Insights from Tandem Mass Tag (TMT) Proteomic Analysis on Protein Network Modification in Control of Yak Hair Follicle Cycle
Abstract
1. Introduction
2. Results
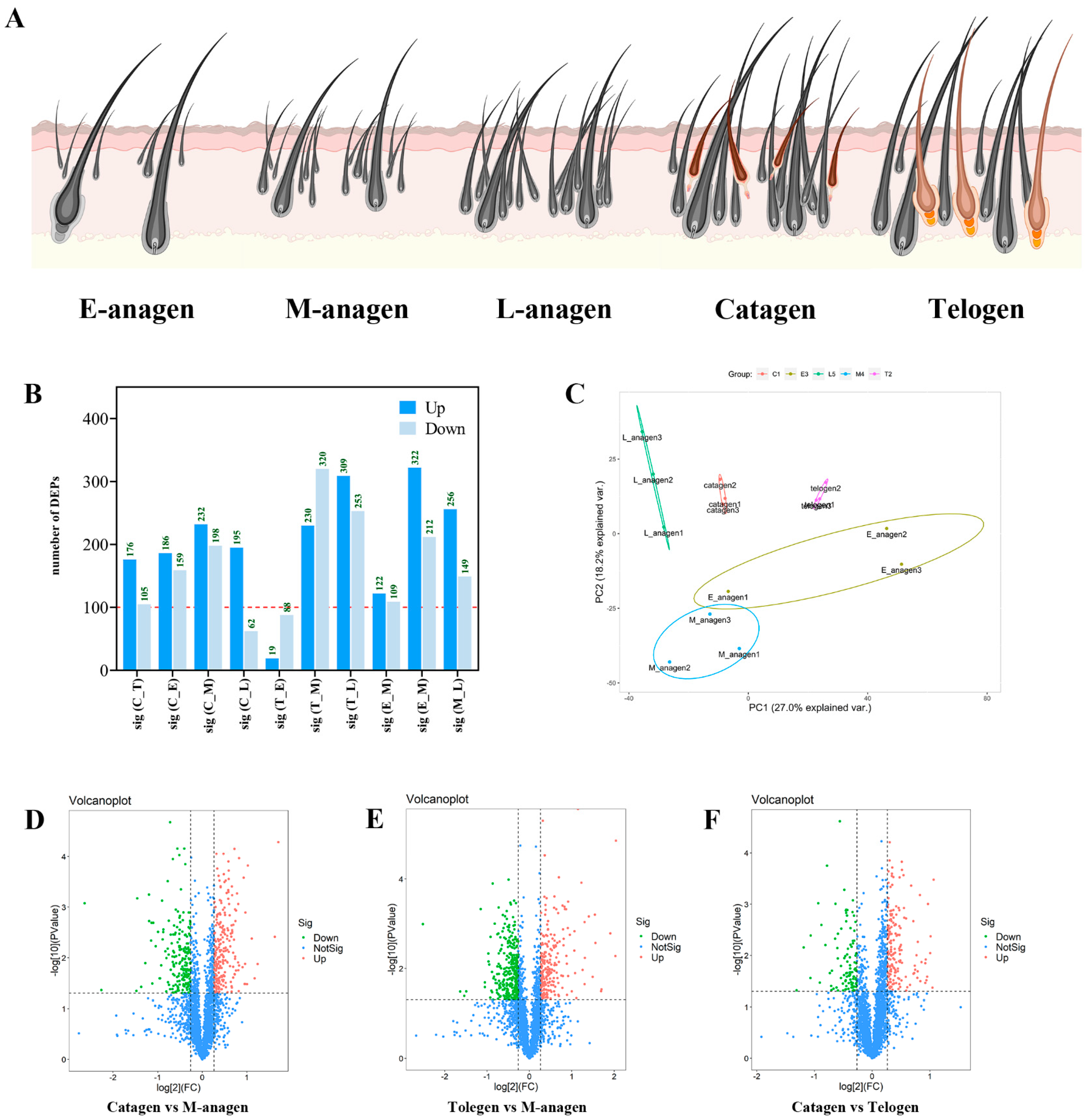
2.1. Yak HFs Cycle Histological Characteristics
2.2. Overview of TMT Proteomics Data
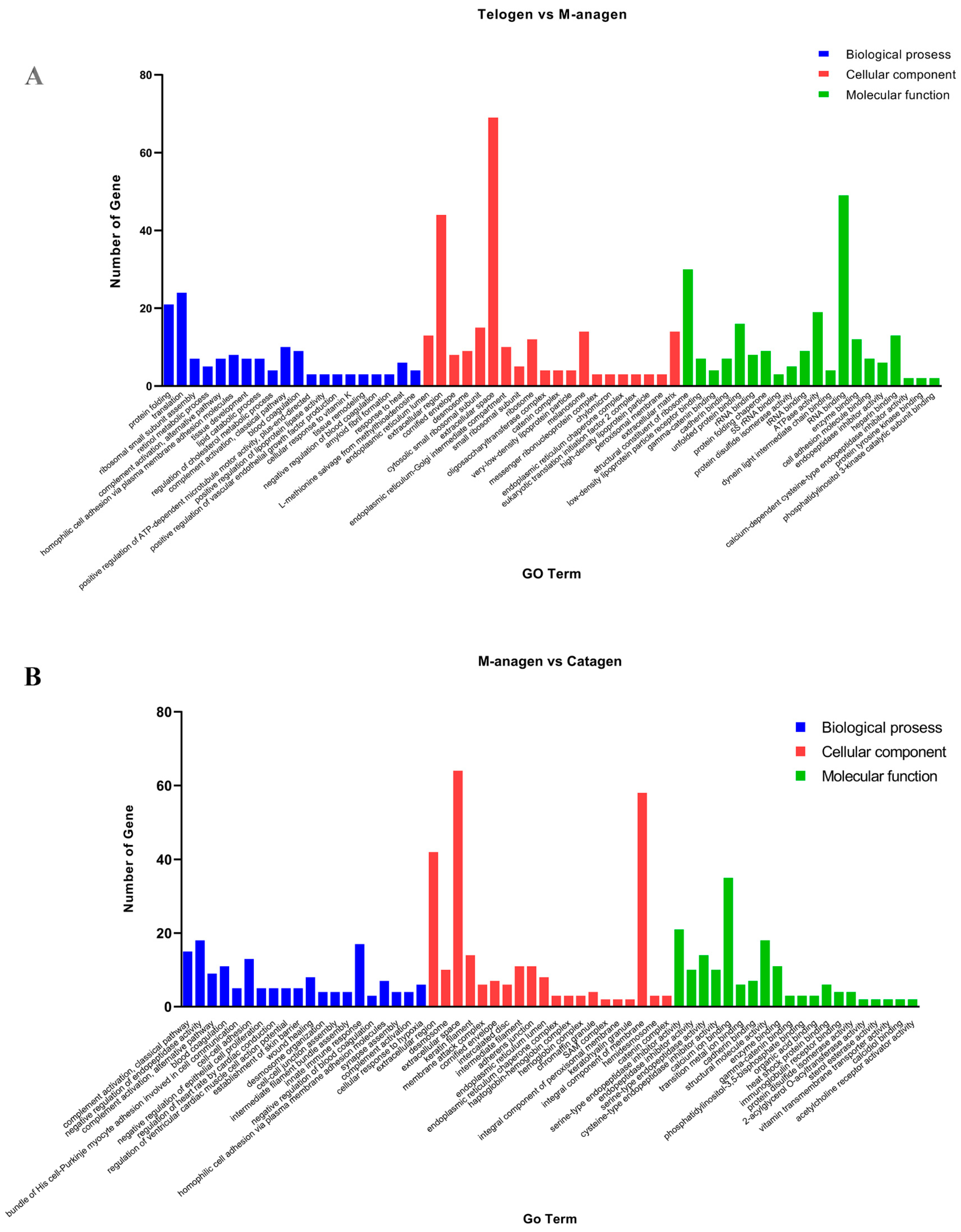
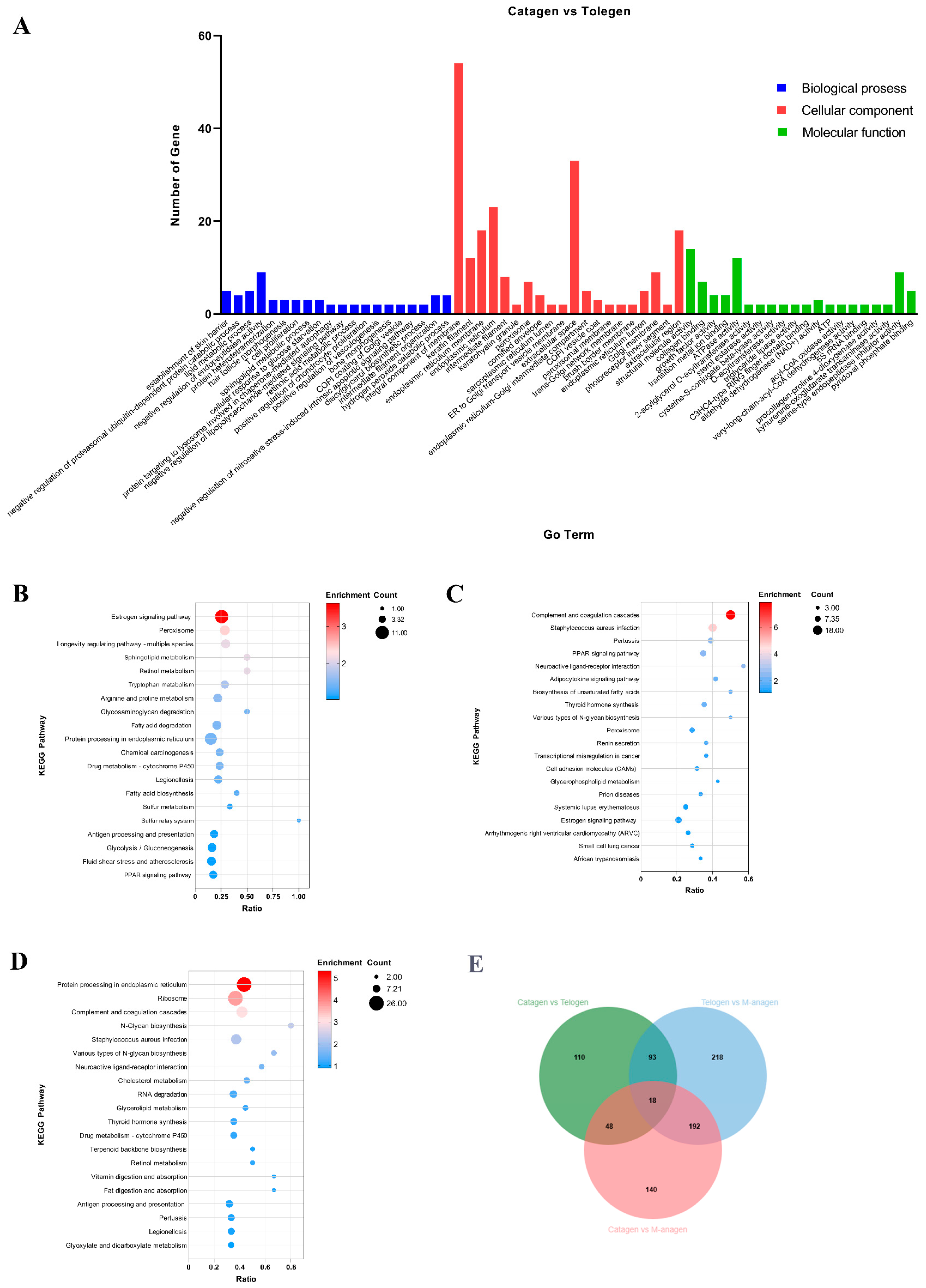
2.3. GO and KEGG Enrichment Analyses of DEGs
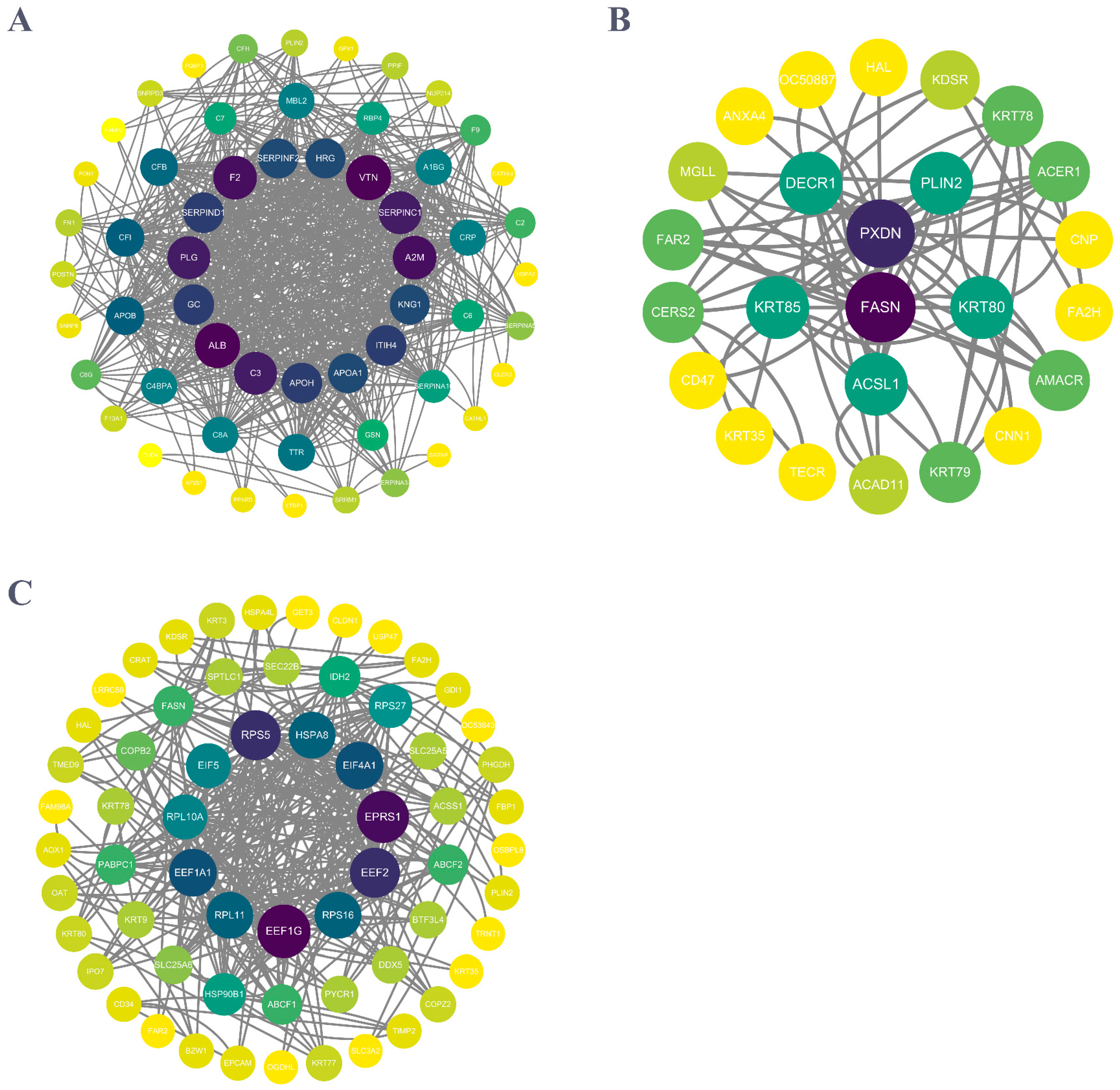
2.4. PPI Network Construction and Analysis
2.5. Protein Validation
3. Discussion
4. Materials and Methods
4.1. Animal and Sample Collection
4.2. Preparation of Tissue Sections and HE Staining
4.3. Protein Extraction and SDS-PAGE
4.4. Protein Sample Digestion and TMT Labelling
4.5. LC–MS/MS Analysis and Database Search
4.6. Bioinformatics Analysis
4.7. Western Blot Analysis
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, B.; Cui, Y.; Yu, S.; He, J.; Yang, X.; Zou, S.; Li, S.; Zhao, P.; Xu, H.; Long, M.; et al. Histological characteristics of hair follicles at different hair cycle and in vitro modeling of hair follicle-associated cells of yak (Bos grunniens). Front. Vet. Sci. 2023, 10, 1277586. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Cui, Y.; Yu, S.; Li, S.; Afedo, S.Y.; Wang, Y.; Bai, X.; He, J. Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell. Signal. 2021, 87, 110140. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Luo, J.; Liu, Y.; Chu, M.; Ren, Q.; Guo, X.; Tang, B.; Ding, X.; Qiu, Q.; Pan, H.; et al. The seasonal development dynamics of the yak hair cycle transcriptome. BMC Genom. 