Phosphatidylcholine Cytidine Transferase α (CCTα) Affects LD Formation Through Fusion and Lipophagy in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Results
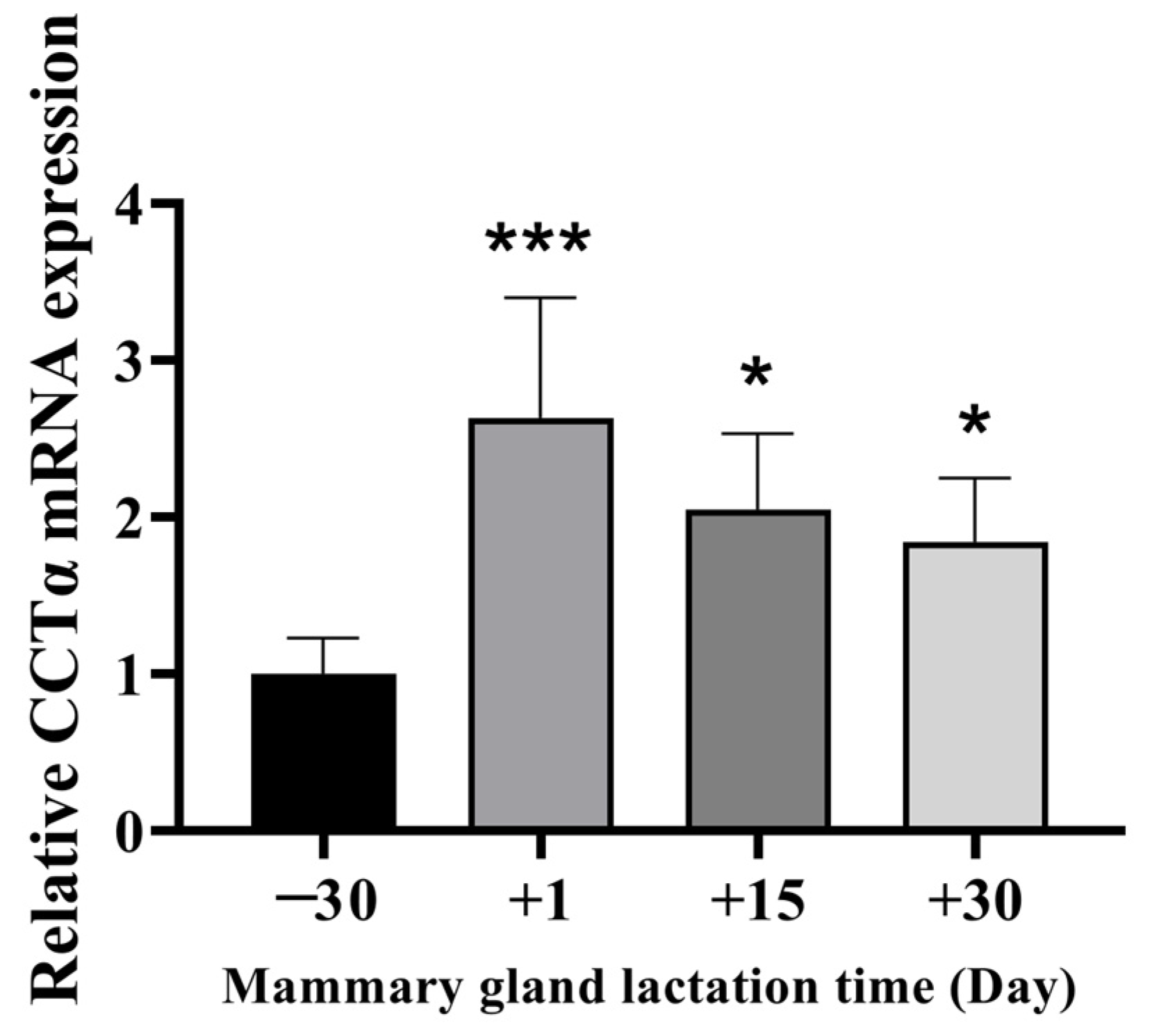
2.1. Expression Levels of CCTα in Bovine Mammary Gland Tissues
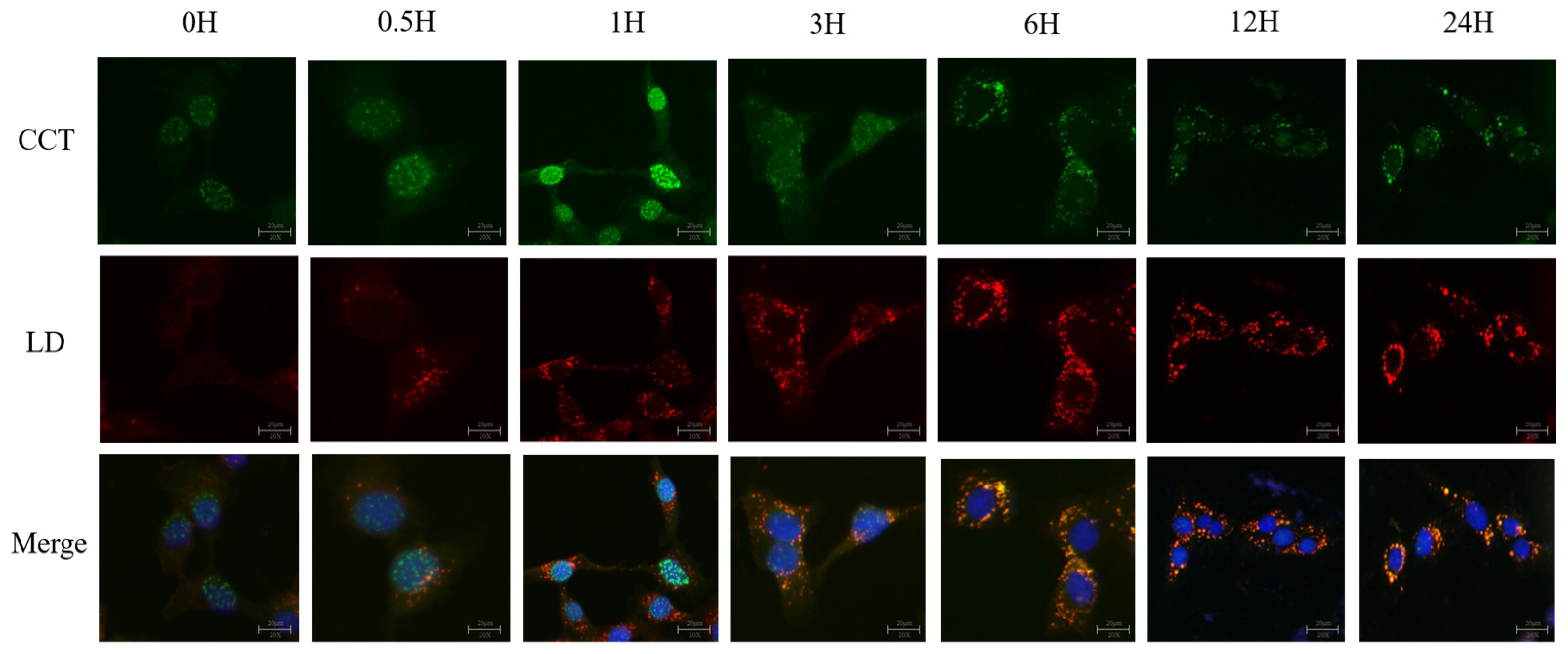
2.2. CCTα Shuttles Between the Nucleus and Cytoplasm
2.3. Construction of CCTα Knockout and Overexpression Cell Lines
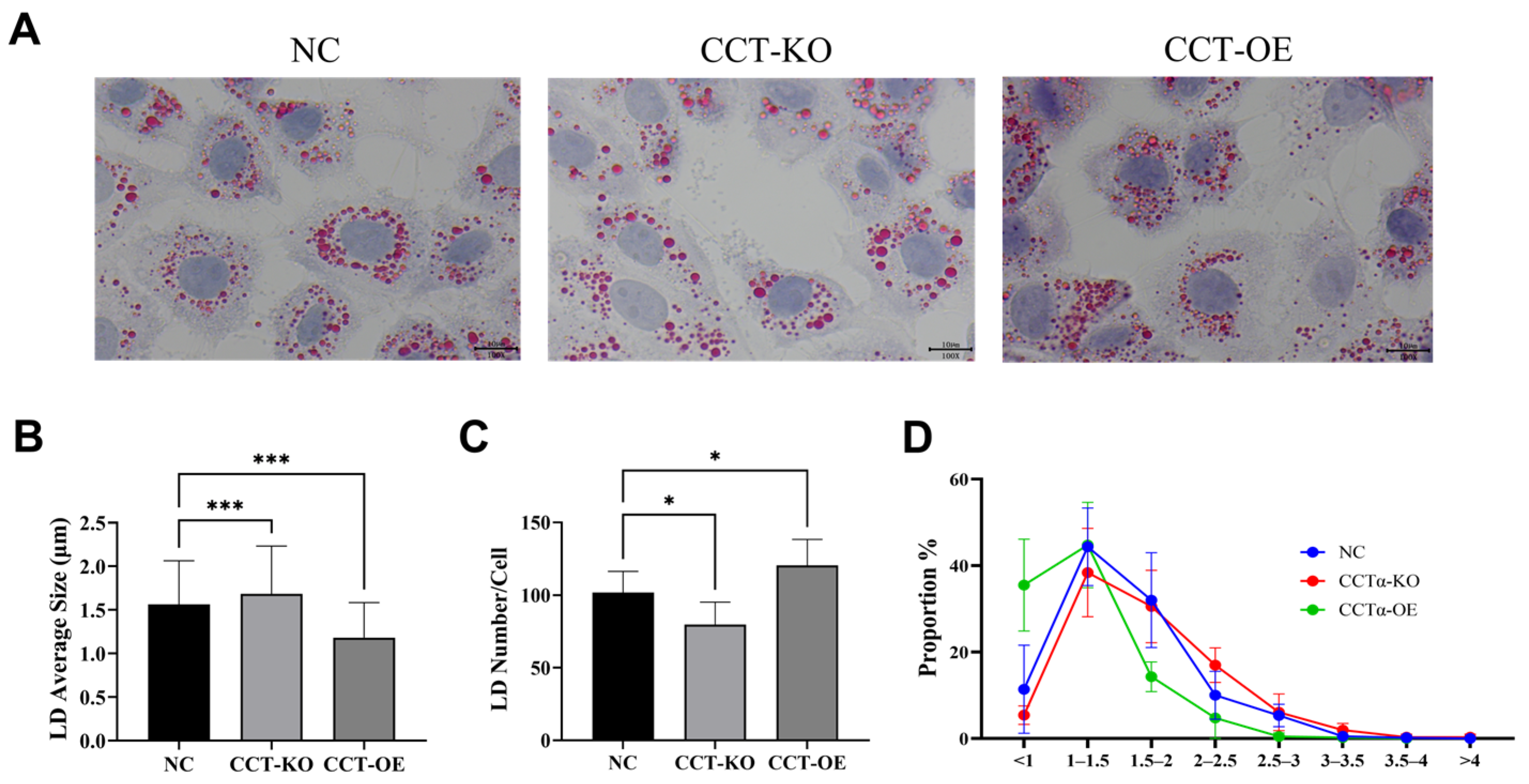
2.4. Effects of CCTα on LD Size and Number
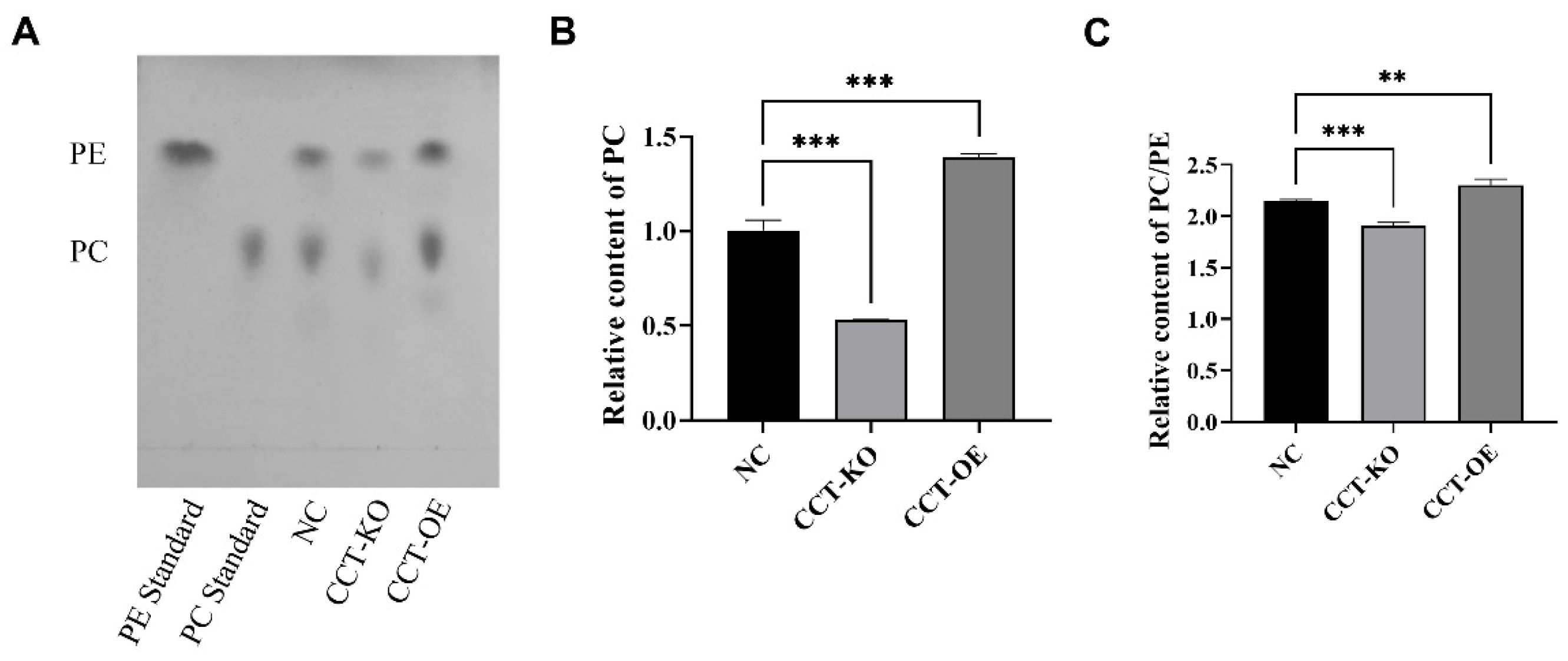
2.5. Effects of CCTα on Phospholipid Synthesis
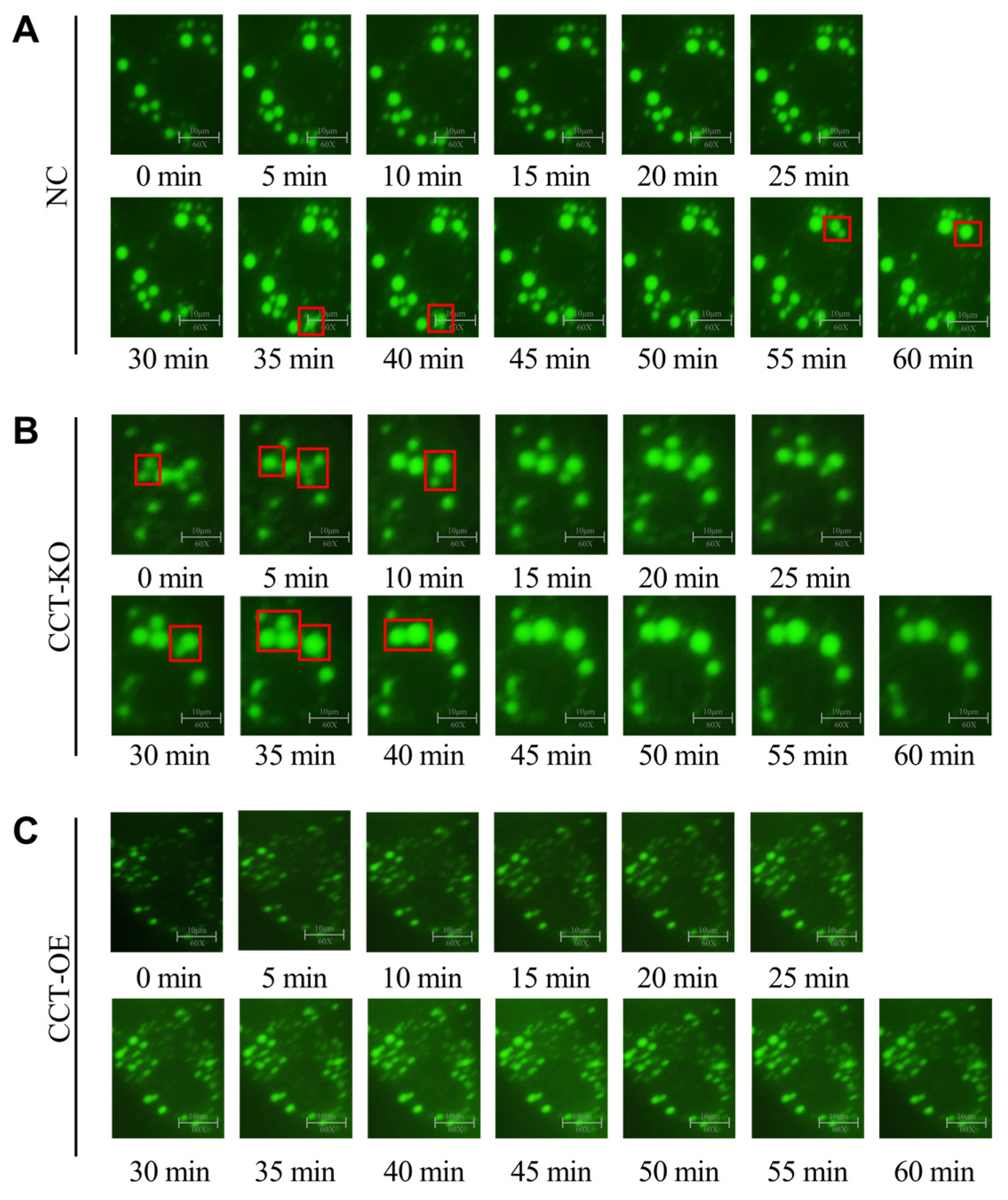
2.6. CCTα Knockout Promotes LD Fusion and Generation of Large LDs
2.7. CCTα Overexpression Enhances Lipophagy
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Bovine Mammary Gland Tissue
4.3. Cell Culture
4.4. Oil Red O Staining
4.5. Immunofluorescence
4.6. Knockout and Overexpression of CCTα
4.7. Western Blotting
4.8. Real-Time Fluorescence Quantification RCR(RT-qPCR)
4.9. Living Cell Observation
4.10. Thin Layer Chromatography
4.11. Transmission Electron Microscopy (TEM)
4.12. Co-Localization of Lysosomes and LDs
