Role of Melatonin in Regulating Rat Skeletal Muscle Tissue Inflammation and Damage Following Carbon Tetrachloride Intoxication
Abstract
:1. Introduction
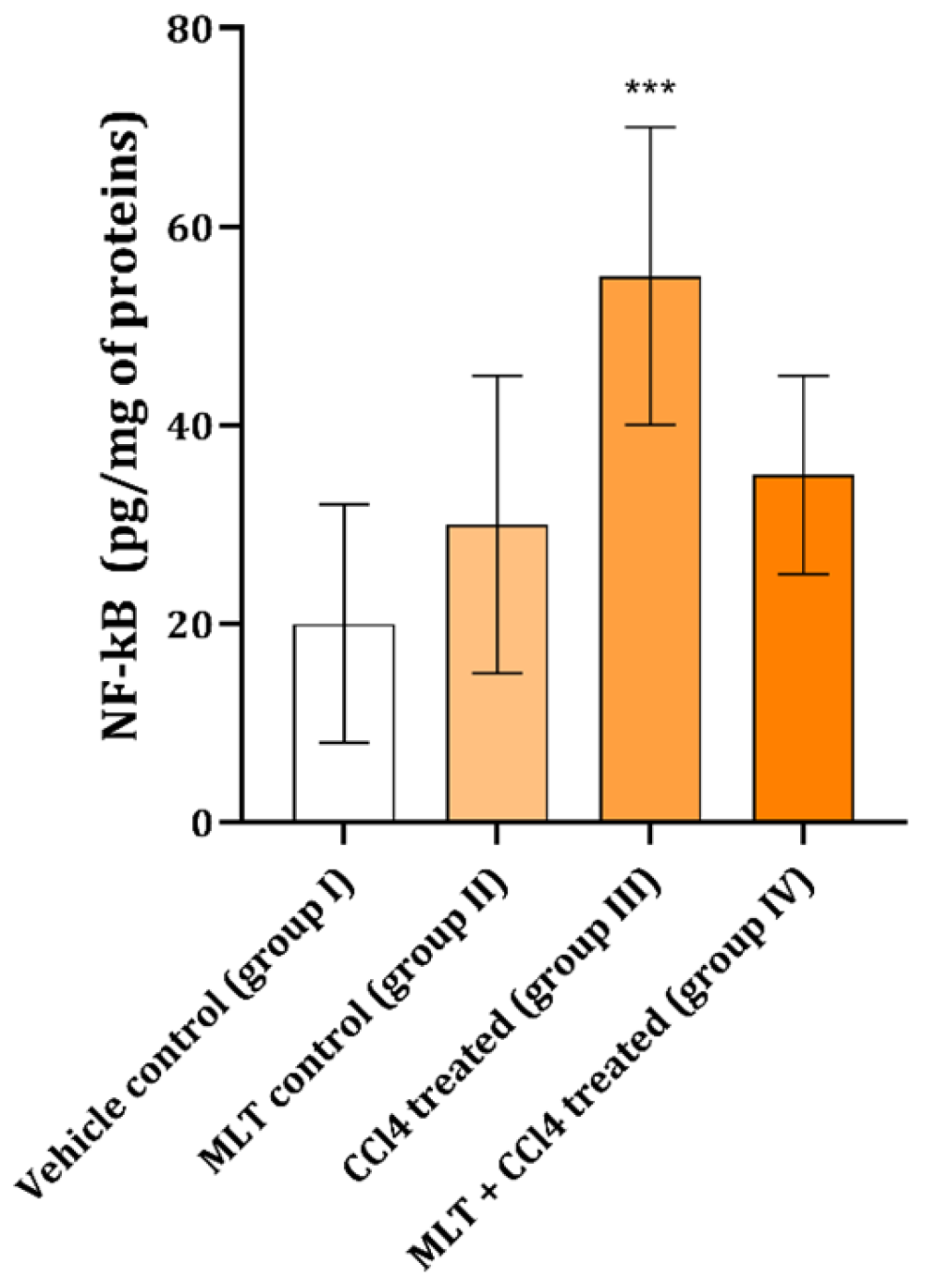
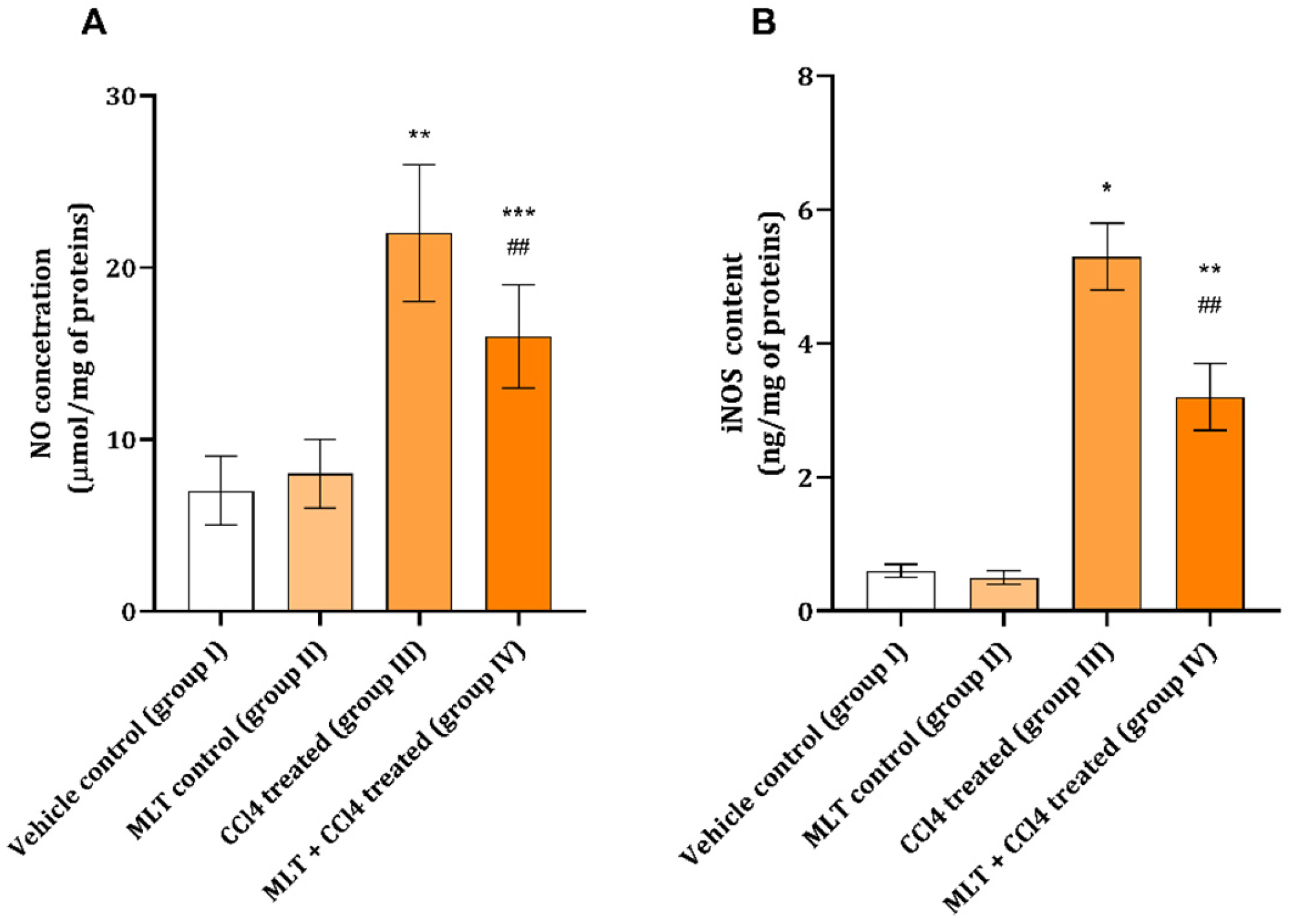
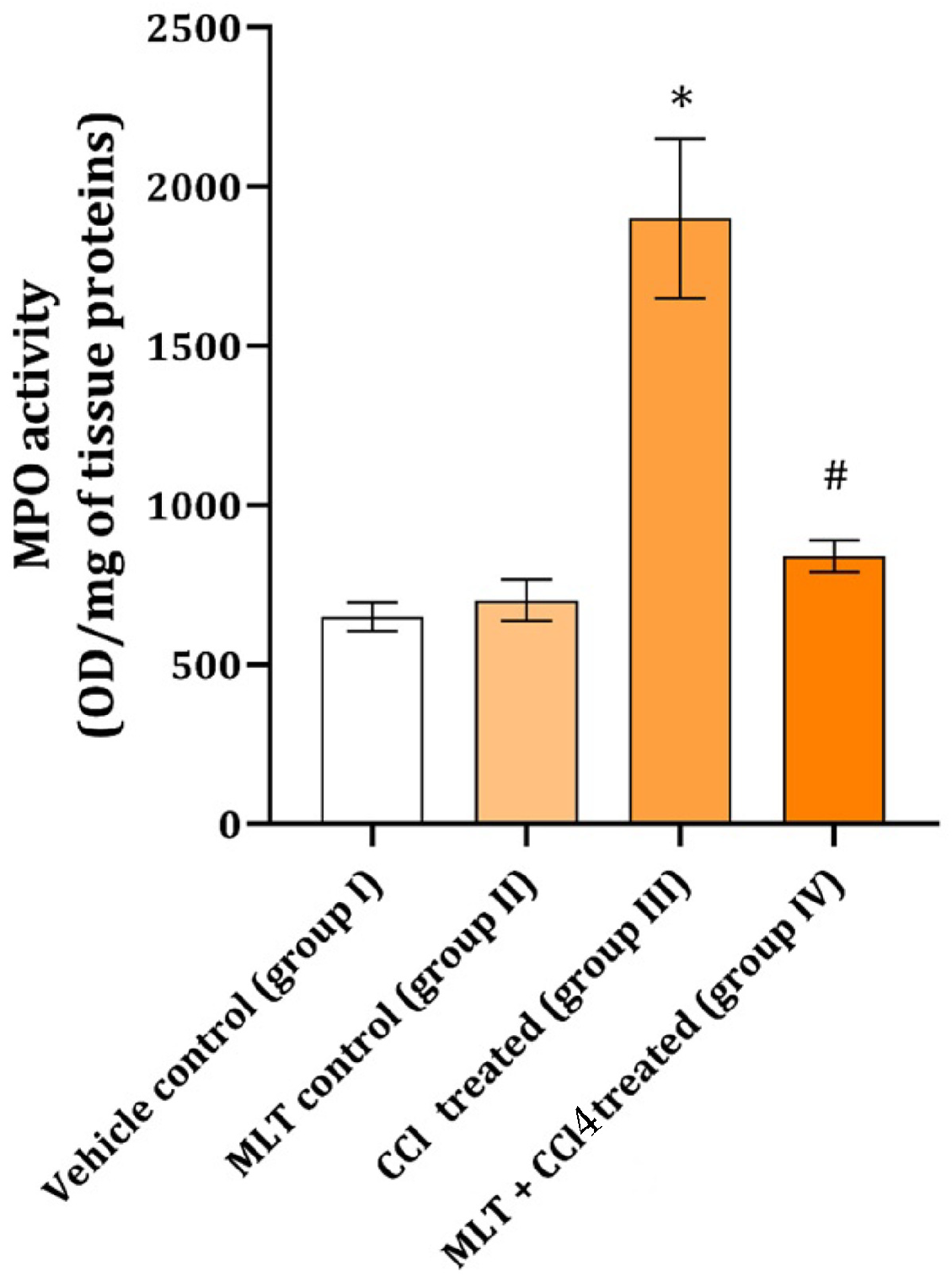
2. Results
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals and Housing
4.3. Experimental Design
4.4. Tissue Collection and Preparation for Biochemical Assays
4.5. Biochemical Parameters Determination
4.5.1. Myeloperoxidase Activity Determination
4.5.2. Nitric Oxide Determination
4.5.3. Inducible Nitric Oxide Synthase Determination
4.5.4. Tissue NF-Kappa-B-Activating Protein
4.5.5. Tissue Processing, Histochemical and Immunohistochemical Staining
4.5.6. Microscopic Tissue Analysis
4.5.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCl4 | Carbon tetrachloride |
| CD11b | Cluster of differentiation 11b |
| CD45 | Cluster of differentiation 45 |
| COX-2 | Cyclooxigenase-2 |
| H&E | Hematoxylin–eosin |
| HPF | High-power field |
| IL-1β | Interleukin 1 beta |
| iNOS | Inducible nitric oxide synthase |
| LCA | Leukocyte common antigen |
| MLT | Melatonin |
| MPO | Myeloperoxidase |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OD | Optical densities |
| PBS | Phosphate-buffered saline |
| TNF-α | Tumor necrosis factor alfa |
| TGF-β1 | Transforming growth factor beta 1 |
References
- Lilić, M.L.; Toskić, D.; Stefanović, R.Ž.; Mekić, B.B.; Ilić, I.R.; Stojanović, N.M. The role of mast cells in carbon tetrachloride induced rat skeletal muscle tissue damage. Acta Medica Median. 2019, 58, 11–15. [Google Scholar] [CrossRef]
