Ocular Surface Changes Differ Significantly Between Oxaliplatin- and Diabetes-Induced Polyneuropathy
Abstract
:1. Introduction
2. Results
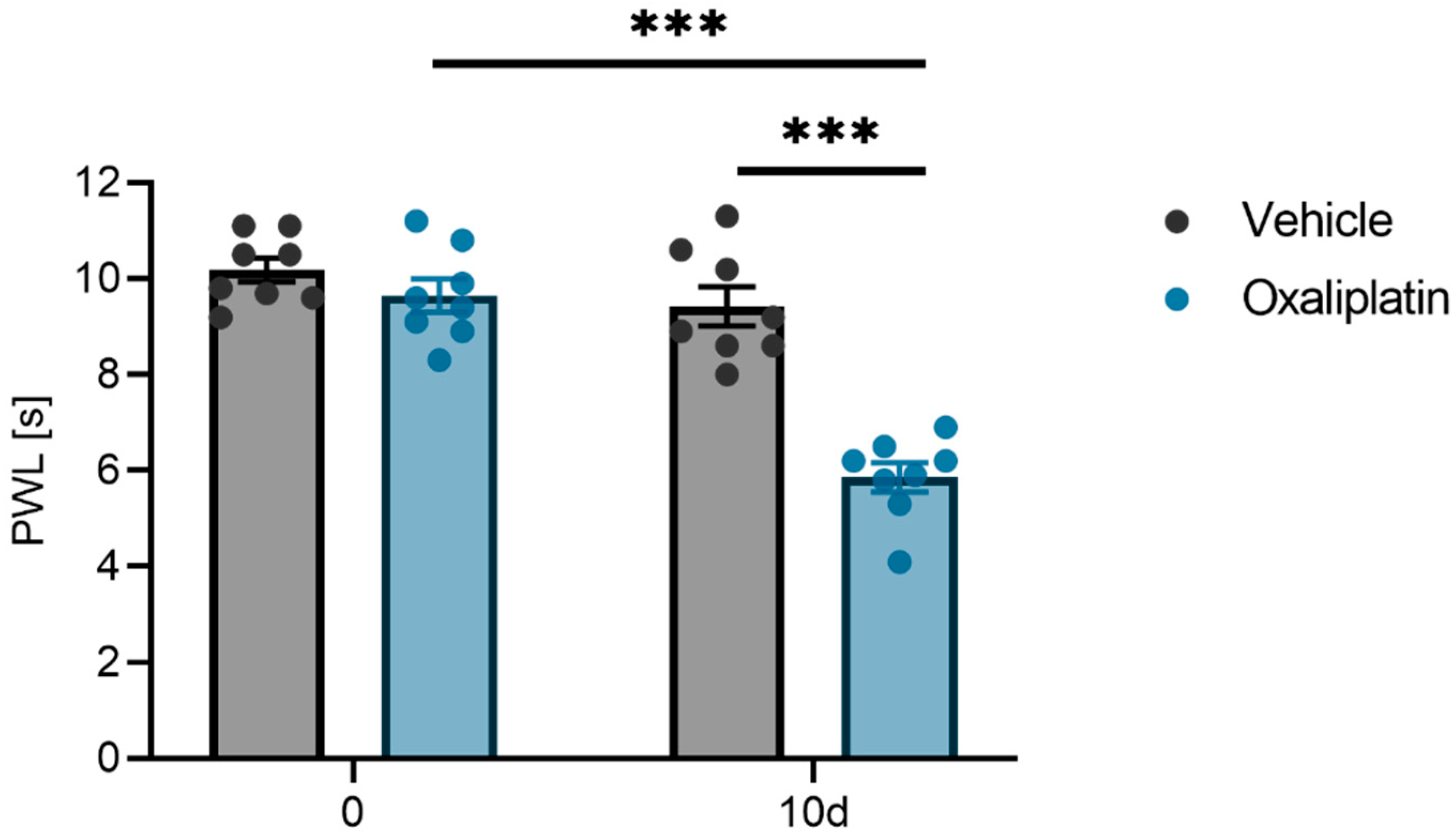
2.1. Mechanical Pain Threshold
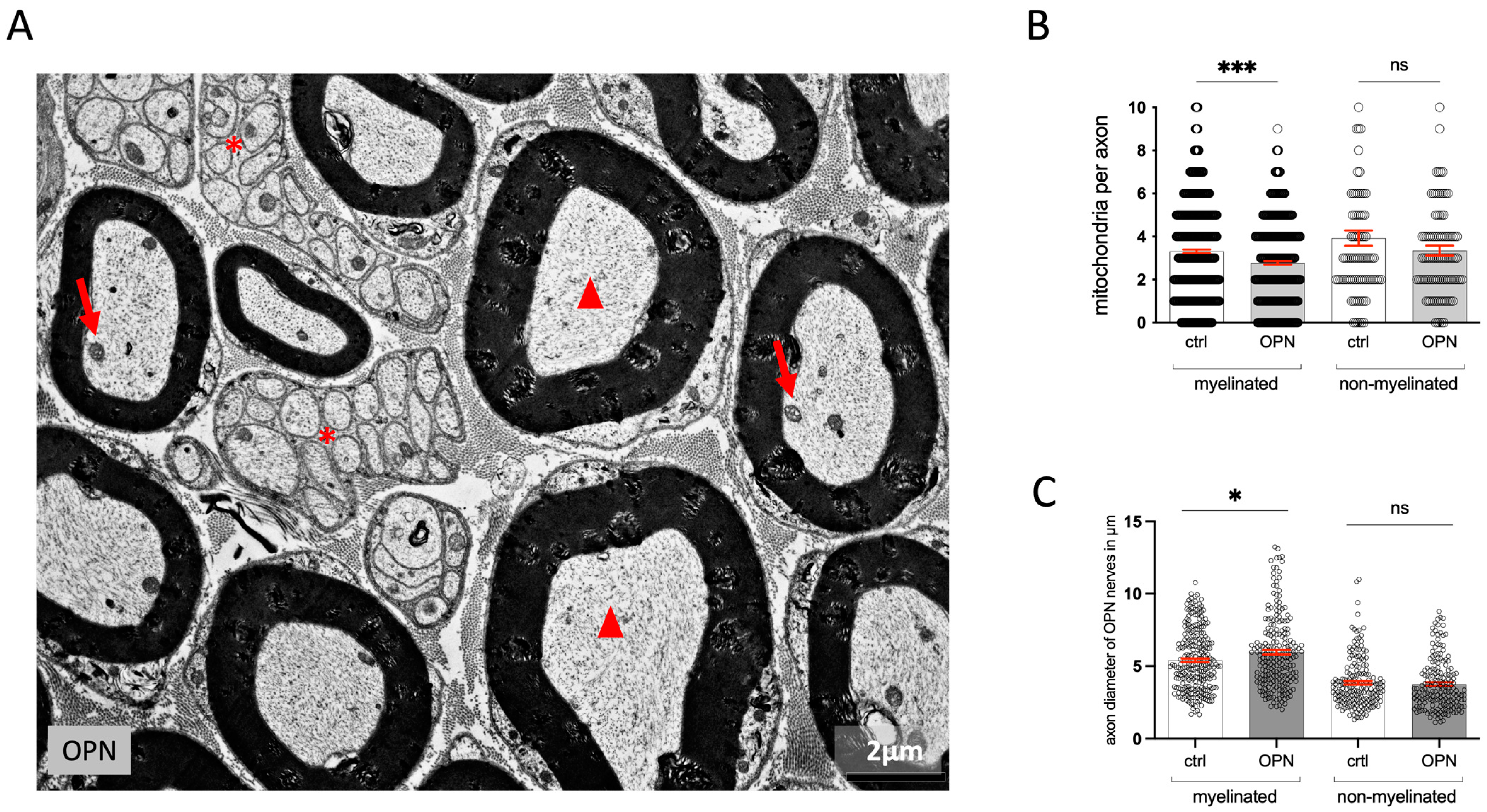
2.2. Quantitative Changes in the Morphology of the Sciatic Nerve
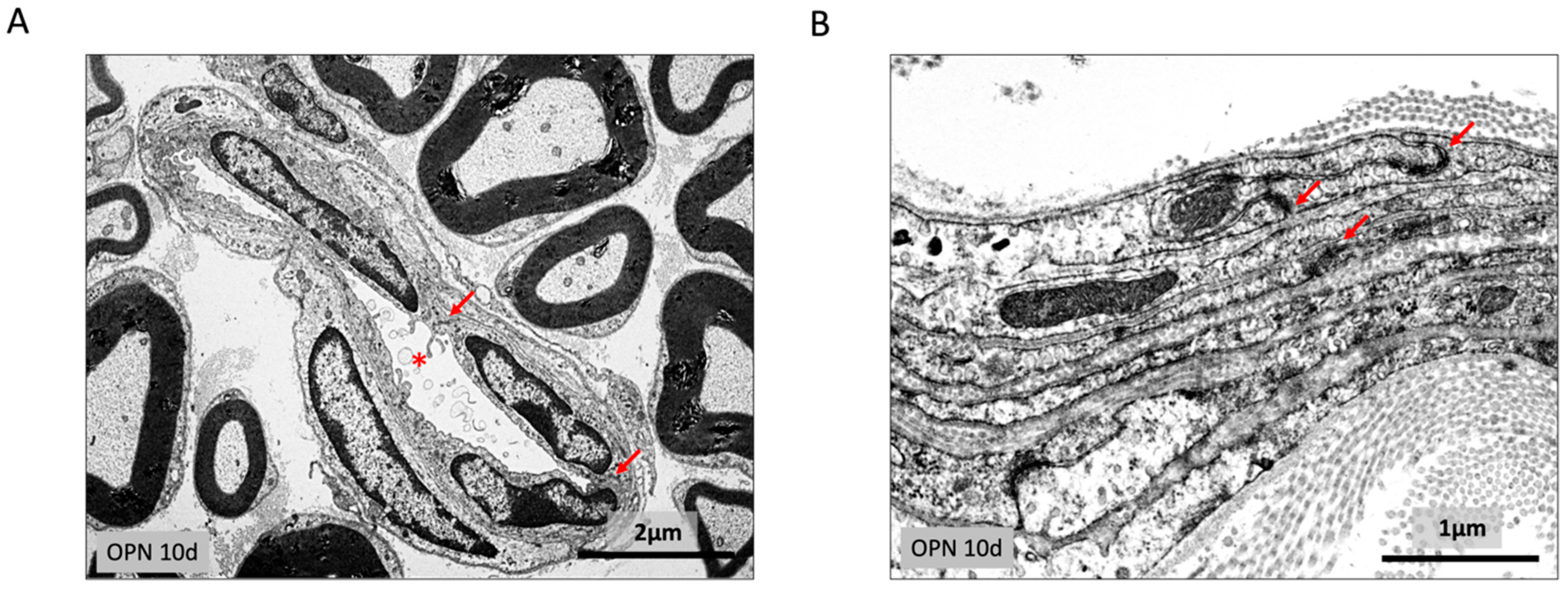
2.3. Endoneurium and Perineural Sheath
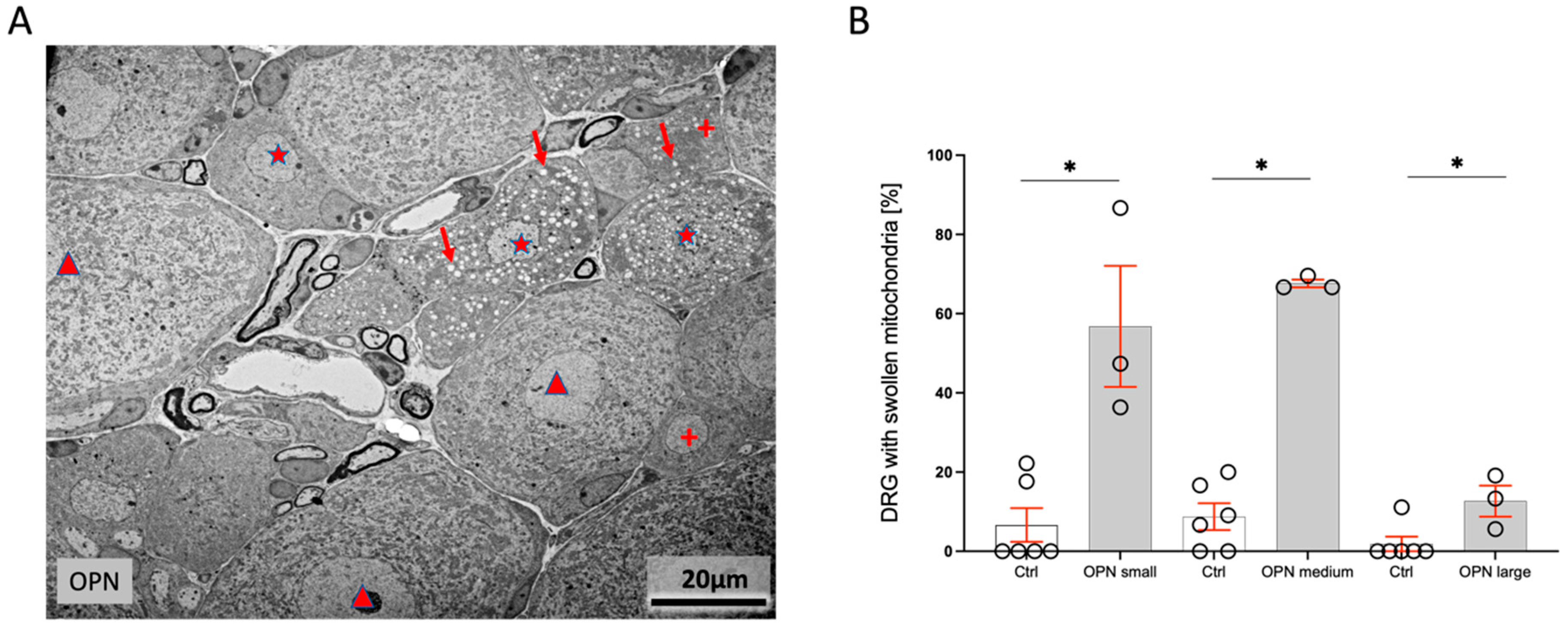
2.4. Dorsal Root Ganglia (DRG)
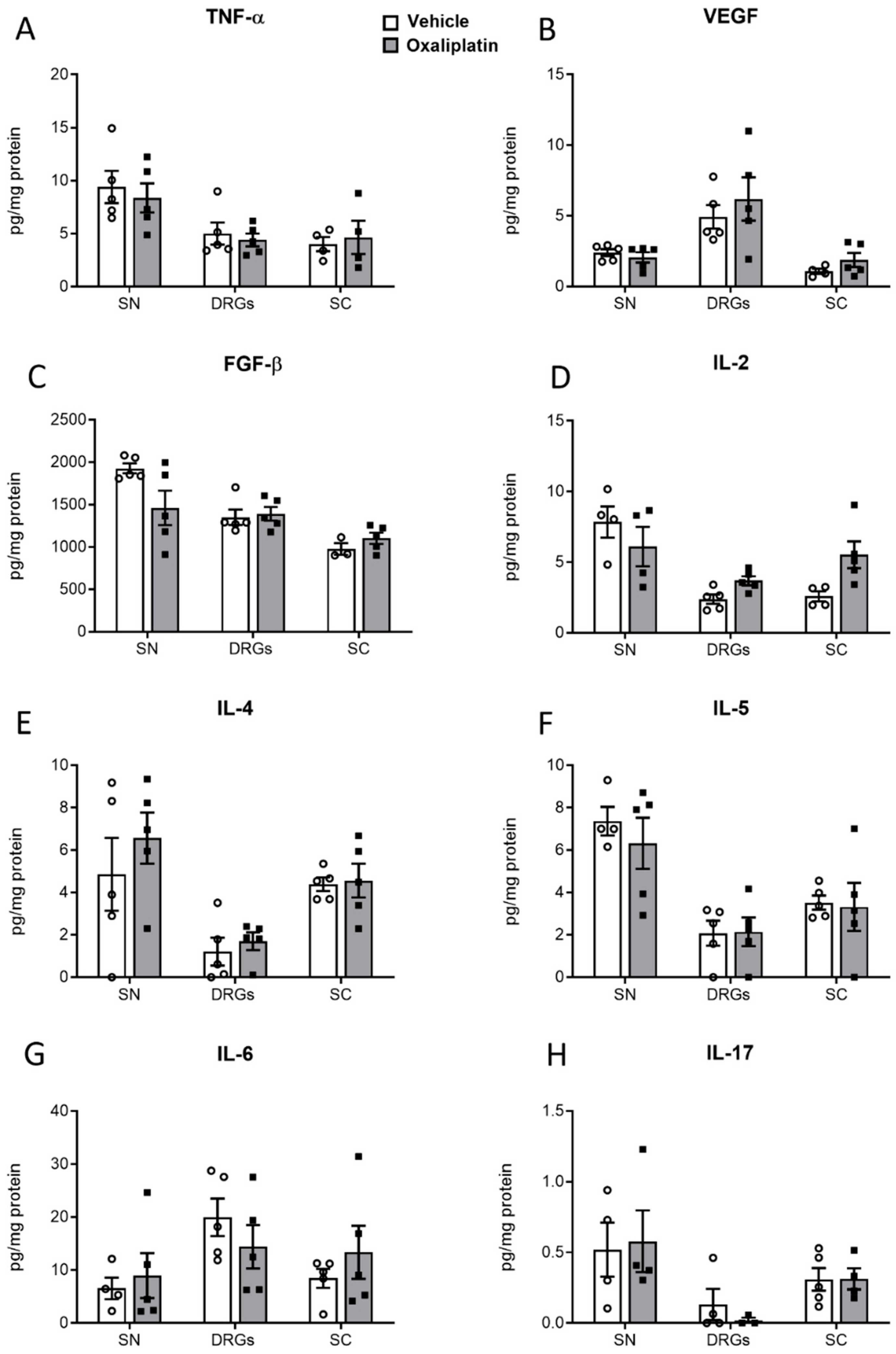
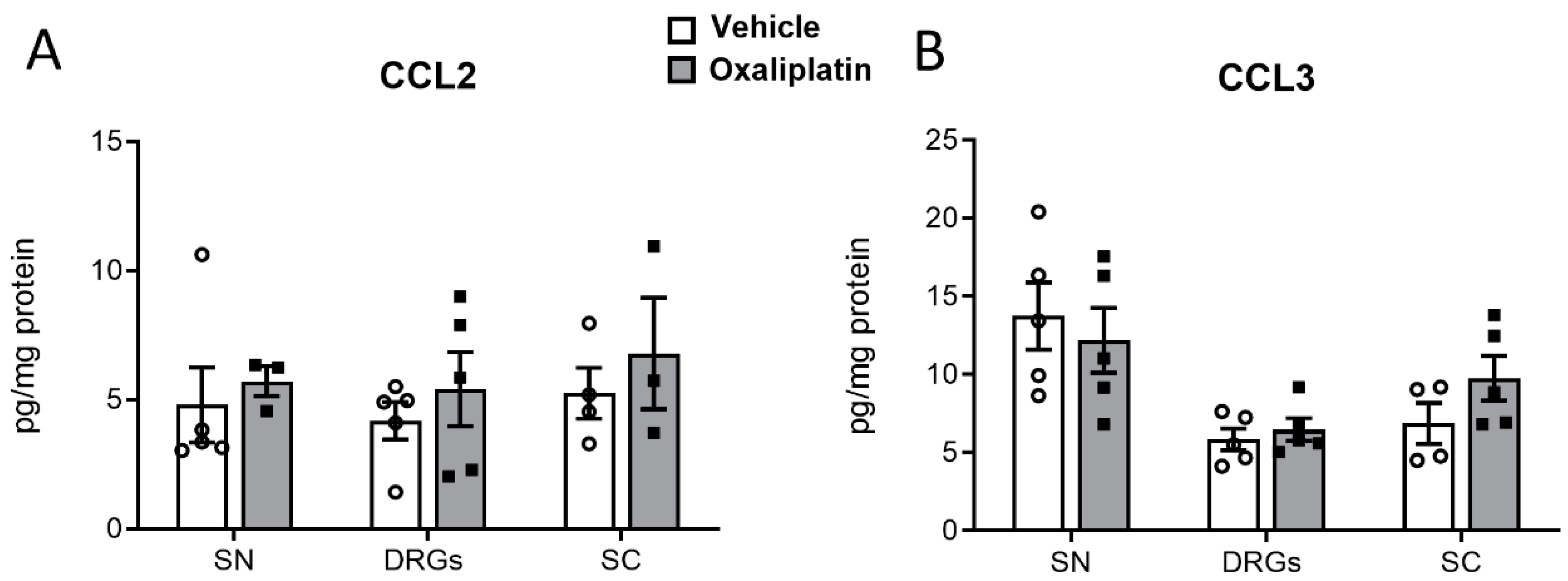
2.5. Concentrations of Proinflammatory Cytokines, Chemokines, and Growth Factors
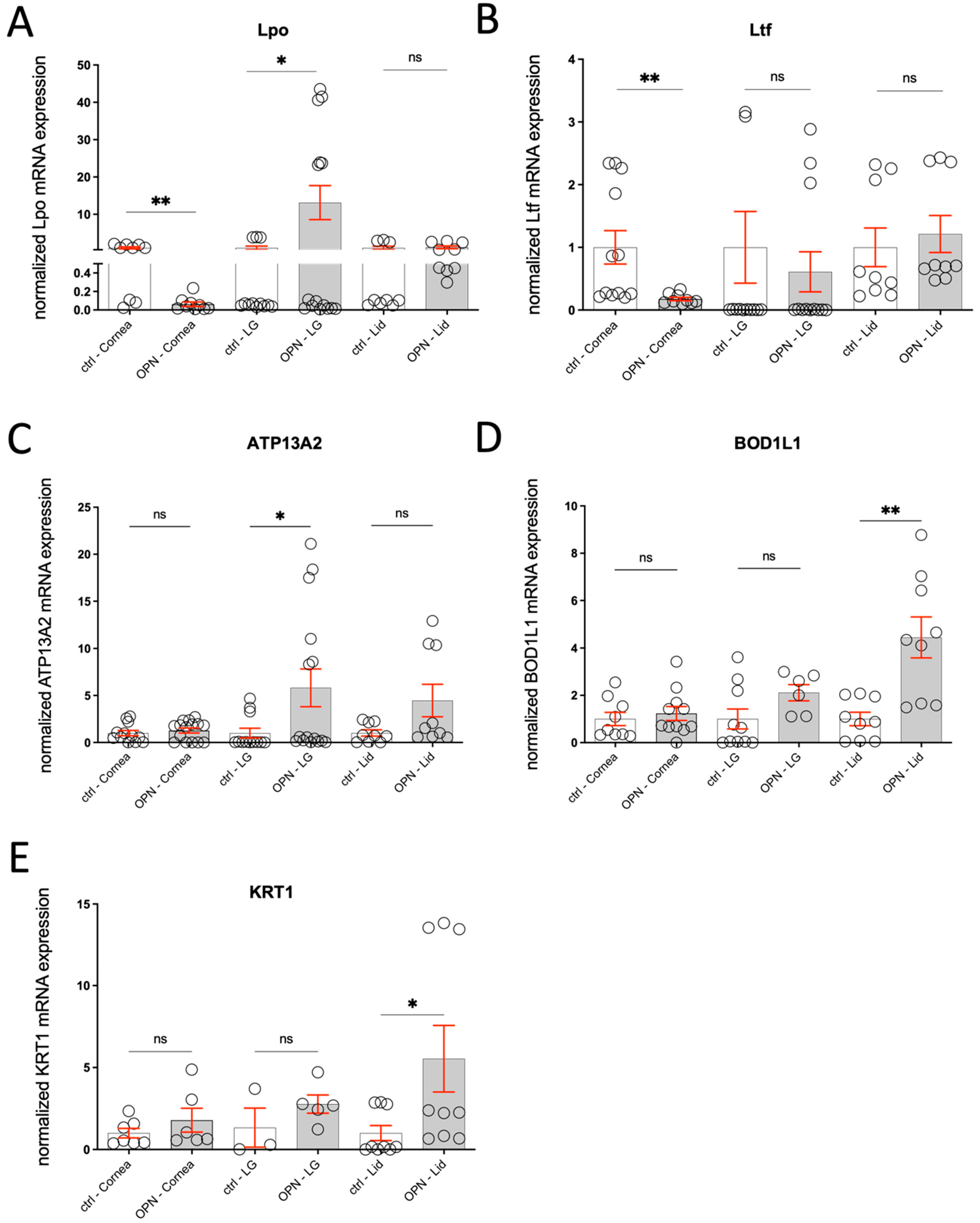
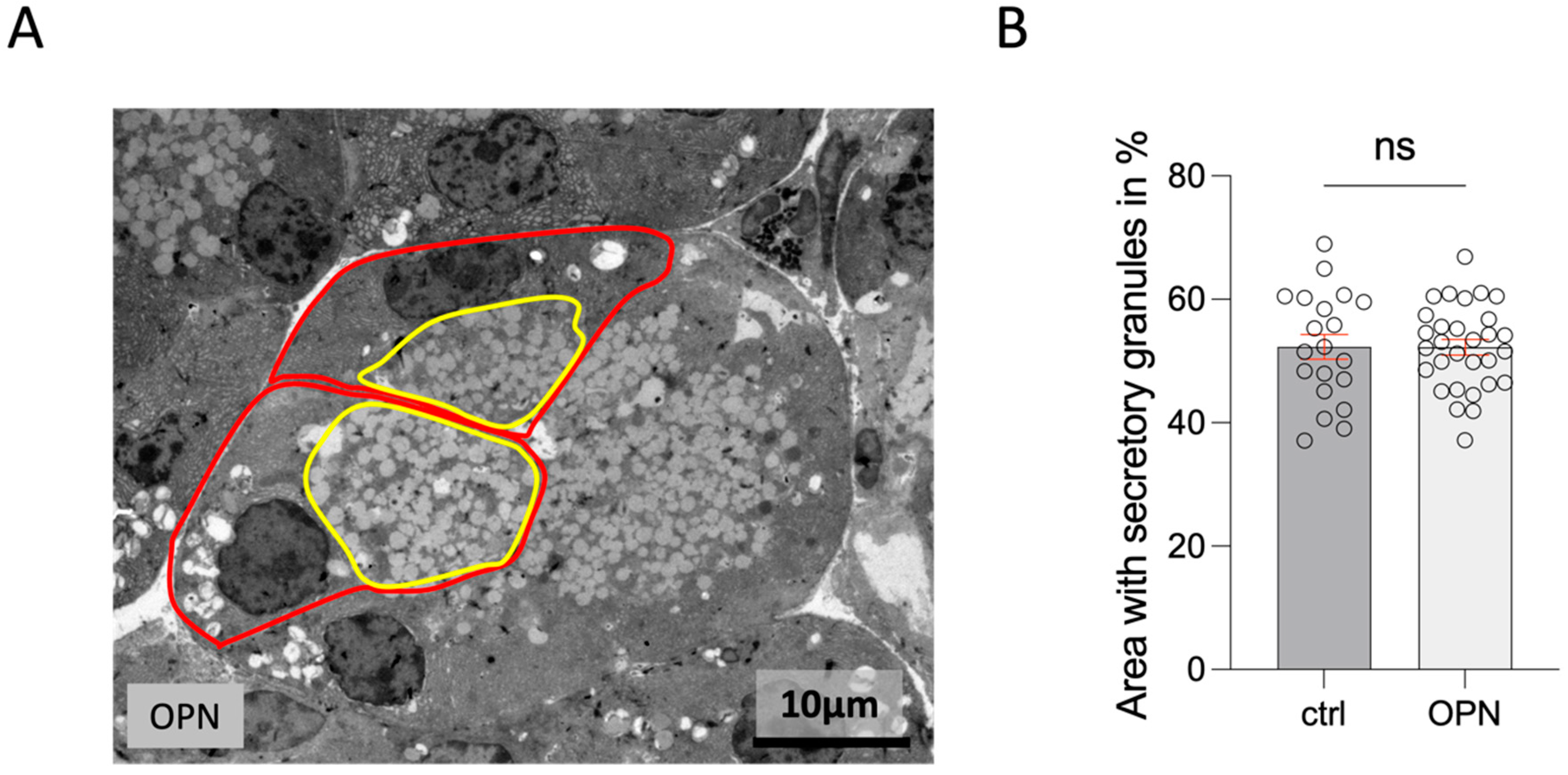
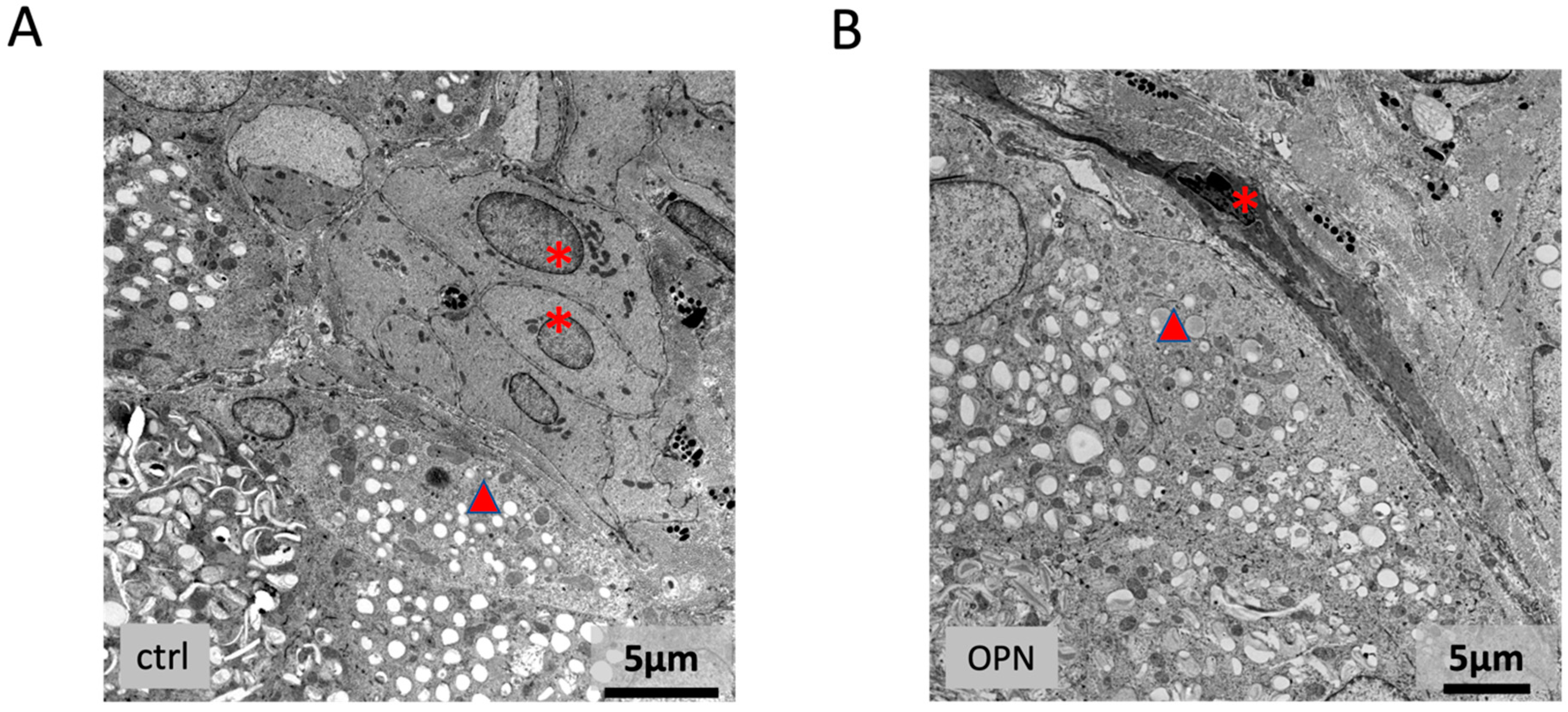
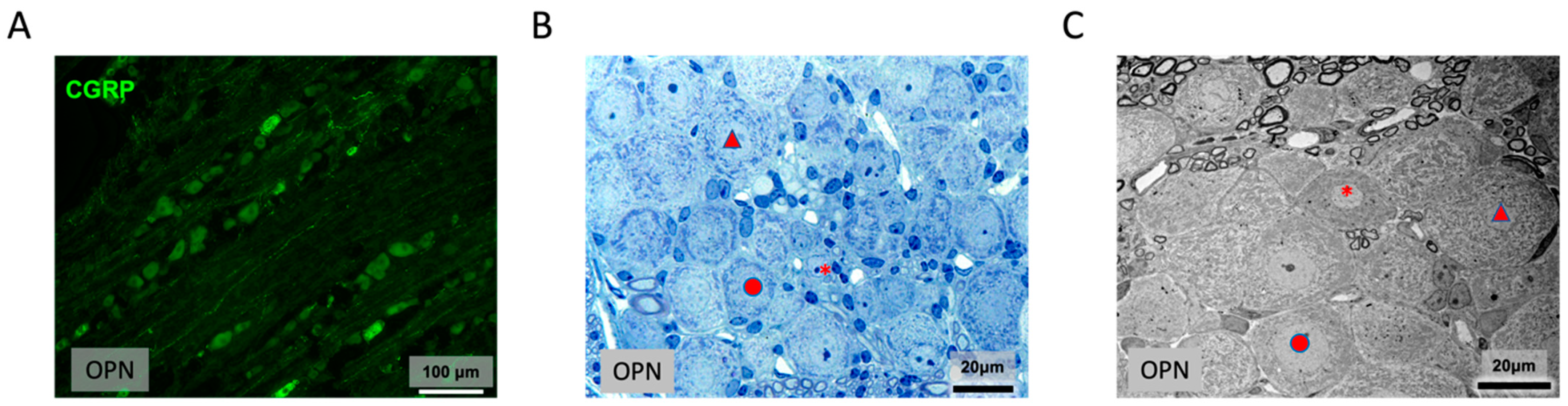
2.6. Ocular Surface Changes
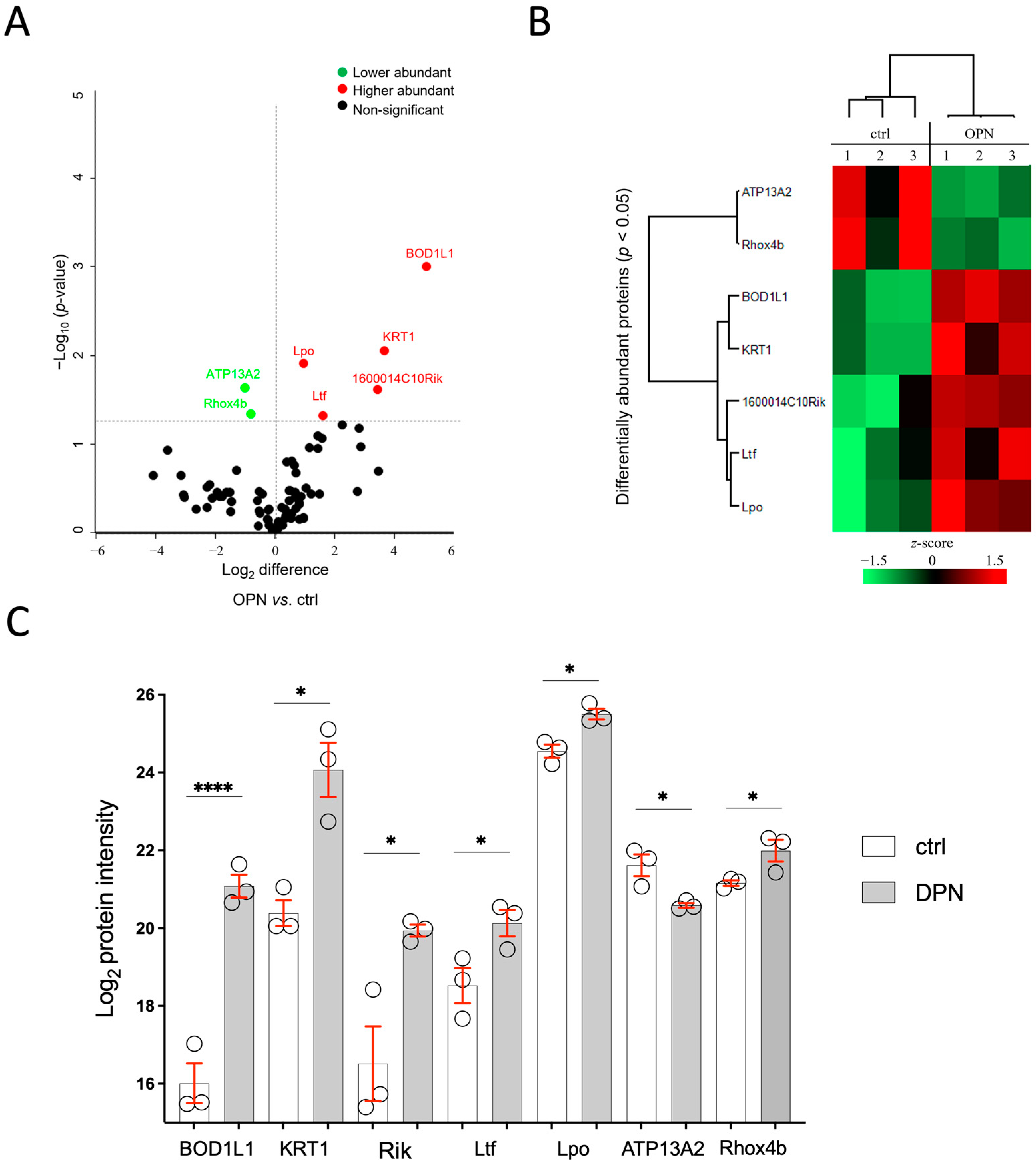
2.6.1. Tear Film Proteome
2.6.2. Immunohistochemistry
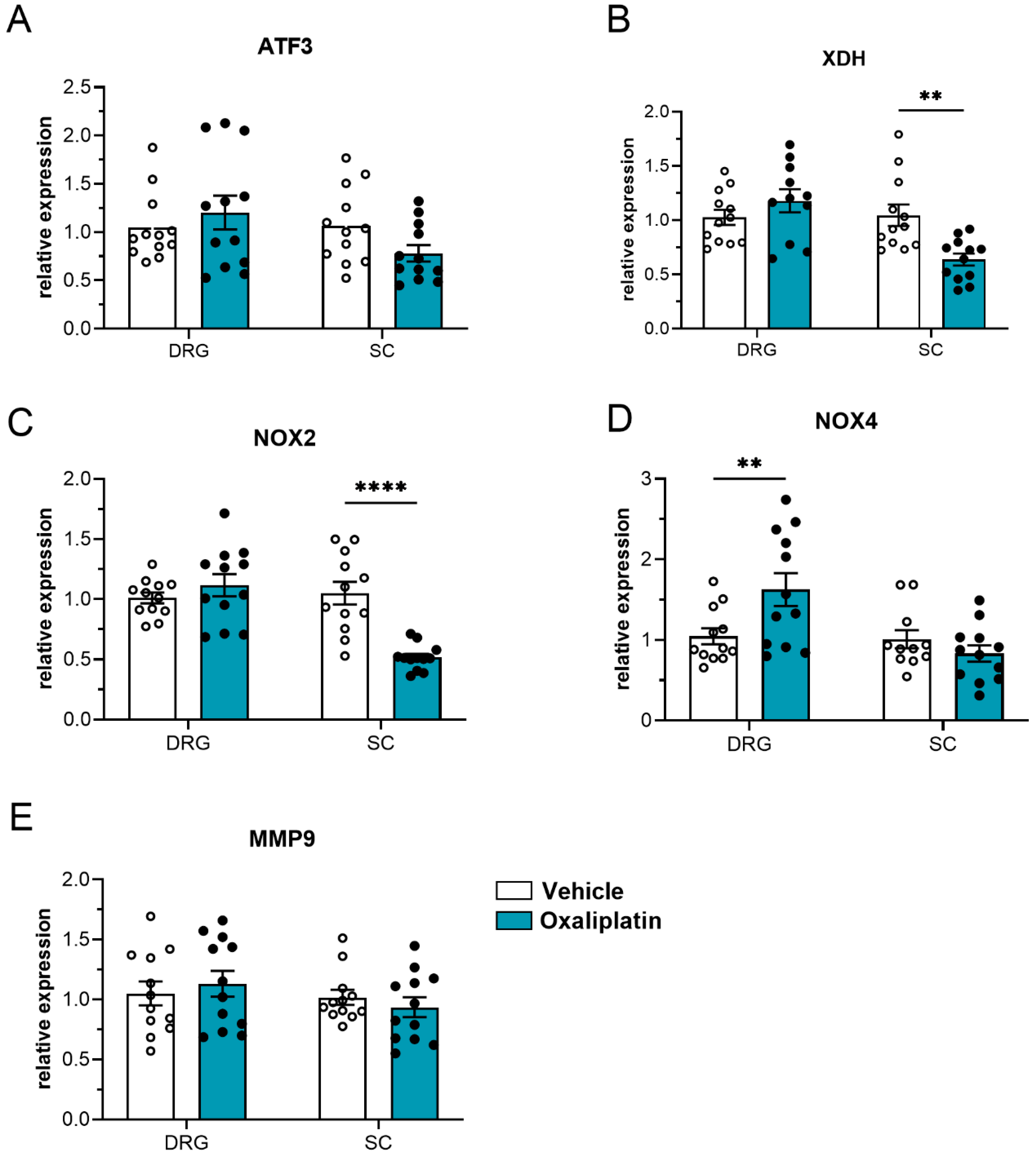
2.6.3. Gene Expression
2.7. Evaluation of Morphological Changes in the Tissues Involved in Tear Film Formation—Qualitative and Quantitative
2.7.1. Conjunctival Goblet Cells
2.7.2. Lacrimal Gland
2.7.3. Meibomian Gland
2.7.4. Cornea
2.7.5. Trigeminal Ganglion
3. Discussion
4. Materials and Methods
4.1. Oxaliplatin-Induced Neuropathic Pain Model
4.2. Behavioral Experiments
4.3. Multiplex Immunoassay
4.4. Murine Tear Sample Preparation and Protein Extraction
4.5. Liquid Chromatography–Electrospray Ionization–MS/MS (LC-ESI-MS/MS) Analysis
4.6. Label-Free Quantification (LFQ) and Statistical Analysis
4.7. Morphology
4.8. Quantitative Evaluation of Goblet Cells
