Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature)
Abstract
1. Introduction
2. History of Lung Cancer
3. Epidemiology of Lung Cancer
4. Pathogenesis of Lung Cancer
- -
- Abnormalities in the regulation of the cell cycle;
- -
- Mutations in proto-oncogenes and tumor suppressor genes;
- -
- Disorders of the DNA repair process;
- -
- Increased expression of growth factors and angiogenesis;
- -
- Avoidance of apoptosis (mutations of anti- and pro-apoptotic genes);
- -
- Increased telomerase activity;
- -
- Tissue invasion and metastasis.
5. Clinical Description of Lung Cancer
6. Diagnosis and Staging of Lung Cancer
- —
- Staging assessment of non-small cell and small cell lung cancer should be carried out using the principles and criteria of the current TNM classification (IV, A).
- —
- In the presence of two lesions suspected of primary cancer, a separate staging assessment should be carried out (III, A).
- —
- In patients with lung cancer with features of mediastinal lymph node involvement, pathomorphological confirmation of the nature of the suspicious lesions should be obtained during imaging studies when qualifying for possible resection of the lung parenchyma (IV, B).
- —
- In patients prior to planned radical treatment, pathomorphological confirmation of the possible presence of neoplasm in single suspicious lesions located in other organs detected by imaging tests is recommended if possible (IV, A).
- —
- In patients with lung cancer who underwent excision of the lung parenchyma and lymph nodes, the final stage is determined on the basis of pathomorphological examination of the surgical material (IV, A).
7. Pathomorphology and Molecular Diagnostics of Lung Cancer
- —
- Rules for handling small specimens and cytological material (especially in advanced forms of NSCLC);
- —
- A new division of adenocarcinomas and squamous cell carcinomas;
- —
- Rhe need to use IHC and genetic tests in pathomorphological diagnostics in order to individualize treatment;
- —
- Diagnosis of large cell carcinoma and other—rare—NSCLCs only in postoperative material;
- —
- Classification in one group of cancers with features of neuroendocrine activity. The classification also presents new rules for determining the degree of differentiation of adenocarcinomas of the lung (grading), and in the group of neuroendocrine tumors, carcinoids are classified as neuroendocrine tumors, while small cell and large cell neuroendocrine carcinomas are classified as neuroendocrine carcinomas.
Pathomorphological and Molecular Evaluation
- —
- Histological assessment of the specimen collected during bronchofiberoscopy;
- —
- Cytological assessment of the smear or bronchial lavage;
- —
- Histological or cytological evaluation of biopsy material through the chest, bronchial or esophageal wall.
- —
- Pap smear examination of pleural effusion and/or needle biopsy of the pleura;
- —
- Needle or surgical biopsy of peripheral lymph nodes;
- —
- Needle biopsy of the metastatic focus;
- —
- Mediastinoskopia;
- —
- Mediastinotomia;
- —
- Thoracoscopy,
- —
- Thoracotomy (after exhaustion of all other options);
- —
- Sputum cytology (a low-sensitivity test, used only when microscopic material cannot be obtained by another method) [66].
8. Risk Factors
9. Smoking
10. Treatment of Patients with Non-Small Cell Lung Cancer
10.1. Surgical Treatment of Patients with Non-Small Cell Lung Cancer
10.2. Treatment of Patients with Early (I–II) and Locally Advanced (III) Non-Small Cell Lung Cancer
10.3. Treatment of Patients with Advanced Stage (IV) Non-Small Cell Lung Cancer
11. Limitations
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vicidomini, G. Current Challenges and Future Advances in Lung Cancer: Genetics, Instrumental Diagnosis and Treatment. Cancers 2023, 15, 3710. [Google Scholar] [CrossRef]
- Rina, A.; Maffeo, D.; Minnai, F.; Esposito, M.; Palmieri, M.; Serio, V.B.; Rosati, D.; Mari, F.; Frullanti, E.; Colombo, F. The Genetic Analysis and Clinical Therapy in Lung Cancer: Current Advances and Future Directions. Cancers 2024, 16, 2882. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, R.; Sabalic, A.; Spaggiari, L. The Early Diagnosis of Lung Cancer: Critical Gaps in the Discovery of Biomarkers. J. Clin. Med. 2023, 12, 7244. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Jiang, Y.; Fu, W.; Liu, X.; Zhong, R.; Cheng, B.; Zhu, F.; Xiang, Y.; He, J.; et al. Advances in lung cancer screening and early detection. Cancer Biol. Med. 2022, 19, 591–608. [Google Scholar] [CrossRef]
- Dama, E.; Colangelo, T.; Fina, E.; Cremonesi, M.; Kallikourdis, M.; Veronesi, G.; Bianchi, F. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers 2021, 13, 3919. [Google Scholar] [CrossRef] [PubMed]
- Chudgar, N.P.; Bucciarelli, P.R.; Jeffries, E.M.; Rizk, N.P.; Park, B.J.; Adusumilli, P.S.; Jones, D.R. Results of the national lung cancer screening trial: Where are we now? Thorac. Surg. Clin. 2015, 25, 145–153. [Google Scholar] [CrossRef]
- Cui, J.W.; Li, W.; Han, F.J.; Liu, Y.D. Screening for lung cancer using low-dose computed tomography: Concerns about the application in low-risk individuals. Transl. Lung Cancer Res. 2015, 4, 275–286. [Google Scholar]
- Pinsky, P.F.; Church, T.R.; Izmirlian, G.; Kramer, B.S. The National Lung Screening Trial: Results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013, 119, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [PubMed]
- Zhao, Y.; Xie, Y.; Jia, D.; Ma, C.; Wei, D.; Zhang, X. Application of gene polymorphisms to predict the sensitivity of patients with locally advanced non-small cell lung cancer undergoing chemoradiotherapy. Am. J. Transl. Res. 2021, 13, 7382–7387. [Google Scholar]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Greenberg, M.; Selikoff, I.J. Lung cancer in the Schneeberg mines: A reappraisal of the data reported by Harting and Hesse in 1879. Ann. Occup. Hyg. 1993, 37, 5–14. [Google Scholar] [PubMed]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Warren, G.W.; Cummings, K.M. Tobacco and lung cancer: Risks, trends, and outcomes in patients with cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 359–364. [Google Scholar] [CrossRef]
- Proctor, R.N. The history of the discovery of the cigarette-lung cancer link: Evidentiary traditions, corporate denial, global toll. Tob. Control 2012, 21, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Debakey, M. Carcinoma of the lung and tobacco smoking: A historical perspective. Ochsner J. 1999, 1, 106–108. [Google Scholar]
- Tindle, H.A.; Stevenson Duncan, M.; Greevy, R.A.; Vasan, R.S.; Kundu, S.; Massion, P.P.; Freiberg, M.S. Lifetime Smoking History and Risk of LungCancer: Results from the Framingham Heart Study. J. Natl. Cancer Inst. 2018, 110, 1201–1207. [Google Scholar]
