MGST1 Protects Pancreatic Ductal Cells from Inflammatory Damage in Acute Pancreatitis by Inhibiting Ferroptosis: Bioinformatics Analysis with Experimental Validation
Abstract
1. Introduction
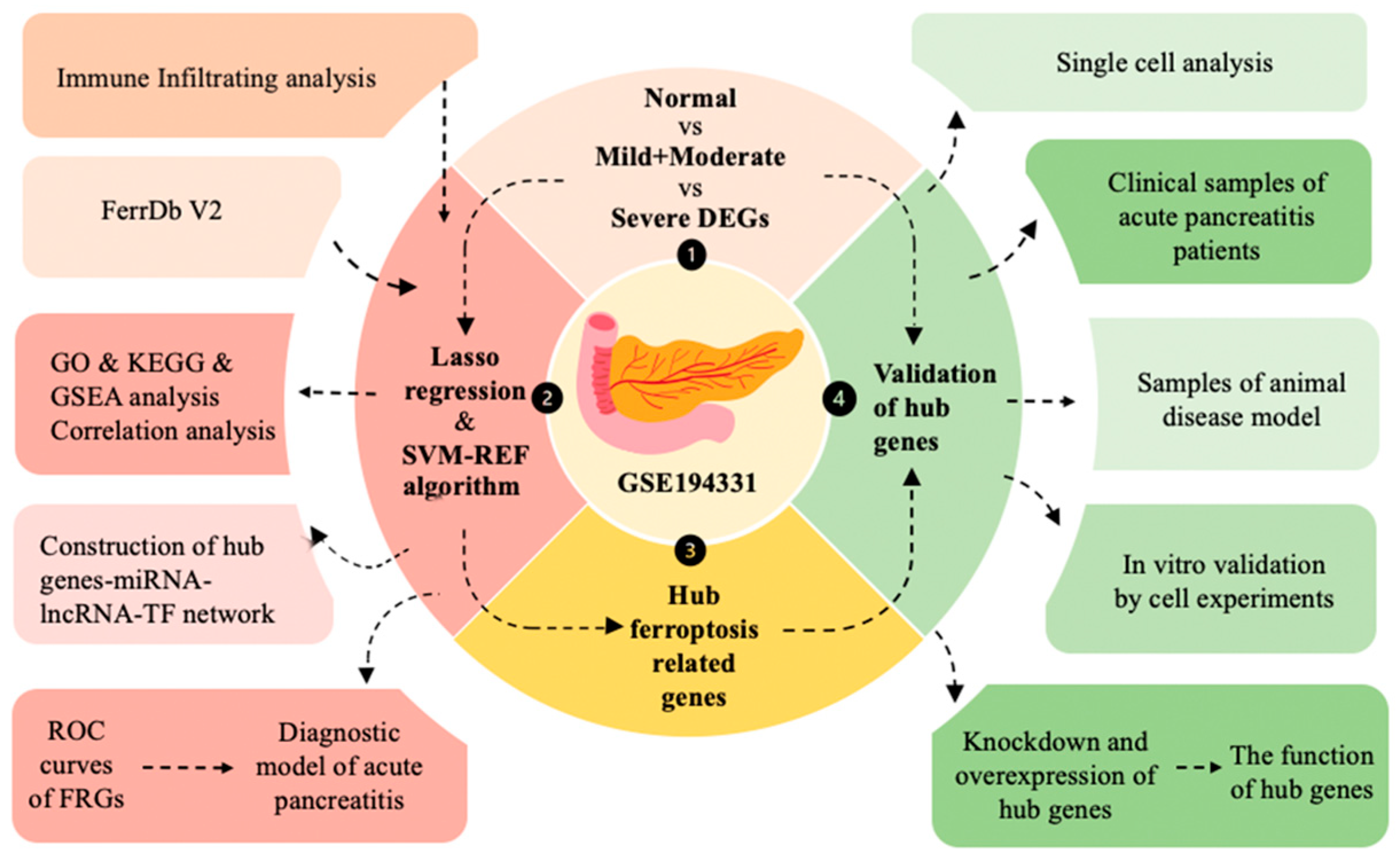
2. Results
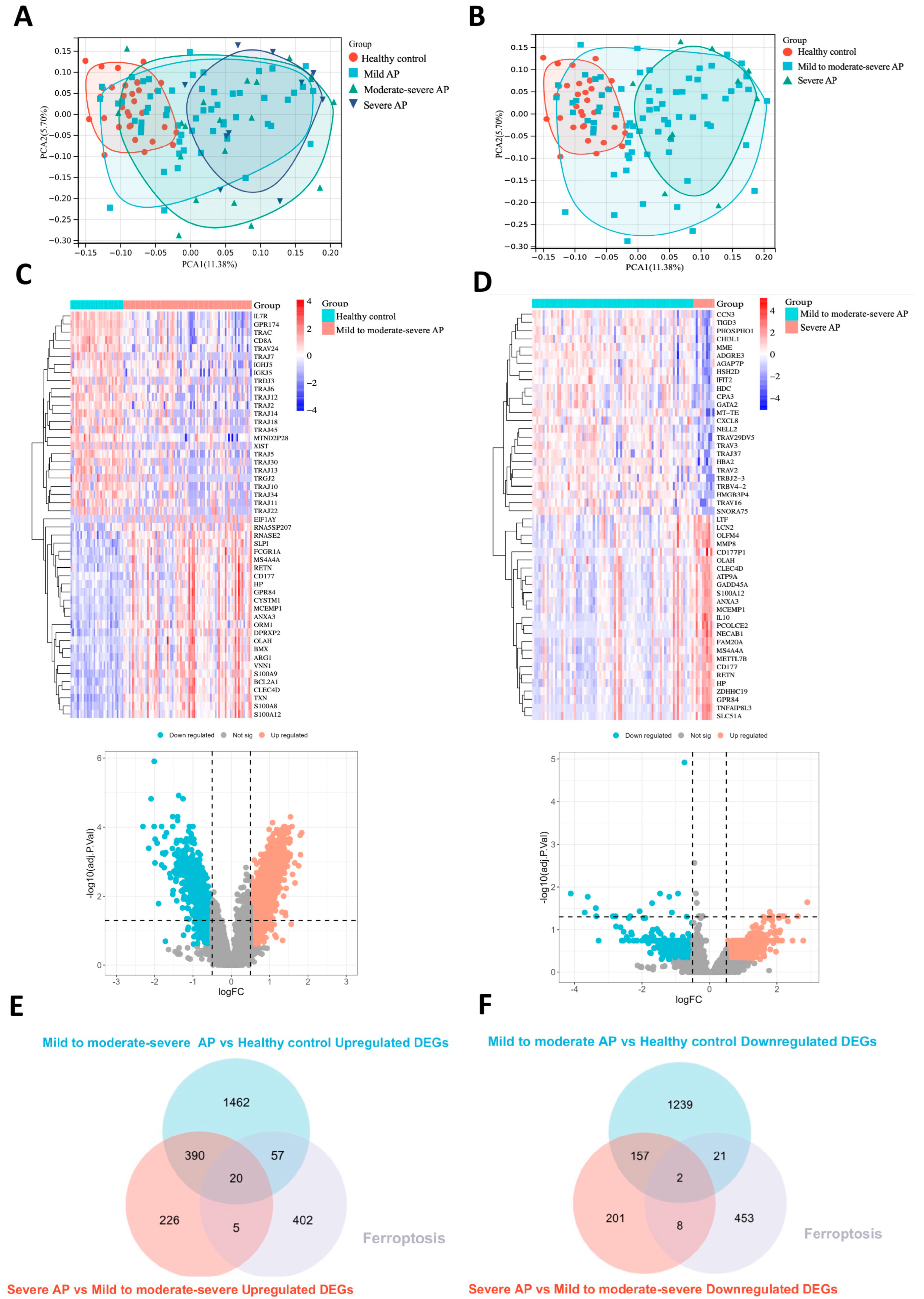
2.1. Identification of DEGs and Ferroptosis-Related Driving Genes in Acute Pancreatitis
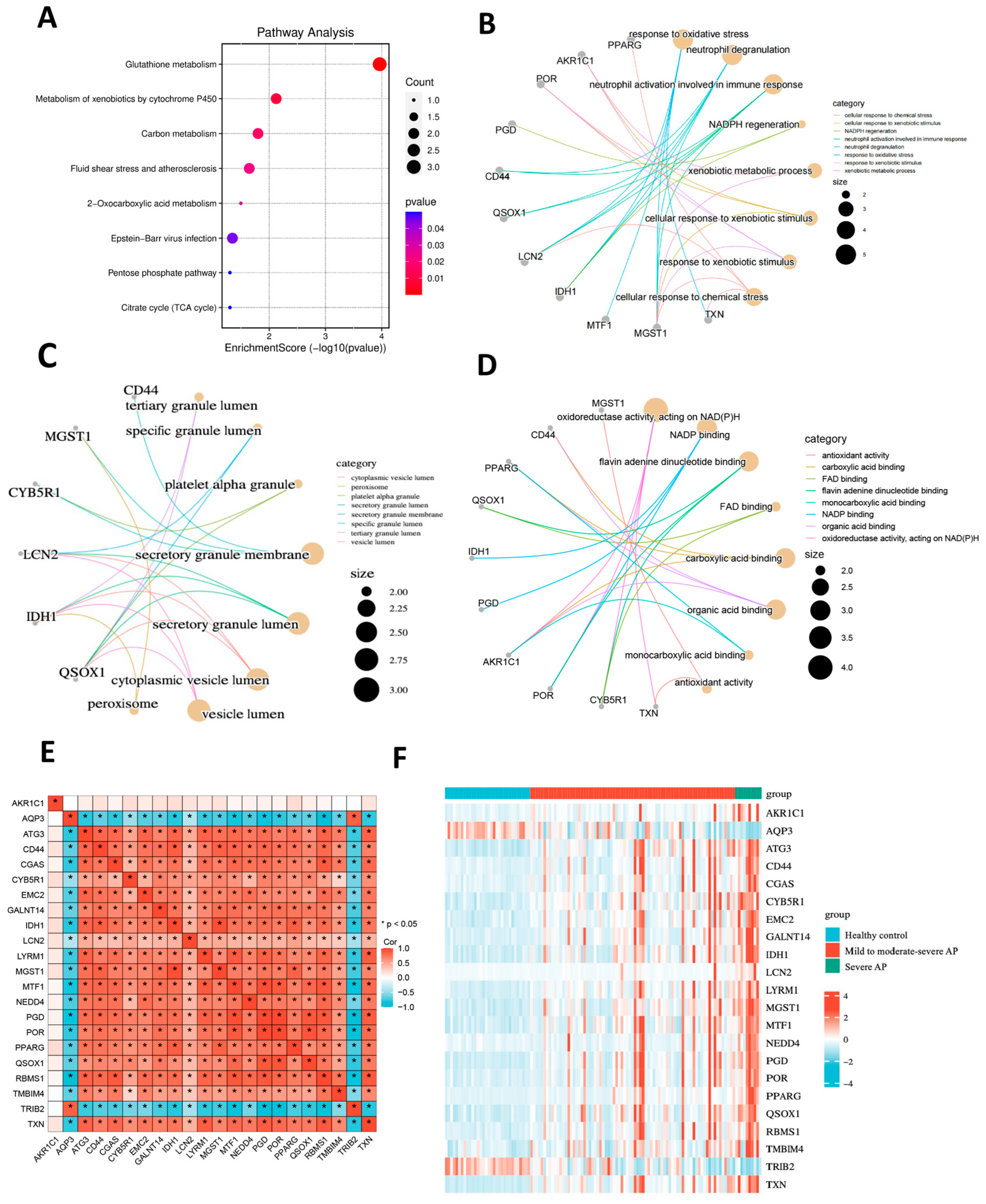
2.2. Enrichment Analysis of Ferroptosis-Related Driving Genes and Correlation Analysis
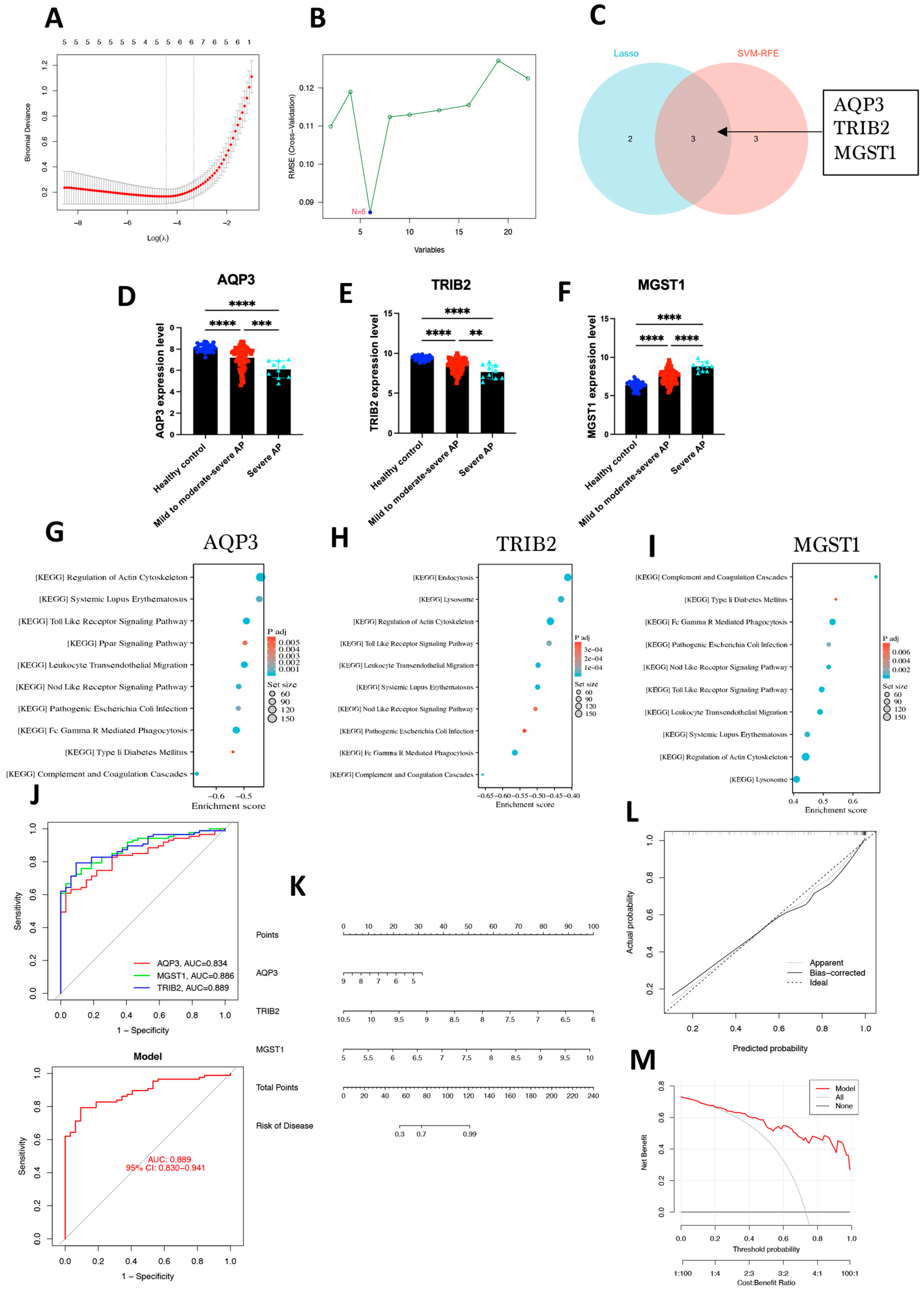
2.3. Identification of Hub Genes Using Machine Learning Methods and Construction of a Diagnostic Model
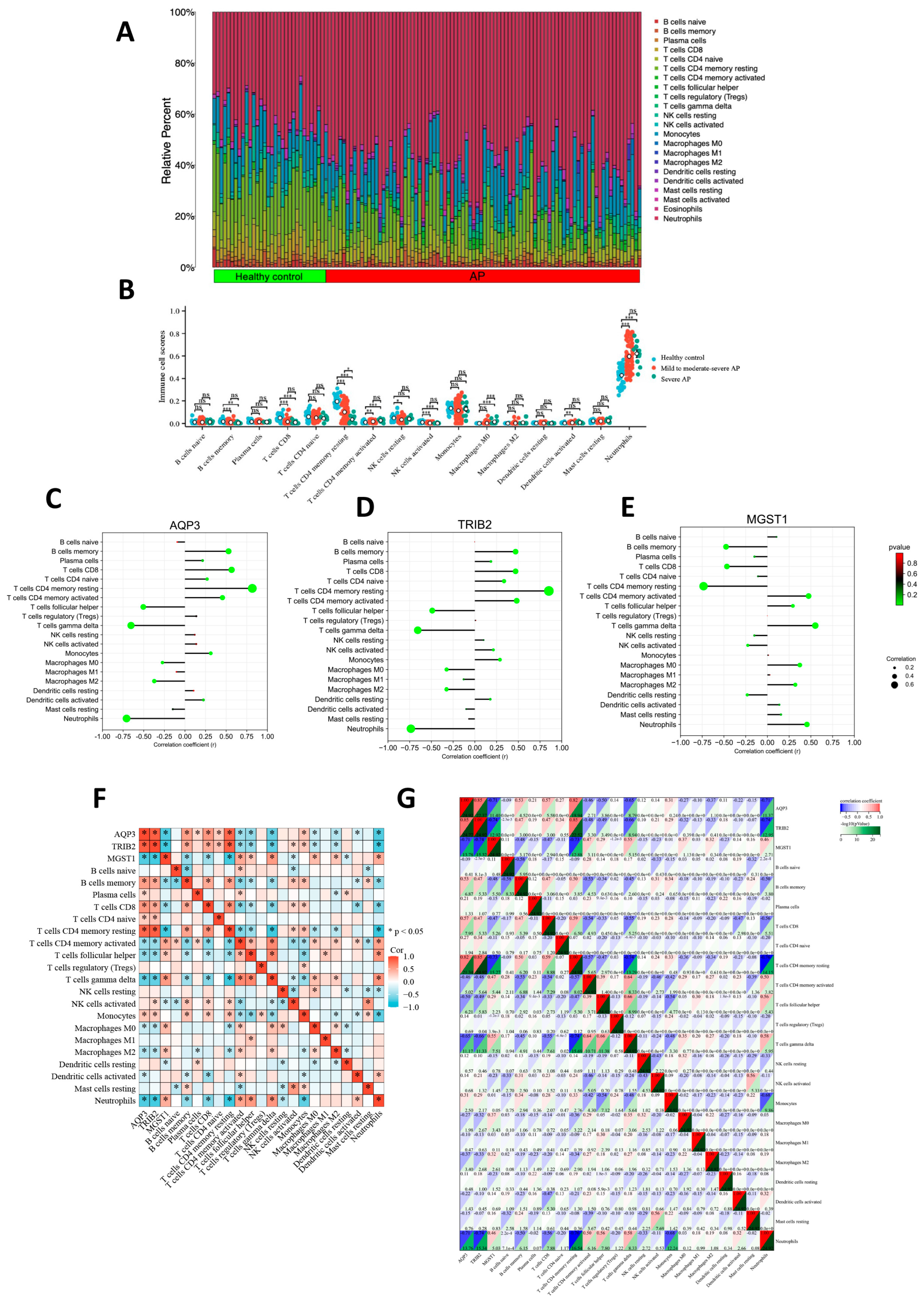
2.4. Association Between Hub Genes and Immune Cell Infiltration
2.5. miRNA, lincRNA, and TF Networks of Hub Genes
2.6. Potential Roles of the Three Hub Genes in Pancreatic Tissue Inferred Through Single-Cell Analysis
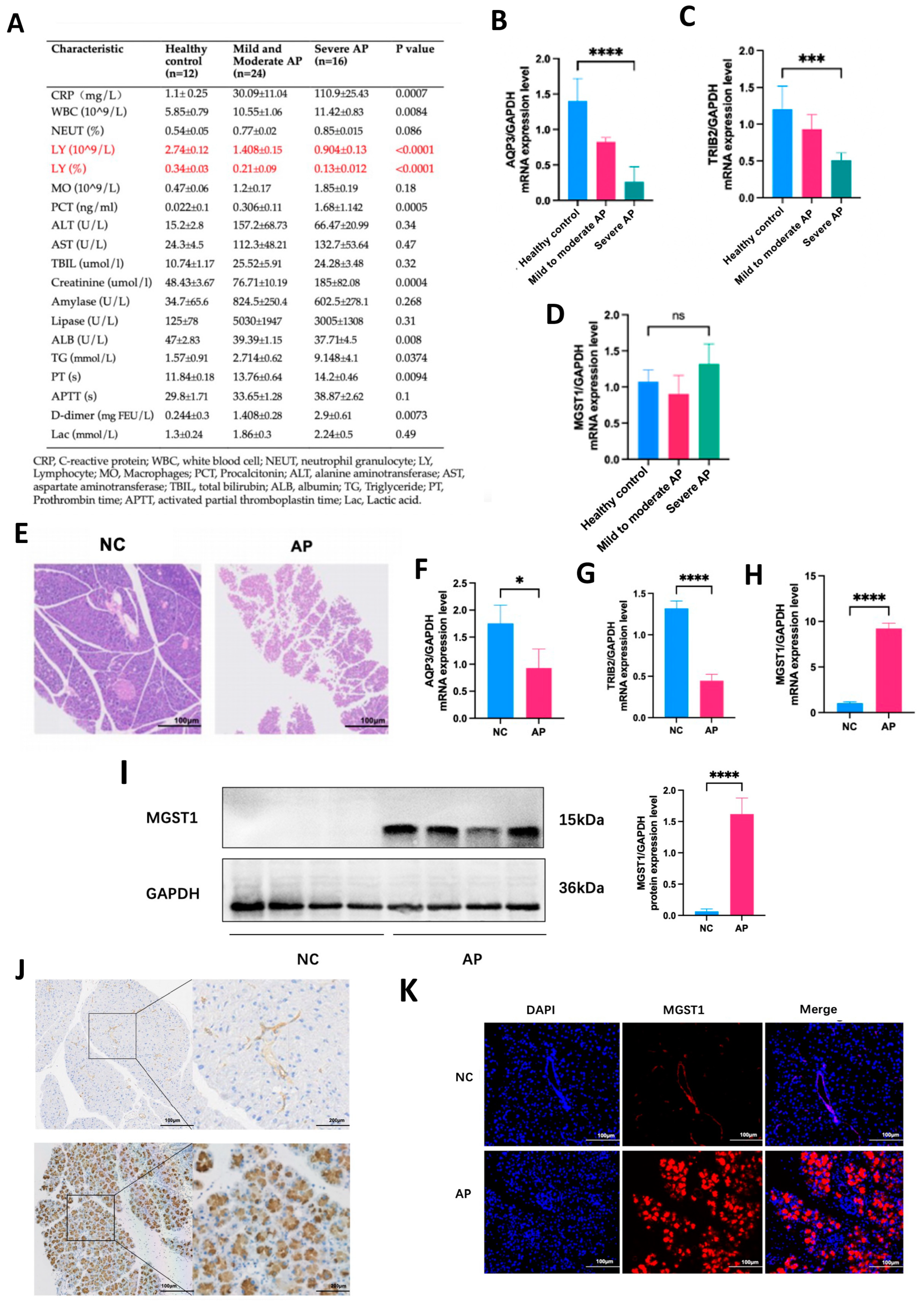
2.7. Validation of Hub Genes Through Clinical Samples and Animal Models
2.8. MGST1 Is Upregulated During Ferroptosis in Human Pancreatic Duct Cells
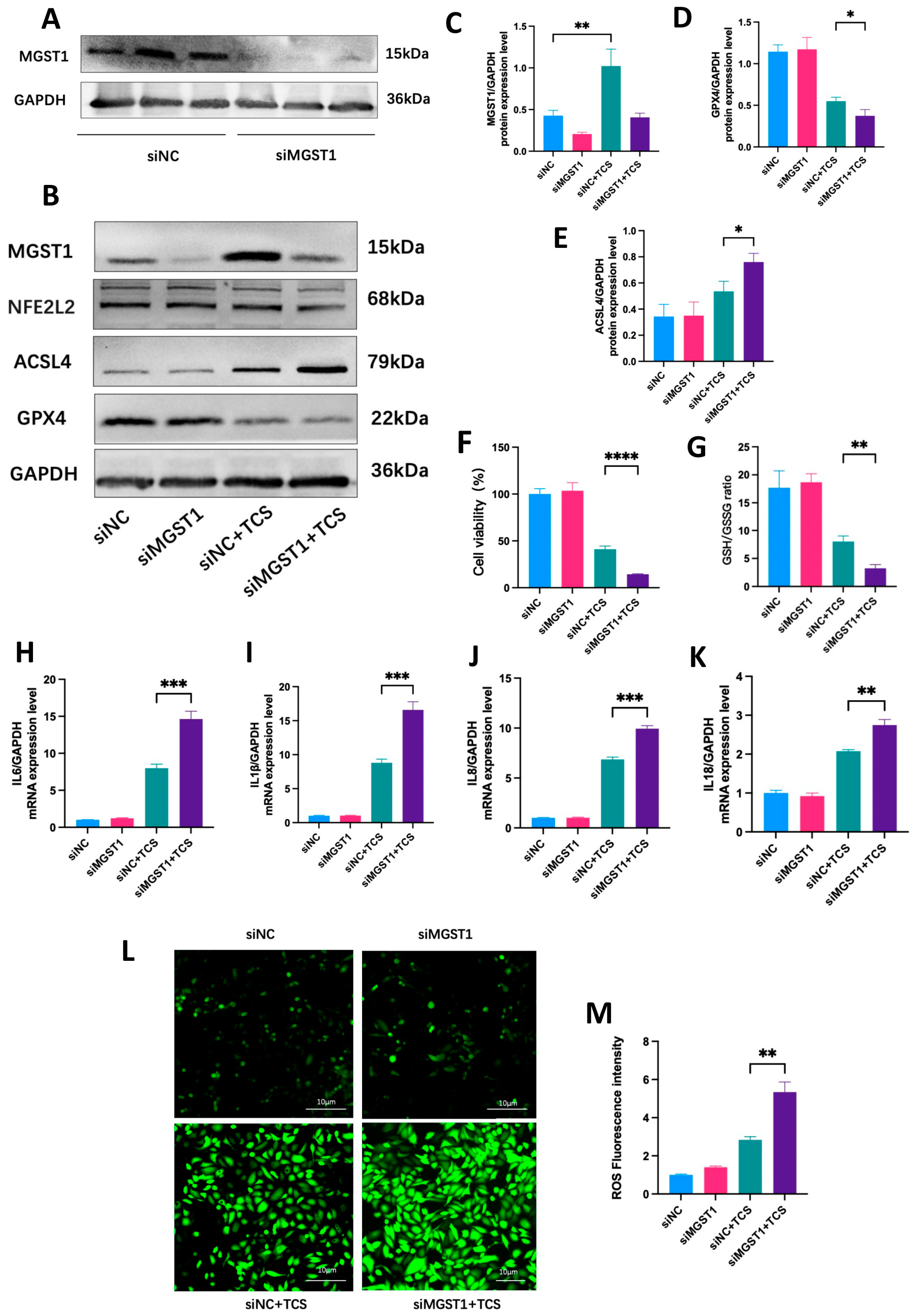
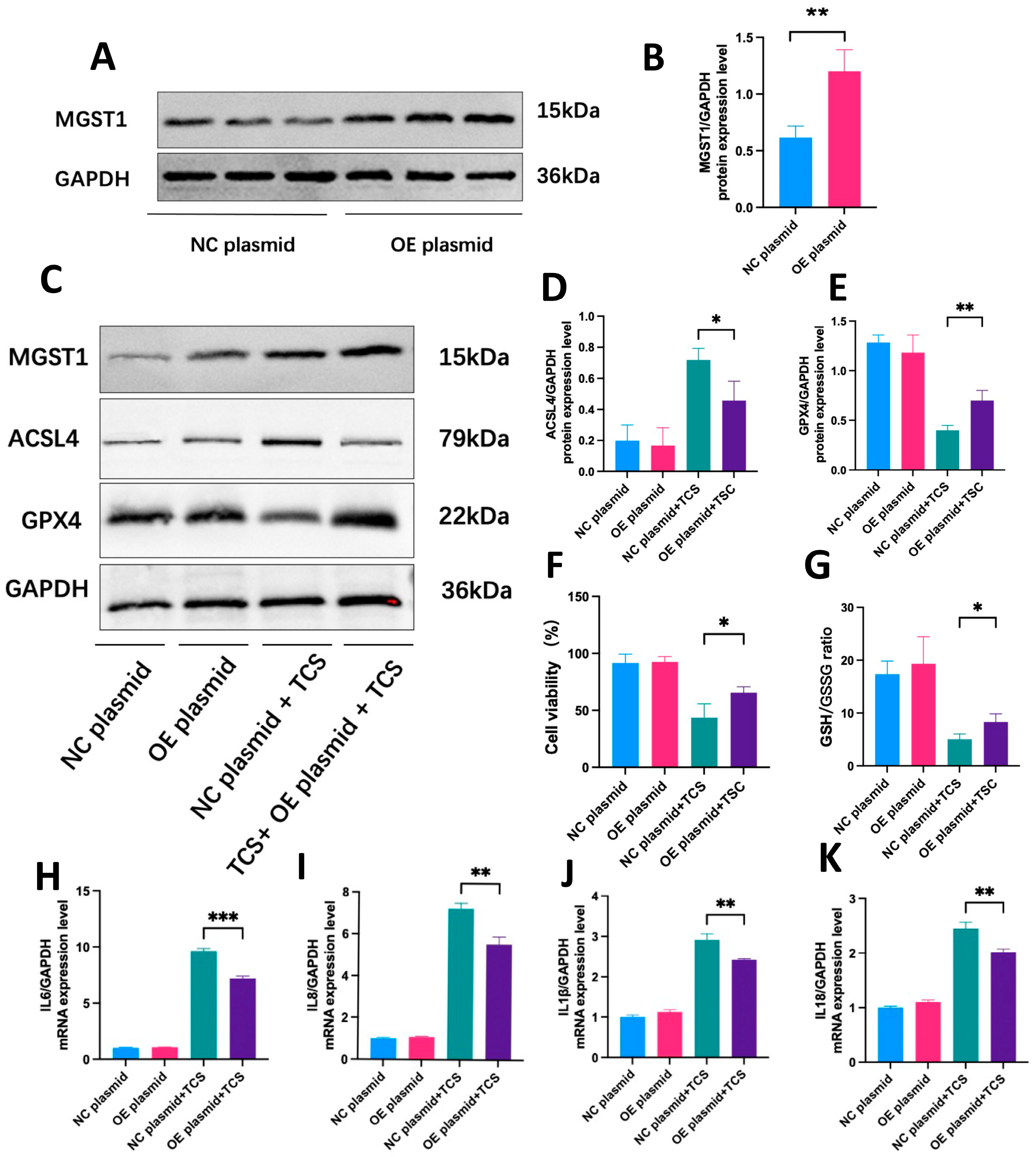
2.9. MGST1 Negatively Regulates Ferroptosis and Protects Human Pancreatic Ductal Cells from Inflammatory Damage
3. Discussion
4. Materials and Methods
4.1. Data Source and Analysis of Differential Expression Genes (DEGs)
4.2. Analysis of Functional Enrichment