- Sokolović, D.T.; Lilić, L.; Milenković, V.; Stefanovic, R.; Ilic Popovic, T.; Mekic, B.; Ilić, I.; Stojanović, N.M.; Ilić, I.R. Effects of melatonin on oxidative stress parameters and pathohistological changes in rat skeletal muscle tissue following carbon tetrachloride application. Saudi Pharm. J. 2018, 26, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, N.M.; Maslovarić, A.; Mihajlović, I.; Marković, A.; Randjelović, P.J.; Sokolović, D. Melatonin treatment prevents carbon-tetrachloride induced rat brain injury. Toxicol. Res. 2023, 12, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Ničković, V.P.; Novaković, T.; Lazarević, S.; Šulović, L.; Živković, Z.; Živković, J.; Mladenović, B.; Stojanović, N.M.; Petrović, V.; Sokolović, D.T. Pre- vs. post-treatment with melatonin in CCl4-induced liver damage: Oxidative stress inferred from biochemical and pathohistological studies. Life Sci. 2018, 202, 28–34. [Google Scholar] [CrossRef]
- Culver, A.; Hamang, M.; Wang, Y.; Jiang, H.; Yanum, J.; White, E.; Gawrieh, S.; Vuppalanchi, R.K.; Chalasani, N.P.; Dai, G.; et al. GDF8 Contributes to Liver Fibrogenesis and Concomitant Skeletal Muscle Wasting. Biomedicines 2023, 1, 1909. [Google Scholar] [CrossRef]
- Hwang, O.K.; Park, J.K.; Lee, E.J.; Lee, E.M.; Kim, A.Y.; Jeong, K.S. Therapeutic Effect of Losartan, an Angiotensin II Type 1 Receptor Antagonist, on CCl4-Induced Skeletal Muscle Injury. Int. J. Mol. Sci. 2016, 17, 227. [Google Scholar] [CrossRef]