2020, 21, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, P.; Zheng, Q.; Chu, M.; Liang, C.; Guo, X.; Wu, X.; He, M.; Pei, C.; Yan, P. Comparative Analysis of mRNA and miRNA Expression between Dermal Papilla Cells and Hair Matrix Cells of Hair Follicles in Yak. Cells 2022, 11, 3985. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Cui, Y.; Yu, S.; Liu, P.; He, J. TGF-β and HSP70 profiles during transformation of yak hair follicles from the anagen to catagen stage. J. Cell. Physiol. 2019, 234, 15638–15646. [Google Scholar] [CrossRef]
- Yang, X.; Cui, Y.; Yue, J.; He, H.; Yu, C.; Liu, P.; Liu, J.; Ren, X.; Meng, Y. The histological characteristics, age-related thickness change of skin, and expression of the HSPs in the skin during hair cycle in yak (Bos grunniens). PLoS ONE 2017, 12, e0176451. [Google Scholar] [CrossRef]
- Alexander, R.M.M.; Ruben, P.; Doris, B.; Herwig, P. Porosity at Different Structural Levels in Human and Yak Belly Hair and Its Effect on Hair Dyeing. Molecules 2020, 25, 2143. [Google Scholar] [CrossRef]
- Song, L.-L.; Cui, Y.; Yu, S.-J.; Liu, P.-G.; Liu, J.; Yang, X.; He, J.-F.; Zhang, Q. Expression characteristics of BMP2, BMPR-IA and Noggin in different stages of hair follicle in yak skin. Gen. Comp. Endocrinol. 2018, 260, 18–24. [Google Scholar] [CrossRef]
- Song, L.; Cui, Y.; Xiao, L.; Yu, S.; He, J. DHT and E2 synthesis-related proteins and receptors expression in male yak skin during different hair follicle stages. Gen. Comp. Endocrinol. 2020, 286, 113245. [Google Scholar] [CrossRef]
- Charlie, C.-P.; Oussama El, B.; Louis, D.; Vincent, B.; Valérie, A.; Laurent, R.; Stéphane, B. Regulation of stem cell fate by HSPGs: Implication in hair follicle cycling. npj Regen. Med. 2022, 7, 77. [Google Scholar] [CrossRef]