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Pressly, J.D.; Gurumani, M.Z.; Varona Santos, J.T.; Fornoni, A.; Merscher, S.; Al-Ali, H. Adaptive and maladaptive roles of lipid droplets in health and disease. Am. J. Physiol. Cell Physiol. 2022, 322, C468–C481. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argaman, N. Symposium review: Milk fat globule size: Practical implications and metabolic regulation. J. Dairy Sci. 2019, 102, 2783–2795. [Google Scholar] [CrossRef]
- Thum, C.; Roy, N.C.; Everett, D.W.; McNabb, W.C. Variation in milk fat globule size and composition: A source of bioactives for human health. Crit. Rev. Food Sci. Nutr. 2023, 63, 87–113. [Google Scholar] [CrossRef]
- Han, L.; Huang, Q.; Yang, J.; Lu, W.; Hu, M.; Yang, Y.; Zhu, H.; Pang, K.; Yang, G. Proteomic analysis of milk fat globule membranes from small-sized milk fat globules and their function in promoting lipid droplet fusion in bovine mammary epithelial cells. Food Funct. 2023, 14, 2304–2312. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, T.; Huang, Q.; Xing, Z.; Yang, J.; Lu, W.; Hu, M.; Han, L.Q.; Loor, J.J.; Gao, T.Y. Size, number and phospholipid composition of milk fat globules are affected by dietary conjugated linoleic acid. J. Anim. Physiol. Anim. Nutr. 2023, 107, 995–1005. [Google Scholar] [CrossRef]
- Tzirkel-Hancock, N.; Sharabi, L.; Argov-Argaman, N. Milk fat globule size: Unraveling the intricate relationship between metabolism, homeostasis, and stress signaling. Biochimie 2023, 215, 4–11. [Google Scholar] [CrossRef]
