Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease
Abstract
1. Introduction
2. Results
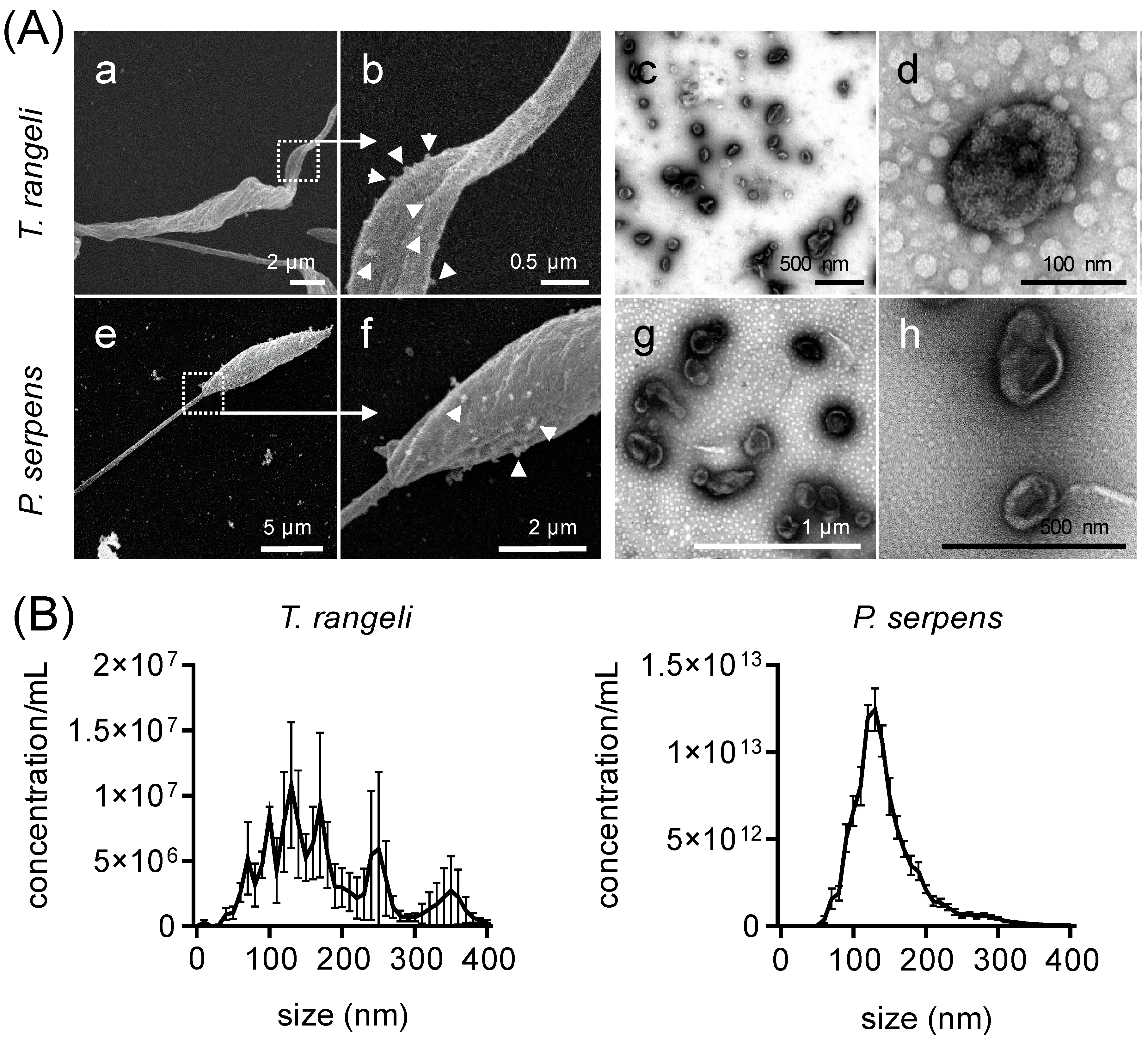
2.1. Non-Pathogenic Trypanosomatids Shed Extracellular Vesicles
2.2. T. rangeli EVs Cross-Reacted with Serum from Both Murine and Human T. cruzi-Infected Individuals
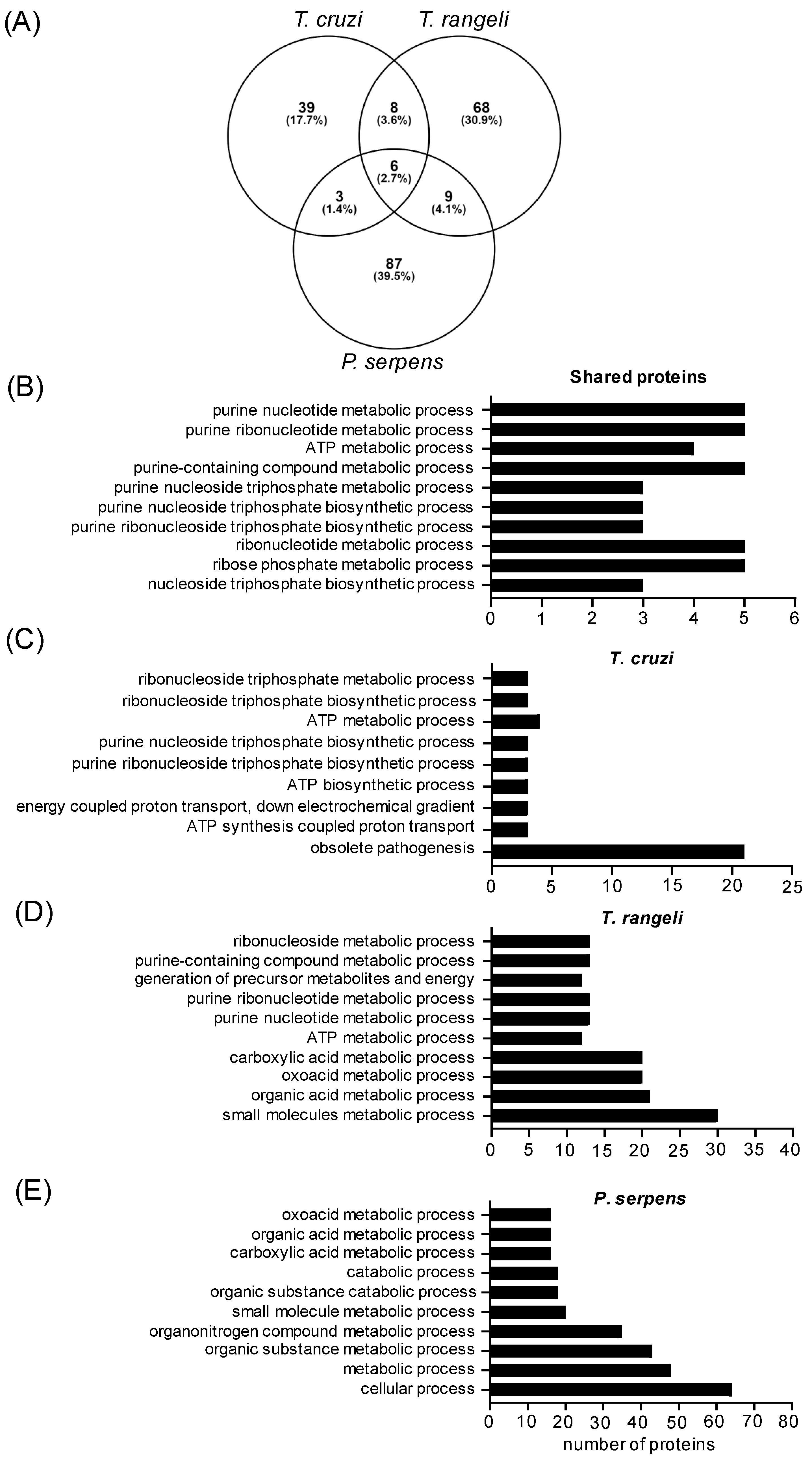
2.3. Common Proteins Shared Among Trypanosomatid EVs
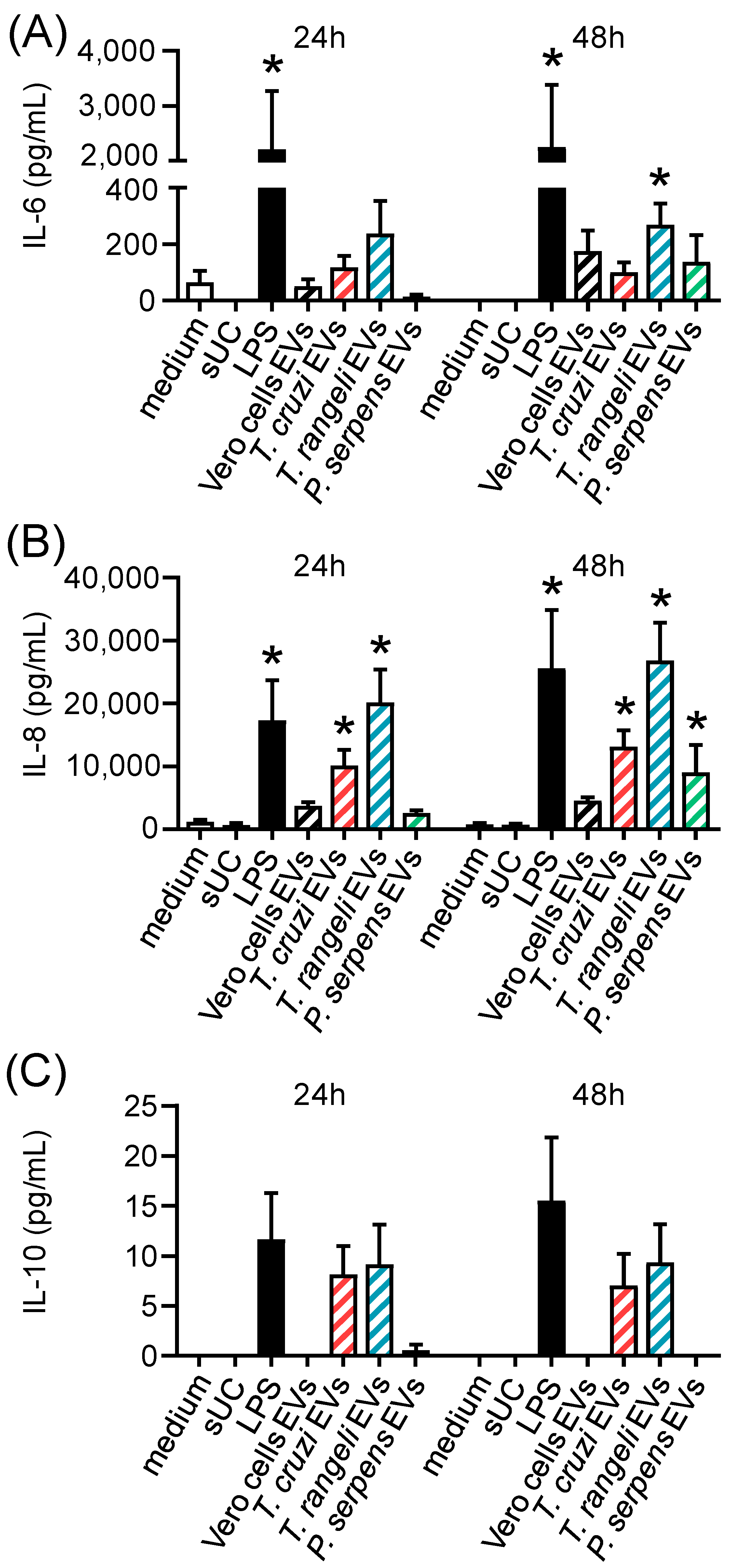
2.4. Trypanosomatid EVs Are Inflammatory but Mildly Modulate CD80 Expression
