Abstract
Water-soluble B vitamins, mainly obtained through dietary intake of fruits, vegetables, grains, and dairy products, act as co-factors in various biochemical processes, including DNA synthesis, repair, methylation, and energy metabolism. These vitamins include B1 (Thiamine), B2 (Riboflavin), B3 (Niacin), B5 (Pantothenic Acid), B6 (Pyridoxine), B7 (Biotin), B9 (Folate), and B12 (Cobalamin). Recent studies have shown that besides their fundamental physiological roles, B vitamins influence oncogenic metabolic pathways, including glycolysis (Warburg effect), mitochondrial function, and nucleotide biosynthesis. Although deficiencies in these vitamins are associated with several complications, emerging evidence suggests that excessive intake of specific B vitamins may also contribute to cancer progression and interfere with therapy due to impaired metabolic and genetic functions. This review discusses the tumor-suppressive and tumor-progressive roles of B vitamins in cancer. It also explores the recent evidence on a comprehensive understanding of the relationship between B vitamin metabolism and cancer progression and underscores the need for further research to determine the optimal balance of B vitamin intake for cancer prevention and therapy.
1. Introduction
B vitamins are a group of eight water-soluble nutrients essential in maintaining various metabolic processes in the body and are necessary for maintaining human health []. The B vitamins include B1 (Thiamine), B2 (Riboflavin), B3 (Niacin), B5 (Pantothenic Acid), B6 (Pyridoxine), B7 (Biotin), B9 (Folate), and B12 (Cobalamin). Each type of B vitamin participates in a specific biochemical reaction as a co-factor and helps with DNA synthesis, repair, methylation, and energy metabolism (Table 1). Since B vitamins play crucial roles in cellular functions and biochemical pathways, it’s no surprise that B vitamins are essential for human health and disease [,,,]. The deficiency of these vitamins could lead to multiple complications, including neurological disturbances, anemia, and skin diseases [,].

Table 1.
Role of B vitamins in various metabolic and cancer-related pathways.
Further, certain drugs and diseases could cause a deficiency of these vitamins and increase complications. For example, prolonged metformin use has been shown to cause thiamine and cobalamin deficiency [,,,,]. Similarly, corticosteroids could cause pyridoxine, folate, and cobalamin deficiency [,,]. Moreover, several drugs, such as proton pump inhibitors [,], anticonvulsants [,], antibiotics [], and chemotherapeutic drugs [,,], have been shown to cause deficiency of specific B vitamins. B vitamins also play a role in cancer development and progression. In certain cancers, the deficiency of B vitamins was also observed.
The involvement of B vitamins in cancer is a complicated process. Generally, B vitamins play an important role in cancer prevention and progression by altering cellular metabolism and DNA synthesis, repair, and methylation [,]. For example, adequate folate, cobalamin, and pyridoxine levels support genomic stability by controlling the DNA strand breaks, mutations, and genomic instability. In addition, niacin contributes to NAD+ production, which is essential for DNA repair and energy balance. Although sufficient B vitamin intake could reduce the cancer progression and risk, excessive supplementation, specifically with vitamins B9, B12, and B6, has been associated with accelerated tumor growth in certain cancers, such as colorectal, prostate, and lung cancer. Further, B vitamins could also interact with oncogenic regulators such as HIF-1α, MYC, and AMPK, which influence cancer cell metabolism. Since B vitamins play a key role in normal cellular function, understanding their role in cancer is highly complex and requires a personalized approach to intake and supplementation, especially in cancer patients.
Further, recent studies also suggest that vitamin deficiencies and excessive intake of vitamins could be associated with cancer development and therapeutic resistance [,,,]. The deficiencies seen in B vitamins could disrupt processes such as DNA repair and immune regulation that cause genomic instability and an increased susceptibility to cancer. On the other hand, excessive supplementation of B vitamins has been associated with the risk of cancer progression [,]. This suggests that maintaining a balanced intake of vitamins and knowing the vitamin deficiencies are very important for maintaining a healthy lifestyle.
This review discusses the importance of water-soluble B vitamins in cancer prevention and progression. Further, we aimed to provide a comprehensive understanding of how these essential nutrients can influence cancer outcomes. We searched PubMed and Google Scholar to find articles published in the last 10 years or so, using keywords including various types of B vitamins, cancer, melanoma, leukemia, breast cancer, lung cancer, colon cancer, and other cancer types. We included various research articles, comprehensive narrative reviews, meta-analytical studies, systematic reviews, clinical studies, and pre-clinical studies to discuss their findings. We did not include studies on other fat-soluble and water-soluble vitamins, such as A and C. This narrative review article discusses only the role of B vitamins and their therapeutic significance in various cancers.
2. Vitamin B1: Thiamine
Vitamin B1 (thiamine) is a water-soluble vitamin in plant and animal-derived foods, such as meats, eggs, and dairy []. Thiamine maintains cellular viability by participating in critical metabolic pathways like glycolysis, the pentose phosphate pathway, and Kreb’s cycle. These pathways are essential for producing ATP and NADPH to sustain metabolic functions in the body []. Thiamine is particularly significant for aerobic metabolism, as it acts as a primary co-factor for pyruvate dehydrogenase in its active form, thiamine pyrophosphate (TPP). TPP also serves as a co-factor for alpha-keto dehydrogenase and transketolase, which convert ribulose-5-phosphate into RNA and DNA substrates and facilitate the conversion of amino acids and fatty acids into acetyl-CoA. Thus, thiamine directly contributes to ribose synthesis, a necessary substrate for cell proliferation, and provides metabolites for gluconeogenesis and NADPH synthesis [,].
The impact of thiamine deficiency can be profound, leading to severe conditions such as Beri-Beri disease and Wernicke-Korsakoff syndrome [,]. In cancer patients, thiamine deficiency has been strongly linked to the manifestation of delirium, highlighting its critical role in maintaining nervous system health []. Thiamine deficiency can also result in widespread neurological, cardiac, and gastrointestinal dysfunctions, which raises important questions about its role in cancer cell proliferation and progression [,,,].
A metabolic shift known as the Warburg effect (Figure 1) is often observed in the initial stages of cancer cell proliferation. This phenomenon, which has become a focus of alternative chemotherapeutic strategies, describes how cancer cells rely on glycolysis and fermentation of lactic acid for energy production, even in the oxygen presence [,]. This preference for glycolysis provides tumor cells with the metabolic intermediates necessary for rapid cell growth and survival. The role of TPP in the Warburg effect is shown in Figure 1. In addition, the individual roles of B vitamins on the Warburg effect are shown in Table 2. The overexpression of pyruvate dehydrogenase kinase (PDK) in cancer cells inactivates pyruvate dehydrogenase (PDH), effectively disconnecting glycolysis from the Krebs cycle and enhancing glycolytic activity []. The inhibition of PDK by TPP has shown promise in triggering apoptosis and reducing tumor cell proliferation by restoring PDH activity [,,]. Through its role in maintaining PDH complex activity, thiamine has been shown to decrease tumor cell proliferation and glycolytic activity by countering PDK-induced PDH inactivation [].
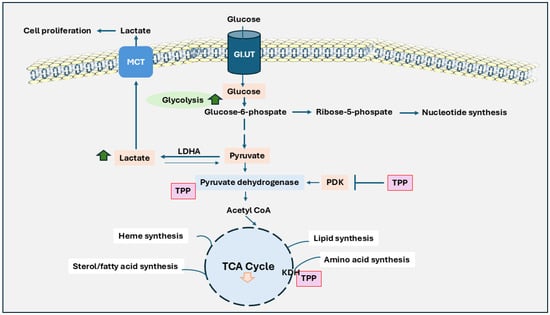
Figure 1.
The role of thiamine in Warburg effect and its impact on cancer cell metabolism. Unlike normal cells, cancer cells heavily depend on glycolysis for energy production, even during aerobic conditions, and produce lactic acid instead of relying on oxidative phosphorylation. Glucose is taken up by the cell via GLUT transporters and converted through glycolysis into pyruvate. Instead of entering the TCA cycle within the mitochondria, pyruvate is converted to lactate in the cytoplasm by lactate dehydrogenase A (LDHA). Lactate is transported by monocarboxylate Transporters (MCT) and causes cancer cell proliferation. Further, the pyruvate dehydrogenase complex is inhibited by pyruvate dehydrogenase kinase (PDK), limiting pyruvate’s conversion to acetyl-CoA and reducing the flow into the TCA cycle. Thymine pyrophosphate (TPP) plays a critical role in restoring PDH activity and counteracting the inhibitory effects of PDK. This pathway also integrates alternative pathways, such as the pentose-phosphate pathway, hexosamine biosynthesis, and lipid synthesis, further supporting cancer cell growth and survival. The activity of the TCA cycle is generally reduced, which limits energy production from mitochondria. This metabolic adaptation provides rapid energy and intermediates needed for biosynthesis. As a result, cancer cells can grow and divide rapidly, even in challenging conditions like low oxygen levels.

Table 2.
Role of B vitamins in Warburg effect. Vitamins B6, B9, and B12 are not directly involved in the Warburg effect. They are directly involved in amino acid metabolism, one-carbon metabolism, and nucleic acid synthesis.
Further evidence of thiamine’s role in cancer metabolism comes from studies involving dichloroacetate, an analog of acetic acid known for its potential to increase aerobic metabolism. Although typically avoided due to its toxic side effects, dichloroacetate showed the action of reducing colorectal cancer cell growth by enhancing oxidative phosphorylation [,]. Thiamine’s involvement in cancer is also evident in studies where cancer cells maintained a base level of TPP despite thiamine-depleting vitamin stores in tissues. When keeping TPP levels low, cancer cells could produce the essential intermediates for growth. However, increasing TPP concentrations shifted metabolism towards PDH activation and mitochondrial respiration, stunting cancer cell proliferation [,]. Additionally, thiamine analog, oxythiamine has been shown to prevent cancer cell growth [,]. Further, it has been shown to reduce DNA and RNA synthesis by inhibiting ribose-5-phosphate and thereby reducing tumor cell growth []. Thiamine at high doses has been shown to prevent the growth of MCF-7 breast cancer cells []. In these cells, thiamine has also been shown to increase PDH activity and reduce glycolysis in the cancer cells. The expression of thiamine transporter SLC19A3 has been shown to be significantly expressed in chronic hypoxia-induced breast cancer cell lines (BT474) []. This increase in the transporter expression is correlated with the increased uptake of thiamine by the breast cancer cells. However, Liu et al. [] have shown that SLC19A3 mRNA levels were downregulated in human breast cancer tumor tissues compared to normal tissues. They have suggested that decreased expression of this transporter could cause breast cancer cells resistance to apoptosis. Similarly, hypoxia has been shown to inhibit the uptake of microbiota-generated thiamine and TPP by the NCM460 human colon epithelial cells by reducing the expression of transporters such as SLC44A4, SLC19A2, and SLC19A3 []. Further, benfotiamine, a lipid-soluble derivative of thiamine, has been shown to control the proliferation of cancer cells and growth in a nude mice xenograft model [].
Thiamine deficiency is most commonly seen in patients with diabetes [,], and diabetic patients are at risk of many complications. Further, some cancer patients have also been shown to have a deficiency of thiamine [,,]. Therefore, diabetic patients with cancer have a significant risk of developing thiamine deficiency and energy metabolism, and supplementation of thiamine in such patients could prevent the cancer progression. Further, thiamine has been shown to regulate the expression of PKC, NF-κB, and advanced glycation end products (AGEs) in diabetics, and these signaling pathways are also critical in cancer progression [,]. Thiamine has been shown to prevent the activation of NF-κB and matrix metalloproteinases (MMPs) []. MMP activation is associated with tumor invasion and metastasis by modulating extracellular matrix remodeling [,]. Similarly, thiamine could also prevent the expression of prostaglandins and activation of COX-2 [], which are involved in tumor growth. Similarly, thiamine has been shown to affect tumor growth by inhibiting the generation of reactive oxygen species (ROS) and reactive nitrogen species [].
Thus, recent studies suggest that the impact of thiamine on cancer progression remains complex and variable (Figure 2). For instance, increased intake of thiamine has been linked with a significant risk of bladder cancer in men, whereas in women, it appears to have a protective effect []. This study also suggests that gender differences are influenced by dietary habits, where men typically consume more meat products leading to a risk of developing bladder cancer. On the other hand, women consuming more dairy products have a weaker association with bladder cancer. However, further research is needed to understand the gender-associated thiamine’s role in cancer. Thus, depending on the type of cancer and metabolic state, thiamine can have both tumor-promoting and tumor-suppressive effects. For example, thiamine supports tumor growth by enhancing glycolysis, the pentose phosphate pathway, and mitochondrial function and providing ATP, NADPH, and biosynthetic precursors essential for rapid tumor cell proliferation. However, in some conditions, thiamine deficiency could cause metabolic stress and oxidative DNA damage, potentially suppressing tumor growth. Although adequate dietary thiamine is necessary for normal function, excessive supplementation could promote tumor progression in susceptible cancers, and personalized approaches are needed when using thiamine in cancer therapy.
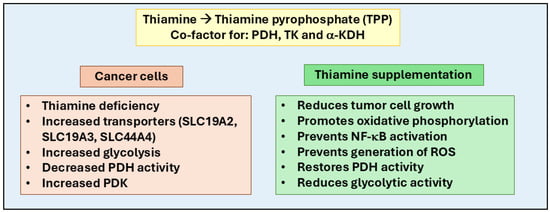
Figure 2.
Role of thiamine in cancer progression and therapy. Thiamine (Vitamin B1) plays a crucial role in cellular metabolism by converting into thiamine pyrophosphate (TPP), a co-factor for enzymes like pyruvate dehydrogenase (PDH), transketolase (TK), and alpha-keto dehydrogenase (α-KDH). These enzymes regulate glycolysis, the pentose phosphate pathway, and the Krebs cycle, essential for ATP and NADPH production, DNA/RNA synthesis, and oxidative stress reduction. In cancer cells, thiamine influences the Warburg effect, where cancer cells rely on glycolysis even in oxygen-rich environments, often due to pyruvate dehydrogenase kinase (PDK) overexpression, which inhibits oxidative phosphorylation. Thiamine supplementation counters this by restoring PDH activity, reducing glycolytic dependence, and promoting oxidative metabolism. Cancer cells modulate thiamine uptake via transporters like SLC19A2, SLC19A3, and SLC44A4. Therapeutically, supplementation with thiamine and its derivatives (such as benfotiamine and oxythiamine) could inhibit tumor growth, reduce oxidative stress, prevent NF-κB-mediated inflammatory signaling, reduce glycolytic activity, and promote oxidative phosphorylation.
3. Vitamin B2: Riboflavin
Vitamin B2, or riboflavin, is primarily obtained from food sources like thiamine, with milk and dairy products among the most significant contributors [,]. Riboflavin is a coenzyme in various oxidation and reduction reactions in the body, mediating electron transfer and maintaining the integrity of several tissues, particularly neural tissues []. Like thiamine, riboflavin plays a critical role in cellular function by helping to keep glutathione in its reduced form, neutralizing reactive oxidative species, and protecting the body from free radical damage. Riboflavin also metabolizes other essential vitamins, including folate, vitamin B6, and B12 [,].
Riboflavin deficiency, known as ariboflavinosis, can lead to a marked increase in oxidative stress, with further adverse effects such as migraines, anemia, diabetes mellitus, hyperglycemia, and hypertension [,,]. In severe cases, deficiency can result in growth retardation, anemia, renal damage, and degenerative effects on the nervous system []. While the extent of riboflavin’s influence on various cancer types varies, it is well-established that riboflavin is a crucial precursor to the coenzymes FAD and FMN, which regulate redox reactions in the TCA cycle and control levels of reactive oxygen species (ROS) in the body []. Riboflavin has been shown to regulate cellular ROS levels and increase the therapeutic efficacy of anti-cancer drugs [,,]. A few studies also suggest that moderate riboflavin levels could trigger the extrinsic pathway of apoptosis and autophagy [,,]. At the same time, higher doses could also activate specific apoptotic mechanisms by downregulating anti-apoptotic factors and promoting pro-apoptotic factors [,].
