Effects of Alexandrium pacificum Exposure on Exopalaemon carinicauda: Hepatopancreas Histology, Antioxidant Enzyme Activity, and Transcriptome Analysis
Abstract
:1. Introduction
2. Results
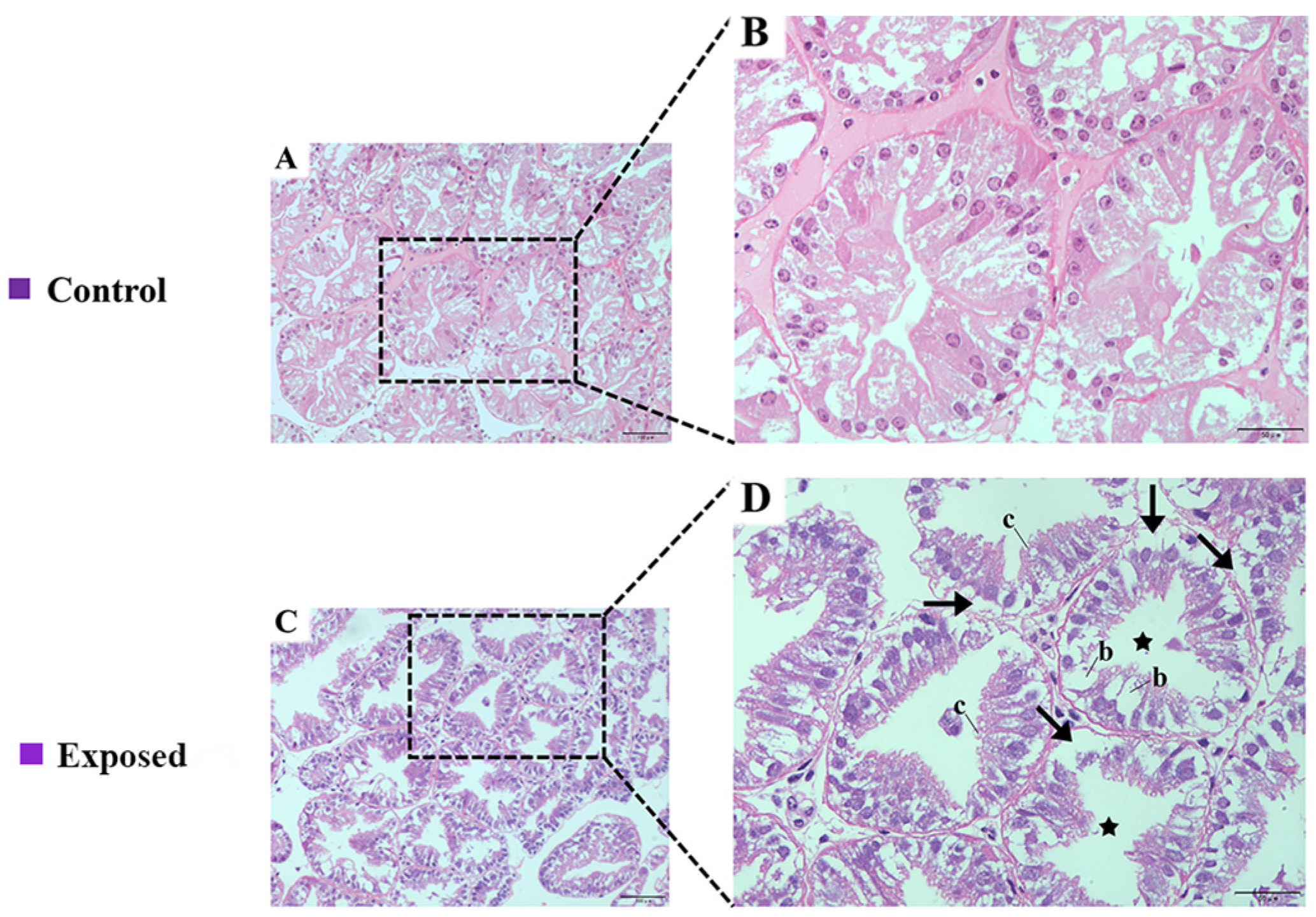
2.1. Histological Assays
2.2. Antioxidant Parameters
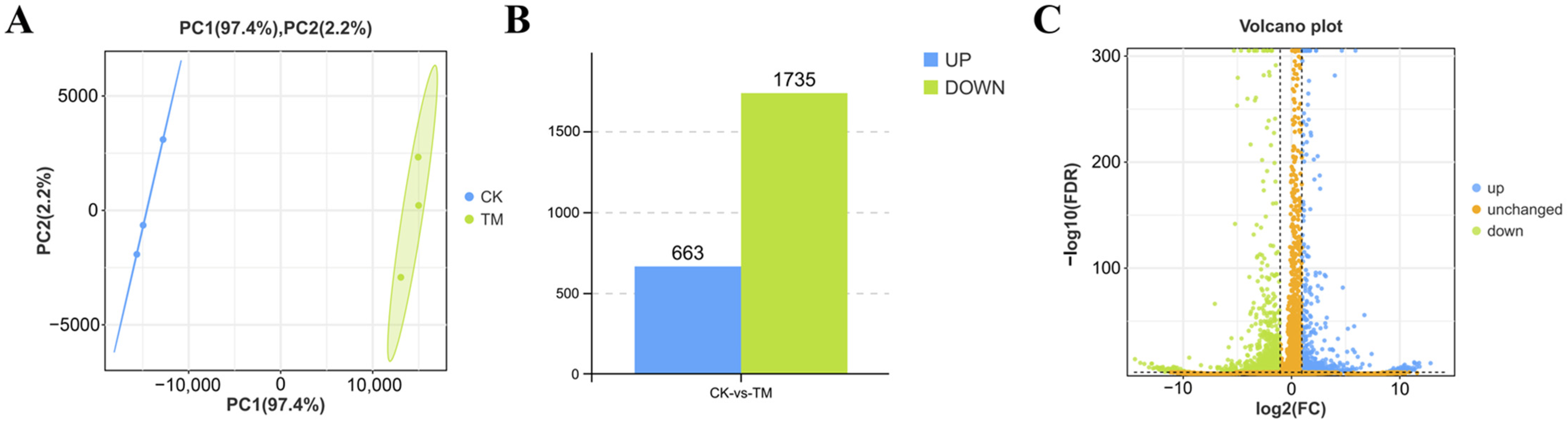
2.3. DEGs in the Hepatopancreas After A. pacificum Exposure
2.4. GO Enrichment Analysis
2.5. KEGG Enrichment Analysis
2.6. Differentially Expressed Genes Involved in Different Functions
2.7. Validation of Gene Expression Through Quantitative Reverse-Transcription PCR (qRT-PCR)
3. Discussion
3.1. Key Genes Associated with Protein Processing and Mitochondrial Function
3.2. Key Genes Related to Energy Metabolism
4. Materials and Methods
4.1. Shrimp Selection and Culture Conditions
4.2. Alexandrium Pacificum Culture
4.3. Experimental Design and Sample Collection
4.4. Histopathological Analyses
4.5. Measurement of Hepatopancreas Antioxidant Parameters
4.6. Transcriptome Sequencing in Hepatopancreas
4.7. Quantitative Reverse-Transcription PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J. Comparative Transcriptomic and Proteomic Analysis of Exopalaemon carinicauda in Response to Alkalinity Stress. Front. Mar. Sci. 2021, 8, 759923. [Google Scholar]
- Feng, Y.; Zhai, Q.; Wang, J.; Li, J.; Li, J. Comparison of florfenicol pharmacokinetics in Exopalaemon carinicauda at different temperatures and administration routes. J. Vet. Pharmacol. Therap. 2019, 42, 230–238. [Google Scholar] [CrossRef]
- Ge, Q.; Li, J.; Wang, J.; Li, Z.; Li, J. Characterization, functional analysis, and expression levels of three carbonic anhydrases in response to pH and saline–alkaline stresses in the ridgetail white prawn Exopalaemon carinicauda. Cell Stress Chaperones 2019, 24, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, P.; Liu, P.; Chen, P.; Li, J. The roles of Na+/K+-ATPase α-subunit gene from the ridgetail white prawn Exopalaemon carinicauda in response to salinity stresses. Fish Shellfish. Immunol. 2015, 42, 264–271. [Google Scholar] [CrossRef]
- Zhang, X.-W. Cloning and characterization of two different ficolins from the giant freshwater prawn Macrobrachium rosenbergii. Dev. Comp. Immunol. 2014, 44, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Meng, H.; Gu, D.; Li, Y.; Jia, M. Molecular mechanisms of Vibrio parahaemolyticus pathogenesis. Microbiol. Res. 2019, 222, 43–51. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.H.; Choi, S.-K.; Jeon, H.J.; Lee, S.H.; Kim, B.K.; Kim, Y.K.; Lee, K.-J.; Han, J.E. Detection of infectious white spot syndrome virus in red claw crayfish (Cherax quadricarinatus) and red swamp crayfish (Procambarus clarkii) imported into Korea. Aquaculture 2021, 544, 737117. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Wang, B.; Xiang, J. A new shrimp peritrophin-like gene from Exopalaemon carinicauda involved in white spot syndrome virus (WSSV) infection. Fish Shellfish. Immunol. 2013, 35, 840–846. [Google Scholar] [CrossRef]
