Impact of Dietary Fiber on Inflammation in Humans
Abstract
1. The Long-Term Benefits of Dietary Fiber in Past and Present
2. Effects of Certain Fiber-Rich Diets on Inflammatory Outcomes in Cohort Studies and RCTs
3. Impact of Specific High- and Low-Fiber Foods on Inflammatory Outcomes
3.1. Whole Grain
3.2. Nuts, Seeds, and Legumes
3.3. Fruits and Vegetables
3.4. Other Relevant Foods Within Fiber-Rich Diets
4. Which Confounding Nutrients of Fiber-Rich Diets Trigger or Antagonize Inflammation?
5. Interventional Evidence for Anti-Inflammatory Properties of Specific Types of Fiber
5.1. Insoluble Fiber
5.2. Prebiotic (Fermentable) Fiber and Synbiotics
5.3. Inulin
5.4. Resistant Starch
5.5. Other Types of Soluble Fiber
6. Putative Anti-Inflammatory Mechanisms of Dietary Fiber
7. Comprehension and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAM | cell-adhesion molecule |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| GI | glycemic index |
| GIP | glucose-dependent insulinotropic peptide |
| GL | glycemic load |
| ICAM | intercellular cell adhesion molecule |
| IFG | impaired fasting glucose |
| IGT | impaired glucose tolerance |
| IL-1 | interleukin 1 |
| IL-1ß | interleukin 1 beta |
| IL-2 | interleukin 2 |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-10 | interleukin 10 |
| MCP | monocyte chemoattractive protein |
| NAFLD | non-alcoholic fatty liver disease |
| RCT | randomized-controlled trial |
| RS | resistant starch |
| T2DM | type 2 diabetes mellitus |
| TNF-alpha | tumor-necrosis factor alpha |
| VCAM | vascular cell adhesion molecule |
References
- Davis, D.R. Declining Fruit and Vegetable Nutrient Composition: What Is the Evidence? HortSci. Horts 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Mayer, A.-M. Historical changes in the mineral content of fruits and vegetables. Br. Food J. 1997, 99, 207–211. [Google Scholar] [CrossRef]
- Shewry, P.R.; Prins, A.; Kosik, O.; Lovegrove, A. Challenges to Increasing Dietary Fiber in White Flour and Bread. J. Agric. Food Chem. 2024, 72, 13513–13522. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; MacAuley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002, 41, 222–227. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Su, C.; Zhang, J.; Zhai, F.Y.; Zhang, B. Trends in dietary fiber intake in Chinese aged 45 years and above, 1991–2011. Eur. J. Clin. Nutr. 2014, 68, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Quan, J.; El Helali, A.; Lam, W.W.T.; Pang, H. Global trends indicate increasing consumption of dietary sodium and fiber in middle-income countries: A study of 30-year global macrotrends. Nutr. Res. 2023, 118, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. Int. Rev. J. 2019, 10, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fibre consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Hollænder, P.L.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, S.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Gu, Y.; Sun, S.; Wang, X.; et al. Insoluble dietary fibre intake is associated with lower prevalence of newly-diagnosed non-alcoholic fatty liver disease in Chinese men: A large population-based cross-sectional study. Nutr. Metab. 2020, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wang, D.; Long, J.; Yang, F.; Si, L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am. J. Cardiol. 2015, 115, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, W.G.; Yang, Y.; Zhang, Q.L.; Zheng, J.L.; Xiang, Y.B. Association between whole grain intake and all-cause mortality: A meta-analysis of cohort studies. Oncotarget 2016, 7, 61996–62005. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, G.; Tan, M.; Zhao, L.; Jin, L.; Tang, X.; Jiang, G.; Zhong, K. Consumption of whole grains in relation to mortality from all causes, cardiovascular disease, and diabetes: Dose-response meta-analysis of prospective cohort studies. Medicine 2016, 95, e4229. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Q.; Shi, W.; Yang, L.; Chen, J.; Lan, Q. Meta-Analysis of the Association Between Whole and Refined Grain Consumption and Stroke Risk Based on Prospective Cohort Studies. Asia Pac. J. Public Health 2016, 28, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Lu, Q.; Gong, B.; Li, L.; Chang, L.; Fu, L.; Zhao, Y. Stroke and food groups: An overview of systematic reviews and meta-analyses. Public Health Nutr. 2018, 21, 766–776. [Google Scholar] [CrossRef]
- Lei, Q.; Zheng, H.; Bi, J.; Wang, X.; Jiang, T.; Gao, X.; Tian, F.; Xu, M.; Wu, C.; Zhang, L.; et al. Whole Grain Intake Reduces Pancreatic Cancer Risk: A Meta-Analysis of Observational Studies. Medicine 2016, 95, e2747. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- InterAct Consortium. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 2015, 58, 1394–1408. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.; Kim, J. Association Between Dietary Nutrient Intake and Chronic Obstructive Pulmonary Disease Severity: A Nationwide Population-Based Representative Sample. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2020, 59, 1869–1879. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fibre intake and asthma (symptoms and control): Results from the French national e-cohort NutriNet-Santé. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef]
- Rubin, K.H.; Rasmussen, N.F.; Petersen, I.; Kopp, T.I.; Stenager, E.; Magyari, M.; Hetland, M.L.; Bygum, A.; Glintborg, B.; Andersen, V. Intake of dietary fibre, red and processed meat and risk of late-onset Chronic Inflammatory Diseases: A prospective Danish study on the “diet, cancer and health” cohort. Int. J. Med. Sci. 2020, 17, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Ebrahimi-Daryani, N.; Dieleman, L.A.; Larijani, B.; Esmaillzadeh, A. Association of Dietary Fiber, Fruit, and Vegetable Consumption with Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2021, 12, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Dourado, E.; Ferro, M.; Sousa Guerreiro, C.; Fonseca, J.E. Diet as a Modulator of Intestinal Microbiota in Rheumatoid Arthritis. Nutrients 2020, 12, 3504. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Akther, M.; Haque, M.E.; Kopalli, S.R. Potential Nutrients from Natural and Synthetic Sources Targeting Inflammaging-A Review of Literature, Clinical Data and Patents. Nutrients 2021, 13, 4058. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Lee, C.L.; Liu, W.J.; Wang, J.S. Associations of low-carbohydrate and low-fat intakes with all-cause mortality in subjects with prediabetes with and without insulin resistance. Clin. Nutr. 2021, 40, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Gribbin, S.; Enticott, J.; Hodge, A.M.; Moran, L.; Thong, E.; Joham, A.; Zaman, S. Association of carbohydrate and saturated fat intake with cardiovascular disease and mortality in Australian women. Heart 2021, 108, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Kagan, A.; Garcia-Palmieri, M.; Kannel, W.B.; Zukel, W.J.; Tillotson, J.; Sorlie, P.; Hjortland, M. Diet and its relation to coronary heart disease and death in three populations. Circulation 1981, 63, 500–515. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Yudkin, J. Dietary fat and dietary sugar in relation to ischaemic heart-disease and diabetes. Lancet 1964, 2, 4–5. [Google Scholar] [CrossRef]
- Cleave, T.L. Sucrose intake and coronary heart-disease. Lancet. 1968, 2, 1187. [Google Scholar] [CrossRef]