Further, the anti-inflammatory properties of riboflavin also help in cancer therapy. Notably, when combined with the chemotherapeutic drug cisplatin, riboflavin has been shown to mitigate cisplatin’s toxic effects by reducing the inflammatory response and replenishing antioxidant enzymes and cellular reductants [,]. Regulation of FAD/FMN levels, control of ROS production, and induction of apoptosis are major pathways where riboflavin is associated with increased cancer risk (Figure 3). Although some processes have been established, further research is needed to understand riboflavin’s dose-dependent and cancer-specific effects. However, few studies have indicated a correlation between riboflavin intake and cancer development [,,]. Like thiamine, overconsumption and underconsumption of riboflavin can alter cancer risk, with the impact varying depending on the cancer type and affected region [,,,]. Riboflavin has been shown to prevent the growth of MCF-7 and MDA-MB-231 breast cancer cells, but not normal cells (L929) growth under the influence of visible light []. Similarly, Sturm et al. [] have shown that riboflavin in combination with gemcitabine decreases the growth of bladder cancer cells growth in the presence of blue light at a wavelength of 453 nm. Another study by Chiu et al. [] has shown that blue light and violet light illumination of riboflavin prevents the growth of colon cancer cells. In addition, radiated riboflavin prevents growth and increases apoptosis of C6 glioblastoma cells when compared to non-radiated riboflavin. Further, a few studies also indicate the role of riboflavin in prostate cancer. A recent study by Lv et al. [] has shown a relationship between riboflavin intake and prostate-specific antigen (PSA) levels detected in American men. They have shown that there is an inverse relationship between riboflavin intake and PSA detection levels. Another study by Zhao et al. [] has shown that riboflavin, by inhibiting the TGF-β signaling, could prevent pancreatic cancer metastasis. These studies suggest that a higher riboflavin intake may suggest a greater risk of diagnosing patients with advanced prostate cancer. Similarly, Gunathilake et al. [], in a Korean population-based study, indicates that a higher riboflavin intake lowers the risk of developing colorectal cancer. Specifically, they have shown that males homozygous for the major alleles of MTRR rs1801394 and MTR rs1805087 polymorphisms and who had a higher intake of vitamin B2 had a significantly lower colorectal cancer risk. In a Chinese health study, Paragomy et al. [] have shown that the increased riboflavin levels in the serum are correlated with the increased risk of pancreatic cancer. Similarly, Ma et al. [] have also shown that increased riboflavin levels in the serum are associated with increased colorectal cancer.
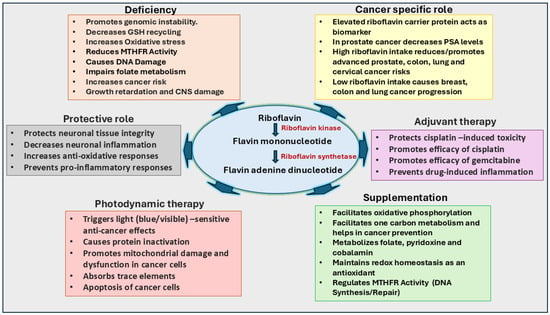
Figure 3.
Significance of riboflavin in cancer growth and therapy. Riboflavin functions as a precursor for flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), essential coenzymes in redox reactions, the TCA cycle, and reactive oxygen species (ROS) regulation. Riboflavin supports neural health, vitamin metabolism, and glutathione maintenance. Its deficiency, termed ariboflavinosis, can increase oxidative stress and promote cancer risk by impairing folate metabolism and DNA synthesis. In cancer therapy, riboflavin regulates ROS to inhibit tumor growth, promotes apoptosis, and reduces inflammation, enhancing the efficacy of cisplatin and gemcitabine. Photosensitive properties of riboflavin increase its anti-cancer potential. Elevated riboflavin intake correlates with reduced risks for colorectal, lung, and cervical cancers, while high serum riboflavin levels may increase risks for pancreatic and colorectal cancers.
As a precursor to FAD and FMN, riboflavin is linked to folate metabolism. Consequently, low riboflavin intake can exacerbate the effects of low dietary folate, which is necessary for DNA methylation and purines and pyrimidine synthesis. These factors are needed for cell growth and repair mechanisms [,]. Further, FAD also serves as a co-factor for the folate-metabolizing enzyme methylenetetrahydrofolate reductase (MTHFR). It catalyzes the one-carbon metabolism of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, eventually converting homocysteine to methionine []. Few studies have also shown that riboflavin deficiency is associated with reduced MTHFR activity and elevated homocysteine levels, which could also serve as a risk factor for cancer development [,].
Further exploration of riboflavin’s role in cancer in the context of breast cancer has shown elevated riboflavin carrier protein (RCP) levels in patients with breast adenocarcinoma []. Since RCP is estrogen-induced, its elevated levels in breast cancer patients suggest that RCP could serve as a predictive marker for breast cancer [] and may be involved in prostate cancer []. Additionally, riboflavin intake has been shown to decrease the risk of colorectal cancer in women, where deficiency disrupts MTHFR enzyme activity, contributing to cancer progression [].
In esophageal cancer, riboflavin deficiency has been linked to changes in the esophageal epithelium, increasing the risk of cancer development [,]. Supplementation with riboflavin and niacin has shown effectiveness in reducing esophageal cancer incidence in regions with high rates of the disease []. Higher riboflavin intake, especially among female non-smokers, has also been associated with a reduced risk of lung cancer []. Riboflavin deficiency is also linked to cervical dysplasia, a precursor to cervical cancer []. In addition, riboflavin, combined with other B vitamins, such as thiamine, folate, or cobalamin, could offer protection against the progression of cancer [,,]. Indeed, a recent study suggests that intake of riboflavin at a dose of 1.2 to 2.4 mg/day could contribute to a reduced risk of cervical cancer in Korean women []. Thus, recent studies suggest that riboflavin could be adjuvant therapy to increase the efficacy of anti-cancer drugs. However, its individual effects on cancer prevention and treatment need additional studies.
4. Vitamin B3: Niacin
Vitamin B3, known as niacin, is primarily derived from nicotinic acid, nicotinamide, and tryptophan. It is essential for generating coenzymes such as nicotinamide adenine dinucleotides (NAD and NADPH). These molecules are necessary for several metabolic pathways, including glycolysis, the Krebs Cycle, oxidative phosphorylation, and the hexose monophosphate (HMP) shunt []. The conversion of tryptophan to niacin, and subsequently to NAD, is catalyzed by a dioxygenase enzyme, which is upregulated by cortisol and tryptophan in the liver []. However, only a small amount of dietary tryptophan is typically converted to niacin, with most niacin obtained through other methods (Figure 4).
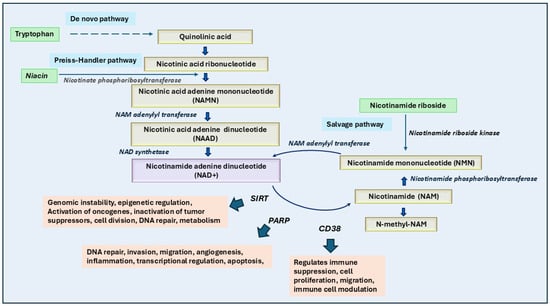
Figure 4.
Role of niacin and its cofactors in cancer growth and therapy. The biosynthesis and metabolism of NAD+ (nicotinamide adenine dinucleotide) occur through three main pathways: the de novo pathway (from tryptophan to quinolinic acid and eventually NAD+), the Preiss-Handler pathway (from niacin to NAD+ via intermediates like NAMN and NAAD), and the salvage pathway (from nicotinamide riboside or nicotinamide to NAD+ via NMN). Enzymatic reactions, such as those catalyzed by nicotinamide riboside kinase and nicotinate phosphoribosyltransferase, play critical roles in these conversions. NAD+ serves as a cofactor for key enzymes like sirtuins (SIRTs), Poly (ADP-ribose) polymerases (PARPs), and CD38, which are involved in genomic stability, DNA repair, cell proliferation, and immune suppression, which eventually lead to cancer growth. NAMPT inhibitors, when combined with niacin, effectively block NAD salvage pathways crucial for tumor growth. High dietary niacin intake could correlate with improved survival and lower mortality in cancer patients, supporting its potential as a chemopreventive agent.
Further, niacin, through its precursor nicotinamide (NAM), contributes to the production of NAD+, which in turn acts as a substrate for key enzymes like sirtuin 1 (SIRT1) and poly ADP-ribose polymerase 1 (PARP1) []. These enzymes play a role in DNA repair, apoptosis, and carcinogenesis [,,]. Thus, the therapies targeting NAD+ metabolism, such as PARP and NAMPT inhibitors, could enhance cancer cell death by blocking NAD+ dependency. Thus, the modulation of NAD+/NADH balance demonstrates a promising therapeutic strategy, but its effects depend on the specific cancer type and treatment approach. As with all essential nutrients, niacin deficiencies can significantly affect human health. The most severe effects of niacin deficiency are manifest in Pellagra, a disease characterized by the “three D’s”: dermatitis (often presenting as a casal necklace), diarrhea, and dementia []. Pellagra can also occur in Hartnup disease, where the failure to absorb amino acids like tryptophan through the intestine and kidneys leads to a secondary niacin deficiency [].
Nikas et al. [] have shown that PARP1 is activated by DNA strand breaks, initiates DNA repair mechanisms, and maintains genomic stability. However, excessive DNA damage can over-activate PARP1, leading to the depletion of NAD+ and other metabolic factors, which may result in necrosis. NAM is notable for inhibiting SIRT1 and PARP1 through negative feedback mechanisms, thus preventing over-regulated replication processes [,].
NAM has been shown to prevent carcinogenic events in melanoma [,]. For example, NAM has been shown to prevent UV-radiation-induced damage to the keratinocytes []. It promotes DNA repair and cellular energy production and decreases inflammation and cell death. In a randomized phase-III clinical trial, Chen et al. suggest that oral NAM reduces the risk of developing new nonmelanoma skin cancers and actinic keratoses in high-risk patients []. Similarly, Carneiro et al. [] have also suggested that NAM is preventive to the development of non-melanoma skin cancers in cancer patients. However, a meta-analytical systemic study by Tosti et al. [] suggests no significant chemopreventive effect of NAM supplementation in skin cancers. Along similar lines, a comprehensive epidemiological study indicates that niacin supplementation provided partial benefit from developing SCC but had no effect on BCC and melanoma []. However, a recent study indicates that NAM prevents melanoma cell growth in culture as well as in mice models [].
Recent studies also suggest that niacin deficiency regulates DNA damage repair caused by nicotine-derived nitrosamine ketones by modulating the genes involved in the cancer progression []. This study indicates that niacin deficiency could be a risk for cigarette smoke-induced genomic instability and cancer development. Moreover, Nicotinamide phosphoribosyltransferase (NAMPT) is a key enzyme involved in the production of NAD in the salvage pathway required for cancer cell growth and progression [] (Figure 3). Overexpression of NAMPT has been shown in many cancer types and inhibitors of NAMPT have been shown to prevent cancer growth []. Cole et al. have also indicated a possible treatment option of coadministration of NAMPT along with niacin for small-cell lung cancer and neuronal cancers []. Similarly, Nomura et al. [] have shown that reducing the levels of nicotinic acid riboside could enhance the efficacy of NAMPT inhibitors in treating neuroendocrine cancers.
In addition, Tabrizi and Abyar [] have shown that copper-based compounds containing vitamins B3 and B4 prevent the growth of breast cancer cells such as MCF7 and MDA-MB-231. Comparably, Abdel-Mohsen et al. [] have also suggested that the copper(I) nicotinate complex, by regulating Notch1 signaling, could prevent triple-negative breast cancer cell growth. NAM has been shown to prevent triple-breast cancer cells by reducing the mitochondrial membrane potential and ATP production and increasing reverse electron transport, lipid metabolism, and reactive oxygen species []. Interestingly, a curcumin derivative containing two molecules of niacin has been shown to prevent the growth of colon, breast, and nasopharyngeal cancer cells by promoting P53-mediated apoptosis and cell cycle arrest []. In addition, Kim et al. [] have also shown that niacin prevents TRAIL-induced apoptotic cell death by activating the autophagy flux in human colon cancer cells.
Similarly, niacin has been shown to prevent glioblastoma in pre-clinical studies. Sarkar et al. [] have shown that niacin-stimulated monocytes produced by interferon-alpha14 inhibit the growth of brain tumor-initiating cells. In the same study, authors have found that in a mouse model of glioblastoma, niacin prevents tumor growth. NAM has been shown to prevent melanoma growth in culture as well as in mouse models, maybe by inhibiting the activation of SIRT2 [,]. Selvanesan et al. [] have also shown in a mouse model that a combination of gemcitabine with nicotinamide (NAM) prevents pancreatic cancer by decreasing the tumor-associated macrophages and myeloid-derived suppressor cells. Similarly, Shu et al. [] have shown that the niacin-ligated platinum (iv) and ruthenium (ii) chimeric complex prevents metastasis as well as the growth of cancer cells in vivo.
Accordingly, current evidence suggests that niacin could be a potent chemopreventive agent in controlling the growth of various cancer types [,]. Further, niacin, along with chemotherapeutic drugs, has been shown to increase overall survival and efficacy. Indeed, a recent National Health and Nutrition Examination Survey from 1999–2014 conducted by Ying et al. [] has suggested that higher dietary vitamin B3 intake is associated with an improved survival rate and lowered mortality in cancer patients.
5. Vitamin B5: Pantothenic Acid
Pantothenic acid (vitamin B5 or pantothenate) in its anionic form, is a precursor to coenzyme A (CoA) and is an essential micronutrient found generally in a wide variety of foods, including animal products, mushrooms, potatoes, and oats []. A portion of pantothenic acid is also synthesized de novo by the gut microbiota after a three-step enzymatic reaction, which concludes with an adenosine triphosphate (ATP)-dependent condensation of β-alanine and pantoate by pantothenate synthetase []. Along with cysteine and four ATP molecules, pantothenic acid is necessary for the biosynthesis of CoA in a five-step enzymatic reaction []. CoA is then utilized as a fundamental coenzyme in numerous metabolic processes, most notably in the pyruvate oxidation in the Krebs cycle for energy production and during both the synthesis and oxidation of fatty acids.
Despite its essential role in metabolism, pantothenic acid could have multiple effects on cancer progression and prevention. Few studies have shown its cancer-promoting actions. For example, a recent study by Heckmann et al. [] has demonstrated that pantothenic acid increases the levels of CoA in fibroblasts. []. Murine models, including TLX-5 lymphoma, sarcoma 180, and fibrosarcoma, also demonstrated lower levels of pantothenate, CoA, and acetyl-CoA compared to healthy mice. Miallot et al. [] have indicated that pantothenic acid increases the polarization of myeloid and dendritic cells and antigen presentation in sarcoma. Interestingly, these tumor models showed an increase in 4-phosphopantothenate levels, an intermediate in CoA synthesis catalyzed by pantothenate kinase. This abnormal level may result from CoA degradation or increased pantothenate kinase activity rather than an increase in CoA itself [].
However, some human studies have shown no significant association between a high pantothenic acid-containing diet and the risk of esophageal squamous cell carcinoma []. Similarly, studies on urothelial cell carcinoma and gastric cancer revealed no statistically significant correlation between pantothenic acid intake and cancer risk [,]. In breast cancer, a cohort study involving 27,853 women aged 45 years or older, with 462 cases of diagnosed breast cancer, found no association between pantothenic acid intake over 12 months and breast cancer development []. Furthermore, in a study involving five patients with advanced carcinomas (three with stage 4 cervical carcinoma and two with recurrent carcinoma of the floor of the mouth), a liquid diet deficient in pantothenic acid for 4 to 10 weeks did not alter the tumor growth rate compared to periods when the diet included pantothenic acid. Although urinalysis confirmed a decrease in pantothenic acid excretion, suggesting lower body levels, no symptoms of pantothenic acid deficiency were observed, likely due to endogenous production from intestinal microbiota [].
Further, tumor-promoting effects of pantothenic acid have been shown in certain cancer cell types. For example, in breast cancer, increased pantothenic acid concentrations correlated with enhanced glycolytic activity and cell migration, particularly in the MCF-7 luminal gene cluster cell line, the JIMT-1 basal A cell line, and the MDA-MB-231, MDA-MB-435, and MDA-MB-436 basal B cell lines []. Similarly, Kreuzaler et al. [] have shown that pantothenic acid is involved in the upregulation of c-MYC and SLC5A6, which promote cancer growth. Further, dietary restriction of pantothenic acid prevents tumor growth by reversing the c-MYC-modulated metabolic changes. Similarly, pantothenic acid and its metabolites have demonstrated protective qualities in specific cancer cells. For example, in Ehrlich ascites tumor cells grown in the peritoneal cavity of Swiss female mice, pantothenic acid and its derivatives (pantothenol and pantethine) decreased plasma membrane and mitochondrial outer membrane permeability. This protective effect is thought to result from the biosynthetic increase in cholesterol, which counters the leakage induced by digitonin, a compound that disrupts membrane integrity by complexing with cholesterol []. Additionally, pantothenic acid and its derivatives protected Ehrlich ascites tumor cells against lipid peroxidation induced by free oxygen radicals, further supporting its role in promoting cellular repair mechanisms [].
