Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment
Abstract
1. Introduction
2. Results
2.1. Material Characterization
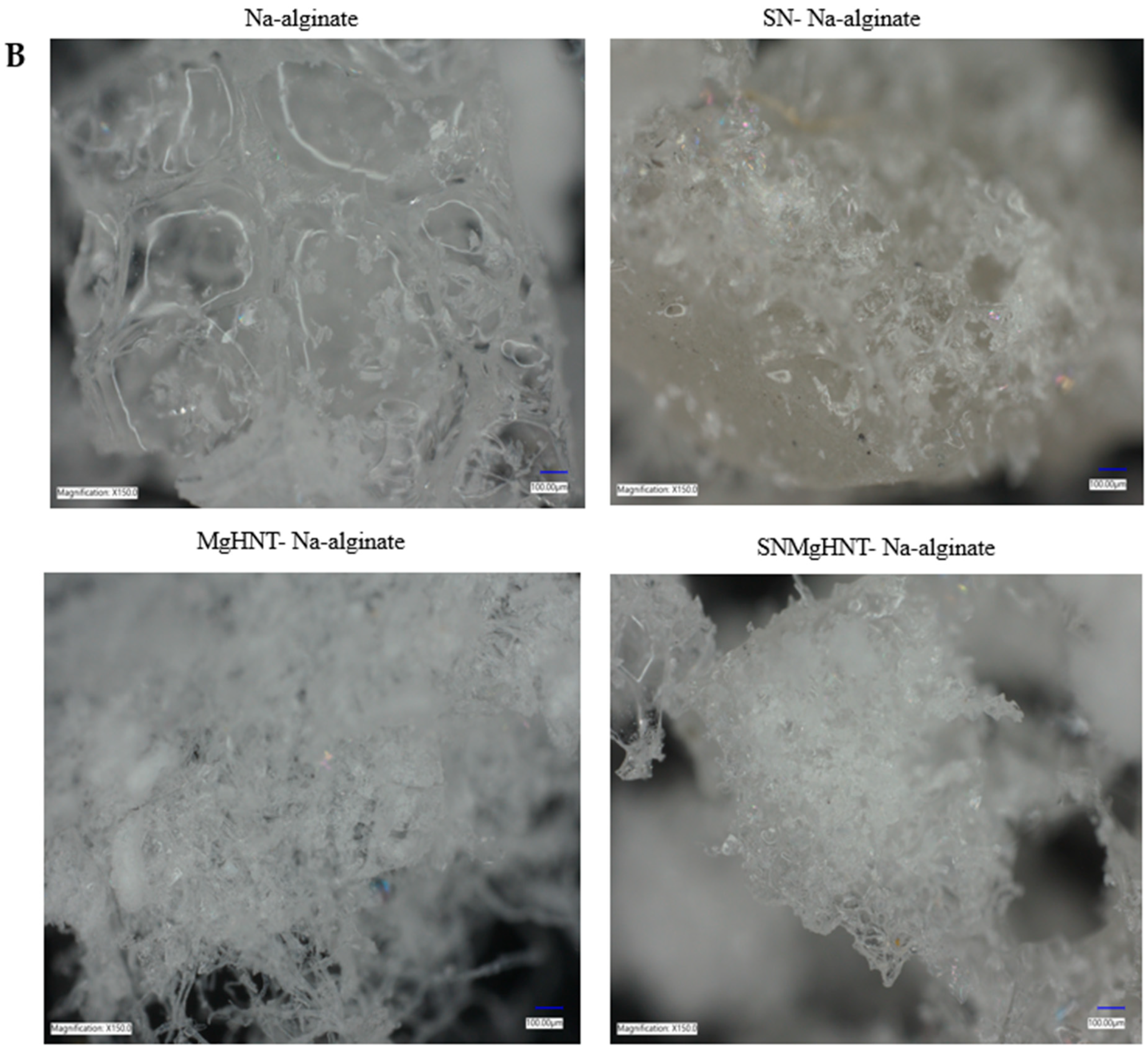
2.1.1. MgHNTs and Nanocomposite Hydrogel Patch Surface Analysis
2.1.2. Surface Area and Pore Size Analysis
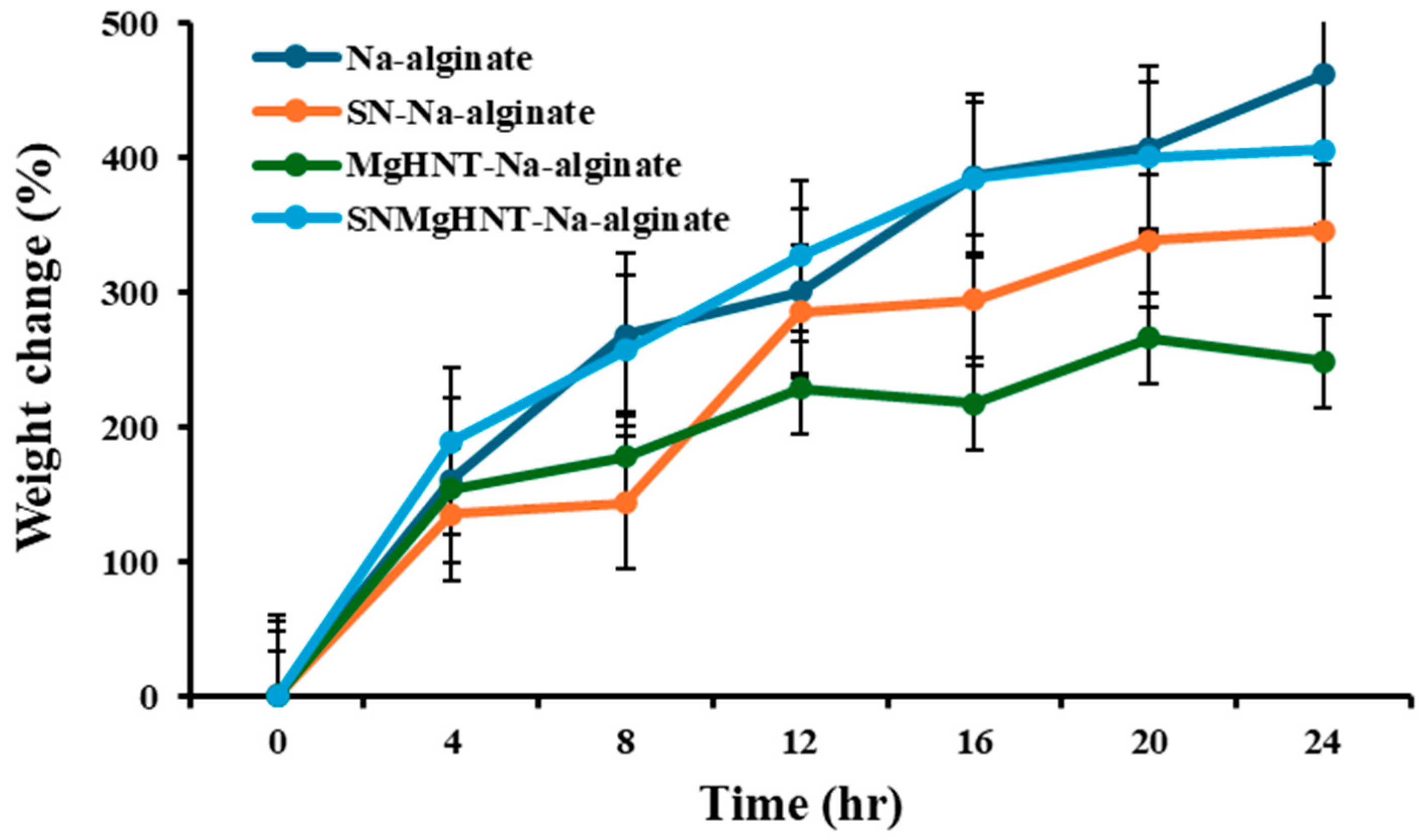
2.1.3. Swelling Behavior Analysis
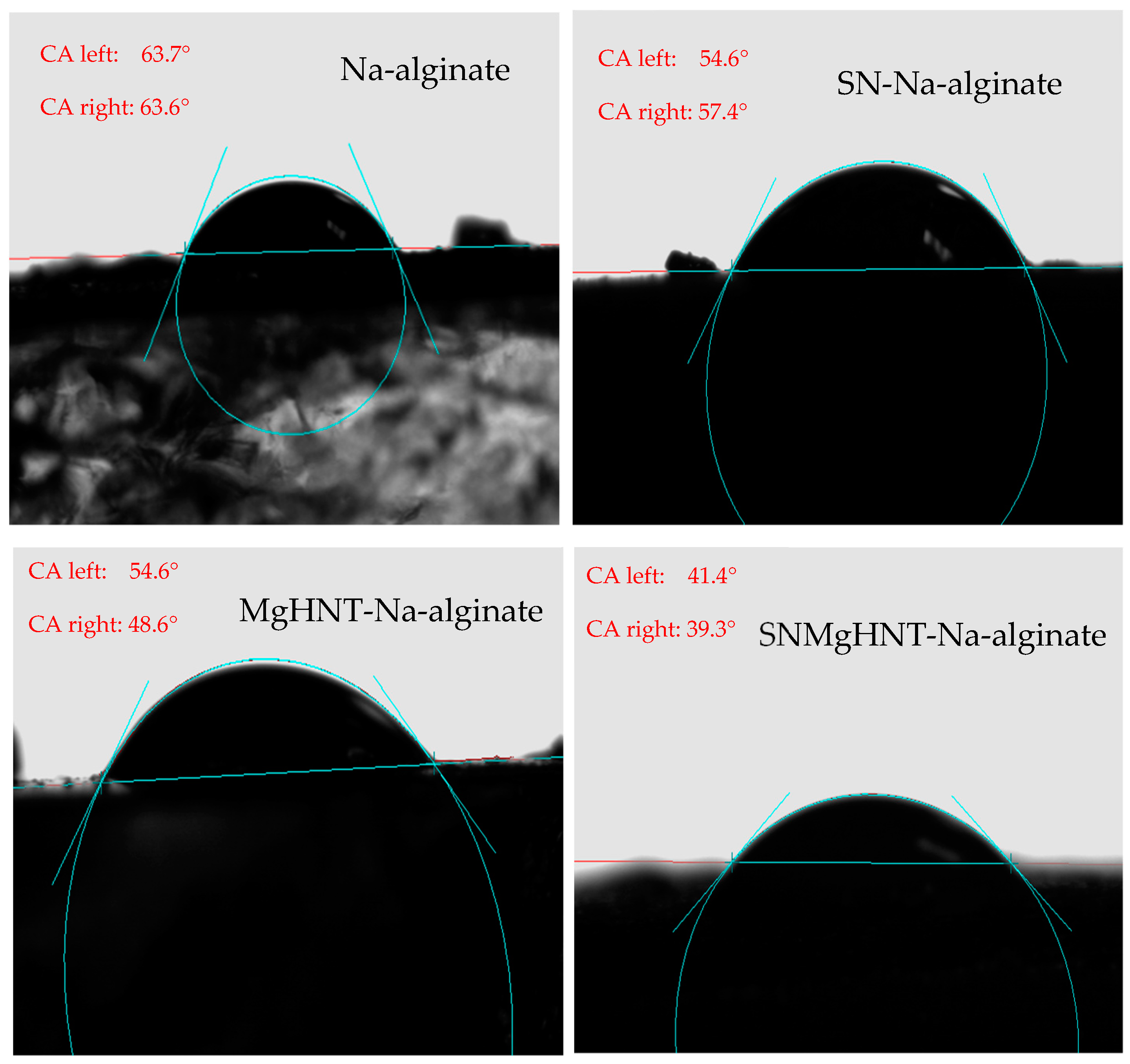
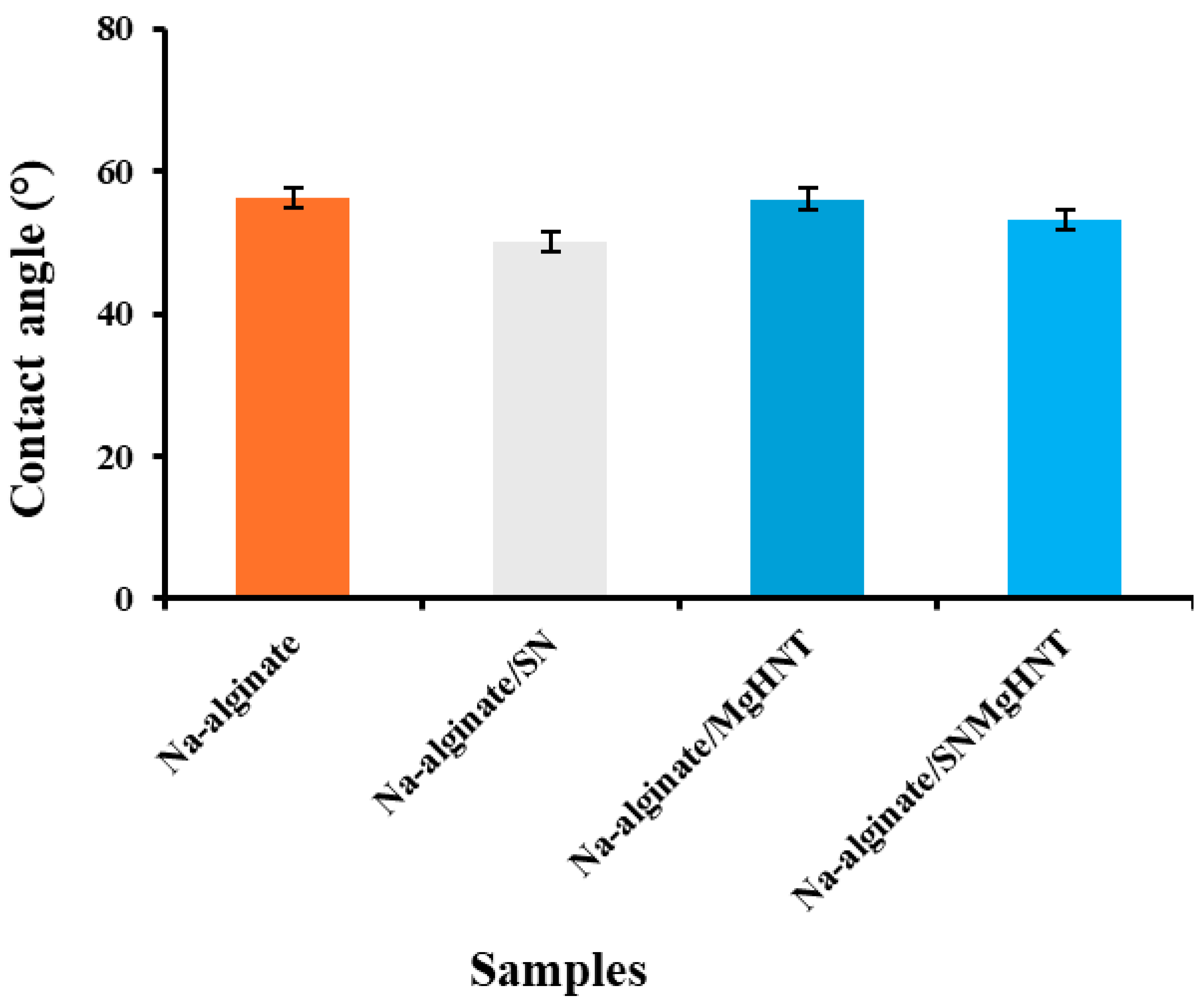
2.1.4. Wettability Analysis
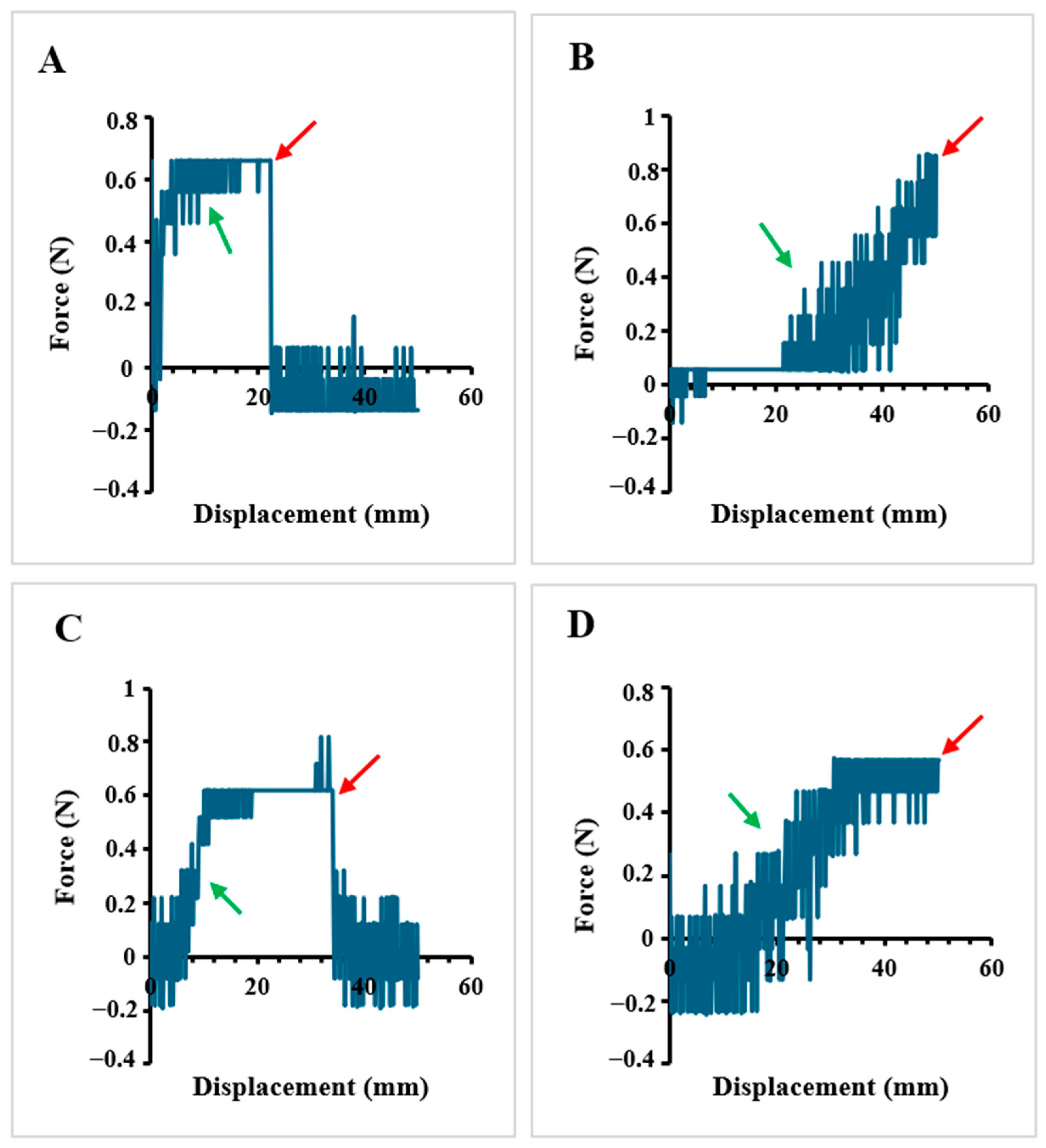
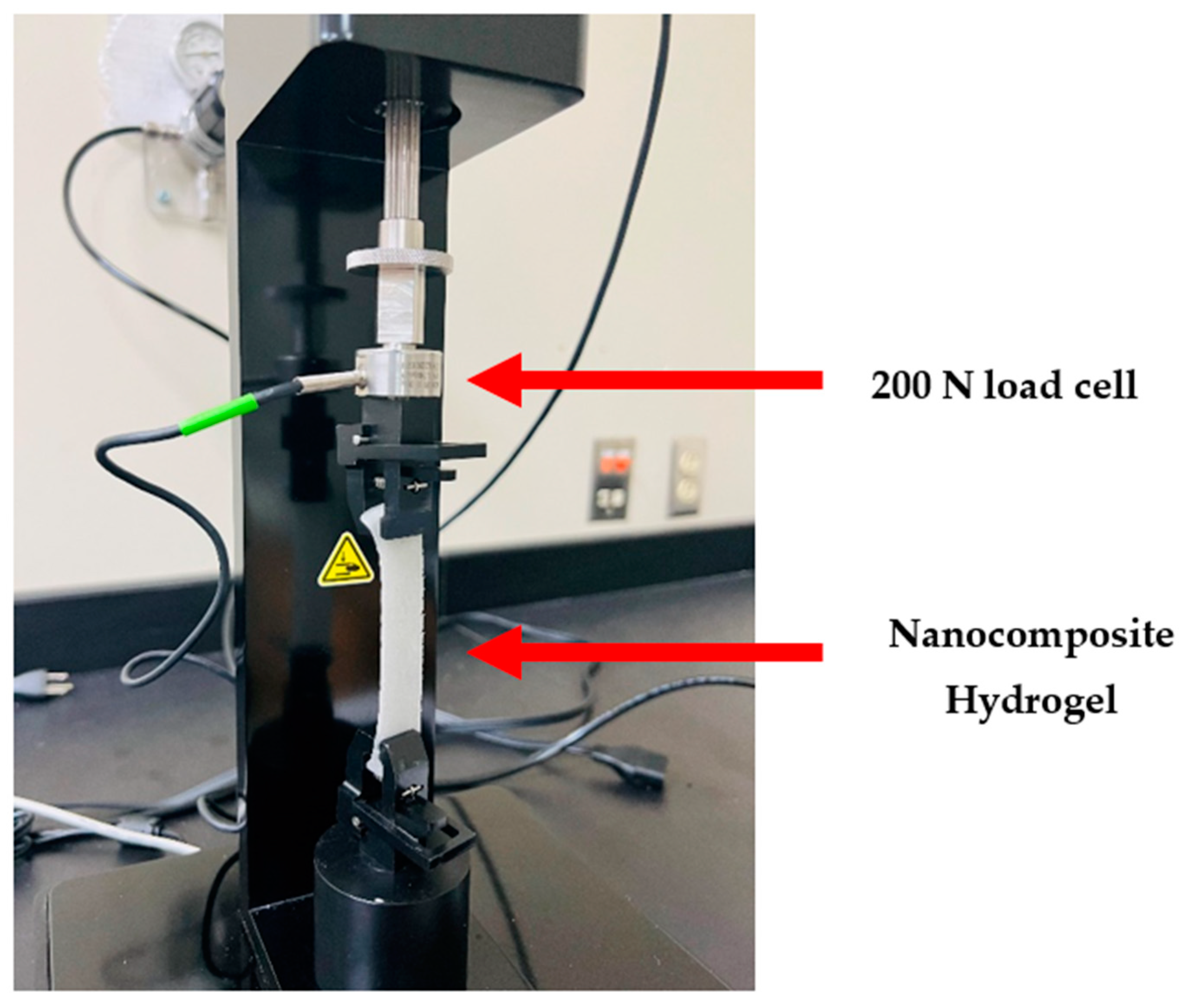
2.1.5. Tensile Properties
2.2. Hydrogel Cellular Response
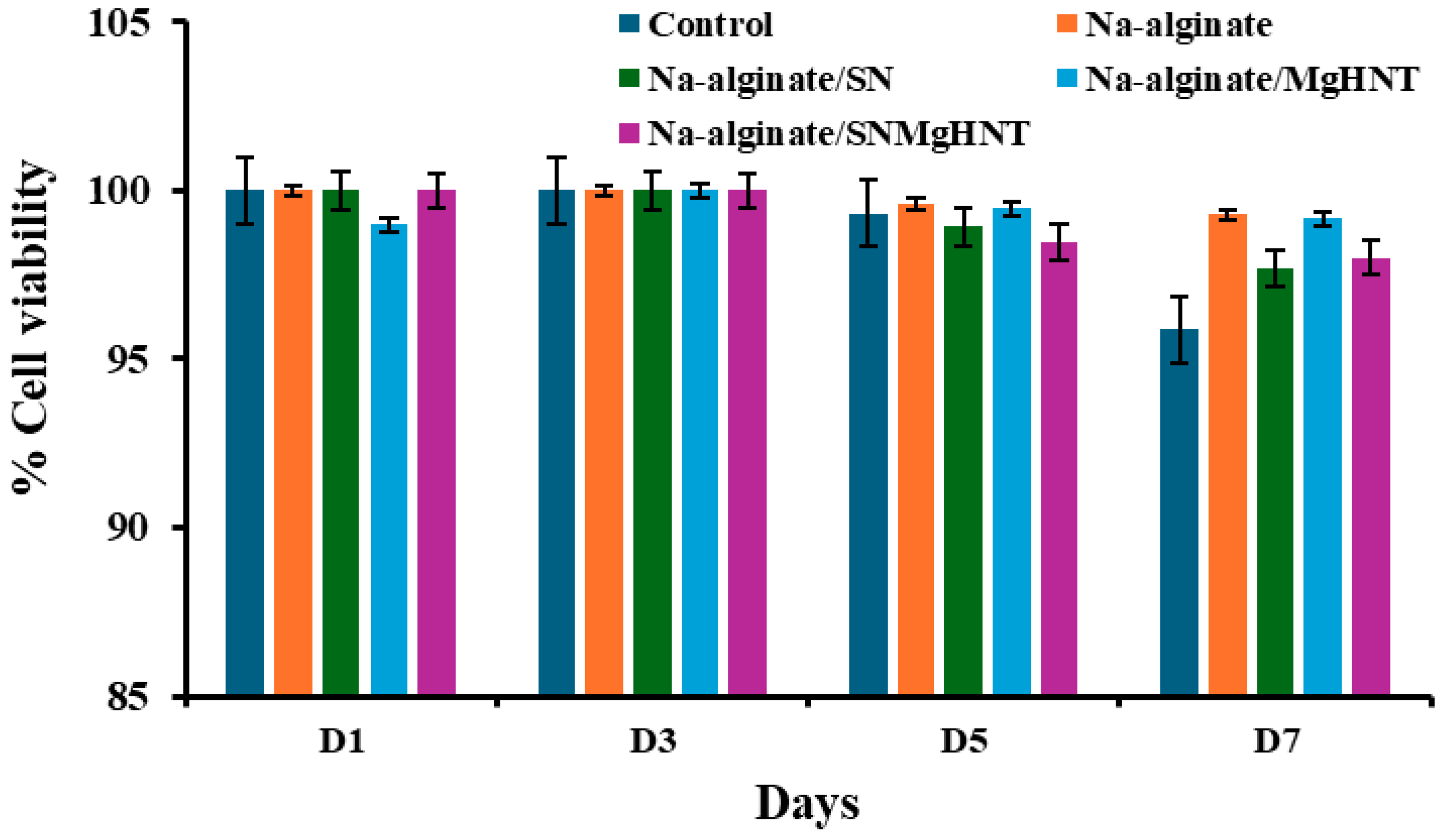
2.2.1. Cell Viability Analysis
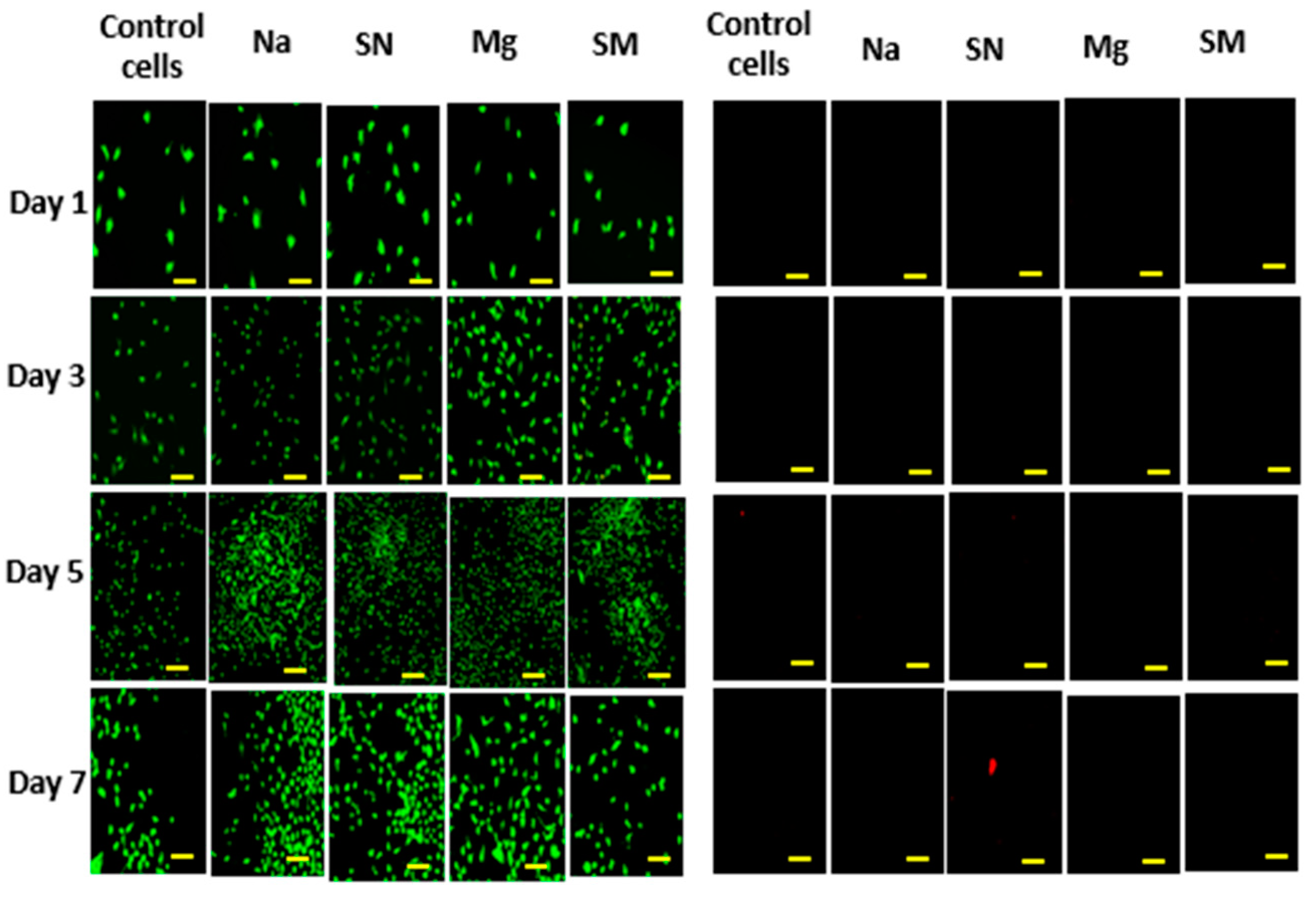
2.2.2. Cell Proliferation Assay
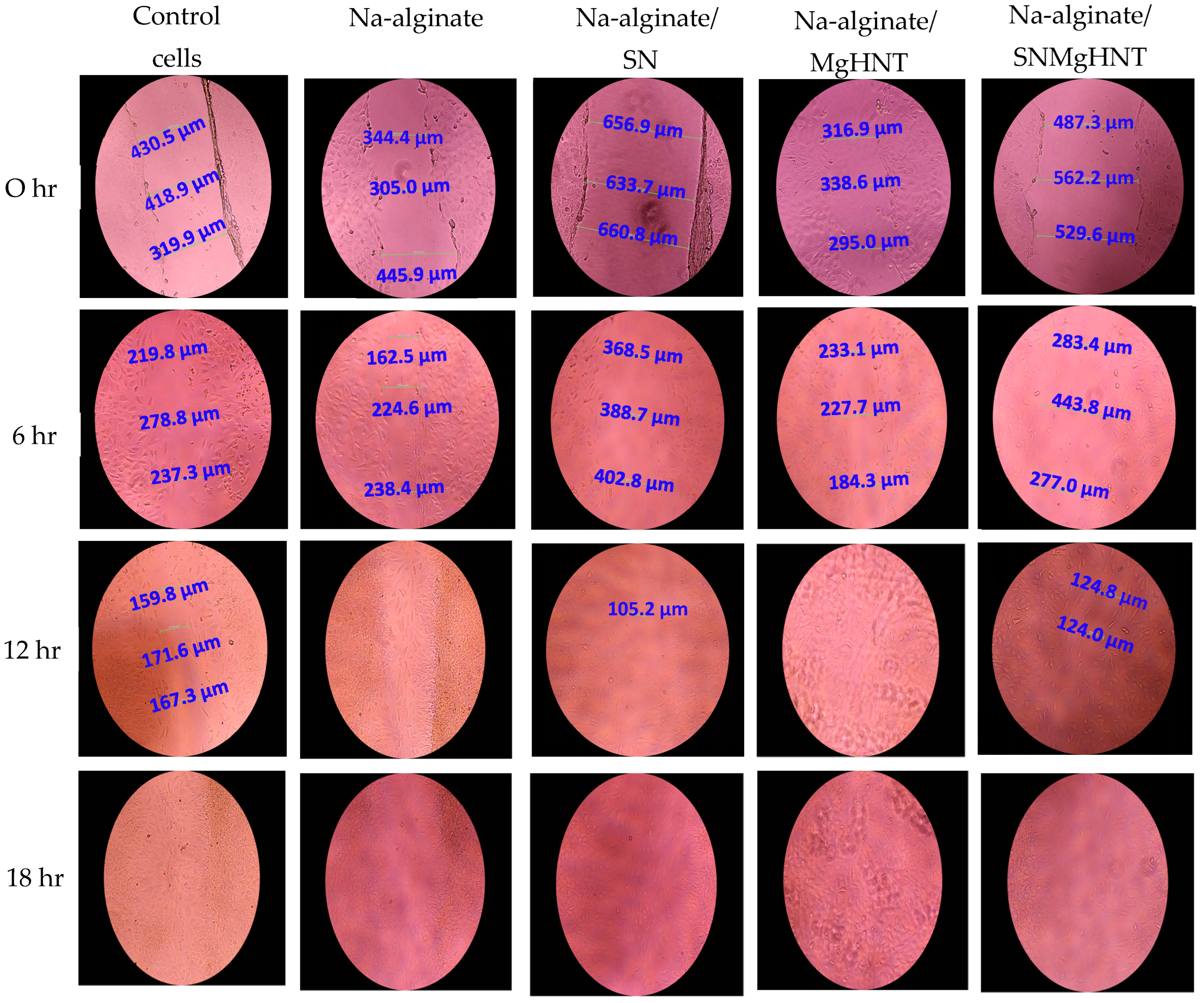
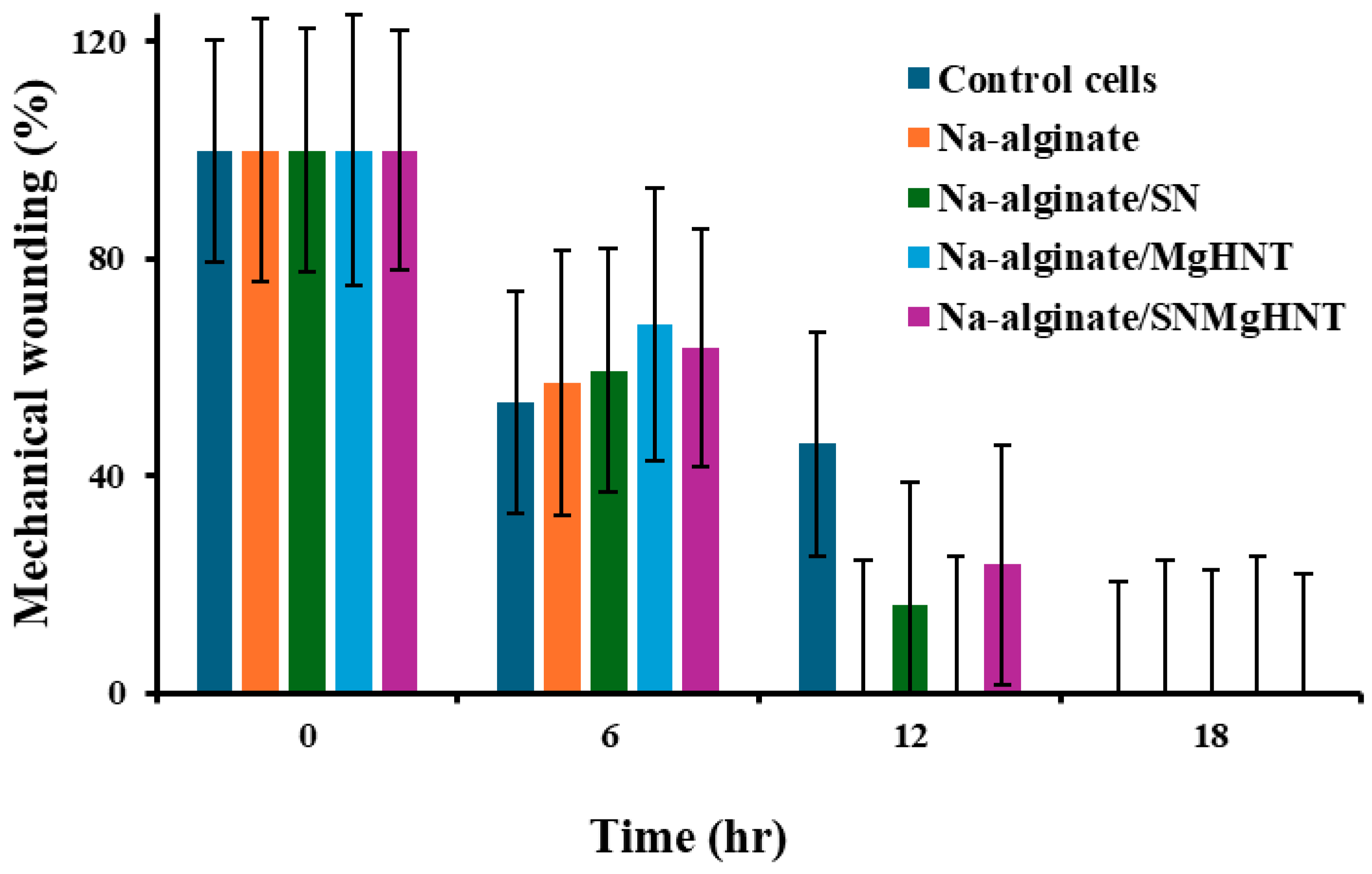
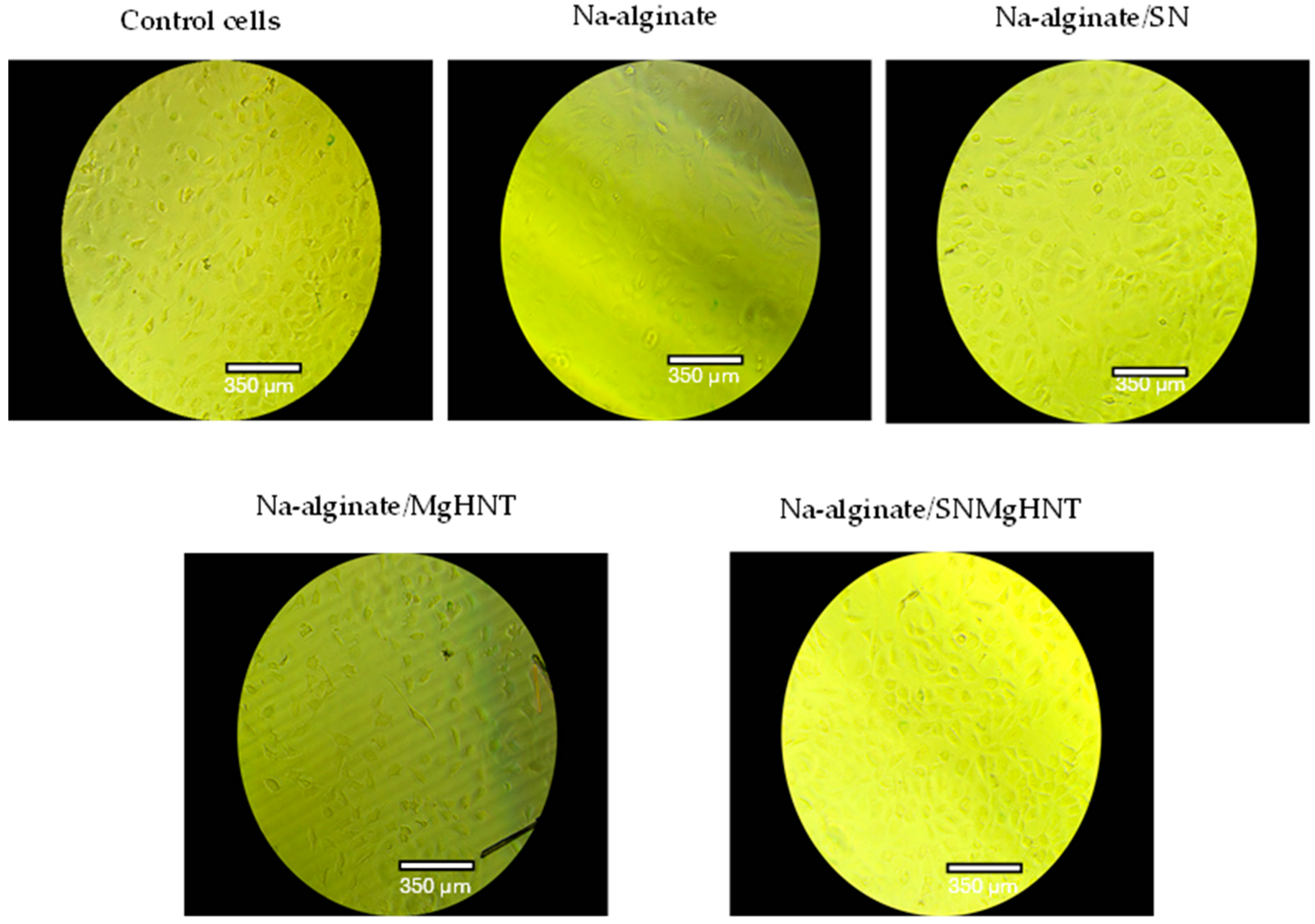
2.2.3. Wound Healing Analysis
2.2.4. MEF Migration Analysis
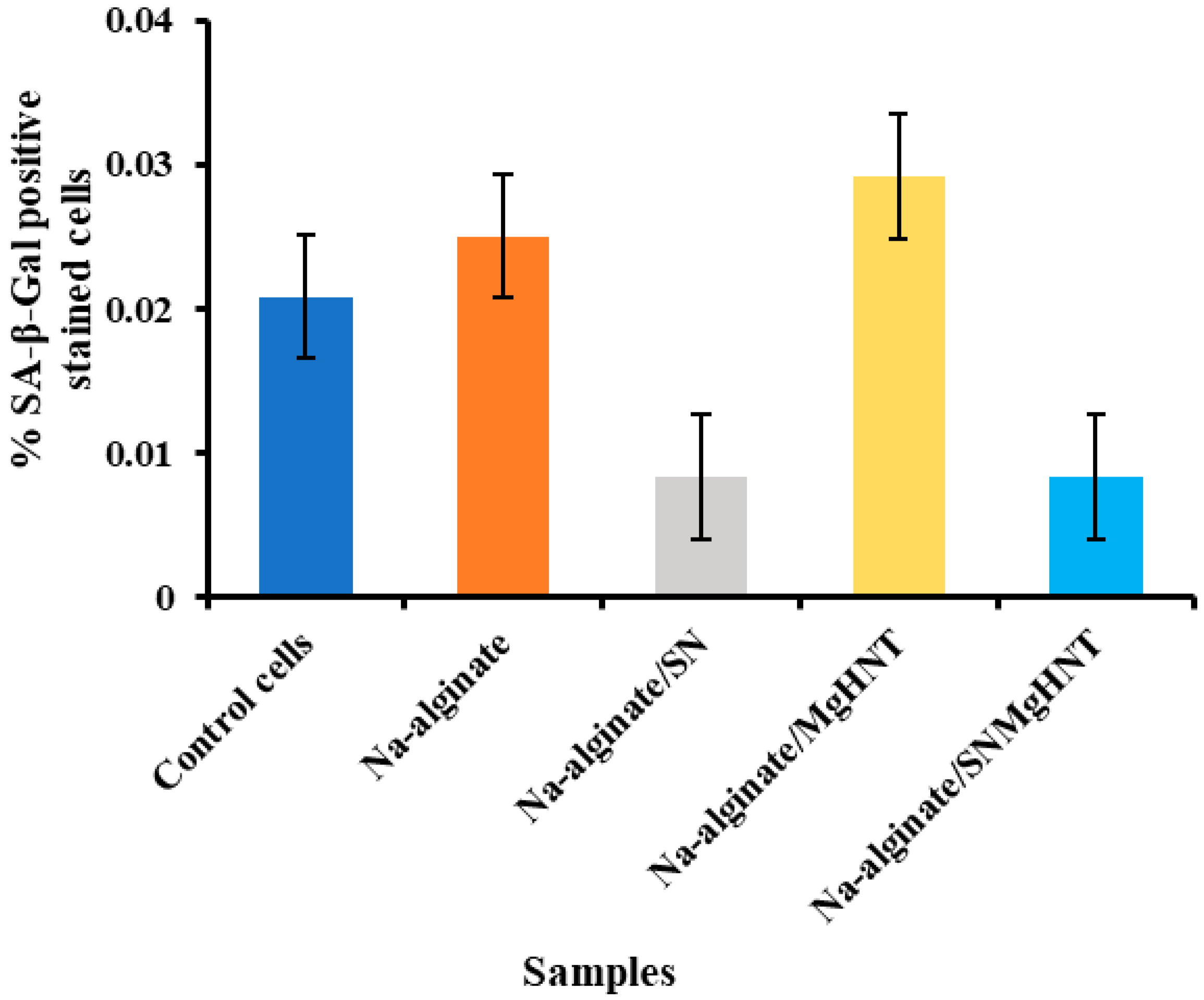
2.2.5. Senescence Analysis
2.2.6. Sustain Release of Gentamicin Sulfate Analysis
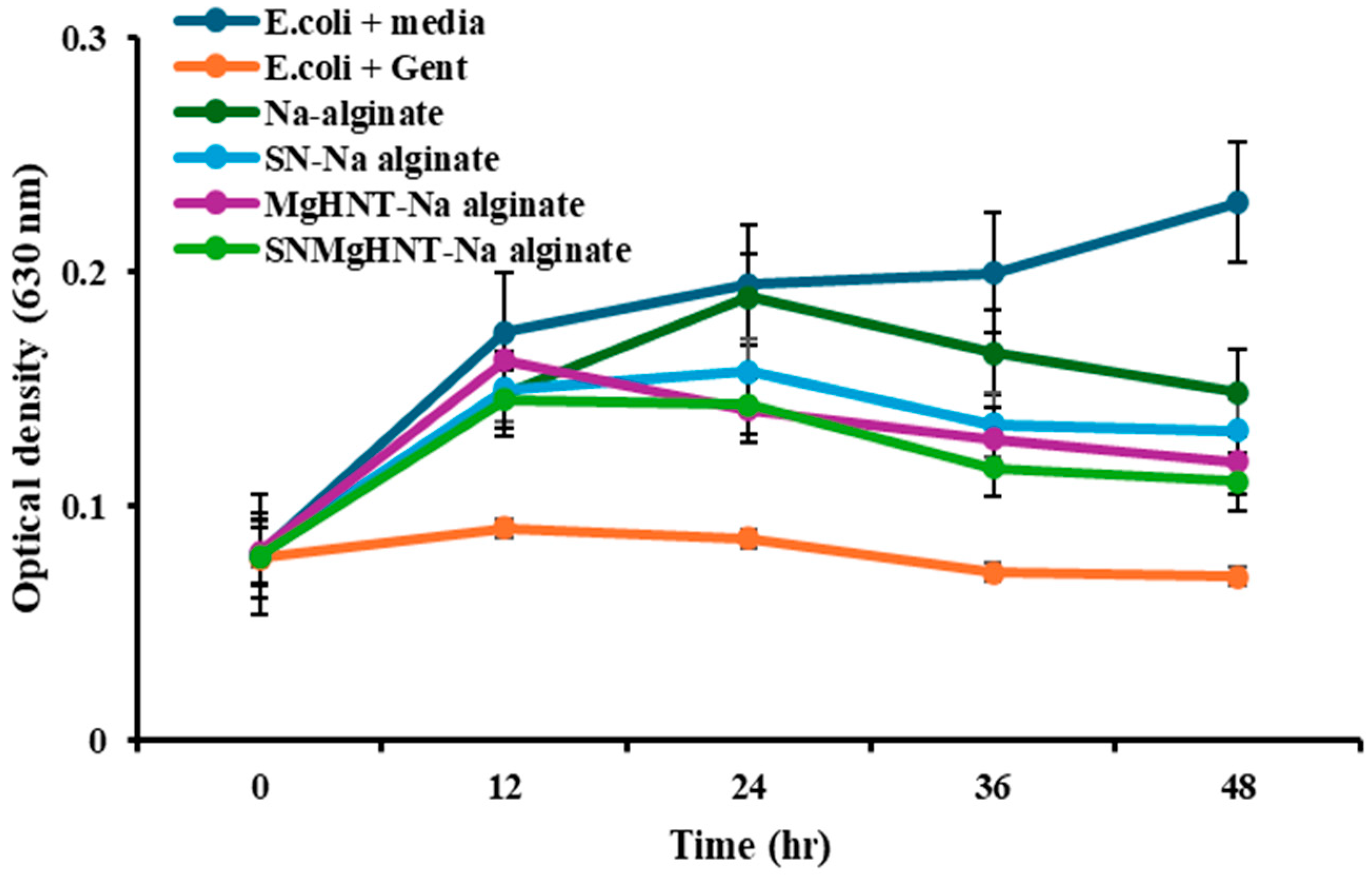
2.3. Antimicrobial Susceptibility Analysis
3. Discussion
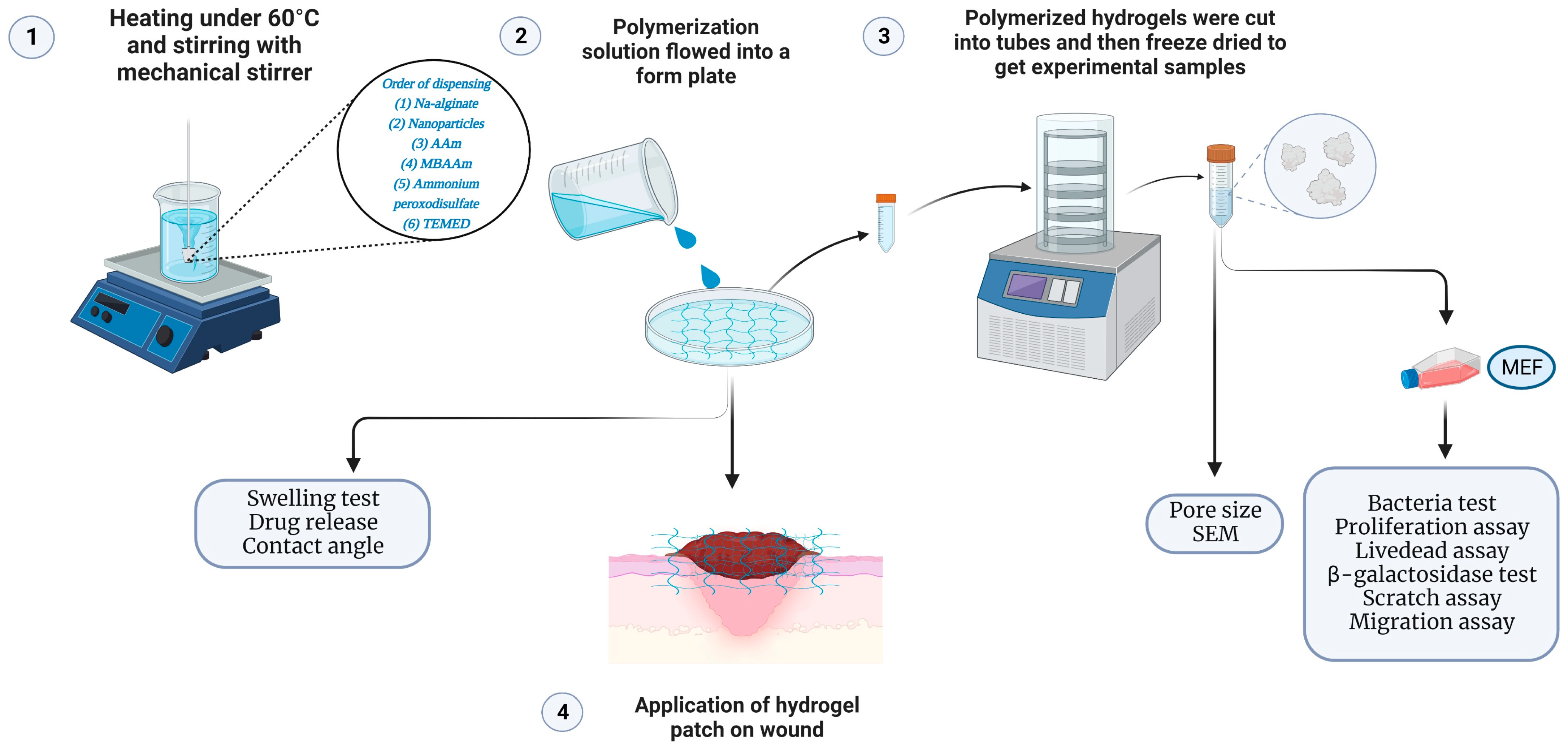
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Metalized HNT (MgHNT)
4.3. Fabrication of Biodegradable Nanocomposite Hydrogel Patch
4.4. Materials Characterization
4.4.1. Morphology and Surface Characterization of MgHNTs and Nanocomposite Hydrogel Patch
4.4.2. Multi BET/Pore Size Testing
4.4.3. Swelling Test
4.4.4. Contact Angle Measurement
4.4.5. Tensile Properties Testing
4.5. Evaluation of In Vitro Fibroblast Response
4.5.1. Cell Culture and Culture Medium
4.5.2. Conditioning of Nanocomposite Hydrogels
4.5.3. Proliferation Assay
4.5.4. Cytotoxicity Assay
4.5.5. Scratch Assay
4.5.6. Migration Assay
4.5.7. β- Galactosidase Staining
4.6. In Vitro Drug Release Study
4.7. Antimicrobial Testing of Nanocomposite Hydrogel Patch
