Chr23-miR-200s and Dmrt1 Control Sexually Dimorphic Trade-Off Between Reproduction and Growth in Zebrafish
Abstract
1. Introduction
2. Results
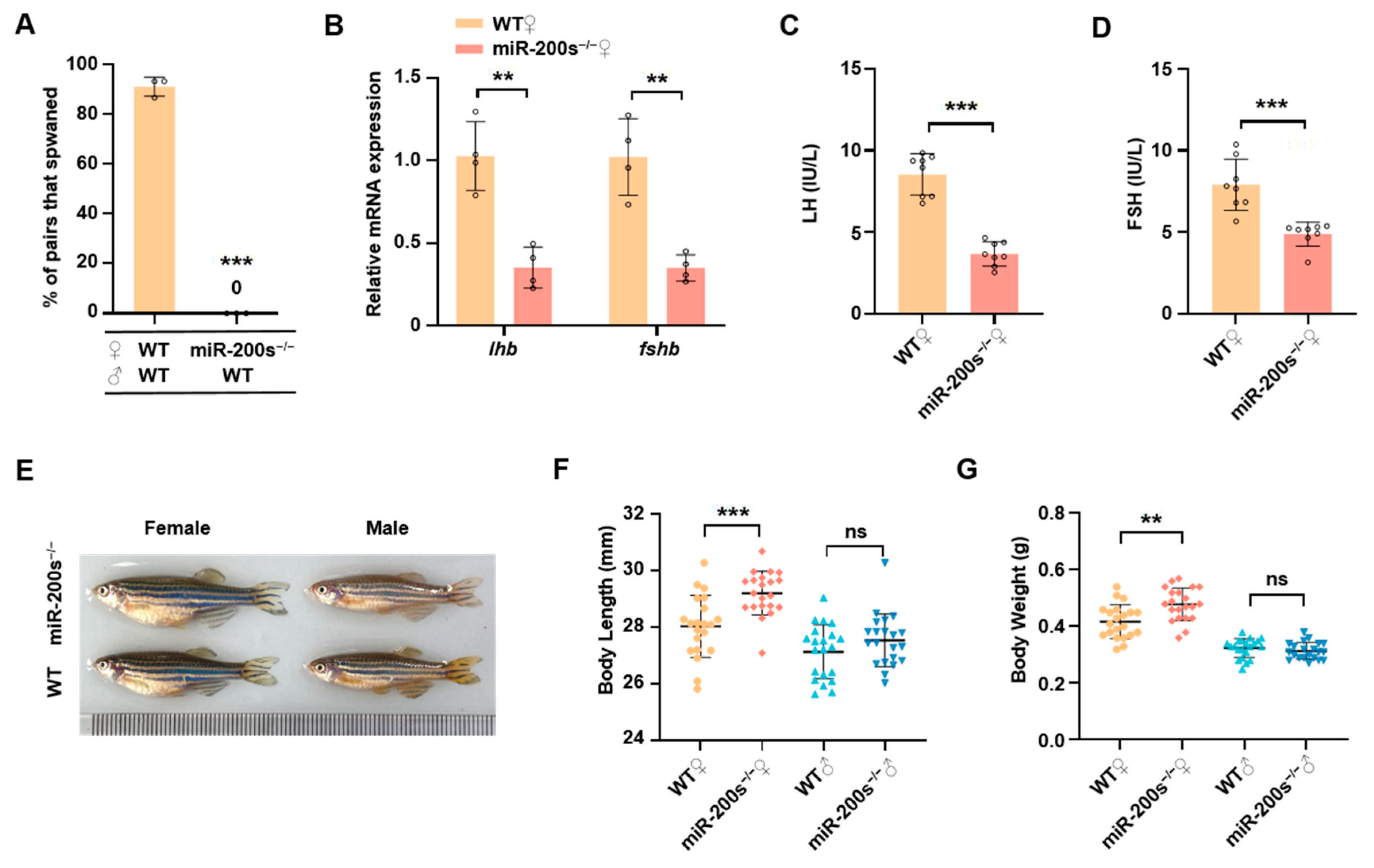
2.1. Chr23-miR-200s Knockout Leads to the Impaired Reproduction and Increased Growth in Female Zebrafish
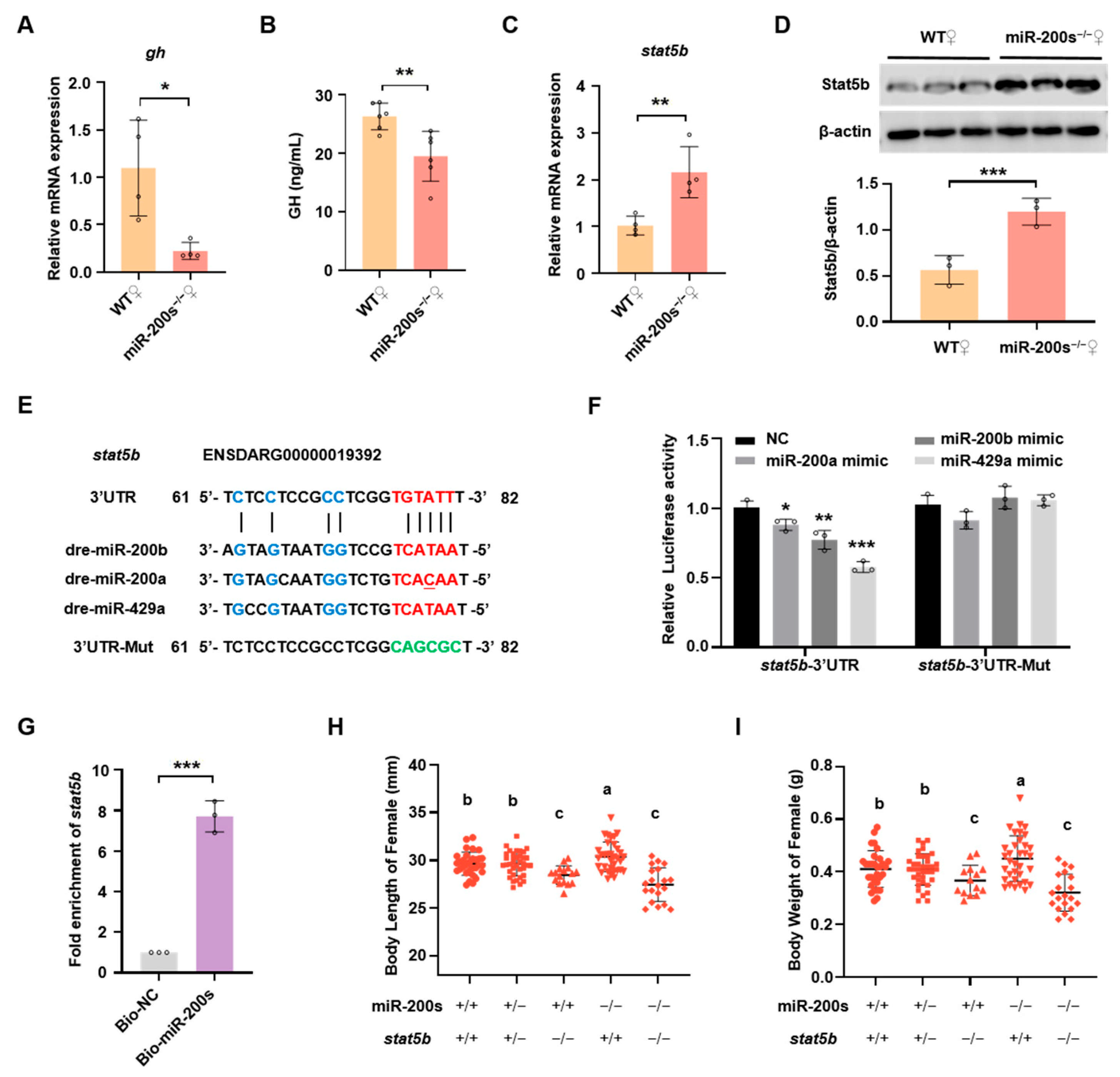
2.2. Chr23-miR-200s Regulate Growth Trait of Female Zebrafish by Directly Targeting stat5b
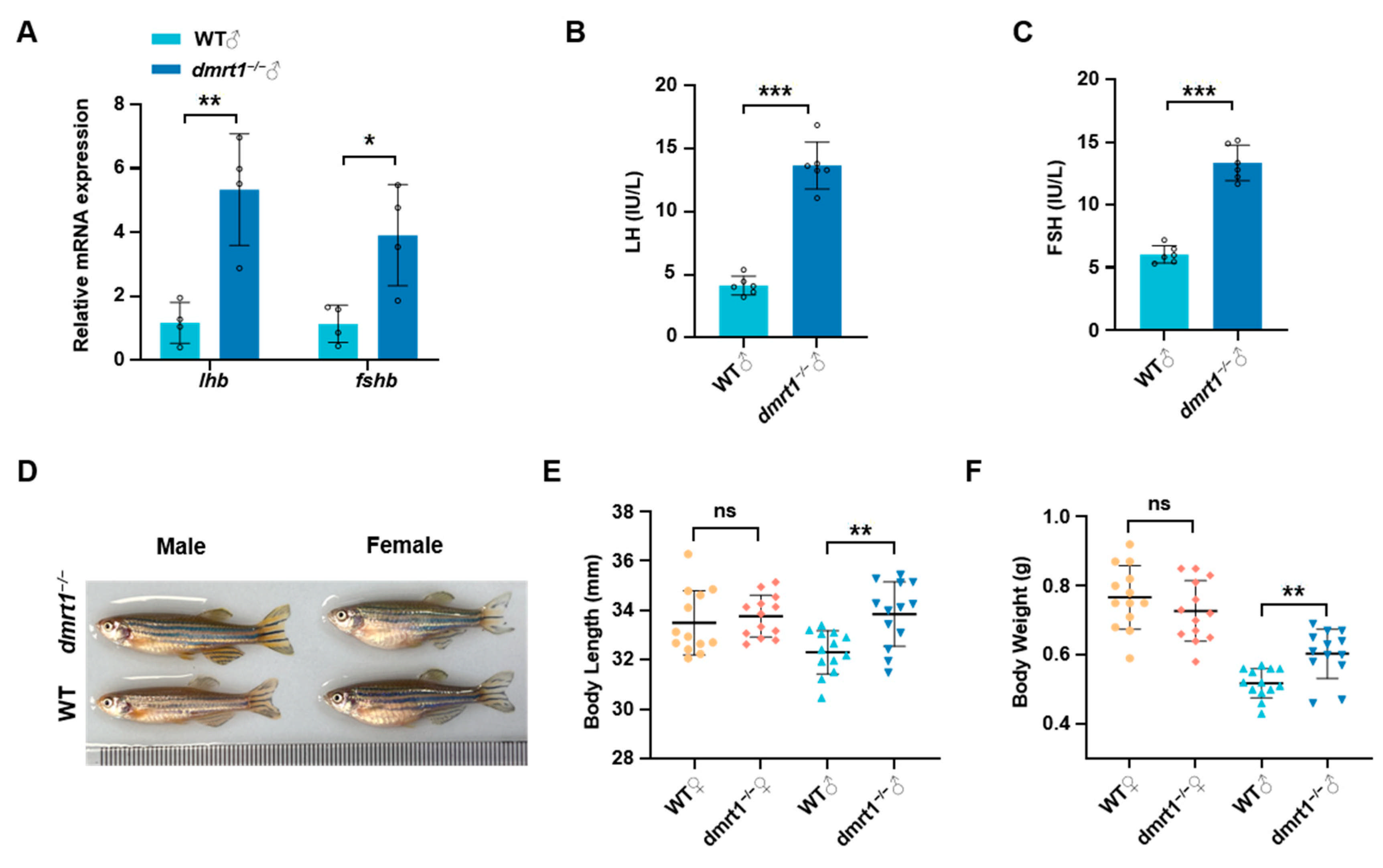
2.3. Dmrt1 Knockout Results in Impaired Reproduction and Promoted Growth in Male Zebrafish
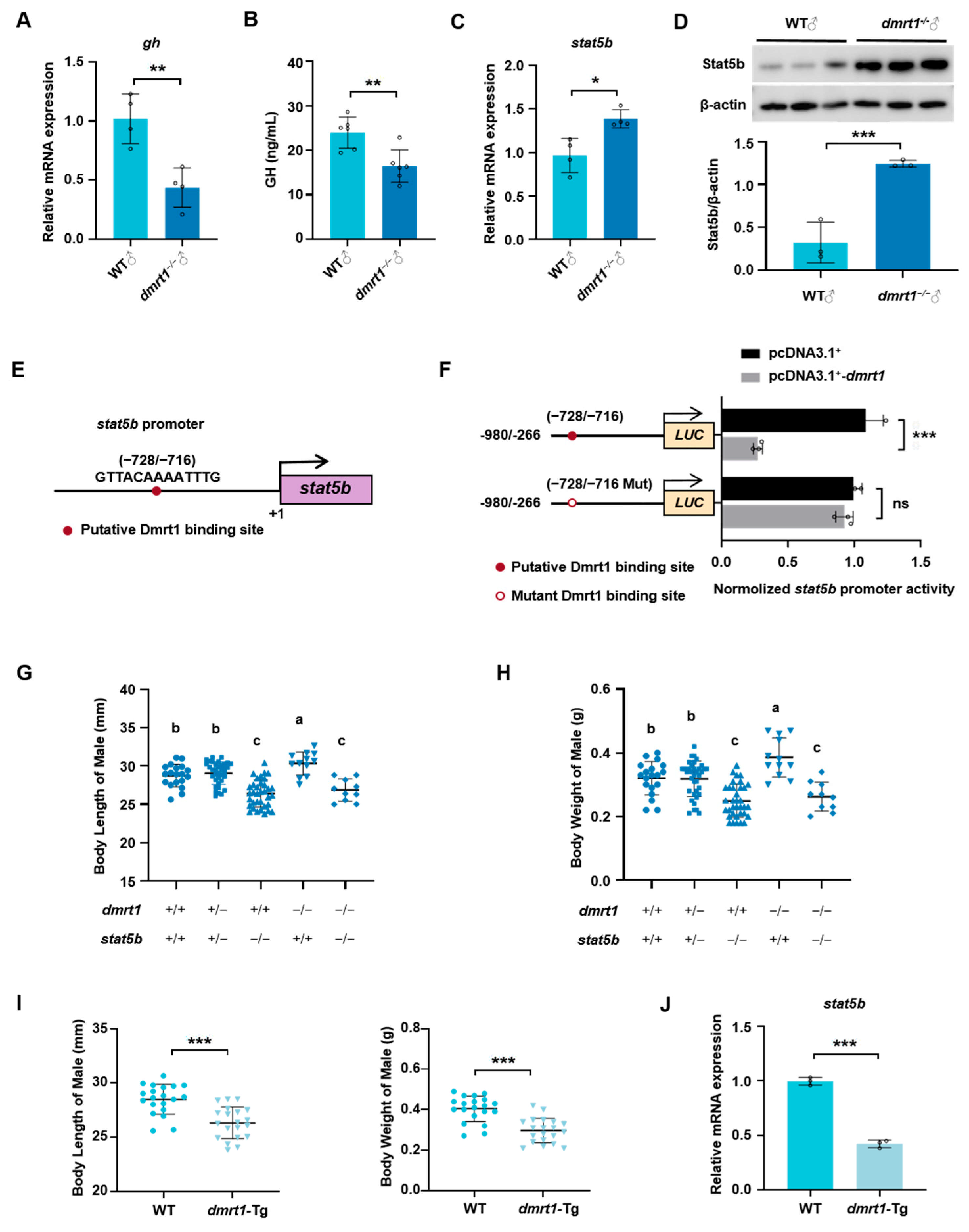
2.4. Dmrt1 Controls Somatic Growth by Transcriptionally Mediating stat5b Expression in Male Zebrafish
3. Discussion
4. Materials and Methods
4.1. Zebrafish Line and Maintenance
4.2. Quantitative Real-Time PCR
4.3. ELISA Analysis of Plasma Hormone
4.4. Western Blot
4.5. Histological Analysis and Immunofluorescence Staining
4.6. Biotinylated miRNA Pulldown
4.7. Luciferase Reporter Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garland, T. Trade-offs. Curr. Biol. 2014, 24, R60–R61. [Google Scholar] [CrossRef] [PubMed]
- Farahpour, F.; Saeedghalati, M.; Brauer, V.S.; Hoffmann, D. Trade-off shapes diversity in eco-evolutionary dynamics. eLife 2018, 7, e36273. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.J.; He, Y.; Yin, X.; Zhong, X.B.; Yan, B.X.; Wu, Y.; Chen, J.; Li, X.Y.; Zhai, K.R.; Huang, Y.F.; et al. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 2021, 184, 5391–5404.e17. [Google Scholar] [CrossRef]
- Guo, T.; Lu, Z.Q.; Xiong, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Kan, Y.; Yang, Y.B.; Zhao, H.Y.; Yu, H.X.; et al. Optimization of rice panicle architecture by specifically suppressing ligand-receptor pairs. Nat. Commun. 2023, 14, 1640. [Google Scholar] [CrossRef]
- Forderer, A.; Chai, J.J. Die another day: Phytosulfokine at the molecular trade-off between growth and defense in plants. EMBO J. 2023, 42, e113540. [Google Scholar] [CrossRef]