Elevated levels of pantothenic acid levels were also found in the MKN-28 and AGS cell lines of gastric cancer cells, suggesting that increased phospholipid production from reactive oxygen species could contribute to both the rise in pantothenic acid levels and the proliferation of these cells []. A case-control study by Secchi et al. [] in Cordoba, Argentina, revealed that high dietary intake of pantothenic acid was statistically significant in increasing the risk of oral squamous cell carcinoma.
Pantothenic acid and its metabolites have also been implicated in treating various cancers in some clinical studies. For example, higher pre-treatment plasma levels of pantothenic acid in patients with Stage III or IV melanoma correlated with an increased response to anti-programmed cell death protein 1 (PD1) antibody therapy. Similarly, pre-treatment with pantothenate injections enhanced the efficacy of anti-PD1 antibody therapy in the murine colon carcinoma model MC38 []. In cancers that rely heavily on glycolysis and lactic acid fermentation for growth, such as soft tissue sarcoma (STS), targeting pantetheine, a degradative product of CoA, has shown promise in reducing tumor growth. Treatment with Vnn1 pantetheinase, which degrades pantetheine into pantothenate and cysteamine, led to increased mitochondrial CoA levels and a move from glycolysis to oxidative phosphorylation, thereby suppressing tumor growth (Figure 5) []. Pantethine, a disulfide bridge-linked molecule of two pantetheine molecules, has also shown potential in treating multidrug-resistant cancers by inhibiting the release of microparticles that protect cancer cells from treatment []. In mouse models of ovarian tumors derived from human ovarian tumor xenografts, pantetheine treatment decreased metastasis and ascites formation [].
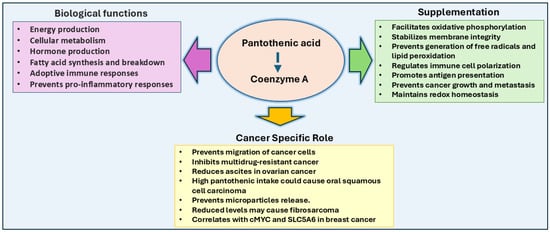
Figure 5.
Pantothenic acid in cancer: Pantothenic acid is a precursor to Coenzyme A (CoA) and is vital for numerous metabolic processes, including the citric acid cycle, fatty acid metabolism, and hormone production. Increased pantothenic acid levels correlate with enhanced glycolytic activity and cancer cell migration, particularly in breast and gastric cancer cells, and its association with c-MYC and SLC5A6 expression promotes breast cancer growth. Pantothenic acid supplementation could enhance immune responses by increasing antigen presentation, shifting tumor metabolism from glycolysis to oxidative phosphorylation, and preventing tumor growth. High intake has been linked to oral squamous cell carcinoma risk, and pantothenate supplementation has been shown to treat multidrug-resistant cancers and reduce metastasis.
6. Vitamin B6: Pyridoxine
Vitamin B6 is an essential water-soluble vitamin that plays a role in over 140 different biochemical reactions within the body. These reactions include the synthesis of heme precursor δ-aminolevulinic acid, sphingoid bases essential for myelin production, and several neurotransmitters such as serotonin, norepinephrine, epinephrine, and γ-aminobutyrate (GABA) []. Vitamin B6 is also a cofactor for several enzymatic reactions involved in amino acid metabolism, glycolysis, gluconeogenesis, glycogenesis, trans-sulfuration, immune response, and polyamine biosynthesis []. Structurally, vitamin B6 encompasses six chemically similar compounds, all containing a pyridine ring with varying groups at the 4′ position, and these compounds are interconvertible. The most bioactive form is pyridoxal 5′-phosphate (PLP), with other forms including pyridoxal, pyridoxamine, pyridoxamine 5′-phosphate, pyridoxine, and pyridoxine 5′-phosphate []. Failure to consume adequate amounts of vitamin B6, as well as conditions such as alcoholism and renal or liver complications, can lead to various health issues, including sideroblastic anemia, seizures, peripheral neuropathy, mood changes, and seborrheic dermatitis [].
Further, pyridoxine also plays an important role in amino acid metabolism. It acts as a coenzyme in transamination, decarboxylation, and one-carbon metabolism, which supports nucleotide biosynthesis and cellular redox balance []. Further, PLP is crucial for protein synthesis, neurotransmitter production, and glutathione-mediated antioxidant defense. Pyridoxine influences cell proliferation in cancer cells by increasing amino acid metabolism and nucleotide synthesis. It also modulates apoptosis by regulating oxidative stress, redox balance, and epigenetic alterations. Thus, the deficiency of pyridoxine could promote DNA damage and tumorigenesis, while an excess of pyridoxine could promote tumor growth by modulating the redox-mediated metabolic pathways. Therefore, pyridoxine seems to play a major role in cancer progression as solid tumors rely on high amino acid turnover for survival and growth.
On the other hand, vitamin B6 has also been linked to a reduced risk of developing and proliferating diverse types of cancer. A meta-analysis study that monitored the dietary intake of vitamin B6 across 1,959,417 individuals, including 98,975 cancer cases, revealed that high intake of vitamin B6 was associated with a reduced risk of cancer at multiple sites, including the breast, colorectal area, ovary, prostate, immune system, endometrium, lung, stomach, esophagus, pancreas, kidney, bladder, oral cavity, nasopharynx, larynx, cervix, liver, and brain []. Specifically concerning breast cancer, a case-control study nested in the Multiethnic Cohort in Hawaii and Southern California indicates that increased circulating levels of PLP are associated with a reduced risk of invasive postmenopausal breast cancer []. Another cohort study suggested that vitamin B6 intake over 12 months had a protective effect against breast cancer development []. Additionally, a meta-analysis of eighteen studies (11 prospective studies and seven case-control studies) on the association of dietary vitamin B6 intake with pancreatic cancer showed that high blood levels of pyridoxal 5′-phosphate might protect against the development of pancreatic cancer []. While vitamin B6 supplementation has shown inconsistent and mostly nonsignificant associations with colorectal cancer development, high blood levels of pyridoxal 5′-phosphate have consistently demonstrated a 30–50% reduction in colorectal cancer risk []. Another meta-analysis found variability in the association between dietary vitamin B6 intake and colorectal cancer risk across nine studies but confirmed an inverse relationship between blood pyridoxal 5′-phosphate levels and colorectal cancer risk across four studies []. A recent meta-analysis study also indicates the risk of developing colon cancer is negatively correlated with the vitamin B6 and PLP levels []. Interestingly, Holowatyj et al. [] have indicated that high preoperative vitamin B6 is linked to increased survival in stage 1-III colorectal cancer patients. Similarly, Li et al. [] have also indicated that high PLP levels but not Par could be associated with the increased survival of colon cancer patients. Along similar lines, Xu et al. [] have also suggested that increased PLP and pyridoxal are associated with colorectal cancer risk in the Chinese population. Thus, the protective effects of pyridoxal 5′-phosphate are thought to be due to its involvement in one-carbon metabolism, which is related to DNA synthesis and methylation, and its role in reducing inflammation, proliferation, and oxidative stress []. Indeed, Wu et al. [] have indicated that Vitamin B6 is a cofactor associated with enzymes, like serine hydroxymethyltransferase (SHMT), methionine synthase reductase (MTRR), and methionine synthase (MS) and that it also plays a regulatory role in regulating genomic stability and cell viability in breast cancer patients.
The ability of vitamin B6 to interact with nuclear receptors and express antioxidant, pro-apoptotic, and anti-angiogenic effects in cells has been further elucidated. Vitamin B6 conjugates with RIP140, a receptor-interacting protein, enhancing its transcriptional co-repressive activity by increasing its interaction with histone deacetylases and retaining RIP140 in the nucleus [].
Further, vitamin B6 deficiency has been linked to an increased risk of various cancers. For example, a recent study indicates that PLP deficiency leads to malignant tumors in a Drosophila model []. Similarly, Yasuda et al. [] have shown that vitamin B6 deficiency is common in patients with primary and secondary myelofibrosis. Moreover, vitamin B6 deficiency has been linked to sarcopenia, a condition in which muscle loss is due to factors such as aging or immobility []. Spinneker et al. [] have suggested an increase in colon tumorigenesis in vitamin B6-deficient mice, while vitamin B6 deficiency in rats showed signs of chronic pancreatitis, a condition known to increase the risk of pancreatic cancer. Several mechanisms have been proposed to explain the correlation between vitamin B6 deficiency and carcinogenesis. In DNA synthesis, vitamin B6 deficiency reduces serine hydroxy-methyltransferase activity, leading to a lack of methylene groups for 5,10-methylenetetrahydrofolate. This deficiency causes inadequate methylation of deoxy-uridylate to deoxy-thymidylate, resulting in the misincorporation of uracil into DNA instead of thymidine, which leads to chromosome strand breaks and impaired DNA excision repair. Another proposed mechanism involves DNA hypomethylation, given that vitamin B6 is associated with DNA methylation. A third mechanism suggests that vitamin B6 deficiency impairs the activity of detoxifying enzymes such as glutathione S-transferase and glutathione peroxidase, which detoxify carcinogenic compounds. Pyridoxal 5′-phosphate is required for enzymes in the trans-sulfuration pathway to generate cysteine, which is essential for glutathione synthesis (Figure 6). A fourth mechanism proposes that vitamin B6 deficiency increases steroid hormone sensitivity, potentially contributing to the development of breast, uterine, and prostate tumors [].
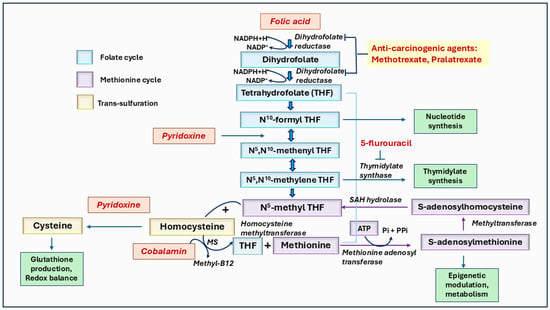
Figure 6.
The role of vitamins B6, B9, and B12 mediated one-carbon metabolism in cancer. Folate (vitamin B9) is converted to dihydrofolate and then to tetrahydrofolate (THF), which undergoes further modifications to form derivatives like N5, N10-methylene THF, and N5-methyl THF. These derivatives are essential for methylation processes, and nucleotide synthesis requires cancer cell growth. The pathway highlights the involvement of pyridoxine (vitamin B6) in converting homocysteine to cysteine via the trans-sulfuration pathway and cobalamin (vitamin B12) in the re-methylation of homocysteine to methionine, which subsequently forms S-adenosylmethionine (SAM), a key methyl donor in several biochemical and epigenetic methylations. Anti-carcinogenic agents, such as methotrexate and pralatrexate, inhibit steps in folate metabolism, while 5-fluorouracil blocks nucleotide synthesis. This pathway underscores the interdependence of folate, pyridoxine, and cobalamin in one-carbon metabolism in essential biological functions like methylation, DNA synthesis, and cellular regulation.
Increased vitamin B6 catabolism, marked by a rise in the PAr index (the ratio of 4-pyridoxic acid, a catabolic product, to the sum of pyridoxal and pyridoxal 5′-phosphate), has been associated with a higher risk of lung cancer. A study of 20 cohorts across four continents found that an increase in the PAr index could serve as a pre-diagnostic marker for lung cancer []. Additionally, a survey of 5364 matched case-control pairs showed that impaired vitamin B6 status was significantly associated with an increased risk of lung cancer, with squamous cell carcinoma being the most common form []. In patients with non-small cell lung cancer, treatment with cytotoxic agents such as cisplatin was less effective if the patient had low expression of pyridoxal kinase, which converts pyridoxal to its more active form, pyridoxal 5′-phosphate []. Huang et al. [] have also shown that low serum B6 vitamers are linked with increased pancreatic cancer risk in the Asian population. Similarly, lower levels of PLP have been associated with the development of pancreatic ductal adenocarcinoma []. These studies suggest that high levels of vitamin B6 could provide protection against various cancers, while low levels or deficiency are a major risk factor for promoting cancer growth.
7. Vitamin B7: Biotin
Vitamin B7, known as biotin, is water-soluble and typically obtained through dietary sources such as beef, eggs, salmon, pork chops, and vegetables like broccoli and spinach []. It acts as a cofactor for various carboxylase enzymes involved in metabolic pathways, including fatty acid synthesis, glucose metabolism, and amino acid metabolism []. Additionally, biotin plays a role in epigenetics and influences gene silencing, DNA repair, and chromatin remodeling []. The importance of biotin is further highlighted by the substantial energy required to produce a single molecule for participation in these biochemical reactions [].
While biotin is a vital cofactor in many biochemical reactions, its broader roles in other pathways are less understood than other B vitamins. However, evidence suggests that biotin may be necessary for skin maintenance. Ogawa et al. [] observed that acrodermatitis enteropathica (AE), partly due to biotin deficiency, led to symptoms similar to those in zinc-deficient diets. Further, biotin deficiency results in more pronounced irritant contact dermatitis, mirroring the effects of zinc deficiency. Therefore, biotin may play a key role in regulating and maintaining skin health, especially in individuals with skin conditions that impact their quality of life [,].
Biotin has also been associated with effects on glycolytic and gluconeogenic enzyme activity. Sugita et al. [] have shown that in biotin treatment in streptozotocin-induced rats, the mRNA levels of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase were decreased, and glucokinase was increased. Similarly, McCarty [] has suggested that a high dose of biotin also alters the expression of glycolytic enzymes. In studies on chronic kidney disease (CKD), the regulation of cGMP, particularly when complexed with specific protein kinases, showed promise for reno-protective functions, potentially aiding in the prevention of CKD []. Moreover, inhibitors of the cGMP pathway, especially when part of the cGMP-cGK1-PDE complex, have shown potential for anti-clotting and anticancer properties. These findings could help to develop new cancer treatment modalities. However, further studies are needed to demonstrate biotin’s role in cGMP activity, signaling, coagulation, and thrombotic events, as well as its potential contributions to cancer cell proliferation.
Indeed, Maiti and Paira [] have indicated that sodium-dependent multivitamin transporter (a biotin transporter) is overexpressed in various cancer cells, including breast, colon, lung, ovarian, and leukemia. Thus, biotin is used in the development of various drug delivery methods to treat cancers. For example, Tang et al. [] have developed biotin-modified liposomes for breast cancer. Raza et al. [] have indicated that the seleno-biotin compound induces apoptosis in ovarian cancer cells. Similarly, Kundu et al. [] have shown the potential use of Chitosan-biotin-conjugated nanoparticles for increasing the chemotherapy potential.
Biotin is well-tolerated, with few noted adverse effects, toxicities, or contraindications. However, biotin deficiency can arise from insufficient dietary intake or an inability to utilize the vitamin within the body. Individuals deficient in biotin typically present symptoms such as skin rashes, hair loss, and fragile nails, along with neurological deficits like depression and paresthesias []. Recent studies mostly concentrated on developing drug delivery methods using biotin conjugates to prevent cancer. However, the connection between biotin deficiency and cancer is not clear, and additional studies are needed to understand the significance of biotin in cancer development and therapy.
8. Vitamin B9: Folate
Vitamin B9, known as folate, is an essential water-soluble vitamin found in legumes and green leafy vegetables. Folate deficiency is associated with developmental and degenerative conditions such as neural tube defects in embryos []. Consequently, vitamin B9 supplementation is recommended for women of childbearing age, starting one month prior to conception to the first trimester of pregnancy. Folate is involved in one-carbon metabolism and has been studied for its role in cancer development [,]. It is required in the methionine pathway to convert homocysteine to methionine, which is converted to S-adenosylmethionine (SAM). SAM plays a fundamental role in DNA and RNA methylation, affecting gene transcription and the expression of tumor suppressors and proto-oncogenes (Figure 6). Additionally, folate is vital in converting deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), which is essential for DNA synthesis and repair. Therefore, folate deficiency can lead to uracil misincorporation instead of thymine, resulting in DNA repair dysfunction, unstable DNA, and strand breaks [,]. On the other hand, excessive folate can also cause tumor progression by supporting rapid cell proliferation. Thus, deficiency and oversupply of folate can influence tumor development and progression.
