Epigenetic Regulation of ZNF687 by miR-142a-3p and DNA Methylation During Osteoblast Differentiation and Mice Bone Development and Aging
Abstract
:1. Introduction
2. Results
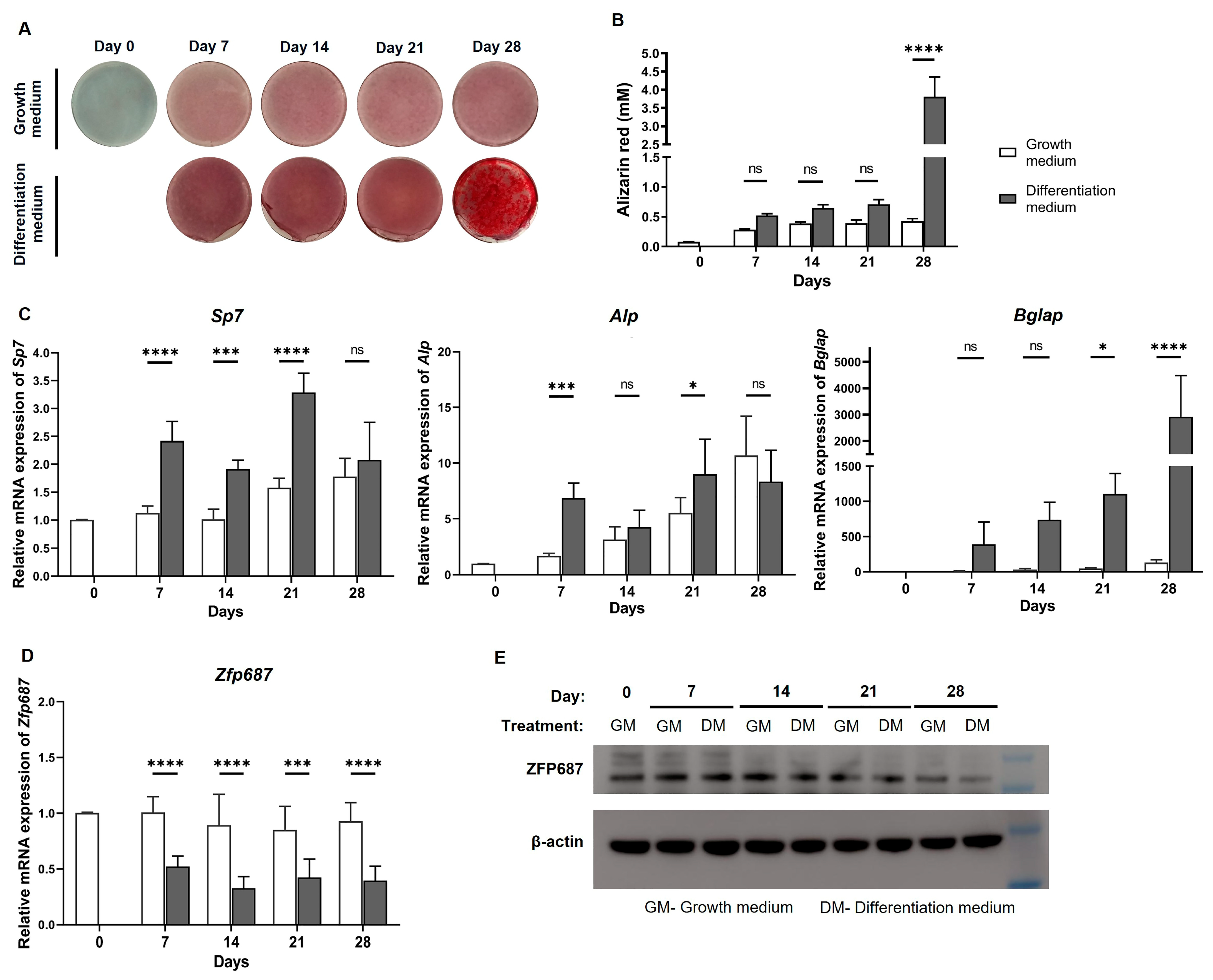
2.1. Mineralization and Expression of Zfp687 and Osteogenic Marker Genes Throughout MC3T3-E1 Osteoblast Differentiation
2.2. Expression of Zfp687 and Bone Marker Genes Throughout Bone Growth/Development
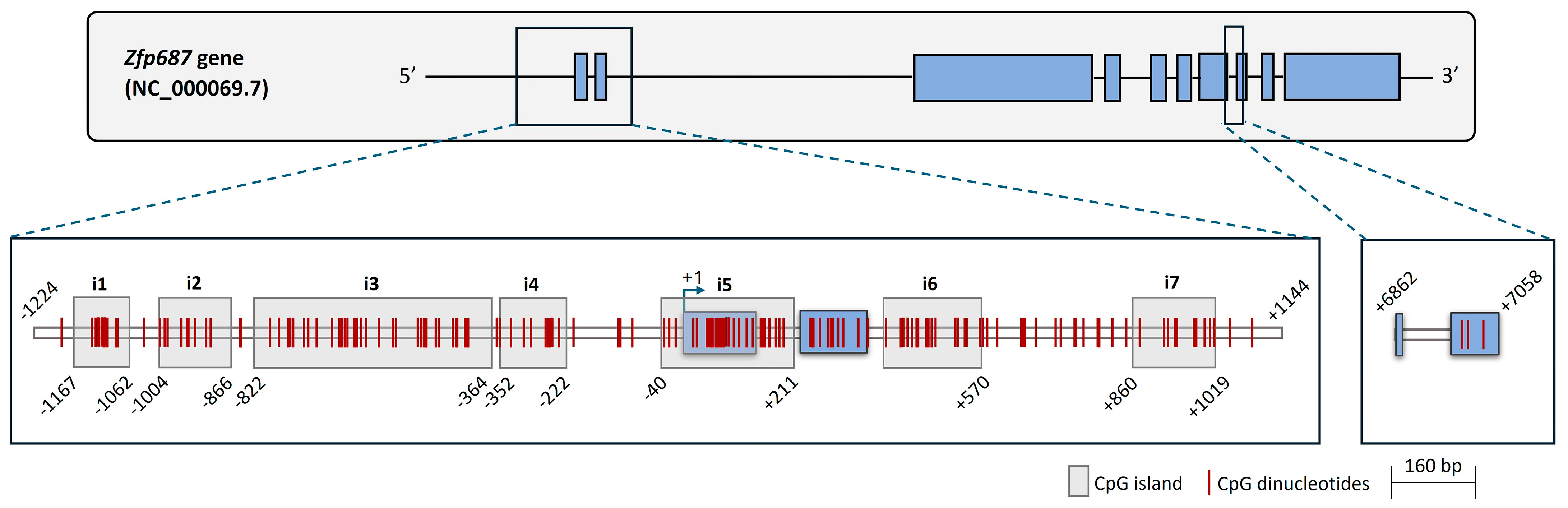
2.3. CpG Sites in the Zfp687 Promoter
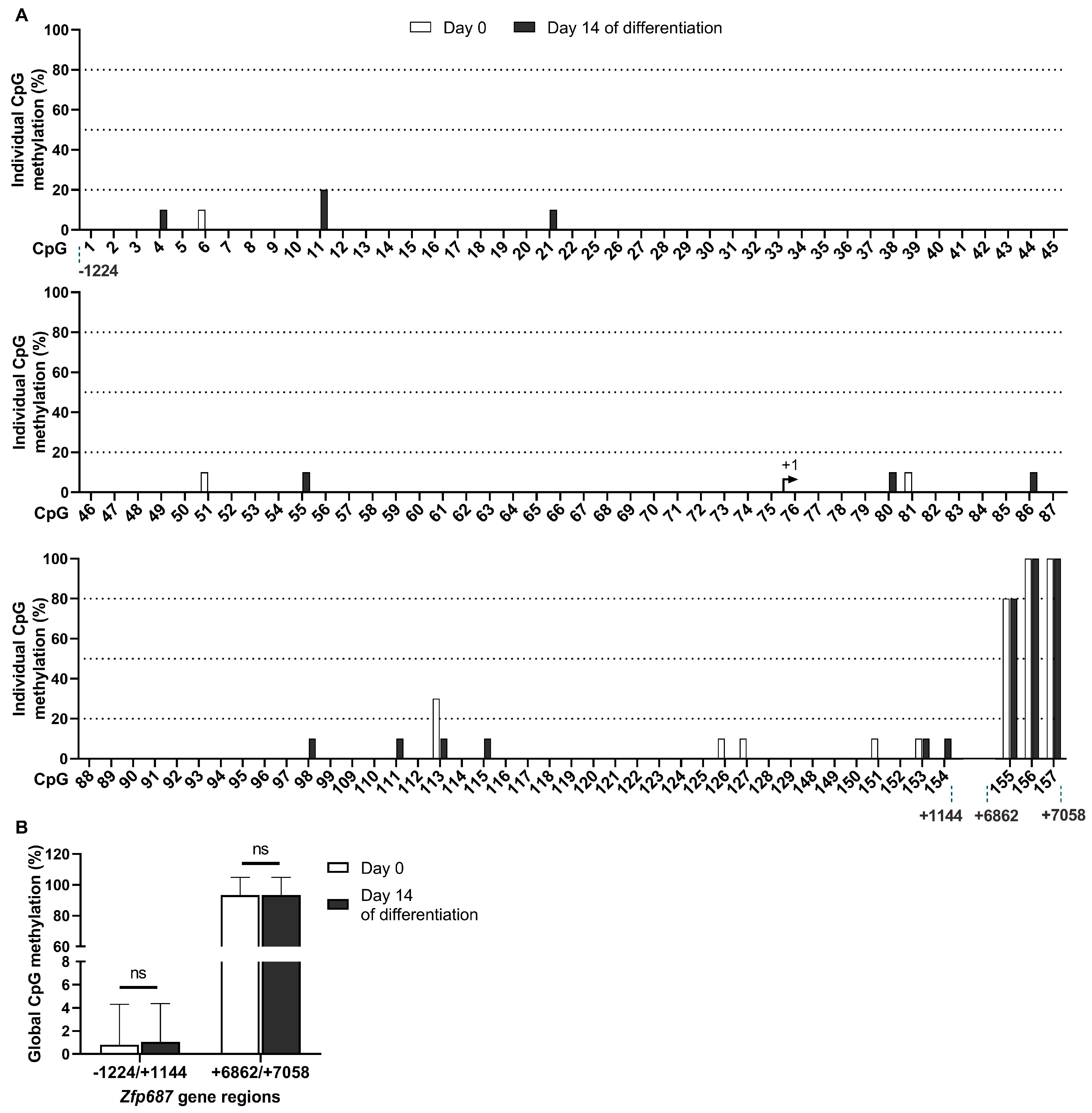
2.4. Methylation Status of Zfp687 During Osteoblastogenesis
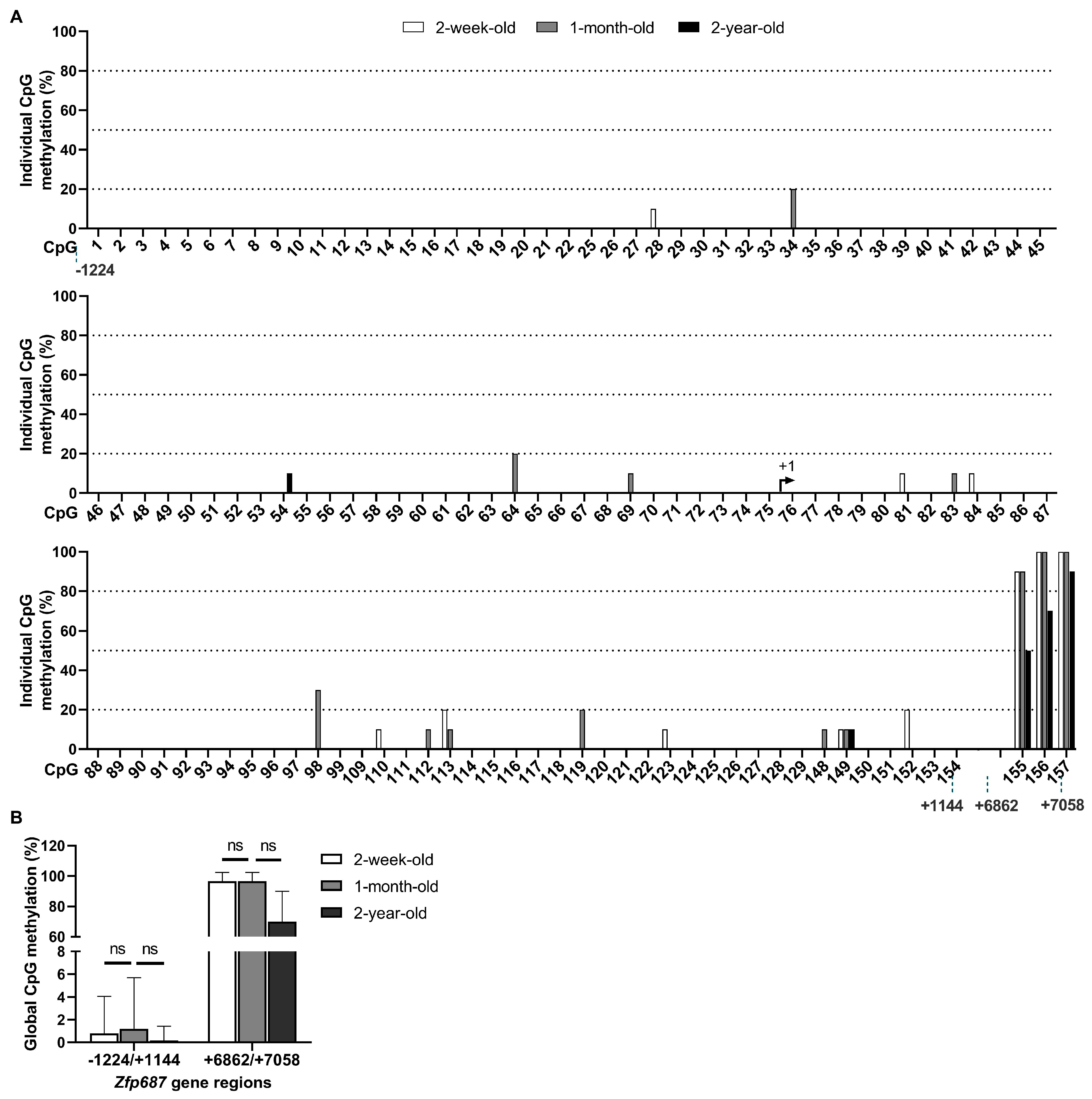
2.5. Methylation Status of Zfp687 Throughout Bone Growth/Development
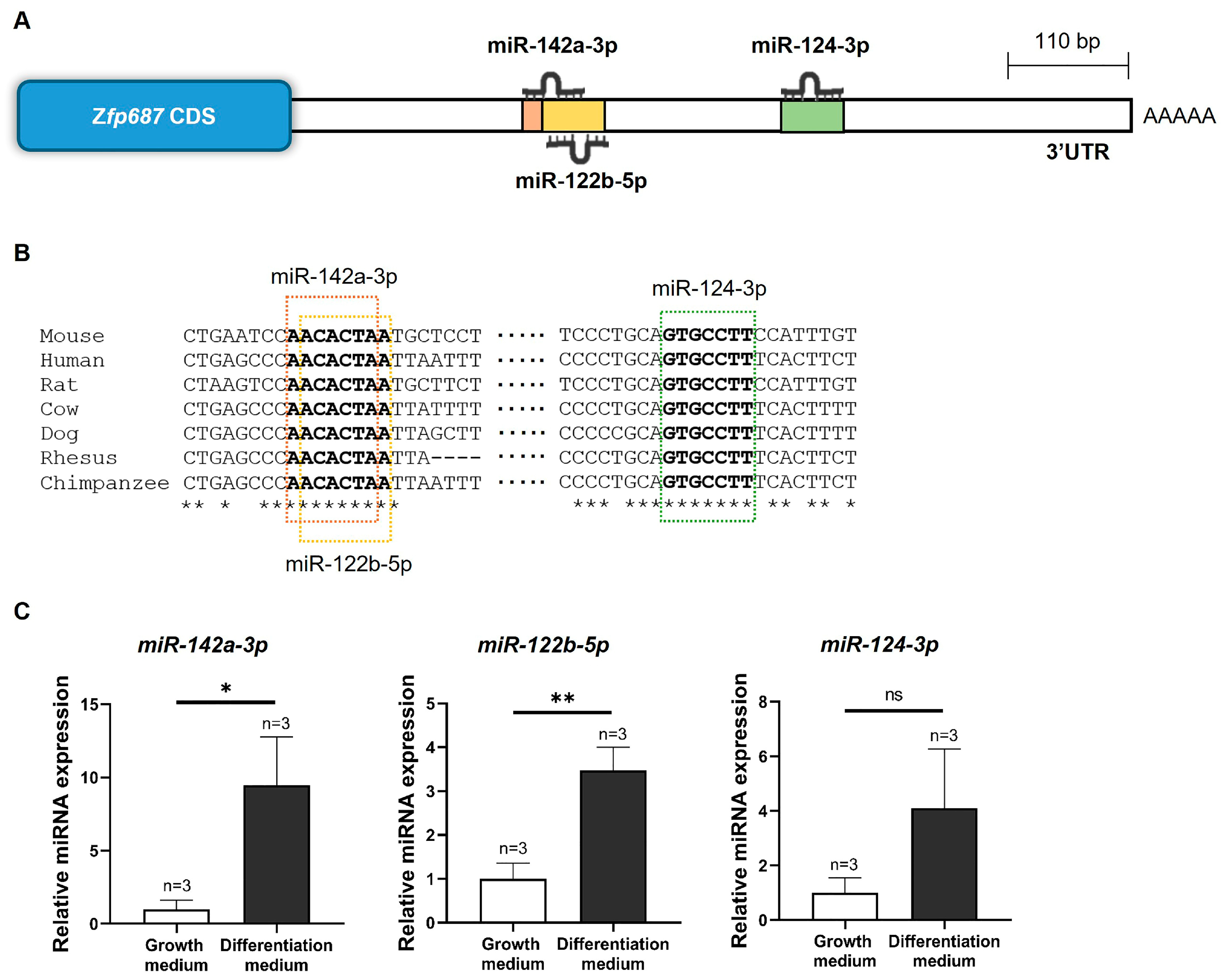
2.6. In Silico Characterization of the Zfp687 3′UTR Regulatory Region and MicroRNA Expression in MC3T3-E1 Osteoblast Differentiation
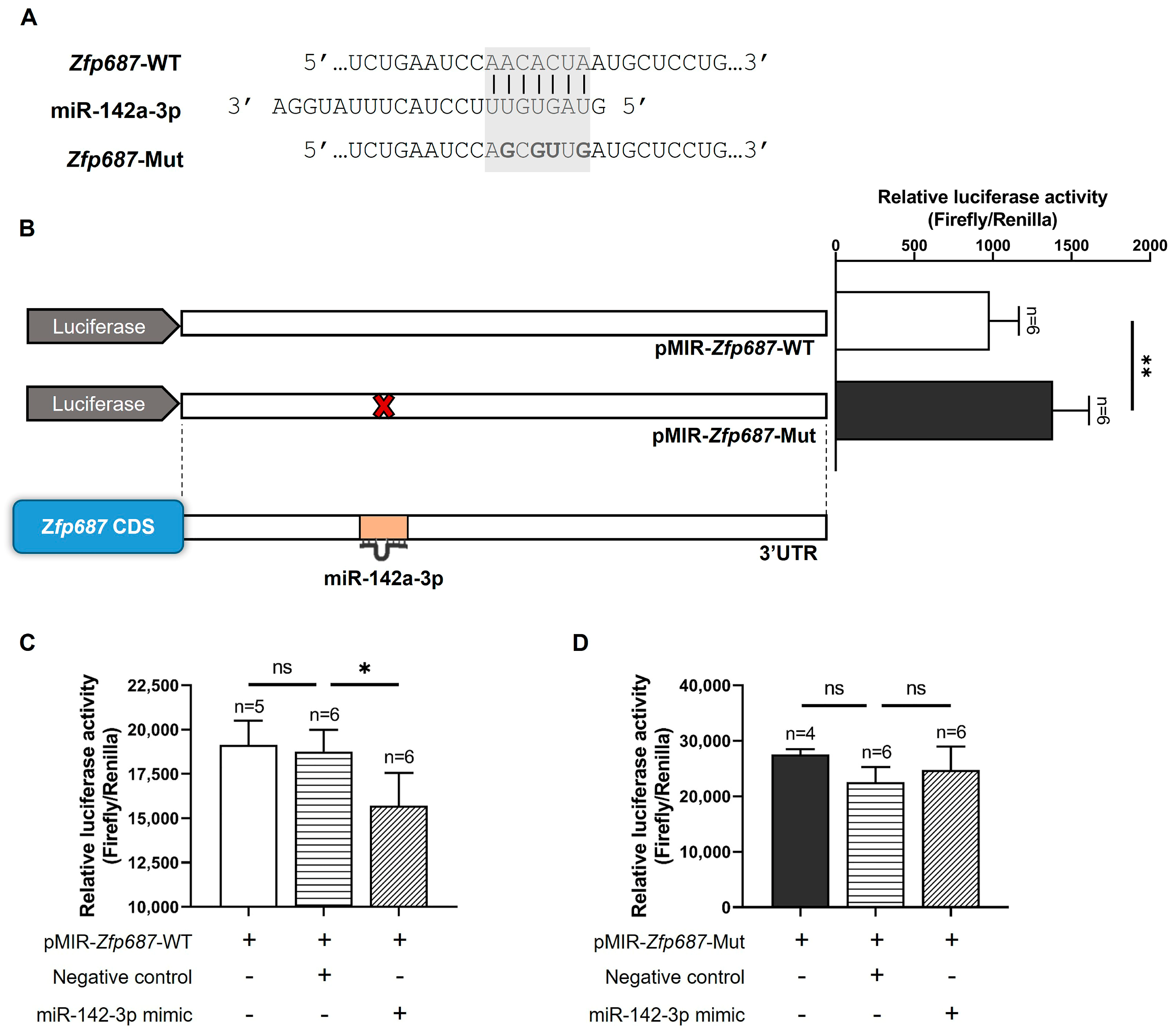
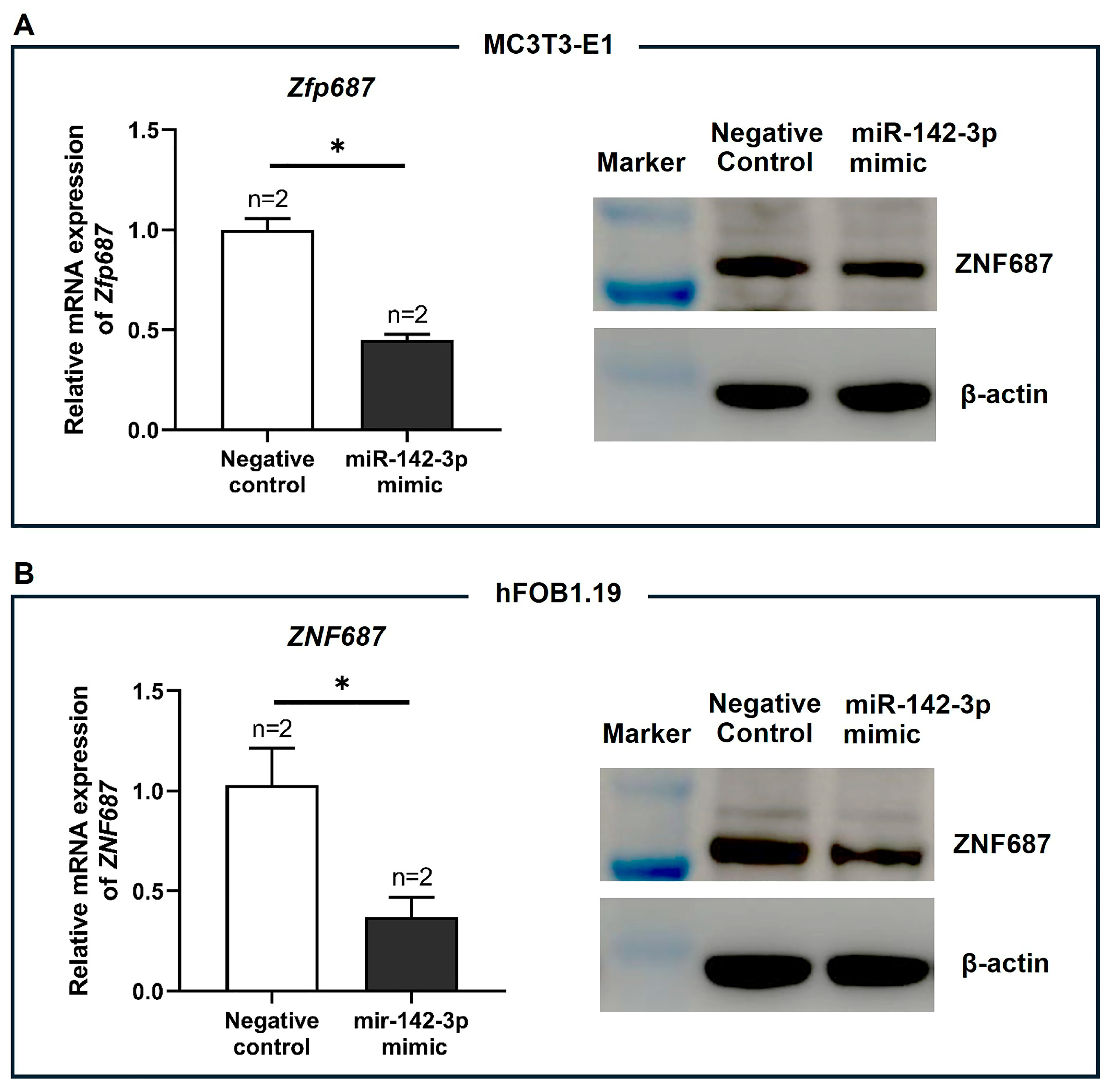
2.7. Zfp687 Regulation by miR-142a-3p
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis of the ZNF687 Gene
4.2. Cell Culture Maintenance
4.3. Osteogenic Differentiation
4.4. Alizarin Red S Staining
4.5. Dissection of Mouse Hindlimb Long Bones
4.6. RNA Extraction and cDNA Synthesis
4.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Protein Extraction and Western Blot
4.9. DNA Extraction and Bisulfite Conversion
4.10. PCR Amplification of Bisulfite-Converted DNA and Cloning into a TOPO Plasmid
4.11. Cloning of the Zfp687 3′UTR into the pMIR-Report Plasmid
4.12. PCR-Based Site-Directed Mutagenesis