- Hörl, G.; Wagner, A.; Cole, L.K.; Malli, R.; Reicher, H.; Kotzbeck, P.; Köfeler, H.; Höfler, G.; Frank, S.; Bogner-Strauss, J.G.; et al. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J. Biol. Chem. 2011, 286, 17338–17350. [Google Scholar] [CrossRef]
- Suzuki, M.; Shinohara, Y.; Ohsaki, Y.; Fujimoto, T. Lipid droplets: Size matters. J. Electron. Microsc. 2011, 60 (Suppl. S1), S101–S116. [Google Scholar] [CrossRef]
- Shi, X.; Li, J.; Zou, X.; Greggain, J.; Rødkær, S.V.; Færgeman, N.J.; Liang, B.; Watts, J.L. Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J. Lipid Res. 2013, 54, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Walther, T.C.; Rao, M.; Stuurman, N.; Goshima, G.; Terayama, K.; Wong, J.S.; Vale, R.D.; Walter, P.; Farese, R.V. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 2008, 453, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Aitchison, A.J.; Arsenault, D.J.; Ridgway, N.D. Nuclear-localized CTP:phosphocholine cytidylyltransferase α regulates phosphatidylcholine synthesis required for lipid droplet biogenesis. Mol. Biol. Cell 2015, 26, 2927–2938. [Google Scholar] [CrossRef]
- Lee, J.; Ridgway, N.D. Phosphatidylcholine synthesis regulates triglyceride storage and chylomicron secretion by Caco2 cells. J. Lipid Res. 2018, 59, 1940–1950. [Google Scholar] [CrossRef]