- He, Z.H.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Ogawa-Ohnishi, M.; Yamashita, T.; Kakita, M.; Nakayama, T.; Ohkubo, Y.; Hayashi, Y.; Yamashita, Y.; Nomura, T.; Noda, S.; Shinohara, H.; et al. Peptide ligand-mediated trade-off between plant growth and stress response. Science 2022, 378, 175–180. [Google Scholar] [CrossRef]
- Qiu, T.; Andrus, R.; Aravena, M.C.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Berveiller, D.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; et al. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 2022, 13, 2381. [Google Scholar] [CrossRef]
- Annunziata, R.; Mele, B.H.; Marotta, P.; Volpe, M.; Entrambasaguas, L.; Mager, S.; Stec, K.; d’Alcala, M.R.; Sanges, R.; Finazzi, G.; et al. Trade-off between sex and growth in diatoms: Molecular mechanisms and demographic implications. Sci. Adv. 2022, 8, eabj9466. [Google Scholar] [CrossRef]
- Pearl Mizrahi, S.; Goyal, A.; Gore, J. Community interactions drive the evolution of antibiotic tolerance in bacteria. Proc. Natl. Acad. Sci. USA 2023, 120, e2209043119. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, X.; Zhang, N.; Ge, H.; Wei, J.; Qian, K.; Zheng, Y.; Park, Y.; Reddy Palli, S.; Wang, J. The pleiotropic AMPK-CncC signaling pathway regulates the trade-off between detoxification and reproduction. Proc. Natl. Acad. Sci. USA 2023, 120, e2214038120. [Google Scholar] [CrossRef] [PubMed]
- Koppik, M.; Baur, J.; Berger, D. Increased male investment in sperm competition results in reduced maintenance of gametes. PLoS Biol. 2023, 21, e3002049. [Google Scholar] [CrossRef] [PubMed]
- Onuma, T.; Yamauchi, T.; Kosakamoto, H.; Kadoguchi, H.; Kuraishi, T.; Murakami, T.; Mori, H.; Miura, M.; Obata, F. Recognition of commensal bacterial peptidoglycans defines Drosophila gut homeostasis and lifespan. PLoS Genet. 2023, 19, e1010709. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.H.; Chang, M.M.; Wang, X.L.; Shi, Z.K.; Raikhel, A.S.; Zou, Z. Ecdysone signaling mediates the trade-off between immunity and reproduction via suppression of amyloids in the mosquito Aedes aegypti. PLoS Pathog. 2022, 18, e1010837. [Google Scholar] [CrossRef]
