Potential Chondroprotective Effect of Artemisia annua L. Water Extract on SW1353 Cell
Abstract
1. Introduction
2. Results
2.1. Antioxidant Activity
2.2. Effect of AWE on the Activation of ROS in IL-1β Induced SW1353 Cell
2.3. Effect of AWE on the Activation of Inflammation in IL-1β-Induced SW1353 Cell
2.4. Effect of AWE on the Activation of MAPKs and NF-κB in IL-1β-Induced SW1353 Cell
2.5. Effect of AWE on the Activation of Collagen II and MMPs in IL-1β-Induced SW1353 Cell
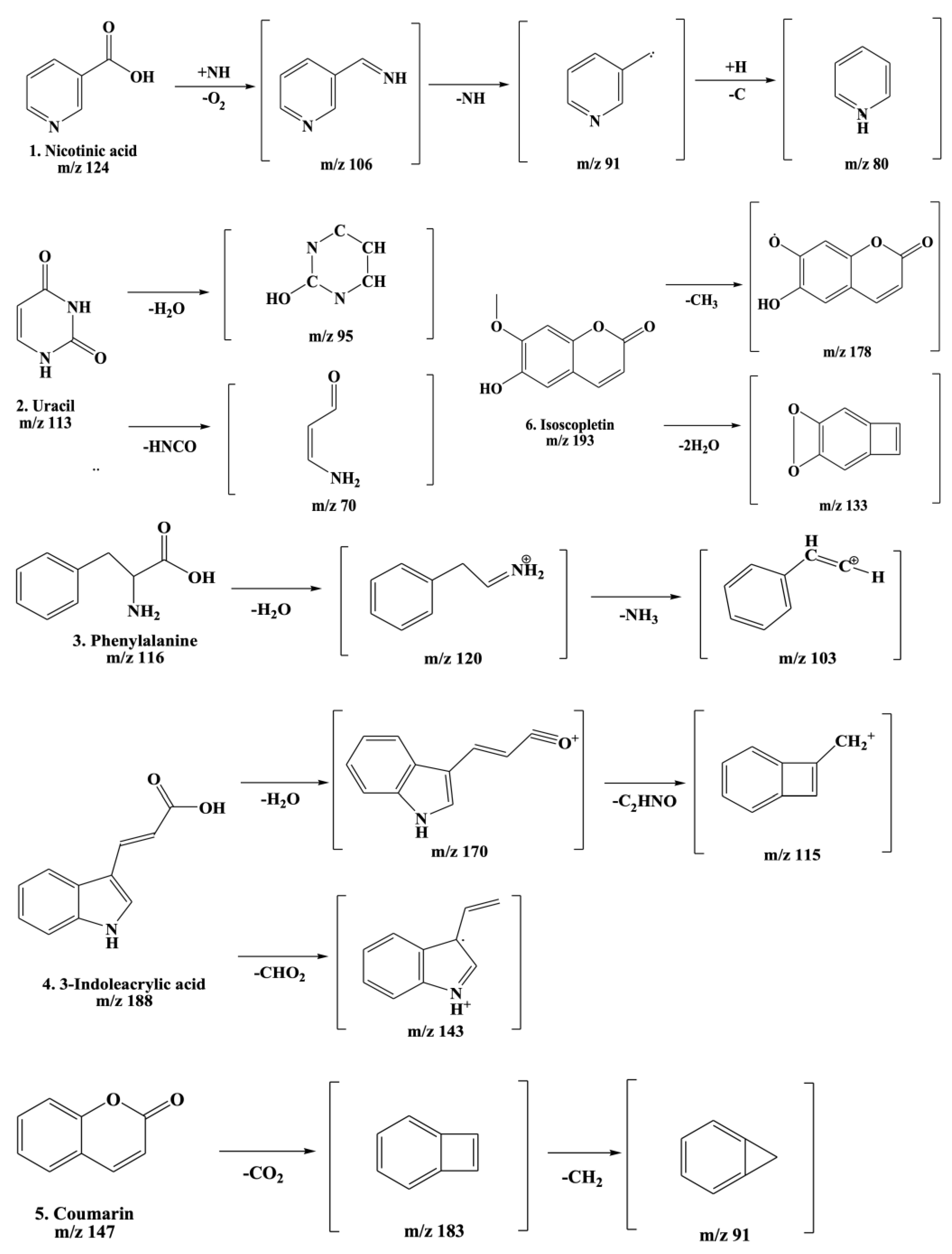
2.6. Ultra Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (UPLC-TOF-MS/MS) Detection of AWE
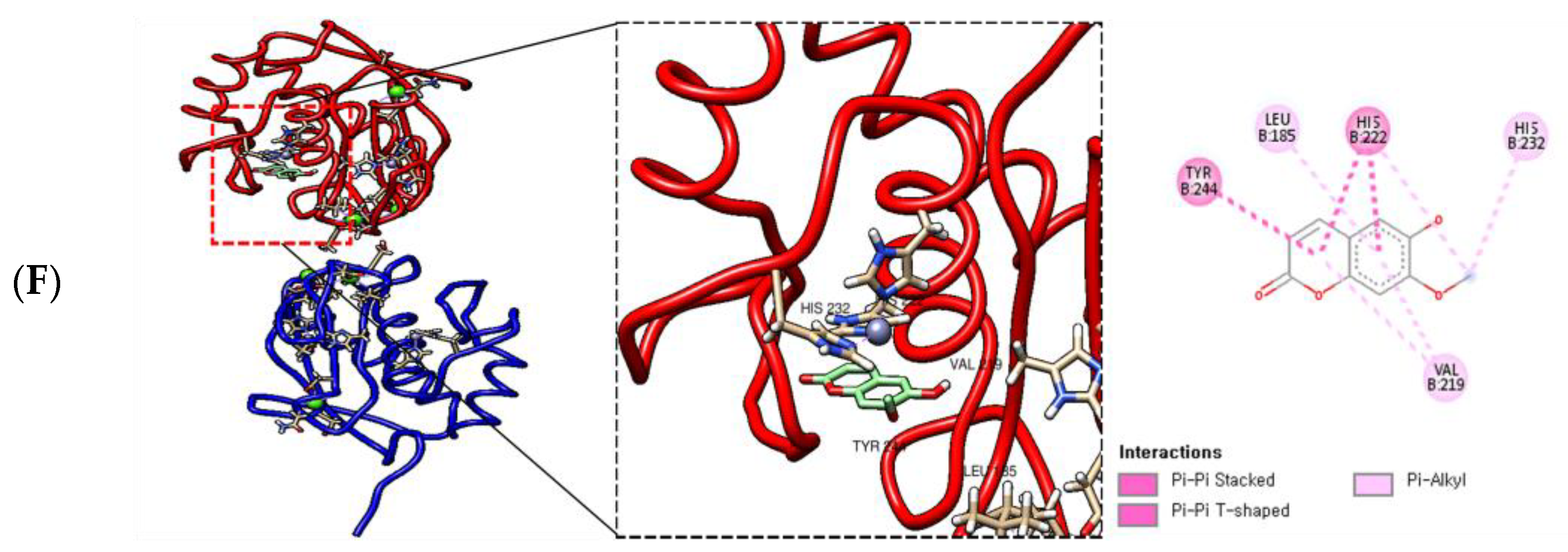
2.7. Molecular Docking of AWE and MMP-13
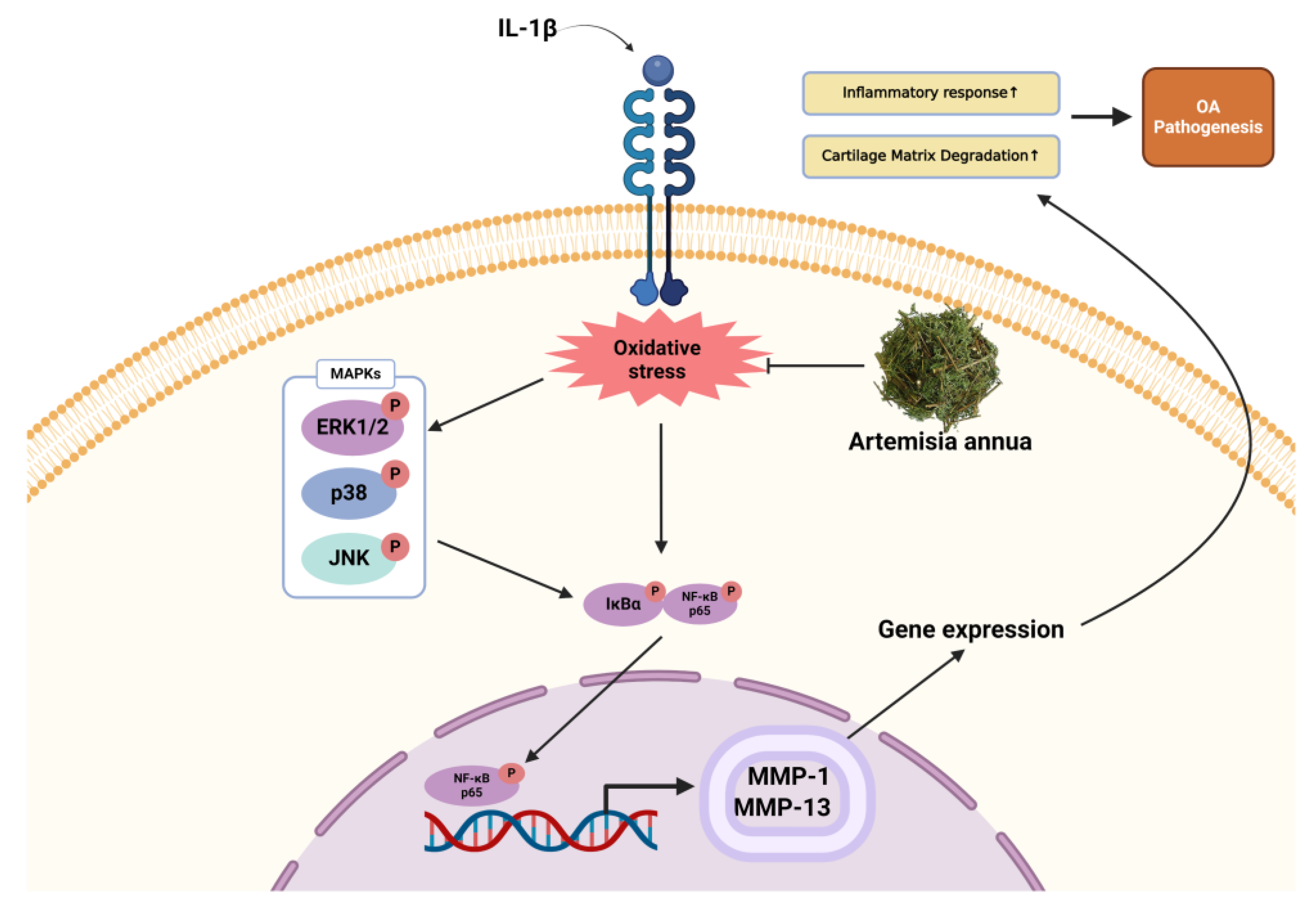
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Total Phenolic and Total Flavonoid Content Determination
4.3. Antioxidant Capacity Analysis
4.4. Cell Culture and Viability Assay
4.5. ROS Assay
4.6. Western Blotting
4.7. UPLC-QTOF-MS Conditions
4.8. Molecular Docking Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part II: Patellofemoral Joint. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Maouche, A.; Boumediene, K.; Baugé, C. Bioactive Compounds in Osteoarthritis: Molecular Mechanisms and Therapeutic Roles. Int. J. Mol. Sci. 2024, 25, 11656. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Zhu, X.; Chan, Y.T.; Yung, P.S.; Tuan, R.S.; Jiang, Y. Subchondral bone remodeling: A therapeutic target for osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef]