4.13. Transient Transfection and Luciferase Reporter Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, G.L. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 2017, 26, E12740. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Peel, N. Bone remodelling and disorders of bone metabolism. Surgery 2009, 27, 70–74. [Google Scholar] [CrossRef]
- Gennari, L.; Rendina, D.; Falchetti, A.; Merlotti, D. Paget’s disease of bone. Calcif. Tissue Int. 2019, 104, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Calero-Paniagua, I.; del Pino-Montes, J. Clinical and genetic advances in Paget’s disease of bone: A review. Clin. Rev. Bone Miner. Metab. 2017, 15, 37–48. [Google Scholar] [CrossRef]
- Silva, I.A.L.; Conceição, N.; Gagnon, É.; Brown, J.P.; Cancela, M.L.; Michou, L. Molecular effect of an OPTN common variant associated to Paget’s disease of bone. PLoS ONE 2018, 13, e0197543. [Google Scholar] [CrossRef]
- Silva, I.A.L.; Varela, D.; Cancela, M.L.; Conceição, N. Zebrafish optineurin: Genomic organization and transcription regulation. Genome 2022, 65, 513–523. [Google Scholar] [CrossRef]
- Divisato, G.; Formicola, D.; Esposito, T.; Merlotti, D.; Pazzaglia, L.; Del Fattore, A.; Siris, E.; Orcel, P.; Brown, J.P.; Nuti, R.; et al. ZNF687 mutations in severe Paget disease of bone associated with giant cell tumor. Am. J. Hum. Genet. 2016, 98, 275–286. [Google Scholar] [CrossRef]
- Scotto di Carlo, F.; Pazzaglia, L.; Mumm, S.; Benassi, M.S.; De Chiara, A.; Franchi, A.; Parafioriti, A.; Righi, A.; Esposito, T.; Whyte, M.P.; et al. ZNF687 mutations in an extended cohort of neoplastic transformations in Paget’s disease of bone: Implications for clinical pathology. J. Bone Miner. Res. 2020, 35, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Scotto di