Effects of Heat Treatment on Physicochemical Properties of Moringa oleifera Lam. Leaf Protein
Abstract
1. Introduction
2. Results
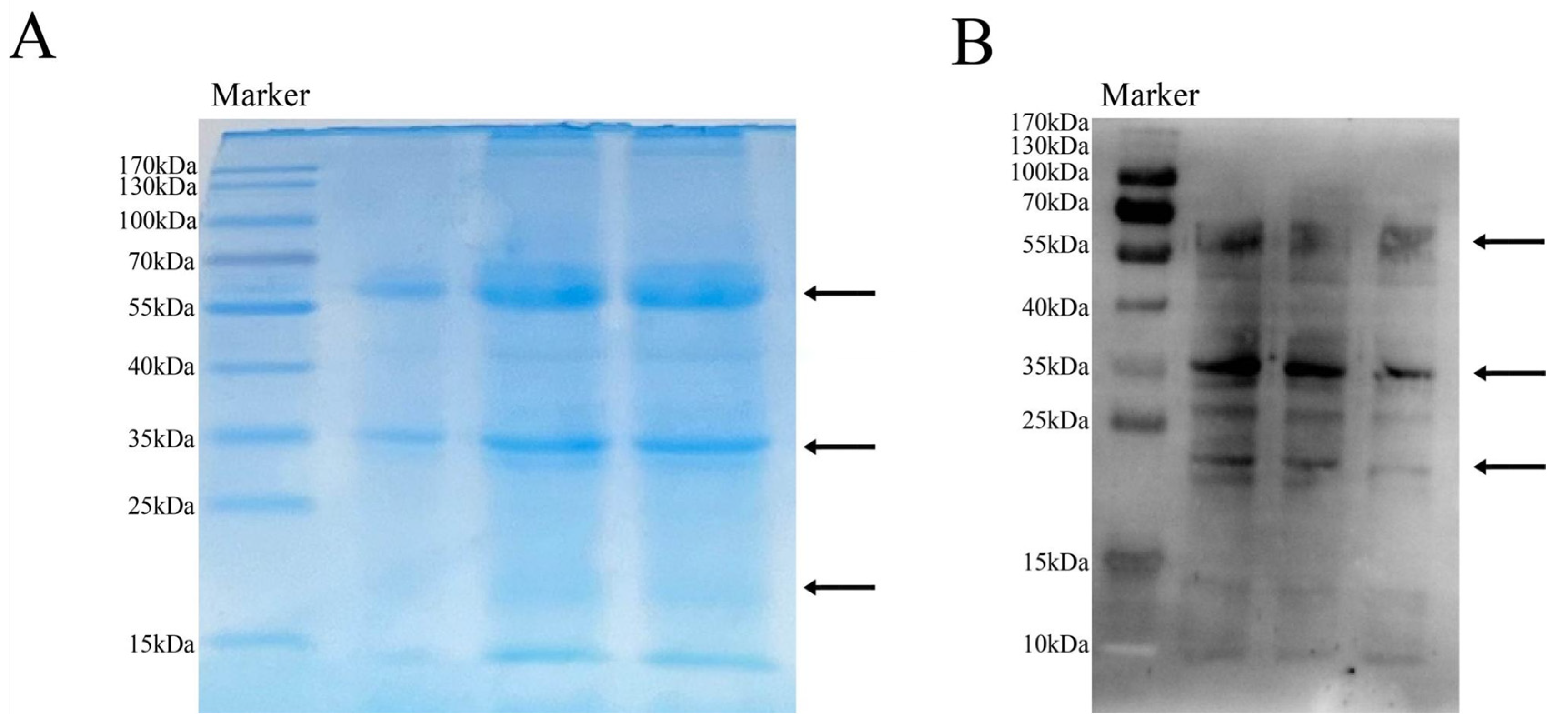
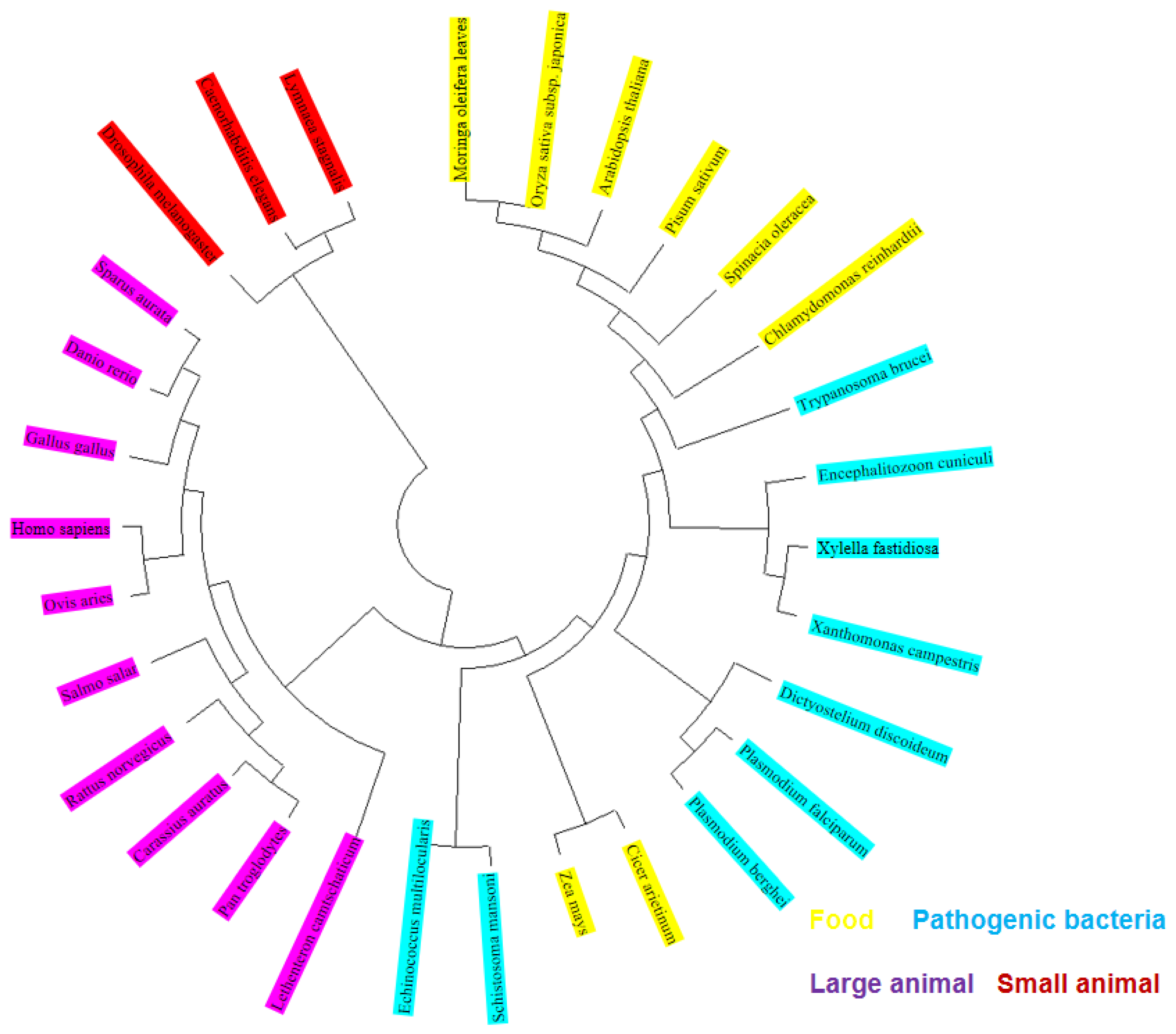
2.1. M. oleifera Leaf Allergen Extraction and Sensitizing Activity Determination
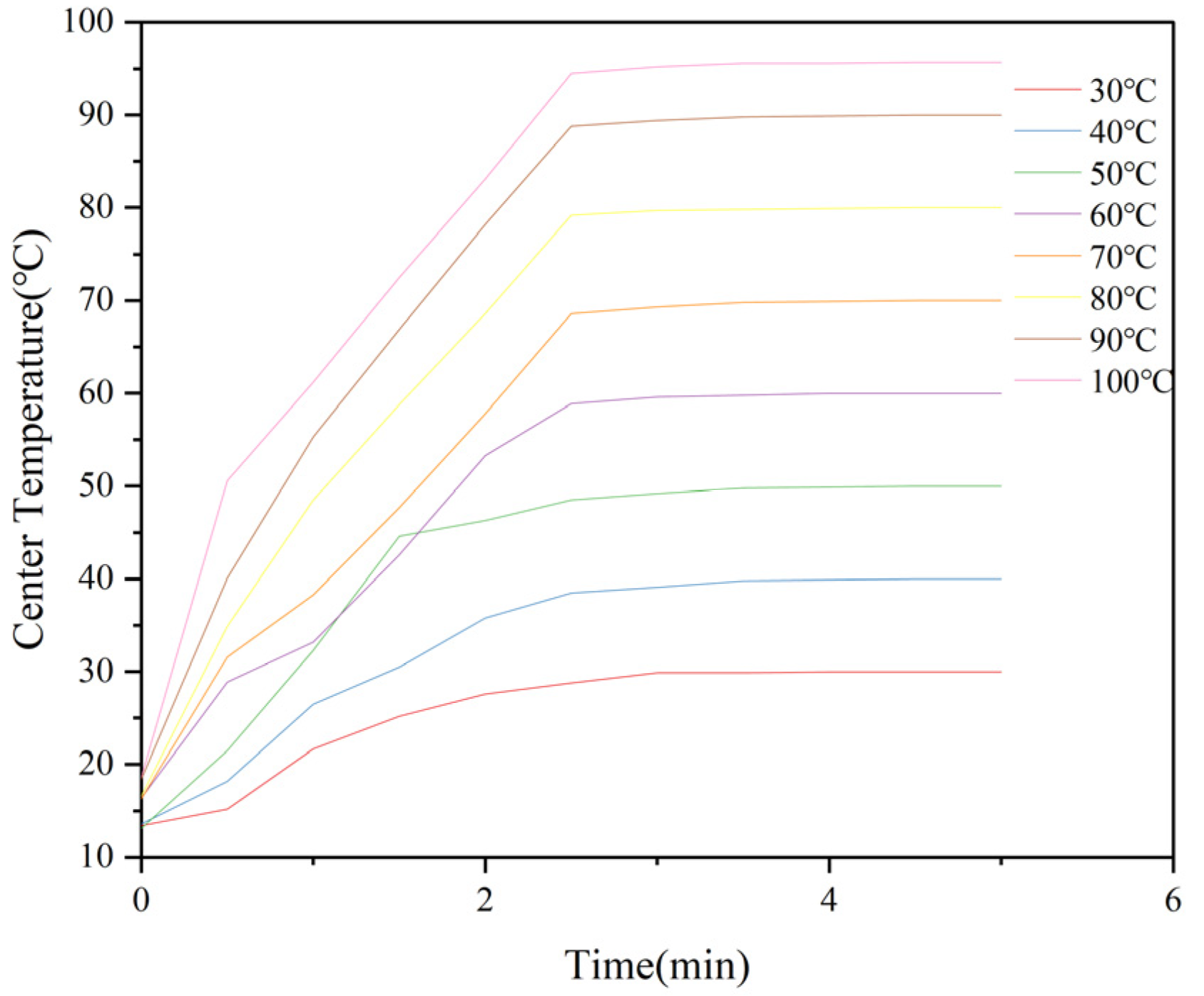
2.2. Determination of Center Temperature in M. oleifera Leaf Allergen
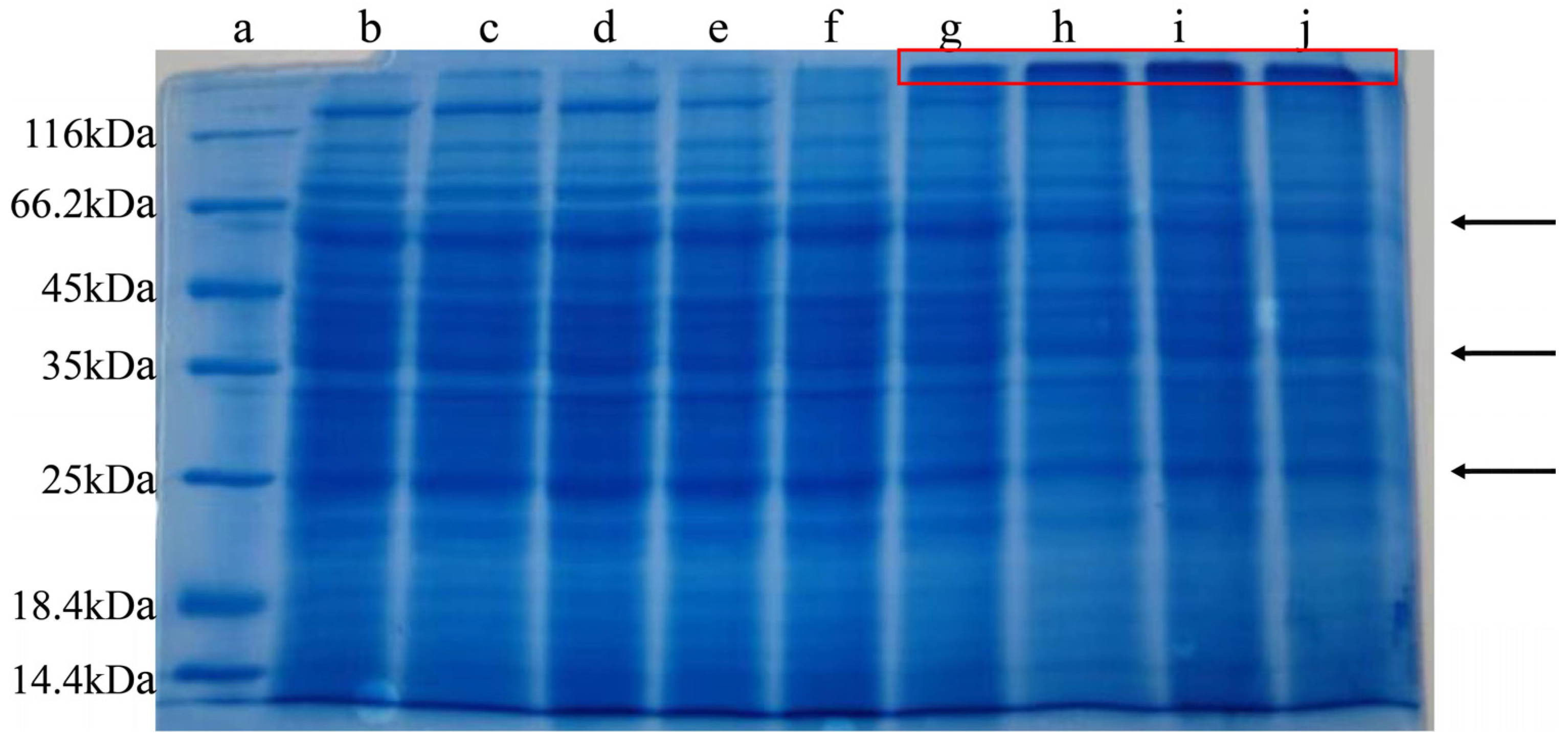
2.3. SDS-PAGE of M. oleifera Leaf Allergen After Heat Treatment
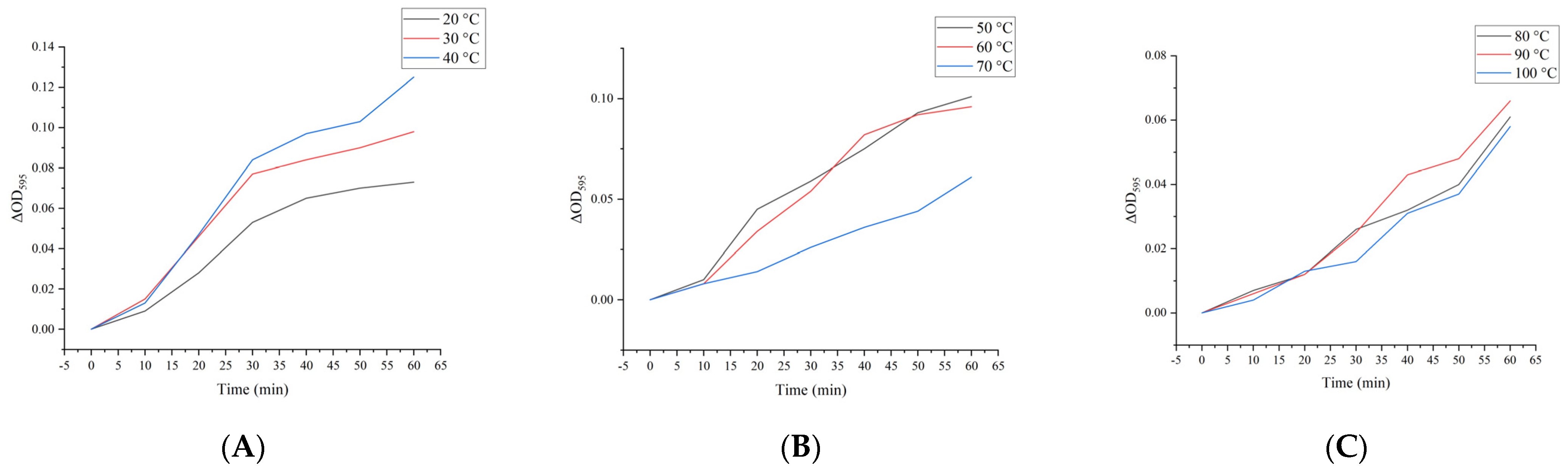
2.4. Effect of Heat Treatment on Hemagglutination Activity of M. oleifera Leaf Allergen
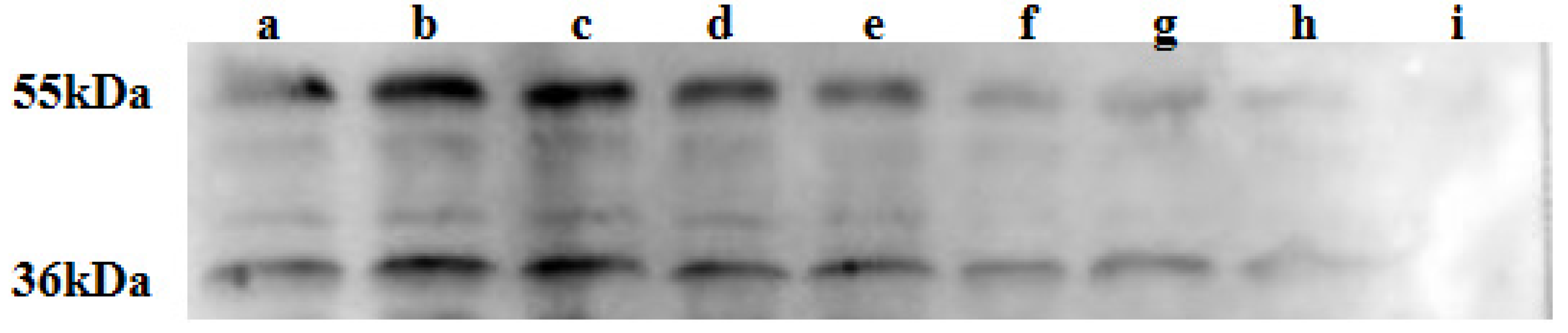
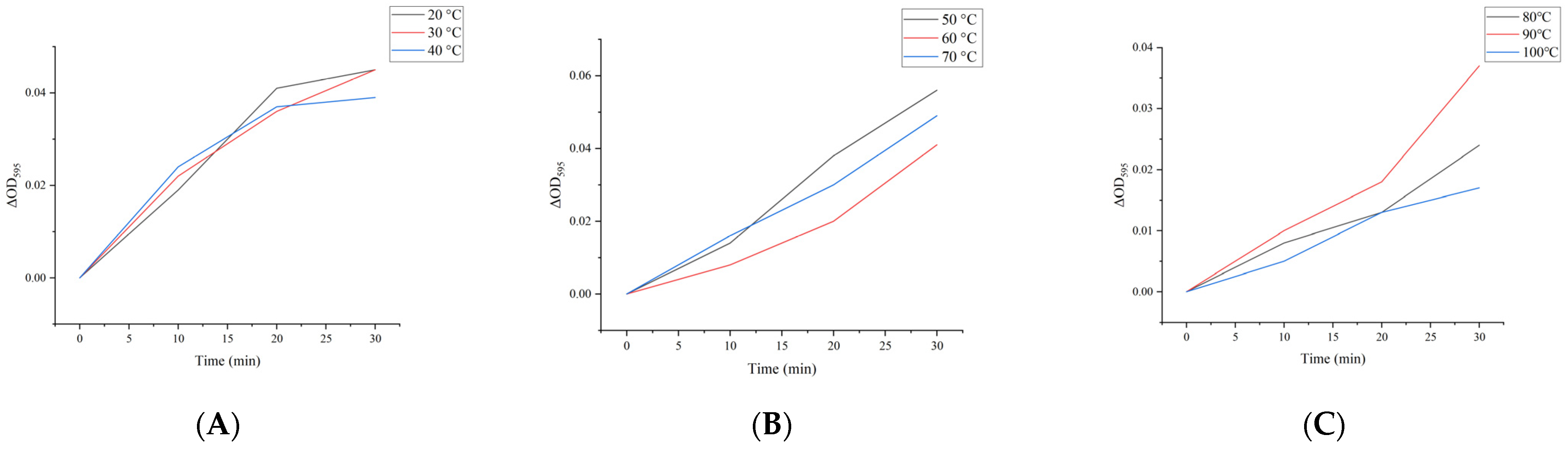
2.5. Effect of Heat Treatment on Sensitizing Activity of M. oleifera Leaf Allergen
2.6. Analysis of M. oleifera Leaf Allergen Denaturation Temperature
2.7. Effect of Heat Treatment on Particle Size and Zeta Potential of M. oleifera Leaf Allergen
2.8. Effect of Heat Treatment on Total Sulfhydryl (TSH) Content of M. oleifera Leaf Allergen
2.9. Effect of Heat Treatment on Surface Hydrophobicity of M. oleifera Leaf Allergen
2.10. Effect of Heat Treatment on Solubility of M. oleifera Leaf Allergen
2.11. Effect of Heat Treatment on Ultraviolet Spectrum (UV) of M. oleifera Leaf Allergen
2.12. Effect of Heat Treatment on Intrinsic Fluorescence Spectrum of M. oleifera Leaf Allergen
2.13. Effect of Heat Treatment on Protein Aggregates of M. oleifera Leaf Allergen
2.14. Effect of Heat Treatment on Simulated Digestibility In Vitro of M. oleifera Leaf Allergen
2.15. Prediction Results of FBA Hydrophilicity
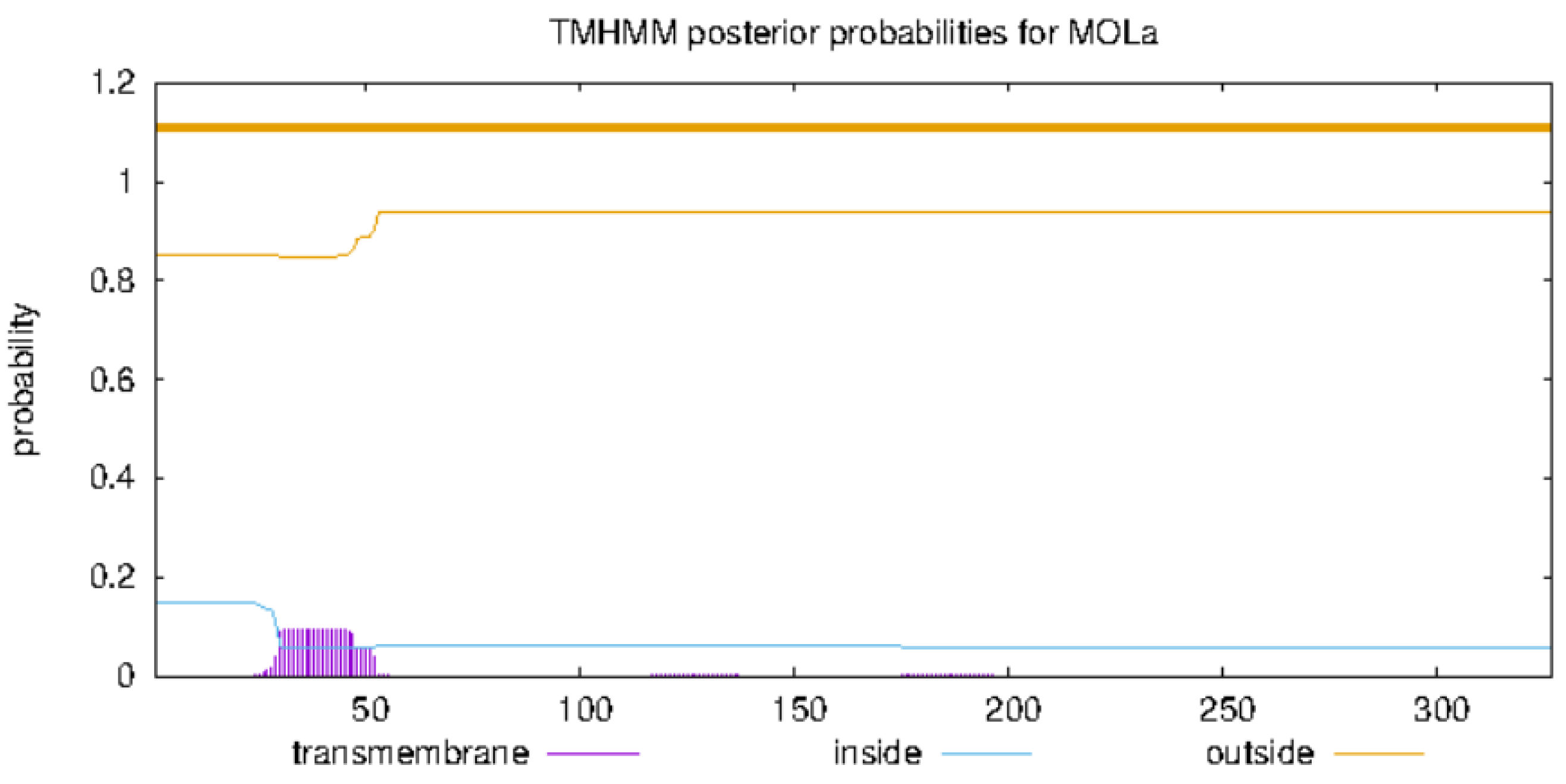