- Mu, C.; Ren, X.; Ge, Q.; Wang, J.; Li, J. Antioxidant response of ridgetail white prawn Exopalaemon carinicauda to harmful dinoflagellate Prorocentrum minimum exposure and its histological change. J. Ocean Univ. China 2017, 16, 285–293. [Google Scholar] [CrossRef]
- Mu, C.; Ren, X.; Li, J. Immune response of the ridgetail white prawn Exopalaemon carinicauda after exposure to the dinoflagellate Prorocentrum minimum. J. Ocean Univ. China 2023, 22, 821–830. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Hoppenrath, M.; Saldarriaga, J.F. Dinoflagellate diversity and distribution. Biodivers. Conserv. 2008, 17, 407–418. [Google Scholar]
- Wang, H.; Kim, H.; Ki, J.-S. Transcriptome survey, molecular identification, and expression analysis of stress-responsive genes in the toxic dinoflagellate Alexandrium pacificum under algicidal agents and metal stresses. J. Appl. Phycol. 2021, 33, 3139–3151. [Google Scholar]
- Alonso-Rodrıguez, R.; Paez-Osuna, F. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: A review with special reference to the situation in the Gulf of California. Aquaculture 2003, 219, 317–336. [Google Scholar] [CrossRef]
- Delgado, J.M.V.; Pólit, P.A.; Panta-Vélez, R.P.; Rodríguez-Díaz, J.M.; Dapena, J.D.; Lozano, A.L.; Maddela, N.R. Identification and composition of Cyanobacteria in ecuadorian shrimp farming ponds—Possible risk to human health. Curr. Microbiol. 2024, 81, 237. [Google Scholar]
- Hadjadji, I. A comparative analysis of Alexandrium catenella/tamarense blooms in Annaba Bay (Algeria) and Thau Lagoon (France); phosphorus limitation as a trigger. Comptes Rendus Biol. 2014, 337, 117–122. [Google Scholar]
- Yan, T.; Zhou, M.; Fu, M.; Yu, R.; Wang, Y.; Li, J. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 2003, 217, 167–178. [Google Scholar]
- Rolton, A.; Rhodes, L.; Hutson, K.S.; Biessy, L.; Bui, T.; MacKenzie, L.; Symonds, J.E.; Smith, K.F. Effects of harmful algal blooms on fish and shellfish species: A case study of New Zealand in a Changing environment. Toxins 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, V. The effect of the toxic dinoflagellate Alexandrium fundyense on the fitness of the calanoid copepod Calanus finmarchicus. Harmful Algae 2016, 51, 56–66. [Google Scholar] [CrossRef]
- Beauclercq, S.; Grenier, O.; Arnold, A.A.; Warschawski, D.E.; Wikfors, G.H.; Genard, B.; Tremblay, R.; Marcotte, I. Metabolomics and lipidomics reveal the effects of the toxic dinoflagellate Alexandrium catenella on immune cells of the blue mussel, mytilus edulis. Harmful Algae 2023, 129, 102529. [Google Scholar]