Epigenetic changes caused by chronic inflammation can also be a factor in cancer development. Further patients with inflammatory bowel diseases are at a higher risk of developing colorectal cancer. Tumors of the gastrointestinal tract, prostate, and liver have also been linked to sites of chronic inflammation. B vitamins, including folate, regulate inflammation and the immune response due to their involvement in nucleic acid, protein synthesis, and methylation. Disruptions in the immune system can occur due to improper cytokine production, faulty antigen presentation, and an unregulated immune response. Inefficient methylation, often caused by vitamin B deficiencies, can lead to hyperhomocysteinemia, a condition associated with oxidative stress and chronic inflammation []. Elevated homocysteine levels and low folate have shown an increased risk of lung, breast, and colorectal cancers [,]. Further, elevated homocysteine and methionine have been suggested to disrupt the epigenetic modification of specific genes that regulate breast cancer progression and initiation, such as RASS-F1 and BRACA1 [,].
Moreover, some case-control studies have shown an inverse correlation between dietary folate intake and the risk of pharyngeal, oral, esophageal, colorectal, pancreatic, laryngeal, and breast cancers [,,,,,]. Additionally, risk estimates for cancers of the endometrium, ovary, prostate, and kidney were below unity, indicating a potential protective effect, while stomach cancer showed no relation to dietary folate intake [,]. Studies using colon cancer cells have demonstrated that folic acid supplementation can inhibit cell proliferation. This inhibition occurs through activating the c-SRC-mediated pathway and amplified levels of the cyclin-dependent kinase (CDK) inhibitor and tumor suppressor p53, leading to G0/G1 cell cycle arrest []. Folate has been shown to be critical in regulating the cancer growth [,,,,,,,,,]. Specifically, colorectal cancer has been one of the most extensively studied cancers concerning folate’s role in carcinogenesis. Most studies have shown a significant inverse relationship between folate status and colorectal cancer risk [,,]. Epidemiologic studies comparing subjects with the highest dietary folate intake to those with the lowest have suggested a reduction of about 40% in the risk of colorectal neoplasms []. Few studies also suggest that even a modest decrease in folate levels can enhance colorectal cancer risk without clinical evidence of folate deficiency [,]. Randomized intervention studies in humans have also shown that folate supplementation in patients with resected colonic adenomas can reverse colorectal cancer biomarkers, including genomic DNA hypomethylation in the rectal mucosa and decreased proliferation of rectal mucosal cells [,].
Although these studies provide evidence of an inverse association between folate levels and colon cancer risk, they are still insufficient to draw definitive conclusions. However, a safe and effective dosage for folate has not been established in humans. Some studies have suggested that excessive folate supplementation could increase cancer risk and promote tumor progression []. Folate supplementation is available in various forms, including tablets, mixtures, and intravenous preparations. Recently, several countries have implemented food fortification programs in grains and cereals to prevent neural tube defects in embryos [].
Further, few studies suggest that the protective effects of folate plateau are beyond a certain level and do not increase indefinitely with higher intake [,]. Colorectal cancer studies observed a decreased cancer risk with moderate folate status but an increased risk with excessive intake []. Similar findings were observed in breast cancer, where high plasma folate concentrations were associated with premenopausal breast cancer. A double-blind study on colorectal polyp recurrence showed that individuals supplemented with 1 mg/day of folate had more multiple and advanced adenomas than the placebo group []. These results suggest that people with prior adenomas might be at a higher risk of developing various or advanced polyps due to folic acid supplementation. The study also indicated a higher rate of invasive prostate cancer among the folic acid group []. Multiple in vitro and animal studies have summarized that increased folate supplementation may promote the progression of established tumors, while folate deficiency might contribute to tumorigenesis [].
9. Vitamin B12: Cobalamin
Vitamin B12 (Cobalamin) is found in animal-derived products such as dairy, red meat, and eggs []. It is not produced by plants, making vegans and vegetarians at a higher risk for B12 deficiency unless they rely on supplementation, as they depend solely on microbial sources. Like folate, vitamin B12 is necessary due to its role in one-carbon metabolism (Figure 6), which involves homocysteine, methionine, and B vitamins []. One-carbon metabolism directs the one-carbon units toward the folate cycle, essential for DNA and RNA synthesis, or toward methionine regeneration, where methionine acts as a precursor to S-adenosylmethionine (SAM). SAM is vital for the methylation of histones, proteins, and DNA. Homocysteine generates methionine through a reaction catalyzed by the enzyme methionine synthase (MeS). The activation of MeS requires cobalamin as a cofactor for converting 5-methyl tetrahydrofolate (THF) to THF. Further, SAM is a potential methyl group donor for DNA, RNA, and protein methylations. Thus, SAM-mediated methylation is also significant for gene expression regulation, genomic stability, and epigenetic modifications, which influence cancer progression. Cobalamin is also vital for nucleotide synthesis, which is required for DNA replication and repair. Thus, a deficiency of cobalamin could lead to DNA damage, uracil misincorporation, and hypomethylation, which increases genomic instability and promotes the risk of developing cancer-causing mutations. Similarly, an excess of B12 could promote tumorigenesis by increasing cell proliferation and modulating cancer cell metabolism.
Further, both vitamin B12 and folate deficiencies are associated with a reduction in a significant antioxidant protein called glutathione, a product of the trans-sulfuration pathway. Glutathione acts as an inhibitor of reactive oxygen species (ROS)-induced oxidative stress and a mediator of redox balance. The impact of folate and vitamin B12 was examined in a study in which mice were treated with Azoxymethane (AOM), a compound known to induce tumors and oxidative stress. The study showed that both vitamins reduced the cytotoxic effects of AOM and mitigated oxidative stress []. A recent study by Obeid et al. [] has indicated high plasma levels of vitamin B12 in various cancer patients. However, the role of B12 in promoting or preventing cancer is still not clear.
Moreover, a few studies also indicated a significant role of vitamin B12 intake and risk of developing colorectal, breast, prostate, malignant melanoma, or squamous cell carcinoma [,,,]. However, the study did reveal a positive correlation between vitamin B12 and the risk of esophageal cancer []. Another nested case-control study examined the association between serum concentrations of one-carbon nutrients, including vitamin B12, and upper gastrointestinal cancers. A statistically significant correlation was noticed between low vitamin B12 serum concentration and an increased risk of non-cardia gastric adenocarcinoma (NCGA). This association is mechanistically understandable due to the growth of the intestinal type of gastric cancer model. Chronic superficial gastritis often progresses to chronic atrophic gastritis and eventually to adenocarcinoma. Chronic atrophic gastritis leads to decreased gastric acid secretion, which diminishes vitamin B12 absorption. This study suggested that vitamin B12 deficiency in these cases was due to malabsorption rather than insufficient dietary intake [].
While vitamin B12 deficiency can contribute to tumor development, over-supplementation may also increase cancer risk. For example, Collin et al. [] have shown that increased intake of folate and vitamin B12 increases the risk of prostate cancer. Similarly, use of vitamin B12 supplements, excluding those from multivitamins, has been associated with a 30–40% increase in lung cancer risk. In contrast, no association was found between folic acid supplementation and cancer risk [,]. Similarly, a Norwegian randomized controlled trial has found an enhanced risk of lung cancer development in individuals who have taken both vitamin B12 and B9 supplements []. Additionally, Fanidi et al. [] have also suggested increased lung cancer risk with high supplementation of vitamins B12 and B6. To further confirm the role of vitamin B12 in lung cancer, various population-based cohort studies measured circulating vitamin B12 concentrations in pre-diagnostic samples [,,,]. These studies found a positive correlation between cancer risk and the circulating concentration of vitamin B12 in cancer patients’ blood samples.
10. Conclusions and Future Perspectives
B vitamins are essential in maintaining cellular functions and metabolic processes. Their balance is very important for cell growth, proliferation, differentiation, and survival. Thus, understanding how B vitamins imbalance could lead to cancer growth and spread will help to control unnecessary dietary intake of excessive vitamin supplements. Further, identifying the role of vitamins is also crucial for developing chemopreventive strategies not only to maximize benefits but also to minimize potential risks. This review focused specifically on the relationship between B vitamins and cancer development or prevention. Several studies provided considerable evidence indicating both beneficial and harmful effects depending on the type of vitamin and cancer type [,,]. While deficiencies in water-soluble B vitamins, such as thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folate, and cobalamin, are generally associated with an increased risk of cancer due to impaired metabolic and cellular functions, emerging research highlights the potential dangers related to excessive intake supplementation of these vitamins. Further, over-supplementation of B vitamins could enhance cancer cell metabolism, DNA repair, and genomic instability which can accelerate tumor progression in cancers with strong metabolic flexibility (Table 3).

Table 3.
Effects of B Vitamin Deficiencies and Over-Supplementation on Cancer Progression and Prevention.
For example, vitamins like folate and B12, which are essential for DNA synthesis and repair, and their excessive supplementation have been linked to increased risks of certain cancers, such as colorectal and lung cancer [,,]. A recent case-control study by Le et al. [] has suggested the risk of esophageal, lung, and breast cancers in patients with low intake of cobalamin and gastric cancer in patients with high intake in Vietnamese population. On the other hand, riboflavin and niacin have been shown to enhance the efficacy of cancer therapies [,]. Premkumar et al. [] have shown that supplementation of riboflavin and niacin along with tamoxifen reduced tumor burden in breast cancer patients. Similarly, rapamycin and niacin combination increases apoptotic cell death in acute myeloid leukemia cells. []. Yuvaraj et al. [] have also shown that combination treatment of Coenzyme-Q, riboflavin and niacin with tamoxifen e increases blood parameters in postmenopausal breast cancer women. However, the impact of vitamins B2 and B3 on cancer prevention and progression depends on dosage and cancer type.
Some risks exist in taking these B vitamins without proper guidance, as they can interact with certain medications and potentially alter their effectiveness. In addition to enhancing the chemotherapeutic effects of some drugs, some B vitamins could also improve the therapeutic effects of general medicines. For example, vitamin B12 supplementation can counteract the deficiency caused by long-term use of metformin []. Similarly, pyridoxine can reduce the efficacy of the anti-epileptic drug levodopa by increasing its metabolism []. Additionally, high doses of niacin have been shown to increase the risk of liver toxicity when taken along with cholesterol-lowering statins []. High niacin levels are also shown to be a risk for cardiovascular complications []. In addition, folic acid can interfere with methotrexate and reduce its effectiveness [].
Further, recent studies also indicated several limitations and knowledge gaps that warrant attention on vitamin supplementation. A significant concern is the potential side effects of high-dose supplementation. A cohort study by Meyer et al. [] has found that a combined high intake of pyridoxine and cobalamin was associated with an increased risk of hip fracture. Similarly, B vitamins might prevent cardiovascular diseases by lowering homocysteine levels; however, they can also alter the efficacy of statin drugs. Another limitation is the inconsistent results from clinical trials. While most observational studies have suggested the protective roles of certain B vitamins in preventing cancer growth, clinical trials have not consistently supported this, and additional studies are required. Further, there is a lack of comprehensive studies understanding the synergetic interactions between different B vitamins in human diseases. For example, taking folic acid alone could mask vitamin B12 deficiency, which could lead to neurological problems []. Thus, some of the discussed limitations point towards the need for more rigorous and well-designed studies to investigate the significance of B vitamins in human health and disease.
The interplay of B vitamins in cancer biology emphasizes the importance of maintaining an equilibrium in vitamin consumption. Careful attention is required to identify the cancer risk in patients with vitamin deficiency. Additional extensive population-based studies are needed to understand the dietary intake of vitamins and cancer progression and therapy. Understanding the role of vitamins in combination with chemotherapeutic drugs and immunotherapeutic drugs is required to enhance the therapeutic efficacy and increase the survival rate.
Further, identifying the molecular mechanisms of how the vitamins-mediated cellular and metabolic pathways are involved in cancer initiation and progression will help identify potential therapeutic strategies. Recent proteomic, metabolomic, and genomic studies will help identify novel biomarkers. Next-generation gene sequencing will also help to identify potential carcinogenic genes regulated by vitamin supplementation. Further studies are also needed to understand the interactions between different B vitamins and their combined effects on cancer progression, which could lead to more targeted and personalized approaches in cancer therapy.
Thus, future research should focus on elucidating the precise mechanisms by which these vitamins influence cancer pathways and identify safe and effective dose levels. Taking over-the-counter vitamin supplementation without a physician’s recommendation is sometimes a risk of developing unnecessary complications and side effects []. Since the requirement of vitamin supplementation depends on age, body metabolic rate, gender, and any prescribed medications, one should not self-diagnose and take supplements that unnecessarily can mask underlying health issues and complicate the disease progression []. Thus, physician input ensures vitamin supplements are safe, effective, and tailored to a patient’s needs. Therefore, always consult a healthcare provider before starting vitamin supplementation, who can recommend suggested doses based on specific medical conditions.
Author Contributions
Z.F., S.B., C.N.A., R.V.P. and K.V.R. drafted the initial manuscript, and analyzed, reviewed, and edited the final draft. K.V.R. conceptualized the idea and topic of the review, drafted, reviewed, and edited. Grammarly online software was used for language editing and improvement. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All the authors declare no conflicts of interest.