2.16. Prediction of Transmembrane Region in FBA
2.17. Prediction Results of FBA Antigenicity Index
2.18. Prediction Results of FBA Calcium Binding Site
2.19. Prediction Results of FBA Secondary Structure
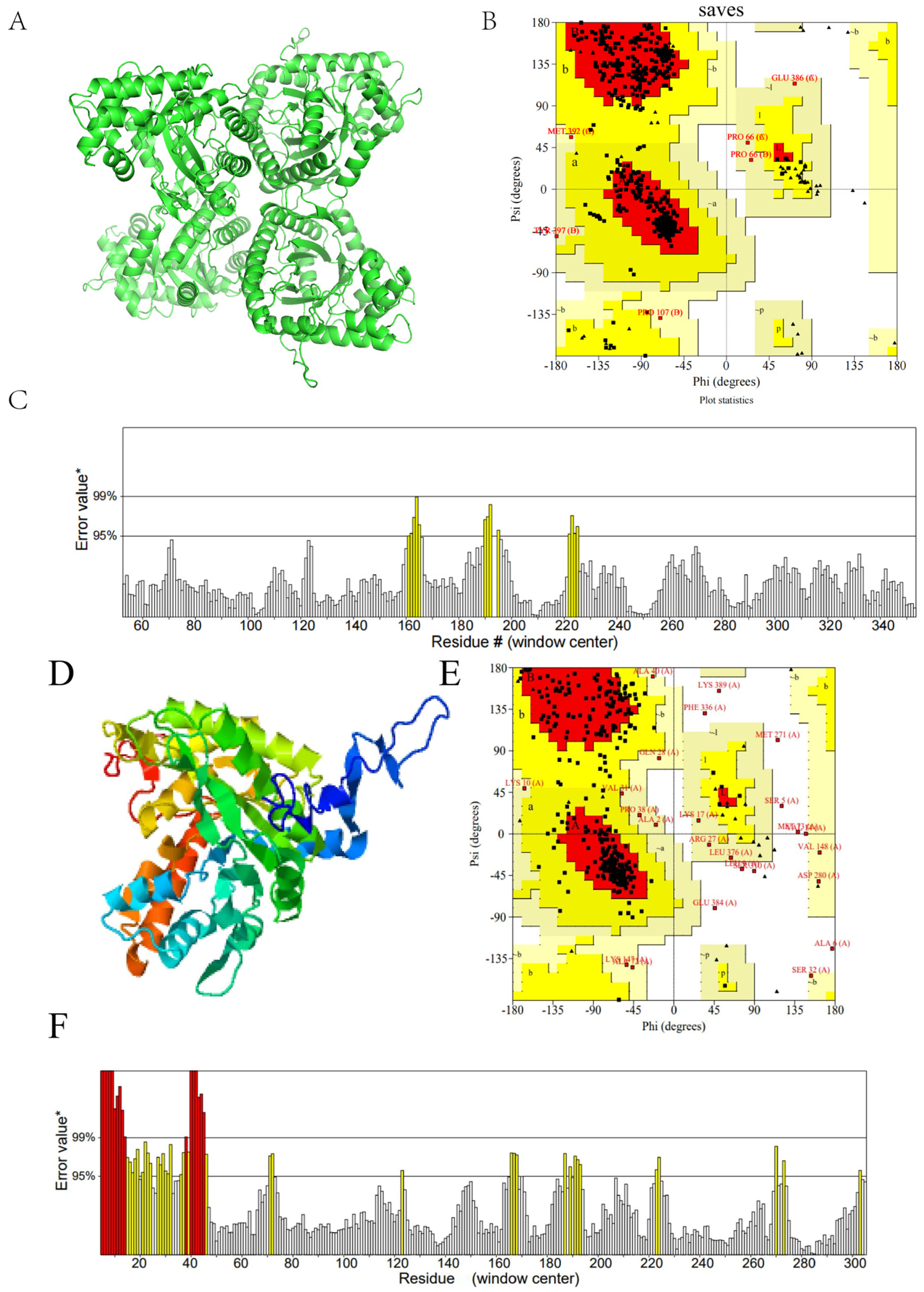
2.20. Prediction Results of FBA Three-Dimensional Structure
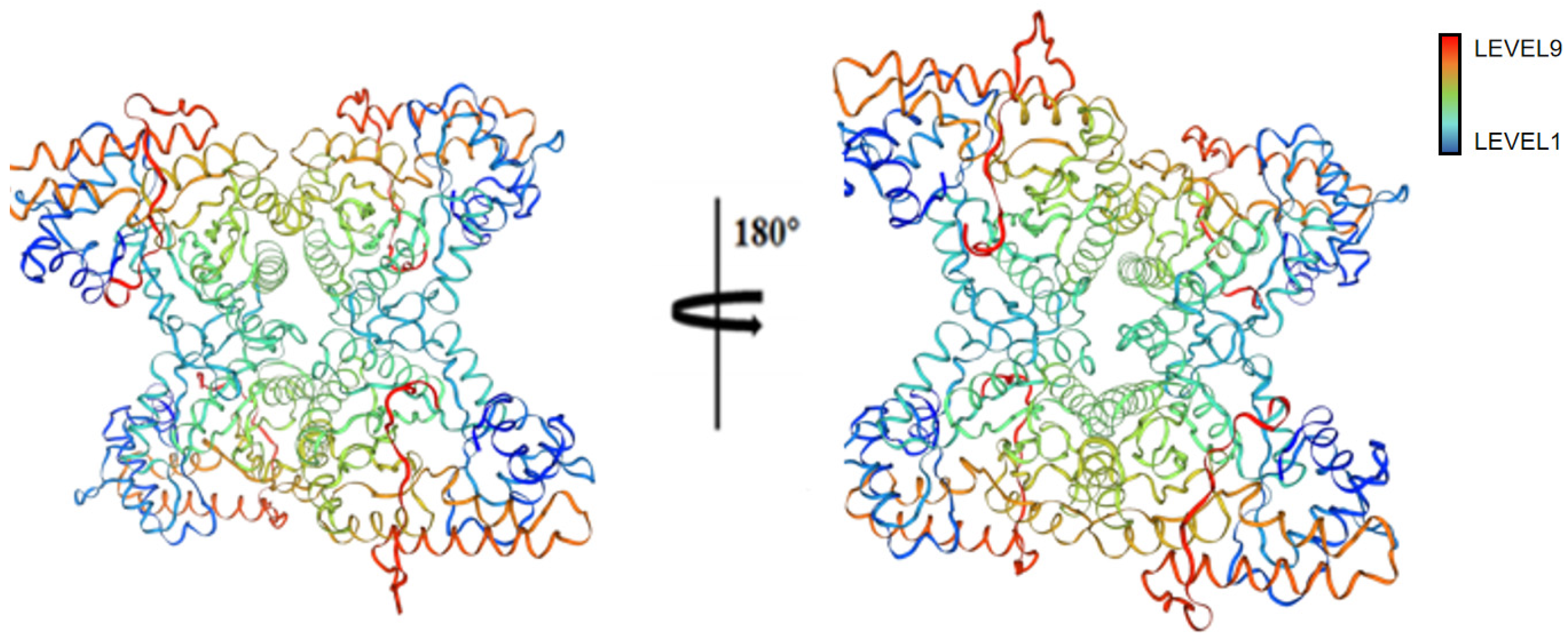
2.21. Conservative Analysis of FBA Three-Dimensional Structure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of M. oleifera Leaf Protein (M. oleifera Leaf Allergen)
4.3. Determination of the Hemagglutination Titer
4.4. Heat Treatment
4.5. SDS-PAGE
4.6. Differential Scanning Calorimetry (DSC)
4.7. Particle Size and Zeta Potential
4.8. Total Sulfhydryl (TSH) Content
4.9. Protein Solubility
4.10. Surface Hydrophobic
4.11. Intrinsic Fluorescence Spectrum
4.12. Ultraviolet Spectrum
4.13. Protein Aggregates
4.14. In Vitro Digestibility
4.14.1. In Vitro Pepsin Digestion
4.14.2. In Vitro Trypsin Digestion
4.15. Prediction of FBA Hydrophilicity
4.16. Prediction of FBA Transmembrane Region
4.17. Prediction of FBA Antigenicity Index
4.18. Prediction of FBA Calcium Binding Site
4.19. Prediction of FBA Secondary Structure
4.20. Prediction of FBA Three-Dimensional Structure
4.21. Immunoblotting
4.22. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandran, C.; Gopalakrishnan, K.V.P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283. [Google Scholar] [CrossRef]
- Hadidi, M.; Hossienpour, Y.; Nooshkam, M.; Mahfouzi, M.; Gharagozlou, M.; Aliakbari, F.S.; Aghababaei, F.; McClement, D.J. Green leaf proteins: A sustainable source of edible plant-based proteins. Crit. Rev. Food Sci. Nutr. 2023, 64, 10855–10872. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. PTR 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci. China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Poussel, M.; Penven, E.; Richard, C.; Jacquenet, S.; Chabot, F.; Paris, C. Occupational asthma to “the miracle tree” (Moringa oleifera): First description. J. Allergy Clin. Immunol. Pract. 2015, 3, 813–814. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, Z.; Zhang, H.; Gao, J.; Wu, Y.; Meng, X.; Zhong, Y.; Chen, H. Potential Allergenicity Response to Moringa oleifera Leaf Proteins in BALB/c Mice. Nutrients 2022, 14, 4700. [Google Scholar] [CrossRef]
- D’Auria, G.; Nitride, C.; Nicolai, M.A.; Mamone, G.; Montesano, D.; Mills, E.N.C.; Ferranti, P. Identification of allergen encoding sequences in a novel food ingredient from Moringa oleifera leaves. Food Chem. 2023, 401, 134185. [Google Scholar] [CrossRef]
- Peumans, W.J.; Van Damme, E.J. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Marothia, D.; Kaur, N.; Jhamat, C.; Sharma, I.; Pati, P.K. Plant lectins: Classical molecules with emerging roles in stress tolerance. Int. J. Biol. Macromol. 2023, 244, 125272. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Panda, P.K.; Sinha, N.; Praharaj, P.P.; Bhol, C.S.; Panigrahi, D.P.; Mahapatra, K.K.; Saha, S.; Patra, S.; Mishra, S.R.; et al. Plant lectins in cancer therapeutics: Targeting apoptosis and autophagy-dependent cell death. Pharmacol. Res. 2019, 144, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Argolo, A.C.; Coelho, L.C.; Paiva, P.M. Detection of water soluble lectin and antioxidant component from Moringa oleifera seeds. Water Res. 2005, 39, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Kwaambwa, H.M.; Maikokera, R. Infrared