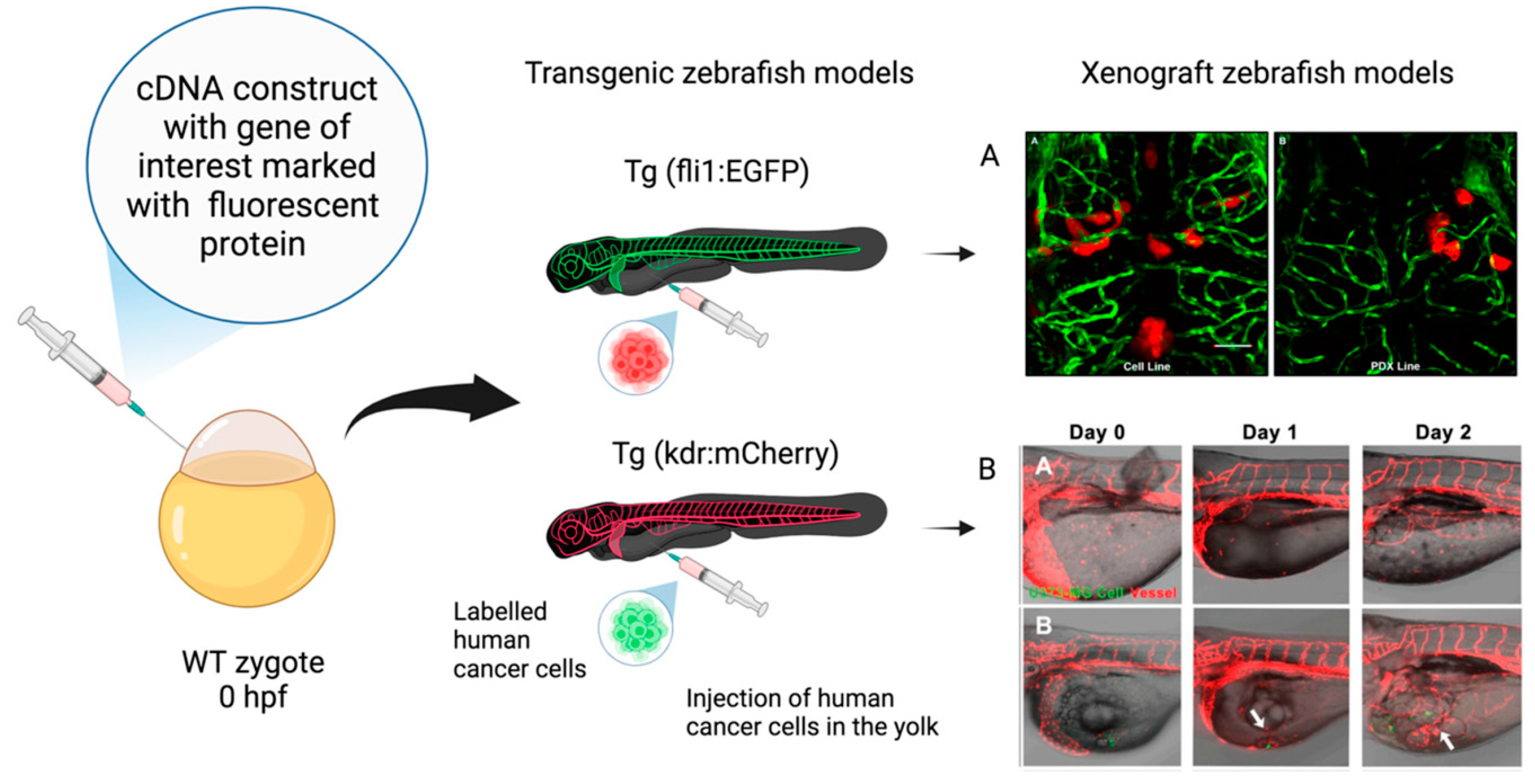
The Zebrafish Model in Animal and Human Health Research
Conflicts of Interest
References
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Amico, M.D.; Caruso Bavisotto, C.; Rappa, F.; Marino Gammazza, A.; Bucchieri, F.; Cappello, F.; Scalia, F.; Szychlinska, M.A. Speeding up Glioblastoma Cancer Research: Highlighting the Zebrafish Xenograft Model. Int. J. Mol. Sci. 2024, 25, 5394. [Google Scholar] [CrossRef] [PubMed]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Ren, J.; Song, G.; Li, Q.; Cui, Z. Transcriptome Analysis Reveals the Molecular Basis of Overfeeding-Induced Diabetes in Zebrafish. Int. J. Mol. Sci. 2023, 24, 11994. [Google Scholar] [CrossRef] [PubMed]
- Umans, R.A.; Ten Kate, M.; Pollock, C.; Sontheimer, H. Fishing for Contact: Modeling Perivascular Glioma Invasion in the Zebrafish Brain. ACS Pharmacol. Transl. Sci. 2020, 4, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Tsai, J.C.; Tseng, Y.T.; Wu, M.S.; Liu, W.S.; Lam, H.I.; Yu, J.H.; Nozell, S.E.; Benveniste, E.N. Small G protein Rac GTPases regulate the maintenance of glioblastoma stem-like cells in vitro and in vivo. Oncotarget 2017, 8, 18031–18049. [Google Scholar] [CrossRef]
- Silva Brito, R.; Canedo, A.; Farias, D.; Rocha, T.L. Transgenic zebrafish (Danio rerio) as an emerging model system in ecotoxicology and toxicology: Historical review, recent advances, and trends. Sci. Total. Environ. 2022, 848, 157665. [Google Scholar] [CrossRef] [PubMed]