- Maksim, V.P.; Ruth, E.B.; Chih Chiang, C.; Clyde, F.; Damon de la, C.; Thomas, A.; Philip, K.M.; Sarah, E.M.; Randall, B.W.; Cheng Ming, C. Self-Organizing and Stochastic Behaviors During the Regeneration of Hair Stem Cells. Science 2011, 332, 586–589. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Z.; Zhao, M.; Mu, Q.; Che, T.; Xie, Y.; Ma, L.; Mi, L.; Li, J.; Zhao, Y. Skin transcriptome reveals the periodic changes in genes underlying cashmere (ground hair) follicle transition in cashmere goats. BMC Genom. 2020, 21, 392. [Google Scholar] [CrossRef]
- Wang, W.-H.; Ramos, R.; Tai, K.-Y.; Wu, Y.-S.; Chang, T.-Y.; Yan, J.-Y.; Plikus, M.V.; Oh, J.W.; Lin, S.-J. Studying Hair Growth Cycle and its Effects on Mouse Skin. J. Investig. Dermatol. 2023, 143, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Wikramanayake, T.; Akhundlu, A.; Haberland, N.I.; Suzuki, T.; Cheret, J.; Nicu, C.; Paus, R. 1433 Epithelially derived, mitochondrial MPZL3 negatively regulates murine and human hair follicle cycling. J. Investig. Dermatol. 2023, 143, S245. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Valentina, G.; Ting, C.; Michael, R.; Markus, S.; Pasolli, H.A.; Nicole, S.; June dela, C.-R.; Elaine, F. A Two-Step Mechanism for Stem Cell Activation during Hair Regeneration. Cell Stem Cell 2009, 4, 464. [Google Scholar] [CrossRef]
- Wang, Q.; Oh, J.W.; Lee, H.-L.; Dhar, A.; Peng, T.; Ramos, R.; Guerrero-Juarez, C.F.; Wang, X.; Zhao, R.; Cao, X.; et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. eLife 2017, 6, e22772. [Google Scholar] [CrossRef]
- Protein Function. In Encyclopedic Dictionary of Genetics, Genomics and Proteomics; Wiley: Hoboken, NJ, USA, 2004. [CrossRef]