- Schott, M.B.; Weller, S.G.; Schulze, R.J.; Krueger, E.W.; Drizyte-Miller, K.; Casey, C.A.; McNiven, M.A. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 2019, 218, 3320–3335. [Google Scholar] [CrossRef]
- Han, L.; Zhang, M.; Xing, Z.; Coleman, D.N.; Liang, Y.; Loor, J.J.; Yang, G. Knockout of butyrophilin subfamily 1 member A1 (BTN1A1) alters lipid droplet formation and phospholipid composition in bovine mammary epithelial cells. J. Anim. Sci. Biotechnol. 2020, 11, 72. [Google Scholar] [CrossRef]
- Hashemi, H.F.; Goodman, J.M. The life cycle of lipid droplets. Curr. Opin. Cell Biol. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Tauchi-Sato, K.; Ozeki, S.; Houjou, T.; Taguchi, R.; Fujimoto, T. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J. Biol. Chem. 2002, 277, 44507–44512. [Google Scholar] [CrossRef]
- Jackowski, S.; Fagone, P. CTP:Phosphocholine cytidylyltransferase: Paving the way from gene to membrane. J. Biol. Chem. 2005, 280, 853–856. [Google Scholar] [CrossRef]
- Haider, A.; Wei, Y.C.; Lim, K.; Barbosa, A.D.; Liu, C.H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress. Dev. Cell 2018, 45, 481–495.e8. [Google Scholar] [CrossRef] [PubMed]
- Lagace, T.A.; Ridgway, N.D. The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum. Mol. Biol. Cell 2005, 16, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, K.; Morton, C.C.; Ridgway, N.D. Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain. J. Lipid Res. 2009, 50, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef]
