Bone and Hemophilia: The Role of Factor VIII—Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
2.2. Identifying Relevant Studies
2.3. Electronic Database Search
- ((“bone and bones”[MeSH Terms] OR (“bone”[All Fields] AND “bones”[All Fields]) OR “bone and bones”[All Fields] OR “bone”[All Fields]) AND (“disease”[MeSH Terms] OR “disease”[All Fields] OR “diseases”[All Fields] OR “disease s”[All Fields] OR “diseased”[All Fields]) AND (“haemophilia”[All Fields] OR “hemophilia a”[MeSH Terms] OR “hemophilia a”[All Fields] OR “hemophilia”[All Fields] OR “haemophilias”[All Fields] OR “hemophilias”[All Fields]) AND (“factor viii”[Supplementary Concept] OR “factor viii”[All Fields] OR “f8 protein human”[Supplementary Concept] OR “f8 protein human”[All Fields] OR “factor viii”[MeSH Terms] OR (“factor”[All Fields] AND “viii”[All Fields]))) AND ((english[Filter]) AND (2012:2023[pdat])) [89];
- AND bone AND disease AND haemophilia AND factor AND VIII AND [2012–2023]/py AND [english]/lim [92].
2.4. Other Sources
2.5. Study Inclusion Criteria
2.6. Data Extraction
2.7. Study Selection
3. Result and Discussion
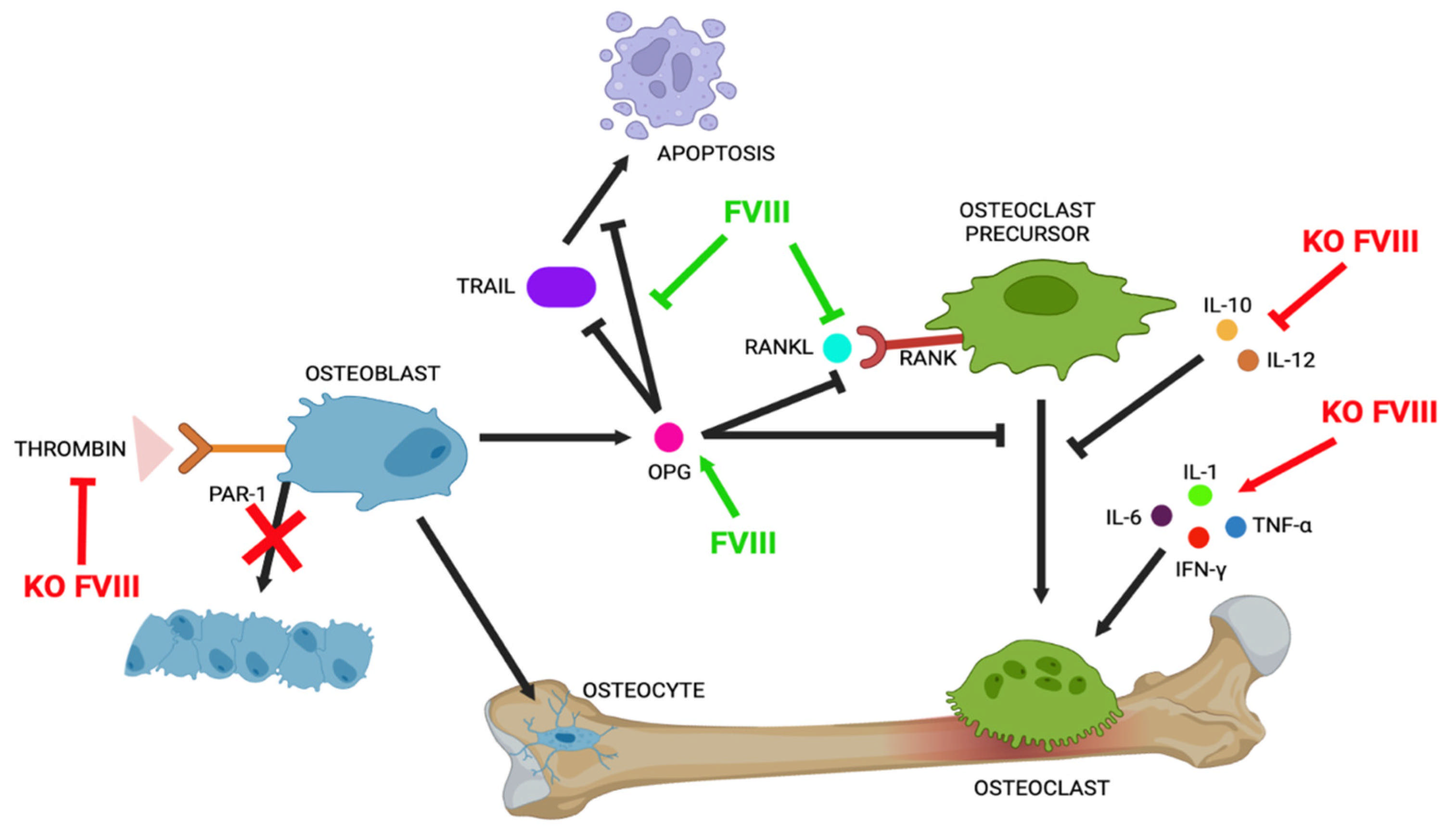
3.1. OPG/RANK/RANKL Pathways in Bone Turnover
3.2. Role of Thrombin/PAR1 in Bone Structure
3.3. Scavenger Receptors in Bone Remodelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Merchan, E.C.; Valentino, L.A. Increased Bone Resorption in Hemophilia. Blood Rev. 2019, 33, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Duplomb, L.; Téletchéa, S.; Charrier, C.; Maillasson, M.; Fouassier, M.; Heymann, D. Factor VIII-von Willebrand Factor Complex Inhibits Osteoclastogenesis and Controls Cell Survival. J. Biol. Chem. 2009, 284, 31704–31713. [Google Scholar] [CrossRef] [PubMed]
- Cadé, M.; Muñoz-Garcia, J.; Babuty, A.; Paré, L.; Cochonneau, D.; Fekir, K.; Chatelais, M.; Heymann, M.-F.; Lokajczyk, A.; Boisson-Vidal, C.; et al. FVIII Regulates the Molecular Profile of Endothelial Cells: Functional Impact on the Blood Barrier and Macrophage Behavior. Cell. Mol. Life Sci. 2022, 79, 145. [Google Scholar] [CrossRef] [PubMed]