4.9. Quantitative Analysis of Semithin and Ultrathin Sections
4.10. Gene Expression Analysis
4.11. TaqMan® Gene Expression Assay System
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comella, P.; Casaretti, R.; Sandomenico, C.; Avallone, A.; Franco, L. Role of oxaliplatin in the treatment of colorectal cancer. Ther. Clin. Risk Manag. 2009, 5, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Kyritsis, A.P.; Makatsoris, T.; Kalofonos, H.P. Chemotherapy-induced peripheral neuropathy in adults: A comprehensive update of the literature. Cancer Manag. Res. 2014, 6, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Park, S.B.; Krishnan, A.V.; Markoulli, M. Corneal nerve changes following treatment with neurotoxic anticancer drugs. Ocul. Surf. 2021, 21, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.F.; Misra, S.L.; Patel, D.V. In Vivo Confocal Microscopy of the Human Cornea in the Assessment of Peripheral Neuropathy and Systemic Diseases. Biomed. Res. Int. 2015, 2015, 951081. [Google Scholar] [CrossRef] [PubMed]
- Tyler, E.F.; McGhee, C.N.J.; Lawrence, B.; Braatvedt, G.D.; Mankowski, J.L.; Oakley, J.D.; Sethi, S.; Misra, S.L. Corneal Nerve Changes Observed by In Vivo Confocal Microscopy in Patients Receiving Oxaliplatin for Colorectal Cancer: The COCO Study. J. Clin. Med. 2022, 11, 4770. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.K.; Smith, E.M.; Beutler, A.S.; Ta, L.E.; Lafky, J.M.; Wagner-Johnston, N.D.; Ruddy, K.J.; Dakhil, S.; et al. Clinical Course of Oxaliplatin-Induced Neuropathy: Results From the Randomized Phase III Trial N08CB (Alliance). J. Clin. Oncol. 