- López-Franco, M.; López-Franco, O.; Murciano-Antón, M.; Cañamero-Vaquero, M.; Fernández-Aceñero, M.; Herrero-Beaumont, G.; Gómez-Barrena, E. Meniscal degeneration in human knee osteoarthritis: In situ hybridization and immunohistochemistry study. Arch. Orthop. Trauma Surg. 2016, 136, 175–183. [Google Scholar] [CrossRef]
- Wang, M.G.; Seale, P.; Furman, D. The infrapatellar fat pad in inflammaging, knee joint health, and osteoarthritis. npj Aging 2024, 10, 34. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage integrity: A review of mechanical and frictional properties and repair approaches in osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the knee. New Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Yavuz, U.; Sökücü, S.; Albayrak, A.; Oztürk, K. Efficacy comparisons of the intraarticular steroidal agents in the patients with knee osteoarthritis. Rheumatol. Int. 2012, 32, 3391–3396. [Google Scholar] [CrossRef]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, 2015, Cd005328. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhang, C.; Ni, L.; Ji, X.; Hong, J.; Chen, Y.; Wang, J.; Li, C.; Lin, J.; Lu, T. Cepharanthine ameliorates chondrocytic inflammation and osteoarthritis via regulating the MAPK/NF-κB-Autophagy pathway. Front. Pharmacol. 2022, 13, 854239. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Na, C.B.; Kim, D.; Han, H.J.; Yun, J.; Park, S.K.; Cho, E. Osteoarthritis improvement effect of Chrysanthemum zawadskii var. latilobum extract in relation to genotype. Int. J. Vitam. Nutr. Res. 2023, 93, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Son, E.; Kim, D.