Pharm. 2016, 512, 186–193. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Vasarri, M.; Marroncini, G.; Barletta, E.; Degl’Innocenti, D. Thymoquinone-Loaded Soluplus®-Solutol® HS15 Mixed Micelles: Preparation, In Vitro Characterization, and Effect on the SH-SY5Y Cell Migration. Molecules 2020, 25, 4707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Mou, Y.H.; He, H.Y.; Yang, D.D.; Qin, L.; Zhang, F.; Zhang, P. Preparation and evaluation of self-assembly Soluplus®-sodium cholate-phospholipid ternary mixed micelles of docetaxel. Drug Dev. Ind. Pharm. 2019, 45, 1788–1798. [Google Scholar] [CrossRef]
- Shi, F.; Chen, L.; Wang, Y.P.; Liu, J.; Adu-Frimpong, M.; Ji, H.; Toreniyazov, E.; Wang, Q.L.; Yu, J.N.; Xu, X.M. Enhancement of oral bioavailability and anti-hyperuricemic activity of aloe emodin via novel Soluplus®-glycyrrhizic acid mixed micelle system. Drug Deliv. Transl. Res. 2021, 12, 603–614. [Google Scholar] [CrossRef]
- Han, S.D.; Jung, S.W.; Jang, S.W.; Jung, H.J.; Son, M.; Kim, B.M.; Kang, M.J. Preparation of Solid Dispersion of Dronedarone Hydrochloride with Soluplus® by Hot Melt Extrusion Technique for Enhanced Drug Release. Chem. Pharm. Bull. 2015, 63, 295–299. [Google Scholar] [CrossRef]
- Nikam, V.K.; Shete, S.K.; Khapare, J.P. Most promising solid dispersion technique of oral dispersible tablet. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 62. [Google Scholar] [CrossRef]
- Althobaiti, A.A.; Ashour, E.A.; Almutairi, M.; Almotairy, A.; Al Yahya, M.; Repka, M.A. Formulation development of curcumin-piperine solid dispersion via hot-melt extrusion. J. Drug Deliv. Sci. Technol. 2022, 76, 103753. [Google Scholar] [CrossRef]
- Darwich, M.; Mohylyuk, V.; Kolter, K.; Bodmeier, R.; Dashevskiy, A. Enhancement of itraconazole solubility and release by hot-melt extrusion with Soluplus®. J. Drug Deliv. 2023, 81, 104280. [Google Scholar] [CrossRef]
- Karami, T.; Ghobadi, E.; Akrami, M.; Haririan, I. Fabrication of a Controlled-Release Core-Shell Floating Tablet of Ketamine Hydrochloride Using a 3D Printing Technique for Management of Refractory Depressions and Chronic Pain. Polymers 2024, 16, 746. [Google Scholar] [CrossRef]
- Li, S.; an Zhang, Z.; Gu, W.; Gallas, M.; Jones, D.; Boulet, P.; Johnson, L.M.; de Margerie, V.; Andrews, G.P. Hot Melt Extruded High-Dose Amorphous Solid Dispersions Containing Lumefantrine and Soluplus. Int. J. Pharm. 2024, 665, 124676. [Google Scholar] [CrossRef]
- Winck, J.; Gottschalk, T.; Thommes, M. Predicting Residence Time and Melt Temperature in Pharmaceutical Hot Melt Extrusion. Pharmaceutics 2023, 15, 1417. [Google Scholar] [CrossRef] [PubMed]
- Darwich, M.; Mohylyuk, V.; Bodmeier, R.; Dashevskiy, A. An approach for pH-independent release of poorly soluble ionizable drugs using hot-melt extrusion. J. Drug Deliv. 2024, 100, 106027. [Google Scholar] [CrossRef]
- Rahman, M.; Radgman, K.; Tarabokija, J.; Ahmad, S.; Bilgili, E. Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug. Nanomaterials 2023, 13, 2419. [Google Scholar] [CrossRef]
- Koleva, I.Z.; Tzachev, C.T. Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F127. Pharmaceuticals 2024, 17, 1156. [Google Scholar] [CrossRef]
- Bajaj, T.; Das Gupta, G.; Singh, C. Spray dried mebendazole-loaded Soluplus-based polymeric micelles for improved biopharmaceutical attributes: In vitro and in vivo studies. Colloid Polym. Sci. 2024, 302, 1067–1080. [Google Scholar] [CrossRef]
- Kushwah, V.; Succhielli, C.; Saraf, I.; Paudel, A. Amorphous Solid Dispersions: Implication of Method of Preparation and Physicochemical Properties of API and Excipients. Pharmaceutics 2024, 16, 1035. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.M.; Adam, M.S.; Wood, J.D. Engineering Advances in Spray Drying for Pharmaceuticals. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 217–240. [Google Scholar] [CrossRef]