and circular dichroism spectroscopic characterisation of secondary structure components of a water treatment coagulant protein extracted from Moringa oleifera seeds. Colloids Surf. B Biointerfaces 2008, 64, 118–125. [Google Scholar] [CrossRef]
- Moura, M.C.; Napoleão, T.H.; Coriolano, M.C.; Paiva, P.M.G.; Figueiredo, R.C.B.Q.; Coelho, L.C.B.B. Water-soluble Moringa oleifera lectin interferes with growth, survival and cell permeability of corrosive and pathogenic bacteria. J. Appl. Microbiol. 2015, 119, 666–676. [Google Scholar] [CrossRef]
- Moura, M.C.; Trentin, D.S.; Napoleão, T.H.; Primon-Barros, M.; Xavier, A.S.; Carneiro, N.P.; Paiva, P.M.G.; Macedo, A.J.; Coelho, L.C.B.B. Multi-effect of the water-soluble Moringa oleifera lectin against Serratia marcescens and Bacillus sp.: Antibacterial, antibiofilm and anti-adhesive properties. J. Appl. Microbiol. 2017, 123, 861–874. [Google Scholar] [CrossRef]
- Araújo, L.C.C.; Aguiar, J.S.; Napoleão, T.H.; Mota, F.V.B.; Barros, A.L.S.; Moura, M.C.; Coriolano, M.C.; Coelho, L.C.B.B.; Silva, T.G.; Paiva, P.M.G. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS ONE 2013, 8, e81973. [Google Scholar] [CrossRef]
- Rolim, L.A.D.M.M.; Macêdo, M.F.S.; Sisenando, H.A.; Napoleão, T.H.; Felzenszwalb, I.; Aiub, C.A.F.; Coelho, L.C.B.B.; Medeiros, S.R.B.; Paiva, P.M.G. Genotoxicity evaluation of Moringa oleifera seed extract and lectin. J. Food Sci. 2011, 76, T53–T58. [Google Scholar] [CrossRef]
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef]
- Barre, A.; Damme, E.J.M.V.; Simplicien, M.; Benoist, H.; Rougé, P. Are Dietary Lectins Relevant Allergens in Plant Food Allergy? Foods 2020, 9, 1724. [Google Scholar] [CrossRef]
- Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro. Molecules 2017, 22, 95. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Zhou, F.; Sun, H.; Cao, X.; Ye, Y.; Li, J. Detection of Lectin Protein Allergen of Kidney Beans (Phaseolus vulgaris L.) and Desensitization Food Processing Technology. J. Agric. Food Chem. 2021, 69, 14723–14741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, L.; Wang, X.; Ding, X.; Li, L.; Tian, Y.; Huang, A. Characterization of a Novel Antimicrobial Peptide Isolated from Moringa oleifera Seed Protein Hydrolysates and Its Membrane Damaging Effects on Staphylococcus aureus. J. Agric. Food Chem. 2022, 70, 6123–6133. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Mckenna, A.B. Reaction kinetics of thermal denaturation of whey proteins in heated reconstituted whole milk. J. Agric. Food Chem. 1996, 44, 422–428. [Google Scholar] [CrossRef]
- Tanford, C. Protein Denaturation. Adv. Protein Chem. 1968, 23, 121–282. [Google Scholar] [PubMed]
- He, F.; Becker, G.W.; Litowski, J.R.; Narhi, L.O.; Brems, D.N.; Razinkov, V.I. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal. Biochem. 2010, 399, 141–143. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef]
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E.G. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar] [CrossRef]
- Demeule, B.; Gurny, R.; Arvinte, T. Detection and characterization of protein aggregates by fluorescence microscopy. Int. J. Pharm. 2007, 329, 37–45. [Google Scholar] [CrossRef]