- Galarza, J.A.; Murphy, L.; Mappes, J. Antibiotics accelerate growth at the expense of immunity. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211819. [Google Scholar] [CrossRef]
- Hu, Y.W.; Wang, S.H.; Tang, Y.; Xie, G.Q.; Ding, Y.J.; Xu, Q.Y.; Tang, B.; Zhang, L.; Wang, S.G. Suppression of yolk formation, oviposition and egg quality of locust (Locusta migratoria manilensis) infected by Paranosema locustae. Front. Immunol. 2022, 13, 848267. [Google Scholar] [CrossRef]
- Gourgoulianni, N.; Schafer, M.A.; Kapun, M.; Busso, J.P.; Blanckenhorn, W.U. Temperature-dependent melanism and phenoloxidase activity in the dimorphic sepsid fly Sepsis thoracica. J. Therm. Biol. 2023, 112, 103473. [Google Scholar] [CrossRef]
- Furness, A.I.; Venditti, C.; Capellini, I. Terrestrial reproduction and parental care drive rapid evolution in the trade-off between offspring size and number across amphibians. PLoS Biol. 2022, 20, e3001495. [Google Scholar] [CrossRef]
- Bargas-Galarraga, I.; Vila, C.; Gonzalez-Voyer, A. High Investment in Reproduction Is Associated with Reduced Life Span in Dogs. Am. Nat. 2023, 201, 163–174. [Google Scholar] [CrossRef]
- Nikkanen, J.; Leong, Y.A.; Krause, W.C.; Dermadi, D.; Maschek, J.A.; Van Ry, T.; Cox, J.E.; Weiss, E.J.; Gokcumen, O.; Chawla, A.; et al. An evolutionary trade-off between host immunity and metabolism drives fatty liver in male mice. Science 2022, 378, 290–295. [Google Scholar] [CrossRef]
- Tang, J.; Huang, M.S.; He, S.; Zeng, J.X.; Zhu, H. Uncovering the extensive trade-off between adaptive evolution and disease susceptibility. Cell Rep. 2022, 40, 111351. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Andreassen, A.H.; Asheim, E.R.; Finnoen, M.H.; Dresler, G.; Brembu, T.; Loh, A.; Miest, J.J.; Jutfelt, F. Reduced physiological plasticity in a fish adapted to stable temperatures. Proc. Natl. Acad. Sci. USA 2022, 119, e2201919119. [Google Scholar] [CrossRef] [PubMed]
