Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles
Abstract
1. Introduction
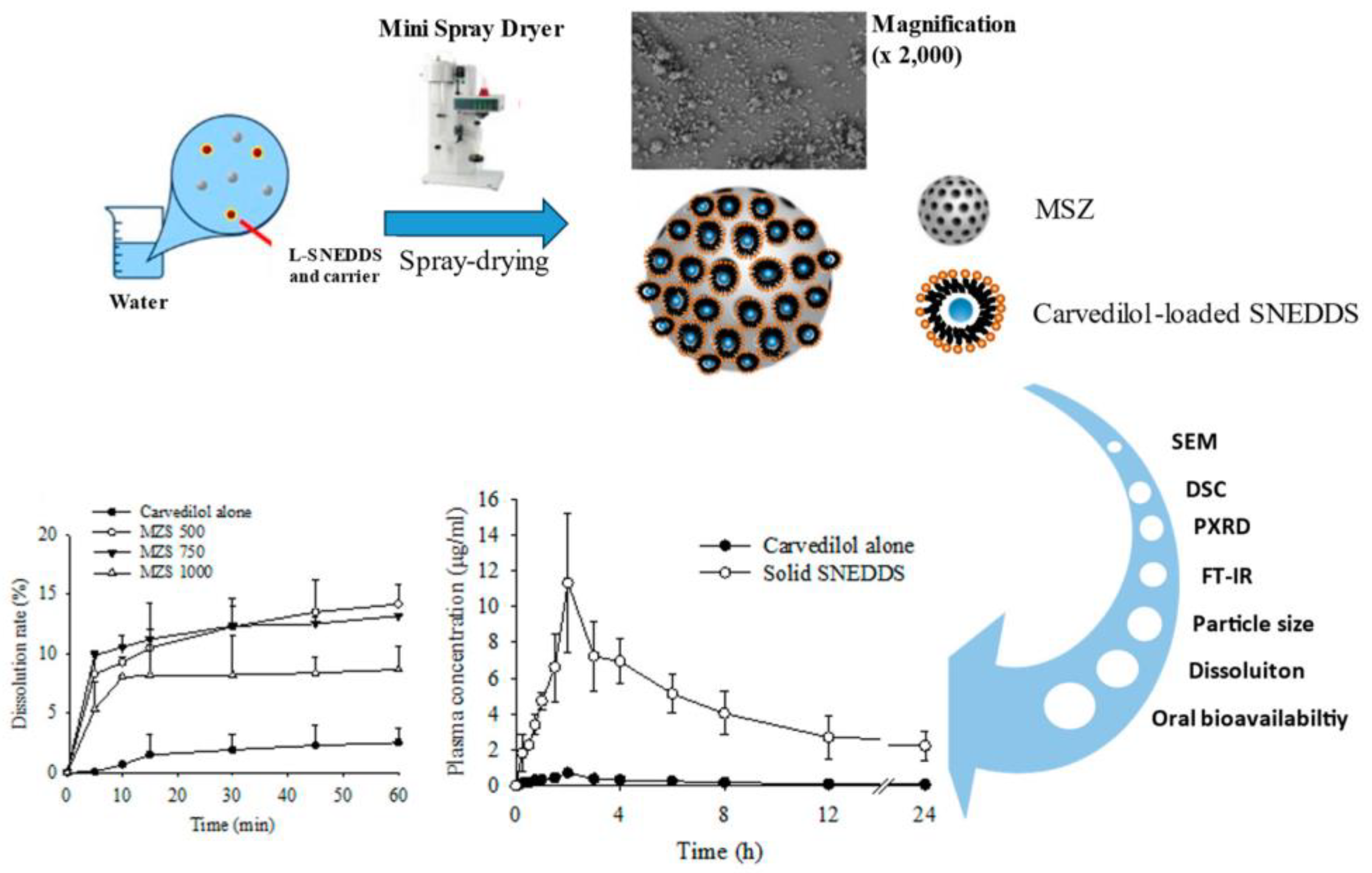
2. Results and Discussion
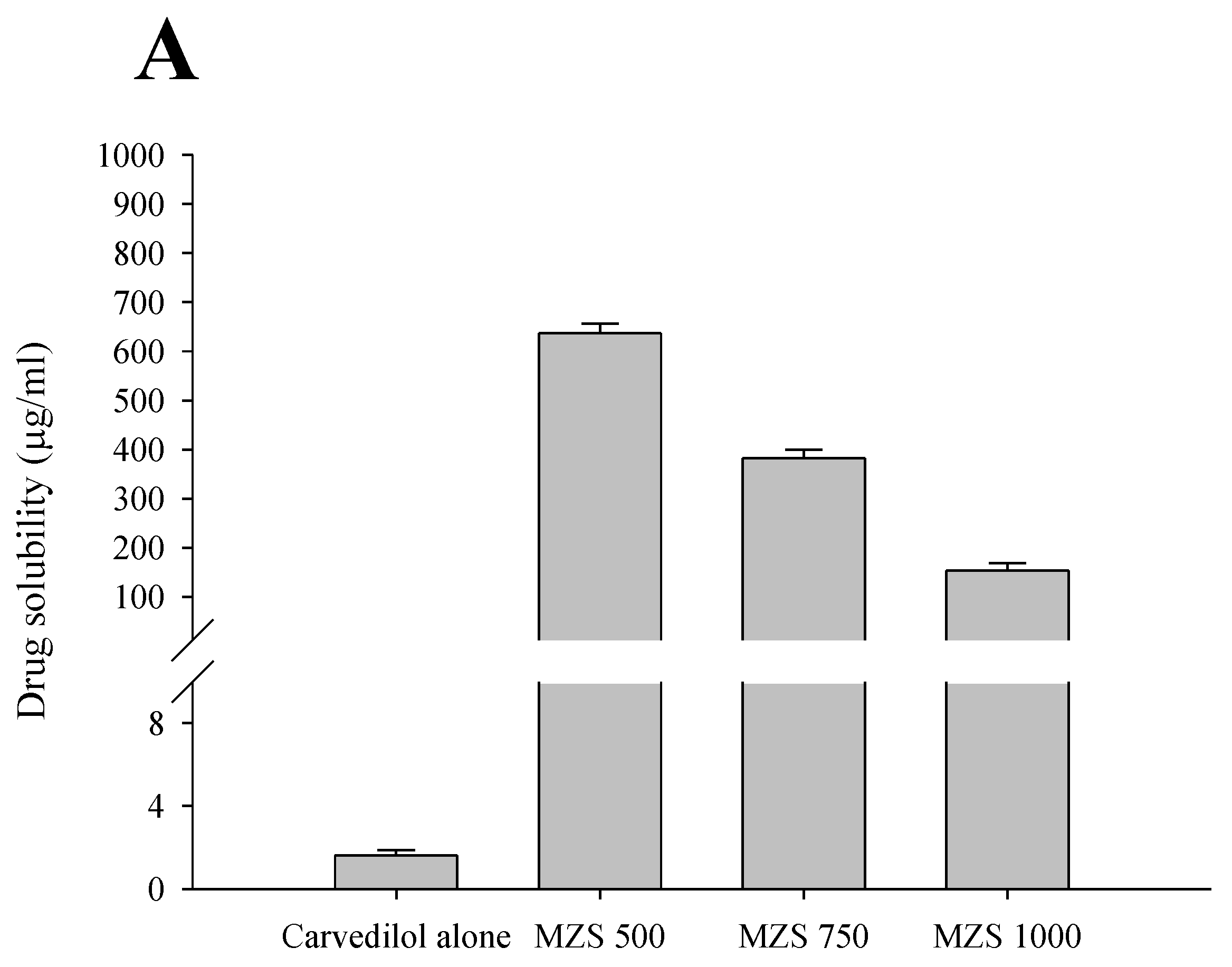
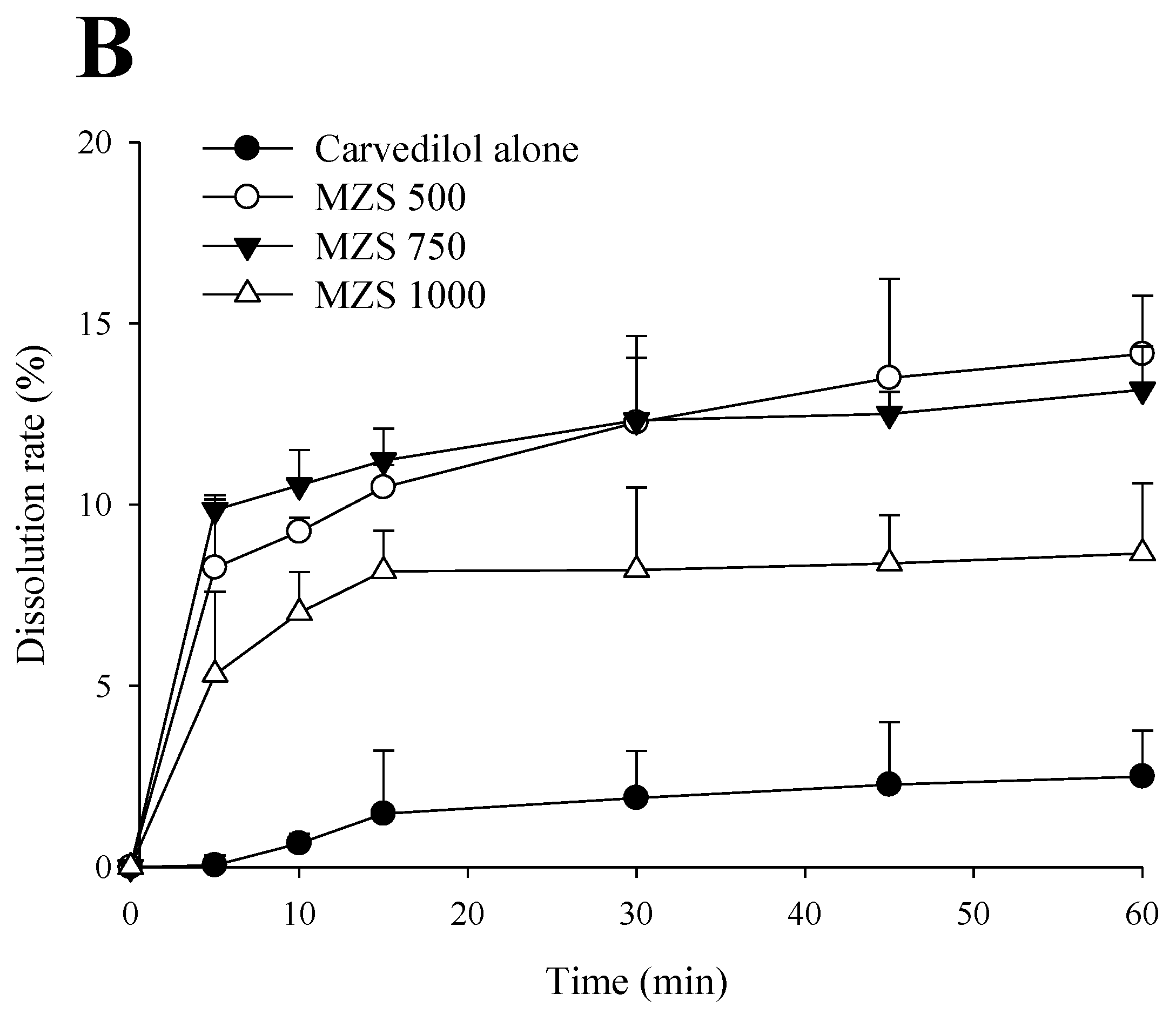
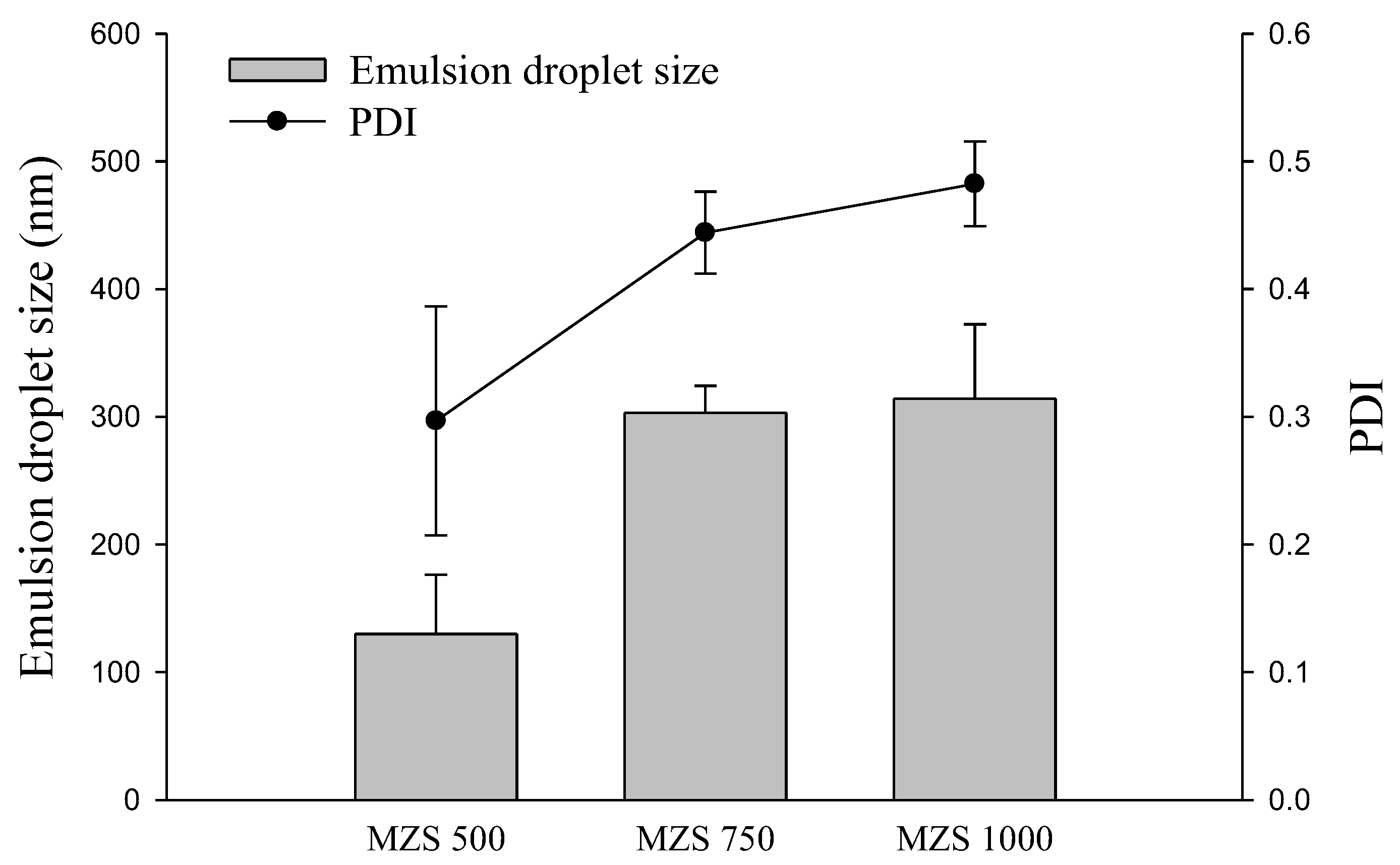
2.1. Impact According to MSN Ratio
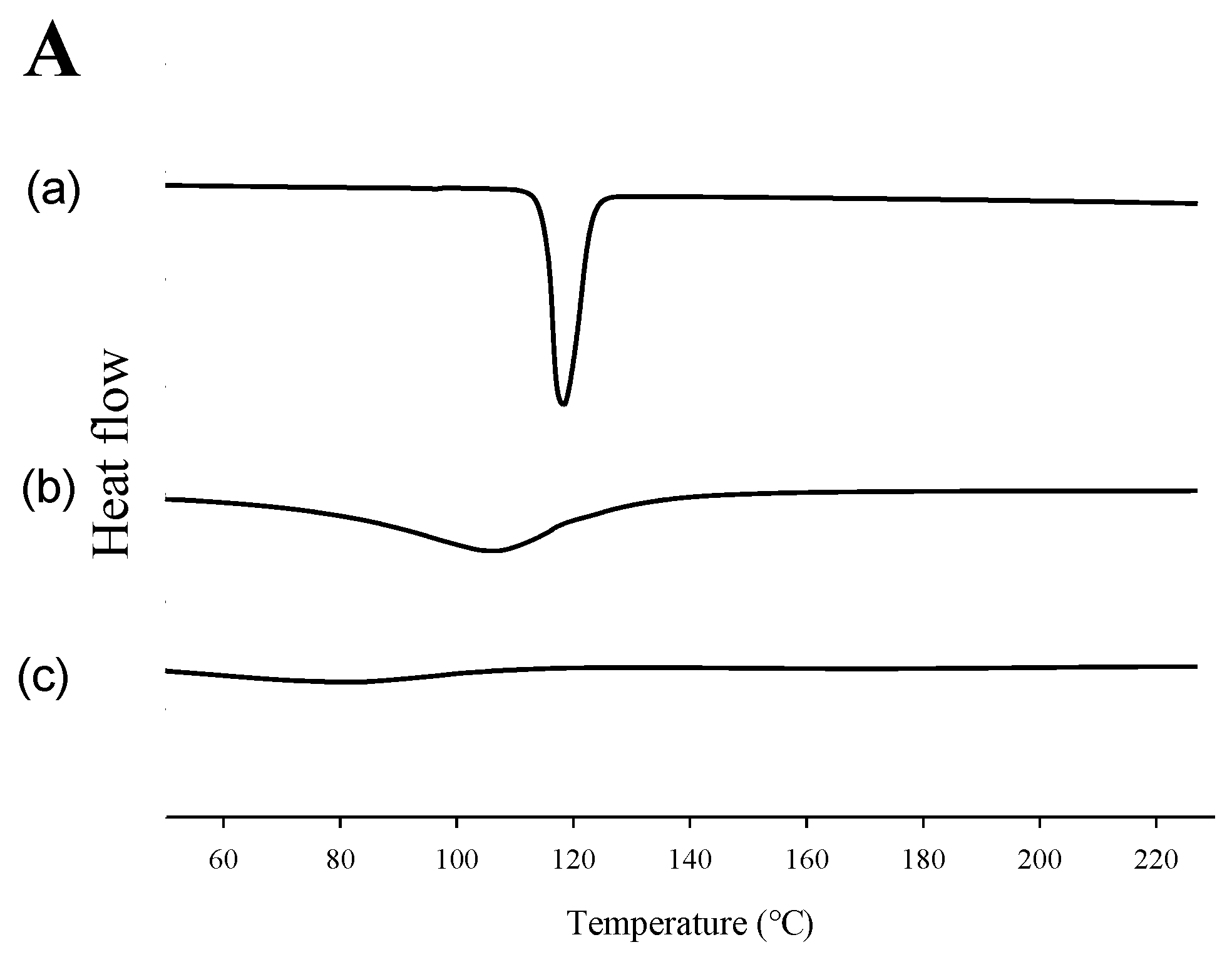
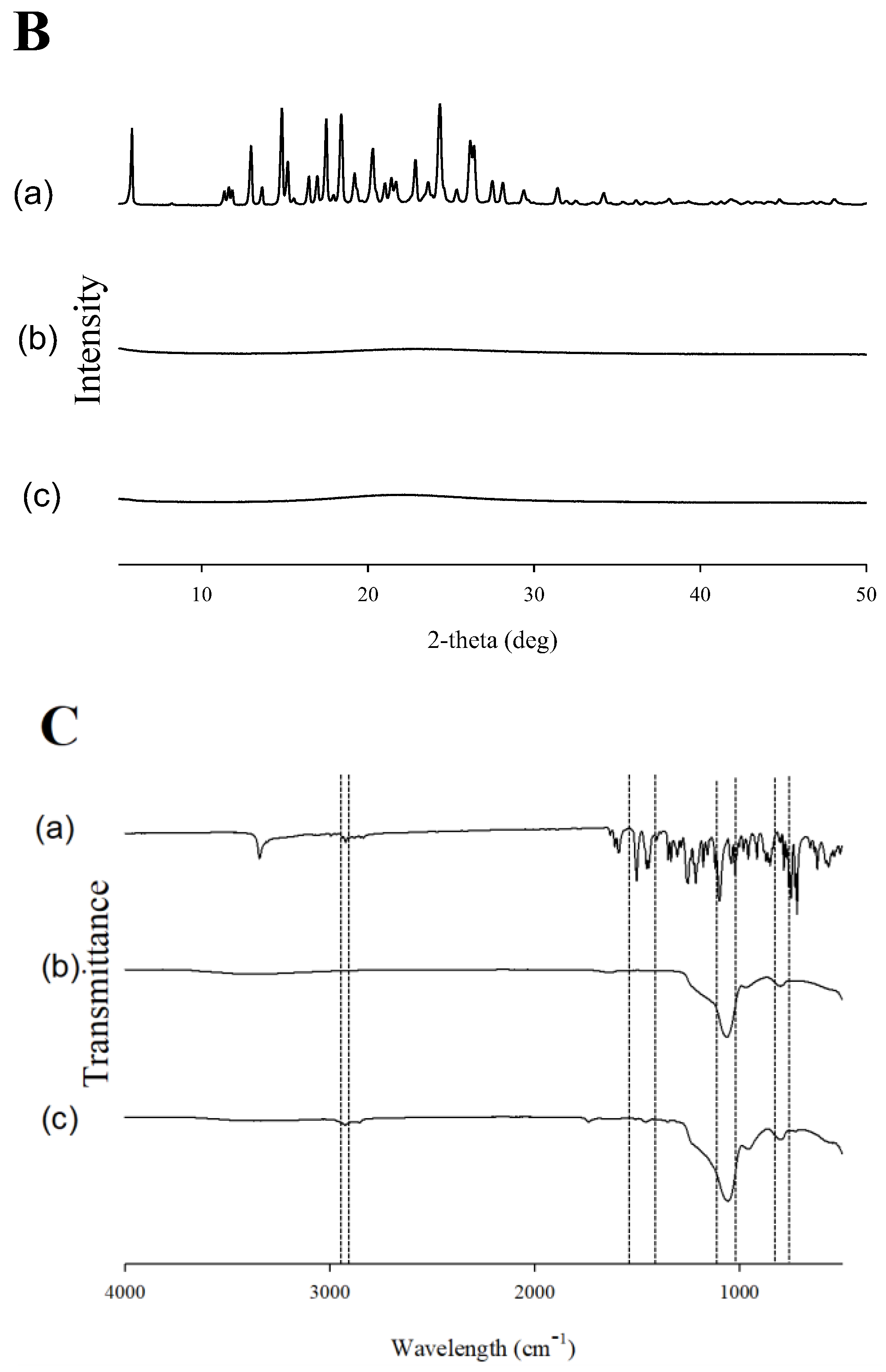
2.2. Evaluation of Physicochemical Properties
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Preparation of S-SNEDDS
3.4. Solubility Studies
3.5. Dissolution Test
3.6. Emulsion Droplet Size
3.7. Pharmacokinetic Studies
3.8. Physicochemical Characterization
3.8.1. DSC
3.8.2. XRD
3.8.3. FT-IR Spectroscopy
3.8.4. SEM
3.8.5. Particle Size Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.E.; Kim, J.S.; Choi, M.J.; Baek, K.; Woo, M.R.; Kim, J.O.; Choi, H.G.; Jin, S.G. Effects of different physicochemical characteristics and supersaturation principle of solidified SNEDDS and surface-modified microspheres on the bioavailability of carvedilol. Int. J. Pharm. 2021, 597, 120377. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, J.S.; Kim, J.; Choi, M.J.; Baek, K.; Kim, J.O.; Choi, H.G.; Jin, S.G. A novel acidic microenvironment microsphere for enhanced bioavailability of carvedilol: Comparison of solvent evaporated and surface-attached system. J. Drug Deliv. Sci. Technol. 2022, 76, 103803. [Google Scholar] [CrossRef]
- Prieto, C.; Evtoski, Z.; Pardo-Figuerez, M.; Lagaron, J.M. Bioavailability enhancement of nanostructured microparticles of carvedilol. J. Drug Deliv. Sci. Technol. 2021, 66, 102780. [Google Scholar] [CrossRef]
