Drug-Induced Liver Injury—Pharmacological Spectrum Among Children
Abstract
1. Introduction
2. Pathogenesis and DILI Subtypes
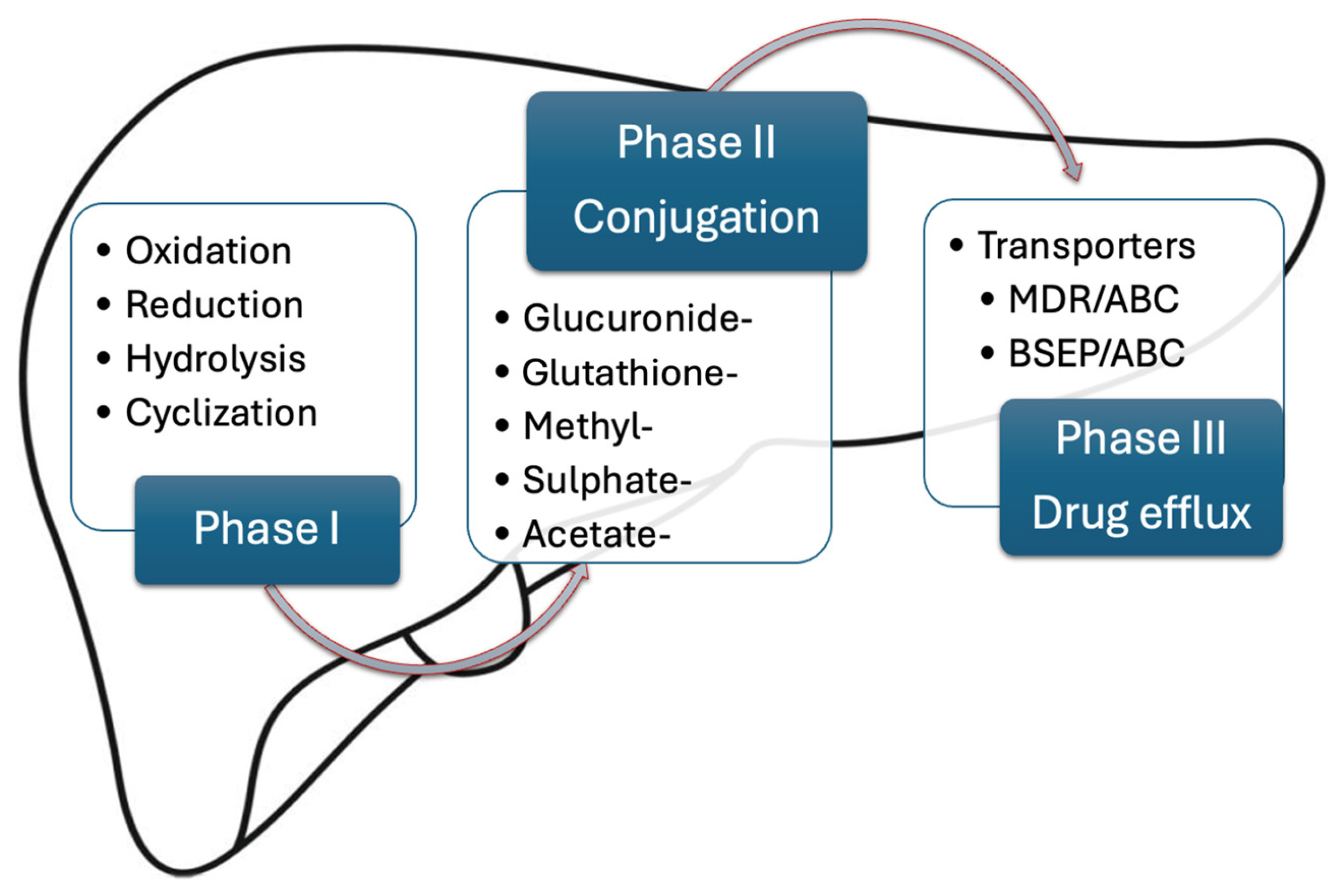
2.1. Drug Pharmacokinetics
2.2. Pathogenesis of DILI Subtypes
2.2.1. Intrinsic DILI
2.2.2. Idiosyncratic DILI
Immune-Mediated Idiosyncratic DILI
Metabolic Idiosyncratic DILI
3. Diagnosis and Management
4. DILI in Children
4.1. Acetaminophen
- Phase 1: within first 24 h patients are either asymptomatic or present with nausea, vomiting, abdominal pain (normal liver tests—transaminase levels begin to rise after 12 h with massive doses).
- Phase 2: 24–72 h after ingestion, patients present right upper quadrant pain (acute hepatitis—elevated liver enzymes, coagulopathy, or renal dysfunction may arise).
- Phase 3: 72–96 h after ingestion patients present jaundice, coagulopathy, encephalopathy, oliguria, edema (peak of liver dysfunction—acute liver failure, kidney failure, multi-organ failure, death).
- Phase 4: beginning with day 4, up to 2 weeks after ingestion, is recovery (delayed histological healing—up to 3 months).
4.2. Antibiotics
4.3. Anti-Tuberculosis Drugs
4.4. Antiepileptic Drugs
4.5. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
4.6. Antineoplastic Agents
4.7. Antimycotic Agents
4.8. Other Drugs
4.8.1. Albendazole
4.8.2. Atomoxetine
4.8.3. Proton Pump Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Babai, S.; Auclert, L.; Le-Louet, H. Safety data and withdrawal of hepatotoxic drugs. Therapies 2021, 76, 715–723. [Google Scholar] [CrossRef]
- Gerussi, A.; Natalini, A.; Antonageli, F.; Mancuso, C.; Agostinetto, E.; Barisani, D.; Di Rosa, F.; Andrade, R.; Invernizzi, P. Immune-Mediated Drug-Induced Liver Injury: Immunogenetics and Experimental Models. Int. J. Mol. Sci. 2021, 22, 4557. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zeng, X.; Liu, Y.; Liu, J.; Li, C.; Chen, L.; Chen, H.; Ouyang, D. The Immunological Mechanisms and Immune-Based Biomarkers of Drug-Induced Liver Injury. Front. Pharmacol. 2021, 12, 723940. [Google Scholar] [CrossRef] [PubMed]
- Jee, A.; Senoskie, S.C.; Uetrecht, J. Idiosyncratic Drug-Induced Liver Injury: Mechanistic and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2954. [Google Scholar] [CrossRef] [PubMed]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, 104–109. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Maddur, H.; Russo, M.W.; Wong, R.J.; Reddy, K.R. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2021, 116, 878–898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaplowitz, N. Mechanisms of drug-induced liver injury. Clin. Liver Dis. 2013, 17, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Tujios, S.; Fontana, R.J. Mechanisms of drug-induced liver injury: From bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Bozic, I.; Kizivat, T.; Bojanic, K.; Mrso, M.; Kralj, E.; Smolic, R.; Wu, G.Y.; Smolic, M. Models of Drug Induced Liver Injury (DILI)—Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef]
- Bergen, A. Immune-Mediated DILI—Predicting the Unpredictable. 16 March 2023. Available online: https://cn-bio.com/immune-mediated-dili-predicting-the-unpredictable/ (accessed on 10 April 2023).