- Zhu, Z.; Qi, J.; Liu, Y.; Sui, Z. The H3K79 methylase DOT1, unreported in photosynthetic plants, exists in Alexandrium pacificum and participates in its growth regulation. Mar. Pollut. Bull. 2023, 190, 114867. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Li, L.; Ge, C.; Jian, L. A comprehensive analysis of hepatopancreas metabolomics and transcriptomics provides insights into the growth of three-year-old crabs (Eriocheir sinensis) under low temperature. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101182. [Google Scholar]
- Jiang, Y.; Liu, X.; Han, H.; Shang, Y.; Li, J.; Gao, B.; Ren, Y.; Meng, X. Temporal and tissue-specific regulation of energy metabolism in the swimming crab Portunus trituberculatus during nitrite stress and recovery. Mar. Pollut. Bull. 2024, 208, 117024. [Google Scholar] [PubMed]
- Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 2019, 280, 1405–1444. [Google Scholar] [CrossRef]
- Shi, K.; Li, J.; Lv, J.; Liu, P.; Li, J.; Li, S. Full-length transcriptome sequences of ridgetail white prawn Exopalaemon carinicauda provide insight into gene expression dynamics during thermal stress. Sci. Total. Environ. 2020, 747, 141238. [Google Scholar]
- Li, W.; Wang, J.; Li, J.; Liu, P.; Fei, F.; Liu, B.; Li, J. The effect of astaxanthin on the alkalinity stress resistance of Exopalaemon carinicauda. Sci. Total. Environ. 2024, 917, 170415. [Google Scholar]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [PubMed]
- Ning, M.; Wei, P.; Shen, H.; Wan, X.; Jin, M.; Li, X.; Shi, H.; Qiao, Y.; Jiang, G.; Gu, W.; et al. Proteomic and metabolomic responses in hepatopancreas of whiteleg shrimp Litopenaeus vannamei infected by microsporidian Enterocytozoon hepatopenaei. Fish Shellfish. Immunol. 2019, 87, 534–545. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Zhuo, H.; Li, J.; Fu, S.; Zhou, X.; Wu, G.; Guo, C.; Liu, J. Integrated histological, physiological, and transcriptome analysis reveals the post-exposure recovery mechanism of nitrite in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2024, 281, 116673. [Google Scholar]
- Duan, Y.; Zhong, G.; Xiao, M.; Yang, Y.; Wang, Y.; Nan, Y. Integrated physiological, energy metabolism, and metabonomic responses indicate the stress response in the hepatopancreas of Litopenaeus vannamei to nitrite stress. Aquat. Toxicol. 2024, 277, 107164. [Google Scholar] [PubMed]
