Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds
Abstract
1. Introduction
| Data from the Literature | Data from Our Laboratory | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Structure | Generic Name | IC50 (μM) | Ref. | Number | Structure | Generic Name | IC50 (μM) | Ref. |
| 1 | 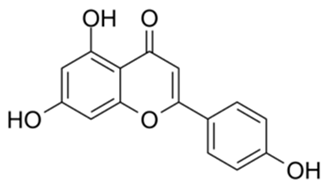 | Apigenin | 84.94 | [25] | 1 | 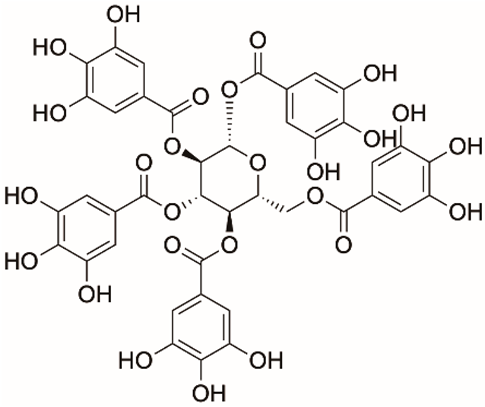 | Pentagalloylglucose | 3.88 | [26] |
| 2 |  | Quercetin | 12.65 | [25] | 2 | 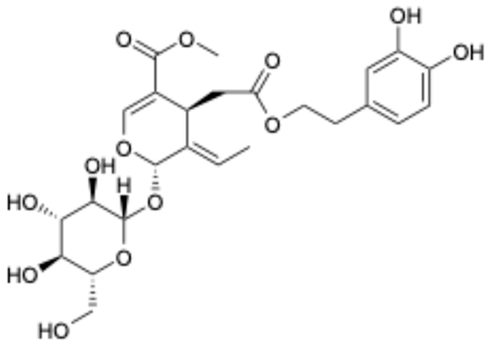 | Oleuropein | 3.50 | |
| 3 | 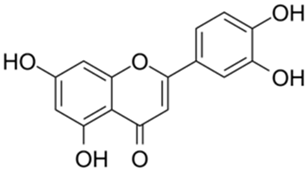 | luteolin | 74.86 | [25] | 3 |  | Methyl rosmarinate | 2.50 | |
| 4 | 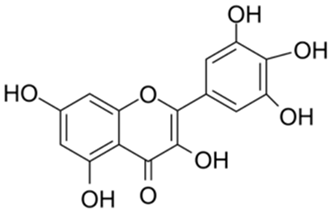 | Myricetin | 0.63 | [27] | 4 | 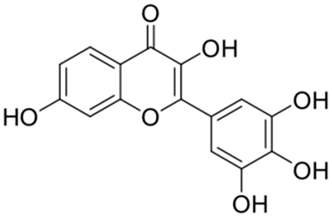 | Robinetin | 0.96 | |
| 5 | 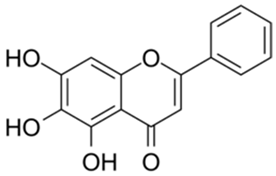 | Baicalein | 0.63 | [27] | 5 | 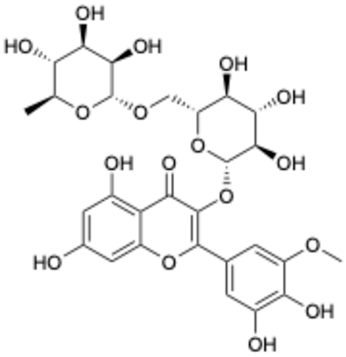 | Laricitrin 3-rutinoside | 3.82 | Unpublished data |
| 6 | 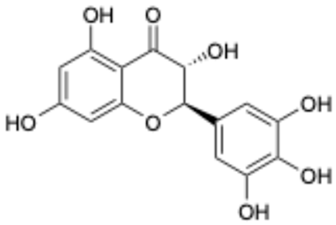 | Dihydromyricetin | 1.14 | [27] | 6 | 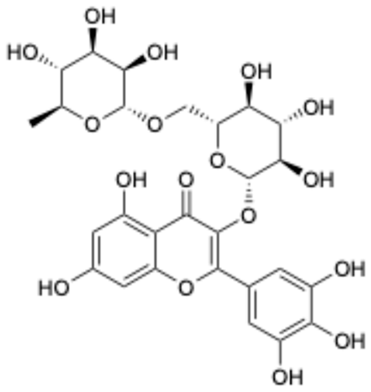 | Myricetin-3-O-rutinoside | 0.85 | |
| 7 | 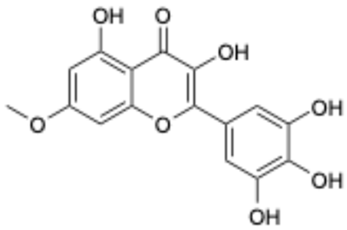 | Compound 3 | 0.30 | [27] | 7 | 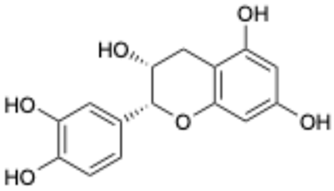 | Epicatechin | 3.30 | |
| 8 | 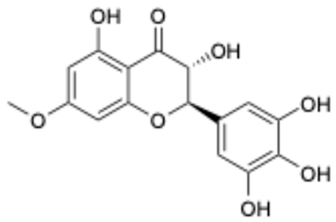 | Compound 7 | 0.26 | [27] | 8 | 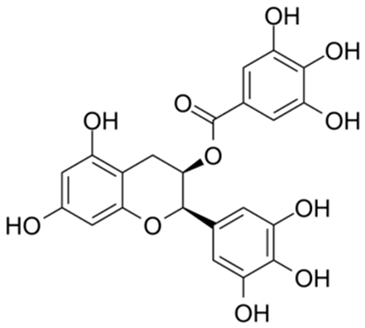 | EGCG | 0.30 | |
| 9 | 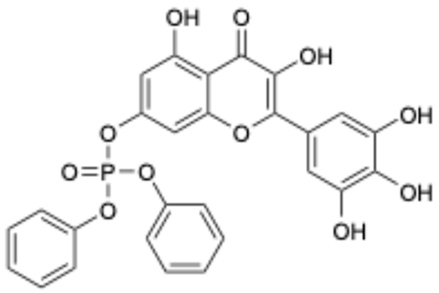 | Compound 9 | 3.13 | [27] | 9 | 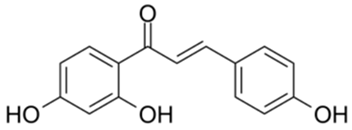 | Isoliquiritigenin | 73.92 | |
| 10 | 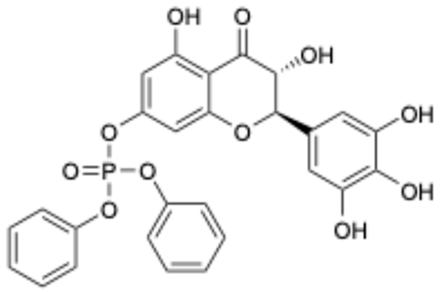 | Compound 10 | 1.84 | [27] | |||||
| Number | Structure | Generic Name | CAS Number | Indications | Phase | Ref. |
|---|---|---|---|---|---|---|