-S.; Shim, K.-S.; Yu, S.H. Evaluating the Anti-Inflammatory and Chondroprotective Effects of Adenocaulon himalaicum Extract Through Network Pharmacology and Experimental Validation. Int. J. Mol. Sci. 2025, 26, 877. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Shin, S.-H.; Park, S.-M.; Choi, M.-S.; Kim, S.; Oh, J.-H.; Yoon, S.; Park, E.-J.; Han, H.-Y. Cytotoxicity-reducing and anti-inflammatory effects of a natural herb mixture extract. Mol. Cell. Toxicol. 2025, 21, 263–269. [Google Scholar] [CrossRef]
- Brown, G.D. The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sudheer, W.N.; Lakshmaiah, V.V.; Mukherjee, E.; Nizam, A.; Thiruvengadam, M.; Nagella, P.; Alessa, F.M.; Al-Mssallem, M.Q.; Rezk, A.A.; et al. Biotechnological Approaches for Production of Artemisinin, an Anti-Malarial Drug from Artemisia annua L. Molecules 2022, 27, 3040. [Google Scholar] [CrossRef]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- Desrosiers, M.R.; Towler, M.J.; Weathers, P.J. Artemisia annua and Artemisia afra essential oils and their therapeutic potential. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 197–209. [Google Scholar]
- Zhang, L.; Reddy, N.; Khoo, C.S.; Koyyalamudi, S.R. Structural Characterization and In-Vitro Antioxidant and Immunomodulatory Activities of Polysaccharide Fractions Isolated from Artemisia annua L. Molecules 2022, 27, 3643. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Z.; Yang, M.-F.; Song, Y.; Xu, H.-M.; Xu, J.; Yue, N.-N.; Zhang, Y.; Tian, C.-M.; Shi, R.-Y.; Liang, Y.-J.; et al. Exploring the efficacy of herbal medicinal products as oral therapy for inflammatory bowel disease. Biomed. Pharmacother. 2023, 165, 115266. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fan, B.; Mao, W.; Kuai, L.; Feng, J.; Wang, Y.; Zhou, M.; Miao, X. Topical application of Artemisia annua L. essential oil ameliorates 2,4-dintrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2024, 333, 118439. [Google Scholar] [CrossRef]
- Baek, A.; Jung, S.H.; Pyo, S.; Kim, S.Y.; Jo, S.; Kim, L.; Lee, E.Y.; Kim, S.H.; Cho, S.-R. 3′-Sialyllactose Protects SW1353 Chondrocytic Cells From Interleukin-1β-Induced Oxidative Stress and Inflammation. Front. Pharmacol. 2021, 12, 609817. [Google Scholar] [CrossRef]
- You, H.; Zhang, R.; Wang, L.; Pan, Q.; Mao, Z.; Huang, X. Chondro-protective effects of shikimic acid on osteoarthritis via restoring impaired autophagy and suppressing the MAPK/NF-κB signaling pathway. Front. Pharmacol. 2021, 12, 634822. [Google Scholar] [CrossRef]
- Stražić, D.; Benković, T.; Gembarovski, D.; Kontrec, D.; Galić, N. Comprehensive ESI-MS and MS/MS analysis of aromatic hydrazones derived from nicotinic acid hydrazide. Int. J. Mass Spectrom. 2014, 371, 54–64. [Google Scholar] [CrossRef]