2015, 33, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Kroigard, T.; Svendsen, T.K.; Wirenfeldt, M.; Schroder, H.D.; Qvortrup, C.; Pfeiffer, P.; Gaist, D.; Sindrup, S.H. Early changes in tests of peripheral nerve function during oxaliplatin treatment and their correlation with chemotherapy-induced polyneuropathy symptoms and signs. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2020, 27, 68–76. [Google Scholar] [CrossRef]
- Schicht, M.; Farger, J.; Wedel, S.; Sisignano, M.; Scholich, K.; Geisslinger, G.; Perumal, N.; Grus, F.H.; Singh, S.; Sahin, A.; et al. Ocular surface changes in mice with streptozotocin-induced diabetes and diabetic polyneuropathy. Ocul. Surf. 2024, 31, 43–55. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011, 232, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn-Gerhardt, W.; Schroder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef] [PubMed]
- Geis, C.; Geuss, E.; Sommer, C.; Schmidt, H.H.; Kleinschnitz, C. NOX4 is an early initiator of neuropathic pain. Exp. Neurol. 2017, 288, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-induced peripheral neuropathy: Clinical features, mechanisms, prevention and treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Egashira, N. Pathological Mechanisms and Preventive Strategies of Oxaliplatin-Induced Peripheral Neuropathy. Front. Pain Res. 2021, 2, 804260. [Google Scholar] [CrossRef] [PubMed]
- Meneray, M.A.; Bennett, D.J.; Nguyen, D.H.; Beuerman, R.W. Effect of sensory denervation on the structure and physiologic responsiveness of rabbit lacrimal gland. Cornea 1998, 17, 99–107. [Google Scholar] [CrossRef]
