Eucommia ulmoides Oliv. Bark Extracts Alleviate MCAO/Reperfusion-Induced Neurological Dysfunction by Suppressing Microglial Inflammation in the Gray Matter
Abstract
1. Introduction
2. Results
2.1. HPLC-MS Analysis of WEU
2.2. WEU Ameliorated the Ischemia-Induced Deficits of Motor Coordination
2.3. WEU Decreased Infarction Size in Post-Stroke Mice
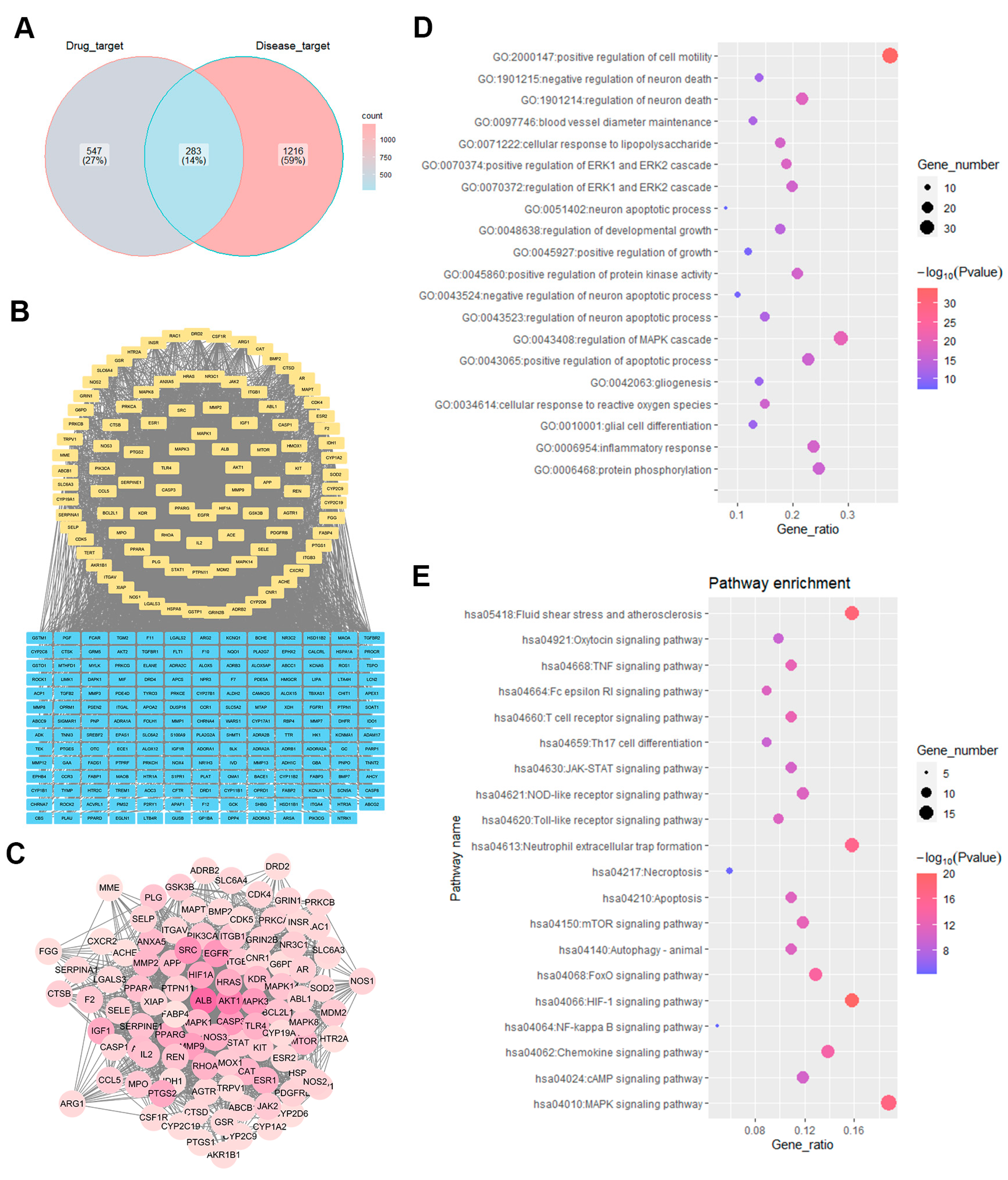
2.4. Identification of WEU Anti-Brain Injury and Dysfunction Target Pathways
2.5. WEU Attenuated the Ischemia-Induced Generation of Pro-Inflammatory Cytokines
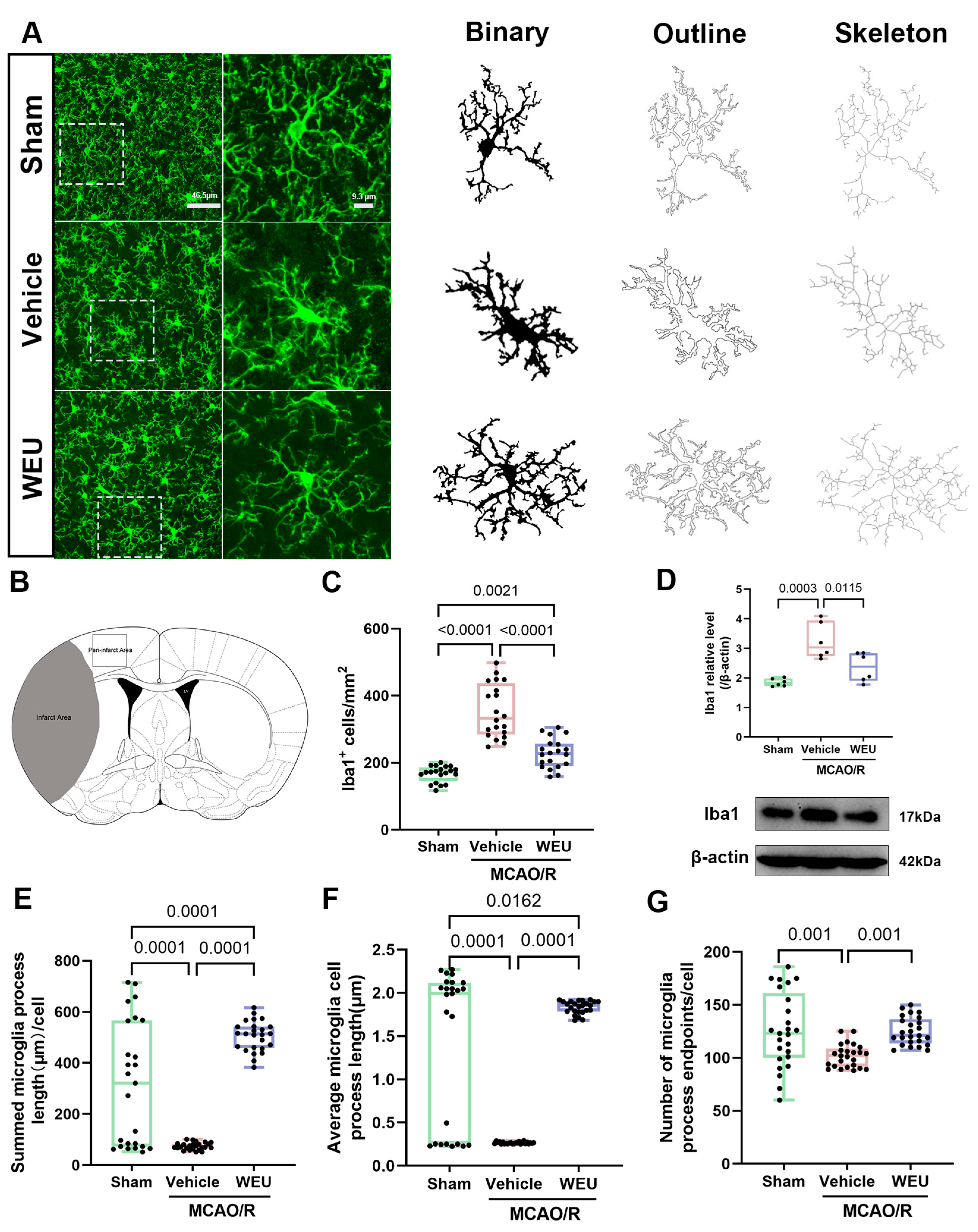
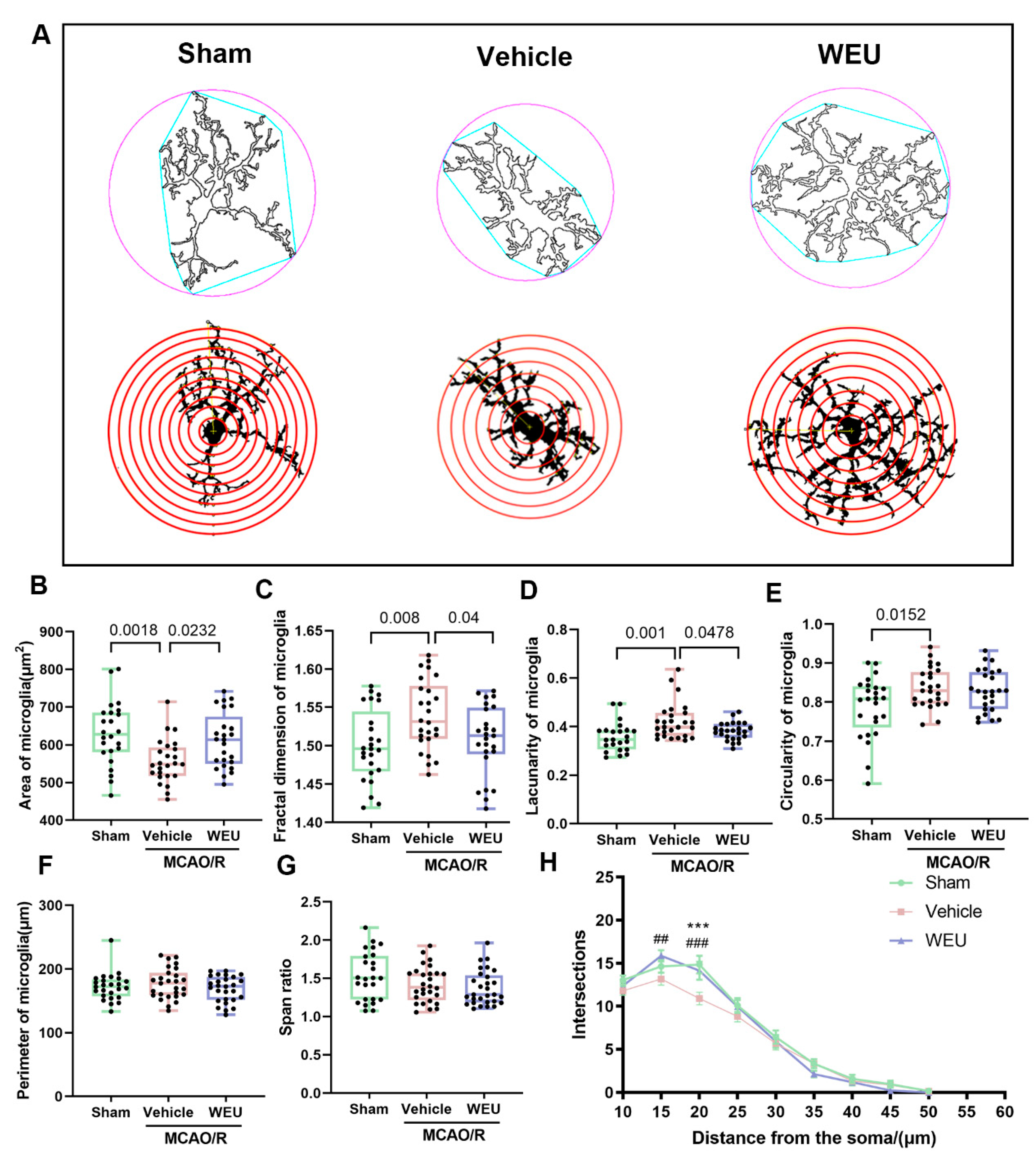
2.6. WEU Suppressed Cerebral Ischemia-Induced Activation of Cortical Microglia
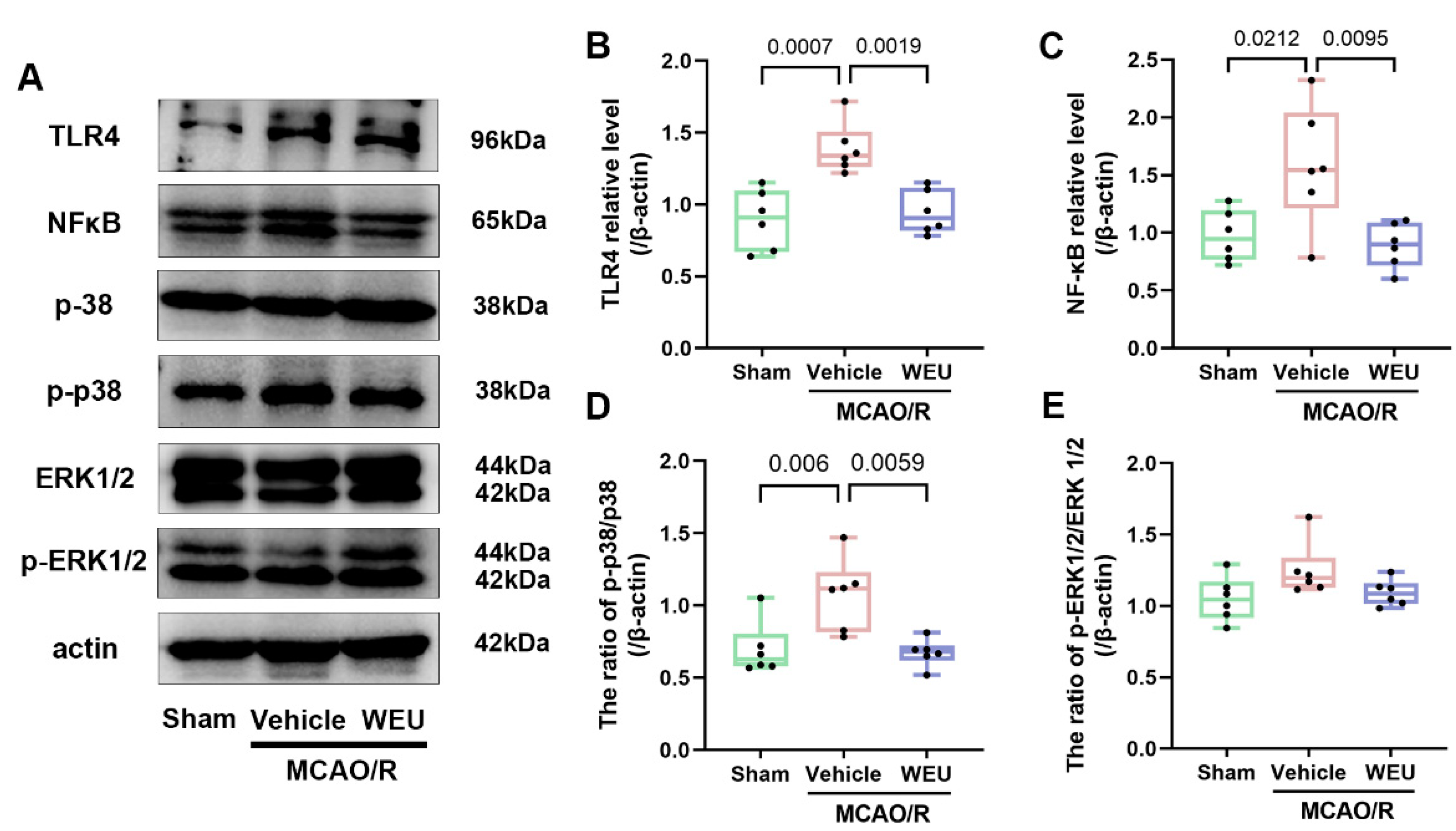
2.7. WEU Inhibited the Ischemia-Induced Activation of the TLR4/p38 MAPK and NF-κB Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Aqueous Extract
4.2. HPLC-MS Analysis
4.3. Animals and Ethical Statement
4.4. Middle Cerebral Artery Occlusion (MCAO) Model
4.5. Experimental Design
4.6. Assessment of Neurological Deficits
4.7. Behavioral Tests
4.7.1. Pole Test
4.7.2. Rotarod Test
4.7.3. Grip and String Tests
4.8. Calculation of Infarct Volume
4.9. Network Pharmacology Analysis
4.10. RNA Extraction and Real-Time Quantitative PCR
4.11. Immunofluorescence Staining
4.12. Microglia Analysis
4.13. Western Blot
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Jia, J.; Yang, L.; Chen, Y.; Zheng, L.; Chen, Y.; Xu, Y.; Zhang, M. The Role of Microglial Phagocytosis in Ischemic Stroke. Front. Immunol. 2021, 12, 790201. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Zhang, Y.; Liu, L.; Yang, L.; Cai, Y.; Zhang, J.; Xu, S. Neuroinflammation in Ischemic Stroke: Focus on MicroRNA-mediated Polarization of Microglia. Front. Mol. Neurosci. 2020, 13, 612439. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Knutsson, L.; Zhou, J.; van Zijl, P.C. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1500–1516. [Google Scholar] [CrossRef]