- Steckhan, N.; Hohmann, C.D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Miraghajani, M.; Khalafi, M.; Sakhaei, M.H.; Rosenkranz, S.K.; Santos, H.O. Effects of low-carbohydrate diets, with and without caloric restriction, on inflammatory markers in adults: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2024, 78, 569–584. [Google Scholar] [CrossRef]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The effect of a ketogenic diet on inflammation-related markers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2025, 83, 40–58. [Google Scholar] [CrossRef]
- Rankin, J.W.; Turpyn, A.D. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J. Am. Coll. Nutr. 2007, 26, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraj, T.; Saadi, H.; Calle, M.C.; Vole, J.S.; Fernandez, M.L. Carbohydrate restriction, as a first-line dietary intervention, effectively reduces biomarkers of metabolic syndrome in Emirati adults. J. Nutr. 2009, 139, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.d.C.; Schofield, G.M.; Zinn, C.; Thornley, S.J.; Crofts, C.; Merien, F.L.R. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: A randomised clinical trial. PeerJ 2019, 7, e6273. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Miller ER 3rd Selvin, E.; Carey, V.J.; Appel, L.J.; Christenson, R.H.; Sacks, F.M. Effect of type and amount of dietary carbohydrate on biomarkers of glucose homeostasis and C reactive protein in overweight or obese adults: Results from the OmniCarb trial. BMJ Open Diabetes Res. Care 2016, 4, e000276. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, P.; Iqbal, N.; Stern, L.; Williams, M.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Gracely, E.J.; Rader, D.J.; Samaha, F.F. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am. J. Med. 2004, 117, 398–405. [Google Scholar] [CrossRef]
- Milajerdi, A.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: A systematic review and meta-analysis of randomized clinical trials. Am. J. Clin. Nutr. 2018, 107, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mejia Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; Wolever, T.M.S.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Wang, X.H.; Adegboye, A.R.A. The Effects of a Low GI Diet on Cardiometabolic and Inflammatory Parameters in Patients with Type 2 and Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Neuenschwander, M.; Hoffmann, G.; Buyken, A.E.; Schlesinger, S. Dietary sugars and cardiometabolic risk factors: A network meta-analysis on isocaloric substitution interventions. Am. J. Clin. Nutr. 2020, 111, 187–196. [Google Scholar] [CrossRef]
- Tantamango-Bartley, Y.; Jaceldo-Siegl, K.; Fan, J.; Fraser, G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol. Biomark. Prev. 2013, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.F.; Hsu, C.C.; Chiu, T.H.; Lee, C.Y.; Liu, T.T.; Tsao, C.K.; Chuang, S.C.; Hsiung, C.A. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: A matched cohort study. Br. J. Nutr. 2015, 114, 1313–1320. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Ferrari, P.; Carayol, M.; Achaintre, D.; Scalbert, A.; Cross, A.J.; Gunter, M.J.; Fensom, G.K.; Appleby, P.N.; et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015, 102, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Hoffmann, G.; Schwingshackl, L.; Schlesinger, S. Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Eur. J. Epidemiol. 2019, 34, 837–852. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Pünsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Kendall, C.W.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; Choo, V.L.; Mejia, S.B.; Stewart, S.E.; Leiter, L.A.; Jenkins, D.J.; et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Jakše, B.; Jakše, B.; Godnov, U.; Pinter, S. Nutritional, Cardiovascular Health and Lifestyle Status of ‘Health Conscious’ Adult Vegans and Non-Vegans from Slovenia: A Cross-Sectional Self-Reported Survey. Int. J. Environ. Res. Public Health 2021, 18, 5968. [Google Scholar] [CrossRef] [PubMed]
- Mądry, E.; Lisowska, A.; Grebowiec, P.; Walkowiak, J. The impact of vegan diet on B-12 status in healthy omnivores: Five-year prospective study. Acta Sci. Pol. Technol. Aliment. 2012, 11, 209–212. [Google Scholar]
- Zazpe, I.; Sanchez-Tainta, A.; Estruch, R.; Lamuela-Raventos, R.M.; Schröder, H.; Salas-Salvado, J.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: The PREDIMED study. J. Am. Diet. Assoc. 2008, 108, 1134–1144, discussion 1145. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Palumbo, E.; Polesel, J.; Hebert, J.R.; Shivappa, N.; Montagnese, C.; Porciello, G.; Calabrese, I.; Luongo, A.; Prete, M.; et al. One-year nutrition counselling in the context of a Mediterranean diet reduced the dietary inflammatory index in women with breast cancer: A role for the dietary glycemic index. Food Funct. 2023, 14, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rejón, A.I.; Castro-Quezada, I.; Ruano-Rodríguez, C.; Ruiz-López, M.D.; Sánchez-Villegas, A.; Toledo, E.; Artacho, R.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; et al. Effect of a Mediterranean Diet Intervention on Dietary Glycemic Load and Dietary Glycemic Index: The PREDIMED Study. J. Nutr. Metab. 2014, 2014, 985373. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.; et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: A meta-analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhu, Y.; Li, Q.; Wang, F.; Ge, X.; Zhou, G.; Miao, L. Association between Mediterranean diet adherence and colorectal cancer: A dose-response meta-analysis. Am. J. Clin. Nutr. 2020, 111, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Yaskolka Meir, A.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.L.; Itsiopoulos, C.; Tierney, A.C.; Kucianski, T.; Radcliffe, J.; Garg, M.; Willcox, J.; Thomas, C.J. Ad libitum Mediterranean diet reduces subcutaneous but not visceral fat in patients with coronary heart disease: A randomised controlled pilot study. Clin. Nutr. ESPEN 2019, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S.A.; Lovegrove, J.A.; Griffin, B.A.; Frost, G.S.; Moore, C.S.; Chatfield, M.D.; Bluck, L.J.; Williams, C.M.; Sanders, T.A.; RISCK Study Group. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: The RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am. J. Clin. Nutr. 2010, 92, 748–758. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; E Manson, J.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Matijevic, N.; Follis, J.L.; Folsom, A.R.; Boerwinkle, E. Associations between dietary patterns and flow cytometry-measured biomarkers of inflammation and cellular activation in the Atherosclerosis Risk in Communities (ARIC) Carotid Artery MRI Study. Atherosclerosis 2010, 212, 260–267. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [CrossRef]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Surkan, P.J.; Esmaillzadeh, A.; Willett, W.C. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J. Nutr. 2011, 141, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Esmaillzadeh, A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm. Metab. Res. 2015, 47, 232–238. [Google Scholar] [PubMed]
- Saneei, P.; Hashemipour, M.; Kelishadi, R.; Esmaillzadeh, A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: A randomized cross-over clinical trial. Ann. Nutr. Metab. 2014, 64, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Shidfar, F.; Tabassi, Z.; Shahaboddin, M.; Esmaillzadeh, A. Association between markers of systemic inflammation, oxidative stress, lipid profiles, and insulin resistance in pregnant women. ARYA Atheroscler. 2013, 9, 172–178. [Google Scholar] [PubMed]
