Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate
Abstract
:1. Introduction
2. Results and Discussion
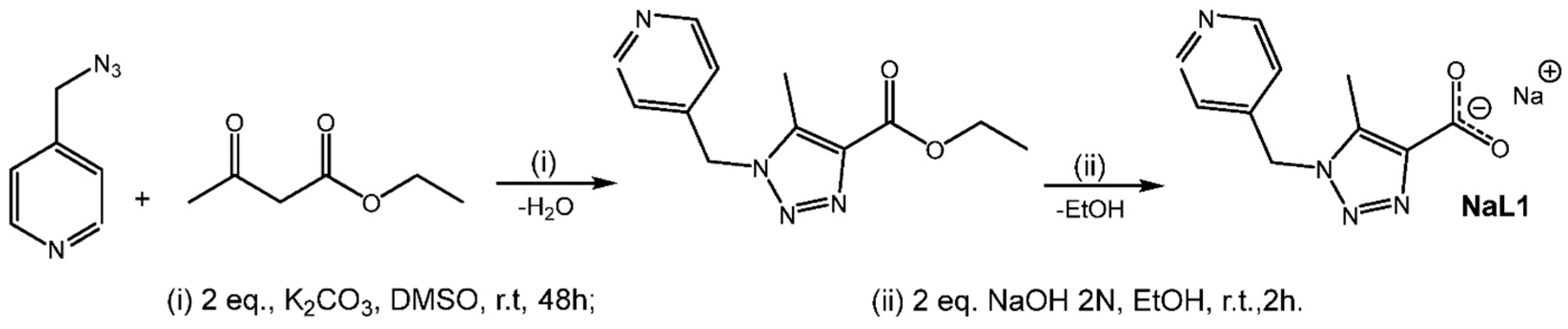
2.1. Synthesis of NaL1
2.2. Syntheses of CP1 and CP2
2.3. Powder X-Ray Diffraction
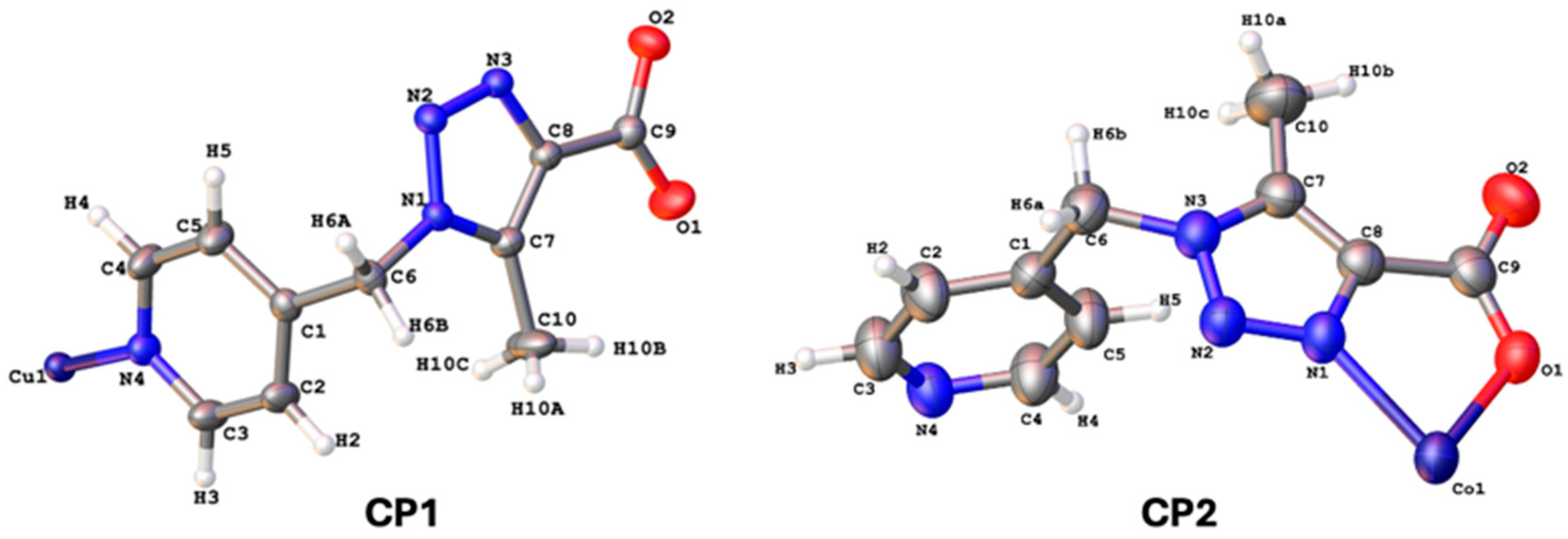
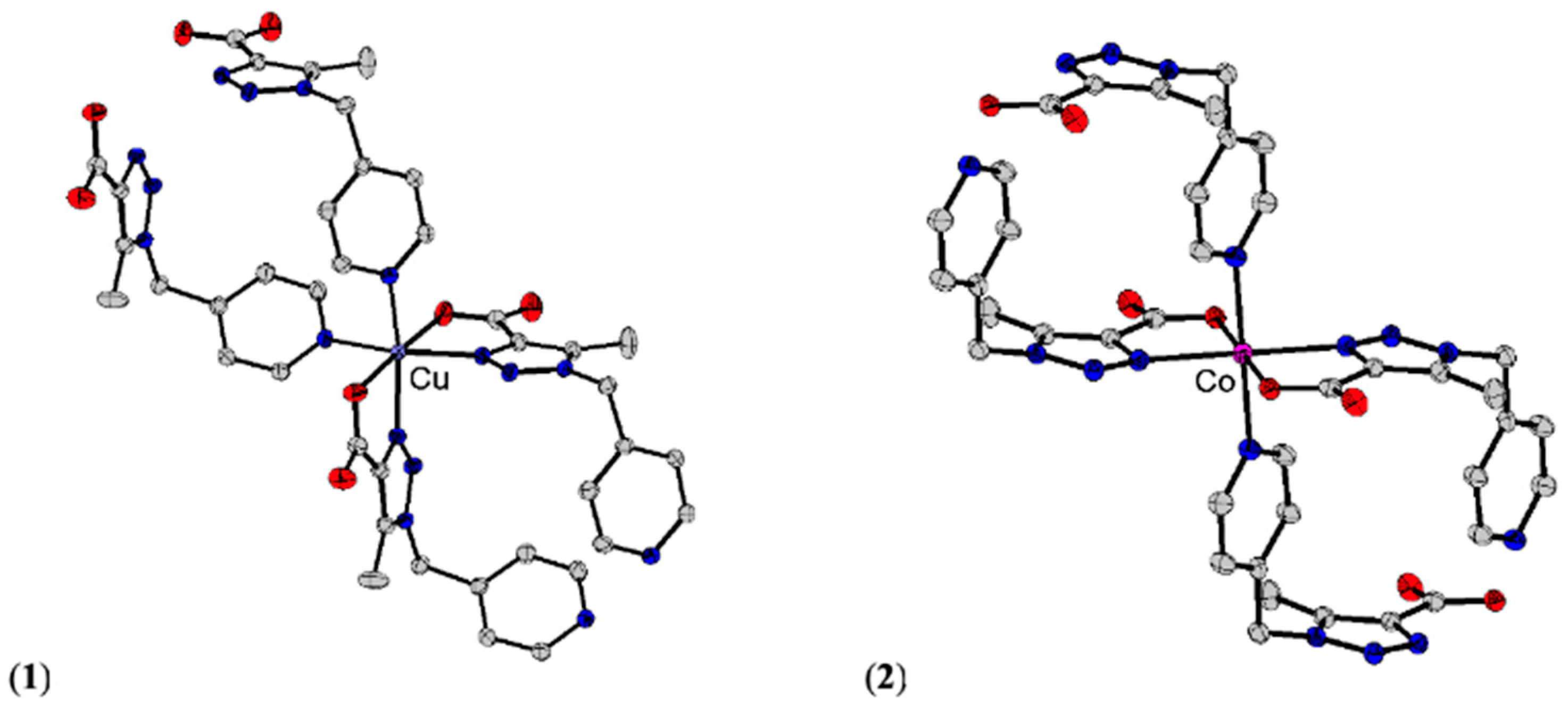
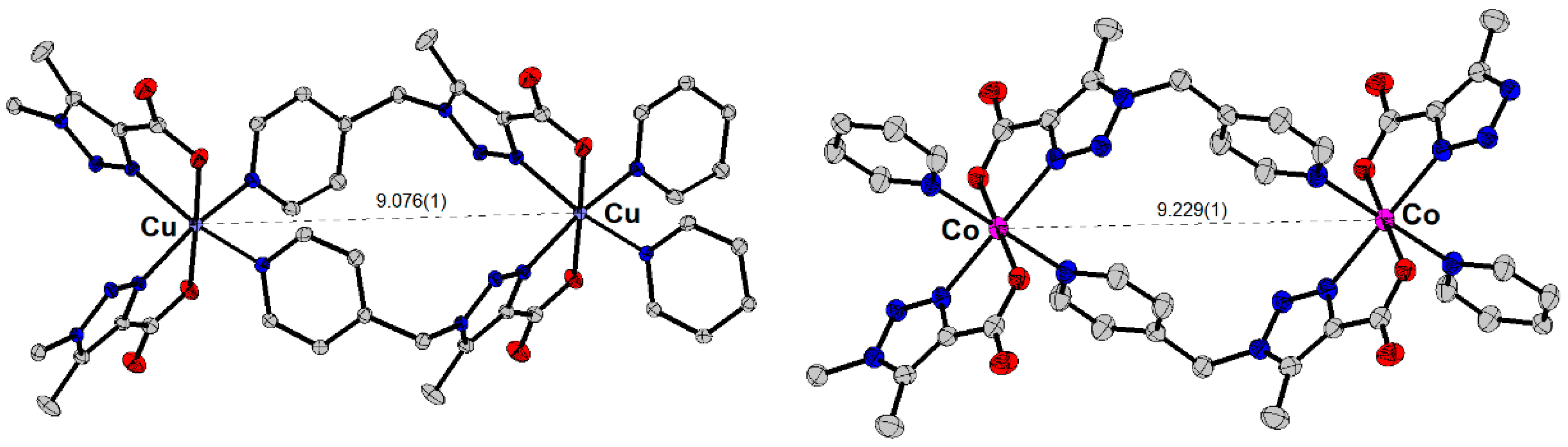
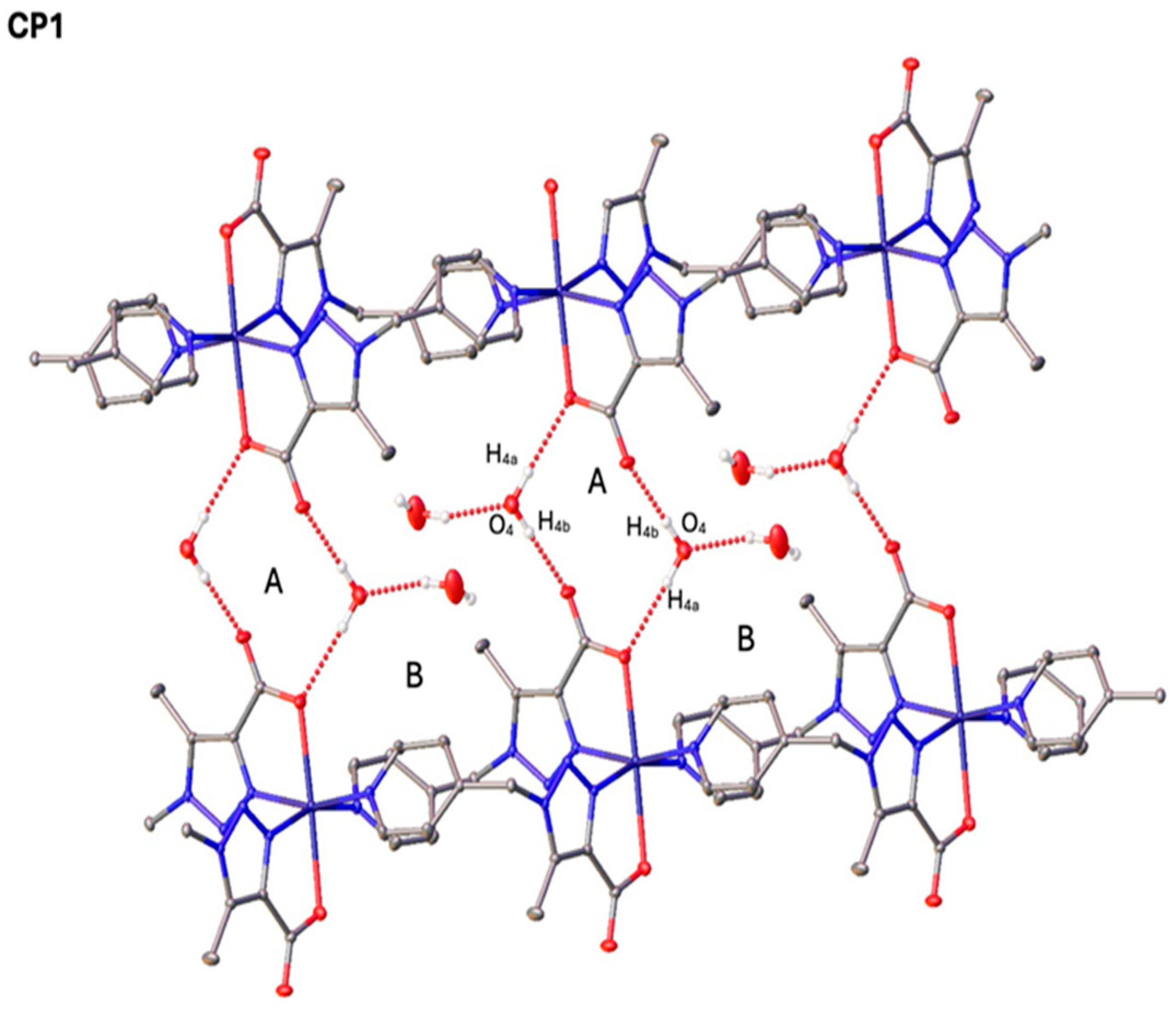
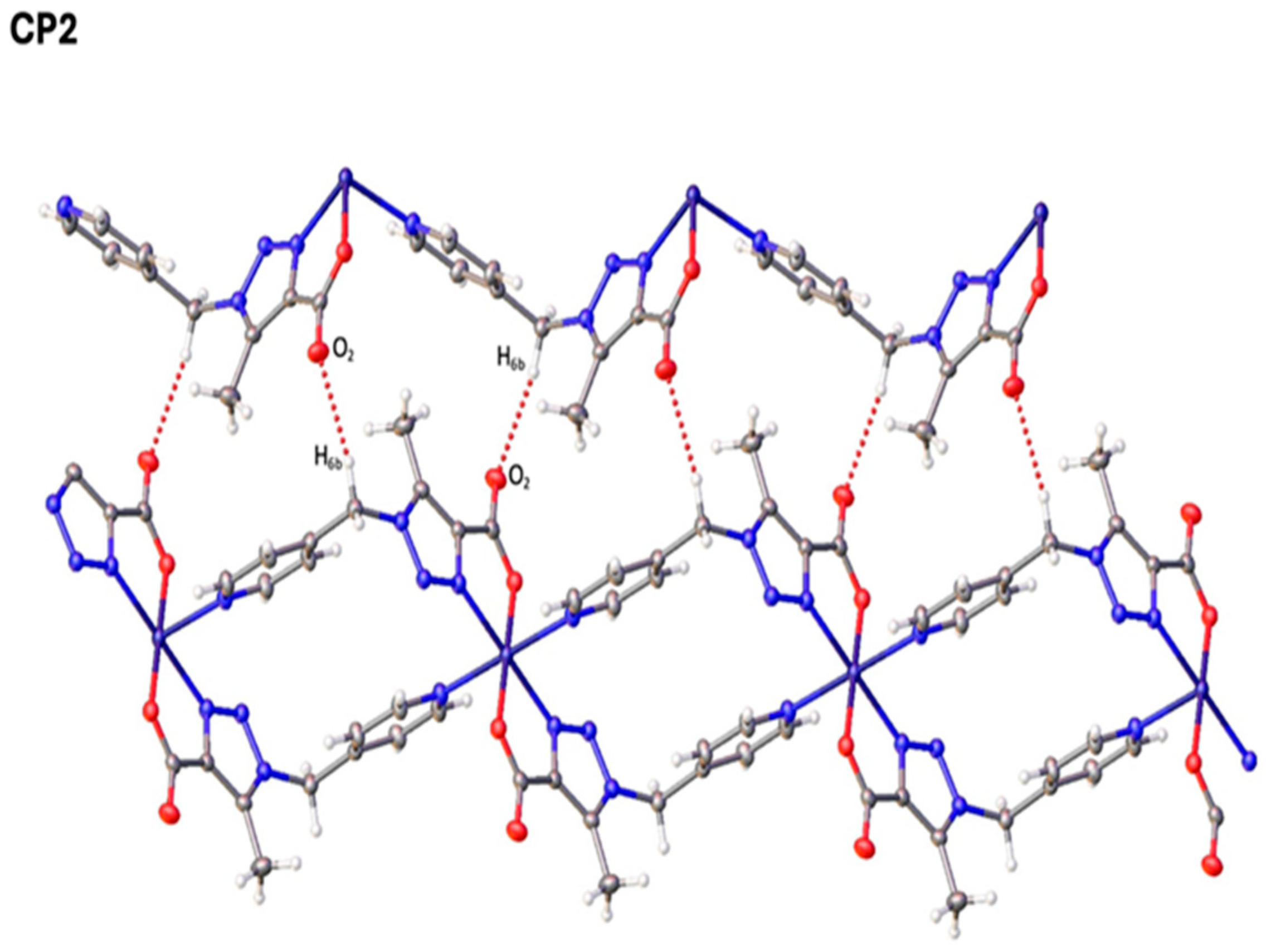
2.4. Single-Crystal X-Ray Diffraction
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Thermal Stability Studies (TGA)
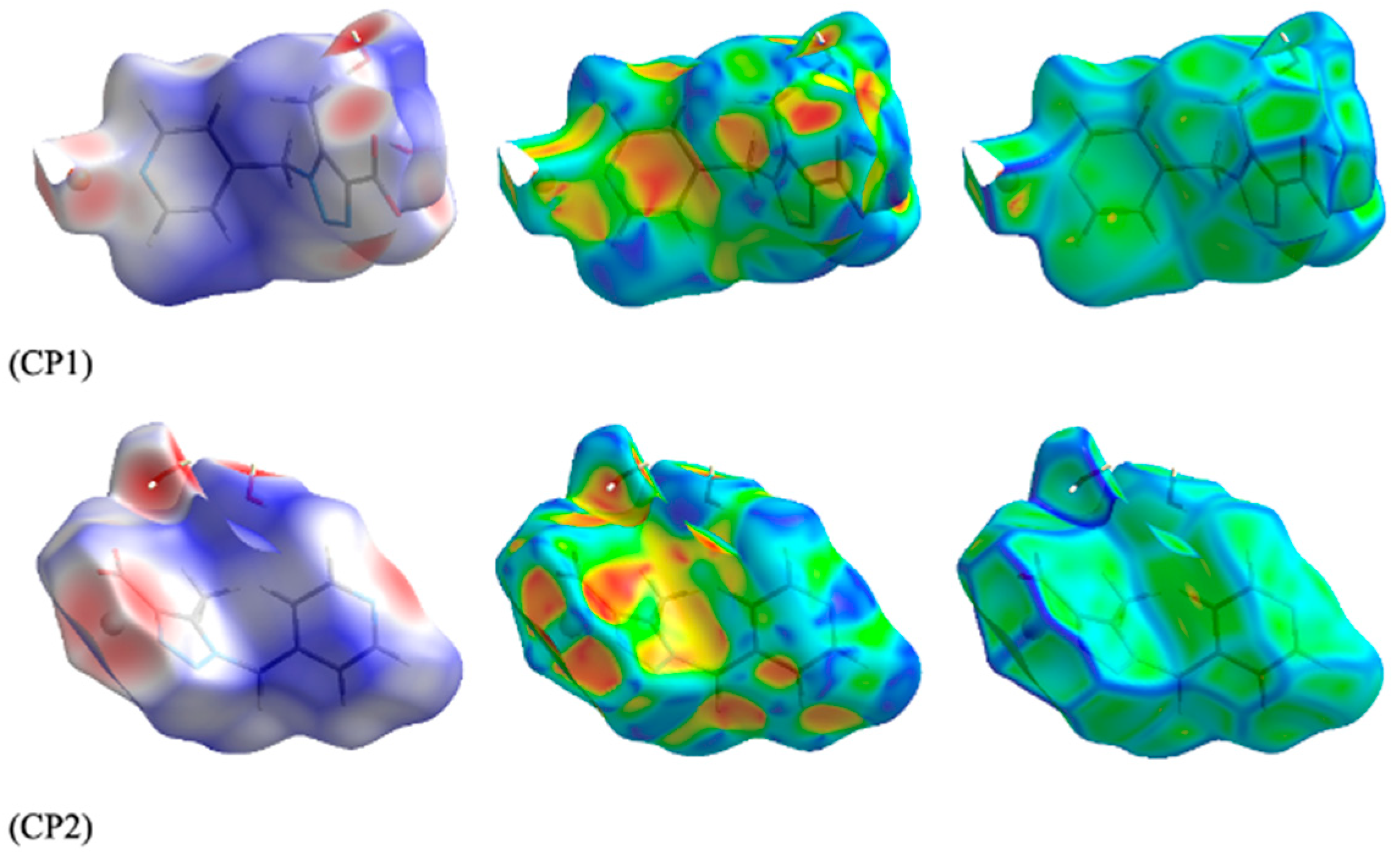
2.7. Hirshfeld Surface Analysis
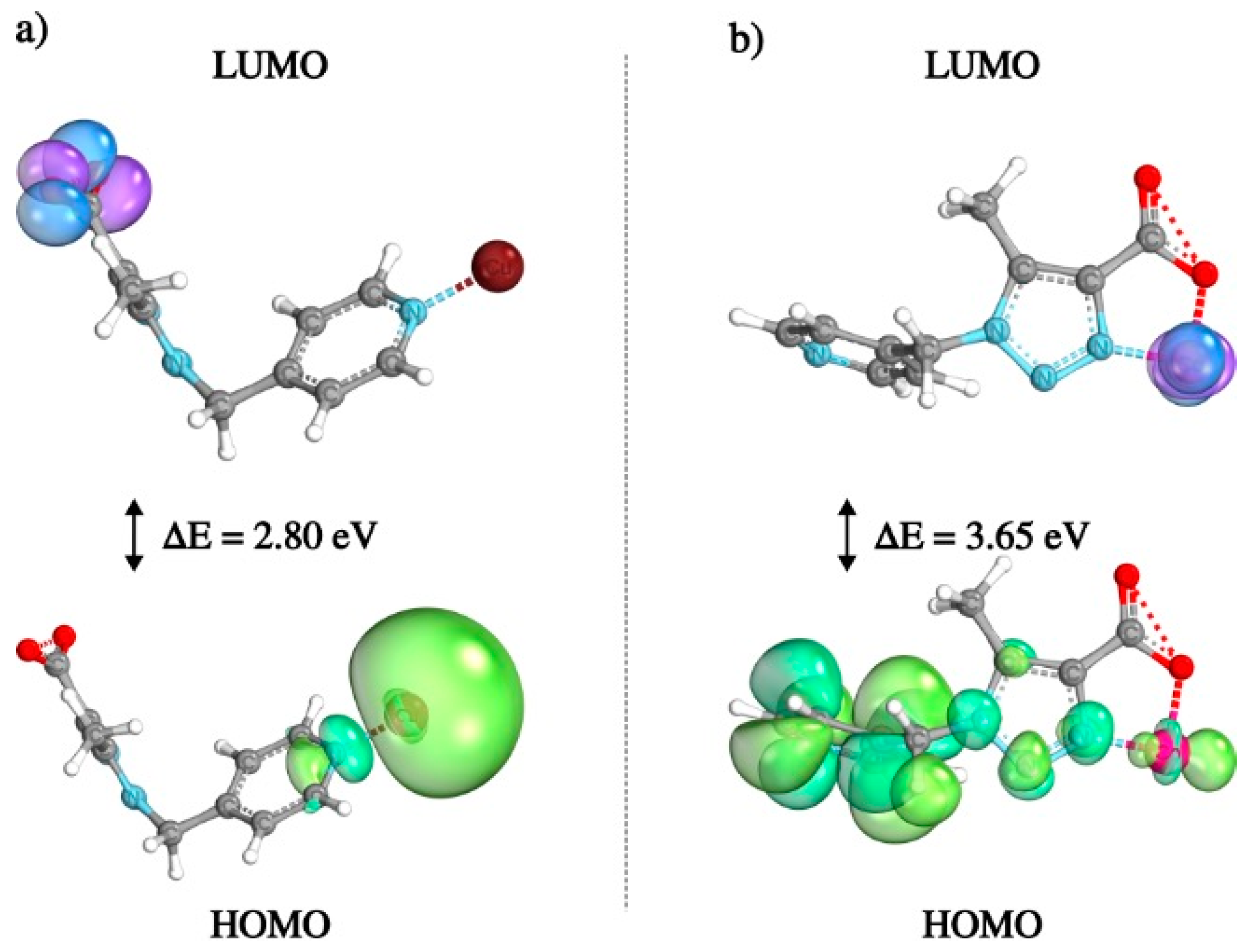
2.8. Density Functional Theory (DFT) Calculations
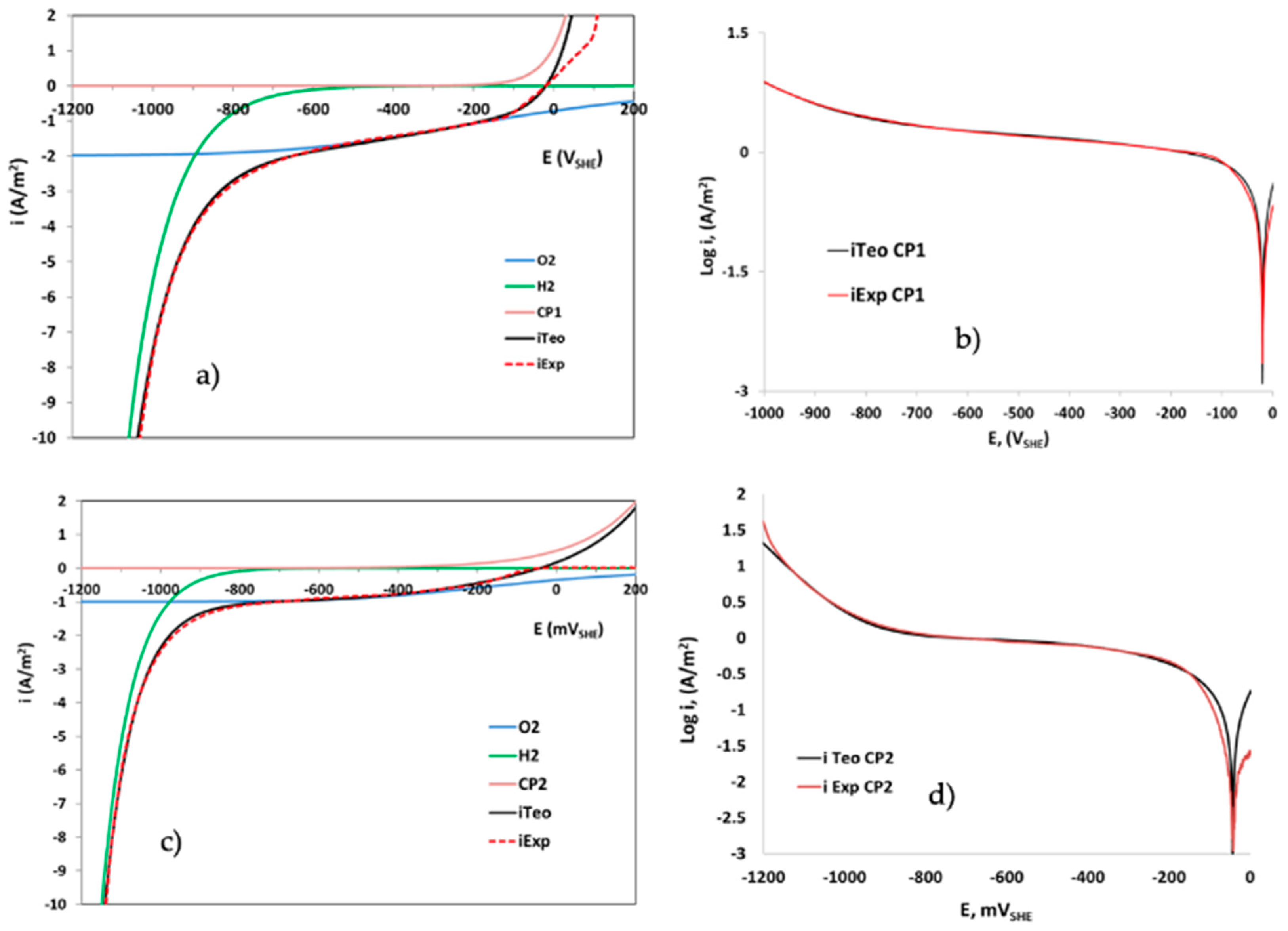
2.9. Electrochemical Analysis of Materials
3. Materials and Methods
3.1. Synthesis of NaL1
3.2. Syntheses of CP1 and CP2
3.3. Powder X-Ray Diffraction
3.4. Single-Crystal X-Ray Diffraction
3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.6. Thermal Stability Studies (TGA)
3.7. Hirshfeld Surface Calculations