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishida, K.; Shimohata, T.; Kanda, Y.; Nguyen, A.Q.; Masuda, R.; Yamazaki, K.; Uebanso, T.; Mawatari, K.; Kashimoto, T.; Takahashi, A. Characteristic Metabolic Changes in Skeletal Muscle Due to Vibrio vulnificus Infection in a Wound Infection Model. Elias JE. mSystems 2023, 8, e00682-22. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Garcia, G.; Rivara, K.; Woodburn, K.; Clemens, L.E.; Simon, S.I. A Designed Host Defense Peptide for the Topical Treatment of MRSA-Infected Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 2143. [Google Scholar] [CrossRef]
- Ranghar, S.; Sirohi, P.; Verma, P.; Agarwal, V. Nanoparticle-based drug delivery systems: Promising approaches against infections. Braz. Arch. Biol. Technol. 2013, 57, 209–222. [Google Scholar] [CrossRef]
- Merlani, M.; Nadaraia, N.; Amiranashvili, L.; Petrou, A.; Geronikaki, A.; Ciric, A.; Glamoclija, J.; Carevic, T.; Sokovic, M. Antimicrobial Activity of Some Steroidal Hydrazones. Molecules. 2023, 28, 1167. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Ribeiro, J.; Silva, V.; Monteiro, A.; Vieira-Pinto, M.; Igrejas, G.; Reis, F.S.; Barros, L.; Poeta, P. Antibiotic Resistance among Gastrointestinal Bacteria in Broilers: A Review Focused on Enterococcus spp. and Escherichia coli. Animals 2023, 13, 1362. [Google Scholar] [CrossRef]
- Varma, A.; Warghane, A.; Dhiman, N.K.; Paserkar, N.; Upadhye, V.; Modi, A.; Saini, R. The role of nanocomposites against biofilm infections in humans. Front Cell Infect. Microbiol. 2023, 13, 1104615. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Deng, L.; Wang, B.; Li, W.; Han, Z.; Chen, S.; Wang, H. Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2022, 217, 77–87. [Google Scholar] [CrossRef]
- Maity, B.; Alam, S.; Samanta, S.; Prakash, R.G.; Govindaraju, T. Antioxidant Silk Fibroin Composite Hydrogel for Rapid Healing of Diabetic Wound. Macromol. Biosci. 2022, 22, e2200097. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin wound healing: Normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021, 26, 4917. [Google Scholar] [CrossRef]
