Development of a Dihydrofolate Reductase Selection System for Saccharomyces boulardii
Abstract
:1. Introduction
2. Results
2.1. DHFR Selection Is Feasible in S. boulardii
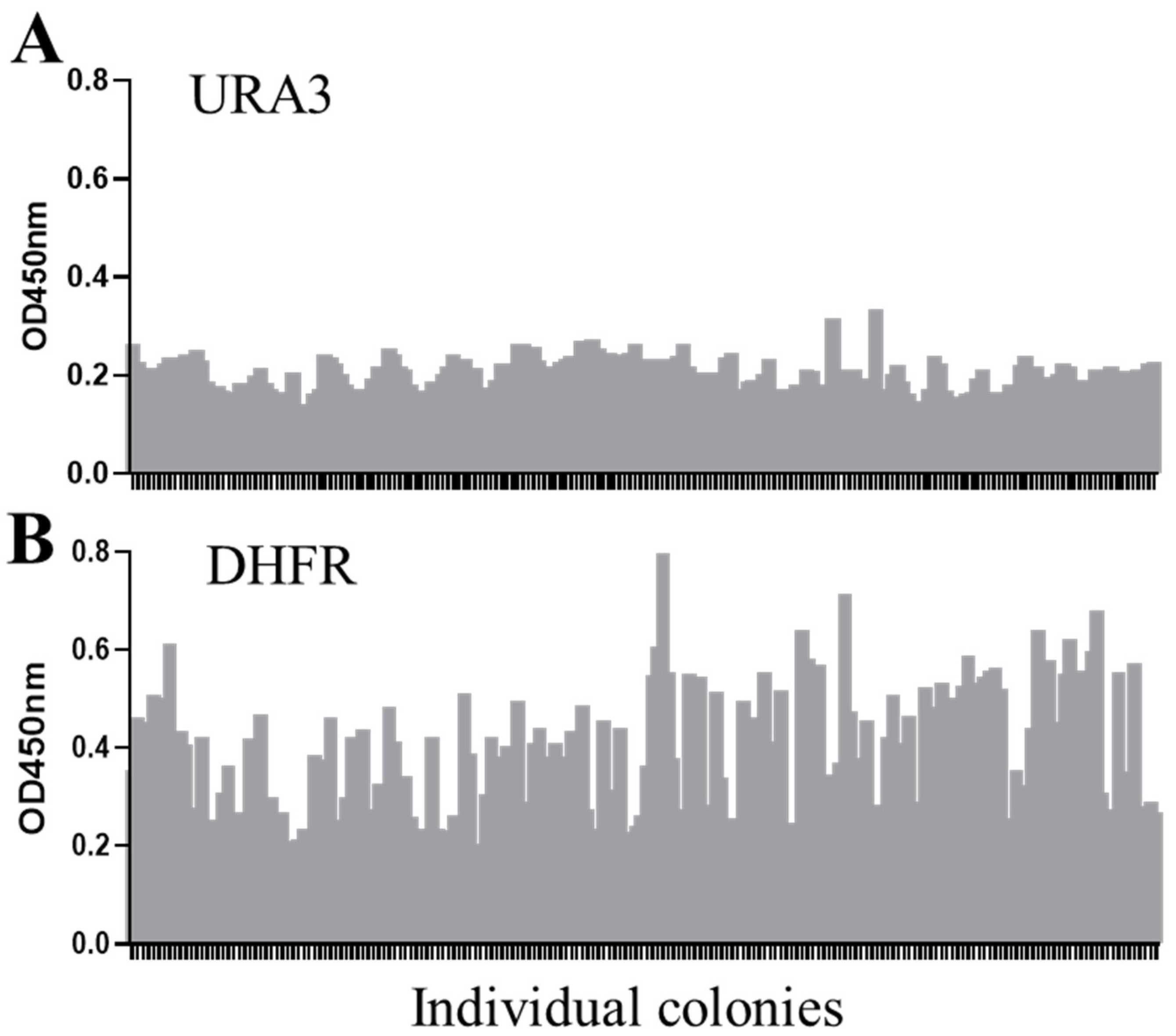
2.2. DHFR Selection Enables Screening for High-Expression Clones in S. boulardii
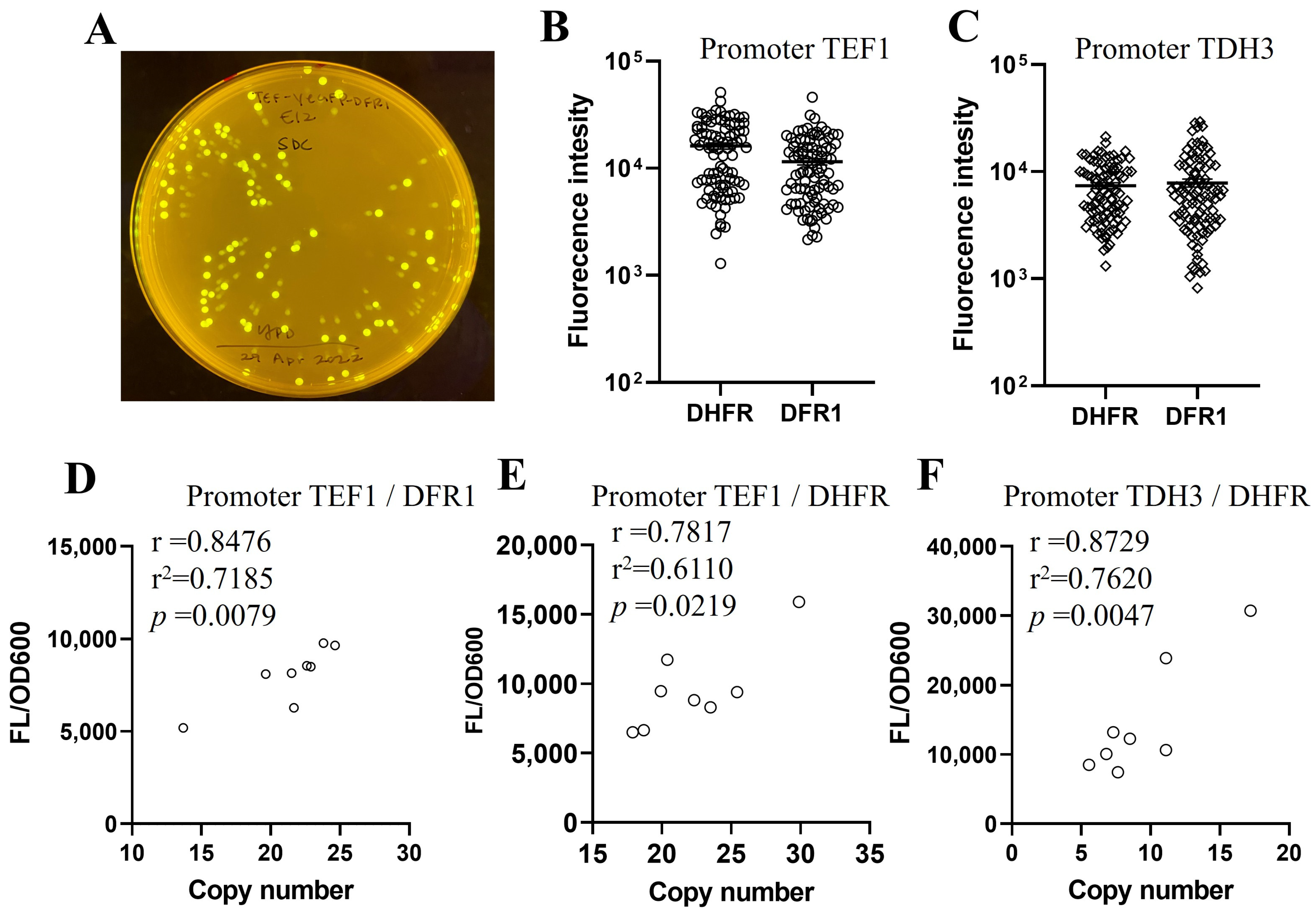
2.3. Endogenous Dihydrofolate Reductase DFR1 Can Be Used as a Selection Marker
2.4. Protein Expression Level Is Correlated with GOI Copy Number in DHFR/DFR1 Selected Transformants
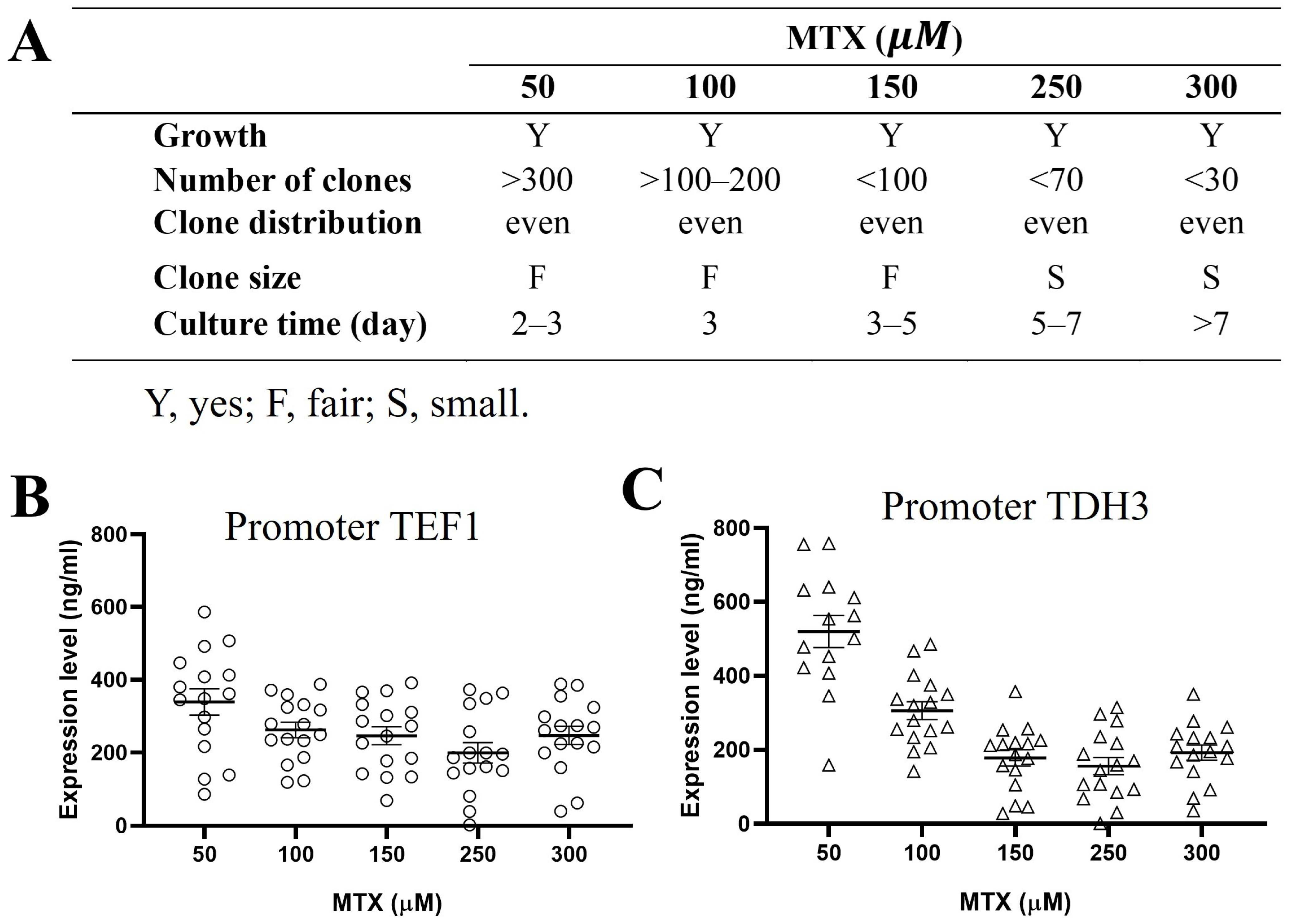
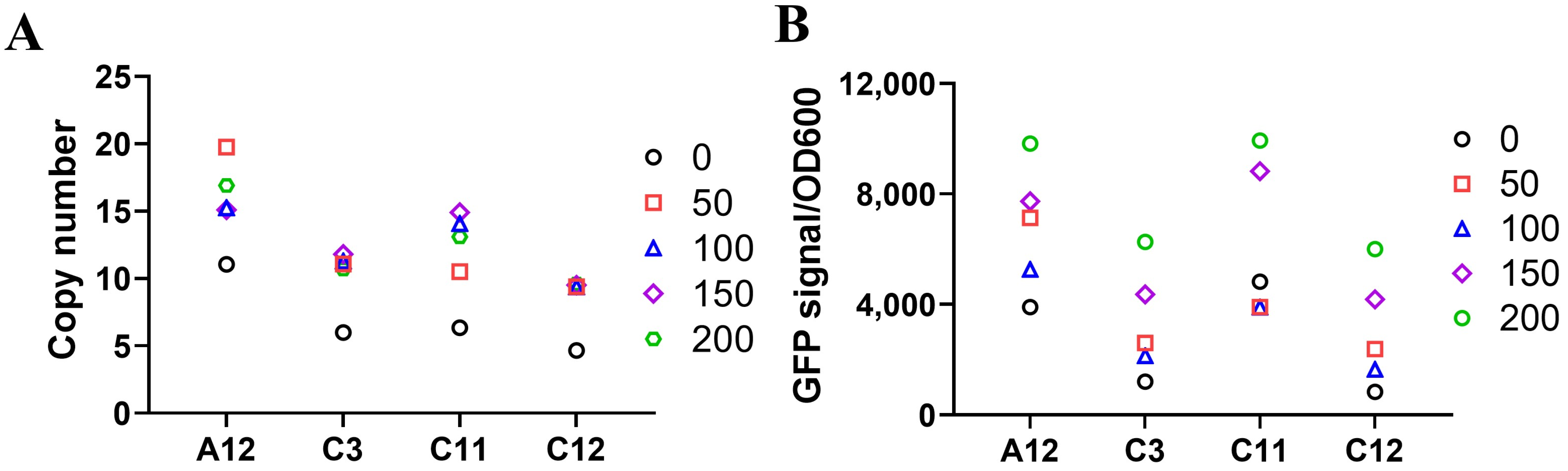
2.5. Gene Amplification Can Be Achieved by MTX in DHFR Selection
3. Discussion
4. Materials and Methods
4.1. Strains, Media, and Culture Conditions
4.2. Plasmid Construction
4.3. Yeast Transformation/Electroporation
4.4. ELISA
4.5. EGFP Expression Studies
4.6. EGFP Copy Number
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.-X.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome evolution across 1011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Heavey, M.K.; Hazelton, A.; Wang, Y.; Garner, M.; Anselmo, A.C.; Arthur, J.C.; Nguyen, J. Targeted delivery of the probiotic Saccharomyces boulardii to the extracellular matrix enhances gut residence time and recovery in murine colitis. Nat. Commun. 2024, 15, 3784. [Google Scholar] [CrossRef]
- Salazar-Parra, M.A.G.; Cruz-Neri, R.U.; Trujillo-Trujillo, X.A.R.; Dominguez-Mora, J.J.; Cruz-Neri, H.I.; Guzmán-Díaz, J.M.; Guzmán-Ruvalcaba, M.J.; Vega-Gastelum, J.O.; Ascencio-Díaz, K.V.; Zarate-Casas, M.F.; et al. Effectiveness of Saccharomyces Boulardii CNCM I-745 probiotic in acute inflammatory viral diarrhoea in adults: Results from a single-centre randomized trial. BMC Gastroenterol. 2023, 23, 229. [Google Scholar] [CrossRef]