- Wang, C.; Yu, J.; Zhang, R.; Wang, W.; Shi, Z.; Liu, Y.; Song, G.; Wang, H.; Han, N.; Huang, L.; et al. Small intestine proteomics coupled with serum metabolomics reveal disruption of amino acid metabolism in Chinese hamsters with type 2 diabetes mellitus. J. Proteom. 2020, 223, 103823. [Google Scholar] [CrossRef]
- Ulrich, O.; Murat, U.; José, I.; Jan Åke, G.; Ralf, P. The Hair Follicle as an Estrogen Target and Source. Endocr. Rev. 2006, 27, 677–706. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, K.; Xu, M.; Andl, T.; Millar, S.E.; Boyce, S.; Zhang, Y. Activating β-catenin signaling in CD133-positive dermal papilla cells increases hair inductivity. FEBS J. 2016, 283, 2823–2835. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Harel, S.; Higgins, C.A.; Cerise, J.E.; Dai, Z.; Chen, J.C.; Clynes, R.; Christiano, A.M. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 2015, 1, e1500973. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xia, W.; Xu, P.; Shen, H.; Dai, X.; Liu, M.; Shi, Y.; Ye, X.; Dang, Y. Lgr4 Deletion Delays the Hair Cycle and Inhibits the Activation of Hair Follicle Stem Cells. J. Investig. Dermatol. 2020, 140, 1706–1712. [Google Scholar] [CrossRef]
- Robert, H.R.; David, M.R.; Hua Sheng, T.; Kathleen, A.S.; Young Jin, L.; John, P.S. Distinguishing Mouse Strains by Proteomic Analysis of Pelage Hair. J. Investig. Dermatol. 2009, 129, 2120–2125. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, N.; Jia, X.; Hua, T.; Zhu, J.; Ding, C.; Yang, D.; Luo, J.; Wang, M.; Luo, J.; et al. Ultra-Strong Regenerated Wool Keratin Fibers Regulating via Keratin Conformational Transition. Adv. Funct. Mater. 2023, 33, 2301447. [Google Scholar] [CrossRef]