- Bromberg, B.B. Autonomic control of lacrimal protein secretion. Invest. Ophthalmol. Vis. Sci. 1981, 20, 110–116. [Google Scholar] [PubMed]
- Ding, C.; Walcott, B.; Keyser, K.T. Sympathetic neural control of the mouse lacrimal gland. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Control of mucin production by ocular surface epithelial cells. Exp. Eye Res. 2004, 78, 173–185. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benitez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.A.; Latremoliere, A.; Basbaum, A.I.; Mogil, J.S.; Porreca, F.; Rice, A.S.C.; Woolf, C.J.; Currie, G.L.; Dworkin, R.H.; Eisenach, J.C.; et al. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain 2016, 157, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Gees, M.; Harrison, S.; De Siena, G.; Materazzi, S.; Moretto, N.; Failli, P.; Preti, D.; Marchetti, N.; Cavazzini, A.; et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011, 152, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, K.; Mulder, G.B. The rotarod. Contemp. Top. Lab. Anim. Sci. 2003, 42, 49. [Google Scholar]
- Sisignano, M.; Angioni, C.; Ferreiros, N.; Schuh, C.D.; Suo, J.; Schreiber, Y.; Dawes, J.M.; Antunes-Martins, A.; Bennett, D.L.; McMahon, S.B.; et al. Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS ONE 2013, 8, e81228. [Google Scholar] [CrossRef]
- Wedel, S.; Mathoor, P.; Rauh, O.; Heymann, T.; Ciotu, C.I.; Fuhrmann, D.C.; Fischer, M.J.M.; Weigert, A.; de Bruin, N.; Hausch, F.; et al. SAFit2 reduces neuroinflammation and ameliorates nerve injury-induced neuropathic pain. J. Neuroinflammation 2022, 19, 254. [Google Scholar] [CrossRef]