- Xiang, X.; Cao, F. Time window and “tissue window”: Two approaches to assist decision-making in strokes. J. Neurol. 2019, 266, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.T.; Lu, Y.; Yan, S.K.; Xiao, X.; Rong, X.L.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, C.; Yang, Y.; Ma, G.; Wei, G.; Liu, S.; Kong, L.; Du, G. Xiao-Xu-Ming decoction prevented hemorrhagic transformation induced by acute hyperglycemia through inhibiting AGE-RAGE-mediated neuroinflammation. Pharmacol. Res. 2021, 169, 105650. [Google Scholar] [CrossRef]
- Yu, P.; Wang, J.; Liu, J.; Zhou, Y.; Luo, F.; Yang, M.; Ai, X. Preparation techniques, structural features, and bioactivities of Eucommia ulmoides polysaccharides: A review. Int. J. Biol. Macromol. 2024, 275, 133686. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Liu, G.; Oladele, O.A.; Rahu, N.; Tossou, M.C.; Yin, Y. Health-Promoting Properties of Eucommia ulmoides: A Review. Evid. -Based Complement. Altern. Med. eCAM 2016, 2016, 5202908. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tang, L.; He, J.W.; Li, J.; Wang, Y.Z. Ethnobotany, Phytochemistry and Pharmacological Properties of Eucommia ulmoides: A Review. Am. J. Chin. Med. 2019, 47, 259–300. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, S.Y.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta(25-35)-induced learning and memory impairments in mice. Neurosci. Lett. 2011, 487, 123–127. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ma, S.X.; Joo, H.J.; Lee, S.Y.; Jang, C.G. Inhibitory Effects of Eucommia ulmoides Oliv. Bark on Scopolamine-Induced Learning and Memory Deficits in Mice. Biomol. Ther. 2013, 21, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yin, Q.; Li, D.; Ma, J.; Li, L.; Chai, S.; Guo, H.; Yang, Z. Anti-neuroinflammatory effects of Eucommia ulmoides Oliv. In a Parkinson’s mouse model through the regulation of p38/JNK-Fosl2 gene expression. J. Ethnopharmacol. 