- Sowmya, S. Deciphering keratin function. Nat. Rev. Mol. Cell Biol. 2008, 9, s17. [Google Scholar] [CrossRef]
- Waleed, R.; Sepideh, A.; Andrew, H.; Eko, R.; Ranjan, K.; Akitsu, H.; Scott, T.M.; Daniel, M.; Jeff, B. Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, X.; Du, J.; Zeng, W.; Wu, W.; Di, J.; Huang, X.; Tian, K. Differential Methylation and Transcriptome Integration Analysis Identified Differential Methylation Annotation Genes and Functional Research Related to Hair Follicle Development in Sheep. Front. Genet. 2021, 12, 735827. [Google Scholar] [CrossRef] [PubMed]
- Frances, S.; Neil, W.; Celia, M.; Patricia, J.C.D.-H.; John, A.M. Compound heterozygous mutations in desmoplakin cause skin fragility and woolly hair. Br. J. Dermatol. 2011, 166, 894–896. [Google Scholar] [CrossRef]
- Bertrand, F.; Nadja, B.; Luca, B. A recessive mutation in the DSP gene linked to cardiomyopathy, skin fragility and hair defects impairs the binding of desmoplakin to epidermal keratins and the muscle-specific intermediate filament desmin. Br. J. Dermatol. 2018, 179, 797–799. [Google Scholar] [CrossRef]
- Lee, J.Y.W.; John, A.M. Mutations in genes encoding desmosomal proteins: Spectrum of cutaneous and extracutaneous abnormalities*. Br. J. Dermatol. 2020, 184, 596–605. [Google Scholar] [CrossRef]
- Hannes, K.; Corinne, D.; Johannes, G.; Hendrik Jan, A.; Peter, F.; Michael, M. 158 The keratin-associated protein epiplakin is down-regulated in psoriasis. J. Investig. Dermatol. 2023, 143, S359. [Google Scholar] [CrossRef]