- Tak, J.W.; Kwon, T.K.; Kim, Y.I.; Cho, J.H.; Kim, J.; Kim, J.O. Development of abiraterone acetate tablets with enhanced oral bioavailability. J. Pharm. Investig. 2024, 54, 345–356. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.S.; Jin, S.G.; Choi, H.G. Development of Novel Tamsulosin Pellet-Loaded Oral Disintegrating Tablet Bioequivalent to Commercial Capsule in Beagle Dogs Using Microcrystalline Cellulose and Mannitol. Int. J. Mol. Sci. 2023, 24, 15393. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to improve drug strength in nasal preparations for brain delivery of low aqueous solubility drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef]
- Nakahara, H.; Koga, K.; Matsuoka, K. Distinct Solubilization Mechanisms of Medroxyprogesterone in Gemini Surfactant Micelles: A Comparative Study with Progesterone. Molecules 2024, 29, 4945. [Google Scholar] [CrossRef]
- Lee, H.I.; Woo, M.R.; Kim, J.S.; Cheon, S.; Park, S.; Woo, S.; Jin, S.G.; Choi, H.G. Development of a novel apixaban-loaded solid self-emulsifying drug delivery system for oral administration: Physicochemical characterization and pharmacokinetics in rats. J. Pharm. Investig. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Kapoor, B.; Jain, S.K.; Gowthamarajan, K.; Zacconi, F.; Chellappan, D.K.; Gupta, G.; et al. Development of mushroom polysaccharide and probiotics based solid self-nanoemulsifying drug delivery system loaded with curcumin and quercetin to improve their dissolution rate and permeability: State of the art. Int. J. Biol. Macromol. 2021, 189, 744–757. [Google Scholar] [CrossRef]