- Payne, F.; Lim, K.; Girousse, A.; Brown, R.J.; Kory, N.; Robbins, A.; Xue, Y.; Sleigh, A.; Cochran, E.; Adams, C.; et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc. Natl. Acad. Sci. USA 2014, 111, 8901–8906. [Google Scholar] [CrossRef]
- Cornell, R.B.; Ridgway, N.D. CTP:phosphocholine cytidylyltransferase: Function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis. Prog. Lipid Res. 2015, 59, 147–171. [Google Scholar] [CrossRef]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef]
- Deevska, G.M.; Nikolova-Karakashian, M.N. The expanding role of sphingolipids in lipid droplet biogenesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862 Pt B, 1155–1165. [Google Scholar] [CrossRef]
- Bartz, R.; Li, W.H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar] [CrossRef]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- Cohen, B.C.; Raz, C.; Shamay, A.; Argov-Argaman, N. Lipid Droplet Fusion in Mammary Epithelial Cells is Regulated by Phosphatidylethanolamine Metabolism. J. Mammary Gland. Biol. Neoplasia 2017, 22, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.C.; Shamay, A.; Argov-Argaman, N. Regulation of lipid droplet size in mammary epithelial cells by remodeling of membrane lipid composition-a potential mechanism. PLoS ONE 2015, 10, e0121645. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Walter, L.; Narayana, V.K.; Fry, R.; Logan, A.; Tull, D.; Leury, B. Milk fat globule size development in the mammary epithelial cell: A potential role for ether phosphatidylethanolamine. Sci. Rep. 2020, 10, 12299. [Google Scholar] [CrossRef]
- Gong, J.; Sun, Z.; Wu, L.; Xu, W.; Schieber, N.; Xu, D.; Shui, G.; Yang, H.; Parton, R.G.; Li, P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 2011, 195, 953–963. [Google Scholar] [CrossRef]
- Walker, A.K.; Jacobs, R.L.; Watts, J.L.; Rottiers, V.; Jiang, K.; Finnegan, D.M.; Shioda, T.; Hansen, M.; Yang, F.; Niebergall, L.J.; et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 2011, 147, 840–852. [Google Scholar] [CrossRef]
- Fei, W.; Shui, G.; Zhang, Y.; Krahmer, N.; Ferguson, C.; Kapterian, T.S.; Lin, R.C.; Dawes, I.W.; Brown, A.J.; Li, P.; et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011, 7, e1002201. [Google Scholar] [CrossRef]
- Mastoridou, E.M.; Goussia, A.C.; Kanavaros, P.; Charchanti, A.V. Involvement of Lipophagy and Chaperone-Mediated Autophagy in the Pathogenesis of Non-Alcoholic Fatty Liver Disease by Regulation of Lipid Droplets. Int. J. Mol. Sci. 2023, 24, 15891. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Liu, J.; He, G.; Zheng, H.; Yang, L.; et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022, 13, 132. [Google Scholar] [CrossRef]