- Huang, M.; Ma, Y.; Che, S.; Shen, L.; Wan, Z.; Su, S.; Ding, S.; Li, X. Nanopolystyrene and phoxim pollution: A threat to hepatopancreas toxicity in chinese mitten crab (Eriocheir sinensis). Aquat. Toxicol. 2024, 276, 107124. [Google Scholar]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitao, M.A.S.; Okamoto, O.K.; Morse, D. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Leng, X. Comparative transcriptome analysis of grapevine in response to copper stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in gill of pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 2024, 586, 740766. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Xu, R.-Q.; Cheng, J.-W.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Hsieh, S.-L. Immunotoxicity and oxidative damage in Litopenaeus vannamei induced by polyethylene microplastics and copper co-exposure. Mar. Pollut. Bull. 2024, 205, 116683. [Google Scholar] [CrossRef] [PubMed]
- Urade, R. Oxidative protein folding in the plant endoplasmic reticulum. Biosci. Biotechnol. Biochem. 2019, 83, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life. Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Cao, P.; Bao, Z.; Xu, Y.; Xu, Z.; Guo, H. Histological, physiological and transcriptomic analysis in hepatopancreas of Procambarus clarkii under heat stress. Ecotoxicol. Environ. Saf. 2024, 289, 117459. [Google Scholar] [CrossRef]
- Zeng, Y.; Deng, B.; Kang, Z.; Araujo, P.; Mjøs, S.A.; Liu, R.; Lin, J.; Yang, T.; Qu, Y. Tissue accumulation of polystyrene microplastics causes oxidative stress, hepatopancreatic injury and metabolome alterations in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2023, 256, 114871. [Google Scholar] [CrossRef]
- Pu, C.; Liu, Y.; Zhu, J.; Ma, J.; Cui, M.; Mehdi, O.M.; Wang, B.; Wang, A.; Zhang, C. Mechanisms insights into bisphenol S-induced oxidative stress, lipid metabolism disruption, and autophagy dysfunction in freshwater crayfish. J. Hazard. Mater. 2024, 479, 135704. [Google Scholar] [CrossRef]
- Ravanelli, S.; den Brave, F.; Hoppe, T. Mitochondrial quality control governed by ubiquitin. Front. Cell Dev. Biol. 2020, 8, 270. [Google Scholar] [CrossRef]
- Durcan, T.M.; Tang, M.Y.; Pérusse, J.R.; Dashti, E.A.; Aguileta, M.A.; McLelland, G.; Gros, P.; Shaler, T.A.; Faubert, D.; Coulombe, B.; et al. USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO. J. 2014, 33, 2473–2491. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Li, H.; Liu, H.; Chen, T.; Lin, Q.; Gong, C.; Yu, F.; Cai, H.; Jin, L.; et al. Mitochondrial homeostatic imbalance-mediated developmental toxicity to H2S in embryonic zebrafish. Environ. Pollut. 2024, 367, 125588. [Google Scholar] [CrossRef]