- Mandić, J.; Kosmač, I.; Kovačević, M.; Hodnik, B.; Hodnik, Ž.; Vrečer, F.; Gašperlin, M.; Perissutti, B.; Pobirk, A.Z. Evaluation of solid carvedilol-loaded SMEDDS produced by the spray drying method and a study of related substances. Int. J. Pharm. 2021, 605, 120783. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Our contributions to applications of mesoporous silica nanoparticles. Acta Biomater. 2022, 137, 44–52. [Google Scholar] [CrossRef]
- Chen, S.; Greasley, S.L.; Ong, Z.Y.; Naruphontjirakul, P.; Page, S.J.; Hanna, J.V.; Redpath, A.N.; Tsigkou, O.; Rankin, S.; Ryan, M.P.; et al. Biodegradable zinc-containing mesoporous silica nanoparticles for cancer therapy. Mater. Today Adv. 2020, 6, 100066. [Google Scholar] [CrossRef]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Ahmad, H.; Abdul Rahman, M.B.; Gill, M.R. Progress in mesoporous silica nanoparticles as drug delivery agents for cancer treatment. Pharmaceutics 2021, 13, 152. [Google Scholar] [CrossRef]
- Schmied, F.P.; Bernhardt, A.; Baudron, V.; Beine, B.; Klein, S. Development and characterization of celecoxib solid self-nanoemulsifying drug delivery systems (S-SNEDDS) prepared using novel cellulose-based microparticles as adsorptive carriers. AAPS PharmSciTech 2022, 23, 213. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Chang, D.; Wei, Z.; Wang, E.; Yu, J.; Xu, Y.; Que, Y.; Chen, Y.; Fan, C.; et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact. Mater. 2023, 24, 81–95. [Google Scholar] [CrossRef]