- Bonakdar, M.A.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus Fibre Production Methods: From Specifics to Technological Convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Srivastava, R.K. Electrospinning of Patterned and 3D Nanofibers. Electrospun Nanofibers 2017, 399–447. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Bonakdar, M.A.; Hamdi, O.; Nazarenko, Y.; Ariya, P.A.; Rodrigue, D. Highly porous biobased membranes via electrospinning of PBS and CTAB. Polymer 2023, 280, 126045. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Younes, H.M.; Kadavil, H.; Ismail, H.M.; Adib, S.A.; Zamani, S.; Alany, R.G.; Al-Kinani, A.A. Overview of Tissue Engineering and Drug Delivery Applications of Reactive Electrospinning and Crosslinking Techniques of Polymeric Nanofibers with Highlights on Their Biocompatibility Testing and Regulatory Aspects. Pharmaceutics 2024, 16, 32. [Google Scholar] [CrossRef]
- Tipduangta, P.; Belton, P.; McAuley, W.J.; Qi, S. The use of polymer blends to improve stability and performance of electrospun solid dispersions: The role of miscibility and phase separation. Int. J. Pharm. 2021, 602, 120637. [Google Scholar] [CrossRef]
- Pisani, S.; Friuli, V.; Conti, B.; Bruni, G.; Maggi, L. Tableted hydrophilic electrospun nanofibers to promote meloxicam dissolution rate. J. Drug Deliv. Sci. Technol. 2021, 66, 102878. [Google Scholar] [CrossRef]
- Gomaa, E.; Attia, M.S.; Ghazy, F.E.S.; Hasssan, A.E.A.; Hasan, A.A. Pump-free electrospraying: A novel approach for fabricating Soluplus®-based solid dispersion nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 67, 103027. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Swain, K.; Pattnaik, S.; Dey, B.K. Quality by Design Based Development of Electrospun Nanofibrous Solid Dispersion Mats for Oral Delivery of Efavirenz. Acta Chim. Slov. 2024, 71, 161–169. [Google Scholar] [CrossRef]
- Batra, A.; Thongsukmak, A.; Desai, D.; Serajuddin, A.T.M. The Effect of Process Variables and Binder Concentration on Tabletability of Metformin Hydrochloride and Acetaminophen Granules Produced by Twin Screw Melt Granulation with Different Polymeric Binders. AAPS PharmSciTech 2021, 22, 154. [Google Scholar] [CrossRef]
- Abid, Z.; Andreoli, F.; Kristensen, M.N.; Petersen, R.S.; Müllertz, A.; Boisen, A.; Keller, S.S. Hot punching for loading of biodegradable microcontainers with budesonide-Soluplus film. Biomed. Microdevices 2021, 23, 37. [Google Scholar] [CrossRef]
- Salawi, A.; Sonju, J.J.; Kamal, M.M.; Abu-Fayyad, A.; Al Hagbani, T.; Nazzal, S. Preparation and characterization of aqueous vitamin E/Soluplus® dispersions for film coating applications. Drug Dev. Ind. Pharm. 2021, 47, 1335–1341. [Google Scholar] [CrossRef]
- Fernández-García, R.; Walsh, D.; O’Connell, P.; Slowing, K.; Raposo, R.; Ballesteros, M.P.; Jiménez-Cebrián, A.; Chamorro-Sancho, M.; Bolás-Fernández, F.; Healy, A.M.; et al. Can amphotericin B and itraconazole be co-delivered orally? Tailoring oral fixed-dose combination coated granules for systemic mycoses. Eur. J. Pharm. Biopharm. 2023, 183, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arora, K.; Mohapatra, H.; Sindhu, R.K.; Bulzan, M.; Cavalu, S.; Paneshar, G.; Elansary, H.O.; El-Sabrout, A.M.; Mahmoud, E.A.; et al. Supersaturation-Based Drug Delivery Systems: Strategy for Bioavailability Enhancement of Poorly Water-Soluble Drugs. Molecules 2022, 27, 2969. [Google Scholar] [CrossRef]
- Dukhan, A.A.M.; Amalina, N.; Oo, M.K.; Sengupta, P.; Doolaanea, A.A.M.; Aljapairai, K.A.S.; Chatterjee, B. Formulation of Dispersed Gliclazide Powder in Polyethylene Glycol–Polyvinyl Caprolactam–Polyvinyl Acetate Grafted Copolymer Carrier for Capsulation and Improved Dissolution. Indian J. Pharm. Educ. Res. 2018, 52, S210–S219. [Google Scholar] [CrossRef]
