Bitter Taste Receptors 38 and 46 Regulate Intestinal Peristalsis
Abstract
1. Introduction
2. Results
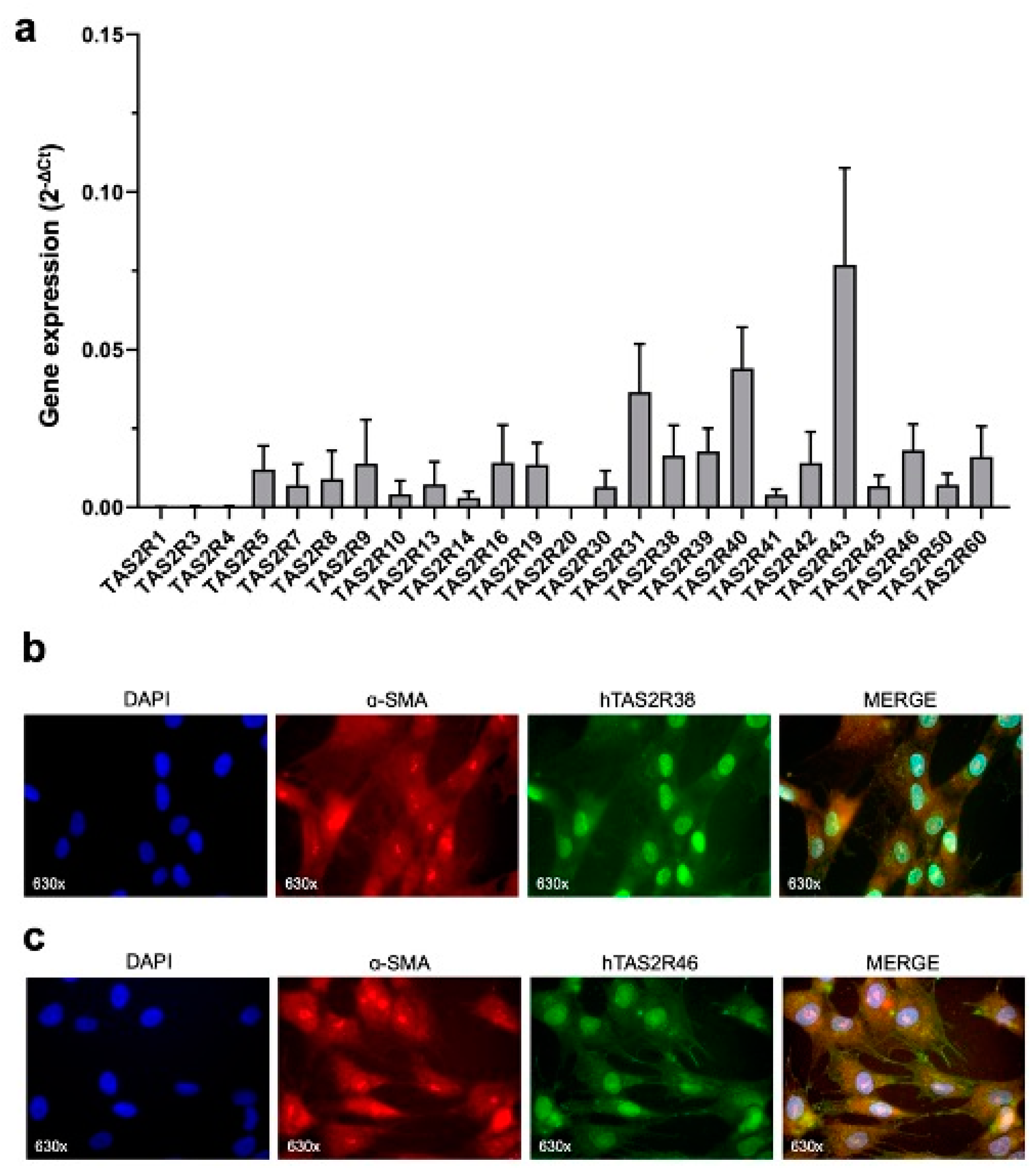
2.1. TAS2R38 and TAS2R46 Expression in HISMCs
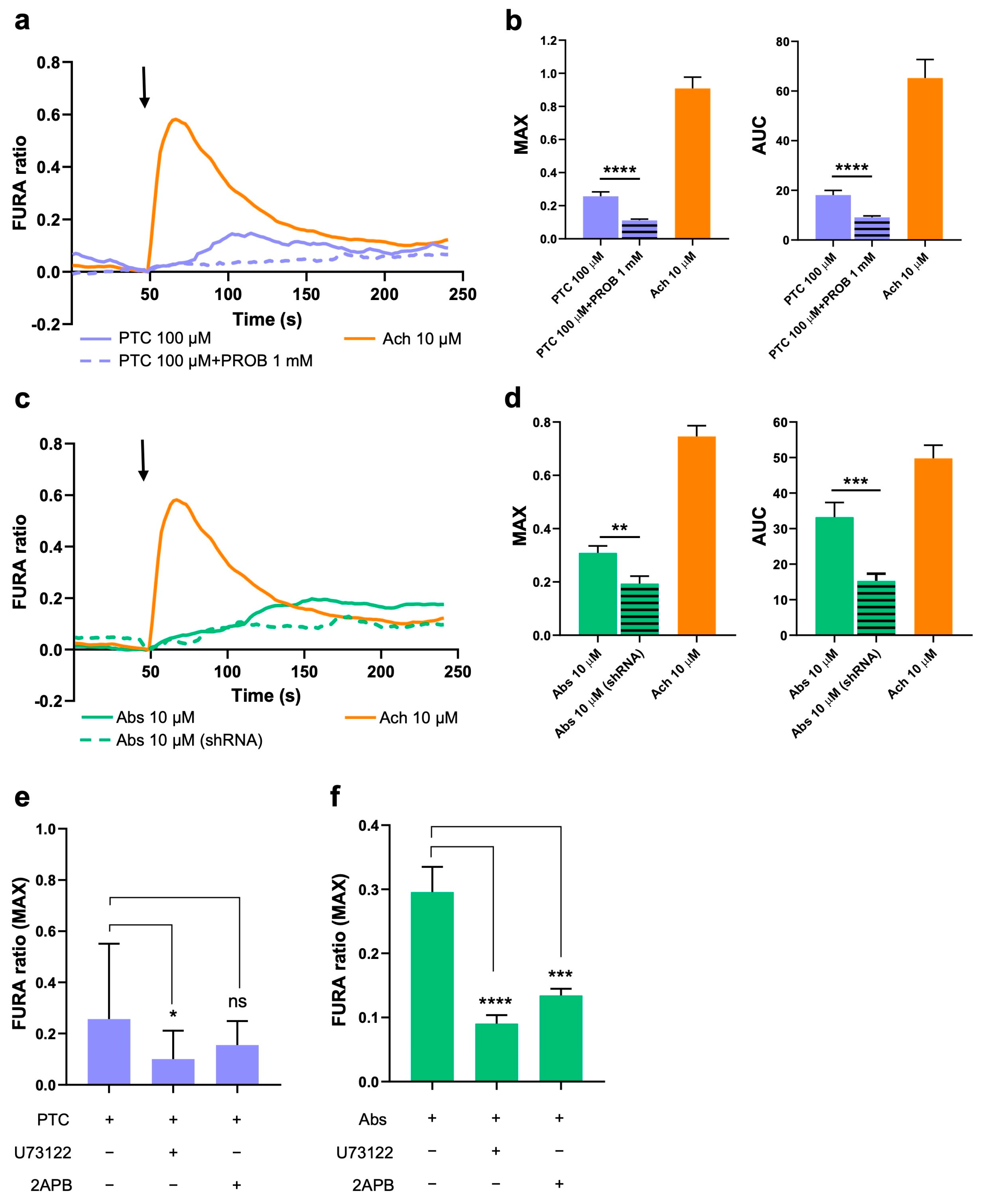
2.2. Activation of TAS2R38 and TAS2R46 Induces Ca2+ Increase in the Cytoplasm
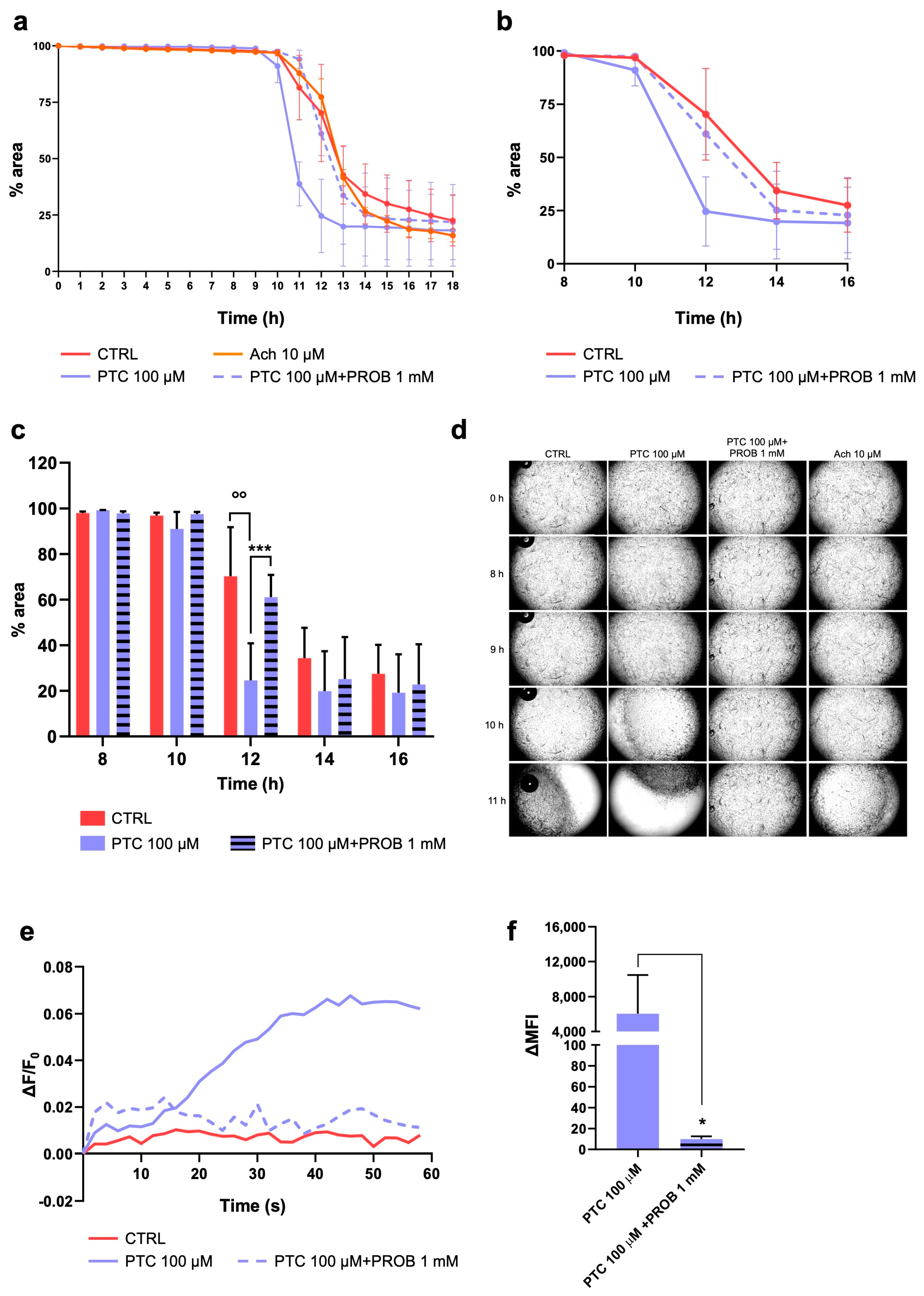
2.3. TAS2R38 Activation Induces HISMCs Contraction and Membrane Depolarization
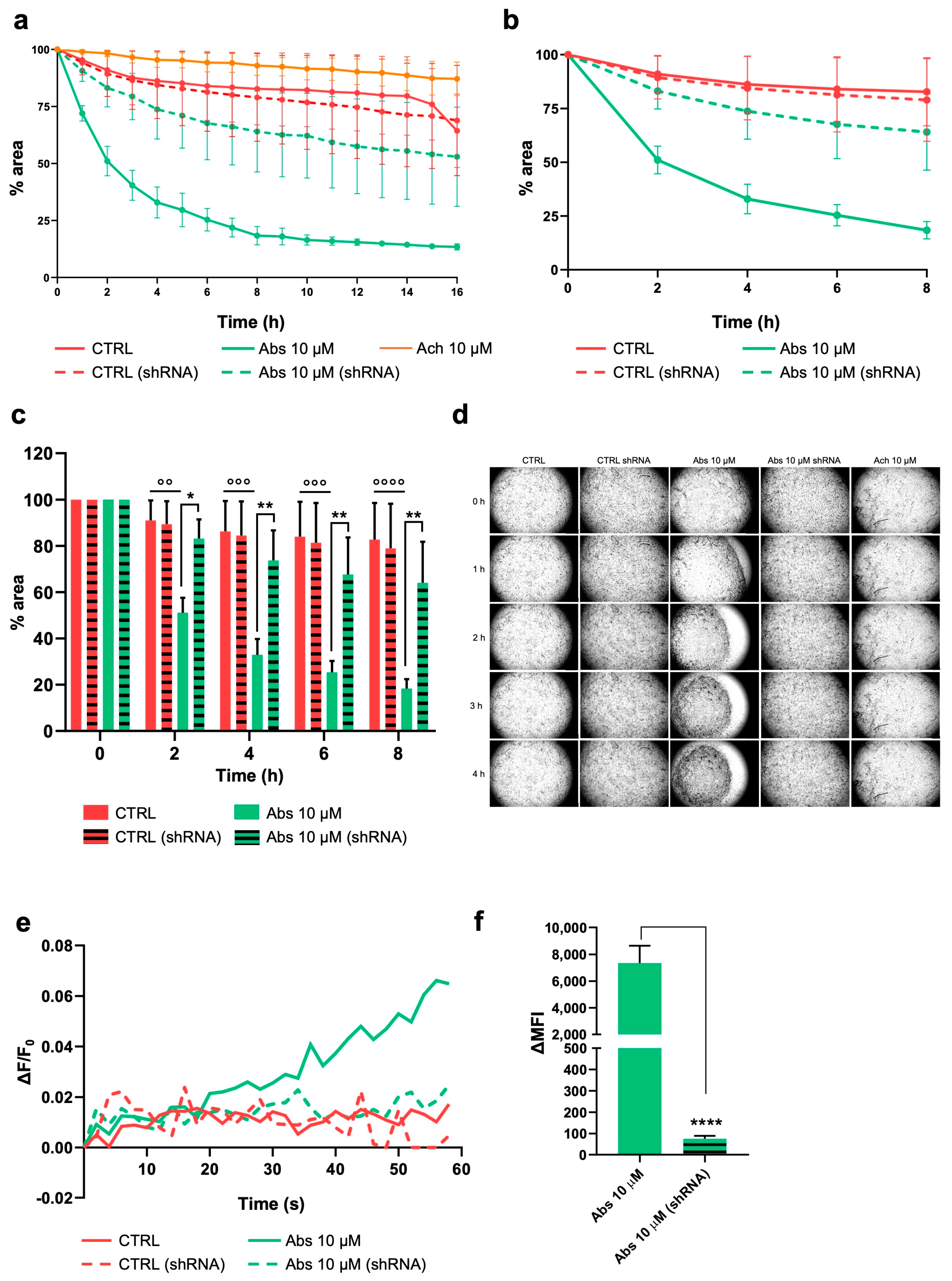
2.4. Activation of TAS2R46 Triggers Rapid Cell Contraction and Membrane Depolarization
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Stimulation
4.3. TAS2R46 Silencing
4.4. Quantitative Real-Time PCR
4.5. Indirect Immunofluorescence
4.6. Contraction Assay
4.7. Calcium Imaging
4.8. Membrane Potential Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abs | absinthin |
| Ach | acetylcholine |
| HISMC | human intestinal smooth muscle cell |
| PROB | probenecid |
| PTC | phenylthiocarbamide |
| SMCM | smooth muscle cell medium |
| TAS2R | bitter taste receptor type 2 |
| [Ca2+]c | cytosolic calcium concentration |
References
- Ji, M.; Su, X.; Su, X.; Chen, Y.; Huang, W.; Zhang, J.; Gao, Z.; Li, C.; Lu, X. Identification of Novel Compounds for Human Bitter Taste Receptors. Chem. Biol. Drug Des. 2014, 84, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Mammalian Bitter Taste Perception. In Chemosensory Systems in Mammals, Fishes, and Insects; Korsching, S., Meyerhof, W., Eds.; Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2009; Volume 47, pp. 77–96. ISBN 978-3-540-69918-7. [Google Scholar]
- Lush, I.E.; Hornigold, N.; King, P.; Stoye, J.P. The Genetics of Tasting in Mice. VII. Glycine Revisited, and the Chromosomal Location of Sac and Soa. Genet. Res. 1995, 66, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter Taste Receptors: Genes, Evolution and Health. Evol. Med. Public Health 2021, 9, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Gustatory and Extragustatory Functions of Mammalian Taste Receptors. Physiol. Behav. 2011, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Vrontakis, M.; Parkinson, F.; Chelikani, P. Functional Bitter Taste Receptors Are Expressed in Brain Cells. Biochem. Biophys. Res. Commun. 