- Monge-Urrea, F.; Monjito-Barrios, E. Drug-induced Liver Injury in Pediatrics. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 391–395. [Google Scholar] [CrossRef]
- Grogan, S.; Preuss, C.V. Pharmacokinetics. 30 July 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557744/ (accessed on 14 January 2024).
- Susa, S.T.; Hussain, A.; Preuss, C.V. Drug Metabolism. 17 August 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442023/ (accessed on 14 January 2024).
- Phang-Lyn, S.; Llerena, V.M. Biochemistry, Biotransformation. 14 August 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544353/ (accessed on 14 January 2024).
- Larson, A.M. Drugs and the Liver: Metabolism and Mechanism of Injury. June 2023. Available online: https://www.uptodate.com/contents/drugs-and-the-liver-metabolism-and-mechanisms-of-injury (accessed on 15 March 2024).
- Curie, G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. JNMT 2018, 46, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Tang, W. Drug metabolism in drug discovery and development. Acta Pharm. Sin. B 2018, 8, 721–732. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K. Paediatric pharmacokinetics and drug doses. Aust. Prescr. 2016, 39, 208–210. [Google Scholar] [CrossRef]
- Benedetti, M.S.; Whomsley, R.; Canning, M. Drug metabolism in the paediatric population and in the elderly. Drug Discov. Today 2007, 12, 599–610. [Google Scholar] [CrossRef]
- Van Groen, B.D.; Reddy, V.P.; Badée, J.; Olivares-Morales, A.; Johnson, T.N.; Nicolaï, J. Pediatric Pharmacokinetics and Dose Predictions: A Report of a Satellite Meeting to the 10th Juvenile Toxicity Symposium. Clin. Translat Sci. 2021, 14, 29–35. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Developmental pharmacokinetics. Semin. Pediatr. Neurol. 2010, 17, 208–213. [Google Scholar] [CrossRef]
- Anderson, G.D. Children Versus Adults: Pharmacokinetic and Adverse-Effect Differences. Epilepsia 2002, 43, 53–59. [Google Scholar] [CrossRef]
- Vander Schaaf, M.; Luth, K.; Townsend, D.M.; Chessman, K.H.; Mills, C.M.; Garner, S.S.; Peterson, Y.K. CYP3A4 drug metabolism considerations in pediatric pharmacotherapy. Med. Chem. Res. 2024, 33, 2221–2235. [Google Scholar] [CrossRef]
- Hosack, T.; Damry, D.; Biswas, S. Drug-induced liver injury: A comprehensive review. Ther. Adv. Gastroenterol. 2023, 16, 17562848231163410. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.A.; Ganey, P.E. Intrinsic versus idiosyncratic drug-induced hepatotoxicity—Two villains or one? J. Pharmacol. Exp. Ther. 2010, 332, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mosedale, M.; Watkins, P.B. Drug-induces liver injury: Advances in mechanistic understanding that will inform risk management. Clin. Pharmacol. Ther. 2017, 101, 469–480. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, X.; Greenhaw, J.; Salminen, A.T.; Russotti, G.M.; Salminen, W.F. Drug-induced liver injury in children: Clinical observations, animal models and regulatory status. Int. J. Toxicol. 2017, 36, 365–379. [Google Scholar] [CrossRef]
- Burns, M.J.; Friedman, S.L.; Larson, A.M. Acetaminophen (Paracetamol) Poisoning in Adults: Pathophysiology, Presentation and Evaluation. December 2023. Available online: https://www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-evaluation (accessed on 14 March 2024).
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar] [CrossRef]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, G.; Franciotta, D.; Perucca, E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia 2007, 48, 1223–1244. [Google Scholar] [CrossRef] [PubMed]
- Merriam-Webster. s.d. Idiosyncracy. Available online: https://www.merriam-webster.com/dictionary/idiosyncracy (accessed on 14 March 2024).
- Uetrecht, J.; Naisbitt, D.J. Idiosyncratic adverse drug reactions: Current concepts. Pharmacol. Rev. 2013, 65, 779–808. [Google Scholar] [CrossRef]
- Sernoskie, S.C.; Jee, A.; Uetrecht, J.P. The innate immune response in idiosyncratic drug reactions. Pharmacol. Rev. 2021, 73, 861–896. [Google Scholar] [CrossRef]
- Parlar, Y.E.; Ayar, S.N.; Cagdas, D.; Balaban, Y.H. Liver immunity, autoimmunity and inborn error of immunity. World J. Hepatol. 2023, 15, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Sanjay, S. Role of immune dysfunction in drug induced liver injury. World J. Hepatol. 2021, 13, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, M.; Quintas, G.; Castell, J.V. The potential role of metabolomics in drug-induced liver injury (DILI) assessment. Metabolites 2022, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Mao, Y.M.; Chen, C.W.; Chen, J.J.; Chen, J.; Cong, W.M.; Ding, Y.; Duan, Z.P.; Fu, Q.C.; Guo, X.Y.; et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 2017, 11, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M. Drug-Induced Liver Injury. July 2022. Available online: https://www.uptodate.com/contents/drug-induced-liver-injury (accessed on 10 April 2023).
- Daneshvar, D.; Haddad, F.; Abergel, J. Carbamazepine-induced immuno-allergic hepatitis. Am. J. Gastroenterol. 2019, 114, 1335. [Google Scholar] [CrossRef]
- De Boer, Y.S.; Kosinski, A.S.; Urban, T.J.; Zhao, Z.; Long, N.; Chalasani, N.; Kleiner, D.E.; Hoofnagle, J.H.; Drug-Induced Liver Injury Network. Drug-induced liver injury network. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin. Gastroenterol. Hepatol. 2017, 15, 103–112. [Google Scholar] [CrossRef]
- Bjornsson, E.S.; Medina-Caliz, I.; Andrade, R.J.; Lucena, I.M. Setting up criteria for drug-induced autoimmune-like hepatitis through a systematic analysis of published reports. Hepatol. Commun. 2022, 6, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef]
- Zeman, M.V.; Hirschfield, G.M. Autoantibodies and liver diseases: Use and abuses. Can. J. Gastroenterol. 2010, 24, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wiler-Normal, C.; Schramm, C. Drug-induced liver injury and its relationship to autoimmune hepatitis. J. Hepatol. 2011, 55, 747–749. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Boer, Y.S.; Liberal, R.; Gerbes, A.; Regev, A.; Regev, A.; Beretta-Piccoli, B.T.; Schramm, C.; Kleiner, D.E.; et al. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report. J. Hepatol. 2023, 79, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Uetrecht, J. Immune mechanisms of idiosyncratic drug-induced liver injury. J. Clin. Transl. Res. 2017, 3, 145–156. [Google Scholar] [CrossRef]
- Lei, S.; Gu, R.; Ma, X. Clinical perspectives of isoniazid-induced liver injury. Liver Res. 2021, 5, 45–52. [Google Scholar] [CrossRef]
- Han, D.; Dara, L.; Win, S.; Than, T.A.; Yuan, L.; Abbasi, S.Q.; Liu, Z.X.; Kaplowitz, N. Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol. Sci. 2013, 34, 243–253. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.J.; Tolosa, L.; Donato, M.T. Metabolic activation and drug-induced liver injury in vitro approaches for the safety risk assessment of new drugs. J. Appl. Toxicol. 2016, 36, 752–768. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Valproate. 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548284/ (accessed on 18 January 2024).