References
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the Nervous System: Current Knowledge of the Biochemical Modes of Action and Synergies of Thiamine, Pyridoxine, and Cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.M.; Esmael, A.; Nassar, A.K.; El-Sherif, M. The Relationship between Exacerbated Diabetic Peripheral Neuropathy and Metformin Treatment in Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-Term Metformin Therapy and Vitamin B12 Deficiency: An Association to Bear in Mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef]
- Fituri, S.; Akbar, Z.; Ganji, V. Impact of Metformin Treatment on Cobalamin Status in Persons with Type 2 Diabetes. Nutr. Rev. 2024, 82, 553–560. [Google Scholar] [CrossRef]
- Al Zoubi, M.S.; Al Kreasha, R.; Aqel, S.; Saeed, A.; Al-Qudimat, A.R.; Al-Zoubi, R.M. Vitamin B12 Deficiency in Diabetic Patients Treated with Metformin: A Narrative Review. Ir. J. Med. Sci. 2024, 193, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chien, H.C.; Yee, S.W.; Giacomini, M.M.; Chen, E.C.; Piao, M.; Hao, J.; Twelves, J.; Lepist, E.I.; Ray, A.S.; et al. Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol. Pharm. 2015, 12, 4301–4310. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, S.Y.; Kim, H. Drug-Induced Vitamin Deficiency. Ann. Clin. Nutr. Metab. 2022, 14, 20–31. [Google Scholar] [CrossRef]
- Björkegren, K.; Svärdsudd, K. Serum cobalamin, folate, methylmalonic acid and total homocysteine as vitamin B12 and folate tissue deficiency markers amongst elderly Swedes-A population-based study. J. Intern. Med. 2001, 249, 423–432. [Google Scholar] [CrossRef]
- Gale, D.P.; Cobbold, J.F.; Chataway, J. Steroid-Responsive Functional B12 Deficiency in Association with Transcobalamin II Polymorphism 776C→G. Eur. J. Haematol. 2006, 76, 75–78. [Google Scholar] [CrossRef]
- Choudhury, A.; Jena, A.; Jearth, V.; Dutta, A.K.; Makharia, G.; Dutta, U.; Goenka, M.; Kochhar, R.; Sharma, V. Vitamin B12 Deficiency and Use of Proton Pump Inhibitors: A Systematic Review and Meta-Analysis. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lerman, T.T.; Cohen, E.; Sochat, T.; Goldberg, E.; Goldberg, I.; Krause, I. Proton Pump Inhibitor Use and Its Effect on Vitamin B12 and Homocysteine Levels Among Men and Women: A Large Cross-Sectional Study. Am. J. Med. Sci. 2022, 364, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Antiepileptic Drugs Reduce Vitamin B12 and Folate Levels. Nat. Rev. Neurol. 2011, 7, 125. [Google Scholar] [CrossRef]
- Cahill, V.; McCorry, D.; Soryal, I.; Rajabally, Y.A. Newer Anti-Epileptic Drugs, Vitamin Status, and Neuropathy: A Cross-Sectional Analysis. Rev. Neurol. 2017, 173, 62–66. [Google Scholar] [CrossRef]
- Prajjwal, P.; Inban, P.; Sai, V.P.; Shiny, K.S.; Lam, J.R.; John, J.; Sulaimanov, M.; Tekuru, Y.; Wasi Ul Haq, M.; Marsool, M.D.M.; et al. The Effects of the Interplay Between Vitamins, Antibiotics, and Gut Microbiota on the Pathogenesis and Progression of Dementia: A Systematic Review and Meta-Analysis. Health Sci. Rep. 2024, 7, e1808. [Google Scholar] [CrossRef] [PubMed]
- Iimura, Y.; Kurokawa, T.; Nojima, M.; Kanemoto, Y.; Yazawa, K.; Tsurita, G.; Kuroda, S. Potential Thiamine Deficiency and Neurological Symptoms in Patients Receiving Chemotherapy for Gastrointestinal Cancer. Int. J. Clin. Pharmacol. Ther. 2020, 58, 139–145. [Google Scholar] [CrossRef]
- Iimura, Y.; Andoh, S.; Kawamata, T.; Sato, A.; Yokoyama, K.; Imai, Y.; Tojo, A.; Nojima, M.; Sugiura, M.; Kuroda, S. Thiamine Deficiency and Neurological Symptoms in Patients with Hematological Cancer Receiving Chemotherapy: A Retrospective Analysis. J. Neurosci. Rural. Pract. 2021, 12, 726–732. [Google Scholar] [CrossRef]
- Renting, L.; Zwart, N.R.K.; Ueland, P.M.; McCann, A.; Ulvik, A.; van Halteren, H.K.; Lubberman, F.J.E.; Winkels, R.M.; Kampman, E.; Kok, D.E. Vitamin B6 Status and Chronic Chemotherapy-Induced Peripheral Neuropathy: A Prospective Cohort Study Among Patients with Non-Metastatic Colorectal Cancer Receiving Oxaliplatin-Based Chemotherapy. BMJ Oncol. 2024, 3, e000462. [Google Scholar] [CrossRef]
- Venturelli, S.; Leischner, C.; Helling, T.; Burkard, M.; Marongiu, L. Vitamins as Possible Cancer Biomarkers: Significance and Limitations. Nutrients 2021, 13, 3914. [Google Scholar] [CrossRef]
- Kune, G.; Watson, L. Colorectal Cancer Protective Effects and the Dietary Micronutrients Folate, Methionine, Vitamins B6, B12, C, E, Selenium, and Lycopene. Nutr. Cancer 2006, 56, 11–21. [Google Scholar] [CrossRef]
- Harnack, L.; Jacobs, D.R., Jr.; Nicodemus, K.; Lazovich, D.; Anderson, K.; Folsom, A.R. Relationship of Folate, Vitamin B6, Vitamin B12, and Methionine Intake to Incidence of Colorectal Cancers. Nutr. Cancer 2002, 43, 152–158. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Hecht, J.L.; Giovannucci, E.; Hankinson, S.E. Intake of Folate and Related Nutrients in Relation to Risk of Epithelial Ovarian Cancer. Am. J. Epidemiol. 2006, 163, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The Importance of Thiamine (Vitamin B1) in Humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.C.; Gutierrez, J.L. Thiamin. Adv. Nutr. 2017, 8, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Júnior, J.R.A.; Costa Pereira, J.P.D.; Pires, L.L.; Maia, C.S. The Dichotomous Effect of Thiamine Supplementation on Tumorigenesis: A Systematic Review. Nutr. Cancer 2022, 74, 1942–1957. [Google Scholar] [CrossRef]
- Mateos-Díaz, A.M.; Marcos, M.; Chamorro, A.J. Wernicke-Korsakoff Syndrome and Other Diseases Associated with Thiamine Deficiency. Med. Clin. 2022, 158, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine Deficiency Disorders: A Clinical Perspective. Ann. N. Y. Acad. Sci. 2021, 1498, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Liu, Z.; Gao, M.; Tu, K.; Xu, Q.; Zhang, Y. Targeting Nutrient Dependency in Cancer Treatment. Front. Oncol. 2022, 12, 820173. [Google Scholar] [CrossRef]
- Onishi, H.; Sato, I.; Uchida, N.; Takahashi, T.; Furuya, D.; Ebihara, Y.; Yoshioka, A.; Ito, H.; Ishida, M. High Proportion of Thiamine Deficiency in Referred Cancer Patients with Delirium: A Retrospective Descriptive Study. Eur. J. Clin. Nutr. 2021, 75, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Ishida, M.; Toyama, H.; Tanahashi, I.; Ikebuchi, K.; Taji, Y.; Fujiwara, K.; Akechi, T. Early Detection and Successful Treatment of Wernicke Encephalopathy in a Patient with Advanced Carcinoma of the External Genitalia During Chemotherapy. Palliat. Support. Care 2016, 14, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H.; Debnam, J.M.; Fuller, G.N.; de Groot, J. Wernicke’s Encephalopathy: An Underrecognized and Reversible Cause of Confusional State in Cancer Patients. Oncology 2009, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Vaze, A.; Lin, S.; Juhaeri, J. Risk of Wernicke’s Encephalopathy and Cardiac Disorders in Patients with Myeloproliferative Neoplasm. Cancer Epidemiol. 2015, 39, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Chen, J.; He, G.; Cai, D.; Giovannetti, E.; Inamura, K.; Liu, S.; Ma, W. Lactic Acid: A Narrative Review of a Promoter of the Liver Cancer Microenvironment. J. Gastrointest. Oncol. 2024, 15, 1282–1296. [Google Scholar] [CrossRef]
- Sutendra, G.; Michelakis, E.D. Pyruvate Dehydrogenase Kinase as a Novel Therapeutic Target in Oncology. Front. Oncol. 2013, 3, 38. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Kinnaird, A.; Stenson, T.H.; Haromy, A.; Parker, J.M.; McMurtry, M.S.; Michelakis, E.D. Mitochondrial Activation by Inhibition of PDKII Suppresses HIF1a Signaling and Angiogenesis in Cancer. Oncogene 2013, 32, 1638–1650. [Google Scholar] [CrossRef]
- Škorja Milić, N.; Dolinar, K.; Miš, K.; Matkovič, U.; Bizjak, M.; Pavlin, M.; Podbregar, M.; Pirkmajer, S. Suppression of Pyruvate Dehydrogenase Kinase by Dichloroacetate in Cancer and Skeletal Muscle Cells Is Isoform Specific and Partially Independent of HIF-1α. Int. J. Mol. Sci. 2021, 22, 8610. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting Pyruvate Dehydrogenase Kinase Signaling in the Development of Effective Cancer Therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef] [PubMed]
- Hanberry, B.S.; Berger, R.; Zastre, J.A. High-Dose Vitamin B1 Reduces Proliferation in Cancer Cell Lines Analogous to Dichloroacetate. Cancer Chemother. Pharmacol. 2014, 73, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Madhok, B.M.; Yeluri, S.; Perry, S.L.; Hughes, T.A.; Jayne, D.G. Dichloroacetate Induces Apoptosis and Cell-Cycle Arrest in Colorectal Cancer Cells. Br. J. Cancer 2010, 102, 1746–1752. [Google Scholar] [CrossRef]
- Roh, J.L.; Park, J.Y.; Kim, E.H.; Jang, H.J.; Kwon, M. Activation of Mitochondrial Oxidation by PDK2 Inhibition Reverses Cisplatin Resistance in Head and Neck Cancer. Cancer Lett. 2016, 371, 20–29. [Google Scholar] [CrossRef]
- Jonus, H.C.; Hanberry, B.S.; Khatu, S.; Kim, J.; Luesch, H.; Dang, L.H.; Bartlett, M.G.; Zastre, J.A. The Adaptive Regulation of Thiamine Pyrophosphokinase-1 Facilitates Malignant Growth During Supplemental Thiamine Conditions. Oncotarget 2018, 9, 35422–35438. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, P.; Wu, Y.; Zhou, H.; Wu, H.; Jin, Y.; Wu, D.; Wu, G. SLC25A19 Is a Novel Prognostic Biomarker Related to Immune Invasion and Ferroptosis in HCC. Int. Immunopharmacol. 2024, 136, 112367. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M.; Czerniecka, M.; Jastrzebska, I.; Ratkiewicz, A.; Wawrusiewicz-Kurylonek, N. In Vitro and In Silico Studies on Cytotoxic Properties of Oxythiamine and 2′-Methylthiamine. Int. J. Mol. Sci. 2024, 25, 4359. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, H.L. A Dose- and Time-Dependent Effect of Oxythiamine on Cell Growth Inhibition in Non-Small Cell Lung Cancer. Cogn. Neurodyn. 2022, 16, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Puigjaner, J.; Cascante, M.; Lee, W.N.; Brandes, J.L.; Bassilian, S.; Yusuf, F.I.; Williams, R.D.; Muscarella, P.; Melvin, W.S.; et al. Oxythiamine and Dehydroepiandrosterone Inhibit the Nonoxidative Synthesis of Ribose and Tumor Cell Proliferation. Cancer Res. 1997, 57, 4242–4248. [Google Scholar]
- Liu, X.; Montissol, S.; Uber, A.; Ganley, S.; Grossestreuer, A.V.; Berg, K.; Heydrick, S.; Donnino, M.W. The Effects of Thiamine on Breast Cancer Cells. Molecules 2018, 23, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.; Paul, A.; Zastre, J. Hypoxia Induced Upregulation and Function of the Thiamine Transporter, SLC19A3 in a Breast Cancer Cell Line. Cancer Biol. Ther. 2010, 10, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Lu, X.; Golinski, M.; Comesse, S.; Watt, D.; Grossman, R.B.; Moscow, J.A. Down-Regulation of Thiamine Transporter THTR2 Gene Expression in Breast Cancer and Its Association with Resistance to Apoptosis. Mol. Cancer Res. 2003, 1, 665–673. [Google Scholar] [PubMed]
- Sabui, S.; Ramamoorthy, K.; Romero, J.M.; Simoes, R.D.; Fleckenstein, J.M.; Said, H.M. Hypoxia Inhibits Colonic Uptake of the Microbiota-Generated Forms of Vitamin B1 via HIF-1α-Mediated Transcriptional Regulation of Their Transporters. J. Biol. Chem. 2022, 298, 101562. [Google Scholar] [CrossRef]
- Jonus, H.C.; Byrnes, C.C.; Kim, J.; Valle, M.L.; Bartlett, M.G.; Said, H.M.; Zastre, J.A. Thiamine Mimetics Sulbutiamine and Benfotiamine as a Nutraceutical Approach to Anticancer Therapy. Biomed. Pharmacother. 2020, 121, 109648. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Ahmed Azmi, M.; Siddiqui, J.A.; Panhwar, G.; Shaikh, F.; Ariff, M. Thiamine Level in Type I and Type II Diabetes Mellitus Patients: A Comparative Study Focusing on Hematological and Biochemical Evaluations. Cureus 2020, 12, e8027. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Mubeen, M.; Chohan, H.K.; Jawed, S.; Jamal, A.; Qamar, J.A.; Chohan, M.K.; Siddiqui, A.A.; Anwar, A.; Hashmi, A.A. Correlation of Fasting Blood Sugar and Glycated Hemoglobin (HbA1c) with Thiamine Levels in Diabetic Patients. Cureus 2023, 15, e46178. [Google Scholar] [CrossRef]
- Boopathy, D.; Grahf, D.; Ross, J.; Hawatian, K.; Rammal, J.A.; Alaimo, K.; Miller, J.B. Thiamine Deficiency Is Common and Underrecognized in Emergency Department Oncology Patients. J. Clin. Med. 2025, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, H.; Levi, R.; Konijn, A.M.; Prokocimer, M. Thiamine Deficiency in Patients with B-Chronic Lymphocytic Leukaemia: A Pilot Study. Postgrad. Med. J. 2001, 77, 582–585. [Google Scholar] [CrossRef]
- Baldo, F.; Drago, E.; Nisticò, D.; Buratti, S.; Calvillo, M.; Micalizzi, C.; Schiaffino, M.C.; Maghnie, M. Severe Lactic Acidosis Caused by Thiamine Deficiency in a Child with Relapsing Acute Lymphoblastic Leukemia: A Case Report. Children 2023, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D.; et al. Benfotiamine Blocks Three Major Pathways of Hyperglycemic Damage and Prevents Experimental Diabetic Retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Allowitz, K.V.; Yoo, J.J.; Taylor, J.R.; Baloch, O.A.; Harames, K.; Ramana, K.V. Therapeutic Potential of Vitamin B1 Derivative Benfotiamine from Diabetes to COVID-19. Future Med. Chem. 2022, 14, 809–826. [Google Scholar] [CrossRef]
- Lubis, B.; Lelo, A.; Amelia, P.; Prima, A. The Effect of Thiamine, Ascorbic Acid, and the Combination of Them on the Levels of Matrix Metalloproteinase-9 (MMP-9) and Tissue Inhibitor of Matrix Metalloproteinase-1 (TIMP-1) in Sepsis Patients. Infect. Drug Resist. 2022, 15, 5741–5751. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix Metalloproteinase-9 (MMP-9) and Its Inhibitors in Cancer: A Minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef] [PubMed]
- Fornieles, G.; Núñez, M.I.; Expósito, J. Matrix Metalloproteinases and Their Inhibitors as Potential Prognostic Biomarkers in Head and Neck Cancer After Radiotherapy. Int. J. Mol. Sci. 2023, 25, 527. [Google Scholar] [CrossRef]
- Ćupić Miladinović, D.; Prevendar Crnić, A.; Peković, S.; Dacić, S.; Ivanović, S.; Santibanez, J.F.; Ćupić, V.; Borozan, N.; Antonijević Miljaković, E.; Borozan, S. Recovery of Brain Cholinesterases and Effect on Parameters of Oxidative Stress and Apoptosis in Quails (Coturnix japonica) After Chlorpyrifos and Vitamin B1 Administration. Chem. Biol. Interact. 2021, 333, 109312. [Google Scholar] [CrossRef] [PubMed]
- Lu’o’ng, K.V.; Nguyễn, L.T. The Role of Thiamine in Cancer: Possible Genetic and Cellular Signaling Mechanisms. Cancer Genom. Proteom. 2013, 10, 169–185. [Google Scholar] [PubMed]
- Boot, I.W.A.; Wesselius, A.; Yu, E.Y.W.; Brinkman, M.; van den Brandt, P.; Grant, E.J.; White, E.; Weiderpass, E.; Ferrari, P.; Schulze, M.B.; et al. Dietary B Group Vitamin Intake and the Bladder Cancer Risk: A Pooled Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2022, 61, 2397–2416. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (Vitamin B-2) and Health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J.; Corfe, B.M.; Nakano, E. Riboflavin in Development and Cell Fate. In Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 56, pp. 229–245. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing Optimal Folate and Related B-Vitamin Status Through the Lifecycle: Health Impacts and Challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Pentieva, K.; Ward, M. Causes and Clinical Sequelae of Riboflavin Deficiency. Annu. Rev. Nutr. 2023, 43, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin Photoactivation by Upconversion Nanoparticles for Cancer Treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivatives as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef]
- Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. [Google Scholar] [CrossRef]
- Juarez, A.V.; Sosa, L.d.V.; De Paul, A.L.; Costa, A.P.; Farina, M.; Leal, R.B.; Torres, A.I.; Pons, P. Riboflavin Acetate Induces Apoptosis in Squamous Carcinoma Cells After Photodynamic Therapy. J. Photochem. Photobiol. B. 2015, 153, 445–454. [Google Scholar] [CrossRef]
- Sau, A.; Sanyal, S.; Bera, K.; Sen, S.; Mitra, A.K.; Pal, U.; Chakraborty, P.K.; Ganguly, S.; Satpati, B.; Das, C.; et al. DNA Damage and Apoptosis Induction in Cancer Cells by Chemically Engineered Thiolated Riboflavin Gold Nanoassembly. ACS Appl. Mater. Interfaces 2018, 10, 4582–4589. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Y.; Song, Z.; Yang, R. Lumiflavin Enhances the Effects of Ionising Radiation on Ovarian Cancer Stem-Like Cells by Inhibiting Autophagy. Anticancer. Agents Med. Chem. 2021, 21, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Manthey, K.C.; Rodriguez-Melendez, R.; Hoi, J.T.; Zempleni, J. Riboflavin Deficiency Causes Protein and DNA Damage in HepG2 Cells, Triggering Arrest in G1 Phase of the Cell Cycle. J. Nutr. Biochem. 2006, 17, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, J.T.; Wu, Y.B.; Gao, K.X.; Xie, M.; Zhou, Z.K.; Tang, J.; Hou, S.S. Riboflavin (Vitamin B2) Deficiency Induces Apoptosis Mediated by Endoplasmic Reticulum Stress and the CHOP Pathway in HepG2 Cells. Nutrients 2022, 14, 3356. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Naseem, I. Riboflavin as Adjuvant with Cisplatin: Study in Mouse Skin Cancer Model. Front. Biosci. (Elite Ed.) 2015, 7, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Naseem, I.; Hassan, I.; Alhazza, I.M.; Chibber, S. Protective Effect of Riboflavin on Cisplatin-Induced Toxicities: A Gender-Dependent Study. J. Trace Elem. Med. Biol. 2015, 29, 303–314. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.Y.; Zhu, Z.L.; Tang, P.Y.; Li, K. Vitamin B2 Intake and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Asian Pac. J. Cancer Prev. 2015, 16, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ben, S.; Du, M.; Ma, G.; Qu, J.; Zhu, L.; Chu, H.; Zhang, Z.; Wu, Y.; Gu, D.; Wang, M. Vitamin B2 Intake Reduces the Risk for Colorectal Cancer: A Dose-Response Analysis. Eur. J. Nutr. 2019, 58, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Jung, S.; Zhang, X.; Ogino, S.; Giovannucci, E.L.; Cho, E. Vitamin B2 Intake and Colorectal Cancer Risk: Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study Cohort. Int. J. Cancer 2016, 139, 996–1008. [Google Scholar] [CrossRef]
- Lu, Y.T.; Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kim, J. Riboflavin Intake, MTRR Genetic Polymorphism (rs1532268), and Gastric Cancer Risk in a Korean Population: A Case-Control Study. Br. J. Nutr. 2022, 127, 1026–1033. [Google Scholar] [CrossRef]
- Xu, L.; Wu, Q.X.; Li, X.; Fang, Y.J.; Zhou, R.L.; Che, M.M.; Ma, T.; Zhang, C.X. Serum Flavin Mononucleotide but Not Riboflavin Is Inversely Associated with the Risk of Colorectal Cancer. Food Funct. 2022, 13, 12246–12257. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tan, Y.; Zhu, L. Dietary Vitamin B2 Intake and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2017, 295, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Banerjee, S.; Paul, S.; Mondal, S.; Mandal, M.; Ghosh, P.; Maity, D.; Anoop, A.; Singh, N.D.P.; Mandal, M. Riboflavin-Induced DNA Damage and Anticancer Activity in Breast Cancer Cells Under Visible Light: A TD-DFT and In Vitro Study. J. Chem. Inf. Model. 2024, 64, 5580–5589. [Google Scholar] [CrossRef] [PubMed]
- Sturm, S.; Niegisch, G.; Windolf, J.; Suschek, C.V. Exposure of Bladder Cancer Cells to Blue Light (λ = 453 nm) in the Presence of Riboflavin Synergistically Enhances the Cytotoxic Efficiency of Gemcitabine. Int. J. Mol. Sci. 2024, 25, 4868. [Google Scholar] [CrossRef]
- Chiu, C.M.; Lee, S.Y.; Chen, P.R.; Zhan, S.Q.; Yuann, J.P.; Huang, S.T.; Wu, M.F.; Cheng, C.W.; Chang, Y.C.; Liang, J.Y. An Investigation of the Influence of Reactive Oxygen Species Produced from Riboflavin-5′-Phosphate by Blue or Violet Light on the Inhibition of WiDr Colon Cancer Cells. Photodiagnosis Photodyn. Ther. 2023, 44, 103810. [Google Scholar] [CrossRef]
- Lv, J.J.; Zhang, L.J.; Kong, X.M.; Zhao, Y.; Li, X.Y.; Wang, J.B.; Yang, X.T.; Cheng, Z.H.; Li, W.Z.; Wang, X.H.; et al. Association Between Vitamin B2 Intake and Prostate-Specific Antigen in American Men: 2003–2010 National Health and Nutrition Examination Survey. BMC Public Health 2024, 24, 1224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Jin, X.; Dong, T.; Gao, X.; Wang, J.; Li, Y.; Ma, E. Riboflavin Protects Against Pancreatic Cancer Metastasis by Targeting TGF-β Receptor 1. Bioorg. Chem. 2024, 146, 107274. [Google Scholar] [CrossRef]
- Gunathilake, M.; Kim, M.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Interactions Between Vitamin B2, the MTRR rs1801394 and MTR rs1805087 Genetic Polymorphisms, and Colorectal Cancer Risk in a Korean Population. Epidemiol. Health 2024, 46, e2024037. [Google Scholar] [CrossRef]
- Paragomi, P.; Wang, R.; Huang, J.Y.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Koh, W.P.; Yuan, J.M.; Luu, H.N. The Association Between Serum Riboflavin and Flavin Mononucleotide with Pancreatic Cancer: Findings from a Prospective Cohort Study. Pancreas 2023, 52, e127–e134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huangfu, Y.; Deng, L.; Wang, P.; Shen, L.; Zhou, Y. High Serum Riboflavin Is Associated with the Risk of Sporadic Colorectal Cancer. Cancer Epidemiol. 2023, 83, 102342. [Google Scholar] [CrossRef] [PubMed]
- Barile, M.; Giancaspero, T.A.; Leone, P.; Galluccio, M.; Indiveri, C. Riboflavin Transport and Metabolism in Humans. J. Inherit. Metab. Dis. 2016, 39, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency—Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Dowey, L.R.C.; Strain, J.J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin Lowers Homocysteine in Individuals Homozygous for the MTHFR 677C→T Polymorphism. Circulation 2006, 113, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, J.; Wang, L.; Li, Y.; Jin, J.; Ai, L.; Li, C.; Li, Z.; Mao, S. MTHFR Genetic Polymorphisms May Contribute to the Risk of Chronic Myelogenous Leukemia in Adults: A Meta-Analysis of 12 Genetic Association Studies. Tumor Biol. 2014, 35, 4233–4245. [Google Scholar] [CrossRef] [PubMed]
- Karande, A.A.; Sridhar, L.; Gopinath, K.S.; Adiga, P.R. Riboflavin Carrier Protein: A Serum and Tissue Marker for Breast Carcinoma. Int. J. Cancer 2001, 95, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Mekseriwattana, W.; Guardia, P.; Herrero, B.T.; de la Fuente, J.M.; Kuhakarn, C.; Roig, A.; Katewongsa, K.P. Riboflavin-Citrate Conjugate Multicore SPIONs with Enhanced Magnetic Responses and Cellular Uptake in Breast Cancer Cells. Nanoscale Adv. 2022, 4, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Ouhtit, A.; Gaur, R.; Fernando, A.; Schwarzenberger, P.; Su, J.; Ismail, M.F.; El-Sayyad, H.I.; Karande, A.; Elmageed, Z.A.; et al. Biochemical Characterization of Riboflavin Carrier Protein (RCP) in Prostate Cancer. Front. Biosci. (Landmark Ed.) 2009, 14, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Xu, Y.W.; Wu, J.Y.; Tan, H.Z.; Wu, Z.Y.; Xue, Y.J.; Zhang, J.J.; Li, E.M.; Xu, L.Y. Plasma Riboflavin Level Is Associated with Risk, Relapse, and Survival of Esophageal Squamous Cell Carcinoma. Nutr. Cancer 2017, 69, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Luo, H.J.; Wu, Z.Y.; Chen, S.Z.; Wang, X.; Yu, S.X.; Wang, J.M.; Lin, S.Y.; Cai, Z.Y.; Gao, Y.L.; et al. Decreased Plasma Riboflavin Is Associated with Poor Prognosis, Invasion, and Metastasis in Esophageal Squamous Cell Carcinoma. Eur. J. Clin. Nutr. 2020, 74, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Taylor, P.R.; Fan, J.H.; Pfeiffer, R.M.; Gail, M.H.; Liang, H.; Murphy, G.A.; Dawsey, S.M.; Qiao, Y.L.; Abnet, C.C. Effects of Nutrition Intervention on Total and Cancer Mortality: 25-Year Post-Trial Follow-Up of the 5.25-Year Linxian Nutrition Intervention Trial. J. Natl. Cancer Inst. 2018, 110, 1229–1238. [Google Scholar] [CrossRef]
- Takata, Y.; Cai, Q.; Beeghly-Fadiel, A.; Li, H.; Shrubsole, M.J.; Ji, B.T.; Yang, G.; Chow, W.H.; Gao, Y.T.; Zheng, W.; et al. Dietary B Vitamin and Methionine Intakes and Lung Cancer Risk Among Female Never Smokers in China. Cancer Causes Control 2012, 23, 1965–1975. [Google Scholar] [CrossRef]
- Aili, A.; Hasim, A.; Kelimu, A.; Guo, X.; Mamtimin, B.; Abudula, A.; Upur, H. Association of the Plasma and Tissue Riboflavin Levels with C20orf54 Expression in Cervical Lesions and Its Relationship to HPV16 Infection. PLoS ONE 2013, 8, e79937. [Google Scholar] [CrossRef][Green Version]
- Hernandez, B.Y.; McDuffie, K.; Wilkens, L.R.; Kamemoto, L.; Goodman, M.T. Diet and Premalignant Lesions of the Cervix: Evidence of a Protective Role for Folate, Riboflavin, Thiamin, and Vitamin B12. Cancer Causes Control 2003, 14, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Buring, J.E.; Manson, J.E. Effect of Combined Folic Acid, Vitamin B6, and Vitamin B12 on Cancer Risk in Women: A Randomized Trial. JAMA 2008, 300, 2012–2021. [Google Scholar] [CrossRef]
- Lee, S.M.; Seol, A.; Cho, H.W.; Min, K.J.; Lee, S.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W. Optimal Dietary Intake of Riboflavin Associated with Lower Risk of Cervical Cancer in Korea: Korean National Health and Nutrition Examination Survey 2010–2021. Life 2024, 14, 529. [Google Scholar] [CrossRef]
- Freese, R.; Lysne, V. Niacin—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Michalowska, M.; Znorko, B.; Kaminski, T.; Oksztulska-Kolanek, E.; Pawlak, D. New Insights into Tryptophan and Its Metabolites in the Regulation of Bone Metabolism. J. Physiol. Pharmacol. 2015, 66, 779–791. [Google Scholar] [PubMed]
- Zhang, X.M.; Jing, Y.P.; Jia, M.Y.; Zhang, L. Negative Transcriptional Regulation of Inflammatory Genes by Group B3 Vitamin Nicotinamide. Mol. Biol. Rep. 2012, 39, 10367–10371. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Sartini, D.; Salvolini, E.; Brisigotti, V.; Molinelli, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers 2021, 13, 4943. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Lu, J.; Li, Y.; Zeng, J.; Du, X.; Yu, A.; Zhao, X.; Chi, F.; Xi, Z.; Cao, S. SIRT1: A Novel Regulator in Colorectal Cancer. Biomed. Pharmacother. 2024, 178, 117176. [Google Scholar] [CrossRef] [PubMed]
- Hołubiec, P.; Leończyk, M.; Staszewski, F.; Łazarczyk, A.; Jaworek, A.K.; Wojas-Pelc, A. Pathophysiology and Clinical Management of Pellagra—A Review. Folia Med. Cracov. 2021, 61, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, R.; Wanders, R.J.A.; van Karnebeek, C.D.M.; Houtkooper, R.H. NAD+ Homeostasis in Human Health and Disease. EMBO Mol. Med. 2021, 13, e13943. [Google Scholar] [CrossRef]
- Nikas, I.P.; Paschou, S.A.; Ryu, H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules 2020, 10, 477. [Google Scholar] [CrossRef]
- Luna, A.; Aladjem, M.I.; Kohn, K.W. SIRT1/PARP1 Crosstalk: Connecting DNA Damage and Metabolism. Genome Integr. 2013, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Scatozza, F.; Moschella, F.; D’Arcangelo, D.; Rossi, S.; Tabolacci, C.; Giampietri, C.; Proietti, E.; Facchiano, F.; Facchiano, A. Nicotinamide Inhibits Melanoma In Vitro and In Vivo. J. Exp. Clin. Cancer Res. 2020, 39, 211. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Li, T.; Wu, S.; Li, W.Q.; Weinstock, M.; Qureshi, A.A.; Cho, E. Niacin Intake and Risk of Skin Cancer in US Women and Men. Int. J. Cancer 2017, 140, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Camillo, L.; Gironi, L.C.; Zavattaro, E.; Esposto, E.; Savoia, P. Nicotinamide Attenuates UV-Induced Stress Damage in Human Primary Keratinocytes from Cancerization Fields. J. Invest. Dermatol. 2022, 142, 1466–1477.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.; Nardone, B.; Kiguradze, T.; Posligua, A.; West, D.P.; Rani, M. Investigating the Potential for Protective Effect Against Non-Melanoma Skin Cancer in Cancer Patients Receiving Oral Niacin. G. Ital. Dermatol. Venereol. 2017, 152, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Tosti, G.; Pepe, F.; Gnagnarella, P.; Silvestri, F.; Gaeta, A.; Queirolo, P.; Gandini, S. The Role of Nicotinamide as a Chemo-Preventive Agent in NMSCs: A Systematic Review and Meta-Analysis. Nutrients 2023, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Lohani, M.; Dhasmana, A.; Haque, S.; Dar, S.A.; Jawed, A.; Wahid, M.; Mandal, R.K.; Akhter, N.; Farasani, A.; Hobani, Y.H.; et al. Niacin Deficiency Modulates Genes Involved in Cancer: Are Smokers at Higher Risk? J. Cell Biochem. 2019, 120, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.S.; Caffa, I.; Monacelli, F.; Nencioni, A. Inhibitors of NAD+ Production in Cancer Treatment: State of the Art and Perspectives. Int. J. Mol. Sci. 2024, 25, 2092. [Google Scholar] [CrossRef] [PubMed]
- Sampath, D.; Zabka, T.S.; Misner, D.L.; O’Brien, T.; Dragovich, P.S. Inhibition of Nicotinamide Phosphoribosyltransferase (NAMPT) as a Therapeutic Strategy in Cancer. Pharmacol. Ther. 2015, 151, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Guiot, M.C.; Gravel, M.; Bernier, C.; Shore, G.C.; Roulston, A. Novel NAPRT Specific Antibody Identifies Small Cell Lung Cancer and Neuronal Cancers as Promising Clinical Indications for a NAMPT Inhibitor/Niacin Co-Administration Strategy. Oncotarget 2017, 8, 77846–77859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, M.; Ohuchi, M.; Sakamoto, Y.; Kudo, K.; Yaku, K.; Soga, T.; Sugiura, Y.; Morita, M.; Hayashi, K.; Miyahara, S.; et al. Niacin Restriction with NAMPT-Inhibition is Synthetic Lethal to Neuroendocrine Carcinoma. Nat. Commun. 2023, 14, 8095. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Abyar, F. De Novo Design of Cu (II) Complex Containing CNC-Pincer-Vitamin B3 and B7 Conjugates for Breast Cancer Application. Mol. Pharm. 2019, 16, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, M.A.; Badawy, A.M.; Abu-Youssef, M.A.; Yehia, M.A.; Abou Shamaa, L.D.; Mohamed, S.A. Influence of Copper(I) Nicotinate Complex on the Notch1 Signaling Pathway in Triple Negative Breast Cancer Cell Lines. Sci. Rep. 2024, 14, 2522. [Google Scholar] [CrossRef]
- Jung, M.; Lee, K.M.; Im, Y.; Seok, S.H.; Chung, H.; Kim, D.Y.; Han, D.; Lee, C.H.; Hwang, E.H.; Park, S.Y.; et al. Nicotinamide (Niacin) Supplement Increases Lipid Metabolism and ROS-Induced Energy Disruption in Triple-Negative Breast Cancer: Potential for Drug Repositioning as an Anti-Tumor Agent. Mol. Oncol. 2022, 16, 1795–1815. [Google Scholar] [CrossRef]