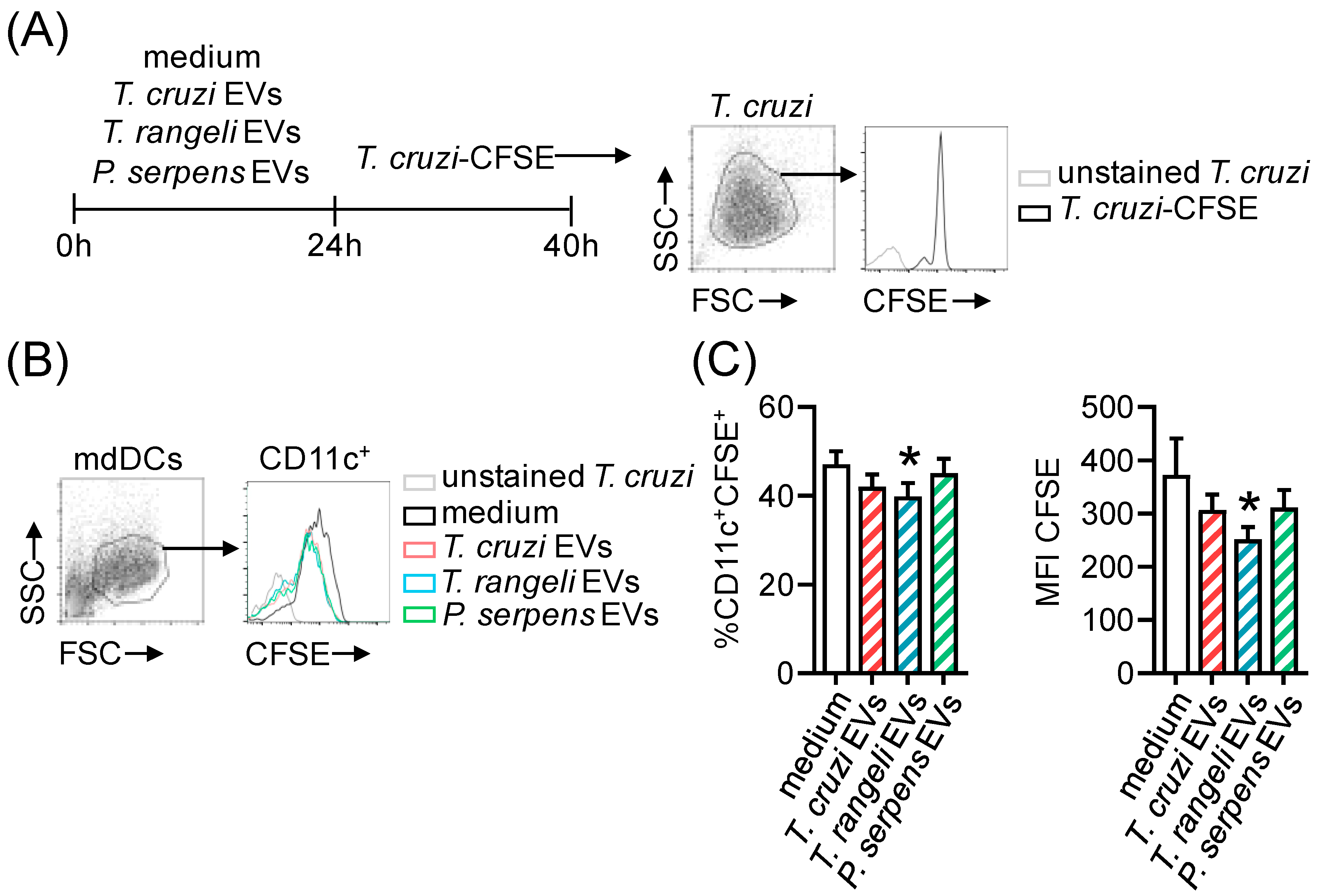
2.5. Prior Stimulation with Trypanosomatid EVs In Vitro Reduces T. cruzi Infection
2.6. T. cruzi-Related Parasite EV Immunization Protects Mice from T. cruzi Infection
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cells
5.2. Parasites
5.3. Extracellular Vesicles
5.4. Electron Microscopy
5.5. ELISA
5.6. Mass Spectrometry-Based Proteomic Analysis (LC-MS/MS)
5.7. Assignment of Proteins Domains and Orthologs
5.8. Extracellular Vesicles In Vitro Stimulation
5.9. Flow Cytometry
5.10. Mice Challenge
5.11. Statistical and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication is key: Extracellular vesicles as mediators of infection and defence during host–microbe interactions in animals and plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Anderson, H.C.; Mulhall, D.; Garimella, R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Investig. 2010, 90, 1549–1557. [Google Scholar] [CrossRef]
- Drurey, C.; Coakley, G.; Maizels, R.M. Extracellular vesicles: New targets for vaccines against helminth parasites. Int. J. Parasitol. 2020, 50, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Hoshina, T.; Matsuzaki, J.; Yoshioka, Y.; Kadota, T.; Hosaka, Y.; Fujimoto, S.; Kawamoto, H.; Watanabe, N.; Sawaki, K.; et al. Early prediction of COVID-19 severity using extracellular vesicle COPB2. J. Extracell. Vesicle 2021, 10, e12092. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 30 August 2024).