| 1 |  | Exifone | 52479-85-3 | Cognitive problems in Parkinson’s disease | Withdrawal a | [28] |
| 2 | 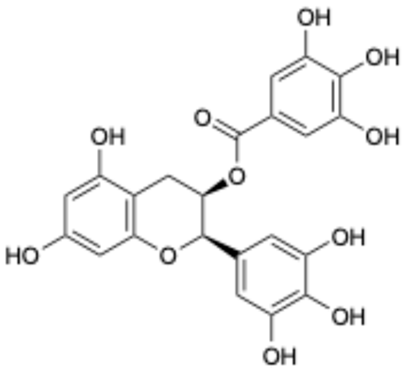 | Epigallocatechin Gallate | 989-51-5 | Multiple sclerosis, Multiple system atrophy, Duchenne muscular dystrophy | Phase II/III | [29,30,31,32] |
| 3 | 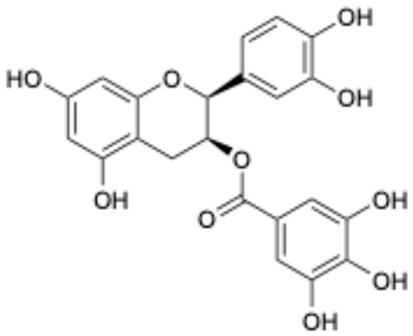 | Epicatechin-3-O-gallate | 1257-08-5 | Prostate cancer prevention, etc. | Phase II | [33,34] |
| 4 | 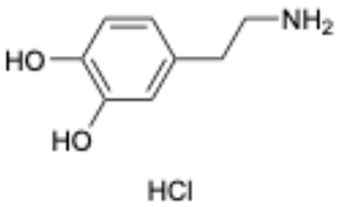 | Dopamine hydrochloride | 62-31-7 | Hypotension, shock, and heart failure | Approved | [35] |
| 5 | 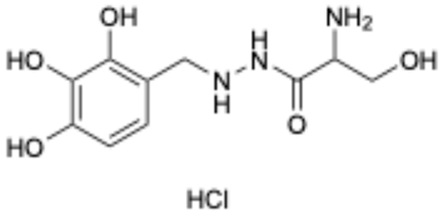 | Benserazide hydrochloride | 4919-77-8 | Parkinson’s disease | Approved | [36,37] |
| 6 |  | Delphinidin-3-glucoside | 6906-38-3 | Hyperlipidemia, etc. | Phase I | [38,39] |
| 7 |  | Delphinidin-3-sambubioside | 53158-73-9 | Hypertension | Phase I | [40] |
| 8 | 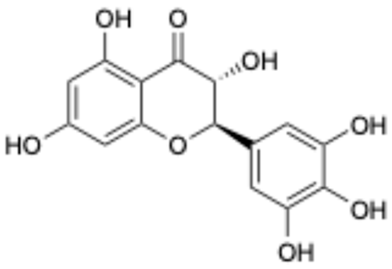 | Dihydromyricetin | 27200-12-0 | Nonalcoholic fatty liver disease | Phase I | [41] |
| 9 | 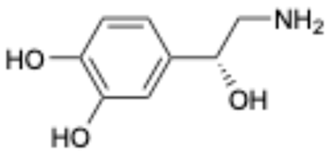 | Norepinephrine | 51-41-2 | Septic shock, etc. | Approved | [42,43] |
2. Results and Analysis
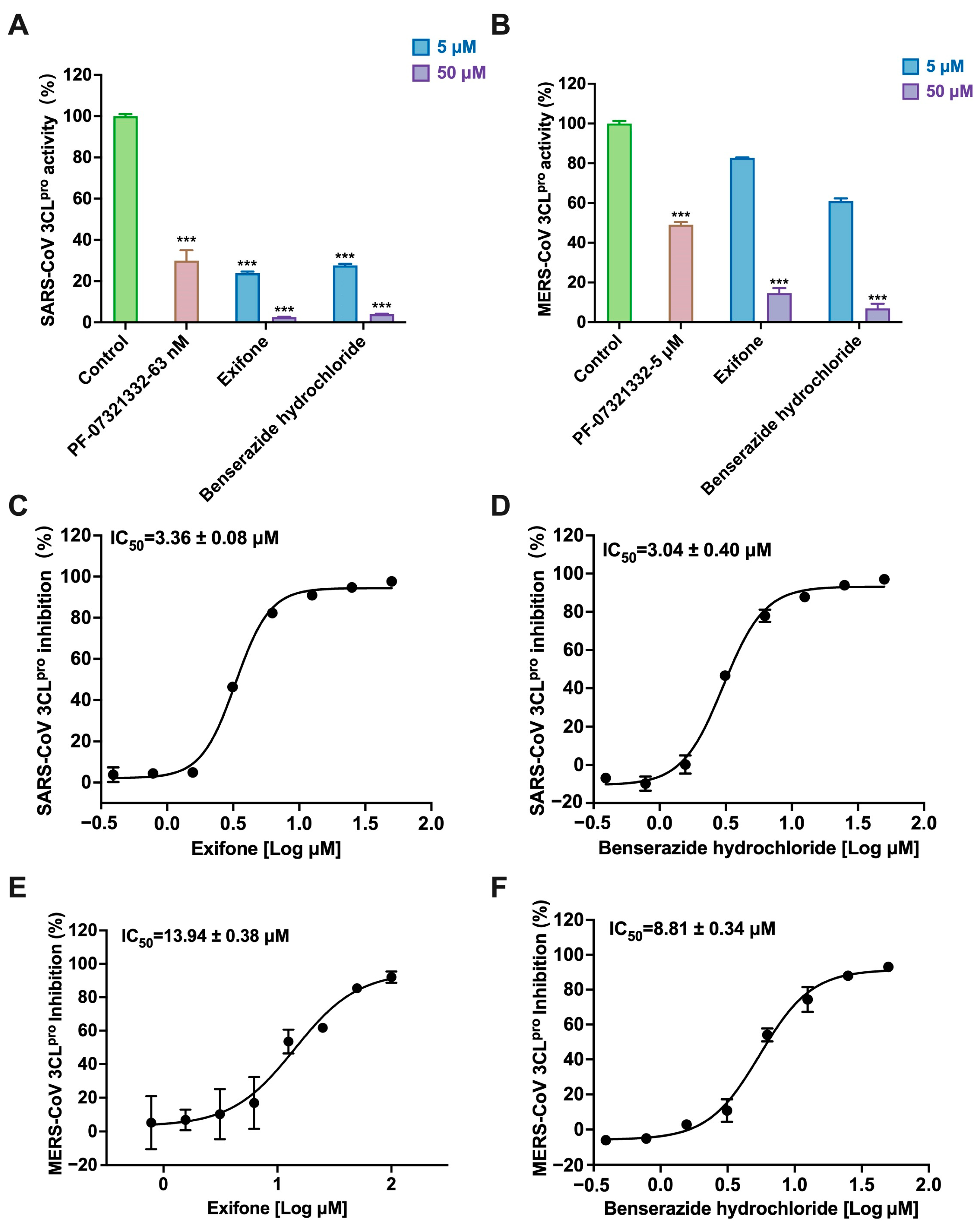
2.1. Inhibition of Five Clinical Drugs Against SARS-CoV-2 3CLpro
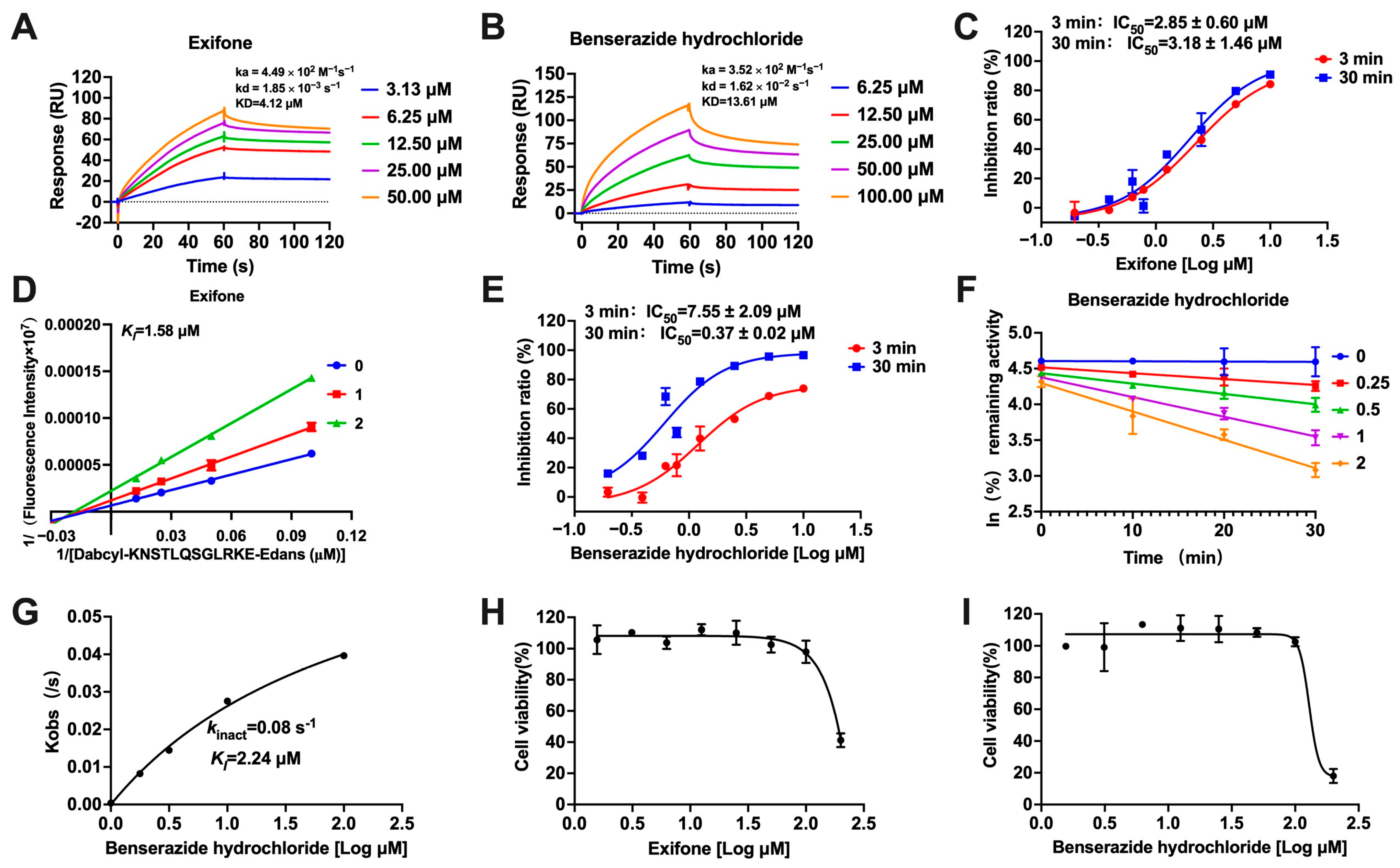
2.2. Mechanisms of Exifone and Benserazide Hydrochloride Against SARS-CoV-2 3CLpro
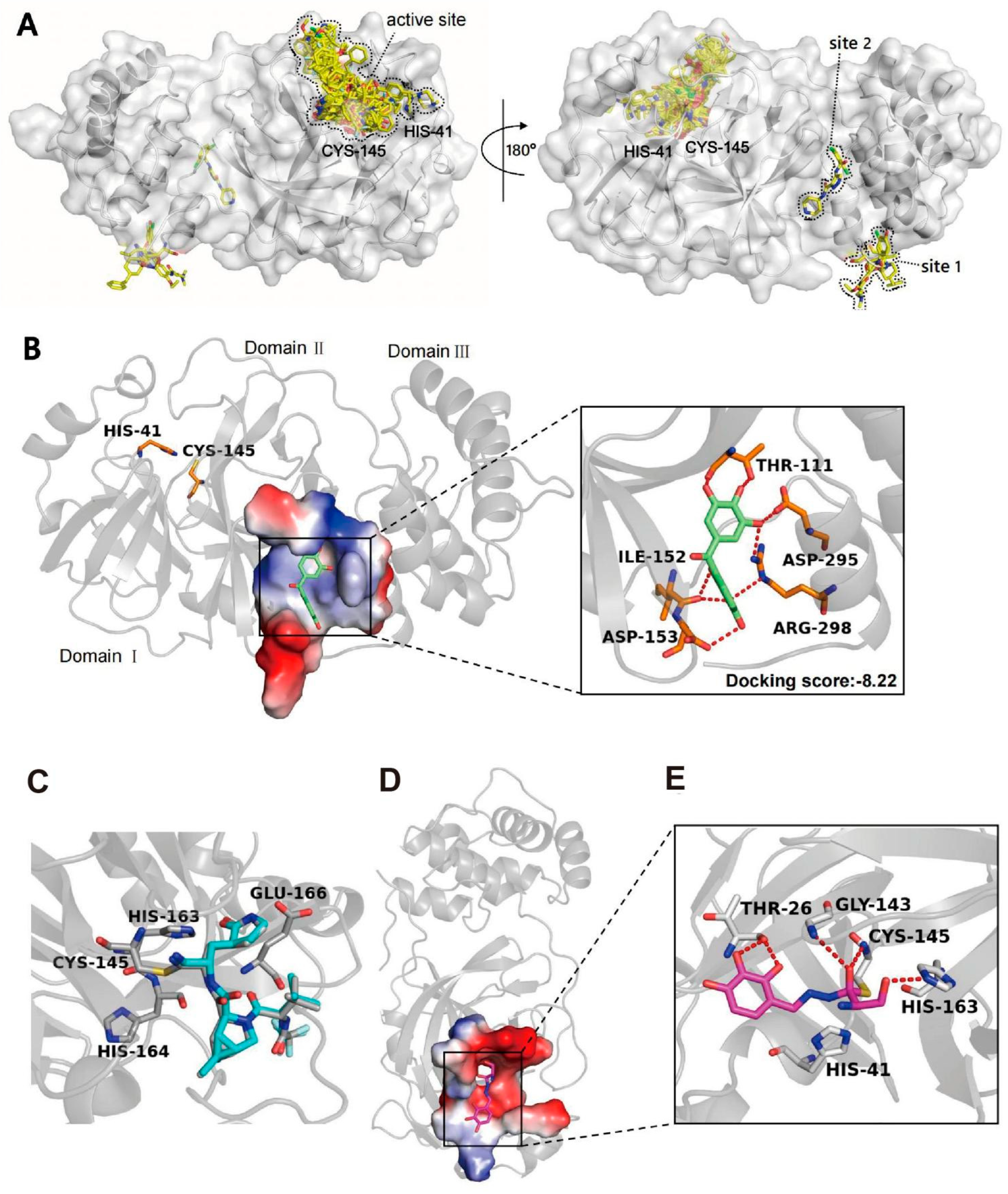
2.3. Binding Modes of Exifone and Benserazide Hydrochloride with SARS-CoV-2 3CLpro
2.4. Exifone and Benserazide Hydrochloride Exhibiting Broad-Spectrum Inhibition Against 3CLpro of Various Coronaviruses
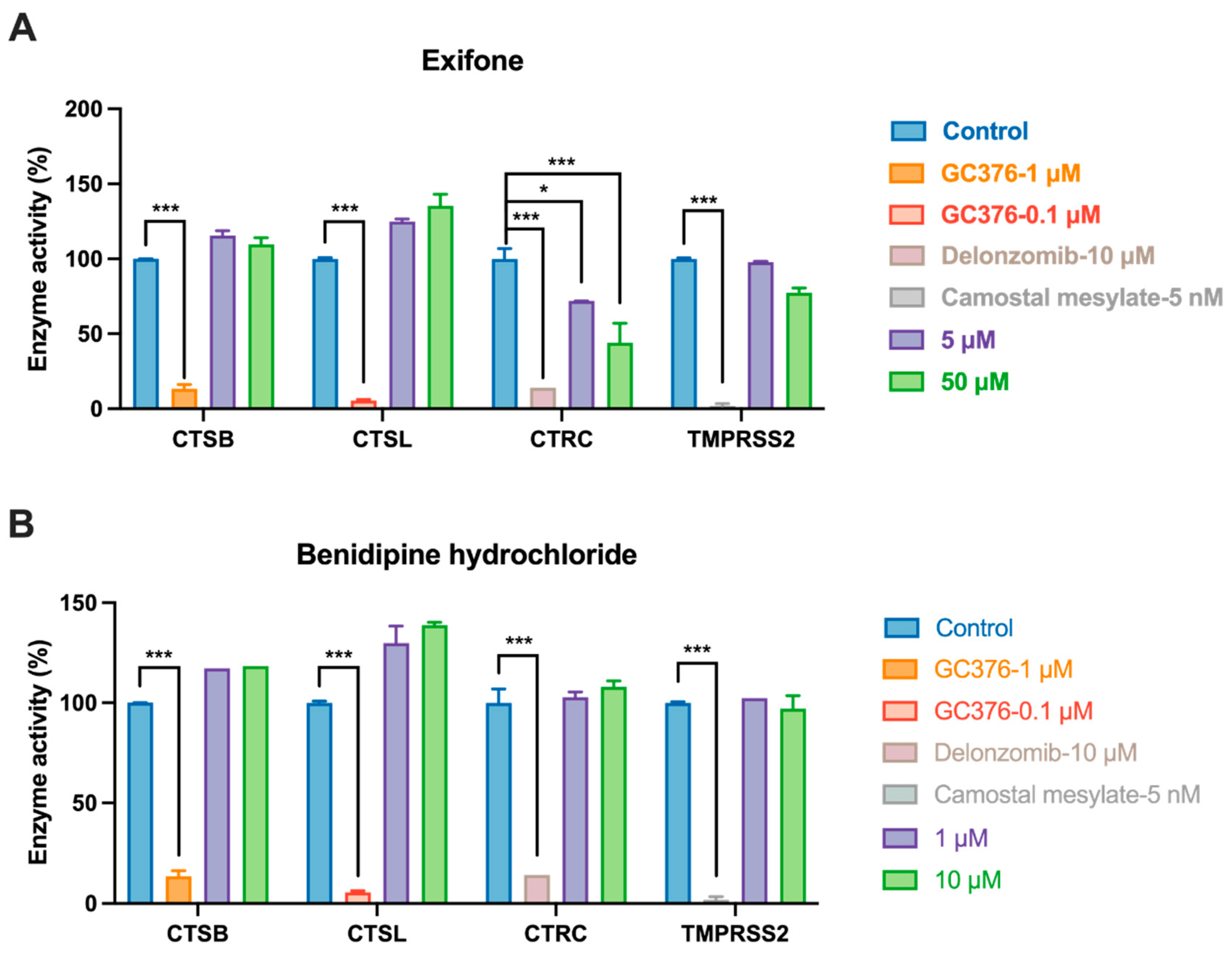
2.5. Evaluation of Host Protease Selectivity of Exifone and Benserazide Hydrochloride
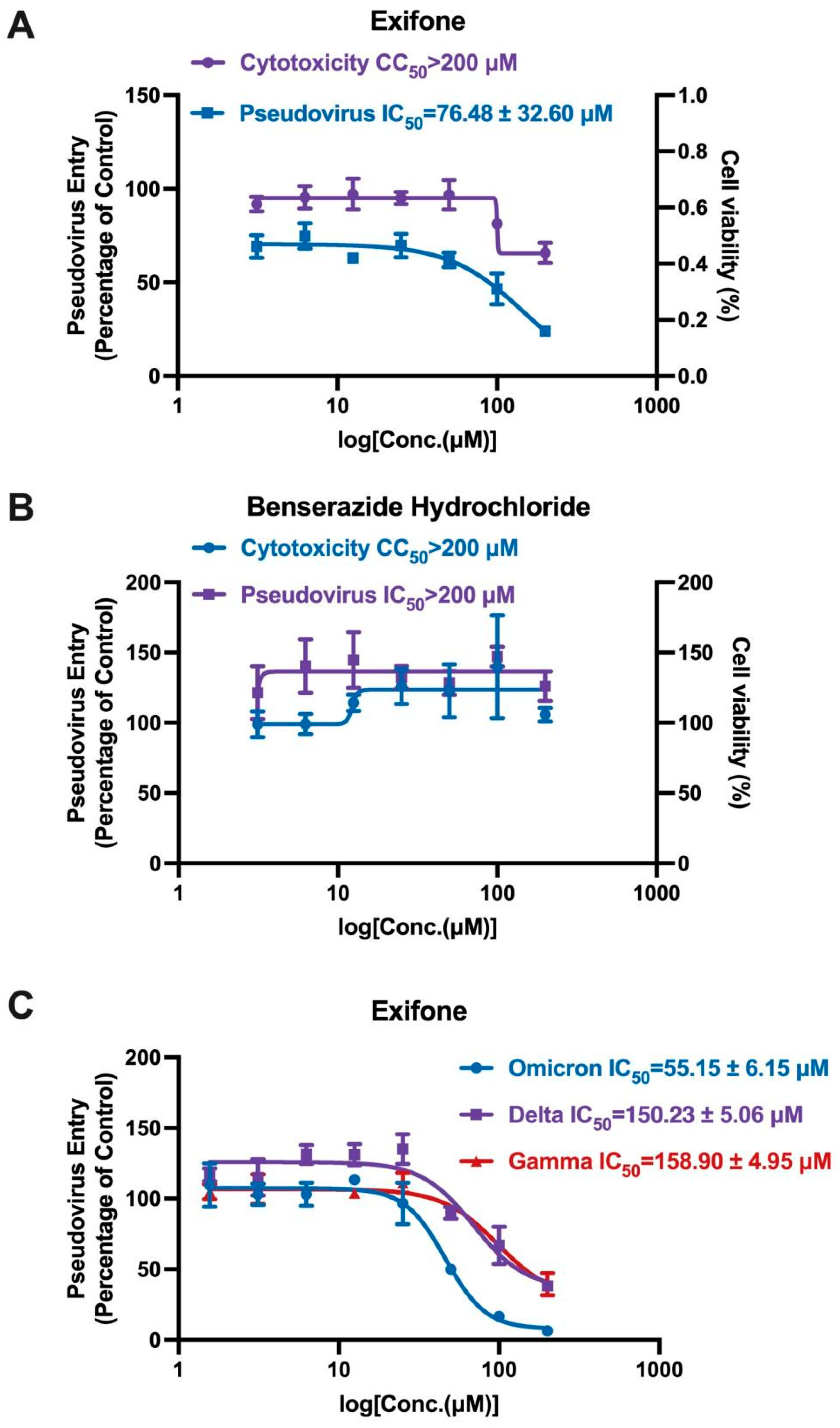