- Huang, T.; Xu, M.; Lee, A.; Cho, S.; Qi, L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: Prospective analysis of 367,442 individuals. BMC Med. 2015, 13, 59. [Google Scholar]
- Xu, Y.; Wan, Q.; Feng, J.; Du, L.; Li, K.; Zhou, Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine 2018, 97, e12995. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Chen, X.; Yu, M.; Pan, Q.; Guo, L. Whole grain food diet slightly reduces cardiovascular risks in obese/overweight adults: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 82. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Haghighatdoost, F. Effects of Whole-Grain Consumption on Selected Biomarkers of Systematic Inflammation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Am. Coll. Nutr. 2019, 38, 275–285. [Google Scholar] [CrossRef]
- Maki, K.C.; Beiseigel, J.M.; Jonnalagadda, S.S.; Gugger, C.K.; Reeves, M.S.; Farmer, M.V.; Kaden, V.N.; Rains, T.M. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. YJADA 2010, 110, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short-and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: A randomized control trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef] [PubMed]
- Rahimlou, M.; Jahromi, N.B.; Hasanyani, N.; Ahmadi, A.R. Effects of Flaxseed Interventions on Circulating Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 1108–1119. [Google Scholar] [CrossRef]
- Askarpour, M.; Karimi, M.; Hadi, A.; Ghaedi, E.; Symonds, M.E.; Miraghajani, M.; Javadian, P. Effect of flaxseed supplementation on markers of inflammation and endothelial function: A systematic review and meta-analysis. Cytokine 2020, 126, 154922. [Google Scholar] [CrossRef]
- Ren, G.Y.; Chen, C.Y.; Chen, G.C.; Chen, W.G.; Pan, A.; Pan, C.W.; Zhang, Y.H.; Qin, L.Q.; Chen, L.H. Effect of Flaxseed Intervention on Inflammatory Marker C-Reactive Protein: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Sabet, H.R.; Ahmadi, M.; Akrami, M.; Motamed, M.; Keshavarzian, O.; Abdollahi, M.; Rezaei, M.; Akbari, H. Effects of flaxseed supplementation on weight loss, lipid profiles, glucose, and high-sensitivity C-reactive protein in patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2024, 47, e24211. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Milajerdi, A.; Reiner, Ž.; Dadgostar, E.; Amirani, E.; Asemi, Z.; Mirsafaei, L.; Mansournia, M.A.; Dana, P.M.; Sadoughi, F.; et al. Effects of flaxseed oil supplementation on biomarkers of inflammation and oxidative stress in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 40, 27–33. [Google Scholar] [CrossRef]
- Khodarahmi, M.; Jafarabadi, M.A.; Moludi, J.; Abbasalizad Farhangi, M. A systematic review and meta-analysis of the effects of soy on serum hs-CRP. Clin. Nutr. 2019, 38, 996–1011. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Saraf-Bank, S.; Bellissimo, N.; Azadbakht, L. Effects of non-soy legume consumption on C-reactive protein: A systematic review and meta-analysis. Nutrition 2015, 31, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Ghasemi, A.; Baradaran, H.R.; Mollanoroozy, E.; Gholami, A. Beneficial effect of soy isoflavones and soy isoflavones plus soy protein on serum concentration of C-reactive protein among postmenopausal women: An updated systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 59, 102715. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Arabzadegan, N.; Daneshzad, E.; Fatahi, S.; Moosavian, S.P.; Surkan, P.J.; Azadbakht, L. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eat. Weight. Disord. 2020, 25, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Ramne, S.; Drake, I.; Ericson, U.; Nilsson, J.; Orho-Melander, M.; Engström, G.; Sonestedt, E. Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk. Nutrients 2020, 12, 3129. [Google Scholar] [CrossRef] [PubMed]
- Aycart, D.F.; Acevedo, S.; Eguiguren-Jimenez, L.; Andrade, J.M. Influence of Plant and Animal Proteins on Inflammation Markers among Adults with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1660. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Collinson, A. Red meat, dietary heme iron, and risk of type 2 diabetes: The involvement of advanced lipoxidation endproducts. Adv. Nutr. 2013, 4, 403–411. [Google Scholar] [CrossRef]
- Davidson, M.H.; Hunninghake, D.; Maki, K.C.; Kwiterovich, P.O., Jr.; Kafonek, S. Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: A long-term, randomized clinical trial. Arch. Intern. Med. 1999, 159, 1331–1338. [Google Scholar] [CrossRef]
- Hunninghake, D.B.; Maki, K.C.; Kwiterovich, P.O., Jr.; Davidson, M.H.; Dicklin, M.R.; Kafonek, S.D. Incorporation of lean red meat into a National Cholesterol Education Program Step I diet: A long-term, randomized clinical trial in free-living persons with hypercholesterolemia. J. Am. Coll. Nutr. 2000, 19, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, N.; Chiu, S.; Williams, P.T.; MKing, S.; Krauss, R.M. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Walton, K.; Batterham, M.; Brock, E.; Langford, K.; McMahon, A.; Roodenrys, S.; Koh, F.; Host, A.; Crowe, R.; et al. Pork and Chicken Meals Similarly Impact on Cognitive Function and Strength in Community-Living Older Adults: A Pilot Study. J. Nutr. Gerontol. Geriatr. 2016, 35, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Parker, B.; Dyer, K.A.; Davis, C.R.; Coates, A.M.; Buckley, J.D.; Howe, P.R. A comparison of regular consumption of fresh lean pork, beef and chicken on body composition: A randomized cross-over trial. Nutrients 2014, 6, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Thomson, R.L.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Effects of eating fresh lean pork on cardiometabolic health parameters. Nutrients 2012, 4, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Keogh, J.B.; Meikle, P.J.; Clifton, P.M. Changes in Lipids and Inflammatory Markers after Consuming Diets High in Red Meat or Dairy for Four Weeks. Nutrients 2017, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Hematdar, Z.; Ghasemifard, N.; Phishdad, G.; Faghih, S. Substitution of red meat with soybean but not non-soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J. Diabetes Metab. Disord. 2018, 17, 111–116. [Google Scholar] [CrossRef]
- Poddar, K.H.; Ames, M.; Hsin-Jen, C.; Feeney, M.J.; Wang, Y.; Cheskin, L.J. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite 2013, 71, 379–387. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Ward, N.C.; Burke, V.; Beilin, L.J.; Puddey, I.B. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J. Nutr. 2007, 137, 363–367. [Google Scholar] [CrossRef]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Xu, J.Y.; Rao, C.P.; Han, S.; Wan, Z.; Qin, L.Q. Effect of whey supplementation on circulating C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2015, 7, 1131–1143. [Google Scholar] [CrossRef]
- Moua, E.D.; Hu, C.; Day, N.; Hord, N.G.; Takata, Y. Coffee Consumption and C-Reactive Protein Levels: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1349. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.Z. Is coffee consumption associated with a lower level of serum C-reactive protein? A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2018, 69, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Osama, H.; Abdelrahman, M.A.; Madney, Y.M.; Harb, H.S.; Saeed, H.; Abdelrahim, M.E.A. Coffee and type 2 diabetes risk: Is the association mediated by adiponectin, leptin, c-reactive protein or Interleukin-6? A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e13983. [Google Scholar] [CrossRef] [PubMed]