- Chou, P.Y.; Fasman, G.D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 1978, 47, 45–148. [Google Scholar]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, I.; Bugajska-Schretter, A.; Linhart, B.; Verdino, P.; Keller, W.; Schulmeister, U.; Sperr, W.R.; Valent, P.; Peltre, G.; Quirce, S.; et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J. Immunol. 2007, 178, 6290–6296. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; Núñez-Gastélum, J.A.; Reyes-Moreno, C.; Ramírez-Wong, B.; López-Cervantes, J. Nutritional Quality of Edible Parts of Moringa oleifera. Food Anal. Methods 2010, 3, 175–180. [Google Scholar] [CrossRef]
- Xi, C.; Li, W.; Liu, X.; Xie, J.; Li, S.; Tian, Y.; Song, S. The Potential Role of Moringa oleifera Lam. Leaf Proteins in Moringa Allergy by Functionally Activating Murine Bone Marrow-Derived Dendritic Cells and Inducing Their Differentiation toward a Th2-Polarizing Phenotype. Nutrients 2024, 16, 7. [Google Scholar] [CrossRef]
- Liu, X.; Xi, C.; Li, W.; Su, H.; Yang, H.; Bai, Z.; Tian, Y.; Song, S. Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions. Int. J. Mol. Sci. 2023, 24, 16425. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Zhao, Q.; Shi, Y.; Chen, Y.; Huang, A. Structural Analysis of a Novel Aspartic-Type Endopeptidase from Moringa oleifera Seeds and Its Milk-Clotting Properties. J. Agric. Food Chem. 2021, 69, 7377–7387. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, S.; Feng, X.; Wang, R.; Zeng, M.; Zhao, Y. Physicochemical state and in vitro digestibility of heat treated water-soluble protein from Pacific oyster (Crassostrea gigas). Food Biosci. 2020, 34, 100528. [Google Scholar] [CrossRef]
- Liu, M.; Han, T.-J.; Huan, F.; Li, M.-S.; Xia, F.; Yang, Y.; Wu, Y.-H.; Chen, G.-X.; Cao, M.-J.; Liu, G.-M. Effects of thermal processing on the allergenicity, structure, and critical epitope amino acids of crab tropomyosin. Food Funct. 2021, 12, 2032–2043. [Google Scholar] [CrossRef]