- Remen, T.; Pintos, J.; Abrahamowicz, M.; Siemiatycki, J. Risk of lung cancer in relation to various metrics of smoking history: A case-control study in Montreal. BMC Cancer 2018, 18, 1275. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Darby, S.; Deo, H.; Silcocks, P.; Whitley, E.; Doll, R. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. BMJ 2000, 321, 323–329. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cooper, W.A.; Lam, D.C.; O’Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S479–S490. [Google Scholar] [PubMed]
- Roh, M.S. Molecular pathology of lung cancer: Current status and future directions. Tuberc. Respir. Dis. 2014, 77, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Boolell, V.; Alamgeer, M.; Watkins, D.N.; Ganju, V. The evolution of therapies in non-small cell lung cancer. Cancers 2015, 7, 1815–1846. [Google Scholar] [CrossRef]
- Massion, P.P.; Carbone, D.P. The molecular basis of lung cancer: Molecular abnormalities and therapeutic implications. Respir. Res. 2003, 4, 12. [Google Scholar] [CrossRef]
- Dakal, T.C.; Dhabhai, B.; Pant, A.; Moar, K.; Chaudhary, K.; Yadav, V.; Ranga, V.; Sharma, N.K.; Kumar, A.; Maurya, P.K.; et al. Oncogenes and tumor suppressor genes: Functions and roles in cancers. MedComm 2024, 5, e582. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef]
- Chen, T.; Ashwood, L.M.; Kondrashova, O.; Strasser, A.; Kelly, G.; Sutherland, K.D. Breathing New Insights into the Role of Mutant P53 in Lung Cancer. Oncogene 2025, 44, 115–129. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sakai, K.; Kenmotsu, H.; Yoh, K.; Daga, H.; Ohira, T.; Ueno, T.; Aoki, T.; Hayashi, H.; Yamazaki, K.; et al. Predictive Value of EGFR Mutation in Non–Small-cell Lung Cancer Patients Treated with Platinum Doublet Postoperative Chemotherapy. Cancer Sci. 2022, 113, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cui, X.; Sidorenkov, G.; Groen, H.J.M.; Vliegenthart, R.; Heuvelmans, M.A.; Liu, S.; Oudkerk, M.; de Bock, G.H. Lung cancer occurrence attributable to passive smoking among never smokers in China: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2020, 9, 204–217. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S31–S49. [Google Scholar] [CrossRef]
- Sarkar, M.; Madabhavi, I.; Niranjan, N.; Dogra, M. Auscultation of the respiratory system. Ann. Thorac. Med. 2015, 10, 158–168. [Google Scholar] [CrossRef]
- Available online: https://www.medicover.pl/en/cancers/lung/ (accessed on 1 January 2015).
- Lazaros, G.; Imazio, M.; Tsioufis, P.; Lazarou, E.; Vlachopoulos, C.; Tsioufis, C. Chronic Pericardial Effusion: Causes and Management. Can. J. Cardiol. 2023, 39, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.C.; Reddy, Y.N.V. Pericardial effusions: Perspective of the acute cardiac care physician. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Klein-Weigel, P.F.; Elitok, S.; Ruttloff, A.; Reinhold, S.; Nielitz, J.; Steindl, J.; Hillner, B.; Rehmenklau-Bremer, L.; Wrase, C.; Fuchs, H.; et al. Superior venacava syndrome. Vasa 2020, 49, 437–448. [Google Scholar] [CrossRef]
- Wright, K.; Digby, G.C.; Gyawali, B.; Jad, R.; Menard, A.; Moraes, F.Y.; Wijeratne, D.T. Malignant Superior Vena Cava Syndrome: A Scoping Review. J. Thorac. Oncol. 2023, 18, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours (WYD 8); John Wiley and Sons Inc.: Oksford, UK, 2016. [Google Scholar]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Panunzio, A.; Sartori, P. Lung Cancer and Radiological Imaging. Curr. Radiopharm. 2020, 13, 238–242. [Google Scholar] [CrossRef]
- Tárnoki, Á.D.; Tárnoki, D.L.; Dąbrowska, M.; Knetki-Wróblewska, M.; Frille, A.; Stubbs, H.; Blyth, K.G.; Juul, A.D. New developments in the imaging of lung cancer. Breathe 2024, 20, 230176. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Chen, Z.; Fan, Y.; Zhou, Y.; Yuan, Z.; Zhang, W. Current treatments for non-small cell lung cancer. Front. Oncol. 2022, 12, 945102. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.; Strange, C.D.; Agrawal, R.; Erasmus, L.T.; Truong, M.T. Approach to Imaging of Mediastinal Masses. Diagnostics 2023, 13, 3171. [Google Scholar] [CrossRef]
- Khan, T.; Usman, Y.; Abdo, T.; Chaudry, F.; Keddissi, J.I.; Youness, H.A. Diagnosis and management of peripheral lung nodule. Ann. Transl. Med. 2019, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Wu, K.C.; Tseng, N.C.; Chen, Y.J.; Chang, C.J.; Yen, K.Y.; Kao, C.H. Differentiation Between Malignant and Benign Pulmonary Nodules by Using Automated Three-Dimensional High-Resolution Representation Learning with Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography. Front. Med. 2022, 9, 773041. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Pollard, J.H.; Saad Eddin, A.; Alatoum, A.; Kandemirli, S.; Gholamrezanezhad, A.; Menda, Y.; Graham, M.M.; Shariftabrizi, A. PET/CT Imaging in Treatment Planning and Surveillance of Sinonasal Neoplasms. Cancers 2023, 15, 3759. [Google Scholar] [CrossRef] [PubMed]
- AlRasheedi, M.; Han, S.; Thygesen, H.; Neilson, M.; Hendry, F.; Alkarn, A.; Maclay, J.D.; Leung, H.Y. A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer. Diagnostics 2023, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Chrabańska, M.; Środa, M.; Kiczmer, P.; Drozdzowska, B. Lung Cancer Cytology: Can Any of the Cytological Methods Replace Histopathology? J. Cytol. 2020, 37, 117. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Kujawa, K.; Yasufuku, K. The Role of Endobronchial Ultrasound versus Mediastinoscopy for Non-Small Cell Lung Cancer. J. Thorac. Dis. 2017, 9, S83–S97. [Google Scholar] [CrossRef]
- Bugalho, A.; de Santis, M.; Szlubowski, A.; Rozman, A.; Eberhardt, R. Trans-Esophageal Endobronchial Ultrasound-Guided Needle Aspiration (EUS-B-NA): A Road Map for the Chest Physician. Pulmonology 2018, 24, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Shikano, K.; Ishiwata, T.; Saegusa, F.; Terada, J.; Sakayori, M.; Abe, M.; Kawasaki, T.; Ikari, J.; Kawata, N.; Tada, Y.; et al. Feasibility and Accuracy of Rapid On-Site Evaluation of Touch Imprint Cytology during Transbronchial Biopsy. J. Thorac. Dis. 2020, 12, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Mayer, N.; Kestenholz, P.; Minervini, F. Surgical Access to the Mediastinum—All Roads Lead to Rome: A Literature Review. Mediastinum 2024, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Ohshimo, S.; Guzman, J.; Costabel, U.; Bonella, F. Differential Diagnosis of Granulomatous Lung Disease: Clues and Pitfalls. Eur. Respir. Rev. 2017, 26, 170012. [Google Scholar] [CrossRef]
- Jackman, D.; Johnson, B. Small-cell lung cancer. Lancet 2005, 366, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Tumours Editorial Board. In Thoracic Tumours, 5th ed.; WHO: Geneva, Switzerland, 2021; Volume 5. [Google Scholar]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [PubMed]
- Kelemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Preobrazhenskaya, E.V.; Orlov, S.V. Current Status of Molecular Diagnostics for Lung Cancer. Explor. Target. Anti-Tumor Ther. 2024, 5, 742–765. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Singhal, S.; Kulkarni, P.; Horne, D.; Malhotra, J.; Salgia, R.; Singhal, S.S. Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. J. Clin. Med. 2024, 13, 4189. [Google Scholar] [CrossRef] [PubMed]
- Brindel, A.; Althakfi, W.; Barritault, M.; Watkin, E.; Maury, J.-M.; Bringuier, P.-P.; Girard, N.; Brevet, M. Uncommon EGFR Mutations in Lung Adenocarcinoma: Features and Response to Tyrosine Kinase Inhibitors. J. Thorac. Dis. 2020, 12, 4643–4650. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.A.; Kamal, I.H.; Osman, A.; Youssef, M.; Ibrahim, A.K. The Prevalence and Clinical Significance of EGFR Mutations in Non-Small Cell Lung Cancer Patients in Egypt: A Screening Study. J. Egypt Natl. Canc. Inst. 2024, 36, 39. [Google Scholar] [CrossRef] [PubMed]
- Zia, V.; Lengyel, C.G.; Tajima, C.C.; de Mello, R.A. Advancements of ALK Inhibition of Non-Small Cell Lung Cancer: A Literature Review. Transl. Lung Cancer Res. 2023, 12, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Fasano, M.; Martinelli, E.; Troiani, T.; Ciardiello, F.; Morgillo, F. Role and Targeting of Anaplastic Lymphoma Kinase in Cancer. Mol. Cancer 2018, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Ritterhouse, L.L. The Role of Plasma Genotyping in ALK- and ROS1-Rearranged Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 2557–2570. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef]
- Chmielewska, I.; Krawczyk, P.; Wójcik-Superczyńska, M.; Grenda, A.; Gil, M.; Stencel, K.; Kieszko, R.; Jankowski, T.; Milanowski, J. Exploring Immunotherapy Efficacy in Non-Small Cell Lung Cancer Patients with BRAF Mutations: A Case Series and Literature Review. Transl. Lung Cancer Res. 2024, 13, 2491–2499. [Google Scholar] [CrossRef]
- Bi, H.; Ren, D.; Ding, X.; Yin, X.; Cui, S.; Guo, C.; Wang, H. Clinical Characteristics of Patients with ROS1 Gene Rearrangement in Non-Small Cell Lung Cancer: A Meta-Analysis. Transl. Cancer Res. 2020, 9, 4383–4392. [Google Scholar] [CrossRef]
- Xu, Y.; Chang, H.; Wu, L.; Zhang, X.; Zhang, L.; Zhang, J.; Li, Y.; Shen, L.; Zhu, X.; Zhou, X.; et al. High Prevalence of ROS1 Gene Rearrangement Detected by FISH in EGFR and ALK Negative Lung Adenocarcinoma. Exp. Mol. Pathol. 2020, 117, 104548. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Kubo, J.; Luo, J.; Desai, M.; Hedlin, H.; Henderson, M.; Chlebowski, R.; Tindle, H.; Chen, C.; Gomez, S.; et al. Active and Passive Smoking in Relation to Lung Cancer Incidence in the Women’s Health Initiative Observational Study Prospective Cohort. Ann. Oncol. 2015, 26, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Brey, C.; Gouveia, F.T.; Silva, B.S.; Sarquis, L.M.M.; Miranda, F.M.D.; Consonni, D. Lung Cancer Related to Occupational Exposure: An Integrative Review. Rev. Gaúcha Enferm. 2020, 41, e20190378. [Google Scholar] [CrossRef]
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.; Lam, S.; Lam, W.L.; Martinez, V.D. Arsenic, Asbestos and Radon: Emerging Players in Lung Tumorigenesis. Environ. Health 2012, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Clofent, D.; Culebras, M.; Loor, K.; Cruz, M.J. Contaminación Ambiental y Cáncer de Pulmón: El Poder Carcinogénico Del Aire Que Respiramos. Arch. Bronconeumol. 2021, 57, 317–318. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.; Kim, B.-G.; Kim, S.-H.; Sohn, J.W.; Yoon, H.J.; Jang, S.H.; Park, D.W. The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea. Cancers 2024, 16, 2063. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Chan, C.P.Y.; Yau, W.-P.; Seow, W.J. Association between Family History of Lung Cancer and Lung Cancer Risk: A Systematic Review and Meta-Analysis. Lung Cancer 2020, 148, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sorscher, S.; LoPiccolo, J.; Heald, B.; Chen, E.; Bristow, S.L.; Michalski, S.T.; Nielsen, S.M.; Lacoste, A.; Keyder, E.; Lee, H.; et al. Rate of Pathogenic Germline Variants in Patients with Lung Cancer. JCO Precis. Oncol. 2023, 7, e2300190. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Lewandowski, T.; Zych, B.; Papp, K.; Zrubcová, D.; Ejder Apay, S.; Nagorska, M. Risk Factors for the Diagnosis of Lung Cancer in Poland: A Large-Scale, Population-Based Case-Control Study. Asian Pac. J. Cancer Prev. 2022, 23, 3299–3307. [Google Scholar] [CrossRef]
- Thompson, A.; Cook, J.; Choquet, H.; Jorgenson, E.; Yin, J.; Kinnunen, T.; Barclay, J.; Morris, A.P.; Pirmohamed, M. Functional validity, role, and implications of heavy alcohol consumption genetic loci. Sci. Adv. 2020, 6, eaay5034. [Google Scholar] [CrossRef]
- Gilham, C.; Rake, C.; Burdett, G.; Nicholson, A.G.; Davison, L.; Franchini, A.; Carpenter, J.; Hodgson, J.; Darnton, A.; Peto, J. Pleural mesothelioma and lung cancer risks in relation to occupational history and asbestos lung burden. Occup. Environ. Med. 2016, 73, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Bosetti, C.; Boccia, S.; Boffetta, P.; La Vecchia, C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: An updated systematic review and a meta-analysis to 2014. Arch. Toxicol. 2014, 88, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Huang, W.; Chen, G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: Focus on China. Chin. J. Cancer 2014, 33, 189–196. [Google Scholar] [CrossRef]
- Mahjub, H.; Sadri, G.H. Meta-analysis of case-referent studies of specific environmental or occupational pollutants on lung cancer. Indian J. Cancer 2006, 43, 169–173. [Google Scholar] [CrossRef]
- Le, T.T.T.; Mendez, D.; Warner, K.E. The Benefits of Quitting Smoking at Different Ages. Am. J. Prev. Med. 2024, 67, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Echeverria, S.E. The Clustering Effects of Current Smoking Status, Overweight/Obesity, and Physical Inactivity with All-Cause and Cause-Specific Mortality Risks in U.S. Adults. Prev. Med. Reports 2024, 42, 102742. [Google Scholar] [CrossRef] [PubMed]
- Jha, P. The Hazards of Smoking and the Benefits of Cessation: A Critical Summation of the Epidemiological Evidence in High-Income Countries. eLife 2020, 9, 49979. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Mitchell, R.; Dorling, D. Time for a Smoke? One Cigarette Reduces Your Life by 11 Minutes. BMJ 2000, 320, 53. [Google Scholar] [CrossRef]
- CNN Health. Available online: https://edition.cnn.com/2025/01/01/health/cigarette-smoking-life-expectancy-study-wellness/index.html (accessed on 26 January 2025).