2.6. Exifone’s Inhibitory Activity on Wild and Mutant SARS-CoV-2-S Pseudovirus
2.7. Mechanistic Insights into Exifone’s Inhibitory Effect on Pseudovirus Entry into Cells
2.8. Binding Modes of Exifone with S-RBD of SARS-CoV-2 Wild-Type and Three Variants
3. Materials and Methods
3.1. Cell Culture
3.2. Protein Expression and Purification
3.3. Enzymatic Inhibition Assay
3.4. SPR
3.5. Determination of kinact/KI Values
3.6. Cell Viability Assay
3.7. Molecular Docking
3.7.1. Searching for Allosteric Sites of SARS-CoV-2 3CLpro
3.7.2. Ligand Preparation
3.7.3. Protein Model Preparation
3.7.4. Docking of Exifone with SARS-CoV-2 3CLpro and Four S-RBDs
3.7.5. Docking of Benserazide Hydrochloride with SARS-CoV-2 3CLpro
3.8. Target Selectivity Tests Toward Host Proteases
3.9. Pseudovirus Production
3.10. Pseudovirus Neutralization Assay
3.11. Flow Cytometry Detecting the Block Activity of the Test Compounds Against SARS-CoV-2 S-RBD/ACE2 Interaction
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Aly, Z.; Agarwal, A.; Alwan, N.; Luyckx, V.A. Long COVID: Long-term health outcomes and implications for policy and research. Nat. Rev. Nephrol. 2023, 19, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, E.-K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef]
- Cannalire, R.; Cerchia, C.; Beccari, A.R.; Di Leva, F.S.; Summa, V. Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: State of the art and future opportunities. J. Med. Chem. 2020, 65, 2716–2746. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Su, H.; Zhao, W.; Xie, H.; Shao, Q.; Xu, Y. What coronavirus 3C-like protease tells us: From structure, substrate selectivity, to inhibitor design. Med. Res. Rev. 2021, 41, 1965–1998. [Google Scholar] [CrossRef]