- Dong, W.; Liao, M.; Zhuang, X.; Huang, L.; Liu, C.; Wang, F.; Yin, X.; Liu, Y.; Liang, Q.; Wang, W. MYC drives autophagy to adapt to stress in Penaeus vannamei. Fish Shellfish. Immunol. 2022, 126, 187–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Cao, L.; Du, J.; Xu, G.; Xu, P. Microcystin-LR-induced autophagy via miR-282–5p/PIK3R1 pathway in Eriocheir sinensis hepatopancreas. Ecotoxicol. Environ. Saf. 2023, 267, 115661. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Dong, J.; Wang, L.; Li, W.; Bao, J.; Jiang, H. Chronic chlorpyrifos exposure induces oxidative stress, neurological damage, and hepatopancreas enrichment in chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 289, 110111. [Google Scholar] [CrossRef]
- Sun, S. Transciptomic and histological analysis of hepatopancreas, muscle and gill tissues of oriental river prawn (Macrobrachium nipponense) in response to chronic hypoxia. BMC Genom. 2015, 16, 491. [Google Scholar] [CrossRef]
- Shang, X.; Geng, L.; Che, X.; Li, W.; Liu, Y.; Li, J.; Teng, X.; Xu, W. Analysis revealed the molecular mechanism of oxidative stress- autophagy-induced liver injury caused by high alkalinity: Integrated whole hepatic transcriptome and metabolome. Front. Immunol. 2024, 15, 1431224. [Google Scholar] [CrossRef]
- Chandel, N.S. Glycolysis. Cold. Spring. Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Histology, physiology, and glucose and lipid metabolism of Lateolabrax maculatus under low temperature stress. J. Therm. Biol. 2022, 104, 103161. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 2023, 145, 155614. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Zhao, H.; Yang, T.; Xue, X.; Zhao, B.; Sun, Y. Prognostic and immunological significance of key gluconeogenesis regulators, PCK1 and PCK2, in human cancers especially kidney renal clear cell carcinoma: Insights from pan-cancer analysis, PCKs signature construction, and in vitro experiments. J. Radiat. Res. Appl. Sci. 2023, 16, 100614. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, Y.; Watari, K.; Lee, M.; Liu, J.; Perez, S.; Thai, M.; Mayfield, J.E.; Zhang, B.; Cunha E Rocha, K.; et al. Fructose-1,6-bisphosphatase is a nonenzymatic safety valve that curtails AKT activation to prevent insulin hyperresponsiveness. Cell Metab. 2023, 35, 1009–1021.e9. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, W.; Zhao, R.; Gu, C.; Shen, H.; Li, H.; Wang, L.; Cheng, J.; Wan, X. Integrated physiological, transcriptome, and metabolome analyses of the hepatopancreas of Litopenaeus vannamei under cold stress. Comp. Biochem. Physiol. Part. D Genomics Proteomics. 2024, 49, 101196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; Liu, T.; Ren, X.; Bai, X. Sex differences in antioxidant ability and energy metabolism level resulting in the difference of hypoxia tolerance in red swamp crayfish (Procambarus clarkii). Comp. Biochem. Physiol. Part D Genomics Proteomics. 2023, 48, 101136. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 688, pp. 1–23. [Google Scholar]