- Cao, S.; Hu, M.; Yang, L.; Li, M.; Shi, Z.; Cheng, W.; Zhang, Y.; Chen, F.; Wang, S.; Zhang, Q. Chemical Constituent Analysis of Ranunculus sceleratus L. Using Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole-Orbitrap High-Resolution Mass Spectrometry. Molecules 2022, 27, 3299. [Google Scholar] [CrossRef]
- Zhang, P.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.T.; Lei, K.M.K.; Poon, T.C.W. Revisiting Fragmentation Reactions of Protonated α-Amino Acids by High-Resolution Electrospray Ionization Tandem Mass Spectrometry with Collision-Induced Dissociation. Sci. Rep. 2019, 9, 6453. [Google Scholar] [CrossRef]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous Determination of Coumarin and Its Derivatives in Tobacco Products by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef]
- Chu, Z.; Liu, Z.; Li, W.; Xu, D.; Pang, L. Simvastatin attenuates delayed encephalopathy induced by carbon monoxide poisoning in rats by regulating oxidative stress, inflammation and NF-κB pathway. Mol. Cell. Toxicol. 2021, 17, 187–193. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, J.; Cai, K.; Liu, Y.; Lu, B.; Zhang, J.; Xu, J.; Gu, C.; Chen, T. From dysfunction to healing: Advances in mitochondrial therapy for Osteoarthritis. J. Transl. Med. 2024, 22, 1013. [Google Scholar] [CrossRef] [PubMed]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Moser, L.B.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Effects of extracellular vesicles from blood-derived products on osteoarthritic chondrocytes within an inflammation model. Int. J. Mol. Sci. 2021, 22, 7224. [Google Scholar] [CrossRef]