- Keno, Y.; Matsuzawa, Y.; Tokunaga, K.; Fujioka, S.; Kawamoto, T.; Kobatake, T.; Tarui, S. High sucrose diet increases visceral fat accumulation in VMH-lesioned obese rats. Int. J. Obes. 1991, 15, 205–211. [Google Scholar] [PubMed]
- Kim, J.Y.; Nolte, L.A.; Hansen, P.A.; Han, D.H.; Kawanaka, K.; Holloszy, J.O. Insulin resistance of muscle glucose transport in male and female rats fed a high-sucrose diet. Am. J. Physiol. 1999, 276, R665–R672. [Google Scholar] [CrossRef] [PubMed]
- Bezpalko, L.; Gavrilyuk, O.; Zayachkivska, O. Inflammatory response in visceral fat tissue and liver is prenatally programmed: Experimental research. J. Physiol. Pharmacol. 2015, 66, 57–64. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels-Ansdell, D.; Schunemann, H.J. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Gögebakan, O.; Kohl, A.; Osterhoff, M.A.; van Baak, M.A.; Jebb, S.A.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Weickert, M.O.; et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (DiOGenes) study: A randomized, controlled trial. Circulation 2011, 124, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Markova, M.; Seebeck, N.; Hornemann, S.; Rosenthal, A.; Lange, V.; Pivovarova-Ramich, O.; Aleksandrova, K. Effects of High and Low Protein Diets on Inflammatory Profiles in People with Morbid Obesity: A 3-Week Intervention Study. Nutrients 2020, 12, 3636. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals with Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef]
- Markova, M.; Koelman, L.; Hornemann, S.; Pivovarova, O.; Sucher, S.; Machann, J.; Rudovich, N.; Thomann, R.; Schneeweiss, R.; Rohn, S.; et al. Effects of plant and animal high protein diets on immune-inflammatory biomarkers: A 6-week intervention trial. Clin. Nutr. 2020, 39, 862–869. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y.; Giovannucci, E.L. Association between dietary fat intake and mortality from all-causes, cardiovascular disease, and cancer: A systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2021, 40, 1060–1070. [Google Scholar] [CrossRef]
- Schwab, U.; Reynolds, A.N.; Sallinen, T.; Rivellese, A.A.; Risérus, U. Dietary fat intakes and cardiovascular disease risk in adults with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wan, Y.; Yang, B.; Huggins, C.E.; Li, D. Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2018, 119, 96–108. [Google Scholar] [CrossRef]
- Langfeld, L.Q.; Du, K.; Bereswill, S.; Heimesaat, M.M. A review of the antimicrobial and immune-modulatory properties of the gut microbiota-derived short chain fatty acid propionate—What is new? Eur. J. Microbiol. Immunol. 2021, 11, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Rozati, M.; Barnett, J.; Wu, D.; Handelman, G.; Saltzman, E.; Wilson, T.; Li, L.; Wang, J.; Marcos, A.; Ordovás, J.M.; et al. Cardio-metabolic and immunological impacts of extra virgin olive oil consumption in overweight and obese older adults: A randomized controlled trial. Nutr. Metab. 2015, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Foshati, S.; Ghanizadeh, A.; Akhlaghi, M. The effect of extra virgin olive oil on anthropometric indices, lipid profile, and markers of oxidative stress and inflammation in patients with depression, a double-blind randomised controlled trial. Int. J. Clin. Pract. 2021, 75, e14254. [Google Scholar] [CrossRef] [PubMed]
- Olalla, J.; García de Lomas, J.M.; Chueca, N.; Pérez-Stachowski, X.; De Salazar, A.; Del Arco, A.; Plaza-Díaz, J.; De la Torre, J.; Prada, J.L.; García-Alegría, J.; et al. Effect of daily consumption of extra virgin olive oil on the lipid profile and microbiota of HIV-infected patients over 50 years of age. Medicine 2019, 98, e17528. [Google Scholar] [CrossRef] [PubMed]
- Weschenfelder, C.; Gottschall, C.B.A.; Markoski, M.M.; Portal, V.L.; Quadros, A.S.; Bersch-Ferreira, Â.C.; Marcadenti, A. Effects of supplementing a healthy diet with pecan nuts or extra-virgin olive oil on inflammatory profile of patients with stable coronary artery disease: A randomised clinical trial. Br. J. Nutr. 2021, 127, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Zahedmehr, A.; Nasrollahzadeh, J. Effect of polyphenol-rich extra-virgin olive oil on lipid profile and inflammatory biomarkers in patients undergoing coronary angiography: A randomised, controlled, clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 548–558. [Google Scholar] [CrossRef]
- Atefi, M.; Pishdad, G.R.; Faghih, S. The effects of canola and olive oils on insulin resistance, inflammation and oxidative stress in women with type 2 diabetes: A randomized and controlled trial. J. Diabetes Metab. Disord. 2018, 17, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition. 2020, 69, 110559. [Google Scholar] [CrossRef]
- Fritsche, K.L. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot. Essent. Fatty Acids. 2008, 79, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Ferns, G.A. Effects of conjugated linoleic acid supplementation on serum C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc. Ther. 2017, 35, 12275. [Google Scholar] [CrossRef]
- Derakhshandeh-Rishehri, S.M.; Rahbar, A.R.; Ostovar, A. Effects of Conjugated Linoleic Acid Intake in the Form of Dietary Supplement or Enriched Food on C-Reactive Protein and Lipoprotein (a) Levels in Humans: A Literature Review and Meta-Analysis. Iran. J. Med. Sci. 2019, 44, 359–373. [Google Scholar] [PubMed]
- Haghighatdoost, F.; Nobakht MGh, B.F. Effect of conjugated linoleic acid on blood inflammatory markers: A systematic review and meta-analysis on randomized controlled trials. Eur. J. Clin. Nutr. 2018, 72, 1071–1082. [Google Scholar] [CrossRef]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Jin, Q.; Wang, X. Effect of dietary alpha-linolenic acid on blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2018, 57, 877–891. [Google Scholar] [CrossRef]
- Khor, B.H.; Narayanan, S.S.; Sahathevan, S.; Gafor, A.H.A.; Daud, Z.A.M.; Khosla, P.; Sabatino, A.; Fiaccadori, E.; Chinna, K.; Karupaiah, T. Efficacy of Nutritional Interventions on Inflammatory Markers in Haemodialysis Patients: A Systematic Review and Limited Meta-Analysis. Nutrients 2018, 10, 397. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef]
- Lin, N.; Shi, J.J.; Li, Y.M.; Zhang, X.Y.; Chen, Y.; Calder, P.C.; Tang, L.J. What is the impact of n-3 PUFAs on inflammation markers in Type 2 diabetic mellitus populations?: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016, 15, 133. [Google Scholar] [CrossRef]
- Malekshahi Moghadam, A.; Saedisomeolia, A.; Djalali, M.; Djazayery, A.; Pooya, S.; Sojoudi, F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singap. Med. J. 2012, 53, 615–619. [Google Scholar]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 7, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Saboori, S.; Shab-Bidar, S.; Speakman, J.R.; Yousefi Rad, E.; Djafarian, K. Effect of vitamin E supplementation on serum C-reactive protein level: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2015, 69, 867–873. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-inflammatory effects of oral supplementation with curcumin: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Djafarian, K.; Mojtahed, A.; Varkaneh, H.K.; Shab-Bidar, S. The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2018, 834, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Li, X.; Li, Z.; Wu, G.R.; Fu, X.F.; Yang, X.M.; Zhang, X.Q.; Gao, X.B. The effect of selenium supplementation on coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2017, 44, 8–16. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Sahebkar, A.; Rodriguez-Moran, M.; Zambrano-Galvan, G.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Plasma C-reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2017, 23, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y.; Murata, M.; Oikawa, S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic. Biol. Med. 2002, 32, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Khayataa, W.; Bashora, G.; Sadeka, M. The effect of phytic acid on rate of starch digestibility in Vicia faba and white wheat bread in vitro. Int. J. Pharm. Sci. Res. 2013, 5, 161–164. [Google Scholar] [CrossRef]