- Desai, P.; Chatterjee, B. Comparison of Two Grafted Copolymers, Soluplus and Kollicoat IR, as Solid Dispersion Carriers of Arteether for Oral Delivery Prepared by Different Solvent-Based Methods. ACS Omega 2023, 8, 45337–45347. [Google Scholar] [CrossRef]
- Nazli, H.; Mesut, B.; Akbal-Dagistan, Ö.; Özsoy, Y. A Novel Semi-Solid Self-Emulsifying Formulation of Aprepitant for Oral Delivery: An In Vitro Evaluation. Pharmaceutics 2023, 15, 1509. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Kshirsagar, S.J. Review on Tablet in Tablet techniques. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1. [Google Scholar] [CrossRef]
- Kinani, A.A.; Taghi, H.S. Formulation and characterization of orodispersible tablet of glimepiride. J. Adv. Pharm. Technol. Res. 2022, 13, 252–260. [Google Scholar] [CrossRef]
- Kanojiya, P.S.; Charde, Y.; Wadertwar, R.N. Solid Dispersion of Artemether in Fast Disintegrating Tablet to Enhance Dissolution Rate and Oral Bioavailability. Indian J. Pharm. Educ. Res. 2022, 56, 153–165. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Xu, X.; Yang, G. Strategies and mechanisms to improve the printability of pharmaceutical polymers Eudragit® EPO and Soluplus®. Int. J. Pharm. 2021, 599, 120410. [Google Scholar] [CrossRef] [PubMed]
- Saydam, M.; Takka, S. Improving the dissolution of a water-insoluble orphan drug through a fused deposition modelling 3-Dimensional printing technology approach. Eur. J. Pharm. Sci. 2020, 152, 105426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Heer, D.; Aggarwal, G.; Kumar, S.L.H. Recent trends of fast dissolving drug delivery system—An overview of formulation technology. Pharmacophore 2013, 4, 1–9. [Google Scholar]
- Browne, E.; Quinn, S.; Cheyne, S.; Healy, A.M. Design and characterisation of an amorphous formulation of nifedipine for the treatment of autonomic dysreflexia. J. Pharm. Pharmacol. 2021, 73, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Shamim, R.; Shafique, S.; Hussain, K.; Abbas, N.; Bukhari, N.I. Surfactant-Assisted Wet Granulation-Based Matrix Tablets without Exceptional Additives: Prolonging Systemic Exposure of Model BCS Class II Ketoprofen. AAPS PharmSciTech 2024, 25, 241. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, Z.; Huo, Y.; Sun, Y.; Zhang, X.; Guan, J.; Mao, S. Influence of drug-carrier compatibility and preparation method on the properties of paclitaxel-loaded lipid liquid crystalline nanoparticles. J. Pharm. Sci 2021, 110, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, Z.; Song, J.; Li, J.; Han, Y.; Hou, W.; Wang, W.; Ruan, B.H. Biodegradable self-assembly micelles significantly enhanced the solubility, biological stability and in vivo antitumor efficacy of Hexylselen. RSC Chem. Biol. 2021, 2, 1669. [Google Scholar] [CrossRef]
- Trivedi, S.; Thool, S.; Wadher, K.; Bhalekar, M.; Bire, P. Self-Assembling Dioscorea bulbifera loaded mixed micelles: Formulation optimization, in-vitro cytotoxicity and in-vivo pharmacokinetics. J. Drug Deliv. Sci. Technol. 2021, 65, 102722. [Google Scholar] [CrossRef]
- Taymouri, S.; Ahmadi, Z.; Mirian, M.M.; Tavakoli, N. Simvastatin nanosuspensions prepared using a combination of pH-sensitive and timed-release approaches for potential treatment of colorectal cancer. Pharm. Dev. Technol. 2021, 26, 335–348. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Ambrus, R.; Csóka, I.; Szabó-Révész, P. Characterizing the Drug-Release Enhancement Effect of Surfactants on Megestrol-Acetate-Loaded Granules. Pharmaceuticals 2022, 15, 113. [Google Scholar] [CrossRef]
- Koromili, M.; Kapourani, A.; Barmpalexis, P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers 2023, 15, 169. [Google Scholar] [CrossRef]