- Ozasa, K.; Katanoda, K.; Tamakoshi, A.; Sato, H.; Tajima, K.; Suzuki, T.; Tsugane, S.; Sobue, T. Reduced Life Expectancy Due to Smoking in Large-Scale Cohort Studies in Japan. J. Epidemiol. 2008, 18, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gallaway, M.S.; Henley, S.J.; Steele, C.B.; Momin, B.; Thomas, C.C.; Jamal, A.; Trivers, K.F.; Singh, S.D.; Stewart, S.L. Surveillance for Cancers Associated with Tobacco Use—United States, 2010–2014. MMWR. Surveill. Summ. 2018, 67, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Graves, B.M.; Johnson, T.J.; Nishida, R.T.; Dias, R.P.; Savareear, B.; Harynuk, J.J.; Kazemimanesh, M.; Olfert, J.S.; Boies, A.M. Comprehensive Characterization of Mainstream Marijuana and Tobacco Smoke. Sci. Rep. 2020, 10, 7160. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, E.; Mielżyńska-Švach, D. Polycyclic Aromatic Hydrocarbons and PAH-Related DNA Adducts. J. Appl. Genet. 2017, 58, 321–330. [Google Scholar] [CrossRef]
- Ma, B.; Stepanov, I.; Hecht, S.S. Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qin, Y.; Zhao, W.; Liang, Z.; Li, M.; Liu, D.; Bai, L.; Chen, Y.; Chen, Y.; Cheng, Y.; et al. International Expert Consensus on Diagnosis and Treatment of Lung Cancer Complicated by Chronic Obstructive Pulmonary Disease. Transl. Lung Cancer Res. 2023, 12, 1661–1701. [Google Scholar] [CrossRef] [PubMed]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic Drivers of Tumourigenesis and Cancer Metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Manić, L.; Wallace, D.; Onganer, P.U.; Taalab, Y.M.; Farooqi, A.A.; Antonijević, B.; Buha Djordjevic, A. Epigenetic Mechanisms in Metal Carcinogenesis. Toxicol. Rep. 2022, 9, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor Initiation and Early Tumorigenesis: Molecular Mechanisms and Interventional Targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- Chaft, J.E.; Rimner, A.; Weder, W.; Azzoli, C.G.; Kris, M.G.; Cascone, T. Evolution of Systemic Therapy for Stages I–III Non-Metastatic Non-Small-Cell Lung Cancer. Nat. Rev. Clin. Oncol. 2021, 18, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Rizzo, S.; Attili, I.; Passaro, A.; Zilli, T.; Martucci, F.; Bonomo, L.; Del Grande, F.; Casiraghi, M.; De Marinis, F.; et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr. Oncol. 2023, 30, 3160–3175. [Google Scholar] [CrossRef]
- dos Santos, P.A.R.; Li, Y.; Ernani, V.; D’Cunha, J.; Aubry, M.-C.; Yang, P. Clinical Outcomes of Stage-IV Non–Small-Cell Lung Cancer in Young Patients and the Impact of Tumor Markers. Cancer Treat. Res. Commun. 2023, 36, 100723. [Google Scholar] [CrossRef]
- Torre, M.; Reda, M.; Musso, V.; Danuzzo, F.; Mohamed, S.; Conforti, S. Diagnostic Accuracy of Endobronchial Ultrasound-Transbronchial Needle Aspiration (EBUS-TBNA) for Mediastinal Lymph Node Staging of Lung Cancer. Mediastinum 2021, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, V.R.; Cardoso, P.F.; Jacomelli, M.; Maia Santos, L.; Minata, M.; Mingarini Terra, R. EBUS-TBNA versus Surgical Mediastinoscopy for Mediastinal Lymph Node Staging in Potentially Operable Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Bras. Pneumol. 2020, 46, e20190221. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Kovacevic, B.; Vilmann, P.; Annema, J.T.; Korevaar, D.A. Performance of EUS-FNA and EUS-B-FNA for the Diagnosis of Left Adrenal Glands Metastases in Patients with Lung Cancer: A Systematic Review and Meta-Analysis. Lung Cancer 2023, 186, 107391. [Google Scholar] [CrossRef] [PubMed]
- Palmero, R.; Vilariño, N.; Navarro-Martín, A.; Nadal, E. Induction Treatment in Patients with Stage III Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 539–554. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; Qin, D.; Cui, Y.; Zhang, H. Advanced Surgical Technologies for Lung Cancer Treatment: Current Status and Perspectives. Eng. Regen. 2023, 4, 55–67. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, L.; Shen, J.; Liu, L.; Wang, J.; He, J.; He, Q.; Jiang, L.; Guo, M.; Chen, X.; et al. Choice of Surgical Procedure—Lobectomy, Segmentectomy, or Wedge Resection—For Patients with Stage T1-2N0M0 Small Cell Lung Cancer: A Population—Based Study. Thorac. Cancer 2019, 10, 593–600. [Google Scholar] [CrossRef]
- Manfredini, B.; Zirafa, C.C.; Filosso, P.L.; Stefani, A.; Romano, G.; Davini, F.; Melfi, F. The Role of Lymphadenectomy in Early-Stage NSCLC. Cancers 2023, 15, 3735. [Google Scholar] [CrossRef] [PubMed]