4.3. Identification of Hub Genes
4.4. ROC Curve Analysis and Diagnostic Model Construction
4.5. Gene Set Enrichment Analysis (GSEA)
4.6. Analysis of Immune Cell Infiltration and Its Correlation with Hub Genes
4.7. Construction of TF and miRNA and lncRNA Hub Gene Regulatory Networks
4.8. Single-Cell Analysis
4.9. Animals Models
4.10. Peripheral Blood Mononuclear Cell (PBMC) Collection
4.11. RT-qPCR and Western Blotting (WB)
4.12. Immunohistochemistry (IHC)
4.13. Immunofluorescence (IF)
4.14. Cell Culture and Treatment
4.15. Cell Transfection
4.16. Enzyme-Linked Immunosorbent Assay (ELISA)
4.17. CCK-8, GSH/GSSG Ratio, and ROS Assays
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.-A.; et al. Global Incidence of Acute Pancreatitis Is Increasing over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New Insights into Acute Pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, N.; Xu, H.; Li, Y.; Sun, T.; Jiang, X.; Fu, D.; You, T.; Diao, S.; Huang, Y.; et al. Caveolin-1 Ameliorates Hepatic Injury in Non-Alcoholic Fatty Liver Disease by Inhibiting Ferroptosis via the NOX4/ROS/GPX4 Pathway. Biochem. Pharmacol. 2024, 230, 116594. [Google Scholar] [CrossRef]
- Dong, C.; Huoshen, W.; Bai, Y.; Liu, J.; Li, B.; Guan, Y.; Luo, P. Uncovering the Molecular Networks of Ferroptosis in the Pathogenesis of Type 2 Diabetes and Its Complications: A Multi-Omics Investigation. Mol. Med. 2024, 30, 268. [Google Scholar] [CrossRef]