- Bustos Fernández, L.M.; Man, F.; Lasa, J.S. Impact of Saccharomyces boulardii CNCM I-745 on Bacterial Overgrowth and Composition of Intestinal Microbiota in Diarrhea-Predominant Irritable Bowel Syndrome Patients: Results of a Randomized Pilot Study. Dig. Dis. 2023, 41, 798–809. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- Sivananthan, K.; Petersen, A.M. Review of Saccharomyces boulardii as a treatment option in IBD. Immunopharmacol. Immunotoxicol. 2018, 40, 465–475. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Mourey, F.; Sureja, V.; Kheni, D.; Shah, P.; Parikh, D.; Upadhyay, U.; Satia, M.; Shah, D.; Troise, C.; Decherf, A. A Multicenter, Randomized, Double-blind, Placebo-controlled Trial of Saccharomyces boulardii in Infants and Children with Acute Diarrhea. Pediatr. Infect. Dis. J. 2020, 39, e347–e351. [Google Scholar] [CrossRef] [PubMed]
- Naghibzadeh, N.; Salmani, F.; Nomiri, S.; Tavakoli, T. Investigating the effect of quadruple therapy with Saccharomyces boulardii or Lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of Helicobacter pylori and treatments adverse effects: A double-blind placebo-controlled randomized clinical trial. BMC Gastroenterol. 2022, 22, 107. [Google Scholar] [CrossRef]
- Vermeersch, S.J.; Vandenplas, Y.; Tanghe, A.; Elseviers, M.; Annemans, L. Economic evaluation of S. boulardii CNCM I-745 for prevention of antibioticassociated diarrhoea in hospitalized patients. Acta Gastro-Enterol. Belg. 2018, 81, 269–276. [Google Scholar]