2011, 406, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, M. Depletion of Bitter Taste Transduction Leads to Massive Spermatid Loss in Transgenic Mice. Mol. Hum. Reprod. 2012, 18, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Moore Simas, T.A.; Delpapa, E.; ZhuGe, R. Bitter Taste Receptors in the Reproductive System: Function and Therapeutic Implications. J. Cell. Physiol. 2024, 239, e31179. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.K.; Bigliardi, P.L.; Stelmashenko, O.; Ramasamy, S.; Postlethwaite, M.; Bigliardi-Qi, M. Functionally Expressed Bitter Taste Receptor TAS2R14 in Human Epidermal Keratinocytes Serves as a Chemosensory Receptor. Exp. Dermatol. 2021, 30, 216–225. [Google Scholar] [CrossRef]
- Behrens, M.; Lang, T. Extra-Oral Taste Receptors—Function, Disease, and Perspectives. Front. Nutr. 2022, 9, 881177. [Google Scholar] [CrossRef]
- Talmon, M.; Camillo, L.; Vietti, I.; Pollastro, F.; Fresu, L.G. Bitter Taste Receptor 46 (hTAS2R46) Protects Monocytes/Macrophages from Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 7325. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral Bitter Taste Receptors as Mediators of Off-Target Drug Effects. FASEB J. 2012, 26, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Rozengurt, E. Taste Receptors in the Gastrointestinal Tract. I. Bitter Taste Receptors and α-Gustducin in the Mammalian Gut. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G171–G177. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Abrial, C.; Fayad-Kobeissi, S.; Brollo, M.; Faisy, C.; Alvarez, J.-C.; Naline, E.; Devillier, P. The Expression and Relaxant Effect of Bitter Taste Receptors in Human Bronchi. Respir. Res. 2013, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Massara, E.; Pruonto, G.; Quaregna, M.; Boccafoschi, F.; Riva, B.; Fresu, L.G. Characterization of a Functional Ca2+ Toolkit in Urine-Derived Stem Cells and Derived Skeletal Muscle Cells. Cell Calcium 2022, 103, 102548. [Google Scholar] [CrossRef]