- Kralova, J.; Pavliuchenko, N.; Fabisik, M.; Ilievova, K.; Spoutil, F.; Prochazka, J.; Pokorna, J.; Sedlacek, R.; Brdicka, T. The receptor-type protein tyrosine phosphatase CD45 promotes onset and severity of IL-1β-mediated autoinflammatory osteomyelitis. J. Biol. Chem. 2021, 297, 101131. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L. The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front. Cell Dev. Biol. 2022, 10, 952249. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Reiter, R.J.; Lupi, L.A. Melatonin as a promising agent to treat ovarian cancer: Molecular mechanisms. Carcingenesis 2017, 38, 945–952. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Liu, S.L.; Degli Esposti, S.; Yao, T.; Diehl, A.M.; Zern, M.A. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 1995, 22, 1474–1481. [Google Scholar] [PubMed]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Dyakova, E.Y.; Kapilevich, L.V.; Shylko, V.G.; Popov, S.V.; Anfinogenova, Y. Physical exercise associated with NO production: Signaling pathways and significance in health and disease. Front. Cell Dev. Biol. 2015, 3, 19. [Google Scholar] [CrossRef]
- Peters, V.B.M.; Matheis, F.; Erdmann, I.; Nemade, H.N.; Muders, D.; Toubartz, M.; Torun, M.; Mehrkens, D.; Geißen, S.; Nettersheim, F.S.; et al. Myeloperoxidase induces monocyte migration and activation after acute myocardial infarction. Front. Immunol. 2024, 15, 1360700. [Google Scholar] [CrossRef]
- Kumar, R.; Coggan, A.R.; Ferreira, L.F. Nitric oxide and skeletal muscle contractile function. Nitric Oxide 2022, 122–123, 54–61. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Lusis, A.J.; Tidball, J.G. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J. Physiol. 2005, 565, 403–413. [Google Scholar] [CrossRef]