Carlo, F.; Maurizi, A.; Fortunato, G.; Teti, A.; Licastro, D.; Settembre, C.; Mello, T.; Gianfrancesco, F. A mutation in the ZNF687 gene that is responsible for the severe form of Paget’s disease of bone causes severely altered bone remodeling and promotes hepatocellular carcinoma onset in a knock-in mouse model. Bone Res. 2023, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Ganss, B.; Jheon, A. Zinc Finger Transcription Factors in skeletal development. Crit. Rev. Oral Biol. Med. 2004, 15, 282–297. [Google Scholar] [CrossRef]
- Varela, D.; Varela, T.; Conceição, N.; Cancela, M.L. Regulation of human ZNF687, a gene associated with Paget’s disease of bone. Int. J. Biochem. Cell Biol. 2023, 154, 106332. [Google Scholar] [CrossRef] [PubMed]
- Park-Min, K.H. Epigenetic Regulation of Bone cells. Connect. Tissue Res. 2017, 58, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Diboun, I.; Wani, S.; Ralston, S.H.; Albagha, O.M.E. Epigenetic DNA methylation signatures associated with the severity of Paget’s disease of bone. Front. Cell Dev. Biol. 2022, 10, 903612. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.G. MicroRNAs: Hidden in the Genome Genes for tiny RNAs have been found to be plentiful. Curr. Biol. 2002, 12, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.E.; Wheeler-Jones, C.P.D.; Orriss, I.R. Regulation of mineralisation in bone and vascular tissue: A comparative review. J. Endocrinol. 2021, 248, R51–R65. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the alizarin red S assay by enhancing mineralization of osteoblasts. Int. J. Mol. Sci. 2023, 24, 723. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, L.M.; Dar, M.S.; Gesty-Palmer, D.; El-Shewy, H.M.; Robinson, K.M.; Haycraft, C.J.; Barth, J.L. Transcriptomic characterization of signaling pathways associated with osteoblastic differentiation of MC-3T3E1 cells. PLoS ONE 2019, 14, e0204197. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Deville de Périère, D.; Cuisinier, F.J.; Cunin, F.; Gergely, C. Porous silicon scaffolds for stem cells growth and osteodifferentiation. In Porous Silicon for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 486–506. [Google Scholar] [CrossRef]
- Beck, G.R.; Zerler, B.; Moran, E. Gene array analysis of osteoblast differentiation. Cell Growth Differ. 2001, 12, 61–83. [Google Scholar]
- Jiang, Q.; Nagano, K.; Moriishi, T.; Komori, H.; Sakane, C.; Matsuo, Y.; Zhang, Z.; Nishimura, R.; Ito, K.; Qin, X.; et al. Roles of Sp7 in osteoblasts for the proliferation, differentiation, and osteocyte process formation. J. Orthop. Translat 2024, 47, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Kamath, T.; Mazur, C.M.; Mirzamohammadi, F.; Rotter, D.; Hojo, H.; Castro, C.D.; Tokavanich, N.; Patel, R.; Govea, N.; et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat. Commun. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Wrobel, E.; Leszczynska, J.; Brzoska, E. The characteristics of human bone-derived cells (HBDCS) during osteogenesis in vitro. Cell Mol. Biol. Lett. 2016, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Barros, T.L.; Brito, V.G.B.; Do Amaral, C.C.F.; Chaves-Neto, A.H.; Campanelli, A.P.; Oliveira, S.H.P. Osteogenic markers are reduced in bone-marrow mesenchymal cells and femoral bone of young spontaneously hypertensive rats. Life Sci. 2016, 146, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ezura, Y.; Sekiya, I.; Koga, H.; Muneta, T.; Noda, M. Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ye, Y.; Zhang, W.; Wang, J.; Chen, A.; Guo, F. MiR-142-3p promotes osteoblast differentiation by modulating Wnt signaling. Mol. Med. Rep. 2013, 7, 689–693. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and role of transcription factors in osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Osteoblast differentiation and signaling: Established concepts and emerging topics. Int. J. Mol. Sci. 2021, 22, 6651. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jeong, H.M.; Jin, Y.H.; Li, H.; Yeo, C.Y.; Lee, K.Y. Akt phosphorylates and regulates the osteogenic activity of Osterix. Biochem. Biophys. Res. Commun. 2011, 411, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Divisato, G.; Scotto di Carlo, F.; Petrillo, N.; Esposito, T.; Gianfrancesco, F. ZNF687 mutations are frequently found in pagetic patients from South Italy: Implication in the pathogenesis of Paget’s disease of bone. Clin. Genet. 2018, 93, 1240–1244. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic. Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Somerville, J.M.; Aspden, R.M.; Armour, K.E.; Armour, K.J.; Reid, D.M. Growth of C57Bl/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif. Tissue Int. 2004, 74, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Lynch, M.A.; Stephens, A.L.; Wood, D.J.; Civitelli, R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS ONE 2012, 7, e34980. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Venton, L.; Sakata, T.; Halloran, B.P. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J. Bone Miner. Res. 2003, 18, 270–277. [Google Scholar] [CrossRef]