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K.; et al. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J. Sci. Food Agric. 2020, 100, 1598–1607. [Google Scholar] [CrossRef]
- Yoon, J.H.; Thompson, L.U.; Jenkins, D.J. The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am. J. Clin. Nutr. 1983, 38, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharmacother. 2017, 87, 443–450. [Google Scholar] [CrossRef]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and cancer risk—A systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, Y.; Zhang, T.; Sun, Q.; Zhang, Y.; Zhang, P.; Wang, G.; Zhang, J.; Wu, J. Associations of dietary total, heme, non-heme iron intake with diabetes, CVD, and all-cause mortality in men and women with diabetes. Heliyon 2024, 10, e38758. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.O.; Gerez, J.R.; Hohmann, M.S.N.; Verri, W.A., Jr.; Bracarense, A.P.F.R.L. Phytic Acid Decreases Oxidative Stress and Intestinal Lesions Induced by Fumonisin B₁ and Deoxynivalenol in Intestinal Explants of Pigs. Toxins 2019, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, L.H.; Ogrodowczyk, A.M.; Wiczkowski, W.; Wróblewska, B. Phytate and Butyrate Differently Influence the Proliferation, Apoptosis and Survival Pathways in Human Cancer and Healthy Colonocytes. Nutrients 2021, 13, 1887. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Yang, F.; Li, X.; Cheng, L.; Song, Y. Phytic acid improves intestinal mucosal barrier damage and reduces serum levels of proinflammatory cytokines in a 1,2-dimethylhydrazine-induced rat colorectal cancer model. Br. J. Nutr. 2018, 120, 121–130. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katayama, T. Dietary phytic acid modulates characteristics of the colonic luminal environment and reduces serum levels of proinflammatory cytokines in rats fed a high-fat diet. Nutr. Res. 2014, 34, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, K.; Parfiniewicz, B.; Bednarek, I.; Swiatkowska, L.; Jezienicka, E.; Kierot, J.; Weglarz, L. The influence of phytic acid on TNF-alpha and its receptors genes’ expression in colon cancer Caco-2 cells. Acta Pol. Pharm. 2008, 65, 75–79. [Google Scholar]
- Wawszczyk, J.; Kapral, M.; Hollek, A.; Weglarz, L. The effect of phytic acid on the expression of NF-kappaB, IL-6 and IL-8 in IL-1beta-stimulated human colonic epithelial cells. Acta Pol. Pharm. 2012, 69, 1313–1319. [Google Scholar] [PubMed]
- Wawszczyk, J.; Orchel, A.; Kapral, M.; Hollek, A.; Weglarz, L. Phytic acid down-regulates IL-8 secretion from colonic epithelial cells by influencing mitogen-activated protein kinase signaling pathway. Acta Pol. Pharm. 2012, 69, 1276–1282. [Google Scholar]
- Armah, S.M. Association between Phytate Intake and C-Reactive Protein Concentration among People with Overweight or Obesity: A Cross-Sectional Study Using NHANES 2009/2010. Int. J. Environ. Res. Public Health 2019, 16, 1549. [Google Scholar] [CrossRef]
- Hanson, L.N.; Engelman, H.M.; Alekel, D.L.; Schalinske, K.L.; Kohut, M.L.; Reddy, M.B. Effects of soy isoflavones and phytate on homocysteine, C-reactive protein, and iron status in postmenopausal women. Am. J. Clin. Nutr. 2006, 84, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Wang, Y.Y.; Wang, L.L.; Ojo, O.; Feng, Q.Q.; Jiang, X.S.; Wang, X.H. Effect of dietary fiber on gut barrier function, gut microbiota, short-chain fatty acids, inflammation, and clinical outcomes in critically ill patients: A systematic review and meta-analysis. JPEN J. Parenter Enteral. Nutr. 2022, 46, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Sadeghi, O.; Sadeghian, M.; Sadeghi, N.; Larijani, B.; Esmaillzadeh, A. The Effect of Whole-Grain Intake on Biomarkers of Subclinical Inflammation: A Comprehensive Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Dürholz, K.; Hofmann, J.; Iljazovic, A.; Häger, J.; Lucas, S.; Sarter, K.; Strowig, T.; Bang, H.; Rech, J.; Schett, G.; et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients 2020, 12, 3207. [Google Scholar] [CrossRef]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, F.; Ni, L.; Sun, Y.; Wu, R.; Zhang, T.; Zhang, J.; Zhong, X.; Li, Y. Sugarcane bagasse dietary fiber as an adjuvant therapy for stable chronic obstructive pulmonary disease: A four-center, randomized, double-blind, placebo-controlled study. J. Tradit. Chin. Med. 2016, 36, 418–426. [Google Scholar] [PubMed]
- Moncada, E.; Bulut, N.; Li, S.; Johnson, T.; Hamaker, B.; Reddivari, L. Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut. Nutrients 2024, 16, 2446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Tan, B.; Li, R. Effect of structural characteristics on the physicochemical properties and functional activities of dietary fiber: A review of structure-activity relationship. Int. J. Biol. Macromol. 2024, 269 Pt 2, 132214. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gögebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Weickert, M.O.; et al. Obesity Does Not Modulate the Glycometabolic Benefit of Insoluble Cereal Fibre in Subjects with Prediabetes-A Stratified Post Hoc Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2726. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Honsek, C.; Kemper, M.; Gerbracht, C.; Meyer, N.M.T.; Arafat, A.M.; Birkenfeld, A.L.; Machann, J.; Dambeck, U.; Osterhoff, M.A.; et al. Effects of Insoluble Cereal Fibre on Body Fat Distribution in the Optimal Fibre Trial. Mol. Nutr. Food Res. 2021, 65, e2000991. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Ostadmohammadi, V.; Lankarani, K.B.; Akbari, M.; Akbari, H.; Vakili, S.; Shokrpour, M.; Kolahdooz, F.; Rouhi, V.; Asemi, Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2019, 852, 254–264. [Google Scholar] [CrossRef]
- da Silva Borges, D.; Fernandes, R.; Thives Mello, A.; da Silva Fontoura, E.; Soares Dos Santos, A.R.; Santos de Moraes Trindade, E.B. Prebiotics may reduce serum concentrations of C-reactive protein and ghrelin in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Farhangi, M.A.; Tavakoli, F.; Aliasgarzadeh, A.; Akbari, A.M. Impact of prebiotic supplementation on T-cell subsets and their related cytokines, anthropometric features and blood pressure in patients with type 2 diabetes mellitus: A randomized placebo-controlled Trial. Complement. Ther. Med. 2016, 24, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar]
- Roshanravan, N.; Alamdari, N.M.; Jafarabadi, M.A.; Mohammadi, A.; Shabestari, B.R.; Nasirzadeh, N.; Asghari, S.; Mansoori, B.; Akbarzadeh, M.; Ghavami, A.; et al. Effects of oral butyrate and inulin supplementation on inflammation-induced pyroptosis pathway in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Cytokine 2020, 131, 155101. [Google Scholar] [CrossRef] [PubMed]
- Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Mascarós, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S.M.; Couto, C.A.; Costa-Silva, L.; Armiliato, G.N.; Pereira, C.A.; Martins, F.S.; Ferrari Mde, L.; Vilela, E.G.; Torres, H.O.; Cunha, A.S.; et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients 2016, 8, 397. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Gholami, A.; Hariri, M. Effect of resistant starch type 2 on inflammatory mediators: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 56, 102597. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, M.; Hosseini, S.A.; Khalatbari Mohseni, G.; Heshmati, J.; Rahimlou, M. Effects of resistant starch interventions on circulating inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.; Meng, Y.; Wang, Q.; Xu, H.; Chen, L. The Effects of Resistant Starch on Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Halajzadeh, J.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Barekat, M.; Mirzaei, H.; Mirhashemi, S.M.; Asemi, Z. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3172–3184. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Dong, X.; Li, X.; Jia, R.; Zhang, H.L. Benefits of resistant starch type 2 for patients with end-stage renal disease under maintenance hemodialysis: A systematic review and meta-analysis. Int. J. Med. Sci. 2021, 18, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Montroy, J.; Berjawi, R.; Lalu, M.M.; Podolsky, E.; Peixoto, C.; Sahin, L.; Stintzi, A.; Mack, D.; Fergusson, D.A. The effects of resistant starches on inflammatory bowel disease in preclinical and clinical settings: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 372. [Google Scholar] [CrossRef]
- González, A.P.; Flores-Ramírez, A.; Gutiérrez-Castro, K.P.; Luévano-Contreras, C.; Gómez-Ojeda, A.; Sosa-Bustamante, G.P.; Caccavello, R.; Barrera-de León, J.C.; Garay-Sevilla, M.E.; Gugliucci, A. Reduction of small dense LDL and Il-6 after intervention with Plantago psyllium in adolescents with obesity: A parallel, double blind, randomized clinical trial. Eur. J. Pediatr. 2021, 180, 2493–2503. [Google Scholar] [CrossRef]
- King, D.E.; Mainous AG 3rd Egan, B.M.; Woolson, R.F.; Geesey, M.E. Effect of psyllium fiber supplementation on C-reactive protein: The trial to reduce inflammatory markers (TRIM). Ann. Fam. Med. 2008, 6, 100–106. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G.; Al-Solaiman, Y.; Jesri, A. Effect of a High-Fiber Diet vs a Fiber-Supplemented Diet on C-Reactive Protein Level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef]
- Kohl, A.; Gögebakan, O.; Möhlig, M.; Osterhoff, M.; Isken, F.; Pfeiffer, A.F.; Weickert, M.O. Increased interleukin-10 but unchanged insulin sensitivity after 4 weeks of (1, 3)(1, 6)-beta-glycan consumption in overweight humans. Nutr. Res. 2009, 29, 248–254. [Google Scholar] [CrossRef]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, R.G.; Slavin, J.L. Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the metabolic syndrome profile by soluble fibre-guar gum-in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Qu, H.; Song, L.; Zhang, Y.; Gao, Z.Y.; Shi, D.Z. The Effect of Prebiotic Products on Decreasing Adiposity Parameters in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis. Curr. Med. Chem. 2021, 28, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; LJenkins, A.; Smircic-Duvnjak, L.; LSievenpiper, J.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Pol, K.; Christensen, R.; Bartels, E.M.; Raben, A.; Tetens, I.; Kristensen, M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013, 98, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J. Effect of the novel functional fibre, polyglycoplex (PGX), on body weight and metabolic parameters: A systematic review of randomized clinical trials. Clin. Nutr. 2015, 34, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Posadzki, P.; Ernst, E. The efficacy of glucomannan supplementation in overweight and obesity: A systematic review and meta-analysis of randomized clinical trials. J. Am. Coll. Nutr. 2014, 33, 70–78. [Google Scholar] [CrossRef]
- Higgins, J.A. Resistant starch and energy balance: Impact on weight loss and maintenance. Crit. Rev. Food Sci. Nutr. 2014, 54, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Guar gum for body weight reduction: Meta-analysis of randomized trials. Am. J. Med. 2001, 110, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.H.; Niu, Y.B.; Sun, Y.; Guo, Z.J.; Li, Q.; Li, C.; Feng, J.; Cao, S.S.; Mei, Q.B. An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-κB pathway in a mouse model of colitis-associated colon cancer. Carcinogenesis 2010, 31, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ishisono, K.; Yabe, T.; Kitaguchi, K. Citrus pectin attenuates endotoxin shock via suppression of Toll-like receptor signaling in Peyer’s patch myeloid cells. J. Nutr. Biochem. 2017, 50, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune modulation by different types of β2→1-fructans is toll-like receptor dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef]
- Beukema, M.; Jermendi, É.; Schols, H.A.; de Vos, P. The influence of calcium on pectin’s impact on TLR2 signalling. Food Funct. 2020, 11, 7427–7432. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Rösch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. Resistant starches differentially stimulate Toll-like receptors and attenuate proinflammatory cytokines in dendritic cells by modulation of intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 1814–1826. [Google Scholar] [CrossRef]
- Van Hung, T.; Suzuki, T. Guar gum fiber increases suppressor of cytokine signaling-1 expression via toll-like receptor 2 and dectin-1 pathways, regulating inflammatory response in small intestinal epithelial cells. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Isken, F.; Klaus, S.; Osterhoff, M.; Pfeiffer, A.F.; Weickert, M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 2010, 21, 278–284. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Kappo, K.A.; Petzke, K.J.; Kipp, A.P.; Blaut, M.; Klaus, S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016, 60, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e1-10. [Google Scholar] [CrossRef]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, M.; O’Dowd, J.F.; Wargent, E.T.; Zaibi, M.S.; Hislop, D.C.; Ngala, R.A.; Smith, D.M.; Cawthorne, M.A.; Stocker, C.J.; Arch, J.R. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br. J. Nutr. 2013, 109, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Tsujimoto, G.; Kimura, I. Regulation of Energy Homeostasis by GPR41. Front. Endocrinol. 2014, 5, 81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation. 2020, 141, 1393–1403. [Google Scholar] [CrossRef]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Kundi, Z.M.; Lee, J.C.; Pihlajamäki, J.; Chan, C.B.; Leung, K.S.; So, S.S.Y.; Nordlund, E.; Kolehmainen, M.; El-Nezami, H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet-Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021, 65, e1900580. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Knudsen, C.; Beaumont, M.; Rodriguez, J.; Neyrinck, A.M.; Bindels, L.B. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: A focus on the gut-liver axis. Proc. Nutr. Soc. 2019, 78, 319–328. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Weickert, M.O.; Hattersley, J.G.; Kyrou, I.; Arafat, A.M.; Rudovich, N.; Roden, M.; Nowotny, P.; von Loeffelholz, C.; Matysik, S.; Schmitz, G.; et al. Effects of supplemented isoenergetic diets varying in cereal fiber and protein content on the bile acid metabolic signature and relation to insulin resistance. Nutr. Diabetes 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Nicolucci, A.C.; Han, J.; Sturzenegger, T.; Shearer, J.; Mickiewicz, B.; Vogel, H.J.; et al. Impact of dietary fiber supplementation on modulating microbiota-host-metabolic axes in obesity. J. Nutr. Biochem. 2019, 64, 228–236. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Houben, T.; Katiraei, S.; Dijk, W.; Boutens, L.; van der Bolt, N.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Mandard, S.; et al. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: A potential role for bile acids. J. Lipid Res. 2017, 58, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Romero, R.; Hellhund, A.; Linne, U.; Bertrams, W.; Pinkenburg, O.; Eldin, H.S.; Binder, K.; Jacob, R.; Walker, A.; et al. Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota. Gut Microbes 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef]