- Yan, A.; Li, Z.; Gao, Y.; Hu, F.; Han, S.; Liu, F.; Liu, Z.; Chen, J.; Yuan, C.; Zhou, C. Isobicyclogermacrenal Ameliorates Hippocampal Ferroptosis Involvement in Neurochemical Disruptions and Neuroinflammation Induced by Sleep Deprivation in Rats. Phytomedicine 2025, 136, 156306. [Google Scholar] [CrossRef]
- Ling, Y.; Yang, Y.; Ren, N.; Xu, H.; Cheng, C.; Lu, D.; Wang, Q.; Yao, X.; Ma, W. Jinwu Jiangu Capsule Attenuates Rheumatoid Arthritis via the SLC7A11/GSH/GPX4 Pathway in M1 Macrophages. Phytomedicine 2024, 135, 156232. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in Infection, Inflammation, and Immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Zou, B.; Li, C.; Zeh, H.J.; Kang, R.; Kroemer, G.; Huang, J.; Tang, D. Trypsin-Mediated Sensitization to Ferroptosis Increases the Severity of Pancreatitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ye, F.; Liu, J.; Klionsky, D.J.; Tang, D.; Kang, R. Extracellular SQSTM1 Exacerbates Acute Pancreatitis by Activating Autophagy-Dependent Ferroptosis. Autophagy 2023, 19, 1733–1744. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, T.; Li, J.; Yu, P.; Mei, Y.; Li, M.; Qi, X.; Liu, F. Serum Iron Fluctuations Link Ferroptosis Process with Mortality and Prognosis of Acute Pancreatitis. iScience 2023, 26, 107774. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of Acute Pancreatitis--2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute Pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Sendler, M.; van den Brandt, C.; Glaubitz, J.; Wilden, A.; Golchert, J.; Weiss, F.U.; Homuth, G.; De Freitas Chama, L.L.; Mishra, N.; Mahajan, U.M.; et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice with Acute Pancreatitis. Gastroenterology 2020, 158, 253–269.e14. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-Dependent T Cell Migration Requires Aquaporin-3-Mediated Hydrogen Peroxide Uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Tanaka, M.; Verkman, A.S.; Yasui, M. Inhibition of Aquaporin-3 in Macrophages by a Monoclonal Antibody as Potential Therapy for Liver Injury. Nat. Commun. 2020, 11, 5666. [Google Scholar] [CrossRef]
- da Silva, I.V.; Cardoso, C.; Martínez-Banaclocha, H.; Casini, A.; Pelegrín, P.; Soveral, G. Aquaporin-3 Is Involved in NLRP3-Inflammasome Activation Contributing to the Setting of Inflammatory Response. Cell. Mol. Life Sci. CMLS 2021, 78, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-G.; Hwang, S.-Y.; Park, J.-I.; Yoon, S.; Bae, H.-R.; Kwak, J.-Y. Role of Aquaporin 3 in Development, Subtypes and Activation of Dendritic Cells. Mol. Immunol. 2011, 49, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.L.; O’Connor, C.; Veiga, J.P.; McCarthy, T.V.; Keeshan, K. TRIB2 Regulates Normal and Stress-Induced Thymocyte Proliferation. Cell Discov. 2016, 2, 15050. [Google Scholar] [CrossRef] [PubMed]