3.8. Density Functional Theory (DFT) Calculations
3.9. Electrochemical Measurements of CP1 and CP2 Electrodes
3.10. Electrochemical and Kinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juríček, M.; Kouwer, P.H.J.; Rowan, A.E. Triazole: A Unique Building Block for the Construction of Functional Materials. Chem. Commun. 2011, 47, 8740–8749. [Google Scholar] [CrossRef] [PubMed]
- Rajeev Kharb, P.C.S.; Yar, M.S. Pharmacological Significance of Triazole Scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Varala, R.; Bollikolla, H.B.; Kurmarayuni, C.M. Synthesis of Pharmacological Relevant 1,2,3-Triazole and Its Analogues-A Review. Curr. Org. Synth. 2021, 18, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination Polymer Networks with O- and N-Donors: What They Are, Why and How They Are Made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Engel, E.R.; Scott, J.L. Advances in the Green Chemistry of Coordination Polymer Materials. Green Chem. 2020, 22, 3693–3715. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, Q. Surfactants as Promising Media for the Preparation of Crystalline Inorganic Materials. Angew. Chem. Int. Ed. 2015, 54, 11616–11623. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, F.-F.; Xiong, W.-W.; Zhang, Q. New Synthetic Strategies to Prepare Metal–Organic Frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Schuster, M.; Kilaru, S.; Steinberg, G. Azoles Activate Type I and Type II Programmed Cell Death Pathways in Crop Pathogenic Fungi. Nat. Commun. 2024, 15, 4357. [Google Scholar] [CrossRef] [PubMed]
- Gumus, A.; D’Agostino, I.; Puca, V.; Crocetta, V.; Carradori, S.; Cutarella, L.; Mori, M.; Carta, F.; Angeli, A.; Capasso, C.; et al. Cyclization of Acyl Thiosemicarbazides Led to New Helicobacter pylori Α-carbonic Anhydrase Inhibitors. Arch Pharm. 2024, 357, e2400548. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hakobyan, E.K.; Petrou, A.; Kartsev, V.G.; Yegoryan, H.A.; Paronikyan, E.G.; Zuppiroli, L.; Jughetsyan, H.V.; et al. New Triazole-Based Hybrids as Neurotropic Agents. RSC Adv. 2024, 14, 32922–32943. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, Y.; Xie, L.; Wang, S.; Ji, Y.; Sun, X.; Zhong, Z.; Peng, W.; Huang, H.; Ye, D. Development of Nitrogen-Rich Hierarchical Porous Carbon for High Selective Adsorption of Methanol in Multi-VOCs Waste Gas Recovery Processes. Sep. Purif. Technol. 2025, 353, 128478. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Z.-L.; Peng, S.; Luo, Z.-L.; Che, Y.-T.; You, L.-Q.; Li, R.-R.; Zhang, H.-X. Catalytic Oxygen Reduction by Pyridylalkylamine Copper Complexes Bearing Triazolylpyridines. Mol. Catal. 2024, 567, 114446. [Google Scholar] [CrossRef]
- Tahghighi, A.; Azerang, P. Click Chemistry beyond Metal-catalyzed Cycloaddition as a Remarkable Tool for Green Chemical Synthesis of Antifungal Medications. Chem. Biol. Drug Des. 2024, 103, e14555. [Google Scholar] [CrossRef] [PubMed]
- Belay, Y.; Muller, A.; Williams, D.B.G. Lanthanum-1,2,3-Triazole-Based 2D Coordination Polymer Is an Efficient Catalyst for the Oxidation of Olefins. Inorg. Chem. 2022, 61, 8226–8232. [Google Scholar] [CrossRef]
- Hernández, B.; Narea, P.; Espinoza, D.; Navarrete, A.; Aguirre, G.; Delgado, G.E.; Cárdenas, A.; Brito, I.; Cisterna, J. Novel Zn(II) and Cd(II) Coordination Polymers Derived from 1,2,3-Triazole-1,3-Diketone Ligand. Syntheses and Structural, Thermal, Computational, and Luminescent Studies. J. Solid State Chem. 2022, 312, 123156. [Google Scholar] [CrossRef]