- Ohlberger, J.; Cline, T.J.; Schindler, D.E.; Lewis, B. Declines in body size of sockeye salmon associated with increased competition in the ocean. Proc. R. Soc. B Biol. Sci. 2023, 290, 20222248. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Levy, M.; Medley, J.K.; Hassan, H.; Miller, B.; Alexander, R.; Wilcock, E.; Yi, K.; Florens, L.; Weaver, K.; et al. Metabolic reprogramming underlies cavefish muscular endurance despite loss of muscle mass and contractility. Proc. Natl. Acad. Sci. USA 2023, 120, e2204427120. [Google Scholar] [CrossRef]
- Krishnan, J.; Seidel, C.W.; Zhang, N.; Singh, N.P.; VanCampen, J.; Peuss, R.; Xiong, S.; Kenzior, A.; Li, H.; Conaway, J.W.; et al. Genome-wide analysis of cis-regulatory changes underlying metabolic adaptation of cavefish. Nat. Genet. 2022, 54, 684–693. [Google Scholar] [CrossRef]
- Pontzer, H.; McGrosky, A. Balancing growth, reproduction, maintenance, and activity in evolved energy economies. Curr. Biol. 2022, 32, R709–R719. [Google Scholar] [CrossRef]
- Cao, M.; Chen, J.; Peng, W.; Wang, Y.; Liao, L.; Li, Y.; Trudeau, V.L.; Zhu, Z.; Hu, W. Effects of growth hormone over-expression on reproduction in the common carp Cyprinus carpio L. Gen. Comp. Endocrinol. 2014, 195, 47–57. [Google Scholar] [CrossRef]
- Emery Thompson, M.; Muller, M.N.; Sabbi, K.; Machanda, Z.P.; Otali, E.; Wrangham, R.W. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc. Natl. Acad. Sci. USA 2016, 113, 7780–7785. [Google Scholar] [CrossRef]
- Williams, T.M.; Carroll, S.B. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat. Rev. Genet. 2009, 10, 883. [Google Scholar] [CrossRef]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef]
- Simmons, L.W.; Sloan, N.S.; Firman, R.C. Sexual Selection Shapes Seminal Vesicle Secretion Gene Expression in House Mice. Mol. Biol. Evol. 2020, 37, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.; Houle, D.; Yan, Z.; Kawecki, T.J.; Keller, L. Evolution under monogamy feminizes gene expression in Drosophila melanogaster. Nat. Commun. 2014, 5, 3482. [Google Scholar] [CrossRef] [PubMed]
- Pointer, M.A.; Harrison, P.W.; Wright, A.E.; Mank, J.E. Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism. PLoS Genet. 2013, 9, e1003697. [Google Scholar] [CrossRef]
- Parsch, J.; Ellegren, H. The evolutionary causes and consequences of sex-biased gene expression. Nat. Rev. Genet. 2013, 14, 83–87. [Google Scholar] [CrossRef]