2020, 260, 113016. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kim, M.J.; Ma, S.X.; You, I.J.; Hwang, J.Y.; Oh, J.H.; Kim, S.Y.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Eucommia ulmoides Oliv. Bark. protects against hydrogen peroxide-induced neuronal cell death in SH-SY5Y cells. J. Ethnopharmacol. 2012, 142, 337–345. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.M.; Lee, H.L.; Go, M.J.; Lee, D.Y.; Kim, C.-W.; Kim, H.-J.; Heo, H.J. Eucommia ulmoides Leaves Alleviate Cognitive Dysfunction in Dextran Sulfate Sodium (DSS)-Induced Colitis Mice through Regulating JNK/TLR4 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4063. [Google Scholar] [CrossRef] [PubMed]
- Cotel, M.C.; Lenartowicz, E.M.; Natesan, S.; Modo, M.M.; Cooper, J.D.; Williams, S.C.; Kapur, S.; Vernon, A.C. Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur. Neuropsychopharmacol. 2015, 25, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Foster, T.D.; Thomas, W.E. Cellular forms and functions of brain microglia. Brain Res. Bull. 1994, 34, 73–78. [Google Scholar] [CrossRef]
- Binley, K.E.; Ng, W.S.; Tribble, J.R.; Song, B.; Morgan, J.E. Sholl analysis: A quantitative comparison of semi-automated methods. J. Neurosci. Methods 2014, 225, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Cramer, S.C. Recovery After Stroke. Continuum 2020, 26, 415–434. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Park, S.Y.; Karthivashan, G.; Ko, H.M.; Cho, D.Y.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-Kim, I.; Choi, D.K. Aqueous Extract of Dendropanax morbiferus Leaves Effectively Alleviated Neuroinflammation and Behavioral Impediments in MPTP-Induced Parkinson’s Mouse Model. Oxidative Med. Cell. Longev. 2018, 2018, 3175214. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ma, S.X.; Hong, S.I.