- Taves, S.; Sun, J.; Livingston, E.W.; Chen, X.; Amiaud, J.; Brion, R.; Hannah, W.B.; Bateman, T.A.; Heymann, D.; Monahan, P.E. Hemophilia A and B Mice, but Not VWF−/−mice, Display Bone Defects in Congenital Development and Remodeling After Injury. Sci. Rep. 2019, 9, 14428. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.; Liel, M.S.; Turner, R.T.; Klein, R.F.; Taylor, J.A. The Bone Disease Associated with Factor VIII Deficiency in Mice Is Secondary to Increased Bone Resorption. Haemophilia 2013, 19, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Samuelson Bannow, B.; Recht, M.; Négrier, C.; Hermans, C.; Berntorp, E.; Eichler, H.; Mancuso, M.E.; Klamroth, R.; O’Hara, J.; Santagostino, E.; et al. Factor VIII: Long-Established Role in Haemophilia A and Emerging Evidence beyond Haemostasis. Blood Rev. 2019, 35, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tudpor, K.; Van Der Eerden, B.C.J.; Jongwattanapisan, P.; Roelofs, J.J.T.H.; Van Leeuwen, J.P.T.M.; Bindels, R.J.M.; Hoenderop, J.G.J. Thrombin Receptor Deficiency Leads to a High Bone Mass Phenotype by Decreasing the RANKL/OPG Ratio. Bone 2015, 72, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, I.; Iannuzzo, G.; Dell’Aquila, F.; Di Minno, M.N.D. Pathophysiological Role of Synovitis in Hemophilic Arthropathy Development: A Two-Hit Hypothesis. Front. Physiol. 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Goldscheitter, G.; Recht, M.; Sochacki, P.; Manco-Johnson, M.; Taylor, J.A. Biomarkers of Bone Disease in Persons with Haemophilia. Haemophilia 2021, 27, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wallny, T.A.; Scholz, D.T.; Oldenburg, J.; Nicolay, C.; Ezziddin, S.; Pennekamp, P.H.; Stoffel-Wagner, B.; Kraft, C.N. Osteoporosis in Haemophilia? An Underestimated Comorbidity? Haemophilia 2007, 13, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, G.; Damiano, M.L.; Tom, A.; Worman, C.; Schultz, W.; Recht, M.; Stopeck, A.T. Prevalence and Risk Factors Associated with Decreased Bone Mineral Density in Patients with Haemophilia. Haemophilia 2009, 15, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Linari, S.; Montorzi, G.; Bartolozzi, D.; Borderi, M.; Melchiorre, D.; Benelli, M.; Morfini, M. Hypovitaminosis D and Osteopenia/Osteoporosis in a Haemophilia Population: A Study in HCV/HIV or HCV Infected Patients. Haemophilia 2013, 19, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Ofotokun, I. Physiological and Pathophysiological Bone Turnover—Role of the Immune System. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.A.; Taylor, J.A. Factor VIII Plays a Direct Role in Osteoblast Development. Blood 2017, 130 (Suppl. S1), 3661. [Google Scholar] [CrossRef]