- Ferguson, V.L.; Ayers, R.A.; Bateman, T.A.; Simske, S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Maupin, K.A.; Childress, P.; Brinker, A.; Khan, F.; Abeysekera, I.; Aguilar, I.N.; Olivos, D.J.; Adam, G.; Savaglio, M.K.; Ganesh, V.; et al. Skeletal adaptations in young male mice after 4 weeks aboard the International Space Station. Microgravity 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Halloran, B.P.; Ferguson, V.L.; Simske, S.J.; Burghardt, A.; Venton, L.L.; Majumdar, S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J. Bone Miner. Res. 2002, 17, 1044–1050. [Google Scholar] [CrossRef]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Arnsdorf, E.J.; Tummala, P.; Castillo, A.B.; Zhang, F.; Jacobs, C.R. The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 2010, 43, 2881–2886. [Google Scholar] [CrossRef]
- Villagra, A.; Gutirrez, J.; Paredes, R.; Sierra, J.; Puchi, M.; Imschenetzky, M.; Van Wijnen, A.; Lian, J.; Stein, G.; Stein, J.; et al. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J. Cell Biochem. 2002, 85, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shen, H.; Deng, H.W. Systemic analysis of osteoblast-specific DNA methylation marks reveals novel epigenetic basis of osteoblast differentiation. Bone Rep. 2017, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Lu, J.; Zhao, Y.; Wang, L.; Li, J.; Qi, B.; Li, H.; Ma, C. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone 2013, 55, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fan, Z. MicroRNAs regulation in osteogenic differentiation of mesenchymal stem cells. Front. Dent. Med. 2021, 2, 747068. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Zhu, Z.; Li, L.; Jin, Y.; Ma, C.; Zhang, W. MiR-27a-3p negatively regulates osteogenic differentiation of MC3T3-E1 preosteoblasts by targeting osterix. Mol. Med. Rep. 2020, 22, 1717–1726. [Google Scholar] [CrossRef]