- Salomé, M.; Magee, A.; Yalla, K.; Chaudhury, S.; Sarrou, E.; Carmody, R.J.; Keeshan, K. A Trib2-p38 axis controls myeloid leukaemia cell cycle and stress response signalling. Cell Death Dis. 2018, 9, 443. [Google Scholar] [CrossRef]
- Liang, K.L.; Paredes, R.; Carmody, R.; Eyers, P.A.; Meyer, S.; McCarthy, T.V.; Keeshan, K. Human TRIB2 Oscillates during the Cell Cycle and Promotes Ubiquitination and Degradation of CDC25C. Int. J. Mol. Sci. 2016, 17, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.-W.; Morgenstern, R.; Townsend, D.M.; Tew, K.D. Microsomal Glutathione Transferase 1 in Cancer and the Regulation of Ferroptosis. Adv. Cancer Res. 2023, 160, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.; Liu, J.; Xie, Y.; Tang, D.; Kang, R. MGST1 Is a Redox-Sensitive Repressor of Ferroptosis in Pancreatic Cancer Cells. Cell Chem. Biol. 2021, 28, 765–775.e5. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, L.; Zhang, J.; Dreij, K.; Spahiu, L.; Holmgren, A.; Abe, H.; Tew, K.D.; Townsend, D.M.; Kelner, M.J.; Morgenstern, R.; et al. MGST1, a GSH Transferase/Peroxidase Essential for Development and Hematopoietic Stem Cell Differentiation. Redox Biol. 2018, 17, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Rustgi, A.K. Pancreatic Ductal Cells in Development, Regeneration, and Neoplasia. J. Clin. Investig. 2011, 121, 4572–4578. [Google Scholar] [CrossRef]