- Pickles, S.; McAllister, E.; McCullagh, G.; Nieroba, T.-J. Quality improvement evaluation of postoperative wound dressings in orthopaedic patients. Int. J. Orthop. Trauma Nurs. 2022, 45, 100922. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Abdel-Rahman, E.M.; Liu, K.D.; Goldstein, S.L.; Agarwal, A.; Okusa, M.D.; Cerda, J. Recovery after critical illness and acute kidney injury. Clin. J. Am. Soc. Nephrol. 2021, 16, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Windisch, P.; Iorio-Siciliano, V.; Palkovics, D.; Ramaglia, L.; Blasi, A.; Sculean, A. The role of surgical flap design (minimally invasive flap vs. extended flap with papilla preservation) on the healing of intrabony defects treated with an enamel matrix derivative: A 12-month two-center randomized controlled clinical trial. Clin. Oral Investig. 2022, 26, 1811–1821. [Google Scholar] [CrossRef]
- Ionescu, A.; Dodi, A.; Petcu, L.C.; Nicolescu, M.I. Open Healing: A Minimally Invasive Protocol with Flapless Ridge Preservation in Implant Patients. Biology 2022, 11, 142. [Google Scholar] [CrossRef]
- van de Wall, B.J.; Beeres, F.J.; Knobe, M.; Link, B.C.; Babst, R. Minimally invasive plate osteosynthesis: An update of practise. Injury. 2021, 52, 37–42. [Google Scholar] [CrossRef]
- Schilde, S.; Delank, K.-S.; Arbab, D.; Gutteck, N. Minimally Invasive vs Open Akin Osteotomy. Foot Ankle Int. 2021, 42, 278–286. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, H.; Zu, Y.; Yin, W. Biodegradable MoOx@MB incorporated hydrogel as light-activated dressing for rapid and safe bacteria eradication and wound healing. RSC Adv. 2022, 12, 8862–8877. [Google Scholar] [CrossRef]
- Seta, M.; Haraźna, K.; Kasarełło, K.; Solarz-Keller, D.; Cudnoch-Jędrzejewska, A.; Witko, T.; Rajfur, Z.; Guzik, M. The Influence of Novel, Biocompatible, and Bioresorbable Poly(3-hydroxyoctanoate) Dressings on Wound Healing in Mice. Int. J. Mol. Sci. 2022, 23, 16159. [Google Scholar] [CrossRef]