- Hardeland, R. Cell polarization, migration and tissue repair: A promising field for future melatonin research. Melatonin Res. 2024, 7, 120–133. [Google Scholar] [CrossRef]
- Motohashi, N.; Uezumi, A.; Yada, E.; Fukada, S.; Fukushima, K.; Imaizumi, K.; Miyagoe-Suzuki, Y.; Takeda, S. Muscle CD31(-) CD45(-) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am. J. Pathol. 2008, 173, 781–791. [Google Scholar] [CrossRef]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [PubMed]
- Phonchai, R.; Phermthai, T.; Kitiyanant, N.; Suwanjang, W.; Kotchabhakdi, N.; Chetsawang, B. Potential effects and molecular mechanisms of melatonin on the dopaminergic neuronal differentiation of human amniotic fluid mesenchymal stem cells. Neurochem. Int. 2019, 24, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Schulz, K.; Kerber, S.; Kelm, M. Reevaluation of the Griess method for determining NO/NO2- in aqueous and protein-containing samples. Nitric Oxide 1999, 3, 225–234. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Mitić, K.V.; Stojnev, S.; Sokolović, D.; Vukelić-Nikolić, M.; Tričković-Vukić, D.; Randjelović, P.J.; Krstić, M.; Radulović, N.S. Functioning of whole bone marrow cell population in a model of L-arginine-induced pancreatitis. Eur. J. Inflamm. 2023, 21, 1721727X231177816. [Google Scholar] [CrossRef]
- Rizo-Roca, D.; Ríos-Kristjánsson, J.G.; Núñez-Espinosa, C.; Ascensão, A.A.; Magalhães, J.; Torrella, J.R.; Pagés, T.; Viscor, G. A semiquantitative scoring tool to evaluate eccentric exercise-induced muscle damage in trained rats. Eur. J. Histochem. 2015, 59, 310–316. [Google Scholar] [CrossRef]