- Emrani, J.; Ahmed, M.; Jeffers-Francis, L.; Teleha, J.C.; Mowa, N.; Newman, R.H.; Thomas, M.D. SARS-COV-2, infection, transmission, transcription, translation, proteins, and treatment: A review. Int. J. Biol. Macromol. 2021, 193, 1249–1273. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Upadhyay, S.; Singh, M.; Raghavendhar, S.; Bhardwaj, M.; Sharma, P.; Patel, A.K. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 164, 2622–2631. [Google Scholar] [CrossRef]
- Liang, P.-H. Characterization and inhibition of SARS-coronavirus main protease. Curr. Top. Med. Chem. 2006, 6, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liao, C.; Yu, L. Molecules for COVID-19 treatment. Chin. Chem. Lett. 2024, 35, 109349. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Lu, H.; Huang, C.; Yang, Y.; Shang, L.; Chen, Z.; Jiang, R.; Liu, Y.; Lin, L. Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19. N. Engl. J. Med. 2024, 390, 230–241. [Google Scholar] [CrossRef]
- Mukae, H.; Yotsuyanagi, H.; Ohmagari, N.; Doi, Y.; Sakaguchi, H.; Sonoyama, T.; Ichihashi, G.; Sanaki, T.; Baba, K.; Tsuge, Y. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: The phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin. Infect. Dis. 2023, 76, 1403–1411. [Google Scholar] [CrossRef]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: Implications for the design of spike-based vaccine immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.W.; Cheng, Y.; Zhang, J.; Jiang, X.M.; Wang, L.; Deng, J.; Wang, P.H. Increasing host cellular receptor—Angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J. Med. Virol. 2020, 92, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Poklar Ulrih, N. Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Su, H.; Yao, S.; Zhao, W.; Zhang, Y.; Liu, J.; Shao, Q.; Wang, Q.; Li, M.; Xie, H.; Shang, W.; et al. Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat. Commun. 2021, 12, 3623. [Google Scholar] [CrossRef]