- Orii, M.; Tsuji, T.; Ogasawara, Y.; Fujimoto, T. Transmembrane phospholipid translocation mediated by Atg9 is involved in autophagosome formation. J. Cell Biol. 2021, 220, e202009194. [Google Scholar] [CrossRef]
- Andrejeva, G.; Gowan, S.; Lin, G.; Wong Te Fong, A.L.; Shamsaei, E.; Parkes, H.G.; Mui, J.; Raynaud, F.I.; Asad, Y.; Vizcay-Barrena, G.; et al. De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy 2020, 16, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Vevea, J.D.; Garcia, E.J.; Chan, R.B.; Zhou, B.; Schultz, M.; Di Paolo, G.; McCaffery, J.M.; Pon, L.A. Role for Lipid Droplet Biogenesis and Microlipophagy in Adaptation to Lipid Imbalance in Yeast. Dev. Cell 2015, 35, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Han, L.Q.; Zhou, Z.; Ma, Y.; Batistel, F.; Osorio, J.S.; Loor, J.J. Phosphorylation of nuclear factor erythroid 2-like 2 (NFE2L2) in mammary tissue of Holstein cows during the periparturient period is associated with mRNA abundance of antioxidant gene networks. J. Dairy. Sci. 2018, 101, 6511–6522. [Google Scholar] [CrossRef] [PubMed]
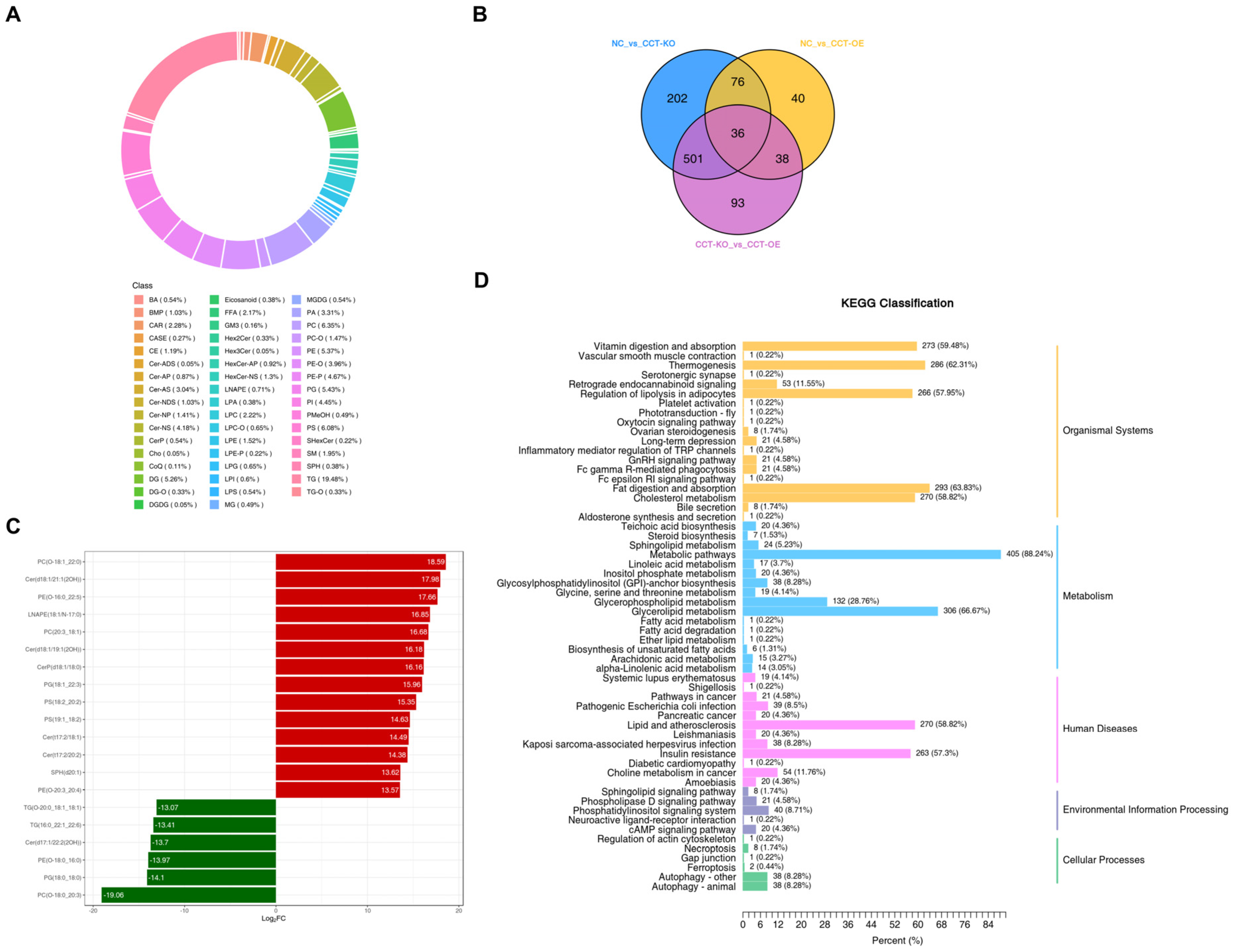

| Group Name | All Significance Difference Lipids | Down | Up |
|---|---|---|---|