- Stachurski, P.; Świątkowski, W.; Ciszewski, A.; Sarna-Boś, K.; Michalak, A. A Short Review of the Toxicity of Dentifrices-Zebrafish Model as a Useful Tool in Ecotoxicological Studies. Int. J. Mol. Sci. 2023, 24, 14339. [Google Scholar] [CrossRef]
- Seli, D.A.; Prendergast, A.; Ergun, Y.; Tyagi, A.; Taylor, H.S. High NaCl Concentrations in Water Are Associated with Developmental Abnormalities and Altered Gene Expression in Zebrafish. Int. J. Mol. Sci. 2024, 25, 4104. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Ricci, S.; Servetto, G.P.; Franceschini, V.; Ruiz-Zepeda, F.; Vigliaturo, R. Altered Morpho-Functional Features of Neurogenesis in Zebrafish Embryos Exposed to Non-Combustion-Derived Magnetite. Int. J. Mol. Sci. 2024, 25, 6459. [Google Scholar] [CrossRef] [PubMed]
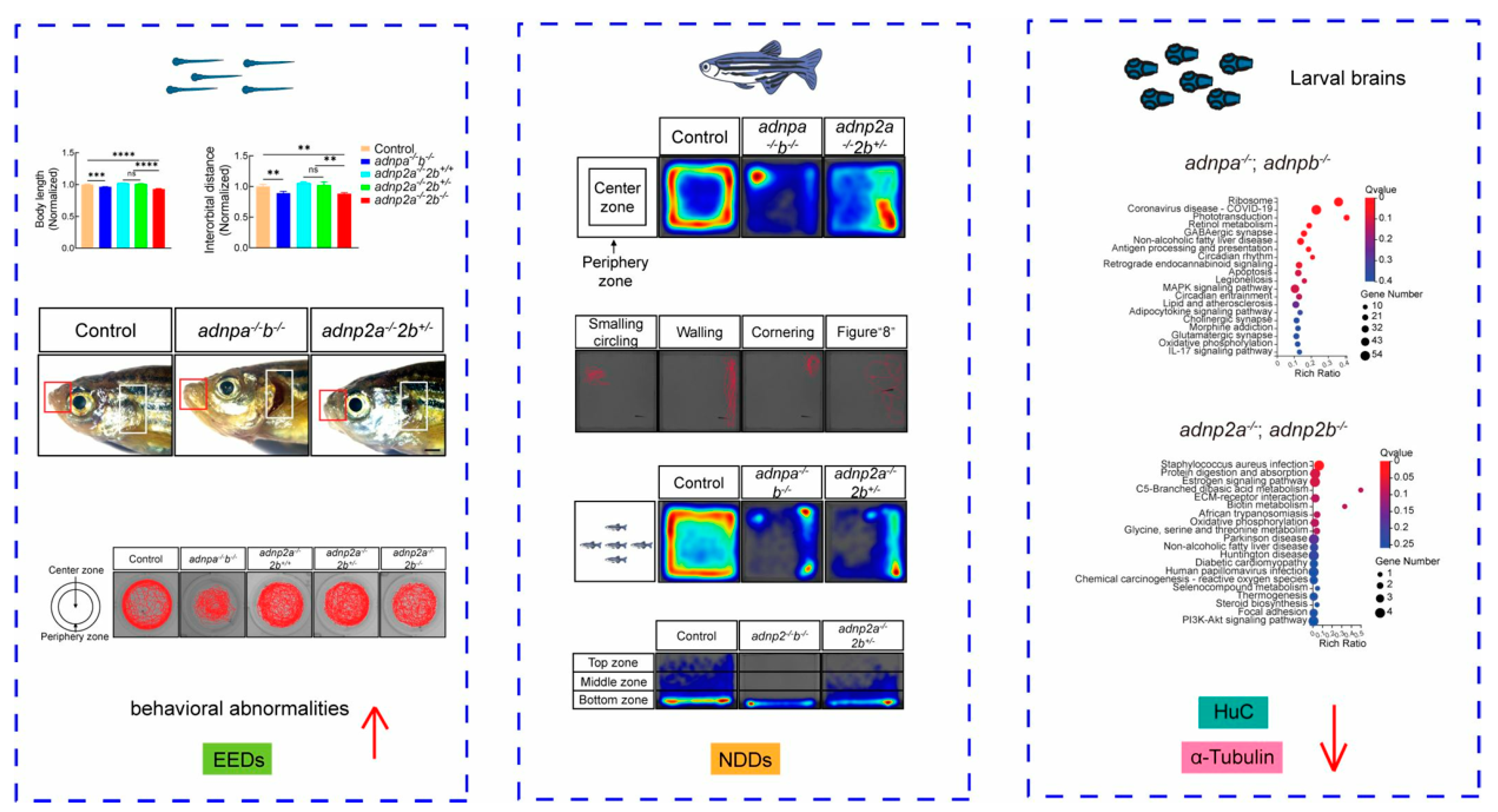
- de Abreu, M.S.; Genario, R.; Giacomini, A.C.V.V.; Demin, K.A.; Lakstygal, A.M.; Amstislavskaya, T.G.; Fontana, B.D.; Parker, M.O.; Kalueff, A.V. Zebrafish as a Model of Neurodevelopmental Disorders. Neuroscience 2020, 445, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Xiong, B.; Duan, M.; Sun, Y. Genetic and Environmental Factors Co-Contributing to Behavioral Abnormalities in adnp/adnp2 Mutant Zebrafish. Int. J. Mol. Sci. 2024, 25, 9469. [Google Scholar] [CrossRef]