- Pallagi, P.; Balla, Z.; Singh, A.K.; Dósa, S.; Iványi, B.; Kukor, Z.; Tóth, A.; Riederer, B.; Liu, Y.; Engelhardt, R.; et al. The Role of Pancreatic Ductal Secretion in Protection against Acute Pancreatitis in Mice. Crit. Care Med. 2014, 42, e177–e188. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Guo, F.; Tao, X.; Zhou, Q.; Xia, S.; Deng, D.; Li, L.; Shang, D. Pancreatic Ductal Deletion of S100A9 Alleviates Acute Pancreatitis by Targeting VNN1-Mediated ROS Release to Inhibit NLRP3 Activation. Theranostics 2021, 11, 4467–4482. [Google Scholar] [CrossRef]
- Molnár, R.; Madácsy, T.; Varga, Á.; Németh, M.; Katona, X.; Görög, M.; Molnár, B.; Fanczal, J.; Rakonczay, Z.; Hegyi, P.; et al. Mouse Pancreatic Ductal Organoid Culture as a Relevant Model to Study Exocrine Pancreatic Ion Secretion. Lab. Investig. J. Tech. Methods Pathol. 2020, 100, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, P.; Hegyi, P.; Rakonczay, Z. The Physiology and Pathophysiology of Pancreatic Ductal Secretion: The Background for Clinicians. Pancreas 2015, 44, 1211–1233. [Google Scholar] [CrossRef]
- Dodson, M.; Anandhan, A.; Zhang, D.D. MGST1, a New Soldier of NRF2 in the Battle against Ferroptotic Death. Cell Chem. Biol. 2021, 28, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Murayama, N.; Kunori, M.; Yamazaki, H. Characterization of Microsomal Glutathione S-Transferases MGST1, MGST2, and MGST3 in Cynomolgus Macaque. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 1621–1625. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, X.; Zhang, C.; Hu, A.; Li, Y. MGST1 Expression Is Associated with Poor Prognosis, Enhancing the Wnt/β-Catenin Pathway via Regulating AKT and Inhibiting Ferroptosis in Gastric Cancer. ACS Omega 2023, 8, 23683–23694. [Google Scholar] [CrossRef] [PubMed]
- Kelner, M.J.; Diccianni, M.B.; Yu, A.L.; Rutherford, M.R.; Estes, L.A.; Morgenstern, R. Absence of MGST1 mRNA and Protein Expression in Human Neuroblastoma Cell Lines and Primary Tissue. Free Radic. Biol. Med. 2014, 69, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Cieszkowski, J.; Warzecha, Z.; Dembiński, A. [Experimental Models of Acute Pancreatitis]. Postepy Hig. Med. Dosw. 2015, 69, 264–269. [Google Scholar] [CrossRef]
- Serra, M.B.; Koike, M.K.; Barbeiro, D.F.; Machado, M.C.C.; de Souza, H.P. Sodium Taurocholate Induced Severe Acute Pancreatitis in C57BL/6 Mice. J. Vis. Exp. JoVE 2021, 172, e61547. [Google Scholar] [CrossRef]


| Datasets | Data Source | Species | Sampling Site | Sample Grouping | Sequencing Type | Sequencing Platform | Dataset Retrieval Date |
|---|---|---|---|---|---|---|---|
| GSE194331 | GEO | Homo | Peripheral blood | Healthy control = 32, Mild AP = 57, Moderately Severe AP = 20, Severe AP = 10 | RNA-seq | GPL16791 | 2024-1-1 |