- Lee, S.A.; Park, B.-R.; Moon, S.-M.; Hong, J.H.; Kim, D.K.; Kim, C.S. Chondroprotective Effect of Cynaroside in IL-1β-Induced Primary Rat Chondrocytes and Organ Explants via NF-κB and MAPK Signaling Inhibition. Oxidative Med. Cell. Longev. 2020, 2020, 9358080. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wei, Z.; Wang, Z.; Xu, S.; Sun, K.; Cheng, P.; Huang, X.; You, H.; Guo, F.; Liang, S.; et al. Mulberroside A alleviates osteoarthritis via restoring impaired autophagy and suppressing MAPK/NF-κB/PI3K-AKT-mTOR signaling pathways. iScience 2023, 26, 105936. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Jo, H.M.; Choi, I.H. Anti-inflammatory activity of Akebia quinata D. extracts by inhibiting MAPK and NF-κB signaling pathways in LPS-induced RAW 264.7 cells according to extraction solvents. Mol. Cell. Toxicol. 2024, 21, 315–323. [Google Scholar] [CrossRef]
- Li, X.; Ellman, M.; Muddasani, P.; Wang, J.H.; Cs-Szabo, G.; van Wijnen, A.J.; Im, H.J. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009, 60, 513–523. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Rong, X.F.; Li, R.H.; Wu, X.Y. Icariin inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017, 15, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J. Mol. Sci. 2023, 24, 9841. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Tan, Q.; Li, F.; Chen, Z.; Zhang, K.; Li, F.; Yang, B.; Xing, Z.; Zhou, F.; Tian, Y. Potential Value of Matrix Metalloproteinase-13 as a Biomarker for Osteoarthritis. Front. Surg. 2021, 8, 750047. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Seo, D.