- He, Y.C.; He, L.; Khoshaba, R.; Lu, F.G.; Cai, C.; Zhou, F.L.; Liao, D.F.; Cao, D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest Through a P53-Mediated Mechanism. Molecules 2019, 24, 4179. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, J.H.; Moon, J.H.; Nazim, U.M.; Lee, Y.J.; Seol, J.W.; Hur, J.; Eo, S.K.; Lee, J.H.; Park, S.Y. Niacin Alleviates TRAIL-Mediated Colon Cancer Cell Death via Autophagy Flux Activation. Oncotarget 2016, 7, 4356–4368. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Yang, R.; Mirzaei, R.; Rawji, K.; Poon, C.; Mishra, M.K.; Zemp, F.J.; Bose, P.; Kelly, J.; Dunn, J.F.; et al. Control of Brain Tumor Growth by Reactivating Myeloid Cells with Niacin. Sci. Transl. Med. 2020, 12, eaay9924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Acklin, S.; Gillenwater, J.; Du, W.; Patra, M.; Yu, H.; Xu, B.; Yu, J.; Xia, F. SIRT2 Promotes Murine Melanoma Progression Through Natural Killer Cell Inhibition. Sci. Rep. 2021, 11, 12988. [Google Scholar] [CrossRef] [PubMed]
- Selvanesan, B.C.; Meena, K.; Beck, A.; Meheus, L.; Lara, O.; Rooman, I.; Gravekamp, C. Nicotinamide Combined with Gemcitabine is an Immunomodulatory Therapy That Restrains Pancreatic Cancer in Mice. J. Immunother. Cancer 2020, 8, e001250. [Google Scholar] [CrossRef]
- Shu, L.; Ren, L.; Wang, Y.; Fang, T.; Ye, Z.; Han, W.; Chen, C.; Wang, H. Niacin-Ligated Platinum (IV)-Ruthenium (II) Chimeric Complexes Synergistically Suppress Tumor Metastasis and Growth with Potentially Reduced Toxicity in Vivo. Chem. Commun. 2020, 56, 3069–3072. [Google Scholar] [CrossRef]
- Giacalone, S.; Spigariolo, C.B.; Bortoluzzi, P.; Nazzaro, G. Oral Nicotinamide: The Role in Skin Cancer Chemoprevention. Dermatol. Ther. 2021, 34, e14892. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Gao, L.; Liao, N.; Xu, X.; Yu, W.; Hong, W. Association Between Niacin and Mortality Among Patients with Cancer in the NHANES Retrospective Cohort. BMC Cancer 2022, 22, 1173. [Google Scholar] [CrossRef] [PubMed]
- Miallot, R.; Millet, V.; Galland, F.; Naquet, P. The Vitamin B5/Coenzyme a Axis: A Target for Immunomodulation? Eur. J. Immunol. 2023, 53, e2350435. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Jackowski, S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus 2007, 2, 10.1128/ecosalplus.3.6.3.4. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, K.; Iuso, A.; Reunert, J.; Grüneberg, M.; Seelhöfer, A.; Rust, S.; Fiermonte, G.; Paradies, E.; Piazzolla, C.; Mannil, M.; et al. Expanding the Genetic and Clinical Spectrum of SLC25A42-Associated Disorders and Testing of Pantothenic Acid to Improve CoA Level In Vitro. JIMD Rep. 2024, 65, 417–425. [Google Scholar] [CrossRef]
- Miallot, R.; Millet, V.; Roger, A.; Fenouil, R.; Tardivel, C.; Martin, J.C.; Tranchida, F.; Shintu, L.; Berchard, P.; Sousa Lanza, J.; et al. The Coenzyme A Precursor Pantethine Enhances Antitumor Immunity in Sarcoma. Life Sci. Alliance 2023, 6, e202302200. [Google Scholar] [CrossRef]
- McAllister, R.; Fixter, L.; Campbell, E. The Effect of Tumor Growth on Liver Pantothenate, CoA, and Fatty Acid Synthetase Activity in the Mouse. Br. J. Cancer 1988, 57, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, B.; Jessri, M.; Akhoondan, M.; Moasheri, S.M.; Rashidkhani, B. Nutrient Patterns and Risk of Esophageal Squamous Cell Carcinoma: A Case-Control Study. Dis. Esophagus 2012, 25, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Dugué, P.A.; Brinkman, M.T.; Hodge, A.M.; Bassett, J.K.; Bolton, D.; Longano, A.; Hopper, J.L.; Southey, M.C.; English, D.R.; Milne, R.L.; et al. Dietary Intake of Nutrients Involved in One-Carbon Metabolism and Risk of Urothelial Cell Carcinoma: A Prospective Cohort Study. Int. J. Cancer 2018, 143, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Dugué, P.A.; Bassett, J.K.; Brinkman, M.T.; Southey, M.C.; Joo, J.E.; Wong, E.M.; Milne, R.L.; English, D.R.; Giles, G.G.; Boussioutas, A.; et al. Dietary Intake of Nutrients Involved in One-Carbon Metabolism and Risk of Gastric Cancer: A Prospective Study. Nutr. Cancer 2019, 71, 605–614. [Google Scholar] [CrossRef]
- Egnell, M.; Fassier, P.; Lécuyer, L.; Zelek, L.; Vasson, M.P.; Hercberg, S.; Latino-Martel, P.; Galan, P.; Deschasaux, M.; Touvier, M. B-Vitamin Intake from Diet and Supplements and Breast Cancer Risk in Middle-Aged Women: Results from the Prospective NutriNet-Santé Cohort. Nutrients 2017, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Ship, A.G.; Schal’Ten, W.E.; Watkin, D.M.; Romine, M. Effects of Pantothenic Acid-Deficient Diets in Patients with Carcinomas. Cancer 1958, 11, 933–937. [Google Scholar] [CrossRef]
- Hutschenreuther, A.; Birkenmeier, G.; Bigl, M.; Krohn, K.; Birkemeyer, C. Glycerophosphoglycerol, Beta-Alanine, and Pantothenic Acid as Metabolic Companions of Glycolytic Activity and Cell Migration in Breast Cancer Cell Lines. Metabolites 2013, 3, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, P.; Inglese, P.; Ghanate, A.; Gjelaj, E.; Wu, V.; Panina, Y.; Mendez-Lucas, A.; MacLachlan, C.; Patani, N.; Hubert, C.B.; et al. Vitamin B5 Supports MYC Oncogenic Metabolism and Tumor Progression in Breast Cancer. Nat. Metab. 2023, 5, 1870–1886. [Google Scholar] [CrossRef] [PubMed]
- Slyshenkov, V.S.; Rakowska, M.; Wojtczak, L. Protective Effect of Pantothenic Acid and Related Compounds Against Permeabilization of Ehrlich Ascites Tumour Cells by Digitonin. Acta Biochim. Pol. 1996, 43, 407–410. [Google Scholar] [CrossRef]
- Slyshenkov, V.S.; Rakowska, M.; Moiseenok, A.G.; Wojtczak, L. Pantothenic Acid and Its Derivatives Protect Ehrlich Ascites Tumor Cells Against Lipid Peroxidation. Free Radic. Biol. Med. 1995, 19, 767–772. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, L.; Lin, S.; Li, Y.; Xiao, S.; Liu, J.; Li, Z.; Cui, Y.; Zhang, J. Metabolic Profiles of Gastric Cancer Cell Lines with Different Degrees of Differentiation. Int. J. Clin. Exp. Pathol. 2018, 11, 869–875. [Google Scholar] [PubMed] [PubMed Central]
- Secchi, D.G.; Aballay, L.R.; Galíndez, M.F.; Piccini, D.; Lanfranchi, H.; Brunotto, M. Red Meat, Micronutrients, and Oral Squamous Cell Carcinoma of Argentine Adult Patients. Nutr. Hosp. 2015, 32, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Saibil, S.D.; Han, S.; Israni-Winger, K.; Lien, S.C.; Laister, R.C.; Sayad, A.; Penny, S.; Amaria, R.N.; Haydu, L.E.; et al. Coenzyme A Fuels T Cell Anti-Tumor Immunity. Cell Metab. 2021, 33, 2415–2427.e6. [Google Scholar] [CrossRef]
- Giessner, C.; Millet, V.; Mostert, K.J.; Gensollen, T.; Vu Manh, T.P.; Garibal, M.; Dieme, B.; Attaf-Bouabdallah, N.; Chasson, L.; Brouilly, N.; et al. Vnn1 Pantetheinase Limits the Warburg Effect and Sarcoma Growth by Rescuing Mitochondrial Activity. Life Sci. Alliance 2018, 1, e201800073. [Google Scholar] [CrossRef] [PubMed]
- Roseblade, A.; Luk, F.; Ung, A.; Bebawy, M. Targeting Microparticle Biogenesis: A Novel Approach to the Circumvention of Cancer Multidrug Resistance. Curr. Cancer Drug Targets 2015, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.F.; Krishnamachary, B.; Wildes, F.; Mironchik, Y.; Mezzanzanica, D.; Podo, F.; de Reggi, M.; Gharib, B.; Bhujwalla, Z.M. Effect of Pantethine on Ovarian Tumor Progression and Choline Metabolism. Front. Oncol. 2016, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, Vitamin B6, and Related Pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J.; Field, M.S. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2017, 109, djw230. [Google Scholar] [CrossRef]
- Lurie, G.; Wilkens, L.R.; Shvetsov, Y.B.; Ollberding, N.J.; Franke, A.A.; Henderson, B.E.; Kolonel, L.N.; Goodman, M.T. Prediagnostic Plasma Pyridoxal 5′-Phosphate (Vitamin B6) Levels and Invasive Breast Carcinoma Risk: The Multiethnic Cohort. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1942–1948. [Google Scholar] [CrossRef]
- Wei, D.H.; Mao, Q.Q. Vitamin B6, Vitamin B12, and Methionine and Risk of Pancreatic Cancer: A Meta-Analysis. Nutr. J. 2020, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H. Vitamin B6 and Colorectal Cancer: Current Evidence and Future Directions. World J. Gastroenterol. 2013, 19, 1005. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Vitamin B6 and Risk of Colorectal Cancer: A Meta-Analysis of Prospective Studies. JAMA 2010, 303, 1077. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Guo, M.; Wang, D.; Liu, K.; Hu, D.; Li, J. Association Between Vitamin B6 and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Nutr. Cancer 2023, 75, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; Ose, J.; Gigic, B.; Lin, T.; Ulvik, A.; Geijsen, A.J.M.R.; Brezina, S.; Kiblawi, R.; van Roekel, E.H.; Baierl, A.; et al. Higher Vitamin B6 Status Is Associated with Improved Survival Among Patients with Stage I–III Colorectal Cancer. Am. J. Clin. Nutr. 2022, 116, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, L.; Ou, Q.J.; Xu, H.; Chen, Y.Y.; Fang, Y.J.; Zhang, C.X. Serum Pyridoxal 5′-Phosphate and Pyridoxic Acid Ratio Index with Prognosis of Colorectal Cancer: A Prospective Cohort Study. Nutrients 2024, 16, 3685. [Google Scholar] [CrossRef]
- Xu, L.; Fang, Y.J.; Che, M.M.; Abulimiti, A.; Huang, C.Y.; Zhang, C.X. Association of Serum Pyridoxal-5′-Phosphate, Pyridoxal, and PAr with Colorectal Cancer Risk: A Large-Scale Case-Control Study. Nutrients 2022, 14, 2389. [Google Scholar] [CrossRef]
- Wu, X.; Xu, W.; Zhou, T.; Cao, N.; Ni, J.; Zou, T.; Liang, Z.; Wang, X.; Fenech, M. The Role of Genetic Polymorphisms as Related to One-Carbon Metabolism, Vitamin B6, and Gene-Nutrient Interactions in Maintaining Genomic Stability and Cell Viability in Chinese Breast Cancer Patients. Int. J. Mol. Sci. 2016, 17, 1003. [Google Scholar] [CrossRef]
- Huq, M.D.M.; Tsai, N.P.; Lin, Y.P.; Higgins, L.; Wei, L.N. Vitamin B6 Conjugation to Nuclear Corepressor RIP140 and Its Role in Gene Regulation. Nat. Chem. Biol. 2007, 3, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Pilesi, E.; Tesoriere, G.; Ferriero, A.; Mascolo, E.; Liguori, F.; Argirò, L.; Angioli, C.; Tramonti, A.; Contestabile, R.; Volontè, C.; et al. Vitamin B6 Deficiency Cooperates with Oncogenic Ras to Induce Malignant Tumors in Drosophila. Cell Death Dis. 2024, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Tsutsui, M.; Ando, J.; Inano, T.; Noguchi, M.; Yahata, Y.; Tanaka, M.; Tsukune, Y.; Masuda, A.; Shirane, S.; et al. Vitamin B6 Deficiency Is Prevalent in Primary and Secondary Myelofibrosis Patients. Int. J. Hematol. 2019, 110, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Kimoto, A.; Zhang, P.; Bumrungkit, C.; Karunaratne, S.; Yanaka, N.; Kumrungsee, T. Relationship of Low Vitamin B6 Status with Sarcopenia, Frailty, and Mortality: A Narrative Review. Nutrients 2024, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; González-Gross, M. Vitamin B6 Status, Deficiency, and Its Consequences—An Overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar]
- Zuo, H.; Ueland, P.M.; Midttun, Ø.; Tell, G.S.; Fanidi, A.; Zheng, W.; Shu, X.; Xiang, Y.; Wu, J.; Prentice, R.; et al. Vitamin B6 Catabolism and Lung Cancer Risk: Results from the Lung Cancer Cohort Consortium (LC3). Ann. Oncol. 2019, 30, 478–485. [Google Scholar] [CrossRef]
- Theofylaktopoulou, D.; Midttun, Ø.; Ueland, P.M.; Meyer, K.; Fanidi, A.; Zheng, W.; Shu, X.O.; Xiang, Y.B.; Prentice, R.; Pettinger, M.; et al. Impaired Functional Vitamin B6 Status Is Associated with Increased Risk of Lung Cancer. Int. J. Cancer 2018, 142, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Senovilla, L.; Olaussen, K.A.; Pinna, G.; Eisenberg, T.; Goubar, A.; Martins, I.; Michels, J.; Kratassiouk, G.; et al. Prognostic Impact of Vitamin B6 Metabolism in Lung Cancer. Cell Rep. 2012, 2, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Butler, L.M.; Midttun, Ø.; Koh, W.P.; Ueland, P.M.; Wang, R.; Jin, A.; Gao, Y.T.; Yuan, J.M. Serum B6 Vitamers (Pyridoxal 5′-Phosphate, Pyridoxal, and 4-Pyridoxic Acid) and Pancreatic Cancer Risk: Two Nested Case-Control Studies in Asian Populations. Cancer Causes Control 2016, 27, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Santos, R.J.; McGuigan, A.J.; Singh, U.; Johnson, P.; Kunzmann, A.T.; Turkington, R.C. The Role of Circulating Protein and Metabolite Biomarkers in the Development of Pancreatic Ductal Adenocarcinoma (PDAC): A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1090–1102. [Google Scholar] [CrossRef]
- Mock, D.M. Biotin: From Nutrition to Therapeutics. J. Nutr. 2017, 147, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- León-Del-Río, A. Biotin in Metabolism, Gene Expression, and Human Disease. J. Inherit. Metab. Dis. 2019, 42, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Sirithanakorn, C.; Cronan, J.E. Biotin, a Universal and Essential Cofactor: Synthesis, Ligation, and Regulation. FEMS Microbiol. Rev. 2021, 45, fuab003. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Sato, T.; Shimada, S.; Kawamura, T. Biotin Is Required for the Zinc Homeostasis in the Skin. Nutrients 2019, 11, 919. [Google Scholar] [CrossRef]
- Zempleni, J.; Hassan, Y.I.; Wijeratne, S.S. Biotin and Biotinidase Deficiency. Expert. Rev. Endocrinol. Metab. 2008, 3, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Shirakawa, H.; Sugimoto, R.; Furukawa, Y.; Komai, M. Effect of Biotin Treatment on Hepatic Gene Expression in Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 2008, 72, 1290–1298. [Google Scholar] [CrossRef]
- McCarty, M.F. In Type 1 Diabetics, High-Dose Biotin May Compensate for Low Hepatic Insulin Exposure, Promoting a More Normal Expression of Glycolytic and Gluconeogenic Enzymes and Thereby Aiding Glycemic Control. Med. Hypotheses 2016, 95, 45–48. [Google Scholar] [CrossRef]
- Shen, K.; Johnson, D.W.; Gobe, G.C. The Role of cGMP and Its Signaling Pathways in Kidney Disease. Am. J. Physiol. Renal Physiol. 2016, 311, F671–F681. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Paira, P. Biotin Conjugated Organic Molecules and Proteins for Cancer Therapy: A Review. Eur. J. Med. Chem. 2018, 145, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Peng, Y.; Yue, Q.; Pu, Y.; Li, R.; Zhao, Y.; Hai, L.; Guo, L.; Wu, Y. Design, Preparation, and Evaluation of Different Branched Biotin-Modified Liposomes for Targeting Breast Cancer. Eur. J. Med. Chem. 2020, 193, 112204. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Singh, A.; Amin, S.; Spallholz, J.E.; Sharma, A.K. Identification and Biotin Receptor-Mediated Activity of a Novel Seleno-Biotin Compound That Inhibits Viability of and Induces Apoptosis in Ovarian Cancer Cells. Chem. Biol. Interact. 2022, 365, 110071. [Google Scholar] [CrossRef]