- Huang, W.; Paul, D.; Calin, G.A.; Bayraktar, R. MiR-142: A master regulator in hematological malignancies and therapeutic opportunities. Cells 2023, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Kelch, S.; Balmayor, E.R.; Seeliger, C.; Vester, H.; Kirschke, J.S.; Van Griensven, M. MiRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017, 7, 15861. [Google Scholar] [CrossRef]
- Letarouilly, J.G.; Broux, O.; Clabaut, A. New insights into the epigenetics of osteoporosis. Genomics 2019, 111, 793–798. [Google Scholar] [CrossRef]
- Fröhlich, L.F. MicroRNAs at the interface between osteogenesis and angiogenesis as targets for bone regeneration. Cells 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Huang, J.; Chen, D. MiRNAs in circulation: Mirroring bone conditions? J. Bone Miner. Res. 2014, 29, 1715–1717. [Google Scholar] [CrossRef]
- Faraldi, M.; Gomarasca, M.; Banfi, G.; Lombardi, G. Free circulating miRNAs measurement in clinical settings: The still unsolved issue of the normalization. Adv. Clin. Chem. 2018, 87, 113–139. [Google Scholar] [CrossRef]
- Evans, C.H. Gene therapy for bone healing. Expert. Rev. Mol. Med. 2010, 12, e18. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.T.; Yang, Y.S.; Xie, J.; Ma, H.; Kim, J.M.; Park, K.H.; Oh, D.S.; Park-Min, K.H.; Greenblatt, M.B.; Gao, G.; et al. WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects. Mol. Ther. 2023, 31, 435–453. [Google Scholar] [CrossRef]