- Shin, Y.B.; Choi, J.Y.; Yoon, M.S.; Yoo, M.K.; Shin, D.H.; Lee, J.W. Evaluation of Anticancer Efficacy of D-α-Tocopheryl Polyethylene-Glycol Succinate and Soluplus® Mixed Micelles Loaded with Olaparib and Rapamycin Against Ovarian Cancer. Int. J. Nanomed. 2024, 19, 7871–7893. [Google Scholar] [CrossRef]
- Kendre, P.; Gite, M.; Jain, S. Solubility enhancement of β-ionone with lipidic, amphiphilic, and inclusion complex: Extensive polymeric biomaterials to develop formulations for poorly soluble drugs. Polym. Bull. 2024, 81, 14479–14498. [Google Scholar] [CrossRef]
- Twal, S.; Jaber, N.; al-Remawi, M.; Hamad, I.; Al-Akayleh, F.; Alshaer, W. Dual stimuli-responsive polymeric nanoparticles combining soluplus and chitosan for enhanced breast cancer targeting. RSC Adv. 2024, 14, 3070. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Ponti, L.; Degl’Innocenti, D.; Bergonzi, M.C. Usnic Acid-Loaded Polymeric Micelles: An Optimal Migrastatic-Acting Formulation in Human SH-SY5Y Neuroblastoma Cells. Pharmaceuticals 2022, 15, 1207. [Google Scholar] [CrossRef] [PubMed]
- Louis, L.; Chee, B.S.; McAfee, M.; Nugent, M.J.D. Design, development and in vitro quantification of novel electrosprayed everolimus-loaded Soluplus®/Polyvinyl alcohol nanoparticles via stability-indicating HPLC method in cancer therapy. Eur. J. Pharm. Biopharm. 2023, 191, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Louis, L.; Simonassi-Paiva, B.; Attallah, O.A.; McAfee, M.; Nugent, M. Freeze-thawed electrosprayed everolimus loaded nanoparticles as a potential drug delivery system in brain tumours: Design and characterisation. J. Drug Deliv. Sci. Technol. 2024, 101, 106209. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Portillo, M.; Tesán, F.; Nicoud, M.; Medina, V.; Moretton, M.; Chiappetta, D.; Zubillaga, M. Design and development of nanoprobes radiolabelled with 99mTc for the diagnosis and monitoring of therapeutic interventions in oncology preclinical research. EJNMMI Radiopharm. Chem. 2024, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.M.; Chaudhari, P.D.; Shaikh, K.S.; Chaudhari, S.Y.; Pathare, S.S.; Saikh, A.A.; Aljarba, N.H.; Kumer, A.; Dhara, B. Dual drug-loaded polymeric mixed micelles for ovarian cancer: Approach to enhanced therapeutic efficacy of albendazole and paclitaxel. J. Cell Mol. Med. 2024, 28, e18389. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Guo, C. Improved anticancer activity of betulinic acid on breast cancer through a grafted copolymer-based micelles system. Drug Deliv. 2021, 28, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Qamar, W.; Kalam, M.A.; Binkhathlan, Z. Soluplus−TPGS Mixed Micelles as a Delivery System for Brigatinib: Characterization and In Vitro Evaluation. ACS Omega 2024, 9, 41830–41840. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Yu, E.; Zhuang, W.; Sun, X.; Wang, H.; Li, Q. Preparation of a camptothecin analog FLQY2 self-micelle solid dispersion with improved solubility and bioavailability. J. Nanobiotechnol. 2022, 20, 402. [Google Scholar] [CrossRef]
- Ali, R.; Kalam, M.A.; Qamar, W.; Alshememry, A.K.; Alhudaithi, S.S.; Binkhathlan, Z. Chrysin-loaded Soluplus-TPGS mixed micelles: Optimization, characterization and anticancer activity against hepatocellular carcinoma cell line. J. Drug Deliv. Sci. Technol. 2024, 102, 106371. [Google Scholar] [CrossRef]
- Fang, J.; Chen, Z.; Li, J.; Li, D.; Wang, W.; Ruan, B.H. Self-Assembled Micellar Glutaminase Allosteric Inhibitor for Effective Therapeutic Intervention. Int. J. Nanomed. 2022, 17, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, R.; Ding, Y.; Wang, C.; Zhang, S.; Sun, Z.; Chen, Y.; Mi, Y.; Gao, M.; Ma, X.; et al. Intelligent triggering of nanomicelles based on a ROS-activated anticancer prodrug and photodynamic therapy (PDT)-synergistic therapy for lung cancers. Eur. J. Med. Chem. 2022, 241, 114622. [Google Scholar] [CrossRef] [PubMed]