-Y.; Park, J.-W.; Kim, S.-H.; Oh, S.-R.; Han, S.-B.; Kwon, O.-K.; Ahn, K.-S. Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study. Int. J. Mol. Sci. 2024, 25, 6908. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic effects of coumarins with different substitution patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]

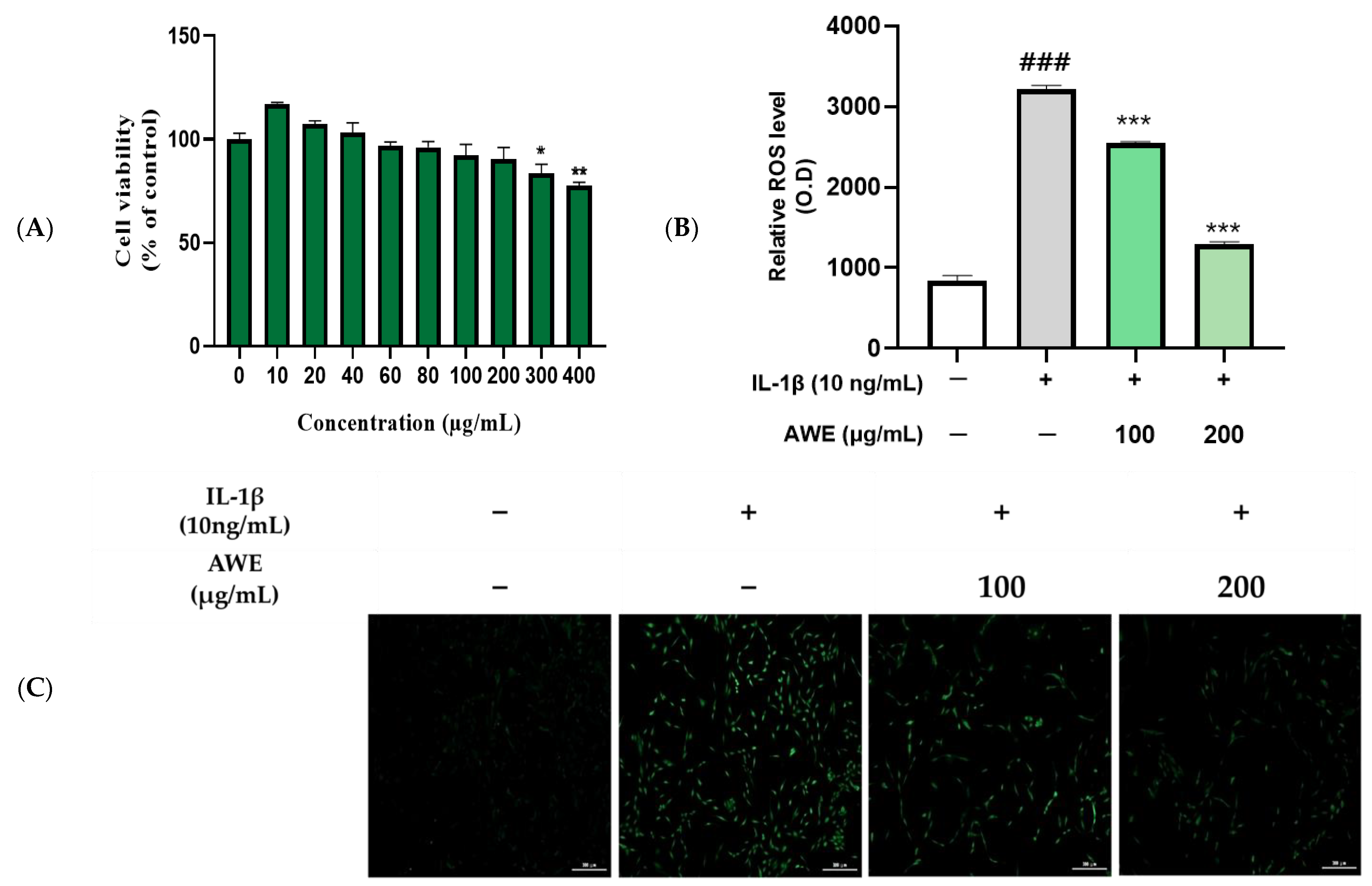
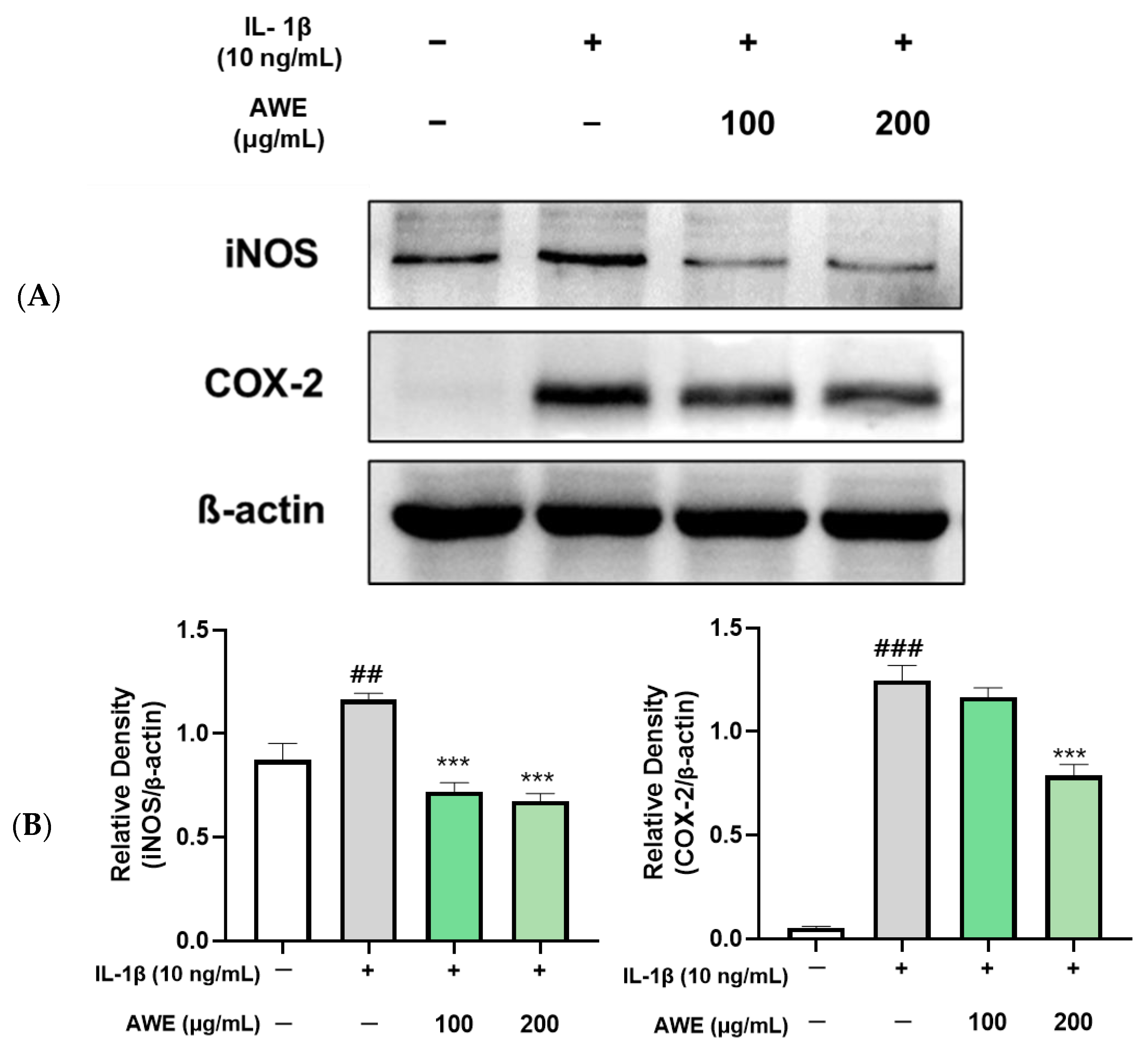

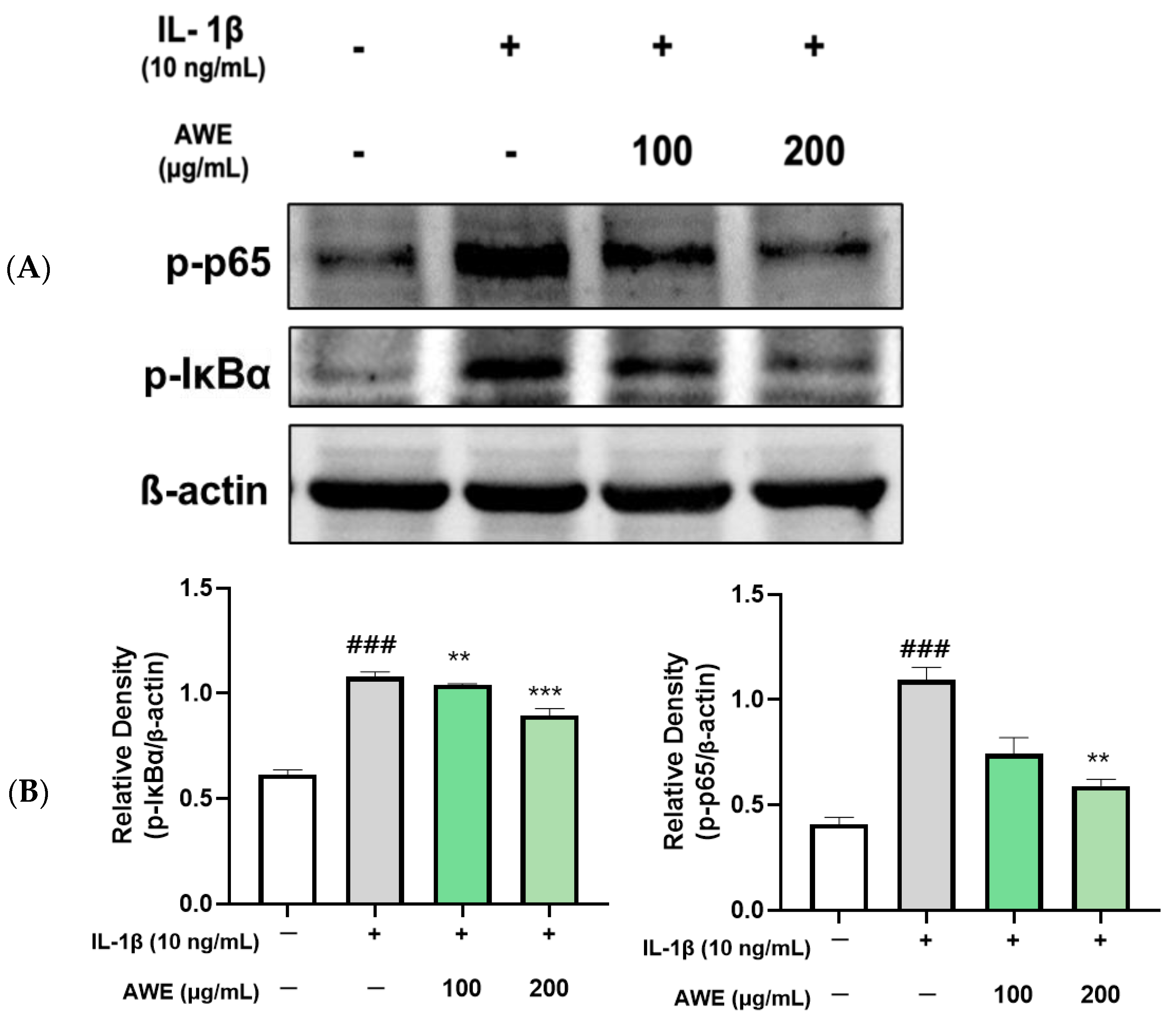
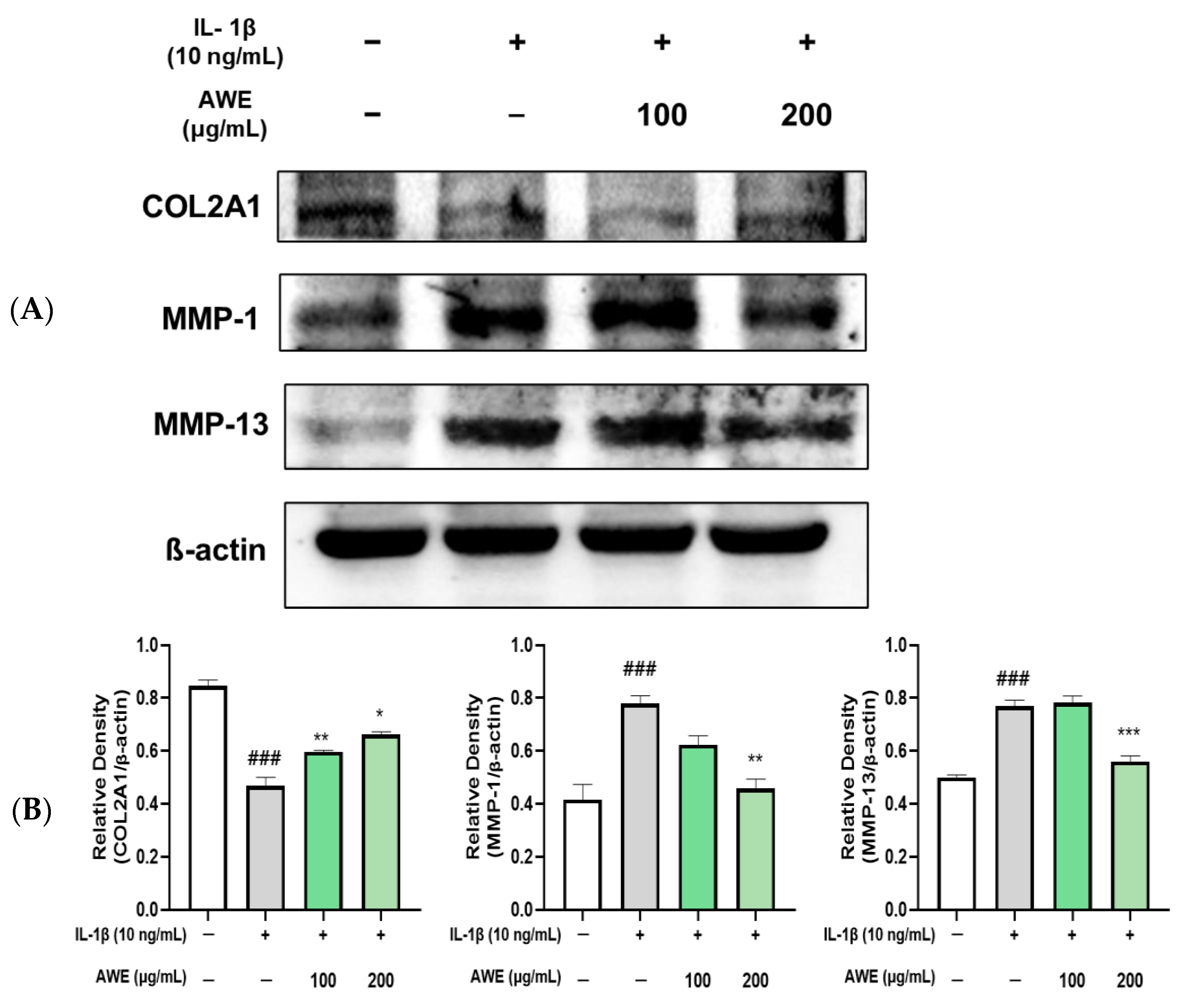


| Items | Concentration | |
|---|---|---|
| AWE | Total polyphenol (GAE mg/g) | 61.136 ± 0.74 |
| Total flavonoid (QE mg/g) | 54.167 ± 1.705 | |
| Peak No. | Retention Time (min) | Compound (Molecular Formula) | MS/MS Fragment (m/z) | Chemical Structures (PubChem CID) |
|---|---|---|---|---|
| 1 | 4.87 | Nicotinic acid (C6H5NO2) | 124,106,96,80 | 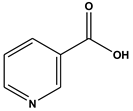 (938) |
| 2 | 5.34 | Uracil (C4H4N2O2) | 113,96,70 | 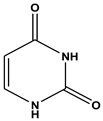 |
| 3 | 9.46 | Phenylalanine (C9H11NO2) | 166,120,103 | (1,174) (6,140) |
| 4 | 12.92 | 3-Indoleacrylic acid (C11H9NO2) | 188,170,143,155 | 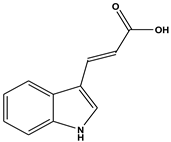 (5,375,048) |
| 5 | 19.32 | Coumarin (C11H9NO2) | 147,103,91 | 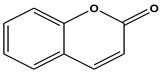 (323) |
| 6 | 23.46 | Isoscopoletin (C10H8O4) | 193,178,133 |  |
| (69,894) |
| Binding Ligand | Amino Acid Residue That Interacts | Docking Score |
|---|---|---|
| Nicotinic acid | MET 252A, LEU 218A | −5.0 kcal/mol |