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Living Lactobacillus–ZnO nanoparticles hybrids as antimicrobial and antibiofilm coatings for wound dressing application. Mater. Sci. Eng. C. 2021, 130, 112457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kim, J.; Hassan, S.; Suo, Z. Self-assembled nanocomposites of high water content and load-bearing capacity. Proc. Natl. Acad. Sci. USA 2022, 119, e2203962119. [Google Scholar] [CrossRef] [PubMed]
- Venkatappa, M.M.; Udagani, C.; Hanumegowda, S.M.; Pramod, S.N.; Venkataramaiah, S.; Rangappa, R.; Achur, R.; Alataway, A.; Dewidar, A.Z.; Al-Yafrsi, M.; et al. Effect of Biofunctional Green Synthesized MgO-Nanoparticles on Oxidative-Stress-Induced Tissue Damage and Thrombosis. Molecules 2022, 27, 5162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Minocha, N.; Sharma, N.; Verma, R.; Kaushik, D.; Pandey, P. Solid Lipid Nanoparticles: Peculiar Strategy to Deliver Bio-Proactive Molecules. Recent Pat. Nanotechnol. 2022, 17, 228–242. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Chen, J.; Liu, X.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Lin, B.; Xi, Z. Selection bioaccumulation of polystyrene nanoplastics in fetal rat brain and damage to myelin development. Ecotoxicol. Environ. Saf. 2024, 278, 116393. [Google Scholar] [CrossRef]
- Zeng, H.; Lv, Z.; Sun, X.; Tong, Y.; Wu, W.; Dong, S.; Mao, L. Predicting Bioaccumulation of Nanomaterials: Modeling Approaches with Challenges. Environ. Health 2024, 2, 189–201. [Google Scholar] [CrossRef]
- Zia, S.; Aqib, A.I.; Muneer, A.; Fatima, M.; Atta, K.; Kausar, T.; Zaheer, C.-N.F.; Ahmad, I.; Saeed, M.; Shafique, A. Insights into nanoparticles-induced neurotoxicity and cope up strategies. Front. Neurosci. 2023, 17, 1127460. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Mu, D.; Zhang, H.; Zhang, G.; Huang, X.; Xiong, P. Current research on ecotoxicity of metal-based nanoparticles: From exposure pathways, ecotoxicological effects to toxicity mechanisms. Front. Public. Health 2024, 12, 1390099. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.L.; Belardi, R.M.; Montoro, S.R. Polymers with Nano-Encapsulated Functional Polymers: Encapsulated Phase Change Materials. Encapsulated Phase Change Materials. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 155–169. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid. Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Shegokar, R. Metal Nanoparticles in Pharma; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Swaminathan, M.; Sharma, N.K. Antimicrobial activity of the engineered nanoparticles used as coating agents. Handb. Ecomater. 2019, 1, 549–563. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.; Matovic, B. Mechanical properties of silicon nitride-based ceramics and its use in structural applications at high temperatures. Mater. Sci. Eng. A 2010, 527, 1314–1338. [Google Scholar] [CrossRef]
- Neumann, A.; Reske, T.; Held, M.; Jahnke, K.; Ragoß, C.; Maier, H.R. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci. Mater. Med. 2004, 15, 1135–1140. [Google Scholar] [CrossRef]
- Kue, R.; Sohrabi, A.; Nagle, D.; Frondoza, C.; Hungerford, D. Enhanced proliferation and osteocalcin production by human osteoblast-like MG63 cells on silicon nitride ceramic discs. Biomaterials 1999, 20, 1195–1201. [Google Scholar] [CrossRef]
- Pezzotti, G.; McEntire, B.J.; Bock, R.; Boffelli, M.; Zhu, W.; Vitale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon Nitride: A Synthetic Mineral for Vertebrate Biology. Sci. Rep. 2016, 6, 2–8. [Google Scholar] [CrossRef]
- Bal, B.; Rahaman, M. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Pezzotti, G.; Bock, R.M.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Marin, E.; McEntire, B.; Bal, B.S.; Mazda, O. Silicon nitride surface chemistry: A potent regulator of mesenchymal progenitor cell activity in bone formation. Appl. Mater. Today 2017, 9, 82–95. [Google Scholar] [CrossRef]