- Su, H.-X.; Yao, S.; Zhao, W.-F.; Li, M.-J.; Liu, J.; Shang, W.-J.; Xie, H.; Ke, C.-Q.; Hu, H.-C.; Gao, M.-N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Luo, C.; Liu, H.; Xu, W.; Chen, G.; Liew, O.W.; Zhu, W.; Puah, C.M.; Shen, X.; et al. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: Structure–activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006, 14, 8295–8306. [Google Scholar] [CrossRef]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs. Front. Chem. 2021, 9, 638187. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.-O.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside inhibits 3CLpro and PLpro of SARS-CoV-2 virus and regulates immune response and inflammation of host cells for the treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, R.; Li, M.; Su, H.; Sun, H.; Ding, Y.; Su, M.; Huang, C.; Chen, X.; Du, Z.; et al. Cocktail polysaccharides isolated from Ecklonia kurome against the SARS-CoV-2 infection. Carbohydr. Polym. 2022, 275, 118779. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of naturally occurring inhibitors against SARS-CoV-2 3CLpro from Ginkgo biloba leaves via large-scale screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, M.; Lei, F.; Liu, J.; Chen, X.; Li, Y.; Wang, Y.; Lu, J.; Yu, D.; Gao, Y.; et al. Methyl rosmarinate is an allosteric inhibitor of SARS-CoV-2 3 CL protease as a potential candidate against SARS-cov-2 infection. Antivir. Res. 2024, 224, 105841. [Google Scholar] [CrossRef]
- Li, H.-H.; Liu, C.-C.; Hsu, T.-W.; Lin, J.-H.; Hsu, J.-W.; Li, A.F.-Y.; Yeh, Y.-C.; Hung, S.-C.; Hsu, H.-S. Upregulation of ACE2 and TMPRSS2 by particulate matter and idiopathic pulmonary fibrosis: A potential role in severe COVID-19. Part. Fibre Toxicol. 2021, 18, 11. [Google Scholar] [CrossRef]