- Conaway, S.; Huang, W.; Hernandez-Lara, M.A.; Kane, M.A.; Penn, R.B.; Deshpande, D.A. Molecular Mechanism of Bitter Taste Receptor Agonist-mediated Relaxation of Airway Smooth Muscle. FASEB J. 2024, 38, e23842. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Wang, W.C.H.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S.B. Bitter Taste Receptors on Airway Smooth Muscle Bronchodilate by Localized Calcium Signaling and Reverse Obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Rossi, S.; Lim, D.; Pollastro, F.; Palattella, G.; Ruffinatti, F.A.; Marotta, P.; Boldorini, R.; Genazzani, A.A.; Fresu, L.G. Absinthin, an Agonist of the Bitter Taste Receptor hTAS2R46, Uncovers an ER-to-Mitochondria Ca2+–Shuttling Event. J. Biol. Chem. 2019, 294, 12472–12482. [Google Scholar] [CrossRef]
- Sharma, P.; Conaway, S.; Deshpande, D. Bitter Taste Receptors in the Airway Cells Functions. In The Pharmacology of Taste; Palmer, R.K., Servant, G., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2021; Volume 275, pp. 203–227. ISBN 978-3-031-06449-4. [Google Scholar]
- Upadhyaya, J.D.; Singh, N.; Sikarwar, A.S.; Chakraborty, R.; Pydi, S.P.; Bhullar, R.P.; Dakshinamurti, S.; Chelikani, P. Dextromethorphan Mediated Bitter Taste Receptor Activation in the Pulmonary Circuit Causes Vasoconstriction. PLoS ONE 2014, 9, e110373. [Google Scholar] [CrossRef] [PubMed]
- Medler, K.F. Calcium Signaling in Taste Cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Tuzim, K.; Korolczuk, A. An Update on Extra-Oral Bitter Taste Receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar] [CrossRef]
- Wu, S.V.; Rozengurt, N.; Yang, M.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Expression of Bitter Taste Receptors of the T2R Family in the Gastrointestinal Tract and Enteroendocrine STC-1 Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.-I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 Regulates Gut Peptide Secretion through Intestinal Bitter Taste Receptor Signaling in Mice. J. Clin. Investig. 2008, 118, 3693–3700. [Google Scholar] [CrossRef]
- Janssen, S.; Laermans, J.; Verhulst, P.-J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter Taste Receptors and α-Gustducin Regulate the Secretion of Ghrelin with Functional Effects on Food Intake and Gastric Emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef]