- Pezzotti, G.; Marin, E.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Sugano, N.; Bock, R.M.; McEntire, B.; Bal, S.B. Bioactive silicon nitride: A new therapeutic material for osteoarthropathy. Sci. Rep. 2017, 7, 44848. [Google Scholar] [CrossRef]
- Boschetto, F.; Rondinella, A.; Marin, E.E. Biological Activity of Silicon Nitride Ceramics: A Critical Review. Materials 2024, 17, 5548. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lee, S.S.; Blugan, G.; Ferguson, S.J. Silicon Nitride as a Biomedical Material: An Overview. Int. J. Mol. Sci. 2022, 23, 6551. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Oba, N.; Zhu, W.; Marin, E.; Rondinella, A.; Boschetto, F.; McEntire, B.; Yamamoto, K.; Bal, B.S. Human osteoblasts grow transitional Si/N apatite in quickly osteointegrated Si3N4 cervical insert. Acta Biomater. 2017, 64, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.M.; McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Boffelli, M.; Pezzotti, G. Surface modulation of silicon nitride ceramics for orthopaedic applications. Acta Biomater. 2015, 26, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: Processing, microstructure, mechanical properties, cytotoxicity. J. Mater. Sci. Mater. Med. 2008, 19, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite nanotubes as a nature’s boon for biomedical applications. BJGP Open 2019, 6, 1849543519863625. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Reis, S.; Veiga, F.; Saleh, M.; Lvov, Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin. Drug Deliv. 2019, 16, 1169–1182. [Google Scholar] [CrossRef]
- Fakhruddin, K.; Hassan, R.; Khan, M.U.A.; Allisha, S.N.; Razak, S.I.A.; Zreaqat, M.H.; Latip, H.F.M.; Jamaludin, M.N.; Hassan, A. Halloysite nanotubes and halloysite-based composites for biomedical applications. Arab J. Chem. 2021, 14, 103294. [Google Scholar] [CrossRef]