- Kratz, J.M.; Gonçalves, K.R.; Romera, L.M.; Moraes, C.B.; Bittencourt-Cunha, P.; Schenkman, S.; Chatelain, E.; Sosa-Estani, S. The translational challenge in Chagas disease drug development. Mem. Inst. Oswaldo Cruz 2022, 117, e200501. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.M. Chagas Disease: Epidemiology and Barriers to Treatment. Am. J. Med. 2020, 133, 1262–1265. [Google Scholar] [CrossRef]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef]
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An Updated View of the Trypanosoma cruzi Life Cycle: Intervention Points for an Effective Treatment. ACS Infect. Dis. 2022, 8, 1107–1115. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Deolindo, P.; de Messias-Reason, I.J.; Arigi, E.A.; Choi, H.; Almeida, I.C.; Evans-Osses, I. Dynamic flux of microvesicles modulate parasite-host cell interaction of Trypanosoma cruzi in eukaryotic cells. Cell Microbiol. 2017, 19, e12672. [Google Scholar] [CrossRef]
- Cornet-Gomez, A.; Retana Moreira, L.; Kronenberger, T.; Osuna, A. Extracellular vesicles of trypomastigotes of Trypanosoma cruzi induce changes in ubiquitin-related processes, cell-signaling pathways and apoptosis. Sci. Rep. 2023, 13, 7618. [Google Scholar] [CrossRef]
- Trocoli Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; e Silva, N.C.; de Almeida Abrahamsohn, I.; Colli, W.; Alves, M.J.M. Trypanosoma. cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 2009, 11, 29–39. [Google Scholar] [CrossRef]
- Lovo-Martins, M.I.; Malvezi, A.D.; Zanluqui, N.G.; Lucchetti, B.F.C.; Tatakihara, V.L.H.; Mörking, P.A.; Oliveira, A.G.D.; Goldenberg, S.; Wowk, P.F.; Pinge-Filho, P. Extracellular Vesicles Shed By Trypanosoma cruzi Potentiate Infection and Elicit Lipid Body Formation and PGE2 Production in Murine Macrophages. Front. Immunol. 2018, 9, 896. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Rodríguez Serrano, F.; Osuna, A. Extracellular vesicles of Trypanosoma cruzi tissue-culture cell-derived trypomastigotes: Induction of physiological changes in non-parasitized culture cells. PLoS Negl. Trop Dis. 2019, 13, e0007163. [Google Scholar] [CrossRef]
- Khosravi, M.; Mirsamadi, E.S.; Mirjalali, H.; Zali, M.R. Isolation and Functions of Extracellular Vesicles Derived from Parasites: The Promise of a New Era in Immunotherapy, Vaccination, and Diagnosis. Int. J. Nanomed. 2020, 15, 2957–2969. [Google Scholar] [CrossRef]
- Lopes, J.D.; Caulada, Z.; Barbieri, C.L.; Camargo, E.P. Cross-reactivity between Trypanosoma cruzi and insect trypanosomatids as a basis for the diagnosos of Chagas’ disease. Am. J. Trop Med. Hyg. 1991, 30, 1183–1188. [Google Scholar] [CrossRef]
- Breganó, J.W.; Picão, R.C.; Graça, V.K.; Menolli, R.A.; Itow Jankevicius, S.; Filho, P.P.; Jankevicius, J.V. Phytomonas serpens, a tomato parasite, shares antigens with Trypanosoma cruzi that are recognized by human sera and induce protective immunity in mice. FEMS Immunol. Med. Microbiol. 2003, 39, 257–264. [Google Scholar] [CrossRef]
- Basso, B.; Castro, I.; Introini, V.; Gil, P.; Truyens, C.; Moretti, E. Vaccination with Trypanosoma rangeli reduces the infectiousness of dogs experimentally infected with Trypanosoma cruzi. Vaccine 2007, 25, 3855–3858. [Google Scholar] [CrossRef] [PubMed]
- O’Daly, J.A.; Carrasco, H.; Fernandez, V.; Rodríguez, M.B. Comparison of chagasic and non-chagasic myocardiopathies by ELISA and immunoblotting with antigens of Trypanosoma cruzi and Trypanosoma rangeli. Acta Trop 1994, 56, 265–287. [Google Scholar] [CrossRef]