- Aronovich, A.; Nur, Y.; Shezen, E.; Rosen, C.; Zlotnikov Klionsky, Y.; Milman, I.; Yarimi, L.; Hagin, D.; Rechavi, G.; Martinowitz, U.; et al. A Novel Role for Factor VIII and Thrombin/PAR1 in Regulating Hematopoiesis and Its Interplay with the Bone Structure. Blood 2013, 122, 2562–2571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haxaire, C.; Hakobyan, N.; Pannellini, T.; Carballo, C.; McIlwain, D.; Mak, T.W.; Rodeo, S.; Acharya, S.; Li, D.; Szymonifka, J.; et al. Blood-Induced Bone Loss in Murine Hemophilic Arthropathy Is Prevented by Blocking the iRhom2/ADAM17/TNF-α Pathway. Blood 2018, 132, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, L.; Schutgens, R.E.G.; van Asbeck, B.S.; Wenting, M.J.; van Veghel, K.; Roosendaal, G.; Biesma, D.H.; Lafeber, F.P.J.G. Identification and Expression of Iron Regulators in Human Synovium: Evidence for Upregulation in Haemophilic Arthropathy Compared to Rheumatoid Arthritis, Osteoarthritis, and Healthy Controls. Haemophilia 2013, 19, e218–e227. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berni, M.; Forlino, A.; Caliogna, L.; De Felice, L.; Di Minno, M.N.D.; Jannelli, E.; Mosconi, M.; Tonelli, F.; Torriani, C.; Pasta, G. Bone and Hemophilia: The Role of Factor VIII—Systematic Review. Int. J. Mol. Sci. 2025, 26, 2172. https://doi.org/10.3390/ijms26052172
Berni M, Forlino A, Caliogna L, De Felice L, Di Minno MND, Jannelli E, Mosconi M, Tonelli F, Torriani C, Pasta G. Bone and Hemophilia: The Role of Factor VIII—Systematic Review. International Journal of Molecular Sciences. 2025; 26(5):2172. https://doi.org/10.3390/ijms26052172
Chicago/Turabian StyleBerni, Micaela, Antonella Forlino, Laura Caliogna, Liliana De Felice, Matteo Nicola Dario Di Minno, Eugenio Jannelli, Mario Mosconi, Francesca Tonelli, Camilla Torriani, and Gianluigi Pasta. 2025. "Bone and Hemophilia: The Role of Factor VIII—Systematic Review" International Journal of Molecular Sciences 26, no. 5: 2172. https://doi.org/10.3390/ijms26052172
APA StyleBerni, M., Forlino, A., Caliogna, L., De Felice, L., Di Minno, M. N. D., Jannelli, E., Mosconi, M., Tonelli, F., Torriani, C., & Pasta, G. (2025). Bone and Hemophilia: The Role of Factor VIII—Systematic Review. International Journal of Molecular Sciences, 26(5), 2172. https://doi.org/10.3390/ijms26052172