- Cisterna, J.; Araneda, C.; Narea, P.; Cárdenas, A.; Llanos, J.; Brito, I. The Positional Isomeric Effect on the Structural Diversity of Cd(II) Coordination Polymers, Using Flexible Positional Isomeric Ligands Containing Pyridyl, Triazole, and Carboxylate Fragments. Molecules 2018, 23, 2634. [Google Scholar] [CrossRef] [PubMed]
- Narea, P.; Hernández, B.; Cisterna, J.; Cárdenas, A.; Llanos, J.; Amo-Ochoa, P.; Zamora, F.; Priego, J.L.; Cortijo, M.; Delgado, G.E.; et al. Heterobimetallic Three-Dimensional 4d-4f Coordination Polymers Based on 5-Methyl-1-(Pyridyn-4-Ylmethyl)-1H-1,2,3-Triazole-3,4-Dicarboxylate. J. Solid State Chem. 2022, 310, 123027. [Google Scholar] [CrossRef]
- Hernández, B.; Narea, P.; Espinoza, D.; Cárdenas, A.; Brito, I.; Delgado, G.E.; Cisterna, J. Synthesis, Crystal Structure, Hirshfeld Surface Analysis, Thermal, Luminescent Properties, and Computational Studies of the New Triazole Derivative 1-(5-Methyl-1-(Pyridin-2-Ylmethyl)-1H-1,2,3-Triazol-4-Yl)Ethan-1-One. J. Mol. Struct. 2023, 1274, 134353. [Google Scholar] [CrossRef]