- Allain, H.; Denmat, J.; Bentue-Ferrer, D.; Milon, D.; Pignol, P.; Reymann, J.M.; Pape, D.; Sabouraud, O.; Van den Driessche, J. Randomized, double-blind trial of exifone versus cognitive problems in Parkinson’s disease. Fundam. Clin. Pharmacol. 1988, 2, 1–12. [Google Scholar] [CrossRef]
- Cuerda-Ballester, M.; Proaño, B.; Alarcón-Jimenez, J.; de Bernardo, N.; Villaron-Casales, C.; Lajara Romance, J.M.; de la Rubia Ortí, J.E. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food Funct. 2023, 14, 1062–1071. [Google Scholar] [CrossRef]
- Cieuta-Walti, C.; Cuenca-Royo, A.; Langohr, K.; Rakic, C.; López-Vílchez, M.Á.; Lirio, J.; González-Lamuño Leguina, D.; González, T.B.; García, J.G.; Roure, M.R.; et al. Safety and preliminary efficacy on cognitive performance and adaptive functionality of epigallocatechin gallate (EGCG) in children with Down syndrome. A randomized phase Ib clinical trial (PERSEUS study). Genet. Med. 2022, 24, 2004–2013. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients With Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, P.; Barrenechea, L.; Labayen, I.; Larrarte, E.; Margareto, J.; Mielgo-Ayuso, J. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Aronson, W.J.; Huang, M.; Zhang, Y.; Lee, R.-P.; Heber, D.; Henning, S.M. Green Tea Polyphenols and Metabolites in Prostatectomy Tissue: Implications for Cancer Prevention. Cancer Prev. Res. 2010, 3, 985–993. [Google Scholar] [CrossRef]
- Wilcox, C.; Aminoff, M. Blood pressure responses to noradrenaline and dopamine infusions in Parkinson’s disease and the Shy-Drager syndrome. Br. J. Clin. Pharmacol. 1976, 3, 207–214. [Google Scholar] [CrossRef]