- de Moraes, M.H.; Guarneri, A.A.; Girardi, F.P.; Rodrigues, J.B.; Eger, I.; Tyler, K.M.; Steindel, M.; Grisard, E.C. Different serological cross-reactivity of Trypanosoma rangeli forms in Trypanosoma cruzi-infected patients sera. Parasites Vectors 2008, 1, 20. [Google Scholar] [CrossRef]
- Saldaña, A.; Sousa, O.E. Trypanosoma rangeli: Epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J. Parasitol. 1996, 82, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Moretti, E.; Fretes, R. Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection. Mem. Inst. Oswaldo Cruz. 2008, 103, 370–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paláu, M.T.; Mejía, A.J.; Vergara, U.; Zúñiga, C.A. Action of Trypanosoma rangeli in infections with virulent Trypanosoma cruzi populations. Mem. Inst. Oswaldo Cruz 2003, 98, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.V.; Malvezi, A.D.; Augusto, L.D.S.; Kian, D.; Tatakihara, V.L.H.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Rizzo, L.V.; Schenkman, S.; Pinge-Filho, P. Oral Exposure to Phytomonas serpens Attenuates Thrombocytopenia and Leukopenia during Acute Infection with Trypanosoma cruzi. PLoS ONE 2013, 8, e68299. [Google Scholar] [CrossRef] [PubMed]
- Graça-de Souza, V.K.; Monteiro-Góes, V.; Manque, P.; Souza, T.A.C.B.; Corrêa, P.R.C.; Buck, G.A.; Ávila, A.R.; Yamauchi, L.M.; Pinge-Filho, P.; Goldenberg, S.; et al. Sera of chagasic patients react with antigens from the tomato parasite Phytomonas serpens. Biol. Res. 2010, 43, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Pinge-Filho, P.; Peron, J.P.S.; De Moura, T.R.; Menolli, R.A.; Graça, V.K.; Estevão, D.; Tadokoro, C.E.; Jankevicius, J.V.; Rizzo, L.V. Protective immunity against Trypanosoma cruzi provided by oral immunization with Phytomonas serpens: Role of nitric oxide. Immunol. Lett. 2005, 96, 283–290. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Hoi, H.; Yoshida, N.; Da Silveira, J.F.; Almeida, I.C. Proteomic analysis of Trypanosoma cruzi secretome: Characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, O.A.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.; et al. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicle 2015, 4, 28734. [Google Scholar] [CrossRef]
- Paranaiba, L.F.; Guarneri, A.A.; Torrecilhas, A.C.; Melo, M.N.; Soares, R.P. Extracellular vesicles isolated from Trypanosoma cruzi affect early parasite migration in the gut of Rhodnius prolixus but not in Triatoma infestans. Mem. Inst. Oswaldo Cruz 2019, 114, e190217. [Google Scholar] [CrossRef]
- Da Silveira, J.F.; Abrahamsohn, P.A.; Colli, W. Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi. Biochim. Biophys. Acta Biomembr. 1979, 550, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.; Umezawa, E.S.; Katzin, A.M.; de Souza, W.; Alves, M.J.; Zingales, B.; Colli, W. Trypanosoma cruzi: Shedding of surface antigens as membrane vesicles. Exp. Parasitol. 1991, 72, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cronemberger-Andrade, A.; Xander, P.; Soares, R.P.; Pessoa, N.L.; Campos, M.A.; Ellis, C.C.; Grajeda, B.; Ofir-Birin, Y.; Almeida, I.C.; Regev-Rudzki, N.; et al. Trypanosoma cruzi-Infected Human Macrophages Shed Proinflammatory Extracellular Vesicles That Enhance Host-Cell Invasion via Toll-Like Receptor 2. Front. Cell Infect. Microbiol. 2020, 10, 99. [Google Scholar] [CrossRef]