- Liu, M.; Huan, F.; Li, M.; Han, T.; Xia, F.; Yang, Y.; Liu, Q.; Chen, G.; Cao, M.; Liu, G. Mapping and IgE-binding capacity analysis of heat/digested stable epitopes of mud crab allergens. Food Chem. 2021, 344, 128735. [Google Scholar] [CrossRef]
- Moraes, A.; Ackerbauer, D.; Kostadinova, M.; Bublin, M.; Oliveira, G.A.; Ferreira, F.; Almeida, F.C.L.; Breiteneder, H.; Valente, A.P. Solution and high-pressure NMR studies of the structure, dynamics and stability of the cross-reactive allergenic cod parvalbumin Gad m 1. World Allergy Organ. J. 2015, 8, A180. [Google Scholar] [CrossRef]
- Chodankar, S.; Aswal, V.K.; Kohlbrecher, J.; Vavrin, R.; Wagh, A.G. Surfactant-induced protein unfolding as studied by small-angle neutron scattering and dynamic light scattering. J. Phys. Condens. Matter 2007, 19, 326102. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Conformational changes and dynamic rheological properties of fish sarcoplasmic proteins treated at various pHs. Food Chem. 2010, 121, 1046–1052. [Google Scholar] [CrossRef]
- Shimada, K.; Cheftel, J.C. Determination of sulfhydryl groups and disulfide bonds in heat-induced gels of soy protein isolate. J. Agric. Food Chem. 1988, 36, 147–153. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]





| Temperature | Hemagglutination Titer/2n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | |
| Control | ++++ | ++++ | +++ | ++ | ++ | + | + | - | - |
| 30 °C | +++ | +++ | +++ | ++ | ++ | + | + | - | - |
| 40 °C | +++ | +++ | ++ | ++ | + | + | - | - | - |
| 50 °C | +++ | +++ | ++ | ++ | + | + | - | - | - |