- Weickert, M.O.; Arafat, A.M.; Blaut, M.; Alpert, C.; Becker, N.; Leupelt, V.; Rudovich, N.; Möhlig, M.; Pfeiffer, A.F. Changes in dominant groups of the gut microbiota do not explain cereal-fiber induced improvement of whole-body insulin sensitivity. Nutr. Metab. 2011, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, J.G.; Pfeiffer, A.F.; Roden, M.; Petzke, K.J.; Hoffmann, D.; Rudovich, N.N.; Randeva, H.S.; Vatish, M.; Osterhoff, M.; Goegebakan, Ö.; et al. Modulation of amino acid metabolic signatures by supplemented isoenergetic diets differing in protein and cereal fiber content. J. Clin. Endocrinol. Metab. 2014, 99, E2599–E2609. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, M.; Fu, H.; Xu, H.; Wei, J.; Wang, T.; Wang, X. Mammalian target of the rapamycin pathway is involved in non-alcoholic fatty liver disease. Mol. Med. Rep. 2010, 3, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Hengstschläger, M.; Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef]
- Mahendran, Y.; Jonsson, A.; Have, C.T.; Allin, K.H.; Witte, D.R.; Jørgensen, M.E.; Grarup, N.; Pedersen, O.; Kilpeläinen, T.O.; Hansen, T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 2017, 60, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef] [PubMed]
- El, K.; Gray, S.M.; Capozzi, M.E.; Knuth, E.R.; Jin, E.; Svendsen, B.; Clifford, A.; Brown, J.L.; Encisco, S.E.; Chazotte, B.M.; et al. GIP mediates the incretin effect and glucose tolerance by dual actions on α cells and β cells. Sci. Adv. 2021, 7, eabf1948. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. S3), 5–29. [Google Scholar] [CrossRef]
- Moede, T.; Leibiger, I.B.; Berggren, P.O. Alpha cell regulation of beta cell function. Diabetologia 2020, 63, 2064–2075. [Google Scholar] [CrossRef]
- Keyhani-Nejad, F.; Irmler, M.; Isken, F.; Wirth, E.K.; Beckers, J.; Birkenfeld, A.L.; Pfeiffer, A.F. Nutritional strategy to prevent fatty liver and insulin resistance independent of obesity by reducing glucose-dependent insulinotropic polypeptide responses in mice. Diabetologia 2015, 58, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Gögebakan, Ö.; Osterhoff, M.A.; Schüler, R.; Pivovarova, O.; Kruse, M.; Seltmann, A.C.; Mosig, A.S.; Rudovich, N.; Nauck, M.; Pfeiffer, A.F. GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: A randomised trial. Diabetologia 2015, 58, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.H.; Keyhani-Nejad, F. High Glycemic Index Metabolic Damage—A Pivotal Role of GIP and GLP-1. Trends Endocrinol. Metab. 2018, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Keyhani-Nejad, F.; Barbosa Yanez, R.L.; Kemper, M.; Schueler, R.; Pivovarova-Ramich, O.; Rudovich, N.; Pfeiffer, A.F.H. Endogenously released GIP reduces and GLP-1 increases hepatic insulin extraction. Peptides 2020, 125, 170231. [Google Scholar] [CrossRef] [PubMed]
- Isken, F.; Pfeiffer, A.F.; Nogueiras, R.; Osterhoff, M.A.; Ristow, M.; Thorens, B.; Tschöp, M.H.; Weickert, M.O. Deficiency of glucose-dependent insulinotropic polypeptide receptor prevents ovariectomy-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E350–E355. [Google Scholar] [CrossRef]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP signaling induces adipose inflammation via a HIF-1α-dependent pathway and impairs insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E414–E425. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.; Rudovich, N.; Weickert, M.O.; Isken, F. Role of the gut Peptide glucose-induced insulinomimetic Peptide in energy balance. Results Probl. Cell Differ. 2010, 52, 183–188. [Google Scholar] [PubMed]
- Fu, Y.; Kaneko, K.; Lin, H.Y.; Mo, Q.; Xu, Y.; Suganami, T.; Ravn, P.; Fukuda, M. Gut Hormone GIP Induces Inflammation and Insulin Resistance in the Hypothalamus. Endocrinology 2020, 161, bqaa102. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, K.S.; Niskanen, L.K.; Liukkonen, K.H.; Poutanen, K.S.; Holst, J.J.; Mykkänen, H.M. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am. J. Clin. Nutr. 2002, 75, 254–262. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Gregersen, S.; Lærke, H.N.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2014, 68, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, K.S.; Laaksonen, D.E.; Autio, K.; Niskanen, L.K.; Holst, J.J.; Savolainen, K.E.; Liukkonen, K.H.; Poutanen, K.S.; Mykkänen, H.M. Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am. J. Clin. Nutr. 2003, 78, 957–964. [Google Scholar] [CrossRef]
- Mofidi, A.; Ferraro, Z.M.; Stewart, K.A.; Tulk, H.M.; Robinson, L.E.; Duncan, A.M.; Graham, T.E. The acute impact of ingestion of sourdough and whole-grain breads on blood glucose, insulin, and incretins in overweight and obese men. J. Nutr. Metab. 2012, 2012, 184710. [Google Scholar] [CrossRef]
- Santaliestra-Pasías, A.M.; Garcia-Lacarte, M.; Rico, M.C.; Aguilera, C.M.; Moreno, L.A. Effect of two bakery products on short-term food intake and gut-hormones in young adults: A pilot study. Int. J. Food Sci. Nutr. 2015, 67, 562–570. [Google Scholar] [CrossRef]
- Hagander, B.; Björck, I.; Asp, N.G.; Lundquist, I.; Nilsson-Ehle, P.; Schrezenmeir, J.; Scherstén, B. Hormonal and metabolic responses to breakfast meals in niddm: Comparison of white and whole-grain wheat bread and corresponding extruded products. Hum. Nutr. Appl. Nutr. 1985, 39, 114–123. [Google Scholar] [PubMed]
- Hagander, B.; Björck, I.; Asp, N.G.; Efendić, S.; Holm, J.; Nilsson-Ehle, P.; Lundquist, I.; Scherstén, B. Rye products in the diabetic diet. Postprandial glucose and hormonal responses in non-insulin-dependent diabetic patients as compared to starch availability in vitro and experiments in rats. Diabetes Res. Clin. Pract. 1987, 3, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Ward, E.; Holst, J.J.; Astrup, A.; Ormsbee, M.J.; Connelly, S.; Arciero, P.J. Resistant starch and protein intake enhances fat oxidation and feelings of fullness in lean and overweight/obese women. Nutr. J. 2015, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, C.E.; Mesa, M.D.; Olza, J.; Buccianti, G.; Pérez, M.; Moreno-Torres, R.; Pérez de la Cruz, A.; Gil, A. Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur. J. Nutr. 2013, 52, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Raben, A.; Tagliabue, A.; Christensen, N.J.; Madsen, J.; Holst, J.J.; Astrup, A. Resistant starch: The effect on postprandial glycemia, hormonal response, and satiety. Am. J. Clin. Nutr. 1994, 60, 544–551. [Google Scholar] [CrossRef]