- Hu, B.; Shen, N.; Li, J.J.; Kang, H.; Hong, J.; Fletcher, J.; Greenberg, J.; Mailick, M.R.; Lu, Q. Genome-wide association study reveals sex-specific genetic architecture of facial attractiveness. PLoS Genet. 2019, 15, e1007973. [Google Scholar] [CrossRef]
- Khramtsova, E.A.; Davis, L.K.; Stranger, B.E. The role of sex in the genomics of human complex traits. Nat. Rev. Genet. 2019, 20, 173–190. [Google Scholar] [CrossRef]
- Johnston, K.J.A.; Ward, J.; Ray, P.R.; Adams, M.J.; McIntosh, A.M.; Smith, B.H.; Strawbridge, R.J.; Price, T.J.; Smith, D.J.; Nicholl, B.I.; et al. Sex-stratified genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2021, 17, e1009428. [Google Scholar] [CrossRef]
- Lagou, V.; Magi, R.; Hottenga, J.J.; Grallert, H.; Perry, J.R.B.; Bouatia-Naji, N.; Marullo, L.; Rybin, D.; Jansen, R.; Min, J.L.; et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat. Commun. 2021, 12, 24. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Jing, J.; Xiong, S.; Li, Z.; Wu, J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5, 15906. [Google Scholar] [CrossRef]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, K.; Zhang, F.; Wu, J.; Zhang, J. The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder. Fishes 2022, 7, 129. [Google Scholar] [CrossRef]
- Xiong, S.; Tian, J.; Ge, S.; Li, Z.; Long, Z.; Guo, W.; Huang, P.; He, Y.; Xiao, T.; Gui, J.F.; et al. The microRNA-200 cluster on chromosome 23 is required for oocyte maturation and ovulation in zebrafish†. Biol. Reprod. 2020, 103, 769–778. [Google Scholar] [CrossRef]
- Kopp, A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 2012, 28, 175–184. [Google Scholar] [CrossRef]
- Lin, Q.; Mei, J.; Li, Z.; Zhang, X.; Zhou, L.; Gui, J.F. Distinct and Cooperative Roles of amh and dmrt1 in Self-Renewal and Differentiation of Male Germ Cells in Zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef]
- Xiong, S.; Ma, W.; Jing, J.; Zhang, J.; Dan, C.; Gui, J.F.; Mei, J. An miR-200 Cluster on Chromosome 23 Regulates Sperm Motility in Zebrafish. Endocrinology 2018, 159, 1982–1991. [Google Scholar] [CrossRef]