- Nathan, J.H.; Jonathan, A.H.; Majid, A.; Francisco, J.; Ralf, P. Deciphering the molecular morphology of the human hair cycle: Wnt signalling during the telogen–anagen transformation. Br. J. Dermatol. 2019, 182, 1184–1193. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; et al. Insights into male androgenetic alopecia using comparative transcriptome profiling: Hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Shigetoshi, S.; Masahiro, K.; Satoshi, T.; Kunihiko, Y.; Junji, T.; Satoshi, I. Two distinct signaling pathways in hair cycle induction: Stat3-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 13824–13829. [Google Scholar] [CrossRef]
- Anandaroop, M.; Suguna Rani, K.; Benjamin, Y. Activated Kras Alters Epidermal Homeostasis of Mouse Skin, Resulting in Redundant Skin and Defective Hair Cycling. J. Investig. Dermatol. 2011, 131, 311–319. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.-P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.-L.; Huang, S.M.; et al. TRP Channel Regulates EGFR Signaling in Hair Morphogenesis and Skin Barrier Formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Liraz, L.; Zeneng, W.; Mary Kathryn, D.; Stanley, L.H.; Noa, N. Saturated fatty acids regulate retinoic acid signalling and suppress tumorigenesis by targeting fatty acid-binding protein 5. Nat. Commun. 2015, 6, 8794. [Google Scholar] [CrossRef]
- Thaddeus, T.S.; Daniel, C.B.; Natacha, S.S.; Skylar, N.T.; Noa, N. Opposing Effects of Retinoic Acid on Cell Growth Result from Alternate Activation of Two Different Nuclear Receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef]
- Susmita, K.; Ana María, C. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy 2016, 12, 432–438. [Google Scholar] [CrossRef]