- Meseguer, E.S.; Elizalde, M.U.; Borobia, A.M.; Ramirez, E. Valproic acid-induced liver injury: A case-control study from a prospective pharmacovigilance program in a tertiary hospital. J. Clin. Med. 2021, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Wai Yue, Y.G.; Wai, C.K.; Peter, W.Y.M.; Tung, W.H.; Yau, C.K. A fatal case of valproate-induced hyperammonemic encephalopathy: An update on proposed pathogenic mechanisms and treatment options. Int. J. Epilepsy 2017, 4, 181–183. [Google Scholar] [CrossRef]
- Fontana, R. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 2014, 146, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Molleston, J.P.; Fontana, R.J.; Lopez, M.J.; Kliner, D.E.; Gu, J.; Chalasani, N. Drug-induced liver injury network. Characteristics of idiosyncratic drug-induced liver injury in children: Results from the DILIN prospective study. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 182–189. [Google Scholar] [CrossRef]
- Bessone, F.; Hernandez, N.; Tagle, M.; Arrese, M.; Parana, R.; Mendez-Sanchez, N.; Ridruejo, E.; Mendizabal, M.; Dagher, L.; Contreras, F.; et al. Drug-induced liver injury: A management position paper from the Latin American Association for study of the liver. Ann. Hepatol. 2021, 24, 100321. [Google Scholar] [CrossRef]
- David, S.; Hamilton, J.P. Drug-induced liver injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar]
- Pop, T.L.; Aldea, C.O.; Delean, D.; Bulata, B.; Boghitoiu, D.; Pacurar, D.; Ulmeanu, C.E.; Grama, A. The role of predictive models in the assessment of the poor outcomes in pediatric acute liver failure. J. Clin. Med. 2022, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Defendi, G.L. Acetaminophen toxicity in children: Diagnosis, clinical assessment and treatment of acute overingestion. Consultant360 2013, 12, 7. [Google Scholar]
- Hogman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef]
- Agrawal, S.; Khazawni, B. Acetaminophen Toxicity. June 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441917/ (accessed on 14 January 2024).
- Allison, R.; Guraja, A.; Shawa, I.T.; Tripathi, G.; Moritz, W.; Kermanizadeh, A. Drug-induced liver injury—A 2023 update. J. Toxicol. Environ. Health—B Crit. Rev. 2023, 26, 442–467. [Google Scholar] [CrossRef]
- LiverTox. Roussel Uclaf Causality Assessment Methos (RUCAM) in Drug-Induced Liver Injury. 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548272 (accessed on 20 March 2024).
- Atallah, E.; Freixo, C.; Alvarez-Alvarez, I.; Cubero, F.J.; Gerbes, A.L.; Kullak-Ublick, G.A.; Aithal, G.P. Biomarkers of idiosyncratic drug-induced liver injury (DILI)—A systematic review. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1327–1343. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Zafra, A.; Di Zeo-Sánchez, D.E.; López-Gómez, C.; Pérez-Valdés, Z.; García-Fuentes, E.; Andrade, R.J.; Lucena, I.M.; Villanueva-Paz, M. Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction. Acta Pharm. Sin. B 2021, 11, 3685–3726. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Genetics of drug-induced liver injury: Current knowledge and future prospects. Clin. Transl. Sci. 2022, 16, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Lewis, J.H. Current and future directions in the treatment and prevention of drug-induced liver injury: A systematic review. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 517–536. [Google Scholar] [CrossRef]
- Teschke, R.; Frenzel, C. Drug-induced liver injury: Do we still need a routine liver biopsy for diagnosis today? Ann. Hepatol. 2014, 13, 121–126. [Google Scholar] [CrossRef]
- Squires, R.H.; Dhawan, A.; Alonso, E.; Narkewicz, M.R.; Shneider, B.L.; Rodriguez-Baez, N.; Dell Olio, D.; Karpen, S.; Bucuvalas, J.; Lobritto, S.; et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: A placebo-controlled clinical trial. Hepatology 2013, 57, 1542–1549. [Google Scholar] [CrossRef]
- Niu, H.; Atallah, E.; Alvarez-Alvarez, I.; Medina-Caliz, I.; Aithal, G.P.; Arikan, C.; Andrade, R.J.; Lucena, I.M. Therapeutic Management of Idiosyncratic Drug-Induced Liver Injury and Acetaminophen Hepatotoxicity in the Paediatric Population: A Systematic Review. Drug Saf. 2022, 45, 1329–1348. [Google Scholar] [CrossRef] [PubMed]
- Grama, A.; Aldea, C.O.; Burac, L.; Delean, D.; Bulata, B.; Sirbe, C.; Duca, E.; Boghitoiu, D.; Coroleuca, A.; Pop, T.L. Etiology and outcome after acute liver failure in children—The experience of a single tertiary care hospital from Romania. Children 2020, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Chidian, A.S.; Buckley, N.A.; Noghrehchi, F.; Cairns, R. Paracetamol (acetaminophen) overdose and hepatotoxicity: Mechanism, treatment, prevention measures, and estimates of burden of disease. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Heard, K.; Dart, R. Acetaminophen (Paracetamol) Poisoning: Management in Adults and Children. December 2023. Available online: https://www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-management-in-adults-and-children (accessed on 25 March 2024).