- Mishina, M.; Ishiwata, K.; Naganawa, M.; Kimura, Y.; Kitamura, S.; Suzuki, M.; Hashimoto, M.; Ishibashi, K.; Oda, K.; Sakata, M.; et al. Adenosine A2A Receptors Measured with [11C]TMSX PET in the Striata of Parkinson’s Disease Patients. PLoS ONE 2011, 6, e17338. [Google Scholar] [CrossRef]
- Mahmud, S.; Uddin, M.A.R.; Paul, G.K.; Shimu, M.S.S.; Islam, S.; Rahman, E.; Islam, A.; Islam, M.S.; Promi, M.M.; Emran, T.B.; et al. Virtual screening and molecular dynamics simulation study of plant-derived compounds to identify potential inhibitors of main protease from SARS-CoV-2. Brief. Bioinf. 2021, 22, 1402–1414. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Song, F.; Zhu, Y.; Shi, Z.; Tian, J.; Deng, X.; Ren, J.; Andrews, M.C.; Ni, H.; Ling, W.; Yang, Y. Plant food anthocyanins inhibit platelet granule secretion in hypercholesterolaemia: Involving the signalling pathway of PI3K–Akt. Thromb. Haemost. 2014, 112, 981–991. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.O.; Saltzman, E.; Blumberg, J.B. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr. 2010, 140, 298–303. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Viviand, X.; Leone, M.; Thirion, X. Effect of norepinephrine on the outcome of septic shock. Crit. Care Med. 2000, 28, 2758–2765. [Google Scholar] [CrossRef]
- Fung, M.; Thornton, A.; Mybeck, K.; Hsiao, J.; Hornbuckle, K.; Muniz, E. Evaluation of the Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999. Ther. Innov. Regul. Sci. 2001, 35, 293–317. [Google Scholar] [CrossRef]