- Huang, P.; Guo, W.; Wang, Y.; Xiong, Y.; Ge, S.; Gong, G.; Lin, Q.; Xu, Z.; Gui, J.F.; Mei, J. Genome-wide association study reveals the genetic basis of growth trait in yellow catfish with sexual size dimorphism. Genomics 2022, 114, 110380. [Google Scholar] [CrossRef]
- Glazier, D. Resource allocation patterns. In Resource Allocation Theory Applied to Farm Animal Production; CABI: Wallingford, UK, 2008; pp. 22–43. [Google Scholar]
- Norbeck, L.A.; Sheridan, M.A. An in vitro model for evaluating peripheral regulation of growth in fish: Effects of 17β-estradiol and testosterone on the expression of growth hormone receptors, insulin-like growth factors, and insulin-like growth factor type 1 receptors in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2011, 173, 270–280. [Google Scholar]
- Hu, Z.; Ai, N.; Chen, W.; Wong, Q.W.; Ge, W. Loss of Growth Hormone Gene (gh1) in Zebrafish Arrests Folliculogenesis in Females and Delays Spermatogenesis in Males. Endocrinology 2019, 160, 568–586. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Fernandes, R.V.; Studzinski, A.L.; Rosa, C.E.; Corcini, C.D.; Junior, A.S.; Marins, L.F. GH overexpression decreases spermatic parameters and reproductive success in two-years-old transgenic zebrafish males. Anim. Reprod. Sci. 2013, 139, 162–167. [Google Scholar] [CrossRef]
- Spicer, L.J. Leptin: A possible metabolic signal affecting reproduction. Domest. Anim. Endocrinol. 2001, 21, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Jarial, K.D.S.; Mahajan, R.; Kapoor, N. Leptin in Energy homeostasis, Male reproduction, and Immune regulation. Chem. Biol. Lett. 2021, 8, 183–191. [Google Scholar]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, I.; Garcia-Clave, E.; Cebrian-Serrano, A.; Le Thuc, O.; Contreras, R.E.; Xu, Y.; Gruber, T.; Schriever, S.C.; Legutko, B.; Lintelmann, J.; et al. Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1. Cell Metab. 2023, 35, 438–455.e7. [Google Scholar] [CrossRef]