- Narea, P.; Hernández, B.; Cisterna, J.; Cárdenas, A.; Amo-Ochoa, P.; Zamora, F.; Delgado, G.E.; Llanos, J.; Brito, I. The Methylene Spacer Matters: The Structural and Luminescent Effects of Positional Isomerism of n-Methylpyridyltriazole Carboxylate Semi-Rigid Ligands in the Structure of Zn(II) Based Coordination Polymers. Polymers 2023, 15, 888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Z.; Bai, S.-Q.; Hor, T.S.A. “Click-and-Click”—Hybridised 1,2,3-Triazoles Supported Cu(i) Coordination Polymers for Azide–Alkyne Cycloaddition. Dalton Trans. 2013, 42, 9437–9443. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 2000. [Google Scholar]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Yang, F.-F.; Yu, X.-Y.; Luo, Y.-H.; Wang, X.-F.; Sun, D.-H.; Zhang, H. Syntheses, Crystal Structures and Luminescent Properties of Six New D10 Transitional Metal Coordination Polymers Based on Bis(Benzimidazole) Ligands. Polyhedron 2015, 85, 337–346. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Commons, C.J.; Hudson, T.A.; Sanchez-Arlt, R. Supramolecular Hydrogen-Bonded Networks Formed from Copper(II) Carboxylate Dimers. Acta Crystallogr. C Struct. Chem. 2024, 80, 239–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.-L.; Zhang, Q.; Yang, S.-Y.; Wei, X.-Q.; Tian, Z.; Shao, D. Supramolecular Porous Frameworks of Two Ni(II) Coordination Polymers with Varying Structures, Porosities, and Magnetic Properties. Polyhedron 2022, 225, 116078. [Google Scholar] [CrossRef]
- Bellagamba, M.; Bencivenni, L.; Gontrani, L.; Guidoni, L.; Sadun, C. Tautomerism in Liquid 1,2,3-Triazole: A Combined Energy-Dispersive X-Ray Diffraction, Molecular Dynamics, and FTIR Study. Struct. Chem. 2013, 24, 933–943. [Google Scholar] [CrossRef]
- El Yacoubi, A.; Massit, A.; El Moutaoikel, S.; Rezzouk, A.; Chafik El Idrissi, B. Rietveld Refinement of the Crystal Structure of Hydroxyapatite Using X-Ray Powder Diffraction. Am. J. Mater. Sci. Eng. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Fullprof, version 7.95; Laboratoire Léon Brillouin (CEA-CNRS): Saclay, France, 2023.