- Dian, C.; Qian, Z.; Ran, M.; Yan, X.; Dian, L. Co-Delivery of Docetaxel and Curcumin Functionalized Mixed Micelles for the Treatment of Drug-Resistant Breast Cancer by Oral Administration. Int. J. Nanomed. 2024, 19, 8603–8620. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Diaz-Gomez, L.; Blanco-Fernandez, B.; Ferreirós, A.; Veiga, F.; Concheiro, A.; Paiva-Santos, A.C.; Alvarez-Lorenzo, C. Cancer cell membrane-modified Soluplus® micelles for gemcitabine delivery to pancreatic cancer using a prodrug approach. Int. J. Pharm. 2024, 662, 124529. [Google Scholar] [CrossRef]
- Alanazi, S.A.; Imam, S.S.; Alshehri, S.; Alanazi, M.M.; Yassin, A.E.B.; Mahdi, W.A.; Alzhrani, R.F.; Al-Agamy, M.H.; Ghoneim, M.M. Optimized freeze-dried solid-dispersions boost naringin solubility, antibacterial and in-vitro anticancer activity. J. Drug Deliv. Sci. Technol. 2024, 102, 106335. [Google Scholar] [CrossRef]
- Alali, A.S.; Kalam, M.A.; Ahmed, M.M.; Aboudzadeh, M.A.; Alhudaithi, S.S.; Anwer, M.K.; Fatima, F.; Iqbal, M. Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers 2022, 14, 4827. [Google Scholar] [CrossRef] [PubMed]
- Riedel, J.; Pibuel, M.; Bernabeu, E.; Poodts, D.; Díaz, M.; Allo, M.; Parola, L.; Hajos, S.; Lázaro-Martinez, J.M.; Salgueiro, M.J.; et al. Glycosylated paclitaxel mixed nanomicelles: Increasing drug brain accumulation and enhancing its in vitro antitumoral activity in glioblastoma cell lines. J. Drug Deliv. Sci. Technol. 2022, 68, 103046. [Google Scholar] [CrossRef]
- Nicoud, M.B.; Ospital, I.A.; Táquez Delgado, M.A.; Riedel, J.; Fuentes, P.; Bernabeu, E.; Rubinstein, M.R.; Lauretta, P.; Martínez Vivot, R.; Aguilar, M.A.; et al. Nanomicellar Formulations Loaded with Histamine and Paclitaxel as a New Strategy to Improve Chemotherapy for Breast Cancer. Int. J. Mol. Sci. 2023, 24, 3546. [Google Scholar] [CrossRef] [PubMed]
- Riedel, J.; Calienni, M.N.; Bernabeu, E.; Calabro, V.; Lázaro-Martinez, J.M.; Prieto, M.J.; Gonzalez, L.; Martinez, C.S.; Del Valle Alonso, S.; Montanari, J.; et al. Paclitaxel and curcumin co-loaded mixed micelles: Improving in vitro efficacy and reducing toxicity against Abraxane®. J. Drug Deliv. Sci. Technol. 2021, 62, 102343. [Google Scholar] [CrossRef]
- Hussain, T.; Paranthaman, S.; Rizvi, S.M.D.; Moin, A.; Gowda, D.V.; Subaiea, G.M.; Ansari, M.; Alanazi, A.S. Fabrication and Characterization of Paclitaxel and Resveratrol Loaded Soluplus Polymeric Nanoparticles for Improved BBB Penetration for Glioma Management. Polymers 2021, 13, 3210. [Google Scholar] [CrossRef]
- Paranthaman, S.; Uthaiah, C.A.; Osmani, R.A.M.; Hani, U.; Ghazwani, M.; Alamri, A.H.; Fatease, A.A.; Madhunapantula, S.V.; Gowda, D.V. Anti-Proliferative Potential of Quercetin Loaded Polymeric Mixed Micelles on Rat C6 and Human U87MG Glioma Cells. Pharmaceutics 2022, 14, 1643. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Di, G.; Wang, J.; Guo, C. Development of quercetin-loaded PVCL–PVA–PEG micelles and application in inhibiting tumor angiogenesis through the PI3K/Akt/VEGF pathway. Toxicol. Appl. Pharmacol. 2022, 437, 115889. [Google Scholar] [CrossRef] [PubMed]