- Chen, M.C.; Wu, S.V.; Reeve, J.R.; Rozengurt, E. Bitter Stimuli Induce Ca2+ Signaling and CCK Release in Enteroendocrine STC-1 Cells: Role of L-Type Voltage-Sensitive Ca2+ Channels. Am. J. Physiol.-Cell Physiol. 2006, 291, C726–C739. [Google Scholar] [CrossRef] [PubMed]
- Bezençon, C.; Fürholz, A.; Raymond, F.; Mansourian, R.; Métairon, S.; Le Coutre, J.; Damak, S. Murine Intestinal Cells Expressing Trpm5 Are Mostly Brush Cells and Express Markers of Neuronal and Inflammatory Cells. J. Comp. Neurol. 2008, 509, 514–525. [Google Scholar] [CrossRef]
- Naveed, A.; Abdullah, S. Impact of Parasitic Infection on Human Gut Ecology and Immune Regulations. Transl. Med. Commun. 2021, 6, 11. [Google Scholar] [CrossRef]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting Extra-Oral Bitter Taste Receptors Modulates Gastrointestinal Motility with Effects on Satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef]
- Feng, P.; Chai, J.; Yi, H.; Redding, K.; Margolskee, R.F.; Huang, L.; Wang, H. Aggravated Gut Inflammation in Mice Lacking the Taste Signaling Protein α-Gustducin. Brain. Behav. Immun. 2018, 71, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Jalševac, F.; Terra, X.; Rodríguez-Gallego, E.; Beltran-Debón, R.; Blay, M.T.; Pinent, M.; Ardévol, A. The Hidden One: What We Know About Bitter Taste Receptor 39. Front. Endocrinol. 2022, 13, 854718. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ro, S.; Ward, S.M. Regulation of Gastrointestinal Motility—Insights from Smooth Muscle Biology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 633–645. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef]
- Chou, W.-L. Therapeutic Potential of Targeting Intestinal Bitter Taste Receptors in Diabetes Associated with Dyslipidemia. Pharmacol. Res. 2021, 170, 105693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, R.; Zhao, Y.; Fang, J.; Li, M.; Kang, S.-G.; Huang, K.; Tong, T. The Gut Odorant Receptor and Taste Receptor Make Sense of Dietary Components: A Focus on Gut Hormone Secretion. Crit. Rev. Food Sci. Nutr. 2024, 64, 6975–6989. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Depoortere, I. The Bitter Truth about Bitter Taste Receptors: Beyond Sensing Bitter in the Oral Cavity. Acta Physiol. 2016, 216, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Anselmi, L.; Rozengurt, E. Enteroendocrine Cells: A Site of ‘Taste’ in Gastrointestinal Chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef]
- Yamazaki, T.; Takahashi, C.; Taniguchi, Y.; Narukawa, M.; Misaka, T.; Ano, Y. Bitter Taste Receptor Activation by Hop-Derived Bitter Components Induces Gastrointestinal Hormone Production in Enteroendocrine Cells. Biochem. Biophys. Res. Commun. 2020, 533, 704–709. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of Sweet, Bitter, and Umami Tastes. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef]
- Wong, G.T.; Gannon, K.S.; Margolskee, R.F. Transduction of Bitter and Sweet Taste by Gustducin. Nature 1996, 381, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Lifshitz, L.M.; Uy, K.F.; Ikebe, M.; Fogarty, K.E.; ZhuGe, R. The Cellular and Molecular Basis of Bitter Tastant-Induced Bronchodilation. PLoS Biol. 2013, 11, e1001501. [Google Scholar] [CrossRef]
- Tazzeo, T.; Bates, G.; Roman, H.N.; Lauzon, A.-M.; Khasnis, M.D.; Eto, M.; Janssen, L.J. Caffeine Relaxes Smooth Muscle through Actin Depolymerization. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L334–L342. [Google Scholar] [CrossRef]
- Zhai, K.; Yang, Z.; Zhu, X.; Nyirimigabo, E.; Mi, Y.; Wang, Y.; Liu, Q.; Man, L.; Wu, S.; Jin, J.; et al. Activation of Bitter Taste Receptors (Tas2rs) Relaxes Detrusor Smooth Muscle and Suppresses Overactive Bladder Symptoms. Oncotarget 2016, 7, 21156–21167. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, M.; Saito, H.; Isono, K.; Goto, T.; Shirakawa, H.; Shoji, N.; Satoh-Kuriwada, S.; Sasano, T.; Okada, R.; Kudoh, K.; et al. Genotyping Analysis of Bitter-Taste Receptor Genes TAS2R38 and TAS2R46 in Japanese Patients with Gastrointestinal Cancers. J. Nutr. Sci. Vitaminol. 2017, 63, 148–154. [Google Scholar] [CrossRef]