- Zheng, D.S.; Wang, X.; Antonson, P.; Gustafsson, J.A.; Li, Z.Y. Genomics of sex hormone receptor signaling in hepatic sexual dimorphism. Mol. Cell. Endocrinol. 2018, 471, 33–41. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; He, Y.; Dawar, F.U.; Xiong, S.; Mei, J. Potential Contributions of miR-200a/-200b and Their Target Gene-Leptin to the Sexual Size Dimorphism in Yellow Catfish. Front. Physiol. 2017, 8, 970. [Google Scholar] [CrossRef]
- Lebeck, J. Editorial: Sexual dimorphism in Glucose and lipid Metabolism. Front. Endocrinol. 2016, 7, 166. [Google Scholar] [CrossRef]
- Schweisgut, J.; Schutt, C.; Wust, S.; Wietelmann, A.; Ghesquiere, B.; Carmeliet, P.; Drose, S.; Korach, K.S.; Braun, T.; Boettger, T. Sex-specific, reciprocal regulation of ER alpha and miR-22 controls muscle lipid metabolism in male mice. EMBO J. 2017, 36, 1199–1214. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Wang, L.X.; Tavakoli, S.; Nguyen, H.N.; Short, J.D.; Asmis, R. Glutaredoxin 1 controls monocyte reprogramming during nutrient stress and protects mice against obesity and atherosclerosis in a sex-specific manner. Nat. Commun. 2022, 13, 790. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Luo, C.; He, Y.; Xu, X.; Yan, X.; Li, Y.; Shu, D.; Qu, H. Effects of the DMRT1 genotype on the body weight and gut microbiota in the broiler chicken. Poult. Sci. 2020, 99, 4044–4051. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. Nutrient regulation of somatic growth in teleost fish. The interaction between somatic growth, feeding and metabolism. Mol. Cell Endocrinol. 2020, 518, 111029. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Le Roith, D. Control of growth by the somatropic axis: Growth hormone and the insulin-like growth factors have related and independent roles. Annu. Rev. Physiol. 2001, 63, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S. Growth hormone and growth? Gen. Comp. Endocrinol. 2013, 190, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Xiong, F.; Pang, S.C.; He, M.D.; Waters, M.J.; Zhu, Z.Y.; Sun, Y.H. Activation of GH signaling and GH-independent stimulation of growth in zebrafish by introduction of a constitutively activated GHR construct. Transgenic Res. 2011, 20, 557–567. [Google Scholar] [CrossRef]