- Batihan, G.; Ceylan, K.C.; Usluer, O.; Kaya, Ş.Ö. Video-Assisted Thoracoscopic Surgery vs Thoracotomy for Non-Small Cell Lung Cancer Greater Than 5 Cm: Is VATS a Feasible Approach for Large Tumors? J. Cardiothorac. Surg. 2020, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Doucet, V.; Perentes, J.Y.; Abdelnour-Berchtold, E.; Zellweger, M.; Faouzi, M.; Bouchaab, H.; Peters, S.; Marcucci, C.; Krueger, T.; et al. Impact of an Enhanced Recovery after Surgery Pathway on Thoracoscopic Lobectomy Outcomes in Non-Small Cell Lung Cancer Patients: A Propensity Score-Matched Study. Transl. Lung Cancer Res. 2021, 10, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M. Role of Chemotherapy and Targeted Therapy in Early-Stage Non–Small Cell Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 630–639. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Crespo, P.C.; Taboada, B.; Gonzalez, A.A.; García, R.G.; Caamaño, A.G.; Reyes, J.C.T.; Rubio, X.M.; Couñago, F. Postoperative Radiotherapy in Resected Non-Small Cell Lung Cancer: The Never-Ending Story. World J. Clin. Oncol. 2021, 12, 833–844. [Google Scholar] [CrossRef]
- Olmetto, E.; Perna, M.; Cerbai, C.; Aquilano, M.; Banini, M.; Mariotti, M.; Livi, L.; Scotti, V. A Narrative Review of Postoperative Adjuvant Radiotherapy for Non-Small Cell Lung Cancer. Mediastinum 2022, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Calvo, V.; Romero, A.; Spicer, J.D.; Cruz-Bermúdez, A. Treatment Sequencing in Resectable Lung Cancer: The Good and the Bad of Adjuvant Versus Neoadjuvant Therapy. Am. Soc. Clin. Oncol. Educ. B. 2022, 42, 711–728. [Google Scholar] [CrossRef]
- Almeldin, D.S.; Malhotra, J.; Patel, M.; Aisner, J.; Jabbour, S.K. Local Treatment of Synchronous Oligometastatic Non-Small Cell Lung Cancer (NSCLC)—Current Consensus and Future Perspectives. J. Thorac. Dis. 2020, 12, 7069–7075. [Google Scholar] [CrossRef]
- Agustoni, F.; Hirsch, F.R. PACIFIC Trial: New Perspectives for Immunotherapy in Lung Cancer. Transl. Lung Cancer Res. 2018, 7, S19–S24. [Google Scholar] [CrossRef]
- Socinski, M.A.; Özgüroğlu, M.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Gray, J.E.; Park, K.; Vincent, M.; et al. Durvalumab After Concurrent Chemoradiotherapy in Elderly Patients with Unresectable Stage III Non–Small–Cell Lung Cancer (PACIFIC). Clin. Lung Cancer 2021, 22, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, B.; Ashley, S.; Norton, A.; Popat, S.; Hughes, S.; Papadopoulos, P.; Priest, K.; O’Brien, M. Early Response to Platinum-Based First-Line Chemotherapy in Non-Small Cell Lung Cancer May Predict Survival. J. Thorac. Oncol. 2007, 2, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ni, J.; Cai, X.; Su, S.; Zhuang, H.; Yang, Z.; Chen, M.; Ma, S.; Xie, C.; Xu, Y.; et al. International Consensus on Radiotherapy in Metastatic Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2022, 11, 1763–1795. [Google Scholar] [CrossRef] [PubMed]
- Comella, P.; Filippelli, G.; De Cataldis, G.; Massidda, B.; Frasci, G.; Maiorino, L.; Putzu, C.; Mancarella, S.; Palmeri, S.; Cioffi, R.; et al. Efficacy of the Combination of Cisplatin with Either Gemcitabine and Vinorelbine or Gemcitabine and Paclitaxel in the Treatment of Locally Advanced or Metastatic Non-Small-Cell Lung Cancer: A Phase III Randomised Trial of the Southern Italy Cooperative Oncology Group (SICOG 0101). Ann. Oncol. 2007, 18, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Boni, L.; Tiseo, M.; Fossella, F.V.; Schiller, J.H.; Paesmans, M.; Radosavljevic, D.; Paccagnella, A.; Zatloukal, P.; Mazzanti, P.; et al. Cisplatin- Versus Carboplatin-Based Chemotherapy in First-Line Treatment of Advanced Non-Small-Cell Lung Cancer: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2007, 99, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Šutić, M.; Vukić, A.; Baranašić, J.; Försti, A.; Džubur, F.; Samaržija, M.; Jakopović, M.; Brčić, L.; Knežević, J. Diagnostic, Predictive, and Prognostic Biomarkers in Non-Small Cell Lung Cancer (NSCLC) Management. J. Pers. Med. 2021, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Parikh, P.; von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III Study Comparing Cisplatin Plus Gemcitabine with Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients with Advanced-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Y.; Li, J.-L. Comparative Review of Drug–Drug Interactions with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for the Treatment of Non-Small-Cell Lung Cancer. Oncol. Targets. Ther. 2019, 12, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Cardarella, S.; Lydon, C.A.; Dahlberg, S.E.; Jackman, D.M.; Jänne, P.A.; Johnson, B.E. Five-Year Survival in EGFR -Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J. Thorac. Oncol. 2016, 11, 556–565. [Google Scholar] [CrossRef]
- Di Noia, V.; D’Aveni, A.; D’Argento, E.; Rossi, S.; Ghirardelli, P.; Bortolotti, L.; Vavassori, V.; Bria, E.; Ceresoli, G.L. Treating Disease Progression with Osimertinib in EGFR-Mutated Non-Small-Cell Lung Cancer: Novel Targeted Agents and Combination Strategies. ESMO Open 2021, 6, 100280. [Google Scholar] [CrossRef]