- Carrai, M.; Steinke, V.; Vodicka, P.; Pardini, B.; Rahner, N.; Holinski-Feder, E.; Morak, M.; Schackert, H.K.; Görgens, H.; Stemmler, S.; et al. Association Between TAS2R38 Gene Polymorphisms and Colorectal Cancer Risk: A Case-Control Study in Two Independent Populations of Caucasian Origin. PLoS ONE 2011, 6, e20464. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Variations in the Bitterness Perception-Related Genes TAS2R38 and CA6 Modify the Risk for Colorectal Cancer in Koreans. Oncotarget 2017, 8, 21253–21265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vascellari, S.; Melis, M.; Cossu, G.; Melis, M.; Serra, A.; Palmas, V.; Perra, D.; Oppo, V.; Fiorini, M.; Cusano, R.; et al. Genetic Variants of TAS2R38 Bitter Taste Receptor Associate with Distinct Gut Microbiota Traits in Parkinson’s Disease: A Pilot Study. Int. J. Biol. Macromol. 2020, 165, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Robino, A.; Rosso, N.; Guerra, M.; Corleone, P.; Casagranda, B.; Giraudi, P.J.; Tiribelli, C.; Simeth, C.; Monica, F.; La Bianca, M.; et al. Taste Perception and Expression in Stomach of Bitter Taste Receptor Tas2r38 in Obese and Lean Subjects. Appetite 2021, 166, 105595. [Google Scholar] [CrossRef]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 Taste Receptor Polymorphisms Underlie Susceptibility to Upper Respiratory Infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef]
- Jeruzal-Świątecka, J.; Fendler, W.; Pietruszewska, W. Clinical Role of Extraoral Bitter Taste Receptors. Int. J. Mol. Sci. 2020, 21, 5156. [Google Scholar] [CrossRef]
- Robinett, K.S.; Koziol-White, C.J.; Akoluk, A.; An, S.S.; Panettieri, R.A.; Liggett, S.B. Bitter Taste Receptor Function in Asthmatic and Nonasthmatic Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2014, 50, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, A.; Zhang, M.; Zeng, H.; Lu, Y.; Liu, L.; Li, J.; Deng, L. Artesunate Attenuates Airway Resistance in Vivo and Relaxes Airway Smooth Muscle Cells in Vitro via Bitter Taste Receptor-dependent Calcium Signalling. Exp. Physiol. 2019, 104, 231–243. [Google Scholar] [CrossRef]