- Shadman, K.A.; Edmonson, M.B.; Coller, R.J.; Sklansky, D.J.; Nacht, C.L.; Zhao, Q.; Kelly, M.M. US Hospital stays in children and adolescents with acetaminophen poisoning. Hosp. Pediatr. 2022, 12, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Alander, S.W.; Dowd, M.D.; Bratton, S.L.; Kearns, G.L. Pediatric Acetaminophen overdose: Risk factors associated with hepatocellular injury. Arch. Pediatr. Adolesc. Med. 2000, 154, 346–350. [Google Scholar] [CrossRef] [PubMed]
- James, L.; Sullivan, J.E.; Roberts, D. The proper use of acetaminophen. J. Paediatr. Child. Health 2011, 16, 544–547. [Google Scholar] [CrossRef]
- Tenenbein, M. Why young children are resistant to acetaminophen poisoning. J. Pediatr. 2000, 137, 891–892. [Google Scholar] [CrossRef]
- Daifallah, A.; Jabr, R.; Al-Tawil, F.; Elkourdi, M.; Salman, Z.; Koni, A.; Samara, A.; Al-Jabi, S.W.; Zyoud, S.H. An assessment of parents’ knowledge and awareness regarding paracetamol use in children: A cross-sectional study from Palestine. BMC Public Health 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Bilenko, N.; Tessler, H.; Okbe, R.; Press, J.; Gorodischer, R. Determinants of antipyretic misuse in children up to 5 years of age: A cross-sectional study. Clin. Ther. 2006, 28, 783–793. [Google Scholar] [CrossRef]
- Serranti, D.; Montagnani, C.; Indolfi, G.; Chiappini, E.; Galli, L.; de Martino, M. Antibiotic induced liver injury: What about children? J. Chemother. 2013, 25, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, S.; Jun, D.W.; Yoon, J.H.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Prevalence and clinical characteristics of antibiotics associated drug-induced liver injury. Ann. Transl. Med. 2021, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Meesters, K.; Chappell, F.; Demirjian, A. Trends in antibiotic use in a large children’s hospital in London (United Kingdom): 5 years of point prevalence surveys. Antibiotics 2024, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Adisa, R.; Orherhe, O.M.; Fakeye, T.O. Evaluation of antibiotic prescriptions and use in under-five children in Ibadan, South Western Nigeria. Afr. Health Sci. 2018, 18, 1189–1201. [Google Scholar] [CrossRef]
- Hernandez, N.; Bessone, F.; Chiodi, D.; Mendizabal, M.; Sanchez, A.; Ridruejo, E.; Bianchi, C.; Pollio, C.; Arrese, M.; Schinoni, M.I.; et al. Amoxicillin-clavulanate induced liver injury: Ten years experience from LATINDILI registry. Ann. Hepatol. 2023, 28, 69–70. [Google Scholar] [CrossRef]
- De Lemos, A.; Ghabril, M.; Rockey, D.C.; Gu, J.Z.; Barnhart, H.X.; Russo, M.W.; Kleiner, D.D.; Bonkovsky, H.L.; Drug-Induced Liver Injury Network (DILIN). Amoxicillin-clavulanate induced DILI: 113 cases from the US Drug-Induced Liver Injury Network (DILIN). Hepatology 2014, 60, 711–712. [Google Scholar] [CrossRef]
- Lucena, M.I.; Molokhia, M.; Shen, Y.; Urban, T.J.; Aithal, G.P.; Andrade, R.J.; Day, C.P.; Ruiz-Cabello, F.; Donaldson, P.T.; Stephens, C.; et al. Susceptibility to Amoxicillin-Clavulanate-Induced Liver Injury Is Influenced by Multiple HLA Class I and II Alleles. Gastroenterology 2011, 141, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Tulkens, P.M. Hepatic safety of antibiotics used in primary care. J. Antimicrob. Chemother. 2011, 66, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Soluyanova, P.; Sanchez-Campos, S.; Castell, J.V.; Jover, R. Molecular mechanisms of hepatotoxic cholestasis by clavulanic acid: Role of NRF2 and FXR pathways. Food Chem. Toxicol. 2021, 158, 112664. [Google Scholar] [CrossRef]
- Ocete Hita, E.; Garcis, J.A.M.; Sanchez, F.G.; Gonzalez, J.C.F.; Molina, A.A.; Escobar, J.S.; Ruiz Extremera, A. Hepatotoxicidad por farmacos o productos naturales en ninos [Hepatotoxicity due to drugs or natural products in children]. An. Pediatr. 2013, 78, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterology 2015, 148, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- CDC. Outpatient Antibiotic Prescriptions—United States. Available online: https://www.cdc.gov/antibiotic-use/data/report-2022.html (accessed on 11 May 2023).
- De Abajo, F.J.; Montero, D.; Madurga, M.; Rodriguez, L.A.G. Acute and clinically relevant drug induced liver injury: A population based case-control study. Brit. J. Clin. Pharmacol. 2004, 58, 71–80. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, P.Y.; Huang, F.L.; Chi, C.S. Reversible oxacillin-associated hepatitis in a 9-month-old boy. J. Paediatr. Child. Health 2008, 44, 146–148. [Google Scholar] [CrossRef]
- Tang, K.; Coombs, S.; Gwee, A. Frequency of drug-induced liver injury in children receiving anti-staphylococcal penicillins. J. Antimicrob. Chemother. 2022, 77, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.G.; Wang, J.B.; Wang, L.F.; Zhao, Y.L.; Bai, Y.F.; Bai, Y.F.; Wang, Z.X.; Li, J.Y.; Xiao, X.H. Causes, features and outcomes of drug-induced liver injury in 69 children from China. Gut Liver 2015, 9, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, F.; Uskudar, O.; Simsek, Z.; Yuksel, I.; Basar, O.; Altinbas, A. Cefuroxime axetil-induced liver failure. Ann. Hepatol. 2010, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Kunze, W.; Streidl, J.P.; Klemm, T.; Lutze, J. Cefuroxime-induced hepatocellular-cholestatic hepatitis with pancytopenia. Open Access Lib. J. 2019, 6, e5036. [Google Scholar] [CrossRef]
- Castellazzi, M.L.; Agostoni, C.V.; Palella, J.; Civeriati, D.; Marchisio, P.; Nebbia, G. Ceftriaxone-induced cholestatic hepatitis in a child: A case report and a review of the literature. Front. Pediatr. 2022, 10, 1051887. [Google Scholar] [CrossRef]
- Thangaraju, P.; Varthya, S.B.; Guruthalingam, M.P.; Venkatesan, S. Cephalosporin’s induced hepatic enzyme derangement—An educational report. J. Family Med. Prim. Care 2020, 9, 2143–2145. [Google Scholar] [CrossRef]
- Mode, L.; Guzman-Cottrill, J.A.; Bassett, M. Cefepime-induced acute liver injury in a 5-year-old boy. Pediatr. Infect. Dis. J. 2024, 43, 78–80. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, C.; Jiang, M.; Chen, K.; Zhong, H.; Chen, Z.; Huang, L.; Li, H.; Zhang, L.; Choonara, I. Safety of ceftriaxone in paediatrics: A systematic review. Arch. Dis. Child. 2020, 105, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Oggiano, A.M.; Clemente, M.G.; Cuzzolin, L.; Locci, C.; Pirdda, C.M.; Schwaz, K.B.; Antonucci, R. Pharmacological treatment of ceftriaxone-related cholelithiasis in children: Is it worthwhile? J. Pediatr. Neonatal Individ. Med. 2019, 8, e080108. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Keeffe, E.B.; Fawaz, K.A. Prolonged cholestasis due to trimethoprim-sulfamethoxazole. Gastroenterology 1992, 102, 2148–2150. [Google Scholar] [CrossRef]
- Fontana, R.J.; Kleiner, D.E.; Chalasani, N.; Bonkovsky, H.; Gu, J.; Barnhart, H.; Li, Y.J.; Hoofnagle, J.H. The impact of patient age and corticosteroids in patients with sulfonamide hepatotoxicity. Am. J. Gastroenterol. 2023, 118, 1566–1575. [Google Scholar] [CrossRef]
- Burgos, R.M.; Reynolds, K.M.; Williams, K.; Li, W.; Yan, C. Trimethoprim-Sulfamethoxazole associated drug-induced liver injury in pediatrics: A systematic review. Pediatr. Infect. Dis. 2020, 39, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Talwalkar, J.; Treeprastetsuk, S.; Kamath, P.S.; Takahashi, N.; Sanderson, S.; Neuhauser, M.; Lindor, K. Drug-induced autoimmune hepatitis: Clinical characteristics and prognosis. Hepatology 2010, 51, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Nitrofurantoin. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548318/ (accessed on 15 March 2024).
- Chalasani, N.; Li, Y.J.; Dellinger, A.; Navarro, V.; Bonkovsky, H.; Fontana, R.J.; Gu, J.; Barnhart, H.; Phillips, E.; Lammert, C.; et al. Clinical features, outcomes and HLA risk factors associated with nitrofurantoin-induced liver injury. J. Hepatol. 2023, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Ferrari, A.; Hernandez, N.; Mendizbal, M.; Ridruejo, E.; Zerega, A.; Reggiardo, M.V.; Vorobioff, J.; Tanno, H.; Arrese, M.; et al. Nitrofurantoin-induced liver injury: Long term follow-up in two prospective DILI registries. Arch. Toxicol. 2023, 97, 593–602. [Google Scholar] [CrossRef]
- Karpman, E.; Kurzrock, E.A. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J. Urol. 2004, 172, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, S.; Akhondi, H. Minocycline. January 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554519/ (accessed on 11 March 2024).