- Giroux-Leprieur, E.; Fallet, V.; Cadranel, J.; Wislez, M. Spotlight on Crizotinib in the First-Line Treatment of ALK-Positive Advanced Non-Small-Cell Lung Cancer: Patients Selection and Perspectives. Lung Cancer Targets Ther. 2016, 7, 83. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-T.; Yu, C.-J.; Yang, J.C.-H.; Shih, J.-Y. Anaplastic Lymphoma Kinase (ALK) Kinase Domain Mutation Following ALK Inhibitor(s) Failure in Advanced ALK Positive Non–Small-Cell Lung Cancer: Analysis and Literature Review. Clin. Lung Cancer 2016, 17, e77–e94. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-Line Pembrolizumab in Advanced Non–Small Cell Lung Cancer Patients with Poor Performance Status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Zhang, L.; Cheng, S.; Yu, J. Network Meta-Analysis of First-Line Immune Checkpoint Inhibitor Therapy in Advanced Non-Squamous Non-Small Cell Lung Cancer Patients with PD-L1 Expression ≥ 50%. BMC Cancer 2023, 23, 791. [Google Scholar] [CrossRef] [PubMed]
- Insa, A.; Martín-Martorell, P.; Di Liello, R.; Fasano, M.; Martini, G.; Napolitano, S.; Vicidomini, G.; Cappabianca, S.; Franco, R.; Morgillo, F.; et al. Which Treatment after First Line Therapy in NSCLC Patients without Genetic Alterations in the Era of Immunotherapy? Crit. Rev. Oncol. Hematol. 2022, 169, 103538. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Bahena, L.; Velasco-Velázquez, M.A. Anti-PD-1 And Anti-PD-L1 Antibodies as Immunotherapy Against Cancer: A Structural Perspective. Rev. Invest. Clin. 2021, 73, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Shah, K.; Malviya, R.; Paliwal, D.; Sagar, S.; Singh, S.; Prajapati, B.G.; Bhattacharya, S. E-Cigarettes and Associated Health Risks: An Update on Cancer Potential. Adv. Respir. Med. 2023, 91, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat Not Burn Tobacco Product-A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 409. [Google Scholar] [CrossRef]
- Nigro, E.; Perrotta, F.; Scialò, F.; D’Agnano, V.; Mallardo, M.; Bianco, A.; Daniele, A. Food, Nutrition, Physical Activity and Microbiota: Which Impact on Lung Cancer? Int. J. Environ. Res. Public Health 2021, 18, 2399. [Google Scholar] [CrossRef] [PubMed]
- Amicizia, D.; Piazza, M.F.; Marchini, F.; Astengo, M.; Grammatico, F.; Battaglini, A.; Schenone, I.; Sticchi, C.; Lavieri, R.; Di Silverio, B.; et al. Systematic Review of Lung Cancer Screening: Advancements and Strategies for Implementation. Healthcare 2023, 11, 2085. [Google Scholar] [CrossRef] [PubMed]

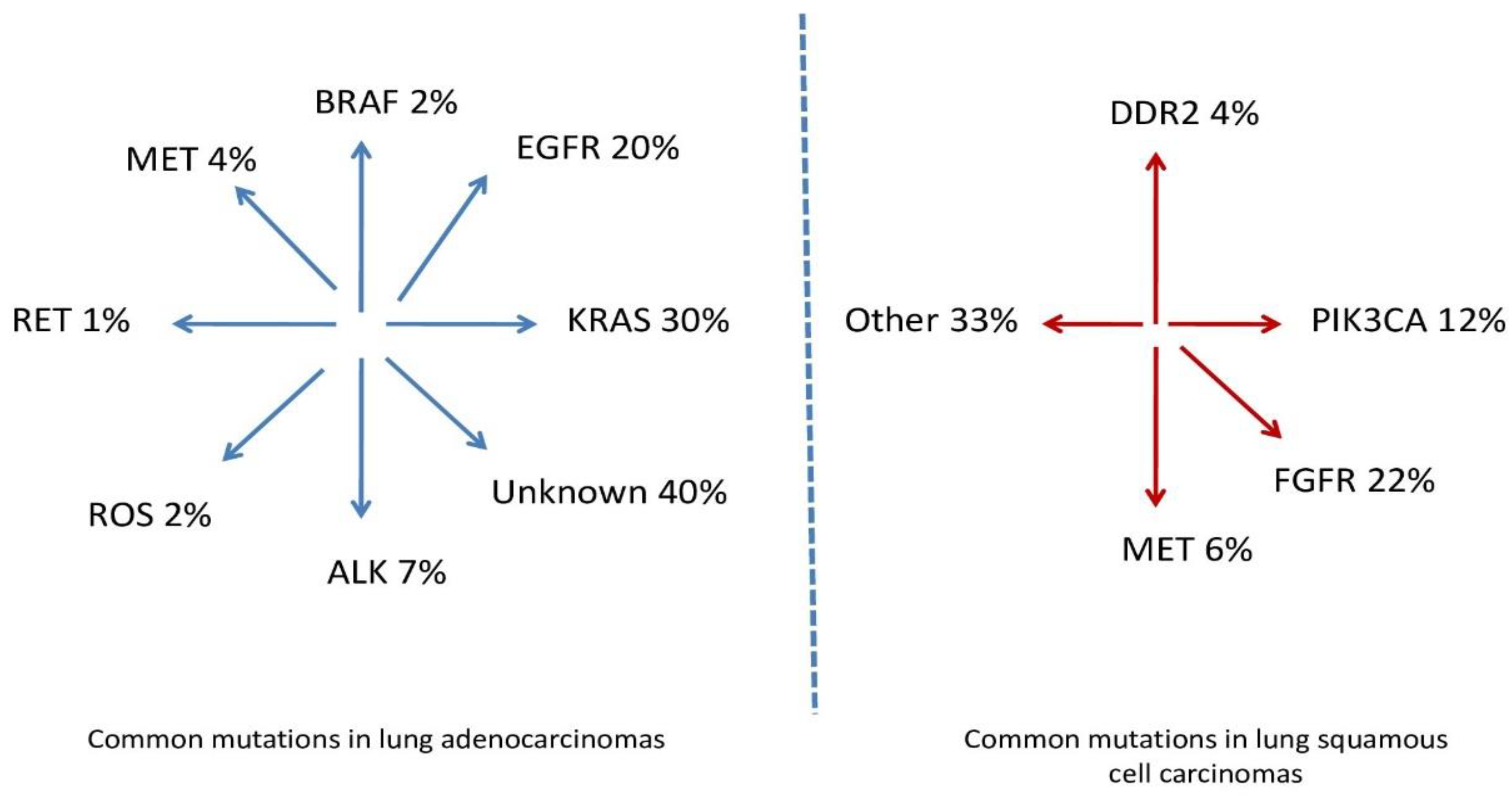
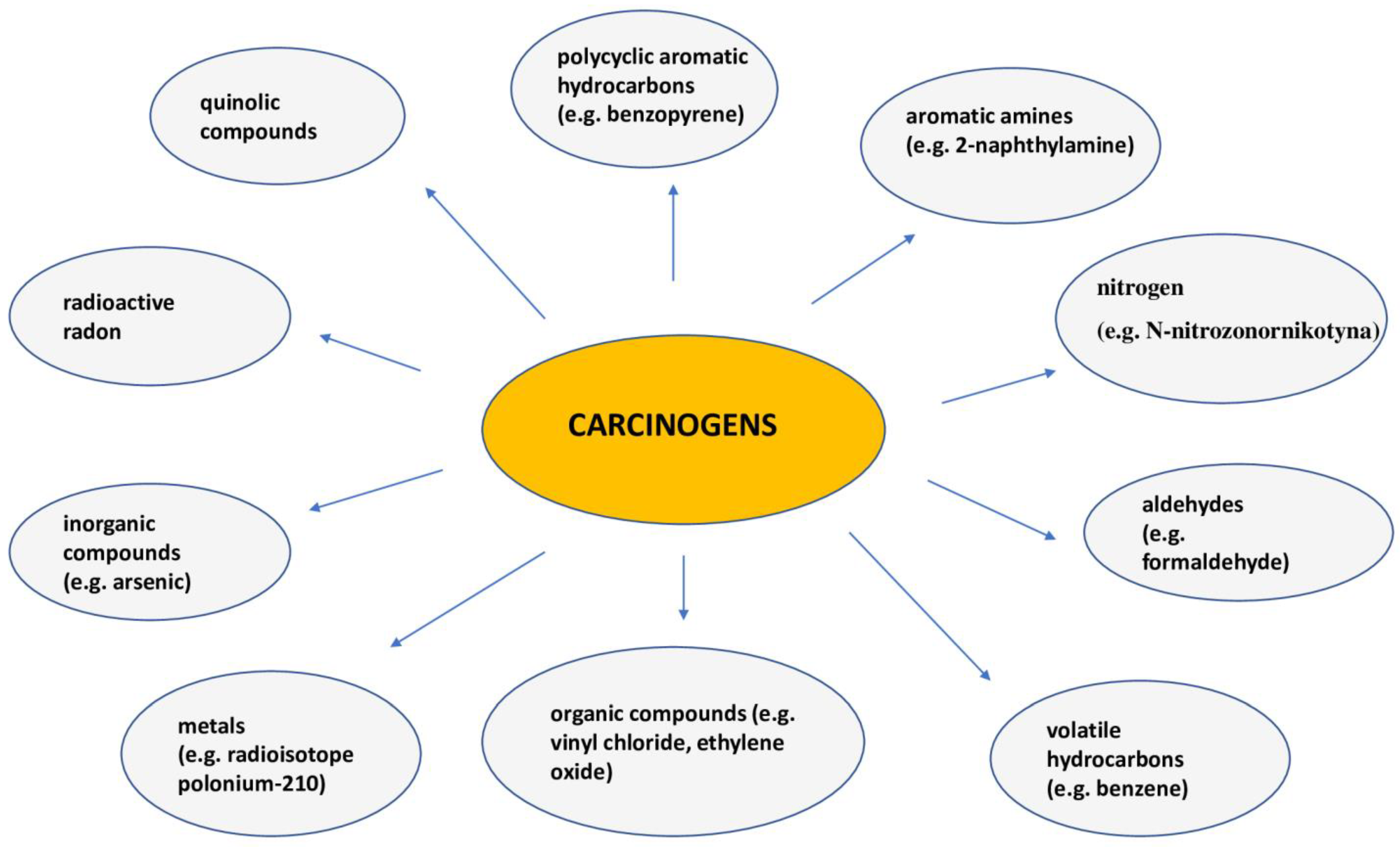

| Local Symptoms of Lung Cancer | General Symptoms of Lung Cancer |
|---|---|
| cough (especially a change in its character) | arthralgia |
| dyspnea | general weakness |
| hemoptysis | weight loss |
| chest pain | weight gain |
| recurrent pneumonia | superficial sensory disorders |
| hoarseness | symptoms of thrombophlebitis |
| swallowing disorder | symptoms of paraneoplastic syndromes: wasting syndrome exhaustion syndrome syndrome of inappropriate antidiuretic hormone secretion (SIADH) Cushing’s syndrome hypercalcemia Lambert–Eaton syndrome retinopathy encephalopathy |
| shoulder pain | |
| superior vena cava syndrome | |
| Horner’s syndrome |
| Primary Tumor Assessment | Lymph Node Assessment | Assessment of Distant Organs |
|---|---|---|
|
|
|
| Feature | Characteristics |
|---|---|
| T | |
| TX | The primary tumor cannot be evaluated or its presence has been demonstrated only on the basis of the presence of tumor cells in the bronchial secretion, without the possibility of visualization by imaging and bronchoscopy |
| T0 | Absent of primary tumor features |
| Tis | carcinoma in situ |
| T1 | Tumor with a diameter of not more than 3 cm, surrounded by pulmonary parenchyma or pulmonary pleura, without infiltration of the main bronchi |
| T1a(mi) | Minimally invasive adenocarcinoma—single tumor—adenocarcinoma ≤ 3 cm, with a predominantly lepidic growth type, with an invasive component ≤ 5 mm in the largest dimension |
| T1a | Tumor with the largest size of 1 cm (also a rare primary tumor spreading superficially, of any dimension, the invasive component of which is limited to the bronchial wall, even if it occurs in the main bronchi) |
| T1b | Tumor with the largest size exceeding 1 cm but not more than 2 cm |
| T1c | Tumor with the largest size exceeding 2 cm but not more than 3 cm |
| T2 | A tumor with a diameter of more than 3 cm but not more than 5 cm, or a tumor with at least one of the following:
|
| T2a | Tumor with a diameter of more than 3 cm but not more than 4 cm |
| T2b | Tumor with a diameter of more than 4 cm but not more than 5 cm |
| T3 | A tumor with a diameter of more than 5 cm but not more than 7 cm, or a tumor of any size with the presence of infiltration of one of the following areas:
Tumor with co-occurrence of satellite lesions in the same lobe of the lung |