- Usta, D.Y.; Olgac, S.; Timur, B.; Teksin, Z.S. Development and pharmacokinetic evaluation of Neusilin® US2-based S-SNEDDS tablets for bosentan: Fasted and fed states bioavailability, IVIS® real-time biodistribution, and ex-vivo imaging. Int. J. Pharm. 2023, 643, 123219. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, J.; Jeon, S.Y.; Han, S.C.; Choi, M.K.; Song, I.S. Pharmacokinetics and Anti-Cancer Activity of Curcumin-and Pluronic P85-Loaded Mesoporous Silica Nanoparticles. Drug Targets Ther. 2024, 3, 121–133. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Mohammed, S.A.; Khan, O.; Ali, H.M.; El-Readi, M.Z.; Mohammed, H.A. Cinnamaldehyde-Based Self-Nanoemulsion (CA-SNEDDS) accelerates wound healing and exerts antimicrobial, antioxidant, and anti-inflammatory effects in rats’ skin burn model. Molecules 2022, 27, 5225. [Google Scholar] [CrossRef] [PubMed]
- Ogadah, C.U.; Mrštná, K.; Matysová, L.; Müllertz, A.; Rades, T.; Niederquell, A.; Šklubalová, Z.; Vraníková, B. Comparison of the liquisolid technique and co-milling for loading of a poorly soluble drug in inorganic porous excipients. Int. J. Pharm. 2024, 650, 123702. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Cheon, S.; Woo, M.R.; Woo, S.; Chung, J.E.; Youn, Y.S.; Oh, K.T.; Lim, S.J.; Ku, S.K.; Nguyen, B.L.; et al. Electrostatic spraying for fine-tuning particle dimensions to enhance oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2024, 19, 100953. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of more efficacious curcumin delivery systems using colloid science: Enhanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Debotton, N.; Garsiani, S.; Cohen, Y.; Dahan, A. Enabling oral delivery of antiviral drugs: Double emulsion carriers to improve the intestinal absorption of zanamivir. Int. J. Pharm. 