- Hammerschmidt, P. CerS6-dependent ceramide synthesis in hypothalamic neurons promotes ER/mitochondrial stress and impairs glucose homeostasis in obese mice. Nat. Commun. 2023, 14, 7824. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Di Camillo, B.; Miner, P.; Huvet, A.; Quillien, V.; Milan, M.; Ferraresso, S.; Pegolo, S.; Patarnello, T.; Bargelloni, L. Understanding the mechanisms involved in the high sensitivity of Pecten maximus larvae to aeration. Aquaculture 2018, 497, 189–199. [Google Scholar] [CrossRef]
- Zhu, T.; Li, S.; Wang, J. Induced sputum metabolomic profiles and oxidative stress are associated with Chronic Obstructive Pulmonary Disease (COPD) severity: Potential use for predictive, preventive, and personalized medicine. EPMA. J. 2020, 11, 645–659. [Google Scholar] [CrossRef]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid. Res. 2014, 55, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Lipopolysaccharide binding protein resists hepatic oxidative stress by regulating lipid droplet homeostasis. Nat. Commun. 2024, 15, 3213. [Google Scholar] [CrossRef]
- Mak, H.Y.; Ouyang, Q.; Tumanov, S.; Xu, J.; Rong, P.; Dong, F. AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway. Nat. Commun. 2021, 12, 6877. [Google Scholar] [CrossRef]
- Yu, K.; Xu, H.; Shi, C.; Wang, C.; Mu, C.; Ye, Y.; Chen, S.; Li, R.; Wu, Q. Overwintering temperature affects lipid and fatty acid metabolism in hepatopancreas and ovary of female mud crab Scylla paramamosain. Aquacult. Rep. 2025, 40, 102563. [Google Scholar]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [PubMed]
- Yang, Y. Acute thiamethoxam exposure induces hepatotoxicity and neurotoxicity in juvenile chinese mitten crab (Eriocheir sinensis). Ecotoxicol. Environ. Saf. 2023, 249, 114399. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar]
- Yuan, J.; Gao, Y.; Zhang, X.; Wei, J.; Liu, C.; Li, F.; Xiang, J. Genome sequences of marine shrimp Exopalaemon carinicauda holthuis provide insights into genome size evolution of caridea. Mar. Drugs 2017, 15, 213. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Bowers, R.M.; Licon, K.S.; Veazey, G.; Read, B. Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol. Immunol. 2009, 46, 1688–1695. [Google Scholar]




| KEGG Pathway | Gene ID | Gene Name | Regulation | Log2FC |
|---|---|---|---|---|
| Protein processing | Unigene0004773 | SEC61 translocon subunit beta (SEC61B) | ↑ | 1.0581 |
| Unigene0035173 | Lectin, Mannose Binding 1 (LMAN1) | ↑ | 1.2096 | |
| Unigene0011351 | DnaJ heat shock protein family (Hsp40) member C3 (DNAJC3) | ↓ | −1.3528 | |
| Unigene0018843 | Cullin 1 (CUL1) | ↓ | −1.6686 | |
| Unigene0019622 | DnaJ heat shock protein family (Hsp40) member C5 (DNAJC 5) | ↓ | −2.2786 | |