- Yang, Y.S.; Xie, J.; Wang, D.; Kim, J.M.; Tai, P.W.L.; Gravallese, E.; Gao, G.; Shim, J.H. Bone-targeting AAV-mediated silencing of Schnurri-3 prevents bone loss in osteoporosis. Nat. Commun. 2019, 10, 2958. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. MiRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Rennie, W.; Liu, C.; Carmack, C.S.; Wolenc, A.; Kanoria, S.; Lu, J.; Long, D.; Ding, Y. STarMir: A web server for prediction of microRNA binding sites. Nucleic Acids Res. 2014, 42, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, D.; Varela, T.; Conceição, N.; Cancela, M.L. Epigenetic Regulation of ZNF687 by miR-142a-3p and DNA Methylation During Osteoblast Differentiation and Mice Bone Development and Aging. Int. J. Mol. Sci. 2025, 26, 2069. https://doi.org/10.3390/ijms26052069
Varela D, Varela T, Conceição N, Cancela ML. Epigenetic Regulation of ZNF687 by miR-142a-3p and DNA Methylation During Osteoblast Differentiation and Mice Bone Development and Aging. International Journal of Molecular Sciences. 2025; 26(5):2069. https://doi.org/10.3390/ijms26052069
Chicago/Turabian StyleVarela, Débora, Tatiana Varela, Natércia Conceição, and M. Leonor Cancela. 2025. "Epigenetic Regulation of ZNF687 by miR-142a-3p and DNA Methylation During Osteoblast Differentiation and Mice Bone Development and Aging" International Journal of Molecular Sciences 26, no. 5: 2069. https://doi.org/10.3390/ijms26052069
APA StyleVarela, D., Varela, T., Conceição, N., & Cancela, M. L. (2025). Epigenetic Regulation of ZNF687 by miR-142a-3p and DNA Methylation During Osteoblast Differentiation and Mice Bone Development and Aging. International Journal of Molecular Sciences, 26(5), 2069. https://doi.org/10.3390/ijms26052069