- Kundu, B.K.; Pragti; Carlton Ranjith, W.A.; Shankar, U.; Kannan, R.R.; Mobin, S.M.; Bandyopadhyay, A.; Mukhopadhyay, S. Cancer-Targeted Chitosan-Biotin-Conjugated Mesoporous Silica Nanoparticles as Carriers of Zinc Complexes to Achieve Enhanced Chemotherapy In Vitro and In Vivo. ACS Appl. Bio Mater. 2022, 5, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Lee, Y.; Vousden, K.H.; Hennequart, M. Cycling back to folate metabolism in cancer. Nat. Cancer 2024, 5, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Linhart, H.G.; Troen, A.; Bell, G.W.; Cantu, E.; Chao, W.H.; Moran, E.; Steine, E.; He, T.; Jaenisch, R. Folate deficiency induces genomic uracil misincorporation and hypomethylation but does not increase DNA point mutations. Gastroenterology 2009, 136, 227–235.e3. [Google Scholar] [CrossRef]
- Duthie, S.J.; Narayanan, S.; Blum, S.; Pirie, L.; Brand, G.M. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr. Cancer 2000, 37, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Prakash, M.D.; Kuol, N.; Nurgali, K.; Stojanovska, L.; Apostolopoulos, V. Anti-Tumor Effects of Vitamin B2, B6, and B9 in Promonocytic Lymphoma Cells. Int. J. Mol. Sci. 2019, 20, 3763. [Google Scholar] [CrossRef]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Naushad, S.M.; Reddy, C.A.; Kumaraswami, K.; Divyya, S.; Kotamraju, S.; Gottumukkala, S.R.; Digumarti, R.R.; Kutala, V.K. Impact of hyperhomocysteinemia on breast cancer initiation and progression: Epigenetic perspective. Cell Biochem. Biophys. 2014, 68, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Beetstra, S.; Suthers, G.; Dhillon, V.; Salisbury, C.; Turner, J.; Altree, M.; McKinnon, R.; Fenech, M. Methionine-dependence phenotype in the de novo pathway in BRCA1 and BRCA2 mutation carriers with and without breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Shrubsole, M.J.; Jin, F.; Dai, Q.; Shu, X.O.; Potter, J.D.; Hebert, J.R.; Gao, Y.T.; Zheng, W. Dietary folate intake and breast cancer risk: Results from the Shanghai Breast Cancer Study. Cancer Res. 2001, 61, 7136–7141. [Google Scholar]
- Xie, H.; Wei, L.; Wang, Q.; Tang, S.; Gan, J. Elevated serum homocysteine levels associated with poor recurrence-free and overall survival in patients with colorectal cancer. Sci. Rep. 2024, 14, 10057. [Google Scholar] [CrossRef]
- Pelucchi, C.; Talamini, R.; Negri, E.; Levi, F.; Conti, E.; Franceschi, S.; La Vecchia, C. Folate intake and risk of oral and pharyngeal cancer. Ann. Oncol. 2003, 14, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, D.; Yang, M.; Pang, Y.; Li, M.; Qin, S.; Cui, K. Folate intake and the risk of endometrial cancer: A dose-response meta-analysis. Medicine 2024, 103, e39775. [Google Scholar] [CrossRef]
- Gersekowski, K.; Ibiebele, T.I.; Australian Ovarian Cancer Study Group; Doherty, J.A.; Harris, H.R.; Goodman, M.T.; Terry, K.L.; Wu, A.H.; Bandera, E.V.; Qin, B.; et al. Folate Intake and Ovarian Cancer Risk among Women with Endometriosis: A Case-Control Study from the Ovarian Cancer Association Consortium. Cancer Epidemiol. Biomark. Prev. 2023, 32, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Shi, W.W.; Gao, H.F.; Zhou, L.; Hou, A.J.; Zhou, Y.H. Folate intake and the risk of breast cancer: A dose-response meta-analysis of prospective studies. PLoS ONE 2014, 9, e100044. [Google Scholar] [CrossRef]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: A meta-analysis. Gastroenterology 2006, 131, 1271–1283. [Google Scholar] [CrossRef]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Folate intake and stomach cancer incidence in a prospective cohort of Swedish women. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Chang, C.; Lee, W.S. Folic acid inhibits COLO-205 colon cancer cell proliferation through activating the FRα/c-SRC/ERK1/2/NFκB/TP53 pathway: In vitro and in vivo studies. Sci. Rep. 2015, 5, 11187. [Google Scholar] [CrossRef]
- Thabet, R.H.; Alessa, R.E.M.; Al-Smadi, Z.K.K.; Alshatnawi, B.S.G.; Amayreh, B.M.I.; Al-Dwaaghreh, R.B.A.; Salah, S.K.A. Folic acid: Friend or foe in cancer therapy. J. Int. Med. Res. 2024, 52, 3000605231223064. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B. Folate and colon cancer: Dietary habits from the distant past coming home to roost. Am. J. Clin. Nutr. 2021, 114, 1–2. [Google Scholar] [CrossRef]
- Bouras, E.; Kim, A.E.; Lin, Y.; Morrison, J.; Du, M.; Albanes, D.; Barry, E.L.; Baurley, J.W.; Berndt, S.I.; Bien, S.A.; et al. Genome-wide interaction analysis of folate for colorectal cancer risk. Am. J. Clin. Nutr. 2023, 118, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Hubner, R.A.; Houlston, R.S. Folate and colorectal cancer prevention. Br. J. Cancer 2009, 100, 233–239. [Google Scholar] [CrossRef]
- Giovannucci, E. Epidemiologic studies of folate and colorectal neoplasia: A review. J. Nutr. 2002, 132, 2350S–2355S. [Google Scholar] [CrossRef]
- Kim, Y.I. Role of folate in colon cancer development and progression. J. Nutr. 2003, 133, 3731S–3739S. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I. Folate and colorectal cancer: An evidence-based critical review. Mol. Nutr. Food Res. 2007, 51, 267–292. [Google Scholar] [CrossRef] [PubMed]
- Catala, G.N.; Bestwick, C.S.; Russell, W.R.; Tortora, K.; Giovannelli, L.; Moyer, M.P.; Lendoiro, E.; Duthie, S.J. Folate, genomic stability and colon cancer: The use of single cell gel electrophoresis in assessing the impact of folate in vitro, in vivo and in human biomonitoring. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 73–80. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.L.; McGlynn, A.P.; McNulty, H.; Reynolds, J.; Wasson, G.R.; Molloy, A.M.; Strain, J.J.; Weir, D.G.; Ward, M.; McKerr, G.; et al. Folic Acid Supplementation in Postpolypectomy Patients in a Randomized Controlled Trial Increases Tissue Folate Concentrations and Reduces Aberrant DNA Biomarkers in Colonic Tissues Adjacent to the Former Polyp Site. J. Nutr. 2016, 146, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification—Its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Wu, K.; Platz, E.A.; Willett, W.C.; Fuchs, C.S.; Selhub, J.; Rosner, B.A.; Hunter, D.J.; Giovannucci, E. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am. J. Clin. Nutr. 2009, 90, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.; Mason, J.B.; Choi, S.W. Too much folate: A risk factor for cancer and cardiovascular disease? Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 30–36. [Google Scholar] [CrossRef]
- Mucha, P.; Kus, F.; Cysewski, D.; Smolenski, R.T.; Tomczyk, M. Vitamin B12 Metabolism: A Network of Multi-Protein Mediated Processes. Int. J. Mol. Sci. 2024, 25, 8021. [Google Scholar] [CrossRef]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted In Vivo Functions and In Vitro Applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Waly, M.I.; Taranikanti, V.; Guizani, N.; Ali, A.; Rahman, M.S.; Al-Attabi, Z.; Al-Malky, R.N.; Al-Maskari, S.N.M.; Al-Ruqaishi, B.R.S.; et al. Folate/Vitamin B12 Supplementation Combats Oxidative Stress-Associated Carcinogenesis in a Rat Model of Colon Cancer. Nutr. Cancer 2019, 71, 100–110. [Google Scholar] [CrossRef]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alternative Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef]
- Banjari, I.; Kožić, S. Dietary intake of vitamin B12 in relation to diet and lifestyle characteristics in a population at high risk for colorectal cancer. Cent. Eur. J. Public. Health 2018, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Khairan, P.; Sobue, T.; Eshak, E.S.; Zha, L.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Shimazu, T.; et al. Association of dietary intakes of vitamin B12, vitamin B6, folate, and methionine with the risk of esophageal cancer: The Japan Public Health Center-based (JPHC) prospective study. BMC Cancer 2021, 21, 982. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fowke, J.H.; Liang, X.; Mozhui, K.; Sen, S.; Bao, W.; Liu, B.; Snetselaar, L.G.; Wallace, R.B.; Shadyab, A.H.; et al. Changes in Dietary Intake of Methionine, Folate/Folic Acid and Vitamin B12 and Survival in Postmenopausal Women with Breast Cancer: A Prospective Cohort Study. Nutrients 2022, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Travis, R.C.; Appleby, P.N.; Albanes, D.; Barricarte Gurrea, A.; Bjørge, T.; Bueno-de-Mesquita, H.B.; Chen, C.; Donovan, J.; Gislefoss, R.; et al. Circulating Folate and Vitamin B12 and Risk of Prostate Cancer: A Collaborative Analysis of Individual Participant Data from Six Cohorts Including 6875 Cases and 8104 Controls. Eur. Urol. 2016, 70, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.; Carsin, A.-E.; Cantwell, M.M.; Anderson, L.A.; Murray, L.J. Intakes of dietary folate and other B vitamins are associated with risks of esophageal adenocarcinoma, Barrett’s esophagus, and reflux esophagitis. J. Nutr. 2013, 143, 1966–1973. [Google Scholar] [PubMed]
- Miranti, E.H.; Stolzenberg-Solomon, R.; Weinstein, S.J.; Selhub, J.; Männistö, S.; Taylor, P.R.; Freedman, N.D.; Albanes, D.; Abnet, C.C.; Murphy, G. Low vitamin B12 increases risk of gastric cancer: A prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int. J. Cancer 2017, 141, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.M.; Metcalfe, C.; Refsum, H.; Lewis, S.J.; Zuccolo, L.; Smith, G.D.; Chen, L.; Harris, R.; Davis, M.; Marsden, G.; et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: A case-control study, systematic review, and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1632–1642. [Google Scholar] [CrossRef]
- Brasky, T.M.; White, E.; Chen, C.L. Long-Term, Supplemental, One-Carbon Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J. Clin. Oncol. 2017, 35, 3440–3448. [Google Scholar] [CrossRef]
- Luu, H.N.; Wang, R.; Jin, A.; Koh, W.P.; Yuan, J.M. The association between dietary vitamin B12 and lung cancer risk: Findings from a prospective cohort study. Eur. J. Cancer Prev. 2021, 30, 275–281. [Google Scholar] [CrossRef]
- Ebbing, M.; Bonaa, K.H.; Nygard, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njolstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Fanidi, A.; Carreras-Torres, R.; Larose, T.L.; Yuan, J.-M.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Pettinger, M.; Cai, Q.; et al. Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer 2019, 145, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.F.H.; Farkas, D.K.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. Cancer Epidemiol. 2016, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.F.H.; Farkas, D.K.; Pedersen, L.; Sørensen, H.T. Elevated plasma vitamin B12 levels and risk of venous thromboembolism among cancer patients: A population-based cohort study. Thromb. Res. 2017, 156, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Amado-Garzon, S.B.; Molina-Pimienta, L.; Vejarano-Pombo, A.; Vélez-Bonilla, M.; Moreno-Chaparro, J.; Buitrago-Lopez, A. Elevated Vitamin B12, Risk of Cancer, and Mortality: A Systematic Review. Cancer Investig. 2024, 42, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.F.H.; Sørensen, H.T.; Horsfall, L.J.; Petersen, I. Elevated Vitamin B12 Levels and Cancer Risk in UK Primary Care: A THIN Database Cohort Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 814–821. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Gatica, F.; Rodríguez-Quintero, P.; Morales, Z.; Romo-Parra, H. A Molecular Approach of Caloric Restriction and Vitamins for Cancer Prevention. Anticancer. Agents Med. Chem. 2023, 23, 571–584. [Google Scholar] [CrossRef]
- Panda, P.K.; Saraf, S.; Verma, A.; Jain, A.; Bidla, P.D.; Raikwar, S.; Kumari, P.; Jain, S.K. Role of Vitamins in Therapeutic and Targeting Approaches for Prostate Cancer: An Overview. Curr. Drug Targets 2024, 25, 934–952. [Google Scholar] [CrossRef]
- Fallah, M.; Karim Dehnavi, M.; Lotfi, K.; Aminianfar, A.; Azadbakht, L.; Esmaillzadeh, A. Folate Biomarkers, Folate Intake, and Risk of Death from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. Rev. 2025, 83, e801–e813. [Google Scholar] [CrossRef]
- Le, N.T.; Pham, Y.T.; Lu, Y.T.; Le, L.T.; Huynh, N.Y.N.; Dao, H.V.; Nguyen, D.D.; Demanelis, K.; Ha, T.H.; Kuchipudi, S.V.; et al. Vitamin B12 Intake and Cancer Risk: Findings from a Case-Control Study in Vietnam. Nutr. Cancer 2025, 77, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.G.; Yuvaraj, S.; Shanthi, P.; Sachdanandam, P. Co-enzyme Q10, riboflavin and niacin supplementation on alteration of DNA repair enzyme and DNA methylation in breast cancer patients undergoing tamoxifen therapy. Br. J. Nutr. 2008, 100, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.G.; Yuvaraj, S.; Sathish, S.; Shanthi, P.; Sachdanandam, P. Anti-angiogenic potential of Coenzyme Q10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascul. Pharmacol. 2008, 48, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Subay, L.B.; Gencer, A.E.B.; Akçok, İ. Rapamycin and Niacin combination induces apoptosis and cell cycle arrest through autophagy activation on acute myeloid leukemia cells. Mol. Biol. Rep. 2024, 52, 75. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, S.; Premkumar, V.G.; Shanthi, P.; Vijayasarathy, K.; Gangadaran, S.G.; Sachdanandam, P. Effect of Coenzyme Q(10), Riboflavin and Niacin on Tamoxifen treated postmenopausal breast cancer women with special reference to blood chemistry profiles. Breast Cancer Res. Treat. 2009, 114, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.T.; Nguyen, N.T.; Do, M.D. Vitamin B12 deficiency in diabetic patients treated with metformin: A cross-sectional study. PLoS ONE 2024, 19, e0302500. [Google Scholar] [CrossRef]
- Wise, A.; Lemus, H.N.; Fields, M.; Swan, M.; Bressman, S. Refractory Seizures Secondary to Vitamin B6 Deficiency in Parkinson Disease: The Role of Carbidopa-Levodopa. Case Rep. Neurol. 2022, 14, 291–295. [Google Scholar] [CrossRef]
- Kothawade, P.B.; Thomas, A.B.; Chitlange, S.S. Novel Niacin Receptor Agonists: A Promising Strategy for the Treatment of Dyslipidemia. Mini Rev. Med. Chem. 2021, 21, 2481–2496. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. High Niacin Levels May Raise Cardiovascular Disease Risk. JAMA. 2024, 331, 1001. [Google Scholar] [CrossRef] [PubMed]
- Nomair, A.M.; Abdelati, A.; Dwedar, F.I.; Elnemr, R.; Kamel, Y.N.; Nomeir, H.M. The impact of folate pathway variants on the outcome of methotrexate therapy in rheumatoid arthritis patients. Clin. Rheumatol. 2024, 43, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.E.; Willett, W.C.; Fung, T.T.; Holvik, K.; Feskanich, D. Association of High Intakes of Vitamins B6 and B12 From Food and Supplements with Risk of Hip Fracture Among Postmenopausal Women in the Nurses’ Health Study. JAMA Netw. Open. 2019, 2, e193591. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Smith, A.; Troen, A.M.; Mason, J.B.; Jacques, P.F.; Selhub, J. Excess Folic Acid and Vitamin B12 Deficiency: Clinical Implications? Food Nutr. Bull. 2024, 45, S67–S72. [Google Scholar] [CrossRef] [PubMed]
- Moses, G. The safety of commonly used vitamins and minerals. Aust. Prescr. 2021, 44, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Marupuru, S.; Axon, D.R.; Slack, M.K. How do pharmacists use and recommend vitamins, minerals, herbals and other dietary supplements? BMC Complement. Altern. Med. 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).