- Manicam, C.; Perumal, N.; Pfeiffer, N.; Grus, F.; Gericke, A. First insight into the proteome landscape of the porcine short posterior ciliary arteries: Key signalling pathways maintaining physiologic functions. Sci. Rep. 2016, 6, 38298. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci. Rep. 2016, 6, 29629. [Google Scholar] [CrossRef]
- Perumal, N.; Manicam, C.; Steinicke, M.; Funke, S.; Pfeiffer, N.; Grus, F.H. Characterization of the human aqueous humour proteome: A comparison of the genders. PLoS ONE 2017, 12, e0172481. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Characterization of lacrimal proline-rich protein 4 (PRR4) in human tear proteome. Proteomics 2014, 14, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Funke, S.; Wolters, D.; Pfeiffer, N.; Grus, F.H. Characterization of human reflex tear proteome reveals high expression of lacrimal proline-rich protein 4 (PRR4). Proteomics 2015, 15, 3370–3381. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Herfurth, A.; Pfeiffer, N.; Manicam, C. Short-Term Omega-3 Supplementation Modulates Novel Neurovascular and Fatty Acid Metabolic Proteome Changes in the Retina and Ophthalmic Artery of Mice with Targeted Cyp2c44 Gene Deletion. Cells 2022, 11, 3494. [Google Scholar] [CrossRef] [PubMed]
- Manicam, C.; Perumal, N.; Wasielica-Poslednik, J.; Ngongkole, Y.C.; Tschabunin, A.; Sievers, M.; Lisch, W.; Pfeiffer, N.; Grus, F.H.; Gericke, A. Proteomics Unravels the Regulatory Mechanisms in Human Tears Following Acute Renouncement of Contact Lens Use: A Comparison between Hard and Soft Lenses. Sci. Rep. 2018, 8, 11526. [Google Scholar] [CrossRef]