| 60 °C | ++ | ++ | + | + | - | - | - | - | - |
| 70 °C | + | + | - | - | - | - | - | - | - |
| 80 °C | + | - | - | - | - | - | - | - | - |
| 90 °C | - | - | - | - | - | - | - | - | - |
| 100 °C | - | - | - | - | - | - | - | - | - |
| Method | α-Helix | β-Sheet | β-Turn | Random Coil |
|---|---|---|---|---|
| PSIPRED | 47.4% (155) | 6.7% (22) | 45.9% (150) | |
| SOPMA | 51.7% (169) | 12.8% (42) | 7.3% (24) | 28.1% (92) |
| NovoPro | 44.0% (144) | 18.3% (60) | 37.6% (123) | |
| Mean | 47.7% | 12.6% (60) | 3.2% | 37.2% (123) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, C.; Li, W.; Xu, Z.; Xie, J.; Gao, X.; Feng, D.; Tian, Y.; Song, S. Effects of Heat Treatment on Physicochemical Properties of Moringa oleifera Lam. Leaf Protein. Int. J. Mol. Sci. 2025, 26, 1647. https://doi.org/10.3390/ijms26041647
Xi C, Li W, Xu Z, Xie J, Gao X, Feng D, Tian Y, Song S. Effects of Heat Treatment on Physicochemical Properties of Moringa oleifera Lam. Leaf Protein. International Journal of Molecular Sciences. 2025; 26(4):1647. https://doi.org/10.3390/ijms26041647
Chicago/Turabian StyleXi, Chuyu, Wenjie Li, Zhiguo Xu, Jing Xie, Xiaoyu Gao, Dan Feng, Yang Tian, and Shuang Song. 2025. "Effects of Heat Treatment on Physicochemical Properties of Moringa oleifera Lam. Leaf Protein" International Journal of Molecular Sciences 26, no. 4: 1647. https://doi.org/10.3390/ijms26041647
APA StyleXi, C., Li, W., Xu, Z., Xie, J., Gao, X., Feng, D., Tian, Y., & Song, S. (2025). Effects of Heat Treatment on Physicochemical Properties of Moringa oleifera Lam. Leaf Protein. International Journal of Molecular Sciences, 26(4), 1647. https://doi.org/10.3390/ijms26041647