2022, 629, 122392. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mehmandar, E.; Jabari, H.; SA, A.R.; Mohammadkhani, R.; Yan, N.; Arjmand, M. Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings. Compos. B Eng. 2021, 212, 108713. [Google Scholar] [CrossRef]
- Baek, K.; Woo, M.R.; Choi, Y.S.; Kang, M.J.; Kim, J.O.; Choi, H.G.; Jin, S.G. Engineering sodium alginate microparticles with different crystallinities for niclosamide repositioning and solubilization to improve solubility and oral bioavailability in rats. Int. J. Biol. Macromol. 2024, 283, 137471. [Google Scholar] [CrossRef]
- Niamnuy, C.; Sungsinchai, S.; Jarernsamrit, P.; Devahastin, S.; Chareonpanich, M. Synthesis and characterization of aluminosilicate and zinc silicate from sugarcane bagasse fly ash for adsorption of aflatoxin B1. Sci. Rep. 2024, 14, 14562. [Google Scholar] [CrossRef]
- Abbasi, M.; Gholizadeh, R.; Kasaee, S.R.; Vaez, A.; Chelliapan, S.; Fadhil Al-Qaim, F.; Deyab, I.F.; Shafiee, M.; Zareshahrabadi, Z.; Amani, A.M.; et al. An intriguing approach toward antibacterial activity of green synthesized Rutin-templated mesoporous silica nanoparticles decorated with nanosilver. Sci Rep. 2023, 13, 5987. [Google Scholar] [CrossRef]
- Woo, M.R.; Kim, J.S.; Cheon, S.; Ji, S.H.; Park, S.; Woo, S.; Kim, J.O.; Jin, S.G.; Choi, H.G. Microneedles integrated with crystallinity control for poorly water-soluble drugs: Enhanced bioavailability and innovative controlled release system. Mater. Des. 2024, 247, 113371. [Google Scholar] [CrossRef]