- Cestari, I.; Ansa-Addo, E.; Deolindo, P.; Inal, J.M.; Ramirez, M.I. Trypanosoma cruzi Immune Evasion Mediated by Host Cell-Derived Microvesicles. J. Immunol. 2012, 188, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.H.; Koo, S.J.; Gupta, S.; Liang, L.Y.; Bahar, B.; Silla, L.; Nuñez-Burgos, J.; Barrientos, N.; Zago, M.P.; Garg, N.J. Gene Expression Profiling and Functional Characterization of Macrophages in Response to Circulatory Microparticles Produced during Trypanosoma cruzi Infection and Chagas Disease. J. Innate Immun. 2017, 9, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lozano, I.M.; De Pablos, L.M.; Longhi, S.A.; Zago, M.P.; Schijman, A.G.; Osuna, A. Immune complexes in chronic Chagas disease patients are formed by exovesicles from Trypanosoma cruzi carrying the conserved MASP N-terminal region. Sci. Rep. 2017, 7, 44451. [Google Scholar] [CrossRef] [PubMed]
- Madeira, R.P.; Dal’Mas Romera, L.M.; De Cássia Buck, P.; Mady, C.; Ianni, B.M.; Torrecilhas, A.C. New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response. J. Immunol. Res. 2021, 2021, 6650670. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, I.C.; Cazzulo, J.J.; Sánchez, D.O. gp63 Homologues in Trypanosoma cruzi: Surface Antigens with Metalloprotease Activity and a Possible Role in Host Cell Infection. Infect. Immun. 2003, 71, 5739–5749. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.M.; Olson, C.L.; Engman, D.M.; McGwire, B.S. Trypanosoma cruzi GP63 proteins undergo stage-specific differential posttranslational modification and are important for host cell infection. Infect. Immun. 2009, 77, 2193–2200. [Google Scholar] [CrossRef]
- Clemente, T.M.; Cortez, C.; Novaes, A.d.S.; Yoshida, N. Surface Molecules Released by Trypanosoma cruzi Metacyclic Forms Downregulate Host Cell Invasion. PLoS Negl. Trop Dis. 2016, 10, e0004883. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.; Nussenzweig, V. A Strain of Trypanosoma cruzi Highly Virulent for Mice. Folia Clin. Biol. 1953, 20, 191–208. [Google Scholar]
- Schottelius, J. Neuraminidase fluorescence test for the differentiation of Trypanosoma cruzi and Trypanosoma rangeli. Trop Med. Parasitol. 1987, 38, 323–327. [Google Scholar] [PubMed]
- Jankevicius, J. Axenic Cultivation of Trypanosomatids Found in Corn (Zea mays) and in Phytophagous Hemipterans (Leptoglossus zonatus Coreidae) and Their Experimental Transmission. J. Eukaryot. Microbiol. 1993, 40, 576–581. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Wowk, P.F.; Zardo, M.L.; Miot, H.T.; Goldenberg, S.; Carvalho, P.C.; Mörking, P.A. Proteomic profiling of extracellular vesicles secreted from Toxoplasma gondii. Proteomics 2017, 17, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef] [PubMed]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop Sao Paulo 1962, 4, 389–396. [Google Scholar] [PubMed]
- Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 16 February 2023).



| T. cruzi ID | T. rangeli ID | P. serpens ID | Ortholog Group | Protein Description |
|---|---|---|---|---|
| Proteins common to T. cruzi and T. rangeli EVs | ||||
| C4B63_231g29 | TRSC58_06706 | - | - | flagellar calcium-binding protein |
| C4B63_50g201 | TRSC58_04178 | - | - | surface protease GP63 * |
| C4B63_18g171 | TRSC58_01540 | - | - | ATP synthase, epsilon chain |
| C4B63_44g221 | TRSC58_06262 | - | - | cytoskeleton-associated protein CAP5.5 |
| C4B63_55g287c | TRSC58_06911 | - | - | cytochrome c |
| C4B63_43g126 | TRSC58_05688 | - | - | ATP synthase F1 subunit gamma protein |
| C4B63_52g93 | TRSC58_07087 | - | - | trans-sialidase *# |
| C4B63_26g286 | TRSC58_00214 | - | - | arginine kinase |
| Proteins common to T. cruzi, T. rangeli, and P. serpens EVs | ||||
| C4B63_13g157 | TRSC58_06854 | CCW59913.1 | OG6_100212 | elongation factor 2 |
| C4B63_351g33c | TRSC58_03454 | CCW64249.1 | OG6_148248 | calpain-like cysteine peptidase |
| C4B63_295g22 | TRSC58_02563 | CCW65611.1 | OG6_100111 | tryparedoxin peroxidase |