- Perumal, N.; Strassburger, L.; Herzog, D.P.; Muller, M.B.; Pfeiffer, N.; Grus, F.H.; Manicam, C. Bioenergetic shift and actin cytoskeleton remodelling as acute vascular adaptive mechanisms to angiotensin II in murine retina and ophthalmic artery. Redox Biol. 2020, 34, 101597. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Strassburger, L.; Schmelter, C.; Gericke, A.; Pfeiffer, N.; Grus, F.H.; Manicam, C. Sample Preparation for Mass-spectrometry-based Proteomics Analysis of Ocular Microvessels. J. Vis. Exp. JoVE 2019, 144, e59140. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Luber, C.A.; Cox, J.; Lauterbach, H.; Fancke, B.; Selbach, M.; Tschopp, J.; Akira, S.; Wiegand, M.; Hochrein, H.; O’Keeffe, M.; et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 2010, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. MCP 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Karnovsky, M.J. Formaldehyde-Glutaraldehyde Fixatives Containing Trinitrocompounds. J. Cell Biol. 1968, 39, 168A–169A. [Google Scholar]
- Welss, J.; Punchago, N.; Feldt, J.; Paulsen, F. The distribution of conjunctival goblet cells in mice. Ann. Anat. 2021, 234, 151664. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



| Antibody | Manufacturer | ID | Kryo(K)/ Wholemount (W) | Dilution |
|---|---|---|---|---|
| Rabbit anti-alpha smooth muscle actin antibody | Abcam, Cambridge, UK | EPR5368 | K | 1:1000 |
| Rabbit anti-ATP13A2/PARK9 | Bioss, Woburn, MA, USA | bs-11708R | K | 1:50 |
| Rabbit anit-cytokeratin 1 | EnoGene, New York, NY, USA | E301319 | K | 1:100 |