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and evaluation of hyaluronic acid-based mucoadhesive self nanoemulsifying drug delivery system (SNEDDS) of tamoxifen for targeting breast cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Sandomierski, M.; Chojnacka, M.; Długosz, M.; Pokora, M.; Zwolińska, J.; Majchrzycki, Ł.; Voelkel, A. Mesoporous silica modified with polydopamine and zinc ions as a potential carrier in the controlled release of mercaptopurine. Materials 2023, 16, 4358. [Google Scholar] [CrossRef] [PubMed]
- Pešić, N.; Dapčević, A.; Ivković, B.; Kachrimanis, K.; Mitrić, M.; Ibrić, S.; Medarević, D. Potential application of low molecular weight excipients for amorphization and dissolution enhancement of carvedilol. Int. J. Pharm. 2021, 608, 121033. [Google Scholar] [CrossRef]
- Mandić, J.; Pobirk, A.Z.; Vrečer, F.; Gašperlin, M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef]
- Patel, V.D.; Rathod, V.; Haware, R.V.; Stagner, W.C. Optimized L-SNEDDS and spray-dried S-SNEDDS using a linked QbD-DM3 rational design for model compound ketoprofen. Int. J. Pharm. 2023, 631, 122494. [Google Scholar] [CrossRef]
- Flores, D.; Almeida, C.M.R.; Gomes, C.R.; Balula, S.S.; Granadeiro, C.M. Tailoring of mesoporous silica-based materials for enhanced water pollutants removal. Molecules 2023, 28, 4038. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.G.; Yun, T.H.; Kim, C.H.; Cho, J.H.; Kim, K.S. The Impact of Polymers on Enzalutamide Solid Self-Nanoemulsifying Drug Delivery System and Improved Bioavailability. Pharmaceutics 2024, 16, 457. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Soliman, G.A.; Shahba, A.A.W.; Aldawsari, M.F.; Alharthy, K.M.; Abdel-Kader, M.S.; Zaatout, H.H. Brain targeting by intranasal drug delivery: Effect of different formulations of the biflavone “Cupressuflavone” from Juniperus sabina L. on the motor activity of rats. Molecules 2023, 28, 1354. [Google Scholar] [CrossRef]
- Un Din, F.; Lee, H.I.; Kim, J.S.; Woo, M.R.; Cheon, S.; Park, S.; Woo, S.; Jin, S.G.; Choi, H.G. Physicochemical characterization and in vivo assessment of novel apixaban-loaded polymeric nano-aggregates. J. Pharm. Investig. 2024, 1, 1–13. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Ahmad, J.; Alharbi, W.S.; Asfour, H.Z. Resveratrol loaded self-nanoemulsifying drug delivery system (SNEDDS) for pancreatic cancer: Formulation design, optimization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102555. [Google Scholar] [CrossRef]
- Elkanayati, R.M.; Omari, S.; Youssef, A.A.A.; Almutairi, M.; Almotairy, A.; Repka, M.; Ashour, E.A. Multilevel categoric factorial design for optimization of raloxifene hydrochloride solid dispersion in PVP K30 by hot-melt extrusion technology. J. Drug Deliv. Sci. Technol. 2024, 92, 105362. [Google Scholar] [CrossRef]
- Abd El-Halim, S.M.; Mamdouh, M.A.; Eid, S.M.; Ibrahim, B.M.; Aly Labib, D.A.; Soliman, S.M. The potential synergistic activity of zolmitriptan combined in new self-nanoemulsifying drug delivery systems: Atr-ftir real-time fast dissolution monitoring and pharmacodynamic assessment. Int. J. Nanomed. 2021, 16, 6395–6412. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, E.; Milanowski, B.; Lulek, J. A systematic approach to the development of cilostazol nanosuspension by liquid antisolvent precipitation (LASP) and its combination with ultrasound. Int. J. Mol. Sci. 2021, 22, 12406. [Google Scholar] [CrossRef] [PubMed]