- Alkathiri, F.A.; Bukhari, S.I.; Imam, S.S.; Alshehri, S.; Mahdi, W.A. Formulation of silymarin binary and ternary solid dispersions: Characterization, simulation study and cell viability assessment against lung cancer cell line. Heliyon 2023, 10, e23221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, R.; Rao, Y.; Liu, S.; Hu, C.; Zhang, Y.; Meng, L.; Wu, Q.; Ouyang, Q.; Liang, H.; et al. Enhancement of the Bioavailability and Anti-Inflammatory Activity of Glycyrrhetinic Acid via Novel Soluplus®—A Glycyrrhetinic Acid Solid Dispersion. Pharmaceutics 2022, 14, 1797. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Calarco, P.; Di Michele, A.; Ceccarini, M.R.; Beccari, T.; Primavilla, S.; Scuota, S.; Marmottini, F.; Ramella, D.; Ricci, M.; et al. Development of sodium carboxymethyl cellulose based polymeric microparticles for in situ hydrogel wound dressing formation. Int. J. Pharm. 2021, 602, 120606. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.; Robertson, J.; Halbert, G.W. Mefenamic acid solid dispersions: Impact of formulation composition on processing parameters, product properties and performance. Int. J. Pharm. 2022, 616, 121505. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Bella, Z.; Gróf, I.; Veszelka, S.; Deli, M.A.; Szucs, K.F.; Sztojkov-Ivanov, A.; Ducza, E.; Gáspár, R.; Kecskeméti, G.; et al. Soluplus® promotes efficient transport of meloxicam to the central nervous system via nasal administration. Int. J. Pharm. 2023, 632, 122594. [Google Scholar] [CrossRef]
- Chhimwal, J.; Dhritlahre, R.K.; Anand, P.; Ruchika; Patial, V.; Saneja, A.; Padwad, Y.S. Amorphous solid dispersion augments the bioavailability of phloretin and its therapeutic efficacy via targeting mTOR/SREBP-1c axis in NAFLD mice. Biomater. 2023, 154, 213627. [Google Scholar] [CrossRef]
- Ashokbhai, M.K.; Ghatole, S.; Gupta, U.; Sanjay, L.R.; Roy, S.; Ravichandiran, V.; Kaity, S. Leveraging solid solubility and miscibility of etoricoxib in Soluplus® towards manufacturing of 3D printed etoricoxib tablets by additive manufacturing. Int. J. Pharm. 2024, 667, 124881. [Google Scholar] [CrossRef] [PubMed]
- Alsmadi, M.M.; AL-Daoud, N.M.; Obaidat, R.M.; Abu-Farsakh, N.A. Enhancing Atorvastatin In Vivo Oral Bioavailability in the Presence of Inflammatory Bowel Disease and Irritable Bowel Syndrome Using Supercritical Fluid Technology Guided by wbPBPK Modeling in Rat and Human. AAPS PharmSciTech 2022, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Anjani, Q.K.; Sabri, A.H.B.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, A.; Domínguez-Robles, J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus®-based dissolving microarray patches loaded with colchicine: Towards a minimally invasive treatment and management of gout. Biomater. Sci. 2022, 10, 5838. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Manzoor, H.; Khan, I.U.; Asghar, S.; Irfan, M.; Albekairi, N.A.; Alshammari, A.; Alqahtani, A.F.; Alotaibi, S.; Munir, R.; et al. Curcumin-loaded soluplus® based ternary solid dispersions with enhanced solubility, dissolution and antibacterial, antioxidant, anti-inflammatory activities. Heliyon 2024, 10, e34636. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-based polypseudorotaxane hydrogels for ophthalmic delivery of flurbiprofen to treat anterior uveitis. Carbohydr. Polym. 2022, 277, 118889. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Demartis, S.; Moreno-Castellanos, N.; Gavini, E.; Donnelly, R.F. Formulation and evaluation of ivermectin-loaded dissolving microarray patches for rosacea disease. J. Pharm. Investig. 2024, 54, 683–698. [Google Scholar] [CrossRef]
- Taha, N.F.; Mahmoud, K.M.; Soliman, A.A.F.; Emara, L.H. Anti-inflammatory and cytoprotective potentials of Meloxicam solid dispersions prepared by different techniques on lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Drug Deliv. Sci. Technol. 2021, 63, 102507. [Google Scholar] [CrossRef]
- Abid, F.; Savaliya, B.; Parikh, A.; Kim, S.; Amirmostofian, M.; Cesari, L.; Song, Y.; Page, S.W.; Trott, D.J.; Garg, S. Nanotechnology and narasin: A powerful combination against acne. Nanoscale 2023, 15, 13728. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, M.; Liu, Y.; Gao, X.; Wu, X.; Shi, D.; Guo, C.; Wang, J. Pterostilbene-Loaded Soluplus/Poloxamer 188 Mixed Micelles for Protection against Acetaminophen-Induced Acute Liver Injury. Mol. Pharm. 2023, 20, 1189–1201. [Google Scholar] [CrossRef]