| Uracil | ALA 186A, HIS 222A, ZN 901A, GLU 223A, LEU 185A, VAL 219A, PRO 242A, ALA 186A | −4.9 kcal/mol |
| Phenylalanine | HIS 222A, GLA 223A, ALA 186A, VAL 219A | −6.8 kcal/mol |
| 3-Indoleacrylic acid | PRO 193A | −5.4 kcal/mol |
| Coumarine | LEU 218B, TYR 244B, VAL 219B, HIS 222B | −7.7 kcal/mol |
| Isoscopoletin | TYP 244B, LEU 185B, HIS 222B, HIS 232B, VAL 219B | −7.7 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Yang, Y.J.; Heo, J.W.; Son, J.-d.; You, Y.Z.; Yang, J.-H.; Park, K.I. Potential Chondroprotective Effect of Artemisia annua L. Water Extract on SW1353 Cell. Int. J. Mol. Sci. 2025, 26, 1901. https://doi.org/10.3390/ijms26051901
Kim MJ, Yang YJ, Heo JW, Son J-d, You YZ, Yang J-H, Park KI. Potential Chondroprotective Effect of Artemisia annua L. Water Extract on SW1353 Cell. International Journal of Molecular Sciences. 2025; 26(5):1901. https://doi.org/10.3390/ijms26051901
Chicago/Turabian StyleKim, Min Jung, Ye Jin Yang, Ji Woong Heo, Jae-dong Son, Young Zoo You, Ju-Hye Yang, and Kwang Il Park. 2025. "Potential Chondroprotective Effect of Artemisia annua L. Water Extract on SW1353 Cell" International Journal of Molecular Sciences 26, no. 5: 1901. https://doi.org/10.3390/ijms26051901
APA StyleKim, M. J., Yang, Y. J., Heo, J. W., Son, J.-d., You, Y. Z., Yang, J.-H., & Park, K. I. (2025). Potential Chondroprotective Effect of Artemisia annua L. Water Extract on SW1353 Cell. International Journal of Molecular Sciences, 26(5), 1901. https://doi.org/10.3390/ijms26051901