- Garriso-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Clemens, V.; Regert, F.; Le Bret, N.; Heuser, I.; Hellman-Regen, J. Anti-inflammatory effects of minocycline are mediated by retinoid signaling. BMC Neurosci. 2018, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Grieco, J.C.; Ciarlone, S.L.; Gieron-Korthals, M.; Schoengerg, M.R.; Smith, A.G.; Philpot, R.M.; Heussler, H.S.; Banko, J.L.; Weeber, E.J. An open-label pilot trial of minocycline in children as a treatament for Angelman syndrome. BMC Neurol. 2014, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef] [PubMed]
- Hauk, L. Acne Vulgaris: Treatment Guidelines from the AAD. Am. Fam. Physician 2017, 95, 740–741. [Google Scholar] [PubMed]
- Ferrajolo, C.; Capuano, A.; Verhamme, K.M.; Schuemie, M.; Rossi, F.; Stricker, B.H.; Sturkenboom, M.C.J.M. Drug-induced hepatic injury in children: A case/non-case study of suspected adverse drug reactions in VigiBase. Br. J. Clin. Pharmacol. 2010, 70, 721–728. [Google Scholar] [CrossRef] [PubMed]
- DiPaola, F.; Molleston, J.P.; Gu, J.; Cirulli, E.T.; Chalasani, N.; Barnhart, H.; Kleiner, D.E.; Hoofnagle, J.H.; Fontana, R.J.; US Drug Induced Liver Injury Network. Antimicrobials and antiepileptics are the leading causes of idiosyncratic drug-induced liver injury in american children. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Harmon, E.G.; McConnie, R.; Kesavan, A. Minocycline-induced autoimmune hepatitis: A rare but important cause of drug-induced autoimmune hepatitis. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 347–350. [Google Scholar] [CrossRef]
- Woodhead, J.L.; Yang, K.; Oldach, D.; MacLauchlin, C.; Fernandes, P.; Watkins, P.B.; Siler, S.Q.; Howell, B.A. Analyzing the mechanisms behind macrolide antibiotic-induced liver injury using quantitative systems toxicology modeling. Pharm. Res. 2019, 36, 48. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Azithromycin. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548434/ (accessed on 22 April 2024).
- Martinez, M.A.; Vuppalanchi, R.; Fontana, R.J.; Stolz, A.; Kleiner, D.E.; Hayashi, P.H.; Gu, J.; Hoofnagle, J.H.; Chalasani, N. Clinical and histologic features of azithromycin-induced liver injury. Clin. Gastroenterol. Hepatol. 2015, 13, 369–376.e3. [Google Scholar] [CrossRef] [PubMed]
- Tchakounte Youngui, B.; Tchounga, B.K.; Graham, S.M.; Bonnet, M. Tuberculosis infection in children and adolescents. Pathogens 2022, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Consolidated Guidelines on Tuberculosis. Module 5: Management of Tuberculosis in Children and Adolescents; Vers. Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004676-4. [Google Scholar]
- Gafar, F.; Arifin, H.; Jurnalis, Y.D.; Yani, F.F.; Fitria, N.; Alffenaar, J.C.; Wilffert, B. Antituberculosis drug-induced liver injury in children: Incidence and risk factors during two-month intensive phase of therapy. Pediatr. Infect. Dis. J. 2019, 38, 50–53. [Google Scholar] [CrossRef]
- Mansukhani, S.; Shah, I. Hepatic dysfunction in children with tuberculosis on treatment with antituberculous therapy. Ann. Hepatol. 2012, 11, 96–99. [Google Scholar] [CrossRef]
- Shang, P.; Xia, Y.; Liu, F.; Wang, X.; Yuan, Y.; Hu, D.; Tu, D.; Chen, Y.; Deng, P.; Cheng, S.; et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ALTI) in China. PLoS ONE 2011, 6, e21836. [Google Scholar] [CrossRef] [PubMed]
- Hotchandani, H.; Moorani, K.N.; Kazi, Y. Anti-tuberculosis therapy induced hepatotoxicity in children. Pak. Pediatr. J. 2013, 37, 117–122. [Google Scholar]
- Chen, F.; Zhang, X.; Zhou, H.; Wang, M. Analysis of status and influencing factors associated with anti-tuberculosis drug-related liver injury in children. Chin. J. Antituberc. 2023, 45, 45–51. [Google Scholar] [CrossRef]
- D’Orazio, J.L. Isoniazid Toxicity. February 2019. Available online: https://emedicine.medscape.com/article/180554-overview#a3 (accessed on 18 May 2024).
- Liu, Y.; Li, H.; Huang, L.; Wan, C.; Wang, H.; Jiao, X.; Zeng, L.; Jia, Z.; Cheng, G.; Zhang, L.; et al. Liver injury in children: Signal analysis of suspected drugs based on the food and drug administration adverse event reporting system. BMC Pediatr. 2023, 23, 492. [Google Scholar] [CrossRef] [PubMed]
- Kuyucu, S.; Caubet, J.C. Hypersensitivity Reactions to Antiepileptic Drugs in Children: Epidemiologic, Pathogenetic, Clinical, and Diagnostic Aspects. J. Allergy Clin. Immunol. Pract. 2018, 6, 1879–1891.e1. [Google Scholar] [CrossRef] [PubMed]
- Muthaffar, O.Y.; Almahmudi, S.M.; Alrabghi, M.O.; Mahfouz, M.M.B.; Alfawaz, N.S. Valproic acid for children below 2 years of age with epilepsy. Neurosci. J. 2021, 26, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin Ahmed, S.; Siddiqi, Z.A. Antiepileptis drugs and liver disease. Seizure—Eur. J. Epilep 2006, 15, 156–164. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Phenytoin. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548889/ (accessed on 17 May 2024).
- LiverTox. Carbamazepine. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548097/ (accessed on 17 May 2024).
- Chalasani, N.; Bonkovsky, H.L.; Stine, J.G.; Gu, J.; Barnhart, H.; Jacobsen, E.; Björnsson, E.; Fontana, R.J.; Kleiner, D.E.; Hoofnagle, J.H.; et al. Clinical characteristics of antiepileptic-induced liver injury in patients from the DILIN prospective study. J. Hepatol. 2022, 76, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Raj, S.; Aradya, V.H.; Rangegowda, V.T.; Veeranna, G.P.; Singh, R.; Reddy, V.; Patil, M. Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: Pateint characteristics, causes and outcome in 36 cases. Hepatology 2016, 63, 993–999. [Google Scholar] [CrossRef]
- Amos, K.; Garcia-Bournissen, F.; Zhao, L.; Taheri, S. Carbamazepine-induced liver injury in an 11-year-old female: Case report and review of the literature. J. Paediatr. Child. Health 2023, 59, 165–168. [Google Scholar] [CrossRef]
- LiverTox. Phenobarbital. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548269/ (accessed on 17 May 2024).
- Star, K.; Edwards, I.R.; Choonara, I. Valproic acid and fatalities in children: A review of individual case safety reports in VigiBase. PLoS ONE 2014, 9, e108970. [Google Scholar] [CrossRef]
- LiverTox. Lamotrigine. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548562/ (accessed on 17 May 2024).