- Kenji, N.; Taichi, M.; Toshihiro, S.; Masashi, M.; Kiyoe, K.; Seiji, K.; Masanori, T.; Tatsuya, T.; Sumiko, Y.; Kojiro, N.; et al. Circulating Apolipoprotein L1 is associated with insulin resistance-induced abnormal lipid metabolism. Sci. Rep. 2019, 9, 14869. [Google Scholar] [CrossRef]
- Tan, Y.; Bian, Y.; Song, Y.; Zhang, Q.; Wan, X. Exosome-Contained APOH Associated with Antiphospholipid Syndrome. Front. Immunol. 2021, 12, 604222. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.C.Y.; Kam-Suen, C.; Cheung, J.T.; Jin, L.; Yan, L.; Yao, W.; William, W.L.F.; Jieling, C.; Samson, W.M.C.; Bernhard, D.; et al. Gut Microbiota-Associated Activation of TLR5 Induces Apolipoprotein A1 Production in the Liver. Circ. Res. 2020, 127, 1236–1252. [Google Scholar] [CrossRef]
- Eric, F.; Jackie, A.F.; Ryan, B.; Schmidt, B.; Matthew, S.R.; Mark, C.H.; Valerie, H. Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Christian, F.G.J.; Maksim, V.P. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163–173. [Google Scholar] [CrossRef]
- Anna, E.; Niklas, M.; Silvia, L.C.; Gaby, Å.; Ingrid, D.; Jurga, L.; Mikael, R. Characterization of the Wnt Inhibitors Secreted Frizzled-Related Proteins (SFRPs) in Human Adipose Tissue. J. Clin. Endocrinol. Metab. 2013, 98, E503–E508. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Gou, M.; Zhu, C.; Guo, W.; Hu, R.; Wang, L.; Liu, X. Potential mechanism of improved cryotolerance of Pediococcus pentosaceus YT2 by cold acclimation based on TMT quantitative proteomics analysis. LWT 2024, 192, 115714. [Google Scholar] [CrossRef]