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. Eucommia ulmoides Oliv. bark. attenuates 6-hydroxydopamine-induced neuronal cell death through inhibition of oxidative stress in SH-SY5Y cells. J. Ethnopharmacol. 2014, 152, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hou, J.; Mo, Y.; Ren, M.; Yang, G.; Qu, Z.; Hu, Y. Geniposidic acid ameliorates spatial learning and memory deficits and alleviates neuroinflammation via inhibiting HMGB-1 and downregulating TLR4/2 signaling pathway in APP/PS1 mice. Eur. J. Pharmacol. 2020, 869, 172857. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef]
- Liu, T.; Ding, Y.; Wen, A. Traditional Chinese medicine for ischaemic stroke. Lancet Neurol. 2018, 17, 745. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory Disequilibrium in Stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 2019, 117, 109102. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.; Franklin, R.J. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain J. Neurol. 2009, 132, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation 2015, 12, 106. [Google Scholar] [CrossRef]
- Srivastava, P.; Cronin, C.G.; Scranton, V.L.; Jacobson, K.A.; Liang, B.T.; Verma, R. Neuroprotective and neuro-rehabilitative effects of acute purinergic receptor P2X4 (P2X4R) blockade after ischemic stroke. Exp. Neurol. 2020, 329, 113308. [Google Scholar] [CrossRef]
- Orr, A.G.; Orr, A.L.; Li, X.J.; Gross, R.E.; Traynelis, S.F. Adenosine A(2A) receptor mediates microglial process retraction. Nat. Neurosci. 2009, 12, 872–878. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Janssens, S.; Beyaert, R. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hyakkoku, K.; Hamanaka, J.; Tsuruma, K.; Shimazawa, M.; Tanaka, H.; Uematsu, S.; Akira, S.; Inagaki, N.; Nagai, H.; Hara, H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 2010, 171, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Belinga, V.F.; Wu, G.J.; Yan, F.L.; Limbenga, E.A. Splenectomy following MCAO inhibits the TLR4-NF-κB signaling pathway and protects the brain from neurodegeneration in rats. J. Neuroimmunol. 