- Manson, M.L.; Säfholm, J.; Al-Ameri, M.; Bergman, P.; Orre, A.-C.; Swärd, K.; James, A.; Dahlén, S.-E.; Adner, M. Bitter Taste Receptor Agonists Mediate Relaxation of Human and Rodent Vascular Smooth Muscle. Eur. J. Pharmacol. 2014, 740, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.C.; Kobs, A.J.; Kramer, A.; Nyquist, M.; Kuroki, M.T.; Osborn, J.; Lidke, D.S.; Low-Nam, S.T.; Blazar, B.R.; Tolar, J. Bone Marrow Stromal and Vascular Smooth Muscle Cells Have Chemosensory Capacity via Bitter Taste Receptor Expression. PLoS ONE 2013, 8, e58945. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ruppert, A.-L.; Meiners, M.; Poharkar, K.; Liu, S.; Mahmoud, W.; Winterberg, S.; Hartmann, P.; Mermer, P.; Perniss, A.; et al. Bitter Tastants Relax the Mouse Gallbladder Smooth Muscle Independent of Signaling through Tuft Cells and Bitter Taste Receptors. Sci. Rep. 2024, 14, 18447. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Massara, E.; Quaregna, M.; De Battisti, M.; Boccafoschi, F.; Lecchi, G.; Puppo, F.; Bettega Cajandab, M.A.; Salamone, S.; Bovio, E.; et al. Bitter Taste Receptor (TAS2R) 46 in Human Skeletal Muscle: Expression and Activity. Front. Pharmacol. 2023, 14, 1205651. [Google Scholar] [CrossRef] [PubMed]
- Bhanarkar, J.; Singh, R.; Siddhanathi, A.U.R.; Avvari, R.K. Small Intestinal Peristalsis: Biomechanics and Clinical Prominence of Digestion. In Advances in Mechatronics and Mechanical Engineering; Pain, P., Banerjee, S., Bose, G.K., Eds.; IGI Global: Hershey, PA, USA, 2022; pp. 179–197. ISBN 978-1-7998-9078-2. [Google Scholar]
- Avvari, R.K. Role of Segmental Contraction in the Small Intestinal Digestion: A Computational Approach to Study the Physics behind the Luminal Mixing and Transport. J. Theor. Biol. 2023, 561, 111418. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Lammers, W.J.E.P. Gut Peristalsis Is Governed by a Multitude of Cooperating Mechanisms. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G1–G8. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; Govender, D. Lymphocytic and Collagenous Colitis: An Overview of so-Called Microscopic Colitis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Drumm, B.T.; Cobine, C.A.; Baker, S.A. Ca2+ Dynamics in Interstitial Cells: Foundational Mechanisms for the Motor Patterns in the Gastrointestinal Tract. Physiol. Rev. 2024, 104, 329–398. [Google Scholar] [CrossRef]