- Liu, X.; Suo, R.; Wang, H.; Wang, W.; Sun, J.; Wang, J. TMT proteomics establishes correlations between solar drying and quality modifications in Penaeus vannamei. Food Chem. 2024, 441, 138330. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.N.; Khalid, M.S.; Arwa, A.; Mai Abdel, J.; Essa, M.S.; Mohammad, A.A.M.; Anas, M.A.R. Dystrophin Protein Quantification as a Duchenne Muscular Dystrophy Diagnostic Biomarker in Dried Blood Spots Using Multiple Reaction Monitoring Tandem Mass Spectrometry: A Preliminary Study. Molecules 2022, 27, 3662. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Cui, Y.; Yu, S.; He, J.; Ma, R.; Liao, B.; Zhao, P.; Wei, P.; Robert, N. Insights from Tandem Mass Tag (TMT) Proteomic Analysis on Protein Network Modification in Control of Yak Hair Follicle Cycle. Int. J. Mol. Sci. 2025, 26, 1532. https://doi.org/10.3390/ijms26041532
Li S, Cui Y, Yu S, He J, Ma R, Liao B, Zhao P, Wei P, Robert N. Insights from Tandem Mass Tag (TMT) Proteomic Analysis on Protein Network Modification in Control of Yak Hair Follicle Cycle. International Journal of Molecular Sciences. 2025; 26(4):1532. https://doi.org/10.3390/ijms26041532
Chicago/Turabian StyleLi, Shijie, Yan Cui, Sijiu Yu, Junfeng He, Rui Ma, Bo Liao, Pengfei Zhao, Pengqiang Wei, and Niayaler Robert. 2025. "Insights from Tandem Mass Tag (TMT) Proteomic Analysis on Protein Network Modification in Control of Yak Hair Follicle Cycle" International Journal of Molecular Sciences 26, no. 4: 1532. https://doi.org/10.3390/ijms26041532
APA StyleLi, S., Cui, Y., Yu, S., He, J., Ma, R., Liao, B., Zhao, P., Wei, P., & Robert, N. (2025). Insights from Tandem Mass Tag (TMT) Proteomic Analysis on Protein Network Modification in Control of Yak Hair Follicle Cycle. International Journal of Molecular Sciences, 26(4), 1532. https://doi.org/10.3390/ijms26041532