| Rabbit anti FAM44A/Bod1l | ThermoFisher Scientific, Rockford, IL, USA | PA559504 | K | 1:100 |
| Rabbit anti-lactoferrin | Invitrogen, ThermoFisher Scientific, Rockford, IL, USA | PA5-95513 | K | 1:300 |
| Rabbit anti lactoperoxidase (LPO) | Abcam, Cambridge, UK | PAA296Mu01 | K | 1:100 |
| Rabbit anti-CGRP | Biotrend, Köln, Germany | ZO5177 | W | 1:500 |
| Mouse antib-3-tubulin | R&D, Minneapolis, MN, USA | MAB1196 | W | 1:100 |
| Gene | Name | Sequence (5′ → 3′) | Product (bp) |
|---|---|---|---|
| Atp13a2 | ms ATP13A2_real_fw | GGC CCT CTA CAG CCT GAC T | 65 |
| ms ATP13A2_real_rev | CCA GGT TGG TGT TGA TTG TG | ||
| Bod1l | ms BOD1L_real_fw | GAG CCA CAA GAT CAG C | 72 |
| ms BOD1L_real_rev | TTG CAC GTG TAG ATG GCT TG | ||
| Krt1 | ms KRT1_real_fw | TTT GCC TCC TTC ATC GAC A | 108 |
| ms KRT1_real_rev | GTT TTG GGT CCG GGT TGT | ||
| LPO | ms LPO_real_fw | CCC CTG CAC ACT GTC TTT TT | 75 |
| ms LPO_real_rev | CCC CTG CAC ACT GTC TTT TT | ||
| Ltf | ms LTF_real_fw | TGG GAA AGG AGT ACG TCA TAG C | 82 |
| ms LTF_real_rev | GTA AGA AAA GCG CAG GCT TC | ||
| 18S | 18S human/mouse real time/SYBR_fw | GGT GCA TGG CCG TTC TTA | 69 |
| 18S human/mouse real time/SYBR_rev | TGC CAG AGT CTC GTT CGT TA | ||
| β-Aktin | β-Aktin_fw | GAT CCT CAC CGA GCG CGG CTA CA | 296 |
| β-Aktin_rev | CGC GAT GTC CAC GTC ACA CTT CA |
| Target | Gene | Article Number | Company |
|---|---|---|---|
| ATF3 | Activating transcription factor 3 | Mm00476033_m1 | Thermo Fisher |
| GAPDH | Glceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 | Thermo Fisher |
| MMP9 | Matrix metallopeptidase 9 | Mm00442991_m1 | Thermo Fisher |
| NOX2 | NADPH oxidase 2 | Mm01287743_m1 | Thermo Fisher |