- Kamitaki, B.K.; Minacapelli, C.D.; Zhang, P.; Wachuku, C.; Gupta, K.; Catalano, C.; Rustgi, V. Drug-induced liver injury associated with antiseizure medications from the FDA adverse event reporting system (FAERS). Epilepsy Behav. 2021, 117, 107832. [Google Scholar] [CrossRef]
- Deng, J.; Fu, Z.R.; Wang, L.; Liu, J.; Chen, C.H.; Fang, F.; Wang, X.L. Acute liver failure associated with lamotrigine in children with epilepsy: A report of two cases and thoughts on pharmacogenomics. Epilepsy Behav. Rep. 2022, 20, 100568. [Google Scholar] [CrossRef] [PubMed]
- Couper, M.R.; Brown, R.M.; Nath, S.; Parida, A.; Kelgeri, C. Periportal necrosis and successful liver transplantation following lamotrigine drug-induced liver injury in a child. BMJ Case Rep. 2023, 16, e255787. [Google Scholar] [CrossRef]
- Arnon, R.; DeVivo, D.; Defelice, A.R.; Kazlow, P.G. Acute hepatic failure in a child treated with lamotrigine. Pediatr. Neurol. 1998, 18, 251–252. [Google Scholar] [CrossRef]
- Petrovic, S.; Kovacevic, M.; Kovacevic, S.V.; Miljkovic, B. Hepatotoxicity of newer antiseizure medications in children: An overview and disproportionality analysis of VigiBase. Expert. Opin. Drug Metab. Toxicol. 2024, 20, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J. Gastroenterol. 2010, 16, 5651–5661. [Google Scholar] [CrossRef] [PubMed]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Antiinflammatory Drugs (NSAIDs). May 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 24 April 2024).
- Traversa, G.; Bianchi, C.; Da Cas, R.; Abraha, I.; Menniti-Ippolito, F.; Venegoni, M. Cohort study of hepatotoxicity associated with simesulide and other non-steroidal anti-inflammatory drugs. BMJ 2003, 327, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C. Nimesulide Must Be Withdrawn Worldwide Due to Serious Liver Damage. 2008. Available online: https://www.ti.ubc.ca/2008/02/04/nimesulide-must-be-withdrawn-worldwide-due-serious-liver-damage/ (accessed on 28 April 2024).
- Meunier, L.; Larrey, D. Recent advancement in hepatotoxicity of non-steroidal anti-inflammatory drugs. Ann. Hepatol. 2018, 17, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Delungahawatta, T.; Pokharel, A.; Paz, R.; Haas, C.J. Topical Diclofenac induced hepatotoxicity. J. Community Hosp. Intern. Med. Perspect. 2023, 13, 21. [Google Scholar] [CrossRef]
- LiverTox. Ibuprofen. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547845/ (accessed on 20 April 2024).
- Zoubek, M.E.; Lucena, M.I.; Andrade, R.J.; Stephens, C. Systematic review: Ibuprofen-induced liver injury. Aliment. Pharmacol. Ther. 2020, 51, 603–611. [Google Scholar] [CrossRef]
- Gui, M.Z.; Ni, M.; Yin, X.D.; Zhang, T.; Li, Z.L. Ibuprofen induced Stevens-Johnson syndrome and liver injury in children: A case report. Transl. Pediatr. 2021, 10, 1737–1742. [Google Scholar] [CrossRef]
- Basturk, A.; Artan, R.; Yılmaz, A.; Gelen, M.T.; Duman, O. Acute vanishing bile duct syndrome after the use of ibuprofen. Arab. J. Gastroenterol. 2016, 17, 137–139. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yang, H.K.; Kim, S.H.; Park, J.H. Ibuprofen associated acute vanishing bile duct syndrome and toxic epidermal necrolysis in an infant. Yonsei Med. J. 2014, 55, 834–883. [Google Scholar] [CrossRef] [PubMed]
- Taghian, M.; Tran, T.A.; Bresson-Hadni, S.; Menget, A.; Felix, S.; Jacquemin, E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J. Pediatr. 2004, 145, 273–276. [Google Scholar] [CrossRef]
- Srivastava, M.; Perez-Atayde, A.; Jonas, M.M. Drug-associated acute-onset vanishing bile duct and Stevens-Johnson syndrome in a child: A case report. Gastroenterology 1998, 115, 743–746. [Google Scholar] [CrossRef]
- Barnett, A.K.; Boyer, E.W. Salicylate (Aspirin) Poisoning: Clinical Manifestations and Evaluation. November 2023. Available online: https://www.uptodate.com/contents/salicylate-aspirin-poisoning-clinical-manifestations-and-evaluation (accessed on 20 April 2024).
- Degnan, L.A. Reye’s Syndrome: A Rare but Serious Pediatric Condition. US Pharm. 2012, 37, HS6–HS8. [Google Scholar]
- Chapman, K.; Arnold, J.K. Reye Syndrome. January 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526101/ (accessed on 24 April 2024).
- Glasgow, J.F.; Middleton, B. Reye syndrome-insights on causation and prognosis. Arch. Dis. Child. 2001, 85, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Su, E.J.; Shieh, J.H.; Hsu, C.C.; Chen, K.T. Reye’s Syndrome Arising from the Treatment of Kawasaki Disease. HK J. Paediatr. 2018, 23, 185–187. [Google Scholar]
- Lee, J.; Kang, J.; Choi, E.; Choi, J. A case of Reye syndrome following treatment of Kawasaki disease with aspirin. Korean J. Pediatr. Infect. Dis. 2012, 19, 79–83. [Google Scholar] [CrossRef]
- Wei, C.M.; Chen, H.L.; Lee, P.I.; Chen, C.M.; Ma, C.Y.; Hwu, W.L. Reye’s syndrome developing in an infant on treatment of Kawasaki syndrome. J. Paediatr. Child. Health 2005, 41, 303–304. [Google Scholar] [CrossRef]
- Banday, A.Z.; Arul, A.; Vignesh, P.; Singh, M.P.; Goyal, K.; Singh, S. Kawasaki disease and influenza—New lessons from old associations. Clin. Rheumatol. 2021, 40, 2991–2999. [Google Scholar] [CrossRef]
- Marchesi, A.; Rigante, D.; Cimaz, R.; Ravelli, A.; de Jacobis, I.T.; Rimini, A.; Cardinale, F.; Cattalini, M.; De Zorzi, A.; Dellepiane, R.M.; et al. Revised recommendations of the Italian Society of Pediatrics about the general management of Kawasaki disease. Ital. J. Pediatr. 2021, 47, 16. [Google Scholar] [CrossRef] [PubMed]
- Belay, E.D.; Bressee, J.S.; Holman, R.C.; Khan, A.S.; Shahriari, A.; Schonbergerm, L.B. Reye’s syndrome in the United States from 1981 through 1997. N. Engl. J. Med. 1999, 340, 1377–1382. [Google Scholar] [CrossRef]
- Kanwar, V.S. Pediatric Acute Lymphoblastic Leukemia. July 2022. Available online: https://emedicine.medscape.com/article/990113 (accessed on 22 June 2024).