- Shabeena, M.; Warale, D.; Prabhu, A.; Kouser, S.; Manasa, D.; Nagaraja, G. Pectin wrapped halloysite nanotube reinforced Polycaprolactone films for potential wound healing application. Int. J. Biol. Macromol. 2024, 262, 130140. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, S.; Liu, J.; Sun, X. Recent Advances of Halloysite Nanotubes in Biomedical Applications. Small 2023, 20, e2306169. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of metal nanoparticle–hydrogel composites in tissue regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Ding, J.; Lin, Y. Hydrogels: A promising therapeutic platform for inflammatory skin diseases treatment. J. Mater. Chem. B. 2024, 12, 8007–8032. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Ahmad, S.A.; Minhaj, M.A.; Ara, T.J.; Nayak, A.K. Nanocomposite Materials for Prosthetic Devices. In Applications of Nanocomposite Materials in Orthopedics; Asiri, I.A.M., Mohammad, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 127–144. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; da Cruz, N.F.; Lynch, D.G.; da Costa, P.F.; Salgado, C.G.; Silva-Júnior, J.O.C.; Rossi, A.; Ribeiro-Costa, R.M. Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals 2024, 17, 1400. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Damiri, F.; Zare, E.N.; Hasan, A.; Neisiany, R.E.; Veiga, F.; Makvandi, P.; Paiva-Santos, A.C. A review on natural biopolymers in external drug delivery systems for wound healing and atopic dermatitis. Int. J. Biol. Macromol. 2024, 263, 130296. [Google Scholar] [CrossRef]
- Jawad, H.; Boccaccini, A.R.; Ali, N.N.; Harding, S.E. Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications. Nanotoxicology 2011, 5, 372–380. [Google Scholar] [CrossRef]
- Sharifi, E.; Sadati, S.A.; Yousefiasl, S.; Sartorius, R.; Zafari, M.; Rezakhani, L.; Alizadeh, M.; Zare, E.N.; Omidghaemi, S.; Ghanavatinejad, F.; et al. Cell loaded hydrogel containing Ag-doped bioactive glass-ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng. Transl. Med. 2022, 7, e10386. [Google Scholar] [CrossRef]