- Fietto, J.L.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef]
- Hudson, L.E.; McDermott, C.D.; Stewart, T.P.; Hudson, W.H.; Rios, D.; Fasken, M.B.; Corbett, A.H.; Lamb, T.J. Characterization of the Probiotic Yeast Saccharomyces boulardii in the Healthy Mucosal Immune System. PLoS ONE 2016, 11, e0153351. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.E.; Fasken, M.B.; McDermott, C.D.; McBride, S.M.; Kuiper, E.G.; Guiliano, D.B.; Corbett, A.H.; Lamb, T.J. Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii. PLoS ONE 2014, 9, e112660. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Saint Fleur, A.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chang, J.H.; Chang, Y.C.; Mou, K.Y. Treatment of murine colitis by Saccharomyces boulardii secreting atrial natriuretic peptide. J. Mol. Med. 2020, 98, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Yamchi, A.; Rahimi, M.; Javan, B.; Abdollahi, D.; Salmanian, M.; Shahbazi, M. Evaluation of the impact of polypeptide-p on diabetic rats upon its cloning, expression, and secretion in Saccharomyces boulardii. Arch. Microbiol. 2023, 206, 37. [Google Scholar] [CrossRef]
- Hedin, K.A.; Zhang, H.; Kruse, V.; Rees, V.E.; Backhed, F.; Greiner, T.U.; Vazquez-Uribe, R.; Sommer, M.O.A. Cold Exposure and Oral Delivery of GLP-1R Agonists by an Engineered Probiotic Yeast Strain Have Antiobesity Effects in Mice. ACS Synth. Biol. 2023, 12, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Rebeck, O.N.; Wallace, M.J.; Prusa, J.; Ning, J.; Evbuomwan, E.M.; Rengarajan, S.; Habimana-Griffin, L.; Kwak, S.; Zahrah, D.; Tung, J.; et al. A yeast-based oral therapeutic delivers immune checkpoint inhibitors to reduce intestinal tumor burden. Cell Chem. Biol. 2024, 32, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Pronk, J.T. Auxotrophic yeast strains in fundamental and applied research. Appl. Environ. Microbiol. 2002, 68, 2095–2100. [Google Scholar] [CrossRef]
- Gaughan, C.L. The present state of the art in expression, production and characterization of monoclonal antibodies. Mol. Divers. 2016, 20, 255–270. [Google Scholar] [CrossRef]
- Liu, J.J.; Kong, I.I.; Zhang, G.C.; Jayakody, L.N.; Kim, H.; Xia, P.F.; Kwak, S.; Sung, B.H.; Sohn, J.H.; Walukiewicz, H.E.; et al. Metabolic Engineering of Probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 2016, 82, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Durmusoglu, D.; Al’Abri, I.S.; Collins, S.P.; Cheng, J.; Eroglu, A.; Beisel, C.L.; Crook, N. In Situ Biomanufacturing of Small Molecules in the Mammalian Gut by Probiotic Saccharomyces boulardii. ACS Synth. Biol. 2021, 10, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Benatuil, L.; Perez, J.M.; Belk, J.; Hsieh, C.M. An improved yeast transformation method for the generation of very large human antibody libraries. Protein Eng. Des. Sel. 2010, 23, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.M. Dihydrofolate reductase inhibitors as antimicrobial agents and their potentiation by sulfonamides. CRC Crit. Rev. Clin. Lab. Sci. 1970, 1, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Taniguchi, N.