- Takale, N.R.; Aji, A.; Jane, K.; Deshmukh, P.R.; Pendharkar, V.V.; Khade, R.R.; Ghule, B.V.; Inamdar, N.N.; Kotagale, N.R. Lumefantrine solid dispersions with piperine for the enhancement of solubility, bioavailability and anti-parasite activity. Int. J. Pharm. 2022, 628, 122354. [Google Scholar] [CrossRef]
- Molina, V.; von Plessing, C.; Romero, A.; Benavides, S.; Troncoso, J.M.; Pérez-Correa, J.R.; Franco, W. Determination of the Dissolution/Permeation and Apparent Solubility for Microencapsulated Emamectin Benzoate Using In Vitro and Ex Vivo Salmo salar Intestine Membranes. Pharmaceuticals 2022, 15, 652. [Google Scholar] [CrossRef]
- Joshi, P.; Sangamwar, A.T. Insights into the Role of Compendial/Biorelevant Media on the Supersaturation Behaviour of Drug Combination (Drug-Drug Interaction) and Precipitation Inhibition by Polymers. AAPS PharmSciTech 2022, 23, 300. [Google Scholar] [CrossRef]
- Paulino, S.; Santos, L.; Rabello, M.; da Silva, P.; Oliveira, J.; do Carmo Lima, M.; Rocha, T.; Albuquerque, M.; Santos, V.; Alves, L.; et al. Development of solid dispersions based on 3-(2,6-difluorobenzyl)-5-(5-bromo-1H-indol-3-ylmethylene) thiazolidine-2,4-dione for schistosomicidal treatment. Exp. Parasitol. 2023, 248, 108455. [Google Scholar] [CrossRef]
- Rocha, T.C.D.; Lima, M.J.D.; Nascimento, J.L.N.; de Oliveira, J.F.; Silva, E.D.; dos Santos, V.H.B.; Aires, A.D.; Sales, V.D.W.; Rosa, T.A.; de Lima, M.D.A.; et al. Development and evaluation of the in vitro schistosomicidal activity of solid dispersions based on 2-(-5-bromo-1-H-indole-3-yl-methylene)-N-(naphthalene-1-ylhydrazine-carbothiamide. Exp. Parasitol. 2023, 256, 108626. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, M.; Yang, J.; Qiu, X.; Xin, L.; Lu, Y.; Huang, H.; Zeng, Z.; Zeng, D. Preparation, Characterization, and Oral Bioavailability of Solid Dispersions of Cryptosporidium parvum Alternative Oxidase Inhibitors. Int. J. Mol. Sci. 2024, 25, 7025. [Google Scholar] [CrossRef]
- Jaligam, M.M.; Takahashi, C.; Heidt, B.; Shen, A.Q. Enhanced antibacterial efficacy: Rapid analysis of silver-decorated azithromycin-infused Soluplus® nanoparticles against E. coli and S. epidermidis biofilms. Nanoscale 2024, 16, 17877. [Google Scholar] [CrossRef]
- Sipos, B.; Földes, F.; Budai-Szucs, M.; Katona, G.; Csóka, I. Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration. Gels 2024, 10, 521. [Google Scholar] [CrossRef]
- Araya, N.; Leiva-Soto, M.A.; Bruna, M.V.; Castro-Munoz, A.; Behrend-Keim, B.; Moraga-Espinoza, D.; Bahamondez-Canas, T.F. Formulation of water-soluble Buddleja globosa Hope extracts and characterization of their antimicrobial properties against Pseudomonas aeruginosa. Front. Pharmacol. 2022, 13, 921511. [Google Scholar] [CrossRef]
- Guillot, A.; Petalas, D.; Skondra, P.; Rico, H.; Garrigues, T.M.; Melero, A. Ciprofoxacin self-dissolvable Soluplus based polymeric flms: A novel proposal to improve the management of eye infections. Drug Deliv. Transl. Res. 2021, 11, 608–625. [Google Scholar] [CrossRef]
- Wang, H.X.; Fan, Y.Z.; Qin, L.; Cheng, Z.P.; Chen, X.Q.; Zeng, S.M.; Liang, S.H.; Tong, Y.; Tao, Z.; Liu, Y.C.; et al. Preparation of Decoquinate Solid Dispersion by Hot-Melt Extrusion as an Oral Dosage Form Targeting Liver-Stage Plasmodium Infection. Antimicrob. Agents Chemother. 2022, 66, e02218-21. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Utomo, E.; Wardoyo, L.A.H.; Larrañeta, E.; Donnelly, R.F. Metronidazole nanosuspension loaded dissolving microarray patches: An engineered composite pharmaceutical system for the treatment of skin and soft tissue infection. Biomater. Adv. 2022, 140, 213073. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Z.; Huang, Z.W.; Fu, F.Q.; Wang, W.; Wu, L.J.; Huang, Y.; Wu, C.B.; Pan, X. Two different protein corona formation modes on Soluplus® nanomicelles. Colloids Surf. B 2022, 218, 112744. [Google Scholar] [CrossRef]