- Liu, J.; Qu, M.; Wang, C.; Xue, Y.; Huang, H.; Chen, Q.; Sun, W.; Zhou, X.; Xu, G.; Jiang, X. A Dual-Cross-Linked Hydrogel Patch for Promoting Diabetic Wound Healing. Small 2022, 18, e2106172. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Jin, W.; Liu, H.; Li, Z.; Nie, P.; Zhao, G.; Cheng, X.; Zheng, G.; Yang, X. Effect of Hydrogel Contact Angle on Wall Thickness of Artificial Blood Vessel. Int. J. Mol. Sci. 2022, 23, 11114. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.S.; Kaplan, D.S.; Çeribaşi, A.O.; Orkmez, M.; Çanak, A.; Tarakçioğlu, M. An Investigation of The Effect of Extracellular Vesicles Isolated from Mouse Embryonic Fibroblasts on Wound Healing in an Experimental Diabetic Mouse Model. An. Acad. Bras. Cienc. 2022, 94, e20201562. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.W., Jr.; Elumalai, A.; Miller, C.C.; Humayun, A.; Mills, D.K. Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System. Polymers 2022, 14, 1603. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alakija, F.B.; Mills, D.K. Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment. Int. J. Mol. Sci. 2025, 26, 1734. https://doi.org/10.3390/ijms26041734
Alakija FB, Mills DK. Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment. International Journal of Molecular Sciences. 2025; 26(4):1734. https://doi.org/10.3390/ijms26041734
Chicago/Turabian StyleAlakija, Femi B., and David K. Mills. 2025. "Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment" International Journal of Molecular Sciences 26, no. 4: 1734. https://doi.org/10.3390/ijms26041734
APA StyleAlakija, F. B., & Mills, D. K. (2025). Fabrication and Characterization of a Stretchable Sodium Alginate Hydrogel Patch Combined with Silicon Nitride and Metalized Halloysite Nanotubes to Develop a Chronic Wound Healing Treatment. International Journal of Molecular Sciences, 26(4), 1734. https://doi.org/10.3390/ijms26041734