| T4 | A tumor with a diameter of more than 7 cm or a tumor of any size with the presence of infiltration of one of the following areas:
Tumor of any size with co-occurrence of satellite lesions in another lobe of the same lung |
| N | |
| NX | Inability to assess the surrounding lymph nodes |
| N0 | Absence of metastases in the surrounding lymph nodes |
| N1 | Metastases in peribronchial and/or hilar lymph nodes on the side of the primary tumor and intrapulmonary (including direct involvement by continuity from the side of the primary tumor) |
| N2 | Metastases in the mediastinal lymph nodes on the side of the primary tumor and/or bifurcation of the trachea |
| N3 | Metastases in the mediastinal lymph nodes or hilum on the contralateral side, under the inclined muscle and/or supraclavicular on the side of the primary tumor or on the opposite side |
| M | |
| MX | Inability to assess metastases to distant organs |
| M0 | Absence of distant metastases |
| M1 | Presence of distant metastases |
| M1a | Satellite lesions in the opposite lung, presence of pleura/pericardial nodules or presence of tumor cells in the pleura/pericardial fluid |
| M1b | Presence of a single distant metastasis in one organ |
| M1c | Multiple metastases in one organ or metastases in different organs |
| Stages | Characteristics | ||
|---|---|---|---|
| Occult cancer | TX | N0 | M0 |
| 0 | Tis | N0 | M0 |
| IA1 | T1a(mi), T1a | N0 | M0 |
| IA2 | T1b | N0 | M0 |
| IA3 | T1c | N0 | M0 |
| IB | T2a | N0 | M0 |
| IIA | T2b | N0 | M0 |
| IIB | T1a, T1b, T1c T2a, T2b T3 | N1 N1 N0 | M0 M0 M0 |
| IIIA | T1a, T1b, T1c, T2a, T2b T3 T4 | N2 N2 N1 N0, N1 | M0 M0 M0 M0 |
| IIIB | T3, T4 T1a, T1b, T1c, T2a, T2b | N2 N3 N3 | M0 M0 M0 |
| IIIC | T3, T4 | N3 | M0 |
| IVA | each T | each N | M1a, M1b |
| IVB | each T | each N | M1c |
| Type | Subtype |
|---|---|
| Adnocarcinoma | wallpapering adenocarcinoma (lepidic adenocarcinoma) acinar adenocarcinoma (acinar adenocarcinoma) papillary adenocarcinoma (papillary adenocarcinoma) small-papillary adenocarcinoma (micropapillary adenocarcinoma) solid adenocarcinoma invasive mucinous adenocarcinoma with mixed mucinous and nonmucinous carcinoma colloid adenocarcinoma fetal adenocarcinoma enteric-type adenocarcinoma minimally invasive adenocarcinoma with carcinoma with mucinous or nonmucinous carcinoma pre-invasive lesions
|
| Squamous cell carcinoma | keratinizing squamous-cell carcinoma non-keratinizing squamous-cell carcinoma squamous-cell carcinoma in situ |
| Neuroendocrine tumors | small-cell carcinoma with combined carcinoma large-cell carcinoma with combined carcinoma typical and atypical carcinoids diffuse idiopathic pulmonary neuroendocrine hyperplasia |
| Large cell carcinoma | |
| Adenoid squamous cell carcinoma | |
| Sarcoma cankers | pleomorphic sarcomatoid carcinoma spindle-cell sarcomatoid carcinoma giant-cell sarcomatoid carcinoma carcinosarcoma pulmonary blastoma |
| Salivary gland type carcinomas | mucoepidermoid carcinoma adenoid-cystic carcinoma |
| Unclassified |
| Risk Factor for Lung Cancer | |
|---|---|
| smoking | Smoking is the cause of 90% of lung cancer cases in men and 80% in women. Smokers have a 30 times higher risk of death from lung cancer than non-smokers. Cigarette smoke hides over 7000 chemical compounds, including over 70 compounds considered carcinogenic. Secondhand smoke is also associated with a higher risk of lung cancer compared to people who are not exposed to tobacco smoke. It is estimated that about 20–50% of “non-smokers” who suffer from lung cancer are passive smokers [85]. |
| alcohol | Studies indicate that people who abused alcohol were more likely to develop lung cancer. Researchers do not provide exact data but estimate that it may be related to another factor: smoking. Studies show that people are more likely to reach for cigarettes when they drink. Researchers at the University of Liverpool studied 125,249 British drinkers and 47,967 Americans. As many as six genes have been identified that, in their opinion, are associated with excessive alcohol consumption and, consequently, with lung cancer [86]. |
| genetic predisposition | The role of genetic factors is still quite poorly understood. The high incidence of lung cancer in some families is associated with a genetically determined tendency to overactivate carcinogenic compounds contained in tobacco smoke or to remove these compounds from the body too slowly. A tendency to slowly repair DNA damage in respiratory epithelial cells after the action of carcinogens is also inherited. To sum up, it can be stated that the hereditary condition is primarily a special susceptibility to the carcinogenic effects of tobacco. This inheritance is the result of the presence of polymorphisms (population variants) in many genes, and there are currently no reliable genetic tests to determine the high risk of developing lung cancer. Research by American specialists shows that the detection of inherited genetic changes (pathogenic germline variants—PGV) is of great importance in predicting the risk of lung cancer. It has been established that it occurs in a fairly large group, i.e., 15% of patients with lung cancer [84]. |