| CCT-KO vs. CCT-OE | 688 | 594 | 74 |
| NC vs. CCT-KO | 815 | 48 | 767 |
| NC vs. CCT-OE | 190 | 50 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fan, Y.; Kang, F.; Yang, Y.; Wang, Y.; Liu, Y.; Han, L. Phosphatidylcholine Cytidine Transferase α (CCTα) Affects LD Formation Through Fusion and Lipophagy in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2025, 26, 2135. https://doi.org/10.3390/ijms26052135
Yang J, Fan Y, Kang F, Yang Y, Wang Y, Liu Y, Han L. Phosphatidylcholine Cytidine Transferase α (CCTα) Affects LD Formation Through Fusion and Lipophagy in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences. 2025; 26(5):2135. https://doi.org/10.3390/ijms26052135
Chicago/Turabian StyleYang, Jingna, Yuxin Fan, Fangyuan Kang, Yanbin Yang, Yueying Wang, Yang Liu, and Liqiang Han. 2025. "Phosphatidylcholine Cytidine Transferase α (CCTα) Affects LD Formation Through Fusion and Lipophagy in Bovine Mammary Epithelial Cells" International Journal of Molecular Sciences 26, no. 5: 2135. https://doi.org/10.3390/ijms26052135
APA StyleYang, J., Fan, Y., Kang, F., Yang, Y., Wang, Y., Liu, Y., & Han, L. (2025). Phosphatidylcholine Cytidine Transferase α (CCTα) Affects LD Formation Through Fusion and Lipophagy in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences, 26(5), 2135. https://doi.org/10.3390/ijms26052135