- Sakurai, M.; Ishitsuka, K.; Ito, R.; Wilkinson, A.C.; Kimura, T.; Mizutani, E.; Nishikii, H.; Sudo, K.; Becker, H.J.; Takemoto, H.; et al. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature 2023, 615, 127–133. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Tajber, L.; Miklaszewski, A.; Cielecka-Piontek, J. Hot Melt Extrusion for Improving the Physicochemical Properties of Polydatin Derived from Polygoni cuspidati Extract; A Solution Recommended for Buccal Applications. Pharmaceuticals 2023, 16, 1226. [Google Scholar] [CrossRef]
- Al-Sudani, B.T.; Al-Musawi, M.H.; Kamil, M.M.; Turki, S.H.; Nasiri-Harchegani, S.; Najafinezhad, A.; Noory, P.; Talebi, S.; Valizadeh, H.; Sharifianjazi, F.; et al. Vasculo-osteogenic keratin-based nanofibers containing merwinite nanoparticles and sildenafil for bone tissue regeneration. Int. J. Pharm. 2024, 667, 124875. [Google Scholar] [CrossRef]
- Fathi, A.M.; Eissa, R.G.; Balata, G.F.; Ghazy, F.E.S.; Eissa, N.G. Intranasal thermosensitive hydrogel of agomelatine solid dispersion for better management of depression. J. Drug Deliv. Sci. Technol. 2023, 88, 104974. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Nishikii, H.; Kimura, T.; Sugiyama-Finnis, A.; Yamazaki, S. Purging myeloma cell contaminants and simultaneous expansion of peripheral blood-mobilized stem cells. Exp. Hematol. 2024, 131, 104138. [Google Scholar] [CrossRef]
- Plantamura, Y.A.S.; Allo, M.; Riedel, J.; Fuentes, P.; Riesco, A.S.; Bernabeu, E.; Garcés, M.; Evelson, P.; Gorzalczany, S.; Carranza, A.; et al. Development of a new micellar formulation of carvedilol and curcumin to enhance blood pressure reduction in a spontaneously hypertensive rat model. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, Y.; Ye, J.; Zhu, Y.; Li, R.; Xu, X.H.; Zhang, Q. Self-assembled micelles enhance the oral delivery of curcumin for the management of alcohol-induced tissue injury. Pharm. Dev. Technol. 2021, 26, 880–889. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Moreno-Castellanos, N.; Adhami, M.; Ramadon, D.; Jangga, J.; Donnelly, R.F. Quercetin loaded polymeric dissolving microarray patches: Fabrication, characterisation and evaluation. Drug Deliv. Transl. Res. 2024, 15, 355–371. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Maw, P.D.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Tacrolimus/hydroxypropyl-β-cyclodextrin-loaded nanoemulsions stabilized by Zein-Soluplus® nanoparticles for retinal diseases. Drug Deliv. Sci. Technol. 2023, 88, 104936. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guembe-Michel, N.; Nguewa, P.; González-Gaitano, G. Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications. Int. J. Mol. Sci. 2025, 26, 1499. https://doi.org/10.3390/ijms26041499
Guembe-Michel N, Nguewa P, González-Gaitano G. Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications. International Journal of Molecular Sciences. 2025; 26(4):1499. https://doi.org/10.3390/ijms26041499
Chicago/Turabian StyleGuembe-Michel, Nerea, Paul Nguewa, and Gustavo González-Gaitano. 2025. "Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications" International Journal of Molecular Sciences 26, no. 4: 1499. https://doi.org/10.3390/ijms26041499
APA StyleGuembe-Michel, N., Nguewa, P., & González-Gaitano, G. (2025). Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications. International Journal of Molecular Sciences, 26(4), 1499. https://doi.org/10.3390/ijms26041499