| occupational factors | Exposure to many occupational factors has consequences in the form of the development of lung diseases, including lung cancer. The most important occupational carcinogens include asbestos, silica, heavy metals and polycyclic aromatic hydrocarbons [87]. All forms of asbestos (chrysotile and amphiboles, including crocidolite, amosite and tremolite) are carcinogenic, although the potency of chrysotile is less than that of other types, likely due to its more effective removal from the lungs. In many underdeveloped countries, occupational exposure to asbestos remains widespread [87,88]. Elevated risk of lung cancer has been reported in several industries and occupations associated with exposure to polycyclic aromatic hydrocarbons, such as aluminum production, coal gasification, coke production, iron and steel foundries, tar distillation, roofing and chimney cleaning. It has also been suggested that people employed in several other industries have increased risk of lung cancer, including shale oil mining, wood impregnation, roofing and carbon electrode manufacturing [88]. |
| environmental factors | Air pollution data show that lung cancer incidence increases by 30–50% in areas with high levels of ambient air pollution compared to areas with lower levels [89,90]. Many studies carried out so far clearly show that the risk of developing lung cancer is much higher in highly urbanized, industrialized regions with a developed transport network, in particular based on the use of internal combustion engines [37]. |
| age | The risk of developing lung cancer also increases with age. The majority of lung cancers occur after the age of 50 (96% of cases in men and 95% of cases in women), with about 50% of cases in both sexes occurring in the population over 65 years of age. The risk of developing lung cancer peaks in men in the eighth decade of life and in women at the turn of the sixth and seventh decades of life [25]. |
| Immunosuppressive Effects | Pro-Inflammatory Effects |
|---|---|
| Effects on dendritic cells and their ability to present antigen Suppression of dendritic cell maturation and cytokine release | Activation of acquired immunity with the involvement of dendritic cells |
| Effects on neutrophils and macrophages Suppression of neutrophil-mediated inflammatory effects Reduction in neutrophil migration and chemotaxis Reduction in macrophage activity towards intracellular organisms | Increase in neutrophil levels in circulation |
| Effects on the T cell population Nicotine inhibits the cellular response associated with the formation of antibodies, interferes with antigen-mediated signaling in T lymphocytes, induces T cell anergy | Polyphenol-rich glycoprotein stimulates peripheral T cell proliferation Increase in the number of circulating T cells Abnormal CD4(+)/CD8(+) ratio Tilting of activity towards the sensitization pathway involving Th2 lymphocytes |
| Effects on B cell populations | |
| Increase in autoreactive B lymphocytes | |
| Effect on humoral response Circulating immunoglobulin reduction | |
| Effects on inflammatory markers and mediators Zahamowanie uwalniania IL-1, IL-2, IL-10, TNF-α i IFN-γ Inhibition of IL-8 release by endothelial cells | Chronic smoking leads to an increase in the concentration of acute phase proteins and pro-inflammatory cytokines, especially TNF-α, TNF-α and IL-6 receptors |
| Other general non-specific mechanisms: Attenuation of IFN signaling | Exposure and release of autoantibodies Release of intracellular antigens due to necrosis induced by tissue hypoxia or toxins |
| Increase in the concentration of free radicals that interact with DNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 2049. https://doi.org/10.3390/ijms26052049
Smolarz B, Łukasiewicz H, Samulak D, Piekarska E, Kołaciński R, Romanowicz H. Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). International Journal of Molecular Sciences. 2025; 26(5):2049. https://doi.org/10.3390/ijms26052049
Chicago/Turabian StyleSmolarz, Beata, Honorata Łukasiewicz, Dariusz Samulak, Ewa Piekarska, Radosław Kołaciński, and Hanna Romanowicz. 2025. "Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature)" International Journal of Molecular Sciences 26, no. 5: 2049. https://doi.org/10.3390/ijms26052049
APA StyleSmolarz, B., Łukasiewicz, H., Samulak, D., Piekarska, E., Kołaciński, R., & Romanowicz, H. (2025). Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). International Journal of Molecular Sciences, 26(5), 2049. https://doi.org/10.3390/ijms26052049