- Bruker AXS Inc. APEX4 Package. In APEX4, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2020. [Google Scholar]
- Méndez-Hernández, D.D.; Tarakeshwar, P.; Gust, D.; Moore, T.A.; Moore, A.L.; Mujica, V. Simple and Accurate Correlation of Experimental Redox Potentials and DFT-Calculated HOMO/LUMO Energies of Polycyclic Aromatic Hydrocarbons. J. Mol. Model. 2013, 19, 2845–2848. [Google Scholar] [CrossRef] [PubMed]
- Boulkroune, M.; Ignatovich, L.; Muravenko, V.; Spura, J.; Chibani, A.; Jouikov, V. Correlation of the Homo–Lumo Gap in Furyl and Thienyl Nitrones and Nitroethenes with Their Electrochemical Redox Potentials*. Chem. Heterocycl. Compd. 2014, 49, 1579–1588. [Google Scholar] [CrossRef]
- Doronin, S.V. Description of Ions Properties Using Molecular Orbital Energy Levels: Trends in Interaction with Model Electrodes and Solvents. Electrochim. Acta 2025, 513, 145541. [Google Scholar] [CrossRef]
- Soliz, A.; Galleguillos-Madrid, F.M.; Cobos-Murcia, J.Á.; Angulo, S.; Salazar-Avalos, S.; Alonso-Fariñas, B.; Guzmán, A. Electrochemical Performance of Ti Gr. 2 as Electrodes in Contact with Saline Suspension of Clays during the Electroflotation Process. Appl. Sci. 2024, 14, 8825. [Google Scholar] [CrossRef]
- Madrid, F.M.G.; Arancibia-Bravo, M.; Cisterna, J.; Soliz, Á.; Salazar-Avalos, S.; Guevara, B.; Sepúlveda, F.; Cáceres, L. Corrosion of Titanium Electrode Used for Solar Saline Electroflotation. Materials 2023, 16, 3514. [Google Scholar] [CrossRef]
- Madrid, F.M.G.; Soliz, A.; Cáceres, L.; Salazar-Avalos, S.; Guzmán, D.; Gálvez, E. Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution. Materials 2023, 16, 6017. [Google Scholar] [CrossRef]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous Dried Extract of Skytanthus Acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions. Metals 2021, 11, 1992. [Google Scholar] [CrossRef]
- Cáceres, L.; Soliz, A.; Galleguillos-Madrid, F.M. Electrochemical Behavior of Carbon Steel ASTM A36 in Diluted Pregnant Leach Solutions from Electrowinning of Copper. Metals 2024, 14, 329. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer, version 17.5; University of Western Austr: Crawley, Australia, 2012.
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths Determined by X-Ray and Neutron-Diffraction. 1. Bond Lengths in Organic-Compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]

| CP1 | CP2 | |
|---|---|---|
| CCDC number | 2377095 | 2377094 |
| Empirical formula | C20H26CuN8O8 | C20H18CoN8O4 |
| Formula weight | 570.04 | 493.35 |
| Temperature/K | 293 | 293 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c (No 15) | P21/n (No 14) |
| a/Å | 19.5486 (16) | 10.9360 (14) |
| b/Å | 9.0762 (6) | 9.2289 (10) |
| c/Å | 14.4365 (11) | 13.3890 (17) |
| β/° | 102.627 (3) | 106.044 (4)) |
| Volume/Å3 | 2499.5 (3) | 1298.7 (3) |
| Z′ | 1 | 1 |
| Z | 4 | 2 |
| ρcalcg/cm3 | 1.515 | 1.262 |
| μ/mm−1 | 0.935 | 0.699 |
| F(000) | 1180 | 507 |
| Crystal size/mm3 Color | 0.11 × 0.21 × 0.30 Blue prism | 0.15 × 0.20 × 0.38 Light pink |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 2.50 to 30.5 | 2.72 to 27.1 |
| Index ranges | −27 ≤ h ≤ 27, −11 ≤ k ≤ 12, −20 ≤ l ≤ 20 | −14 ≤ h ≤ 13, −10 ≤ k ≤ 11, −17 ≤ l ≤ 17 |
| Reflections collected | 23,651 | 21,181 |
| Independent reflections | 3811 [Rint = 0.058] | 2864 [Rint = 0.066] |
| Data/restraints/parameters | 3811/0/175 | 2864/0/153 |
| Goodness-of-fit on F2 | 1.034 | 1.26 |
| Final R indexes [all data] | R1 = 0.0366, wR2 = 0.101 | R1 = 0.066, wR2 = 0.2863 |
| Largest diff. peak/hole/e Å−3 | 0.50/−0.50 | 0.80/−0.70 |
| CP1 | CP2 | ||
|---|---|---|---|
| Bond Distance (Å) | |||
| Cu–O2 2 | 2.404 (14) | Co1–O1 | 2.073 (4) |
| Cu–O2 1 | 2.404 (14) | Co1–O1 1 | 2.074 (4) |
| Cu–N3 1 | 2.028 (13) | Co1–N1 | 2.124 (4) |
| Cu–N3 2 | 2.028 (13) | Co–N1 1 | 2.124 (4) |
| Cu–N4 | 2.032 (14) | Co1–N4 2 | 2.174 (4) |
| Cu–N4 4 | 2.032 (14) | Co–N4 3 | 2.174 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergedahl, M.; Narea, P.; Llanos, J.; Pulido, R.; Naveas, N.; Amo-Ochoa, P.; Zamora, F.; Delgado, G.E.; Galleguillos Madrid, F.M.; León, Y.; et al. Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate. Int. J. Mol. Sci. 2025, 26, 1671. https://doi.org/10.3390/ijms26041671
Bergedahl M, Narea P, Llanos J, Pulido R, Naveas N, Amo-Ochoa P, Zamora F, Delgado GE, Galleguillos Madrid FM, León Y, et al. Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate. International Journal of Molecular Sciences. 2025; 26(4):1671. https://doi.org/10.3390/ijms26041671
Chicago/Turabian StyleBergedahl, Markus, Pilar Narea, Jaime Llanos, Ruth Pulido, Nelson Naveas, Pilar Amo-Ochoa, Félix Zamora, Gerzón E. Delgado, Felipe M. Galleguillos Madrid, Yasna León, and et al. 2025. "Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate" International Journal of Molecular Sciences 26, no. 4: 1671. https://doi.org/10.3390/ijms26041671
APA StyleBergedahl, M., Narea, P., Llanos, J., Pulido, R., Naveas, N., Amo-Ochoa, P., Zamora, F., Delgado, G. E., Galleguillos Madrid, F. M., León, Y., & Brito, I. (2025). Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate. International Journal of Molecular Sciences, 26(4), 1671. https://doi.org/10.3390/ijms26041671