| GSE188819 | GEO | Mouse | pancreatic tissue | Control = 2, AP = 2 | Single-cell-seq | GPL19057 | 2024-1-1 |
| 484 Ferroptosis-related genes | FerrDb V2 | Homo | 2024-1-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Ling, X.; Guo, X.; Ding, Z. MGST1 Protects Pancreatic Ductal Cells from Inflammatory Damage in Acute Pancreatitis by Inhibiting Ferroptosis: Bioinformatics Analysis with Experimental Validation. Int. J. Mol. Sci. 2025, 26, 1899. https://doi.org/10.3390/ijms26051899
Zhang R, Ling X, Guo X, Ding Z. MGST1 Protects Pancreatic Ductal Cells from Inflammatory Damage in Acute Pancreatitis by Inhibiting Ferroptosis: Bioinformatics Analysis with Experimental Validation. International Journal of Molecular Sciences. 2025; 26(5):1899. https://doi.org/10.3390/ijms26051899
Chicago/Turabian StyleZhang, Ruoyi, Xin Ling, Xianwen Guo, and Zhen Ding. 2025. "MGST1 Protects Pancreatic Ductal Cells from Inflammatory Damage in Acute Pancreatitis by Inhibiting Ferroptosis: Bioinformatics Analysis with Experimental Validation" International Journal of Molecular Sciences 26, no. 5: 1899. https://doi.org/10.3390/ijms26051899
APA StyleZhang, R., Ling, X., Guo, X., & Ding, Z. (2025). MGST1 Protects Pancreatic Ductal Cells from Inflammatory Damage in Acute Pancreatitis by Inhibiting Ferroptosis: Bioinformatics Analysis with Experimental Validation. International Journal of Molecular Sciences, 26(5), 1899. https://doi.org/10.3390/ijms26051899