| NOX4 | NADPH oxidase 4 | Mm00479246_m1 | Thermo Fisher |
| XDH | Xanthine dehydrogenase | Mm00442110_m1 | Thermo Fisher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schicht, M.; Sisignano, M.; Farger, J.; Wedel, S.; Phunchago, N.; Perumal, N.; Manicam, C.; Scholich, K.; Geisslinger, G.; Paulsen, F.; et al. Ocular Surface Changes Differ Significantly Between Oxaliplatin- and Diabetes-Induced Polyneuropathy. Int. J. Mol. Sci. 2025, 26, 1884. https://doi.org/10.3390/ijms26051884
Schicht M, Sisignano M, Farger J, Wedel S, Phunchago N, Perumal N, Manicam C, Scholich K, Geisslinger G, Paulsen F, et al. Ocular Surface Changes Differ Significantly Between Oxaliplatin- and Diabetes-Induced Polyneuropathy. International Journal of Molecular Sciences. 2025; 26(5):1884. https://doi.org/10.3390/ijms26051884
Chicago/Turabian StyleSchicht, Martin, Marco Sisignano, Jessica Farger, Saskia Wedel, Nichapa Phunchago, Natarajan Perumal, Caroline Manicam, Klaus Scholich, Gerd Geisslinger, Friedrich Paulsen, and et al. 2025. "Ocular Surface Changes Differ Significantly Between Oxaliplatin- and Diabetes-Induced Polyneuropathy" International Journal of Molecular Sciences 26, no. 5: 1884. https://doi.org/10.3390/ijms26051884
APA StyleSchicht, M., Sisignano, M., Farger, J., Wedel, S., Phunchago, N., Perumal, N., Manicam, C., Scholich, K., Geisslinger, G., Paulsen, F., & Lütjen-Drecoll, E. (2025). Ocular Surface Changes Differ Significantly Between Oxaliplatin- and Diabetes-Induced Polyneuropathy. International Journal of Molecular Sciences, 26(5), 1884. https://doi.org/10.3390/ijms26051884