| Unigene0021323 | ER Degradation Enhancing Alpha-Mannosidase Like Protein 3 (edem3) | ↓ | −1.6412 | |
| Unigene0017803 | DnaJ heat shock protein family (Hsp40) member C10 (DNAJC10) | ↓ | −3.2146 | |
| Unigene0006430 | B Cell Receptor-Associated Protein 29 (Bcap29) | ↓ | −1.0944 | |
| Unigene0020150 | ubiquitination factor E4B (UBE4B) | ↓ | −1.5901 | |
| Unigene0006758 | DnaJ heat shock protein family (Hsp40) member A2 (DNAJA2) | ↓ | −1.1873 | |
| Unigene0006469 | crystallin, alpha B (CRYAB) | ↑ | 1.8274 | |
| Unigene0024253 | Thioredoxin Domain Containing 5 (TXNDC5) | ↓ | −1.1330 | |
| Unigene0042968 | Membrane-bound transcription factor peptidase, site 1 (MBTPS1) | ↓ | −1.5759 | |
| Unigene0018951 | NSFL1 cofactor (NSFL1C) | ↓ | −1.1385 | |
| Unigene0039288 | Endoplasmic Reticulum Lectin 1 (Erlec1) | ↓ | −1.9039 | |
| Unigene0008319 | lethal (2) essential for life [l(2)efl] | ↓ | −2.1341 | |
| Unigene0014712 | Membrane-associated ring-CH-type finger 6 (MARCHF6) | ↓ | −1.0324 | |
| Unigene0017804 | Ubiquitin Recognition Factor In ER-Associated Degradation 1 (Ufd1) | ↓ | −1.2287 | |
| Mitophagy | Unigene0041625 | Sequestosome 1 (SQSTM1) | ↓ | −1.8847 |
| Unigene0033333 | Ubiquitin C (UBC) | ↓ | −1.4974 | |
| Unigene0038070 | Autophagy-related protein 9A (ATG9A) | ↓ | −2.2367 | |
| Unigene0034577 | Muscle RAS Oncogene Homolog (MRAS) | ↓ | −1.5686 | |
| Unigene0009963 | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit (JUN) | ↓ | −1.3923 | |
| Unigene0038584 | GABA type A receptor-associated protein (GABARAP) | ↓ | −1.3066 | |
| Unigene0019289 | TANK binding kinase 1 (tbk1) | ↓ | −1.5371 | |
| Unigene0003988 | Microtubule-associated protein 1 light chain 3 alpha (MAP1LC3A) | ↓ | −1.3475 | |
| Unigene0007421 | Ras oncogene at 85D (Ras85D) | ↓ | −1.1482 | |
| Unigene0043075 | Ubiquitin Specific Peptidase 8 (Usp8) | ↓ | −1.0614 | |
| Unigene0032676 | TBC1 Domain Family Member 15 (TBC1D15) | ↓ | −1.3495 | |
| Sphingolipid metabolism | Unigene0039670 | Sphingomyelin Phosphodiesterase 1 (SMPD1) | ↓ | −1.2652 |
| Unigene0041427 | UDP-glucose ceramide glucosyltransferase (ugcg) | ↓ | −1.9397 | |
| Unigene0000281 | sphingosine-1-phosphate lyase (Sply) | ↓ | −1.3089 | |
| Unigene0020466 | galactose-3-O-sulfotransferase 1 (GAL3ST1) | ↑ | 1.0295 | |
| Unigene0032984 | Ceramide Synthase 6 (CERS6) | ↓ | −1.2039 | |
| Unigene0016910 | Burrows-Wheeler Aligner (bwa) | ↓ | −2.1083 | |
| Glycerophospholipid metabolism | Unigene0003027 | N-myristoyltransferase 2 (NMT2) | ↑ | 1.7817 |
| Unigene0001693 | lipin 2 (LPIN2) | ↓ | −2.5149 | |
| Unigene0005731 | Phospholipase D beta 1 (PLDBETA1) | ↓ | −1.7478 | |
| Unigene0040271 | 1-acylglycerol-3-phosphate O-acyltransferase 4 (AGPAT4) | ↓ | −1.1435 | |