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric acute lymphoblastic leukemia. Version 2.2020. NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef]
- Mudd, T.W.; Guddati, A.K. Management of hepatotoxicity of chemotherapy and targeted agents. Am. J. Cancer Res. 2021, 11, 3461–3474. [Google Scholar]
- Horvath, A.; Papp, Z.E. Chemotherapy induced liver toxicity in children with malignant diseases. Bull. Med. Sci. 2019, 91, 37–41. [Google Scholar] [CrossRef]
- Urrutia-Maldonado, E.; Abril-Molina, A.; Ales-Palmer, M.; Luque, J.M.G.; de Rueda, P.M.; Ocete-Hita, E. Lesion hepatica inducida pot quimioterapia en ninos [Chemotherapy-induced liver injury in children]. An. Pediatr. 2019, 91, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.L.; Sang, G.Y.; Zou, X.Q.; Cheng, D.H. Drug-induced liver injury during consolidation therapy in childhood acute lymphoblastic leukemia as assessed for causality usinf the updated RUCAM. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5914593. [Google Scholar] [CrossRef]
- Lai, R.; Li, X.; Zhang, J.; Chen, J.; Yang, C.; Xie, W.; Yu, Y.; Guo, X.; Zhang, X.; Lu, G.; et al. Drug induced liver injury in children: A nationwide cohort study from China. JHEP Rep. 2024, 6, 101102. [Google Scholar] [CrossRef] [PubMed]
- Kelgeri, C.; Ramakrishna, S.H.; Brown, R.M.; Al-Abadi, E.; Gupte, G.L. Liver injury in children with long-term low-dose methotrexate. Acta Paediatr. 2020, 109, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.; Davidson, D.L.; O’Brien, R.T.; Pearson, H.A. Methotrexate hepatotoxicity in children with leukemia. J. Pediatr. 1977, 90, 1019–1021. [Google Scholar] [CrossRef]
- Hutter, R.V.; Shipkey, F.H.; Tan, C.T.; Murphy, M.L.; Chowdhury, M. Hepatic fibrosis in children with acute leukemia: A complication of therapy. Cancer 1960, 13, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hammerli, C.; Suter-Dick, L. Methotrexate-induced liver injury is associated with oxidative stress, impaired mitochondrial respiration and endoplasmic reticulum stress in vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef]
- Zhou, Y.; He, H.; Ding, L.; Wang, T.; Liu, X.; Zhang, M.; Zhang, A.; Fu, J. Effects of gene polymorphism on delayed MTX clearance, toxicity and metabolomic changes after HD-MTX treatment in children with acute lymphoblastic leukemia. Eur. J. Pediatr. 2024, 183, 581–590. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Mercaptopurine. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548546/ (accessed on 25 June 2024).
- Sacerdotianu, V.M.; Streba, C.T.; Rogoveanu, I.; Streba, L.; Vere, C.C. Oncological Therapy-Associated Liver Injuries. 2022. Available online: https://www.intechopen.com/chapters/83031 (accessed on 20 May 2024).
- Ramadori, G.; Cameron, S. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S. Hepatic Sinusoidal Obstruction Syndrome (Veno-Occlusive Disease) in Children. January 2024. Available online: https://www.uptodate.com/contents/hepatic-sinusoidal-obstruction-syndrome-veno-occlusive-disease-in-children (accessed on 20 May 2024).
- Rakhshan, A.; Kamel, B.R.; Saffaei, A.; Tavakoli-Ardakani, M. Hepatotoxicity induced by azole antifungal agents: A review study. Iran. J. Pharm. Res. 2023, 22, e130336. [Google Scholar] [CrossRef]
- LiverTox. Fluconazole. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548300/ (accessed on 20 May 2024).
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, I.M.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Yin, X.D.; Zhang, Y.; Shao, Q.H.; Mao, X.Y.; Hu, W.J.; Shen, Y.L.; Zhao, B.; Li, Z.L. Antifungal drugs and drug-induced liver injury: A real-world study leveraging the FDA Adverse Event Reporting System database. Front. Pharmacol. 2022, 13, 891336. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Poluzzi, E.; Koci, A.; Caraceni, P.; Ponti, F.D. Assessing liver injury associated with antimycotics: Concise literature review and clues from data mining of the FAERS database. World J. Hepatol. 2014, 6, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Ertem, O.; Gumustekin, M. Voriconazole-induced hepatotoxicity concise up-to-date review. J. Basic. Clin. Health Sci. 2022, 6, 325–334. [Google Scholar]
- Gadour, E.; Kotb, A. Systematic review of antifungal-induced acute liver failure. Cureus 2021, 13, e18940. [Google Scholar] [CrossRef] [PubMed]
- Doss, S.; Potschka, H.; Doss, F.; Mitzner, S.; Sauer, M. Hepatotoxicity of antimycotics used for invasive fungal infections: In vitro results. Biomed. Res. Int. 2017, 2017, 9658018. [Google Scholar] [CrossRef] [PubMed]
- Dongmo Fotsing, L.N.; Bajaj, T. Caspofungin. February 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545140/ (accessed on 25 May 2024).
- LiverTox. Echinocandins. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548299/ (accessed on 25 May 2024).
- Saner, F.; Gensicke, J.; Rath, P.; Fruhauf, N.; Gu, Y.; Paul, A.; Radtke, A.; Malagó, M.; Broelsch, C. Safety profile of concomitant use of caspofungin and cyclosporine or tacrolimus in liver transplant patients. Infection 2006, 34, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Rodriguez, C.; Lopez-Duarte, M.; Jurado, M.; Lopez, J.; Arranz, R.; Cisneros, J.M.; Martino, M.L.; Garcia-Sanchez, P.J.; Morales, P.; Olivé, T.; et al. Safety of the concomitant use of caspofungin and cyclosporin A in patients with invasive fungal infections. Bone Marrow Transplant. 2004, 34, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.; Sung, L.; Allen, U.; Naqvi, A.; Drynan-Arsenault, J.; Dekker, A.; Maloney, A.M.; Dupuis, L.L. Efficacy and safety of caspofungin for the empiric management of fever in neutropenic children. Pediatr. Infect. Dis. J. 2007, 26, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, F.M.; Fleckenstein, J.M. Antiparasitic agents. In Infectious Diseases; Powderly, D.J., Opal, W.G., Cohen, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Laman, M.; Tavul, L.; Karl, S.; Kotty, B.; Kerry, Z.; Kumai, S.; Samuel, A.; Lorry, L.; Timinao, L.; Cade Howard, S.; et al. Mass drug administration of ivermectin, diethylcarbamazepine, plus albendazole for reduction of lymphatic filariasis endemicity in Papua New Guinea: A cluster-randomised trial. Lancet Infect. Dis. 2022, 22, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Dua, A. Albendazole. 20 September 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553082/ (accessed on 10 January 2024).
- Palomares, F.; Palencia, G.; Ambrosio, J.R.; Ortiz, A.; Jung-Cook, H. Evaluation of the efficacy of albendazole sulphoxide and praziquantel in combination on Taenia crassiceps cysts: In vitro studies. J. Antimicrob. Chemother. 2006, 57, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Donhauser, Z.J.; Jobs, W.B.; Binka, E.C. Mechanics of microtubules: Effects of protofilament orientation. Biophys. J. 2010, 99, 1668–1675. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Vinaud, M.C.; Ferreira, C.S.; Lino Junior, R.d.S.; Bezerra, J.C. Taenia crassiceps: Energetic and respiratory metabolism from cysticerci exposed to praziquantel and albendazole in vitro. Exp. Parasitol. 2008, 120, 221–226. [Google Scholar] [CrossRef]
- LiverTox. Albendazole. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548360/ (accessed on 10 January 2024).