- Hughes, R.L.; Horn, W.F.; Wen, A.; Rust, B.; Woodhouse, L.R.; Newman, J.W.; Keim, N.L. Resistant starch wheat increases PYY and decreases GIP but has no effect on self-reported perceptions of satiety. Appetite 2021, 168, 105802. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S.; Rebry, R.M.; Tetlow, I.J.; Emes, M.J.; McKeown, B.; Graham, T.E. Resistant starch intake at breakfast affects postprandial responses in type 2 diabetics and enhances the glucose-dependent insulinotropic polypeptide--insulin relationship following a second meal. Appl. Physiol. Nutr. Metab. 2013, 38, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Emilien, C.H.; Zhu, Y.; Hsu, W.H.; Williamson, P.; Hollis, J.H. The effect of soluble fiber dextrin on postprandial appetite and subsequent food intake in healthy adults. Nutrition 2018, 47, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ekström, L.M.; Björck, I.M.; Östman, E.M. An improved course of glycaemia after a bread based breakfast is associated with beneficial effects on acute and semi-acute markers of appetite. Food Funct. 2016, 7, 1040–1047. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Suzuki, J.; Fukuoka, D.; Tokimitsu, I.; Hase, T. RS4-type resistant starch prevents high-fat diet-induced obesity via increased hepatic fatty acid oxidation and decreased postprandial GIP in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E652–E662. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; King, R.A.; Christophersen, C.T.; Bird, A.R. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr. Metab. 2012, 9, 93. [Google Scholar] [CrossRef]
- Hosoda, Y.; Okahara, F.; Mori, T.; Deguchi, J.; Ota, N.; Osaki, N.; Shimotoyodome, A. Dietary steamed wheat bran increases postprandial fat oxidation in association with a reduced blood glucose-dependent insulinotropic polypeptide response in mice. Food Nutr. Res. 2017, 61, 1361778. [Google Scholar] [CrossRef][Green Version]
- Neyrinck, A.M.; Hiel, S.; Bouzin, C.; Campayo, V.G.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with bifidogenic properties abolishes metabolic disorders induced by western diet in mice. Nutr. Diabetes 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Pluschke, A.M.; Williams, B.A.; Zhang, D.; Anderson, S.T.; Roura, E.; Gidley, M.J. Male grower pigs fed cereal soluble dietary fibres display biphasic glucose response and delayed glycaemic response after an oral glucose tolerance test. PLoS ONE 2018, 13, e0193137. [Google Scholar] [CrossRef]
- Simões Nunes, C.; Malmlöf, K. Glucose absorption, hormonal release and hepatic metabolism after guar gum ingestion. Reprod. Nutr. Dev. 1992, 32, 11–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hooda, S.; Matte, J.J.; Vasanthan, T.; Zijlstra, R.T. Dietary oat beta-glucan reduces peak net glucose flux and insulin production and modulates plasma incretin in portal-vein catheterized grower pigs. J. Nutr. 2010, 140, 1564–1569. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.C.; Philip, N.H.; Hui, L.; Zhou, X.; Franklin, R.A.; Kong, Y.; Medzhitov, R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci. Signal. 2019, 12, eaau1851. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Wu, G.D.; Walters, W.; Compher, C.; Ni, J.; Delaroque, C.; Albenberg, L.; Ley, R.E.; Patterson, A.D.; Lewis, J.D.; et al. Human Intestinal Microbiome Determines Individualized Inflammatory Response to Dietary Emulsifier Carboxymethylcellulose Consumption. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 315–318. [Google Scholar] [CrossRef]
- Monfort-Pires, M.; Ferreira, S.R. Inflammatory and metabolic responses to dietary intervention differ among individuals at distinct cardiometabolic risk levels. Nutrition 2017, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
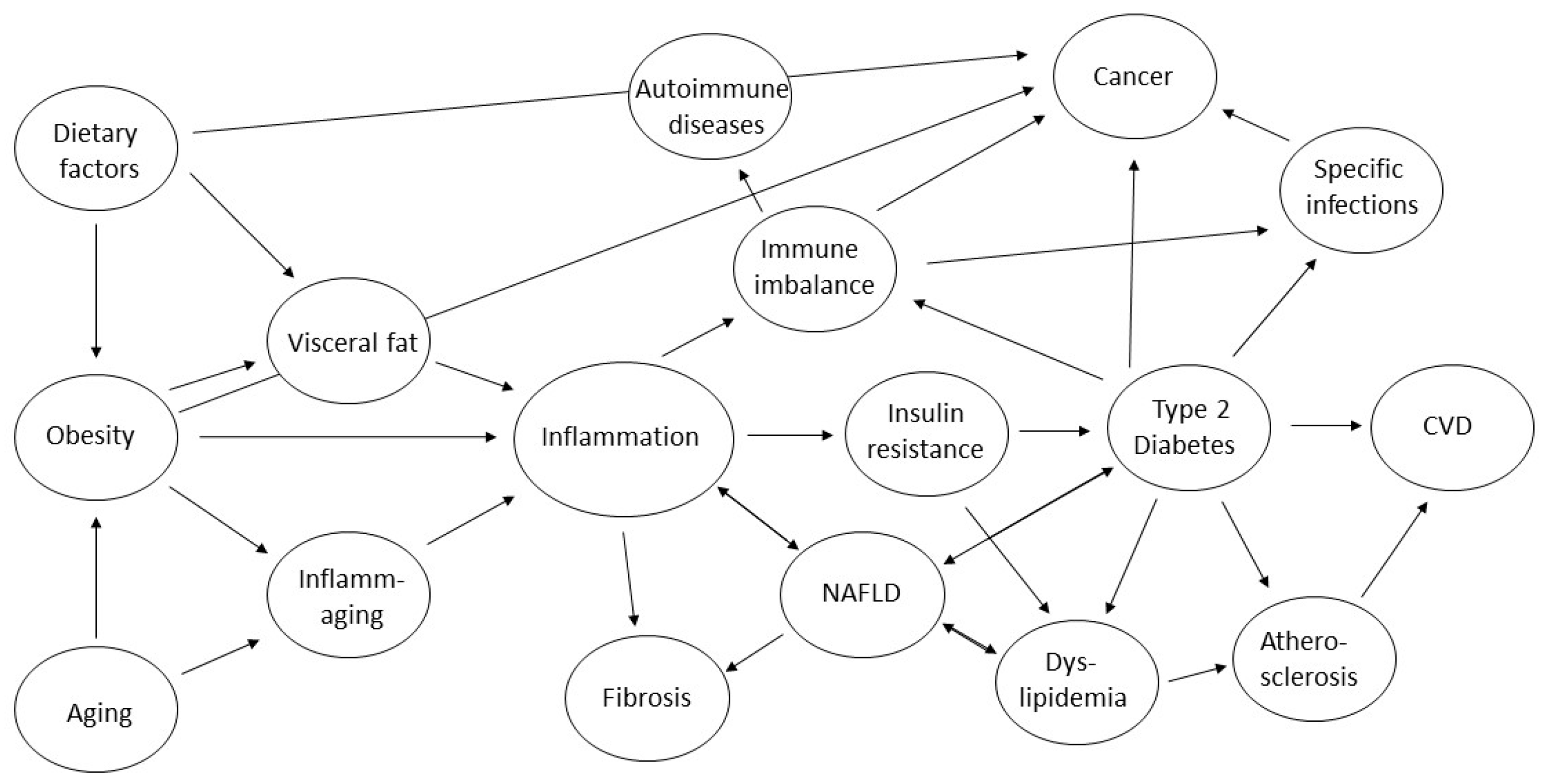
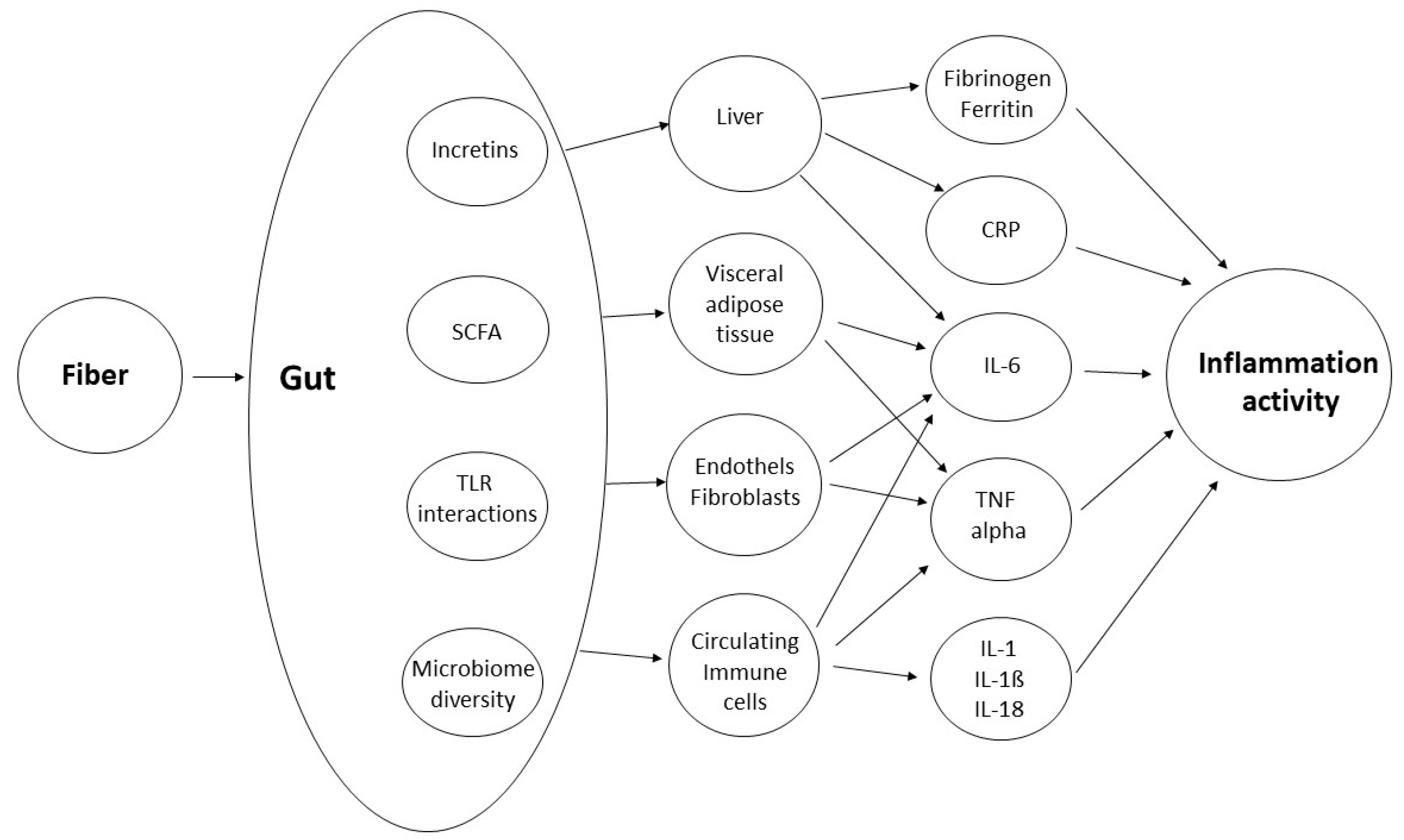
| Type of Fiber | Water-Solubility | Fermentability | Viscosity | Monomers and Structure | Main Food Sources |
|---|---|---|---|---|---|
| Lignin | ↓ | ↓ | ↓ | Lignols, few hundred monomers | cereals and legumes, fruit stones |
| Cellulose | ↓ | ↓ | ↓ | β-(1-4)-linked glucose, unbranched, few thousand monomers | cereals and legumes |
| Cellodextrins | ↑ | ↑ | ↓ | β-(1-4)-linked glucose, unbranched, few hundred monomers | cereals and legumes |
| Chitin | ↓ | ↓ | ↓ | β-(1-4)-linked N-acetylglucosamine, unbranched | crustaceans, arthropods, mushrooms |
| Arabinoxylan | ↓/↑ | ↓/↑ | ↓/↑ | β-(1-4)-linked xylose, arabinose-branches, few hundred monomers | grains, psyllium |
| Arabinoxylan-oligosaccharides | ↑ | ↓/↑ | ↑ | β-(1-4)-linked xylose, arabinose-branches, few dozen monomers | grains, psyllium |
| Other pentose-Hemicelluloses | ↓ | ↓ | ↓ | β-(1-4)-linked pentoses, branched, few hundred monomers | oat, rye |
| Galactomannan | ↑ | ↑ | ↑ | β-(1-4)-linked mannose, galactose-branches, few hundred monomers | various gums (e.g., guar, cassia) |
| Other hexose-Hemicelluloses | ↓ | ↓ | ↓ | β-(1-4)-linked hexoses, branched, few hundred monomers | barley, wheat |
| Xyloglucan | ↑ | ↓ | ↓ | β-(1-4)-linked glucose, xylose-branches, few hundred monomers | fruits, vegetables |
| (mixed-linkage) ß-glucan | ↑ | ↑ | ↑ | β-(1-4)- β-(1-3)-linked glucose, few hundred monomers | barley, oat, rye |
| Resistant starch type 1 | ↓ | ↑ | ↓ | α-(1-4)-linked glucose, α-(1-6)-linked branches, cellular matrix | unprocessed starchy vegetables |
| Resistant starch type 2 | ↓ | ↑ | ↓ | α-(1-4)-linked glucose, α-(1-6)-linked branches, specific conformation | unripe fruits |
| Resistant starch type 3 | ↑ | ↑ | ↓ | α-(1-4)-linked glucose, α-(1-6)-linked branches, retrograded | starchy, protein-containing foods |
| Resistant starch type 4 | ↑/↓ | ↑ | ↓ | α-(1-4)-linked glucose, α-(1-6)-linked branches, chemically altered | synthetic alteration of starch |
| Resistant starch type 5 | ↑/↓ | ↑ | ↓ | α-(1-4)-linked glucose, α-(1-6)-linked branches, in lipid complexes | processed starchy, fatty foods |
| Fructan (e.g., Inulin) | ↑ | ↑↑ | ↑/↓ | β-(2-1)- and/or β-(2-6)-linked fructose, unbranched, dozens of monomers | tubers and roots |
| Raffinoses | ↑ | ↑ | ↑ | (1-6)-linked oligosaccharides of galactose, fructose, glucose, unbranched | legumes, vegetables, grains |
| Pectin | ↑ | ↑↑ | ↑ | α-(1–4)-linked galacturonate, variable substitutes and branches | fruits and vegetables |
| Alginate | ↑ | ↑ | ↑ | (1-4)-linked mannuronate and guluronate, unbranched | brown seaweeds |
| Agar | ↑ | ↑ | ↑ | α-(1-3)/β-(1-4)-linked galactose and 3,6-anhydro-galactose, side-groups | red (and other) algae |
| Carrageenan | ↑ | ↑ | ↑ | sulfated (anhydro-)galactose in various linkage patterns, unbranched | red algae, food additive |
| Guar gum | ↑ | ↑↑ | ↑ | β-(1-4)-linked mannose with α-(1-6)-galactose side chains | guar, food additive |
| Xanthan | ↑ | ↑ | ↑ | β-(1-4)-linked glucose; glucose-mannose-glucuronate-branched | synthesized food additive |
| Polydextrose | ↑ | ↑ | ↑ | Glucose in variable α- and β-linkage; added by sorbitol and citric acid | synthesized food additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabisch, S.; Hajir, J.; Sukhobaevskaia, V.; Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber on Inflammation in Humans. Int. J. Mol. Sci. 2025, 26, 2000. https://doi.org/10.3390/ijms26052000
Kabisch S, Hajir J, Sukhobaevskaia V, Weickert MO, Pfeiffer AFH. Impact of Dietary Fiber on Inflammation in Humans. International Journal of Molecular Sciences. 2025; 26(5):2000. https://doi.org/10.3390/ijms26052000
Chicago/Turabian StyleKabisch, Stefan, Jasmin Hajir, Varvara Sukhobaevskaia, Martin O. Weickert, and Andreas F. H. Pfeiffer. 2025. "Impact of Dietary Fiber on Inflammation in Humans" International Journal of Molecular Sciences 26, no. 5: 2000. https://doi.org/10.3390/ijms26052000
APA StyleKabisch, S., Hajir, J., Sukhobaevskaia, V., Weickert, M. O., & Pfeiffer, A. F. H. (2025). Impact of Dietary Fiber on Inflammation in Humans. International Journal of Molecular Sciences, 26(5), 2000. https://doi.org/10.3390/ijms26052000