- Dash, S.N.; Patnaik, L. Flight for fish in drug discovery: A review of zebrafish-based screening of molecules. Biol. Lett. 2023, 19, 20220541. [Google Scholar] [CrossRef] [PubMed]
- Reina, C.; Cardella, C.; Lo Pinto, M.; Pucci, G.; Acuto, S.; Maggio, A.; Cavalieri, V. Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos. Int. J. Mol. Sci. 2023, 24, 13191. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Kasica, N.; Maciąg, M.; Luca, S.V.; Budzyńska, B.; Fondai, D.; Podlasz, P.; Skalicka-Woźniak, K. Simple Coumarins from Peucedanum luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 8693. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szychlinska, M.A.; Marino Gammazza, A. The Zebrafish Model in Animal and Human Health Research. Int. J. Mol. Sci. 2025, 26, 1945. https://doi.org/10.3390/ijms26051945
Szychlinska MA, Marino Gammazza A. The Zebrafish Model in Animal and Human Health Research. International Journal of Molecular Sciences. 2025; 26(5):1945. https://doi.org/10.3390/ijms26051945
Chicago/Turabian StyleSzychlinska, Marta Anna, and Antonella Marino Gammazza. 2025. "The Zebrafish Model in Animal and Human Health Research" International Journal of Molecular Sciences 26, no. 5: 1945. https://doi.org/10.3390/ijms26051945
APA StyleSzychlinska, M. A., & Marino Gammazza, A. (2025). The Zebrafish Model in Animal and Human Health Research. International Journal of Molecular Sciences, 26(5), 1945. https://doi.org/10.3390/ijms26051945