- Herrington, J.; Smit, L.S.; Schwartz, J.; Carter-Su, C. The role of STAT proteins in growth hormone signaling. Oncogene 2000, 19, 2585–2597. [Google Scholar] [CrossRef]
- Xiong, S.; Mei, J.; Huang, P.; Jing, J.; Li, Z.; Kang, J.; Gui, J.F. Essential roles of stat5.1/stat5b in controlling fish somatic growth. J. Genet. Genom. 2017, 44, 577–585. [Google Scholar] [CrossRef]
- Xiong, Y.; Zheng, X.; Ke, W.; Gong, G.; Wang, Y.; Dan, C.; Huang, P.; Wu, J.; Guo, W.; Mei, J. Function and association analysis of Cyclophilin A gene with resistance to Edwardsiella ictaluri in yellow catfish. Dev. Comp. Immunol. 2020, 113, 103783. [Google Scholar] [CrossRef]
- Pedroso, G.L.; Hammes, T.O.; Escobar, T.D.; Fracasso, L.B.; Forgiarini, L.F.; da Silveira, T.R. Blood collection for biochemical analysis in adult zebrafish. J. Vis. Exp. 2012, 63, 3865. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Liu, Y.; Huang, H.; Yu, J.; Li, X.; Lin, Q.; Huang, P.; Mei, J. Chr23-miR-200s and Dmrt1 Control Sexually Dimorphic Trade-Off Between Reproduction and Growth in Zebrafish. Int. J. Mol. Sci. 2025, 26, 1785. https://doi.org/10.3390/ijms26041785
Ge S, Liu Y, Huang H, Yu J, Li X, Lin Q, Huang P, Mei J. Chr23-miR-200s and Dmrt1 Control Sexually Dimorphic Trade-Off Between Reproduction and Growth in Zebrafish. International Journal of Molecular Sciences. 2025; 26(4):1785. https://doi.org/10.3390/ijms26041785
Chicago/Turabian StyleGe, Si, Ying Liu, Haoran Huang, Jiawang Yu, Xiaohui Li, Qiaohong Lin, Peipei Huang, and Jie Mei. 2025. "Chr23-miR-200s and Dmrt1 Control Sexually Dimorphic Trade-Off Between Reproduction and Growth in Zebrafish" International Journal of Molecular Sciences 26, no. 4: 1785. https://doi.org/10.3390/ijms26041785
APA StyleGe, S., Liu, Y., Huang, H., Yu, J., Li, X., Lin, Q., Huang, P., & Mei, J. (2025). Chr23-miR-200s and Dmrt1 Control Sexually Dimorphic Trade-Off Between Reproduction and Growth in Zebrafish. International Journal of Molecular Sciences, 26(4), 1785. https://doi.org/10.3390/ijms26041785