2016, 293, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, B.; Zhang, X.; Chen, L.; Zhang, L.; Zhao, X.; Bai, X.; Zhu, C.; Cui, L.; Wang, L. Neuroprotective effect of bicyclol in rat ischemic stroke: Down-regulates TLR4, TLR9, TRAF6, NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res. 2013, 1528, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Harari, O.A.; Liao, J.K. NF-κB and innate immunity in ischemic stroke. Ann. N. Y. Acad. Sci. 2010, 1207, 32–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, T.; Liu, G.; Gao, X.; Gao, Y.; Zhuang, Z.; Lu, Y.; Wang, H.; Li, W.; Wu, L.; et al. TRAF3 mediates neuronal apoptosis in early brain injury following subarachnoid hemorrhage via targeting TAK1-dependent MAPKs and NF-κB pathways. Cell Death Dis. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.P.; Jiang, N.; Wang, S.N.; Qi, R.Z.; Wang, L.; Zhao, P.R.; Liang, L.; Yu, B. Eucommia ulmoides Oliv. bark aqueous extract inhibits osteoarthritis in a rat model of osteoarthritis. J. Ethnopharmacol. 2015, 162, 148–154. [Google Scholar] [CrossRef]
- Gibson, C.L.; Murphy, S.P. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J. Cereb. Blood Flow Metab. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Wang, M.; Sun, P.; Li, Z.; Li, J.; Lv, X.; Chen, S.; Zhu, X.; Chai, X.; Zhao, S. Eucommiae cortex polysaccharides attenuate gut microbiota dysbiosis and neuroinflammation in mice exposed to chronic unpredictable mild stress: Beneficial in ameliorating depressive-like behaviors. J. Affect. Disord. 2023, 334, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, M.; Li, Z.; Wei, J.; Liu, F.; Zheng, W.; Zhu, X.; Chai, X.; Zhao, S. Eucommiae cortex polysaccharides mitigate obesogenic diet-induced cognitive and social dysfunction via modulation of gut microbiota and tryptophan metabolism. Theranostics 2022, 12, 3637–3655. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Banti, V.; Perata, P. ROS signaling as common element in low oxygen and heat stresses. Plant Physiol. Biochem. 2012, 59, 3–10. [Google Scholar] [CrossRef]
- Bouët, V.; Freret, T.; Toutain, J.; Divoux, D.; Boulouard, M.; Schumann-Bard, P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007, 203, 555–567. [Google Scholar] [CrossRef]
- Fréchou, M.; Margaill, I.; Marchand-Leroux, C.; Beray-Berthat, V. Behavioral tests that reveal long-term deficits after permanent focal cerebral ischemia in mouse. Behav. Brain Res. 2019, 360, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D. High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J. Neurosurg. 1985, 62, 882–887. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef]
- Fréchou, M.; Zhu, X.; Liere, P.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Dose-dependent and long-term cerebroprotective effects of intranasal delivery of progesterone after ischemic stroke in male mice. Neuropharmacology 2020, 170, 108038. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1.2.1–1.2.12. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, 57648. [Google Scholar] [CrossRef]
- Li, C.; Pan, J.; Sun, P.; Wang, S.; Wang, S.; Feng, W.; Chen, S.; Chai, X.; Zhao, S.; Zhu, X. Ketogenic Diet Alleviates Hypoglycemia-Induced Neuroinflammation via Modulation the Gut Microbiota in Mice. Mol. Nutr. Food Res. 2023, 67, e2200711. [Google Scholar] [CrossRef]


| Compounds | Standard (µg/L) | WEU (µg/L) |
|---|---|---|
| aucubin | 192 | 166 |
| chlorogenic acid | 208 | 17,900 |
| geniposidic acid | 185 | 1360 |
| quercetin | 215 | 160 |
| protocatechuic acid | 146 | 18.6 |
| betulin | 185 | 167 |
| pinoresinol diglucoside | 185 | 86.4 |
| Table | A% | B% |