| C4B63_47g72 | TRSC58_07150 | CCW64268.1 | OG6_100260 | enolase |
| C4B63_51g126 | TRSC58_03588 | CCW60849.1 | OG6_100108 | alpha tubulin |
| C4B63_97g34 | TRSC58_04120 | CCW64916.1 | OG6_100641 | P-type H+-ATPase * |
| Proteins common to T. cruzi and P. serpens | ||||
| C4B63_4g422 | - | CCW60828.1 | - | C-terminal motor kinesin |
| C4B63_19g183 | - | CCW63407.1 | - | cyclophilin a |
| C4B63_292g78c | - | CCW64575.1 | - | calmodulin |
| Proteins common to T. rangeli and P. serpens EVs | ||||
| - | TRSC58_02653 | CCW64813.1 | OG6_100712 | adenylate kinase |
| - | TRSC58_01995 | CCW63656.1 | OG6_111169 | trypanothione synthetase |
| - | TRSC58_03782 | CCW67782.1 | OG6_100076 | ABC transporter |
| - | TRSC58_04008 | CCW64170.1 | OG6_101294 | eukaryotic initiation factor 4a |
| - | TRSC58_03503 | CCW64848.1 | OG6_100294 | threonyl-tRNA synthetase |
| - | TRSC58_03680 | CCW62159.1 | OG6_100353 | cystathione gamma lyase |
| - | TRSC58_04758 | CCW61177.1 | OG6_100715 | S-adenosylhomocysteine hydrolase |
| TRSC58_06901 | CCW63810.1 | OG6_100418 | hexokinase | |
| - | TRSC58_01051 | CCW63217.1 | OG6_103540 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase |
| spp. | ID | Protein Description | TMD | Epitopes |
|---|---|---|---|---|
| T. rangeli | TRSC58_02343 | hypothetical protein | 14 | na |
| TRSC58_06436 | hypothetical protein | 9 | na | |
| TRSC58_04120 | P-type H+-ATPase * | 7 | na | |
| TRSC58_00699 | hypothetical protein | 6 | na | |
| TRSC58_07060 | 40S ribosomal protein S3a | 2 | na | |
| TRSC58_00147 | hypothetical protein | 1 | na | |
| TRSC58_04043 | aspartate aminotransferase, mitochondrial | 1 | na | |
| TRSC58_04178 | surface protease GP63 * | 1 | na | |
| TRSC58_04835 | chaperonin GroEL | 1 | na | |
| TRSC58_05116 | calreticulin | 1 | na | |
| TRSC58_07191 | hypothetical protein | 1 | na | |
| T. cruzi | C4B63_44g146 | vacuolar proton pyrophosphatase 1 | 16 | 5 |
| C4B63_49g193 | trans-sialidase, Group II | 3 | 3 | |
| C4B63_26g29 | trans-sialidase, Group I | 1 | 2 | |
| C4B63_52g93 | trans-sialidase * | 1 | 1 | |
| C4B63_43g9 | serine carboxypeptidase (CBP1) | 1 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggio, J.B.; Vedam, V.V.; Nisimura, L.M.; da Silva, R.V.; Lovo-Martins, M.I.; Borges, B.S.; Mörking, P.A.; Batista, M.; Marchini, F.K.; Yamada-Ogatta, S.F.; et al. Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease. Int. J. Mol. Sci. 2025, 26, 1544. https://doi.org/10.3390/ijms26041544
Aggio JB, Vedam VV, Nisimura LM, da Silva RV, Lovo-Martins MI, Borges BS, Mörking PA, Batista M, Marchini FK, Yamada-Ogatta SF, et al. Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease. International Journal of Molecular Sciences. 2025; 26(4):1544. https://doi.org/10.3390/ijms26041544
Chicago/Turabian StyleAggio, Juliana Bernardi, Verônica Vitória Vedam, Líndice Mitie Nisimura, Rosiane Valeriano da Silva, Maria Izabel Lovo-Martins, Beatriz Santana Borges, Patrícia Alves Mörking, Michel Batista, Fabricio Klerynton Marchini, Sueli Fumie Yamada-Ogatta, and et al. 2025. "Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease" International Journal of Molecular Sciences 26, no. 4: 1544. https://doi.org/10.3390/ijms26041544
APA StyleAggio, J. B., Vedam, V. V., Nisimura, L. M., da Silva, R. V., Lovo-Martins, M. I., Borges, B. S., Mörking, P. A., Batista, M., Marchini, F. K., Yamada-Ogatta, S. F., Pinge-Filho, P., Goldenberg, S., Eger, I., & Wowk, P. F. (2025). Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease. International Journal of Molecular Sciences, 26(4), 1544. https://doi.org/10.3390/ijms26041544