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Cocktail delta-integration: A novel method to construct cellulolytic enzyme expression ratio-optimized yeast strains. Microb. Cell Fact. 2010, 9, 32. [Google Scholar] [CrossRef]
- Hamedi, H.; Misaghi, A.; Modarressi, M.H.; Salehi, T.Z.; Khorasanizadeh, D.; Khalaj, V. Generation of a Uracil Auxotroph Strain of the Probiotic Yeast Saccharomyces boulardii as a Host for the Recombinant Protein Production. Avicenna J. Med. Biotechnol. 2013, 5, 29–34. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Nyasae, L.; Lee, R.; Lu, W.; So, E.; Feng, H.; Yang, Z. Development of a Dihydrofolate Reductase Selection System for Saccharomyces boulardii. Int. J. Mol. Sci. 2025, 26, 2073. https://doi.org/10.3390/ijms26052073
Yu H, Nyasae L, Lee R, Lu W, So E, Feng H, Yang Z. Development of a Dihydrofolate Reductase Selection System for Saccharomyces boulardii. International Journal of Molecular Sciences. 2025; 26(5):2073. https://doi.org/10.3390/ijms26052073
Chicago/Turabian StyleYu, Hua, Lydia Nyasae, Rachel Lee, Wenyan Lu, Edward So, Hanping Feng, and Zhiyong Yang. 2025. "Development of a Dihydrofolate Reductase Selection System for Saccharomyces boulardii" International Journal of Molecular Sciences 26, no. 5: 2073. https://doi.org/10.3390/ijms26052073
APA StyleYu, H., Nyasae, L., Lee, R., Lu, W., So, E., Feng, H., & Yang, Z. (2025). Development of a Dihydrofolate Reductase Selection System for Saccharomyces boulardii. International Journal of Molecular Sciences, 26(5), 2073. https://doi.org/10.3390/ijms26052073