- Grama, A.; Aldea, C.; Burac, L.; Delean, D.; Boghitoiu, D.; Bulata, B.; Nitescu, V.; Ulmeanu, C.; Pop, T.L. Acute liver failure secondary to toxic exposure in children. Arch. Med. Sci. 2019, 18, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Dijmarescu, I.; Guta, O.M.; Brezeanu, L.E.; Dijmarescu, A.D.; Becheanu, C.M.; Pacurar, D. Drug-induced hepatitis in children: The experience of a single center in Romania. Children 2022, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Sarkar, S. Albendazole-induced recurrent hepatitis. Indian Pediatr. 2013, 50, 1064. [Google Scholar] [PubMed]
- Shah, C.; Mahapatra, A.; Shukla, A.; Bhatia, S. Recurrent acute hepatitis caused by albendazole. Trop. Gastroenterol. 2013, 34, 38–39. [Google Scholar] [CrossRef]
- Dragutinovic, N.; Barac, A.; Stevanovic, G.; Dordic, I.; Paglietti, B.; Micic, J.; Aleksić, E.; Stojnić, J.; Martinov Nestorov, J. Acute hepatitis in a paediatric patient: Immune-mediated drug-induced liver injury or albendazole-induced autoimmune hepatitis? J. Infect. Dev. Ctries. 2022, 16, 1660–1663. [Google Scholar] [CrossRef]
- Ayano, G.; Demelash, S.; Gizacheq, Y.; Tsegay, L.; Alati, R. The global prevalence of attention deficit hyperactivity disorder in children and adolescents: An umbrella review of meta-analyses. J. Affect. Disord. 2023, 339, 860–866. [Google Scholar] [CrossRef]
- Cattoi, B.; Alpern, I.; Katz, J.S.; Keepnews, D.; Solanto, M.V. The adverse health outcomes, economic burden and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): A call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J. Atten. Disord. 2022, 26, 807–808. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F., Jr.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical practice guideline for the diagnosis, evaluation and treatment of attention deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019, 144, e201922528. [Google Scholar]
- LiverTox. Atomoxetine. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548671/ (accessed on 25 January 2024).
- Felt, B.T.; Biermann, B.; Christmer, J.G.; Kochhar, P.; Harrison, R.V. Diagnosis and management of ADHD in children. Am. Fam. Physician 2014, 90, 456–464. [Google Scholar] [PubMed]
- Stojanovski, S.D.; Casavant, M.J.; Mousa, H.M.; Baker, P.; Nahata, M.C. Atomoxetine-induced hepatitis in a child. Clin. Toxicol. 2007, 45, 51–55. [Google Scholar] [CrossRef]
- Bangs, M.E.; Jin, L.; Zhang, S.; Desaiah, D.; Allen, A.J.; Read, H.A.; Regev, A.; Wernicke, J.F. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008, 31, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Ozcay, F.; Piskin, E.; Karaman, M.G.; Bilezikci, B.; Calik, M.; Tekin, I.; Haberal, M. Idiosyncratic liver failure probably associated with atomoxetine: A case report. J. Child. Adolesc. Pshychopharmacol. 2011, 21, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Potnis, D.; Wackernah, R.S. Drug-induced liver injury in children: Atomoxetine and nonstimulants for ADHD. Am. J. Pharm. Benefits 2015, 7, e15–e20. [Google Scholar]
- Ahmed, A.; Clarke, J.O. Proton Pump Inhibitors (PPI). May 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557385/ (accessed on 28 May 2024).
- Dipasquale, V.; Cicala, G.; Spina, E.; Romano, C. A narrative review on efficacy and safety of proton pump inhibitors in children. Front. Pharmacol. 2022, 13, 839972. [Google Scholar] [CrossRef]
- Kataria, A.; Stolow, E.; Hubbard, H. Pantoprazole-induced acute hepatocellular and cholestatic hepatitis. Postgrad. Med. J. 2022, 98, 11–12. [Google Scholar] [CrossRef]
- Seife Hassen, S.; Ata, F.; Bilal, A.B.I.; Ali, M.S.; Petkar, M.; Awad Elzouki, A.Y.; Zahid, M. Immune-mediated drug-induced liver injury secondary to Omeprazole: A case report. Clin. Case Rep. 2020, 8, 3421–3426. [Google Scholar] [CrossRef]
- Alhankawi, D.; Sharma, S.; Sun, K.; Theise, N.; Park, J. Proton pump inhibitor-induced liver injury. Am. J. Gastroenterol. 2018, 113, 1349–1350. [Google Scholar] [CrossRef]
- El-Matary, W.; Dalzell, M. Omeprazole-induced hepatitis. Pediatr. Emerg. Care 2005, 21, 529–530. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, X.; Song, Z.; Xie, Y.; Zhang, X.; Du, Z.; Wei, R.; Fan, D.; Liu, Y.; Zhao, O.; et al. Signal detection of potentially drug-induced liver injury in children using electronic health records. Front. Pediatr. 2020, 8, 171. [Google Scholar] [CrossRef] [PubMed]

| Liver Injury | Mechanism | Drugs |
|---|---|---|
| Acute fatty liver | Acute mitochondrial injury Fatty acid beta-oxidation inhibition | VPA |
| Acute hepatic necrosis | Reactive metabolite +/− immune activation | Isoniazid, aspirin |
| Autoimmune-like hepatitis | Anti-drug antibodies Autoantibody production | Nitrofurantoin, minocycline, atomoxetine |
| Cholestatic hepatitis | Immune-mediated injury | Phenytoin, amoxicillin–clavulanate, ibuprofen |
| Fibrosis | Stellate cell activation/chronic endothelial cell injury | Methotrexate |
| Immune allergic hepatitis | Drug hypersensitivity | Trimethoprim–sulfamethoxazole, carbamazepine, phenytoin |
| Vanishing bile duct syndrome (VBDS) | Immune-mediated cholangiocyte injury | Amoxicillin–clavulanate, ibuprofen, sulfonamides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maris, B.R.; Grama, A.; Pop, T.L. Drug-Induced Liver Injury—Pharmacological Spectrum Among Children. Int. J. Mol. Sci. 2025, 26, 2006. https://doi.org/10.3390/ijms26052006
Maris BR, Grama A, Pop TL. Drug-Induced Liver Injury—Pharmacological Spectrum Among Children. International Journal of Molecular Sciences. 2025; 26(5):2006. https://doi.org/10.3390/ijms26052006
Chicago/Turabian StyleMaris, Bianca Raluca, Alina Grama, and Tudor Lucian Pop. 2025. "Drug-Induced Liver Injury—Pharmacological Spectrum Among Children" International Journal of Molecular Sciences 26, no. 5: 2006. https://doi.org/10.3390/ijms26052006
APA StyleMaris, B. R., Grama, A., & Pop, T. L. (2025). Drug-Induced Liver Injury—Pharmacological Spectrum Among Children. International Journal of Molecular Sciences, 26(5), 2006. https://doi.org/10.3390/ijms26052006