| Traced Parameter | Vehicle Control (Group I) | MLT Control (Group II) | CCl4 Treated (Group III) | MLT + CCl4 Treated (Group IV) |
|---|---|---|---|---|
| Score of inflammatory cell infiltration | 0.1 | 0.1 | 1.55 | 0.6 |
| CD45 cell infiltration (number per HPF) | 0 | 0 | 7 ± 1 | 3 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antić, V.M.; Antic, M.; Stojiljkovic, N.; Stanković, N.; Pavlović, M.; Sokolović, D. Role of Melatonin in Regulating Rat Skeletal Muscle Tissue Inflammation and Damage Following Carbon Tetrachloride Intoxication. Int. J. Mol. Sci. 2025, 26, 1718. https://doi.org/10.3390/ijms26041718
Antić VM, Antic M, Stojiljkovic N, Stanković N, Pavlović M, Sokolović D. Role of Melatonin in Regulating Rat Skeletal Muscle Tissue Inflammation and Damage Following Carbon Tetrachloride Intoxication. International Journal of Molecular Sciences. 2025; 26(4):1718. https://doi.org/10.3390/ijms26041718
Chicago/Turabian StyleAntić, Vladimir Milan, Milorad Antic, Nenad Stojiljkovic, Nemanja Stanković, Miljana Pavlović, and Dušan Sokolović. 2025. "Role of Melatonin in Regulating Rat Skeletal Muscle Tissue Inflammation and Damage Following Carbon Tetrachloride Intoxication" International Journal of Molecular Sciences 26, no. 4: 1718. https://doi.org/10.3390/ijms26041718
APA StyleAntić, V. M., Antic, M., Stojiljkovic, N., Stanković, N., Pavlović, M., & Sokolović, D. (2025). Role of Melatonin in Regulating Rat Skeletal Muscle Tissue Inflammation and Damage Following Carbon Tetrachloride Intoxication. International Journal of Molecular Sciences, 26(4), 1718. https://doi.org/10.3390/ijms26041718