| Unigene0033720 | glycerol-3-phosphate acyltransferase 4 (Gpat4) | ↓ | −1.4497 | |
| Unigene0039712 | Glycerophosphocholine Phosphodiesterase 1 (Gpcpd1) | ↓ | −2.1396 | |
| Unigene0019010 | glycerol-3-phosphate dehydrogenase 2 (Gpd2) | ↓ | −2.2879 | |
| Unigene0019229 | Glycerol-3-phosphate dehydrogenase 1 (Gpdh1) | ↓ | −1.1828 | |
| Unigene0012708 | lysophosphatidylglycerol acyltransferase 1 (LPGAT1) | ↓ | −1.5238 | |
| Unigene0001374 | 1-Acylglycerol-3-Phosphate O-Acyltransferase 1 (AGPAT1) | ↓ | −1.6105 | |
| Unigene0008571 | Selenoprotein I (SELENOI) | ↓ | −1.4981 | |
| Glycolysis/Gluconeogenesis | Unigene0001370 | Triosephosphate Isomerase 1a (tpi1a) | ↑ | 1.1835 |
| Unigene0004971 | Aldehyde Dehydrogenase 3 Family Member A2 (Aldh3a2) | ↓ | −1.8675 | |
| Unigene0010840 | Enolase (Eno) | ↑ | 1.0917 | |
| Unigene0039462 | Fructose-1,6-bisphosphatase 1 (FBP1) | ↑ | 1.5572 | |
| Unigene0033523 | Alcohol Dehydrogenase 5 (Class III), Chi Polypeptide (ADH5) | ↑ | 1.4259 | |
| Unigene0013435 | Phosphoglycerate Kinase (Pgk) | ↑ | 1.0731 | |
| Unigene0000916 | Lactate Dehydrogenase (LDH) | ↓ | −1.3764 | |
| Unigene0037089 | Aldehyde Dehydrogenase 3 Family Member A1 (Aldh3a1) | ↓ | −1.4323 | |
| Unigene0002179 | Phosphoenolpyruvate Carboxykinase 2, Mitochondrial (PCK2) | ↓ | −1.6471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Cheng, W.; Fan, M.; Liu, D.; Cao, Y.; Mei, X.; Wan, J.; Hu, G.; Gao, H.; Ji, N. Effects of Alexandrium pacificum Exposure on Exopalaemon carinicauda: Hepatopancreas Histology, Antioxidant Enzyme Activity, and Transcriptome Analysis. Int. J. Mol. Sci. 2025, 26, 1605. https://doi.org/10.3390/ijms26041605
Han W, Cheng W, Fan M, Liu D, Cao Y, Mei X, Wan J, Hu G, Gao H, Ji N. Effects of Alexandrium pacificum Exposure on Exopalaemon carinicauda: Hepatopancreas Histology, Antioxidant Enzyme Activity, and Transcriptome Analysis. International Journal of Molecular Sciences. 2025; 26(4):1605. https://doi.org/10.3390/ijms26041605
Chicago/Turabian StyleHan, Wanyu, Weitao Cheng, Menghao Fan, Dexue Liu, Yanrong Cao, Xuao Mei, Jiaxuan Wan, Guangwei Hu, Huan Gao, and Nanjing Ji. 2025. "Effects of Alexandrium pacificum Exposure on Exopalaemon carinicauda: Hepatopancreas Histology, Antioxidant Enzyme Activity, and Transcriptome Analysis" International Journal of Molecular Sciences 26, no. 4: 1605. https://doi.org/10.3390/ijms26041605
APA StyleHan, W., Cheng, W., Fan, M., Liu, D., Cao, Y., Mei, X., Wan, J., Hu, G., Gao, H., & Ji, N. (2025). Effects of Alexandrium pacificum Exposure on Exopalaemon carinicauda: Hepatopancreas Histology, Antioxidant Enzyme Activity, and Transcriptome Analysis. International Journal of Molecular Sciences, 26(4), 1605. https://doi.org/10.3390/ijms26041605