|---|---|---|
| 0 | 97 | 3 |
| 3 | 97 | 3 |
| 7.5 | 85 | 15 |
| 12 | 85 | 15 |
| 18 | 40 | 60 |
| 21 | 10 | 90 |
| 22 | 10 | 90 |
| 23 | 97 | 3 |
| 25 | 97 | 3 |
| Compound | DP (V) | CE (V) |
|---|---|---|
| Aucubin | −30 | −13 |
| Chlorogenic acid | −70 | −20 |
| Geniposidic acid | −85 | −26 |
| Quercetin | −40 | −12 |
| Protocatechuic acid | −15 | −20 |
| Betulin | −180 | 13 |
| Pinoresinol diglucoside | −30 | −34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Chai, X.; Li, C.; Wu, Y.; Ma, Y.; Wang, S.; Xue, Y.; Zhao, Y.; Chen, S.; Zhu, X.; et al. Eucommia ulmoides Oliv. Bark Extracts Alleviate MCAO/Reperfusion-Induced Neurological Dysfunction by Suppressing Microglial Inflammation in the Gray Matter. Int. J. Mol. Sci. 2025, 26, 1572. https://doi.org/10.3390/ijms26041572
Pan J, Chai X, Li C, Wu Y, Ma Y, Wang S, Xue Y, Zhao Y, Chen S, Zhu X, et al. Eucommia ulmoides Oliv. Bark Extracts Alleviate MCAO/Reperfusion-Induced Neurological Dysfunction by Suppressing Microglial Inflammation in the Gray Matter. International Journal of Molecular Sciences. 2025; 26(4):1572. https://doi.org/10.3390/ijms26041572
Chicago/Turabian StylePan, Jiarong, Xuejun Chai, Cixia Li, Yongji Wu, Yue Ma, Songlin Wang, Yuhuan Xue, Yongkang Zhao, Shulin Chen, Xiaoyan Zhu, and et al. 2025. "Eucommia ulmoides Oliv. Bark Extracts Alleviate MCAO/Reperfusion-Induced Neurological Dysfunction by Suppressing Microglial Inflammation in the Gray Matter" International Journal of Molecular Sciences 26, no. 4: 1572. https://doi.org/10.3390/ijms26041572
APA StylePan, J., Chai, X., Li, C., Wu, Y., Ma, Y., Wang, S., Xue, Y., Zhao, Y., Chen, S., Zhu, X., & Zhao, S. (2025). Eucommia ulmoides Oliv. Bark Extracts Alleviate MCAO/Reperfusion-Induced Neurological Dysfunction by Suppressing Microglial Inflammation in the Gray Matter. International Journal of Molecular Sciences, 26(4), 1572. https://doi.org/10.3390/ijms26041572