| Formulation | MSN 500 | MSN 750 | MSN 500 |
|---|---|---|---|
| Carvedilol (g) | 0.10 | 0.10 | 0.10 |
| Peceol (g) | 0.25 | 0.25 | 0.25 |
| Tween 80 (g) | 0.50 | 0.50 | 0.50 |
| Labrazol (g) | 0.25 | 0.25 | 0.25 |
| MSN (g) | 0.50 | 0.75 | 1.00 |
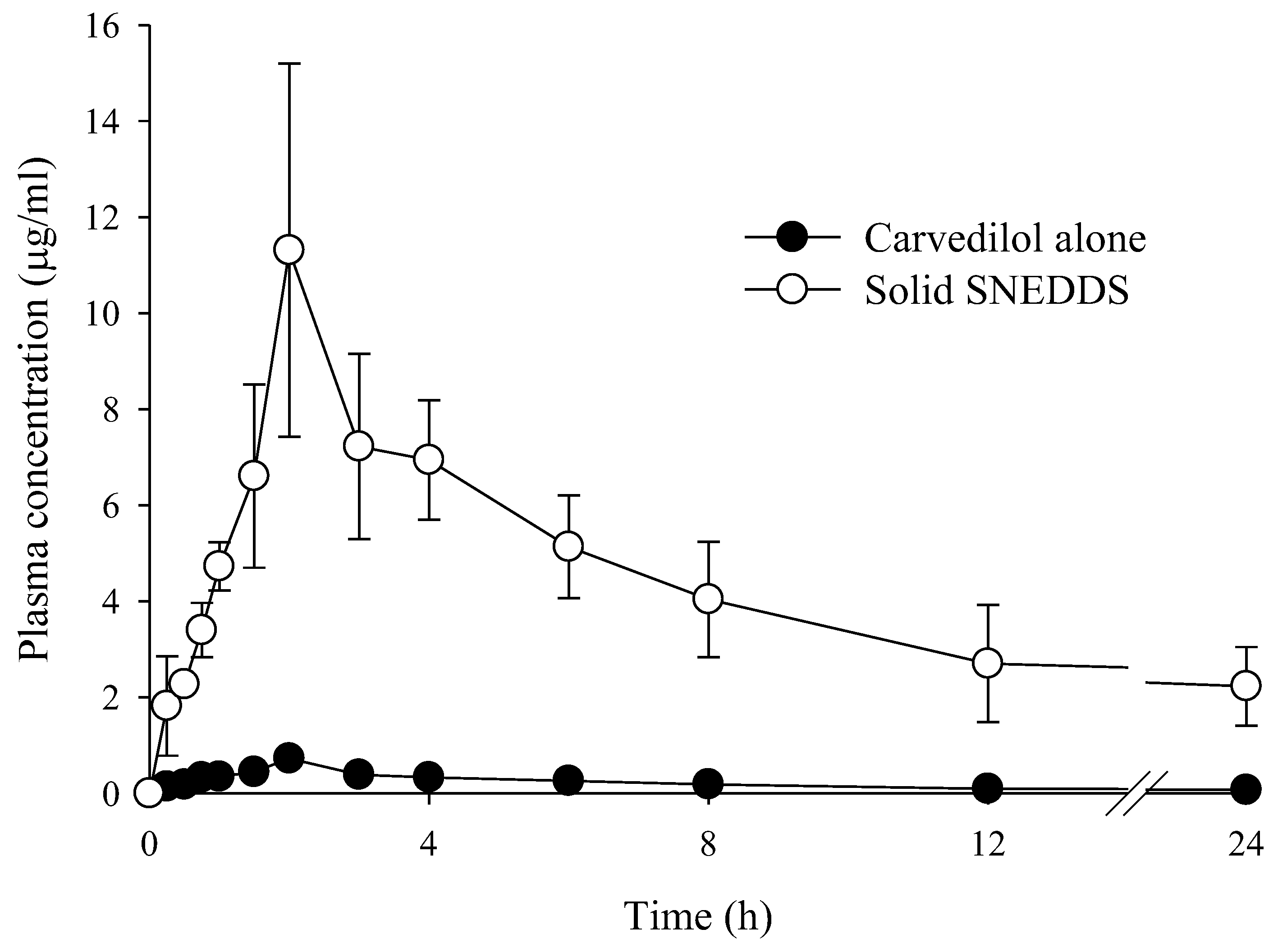
| Parameters | Carvedilol Alone | S-SNEDDS |
|---|---|---|
| AUC (h·µg/mL) | 4.17 ± 1.02 | 90.41 ± 5.91 |
| Cmax (µg/mL) | 0.72 ± 0.11 | 11.31 ± 1.15 |
| Tmax (h) | 2.00 ± 0.00 | 2.00 ± 0.00 |
| t1/2 (h) | 8.50 ± 2.23 | 11.21 ± 4.15 |
| Kel (h−1) | 0.08 ± 0.01 | 0.06 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Kim, N.; Jin, S.G. Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles. Int. J. Mol. Sci. 2025, 26, 1592. https://doi.org/10.3390/ijms26041592
Jang H, Kim N, Jin SG. Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles. International Journal of Molecular Sciences. 2025; 26(4):1592. https://doi.org/10.3390/ijms26041592
Chicago/Turabian StyleJang, Hangeul, Nahyun Kim, and Sung Giu Jin. 2025. "Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles" International Journal of Molecular Sciences 26, no. 4: 1592. https://doi.org/10.3390/ijms26041592
APA StyleJang, H., Kim, N., & Jin, S. G. (2025). Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles. International Journal of Molecular Sciences, 26(4), 1592. https://doi.org/10.3390/ijms26041592