- López-Pingarrón, L.; Almeida, H.; Soria-Aznar, M.; Reyes-Gonzales, M.C.; Rodríguez-Moratinos, A.B.; Muñoz-Hoyos, A.; García, J.J. Interstitial Cells of Cajal and Enteric Nervous System in Gastrointestinal and Neurological Pathology, Relation to Oxidative Stress. Curr. Issues Mol. Biol. 2023, 45, 3552–3572. [Google Scholar] [CrossRef]
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2023, 50, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical Presentation of Crohn’s, Ulcerative Colitis, and Indeterminate Colitis: Symptoms, Extraintestinal Manifestations, and Disease Phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD Immunopathogenesis: A Comprehensive Review of Inflammatory Molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Sun, K.; Sun, S.; Zhang, Y.; Chen, L.; Ni, Y.; Hou, M.; Xu, Z.; Chen, L.; et al. Berberine Ameliorates Dextran Sulfate Sodium -Induced Colitis through Tuft Cells and Bitter Taste Signalling. BMC Biol. 2024, 22, 280. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wölfle, U.; Elsholz, F.; Kersten, A.; Haarhaus, B.; Schumacher, U.; Schempp, C. Expression and Functional Activity of the Human Bitter Taste Receptor TAS2R38 in Human Placental Tissues and JEG-3 Cells. Molecules 2016, 21, 306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camillo, L.; Pollastro, F.; Talmon, M.; Fresu, L.G. Bitter Taste Receptors 38 and 46 Regulate Intestinal Peristalsis. Int. J. Mol. Sci. 2025, 26, 2092. https://doi.org/10.3390/ijms26052092
Camillo L, Pollastro F, Talmon M, Fresu LG. Bitter Taste Receptors 38 and 46 Regulate Intestinal Peristalsis. International Journal of Molecular Sciences. 2025; 26(5):2092. https://doi.org/10.3390/ijms26052092
Chicago/Turabian StyleCamillo, Lara, Federica Pollastro, Maria Talmon, and Luigia Grazia Fresu. 2025. "Bitter Taste Receptors 38 and 46 Regulate Intestinal Peristalsis" International Journal of Molecular Sciences 26, no. 5: 2092. https://doi.org/10.3390/ijms26052092
APA StyleCamillo, L., Pollastro, F., Talmon, M., & Fresu, L. G. (2025). Bitter Taste Receptors 38 and 46 Regulate Intestinal Peristalsis. International Journal of Molecular Sciences, 26(5), 2092. https://doi.org/10.3390/ijms26052092