- Shi, J.; Sivaraman, J.; Song, J. Mechanism for Controlling the Dimer-Monomer Switch and Coupling Dimerization to Catalysis of the Severe Acute Respiratory Syndrome Coronavirus 3C-Like Protease. J. Virol. 2008, 82, 4620–4629. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, T.J.; Lutomski, C.A.; Kantsadi, A.L.; Malla, T.R.; John, T.; Mikhailov, V.; Bolla, J.R.; Schofield, C.J.; Zitzmann, N.; Vakonakis, I. Allosteric inhibition of the SARS-CoV-2 main protease: Insights from mass spectrometry based assays. Angew. Chem. Int. Ed. 2020, 59, 23544–23548. [Google Scholar] [CrossRef]
- Kneller, D.W.; Li, H.; Phillips, G.; Weiss, K.L.; Zhang, Q.; Arnould, M.A.; Jonsson, C.B.; Surendranathan, S.; Parvathareddy, J.; Blakeley, M.P. Covalent narlaprevir-and boceprevir-derived hybrid inhibitors of SARS-CoV-2 main protease. Nat. Commun. 2022, 13, 2268. [Google Scholar] [CrossRef]
- Ma, C.; Xia, Z.; Sacco, M.D.; Hu, Y.; Townsend, J.A.; Meng, X.; Choza, J.; Tan, H.; Jang, J.; Gongora, M.V.; et al. Discovery of Di- and Trihaloacetamides as Covalent SARS-CoV-2 Main Protease Inhibitors with High Target Specificity. J. Am. Chem. Soc. 2021, 143, 20697–20709. [Google Scholar] [CrossRef]
- Piva, R.; Ruggeri, B.; Williams, M.; Costa, G.; Tamagno, I.; Ferrero, D.; Giai, V.; Coscia, M.; Peola, S.; Massaia, M.; et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood 2008, 111, 2765–2775. [Google Scholar] [CrossRef]
- Xiong, J.; Xiang, Y.; Huang, Z.; Liu, X.; Wang, M.; Ge, G.; Chen, H.; Xu, J.; Zheng, M.; Chen, L. Structure-based virtual screening and identification of potential inhibitors of SARS-CoV-2 S-RBD and ACE2 interaction. Front. Chem. 2021, 9, 740702. [Google Scholar] [CrossRef]
- Zarei, A.; Ramazani, A.; Rezaei, A.; Moradi, S. Screening of honey bee pollen constituents against COVID-19: An emerging hot spot in targeting SARS-CoV-2-ACE-2 interaction. Nat. Prod. Res. 2023, 37, 974–980. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhai, G.; Li, Y.; Wang, M.; Chen, X.; Wang, R.; Xie, H.; Zhang, W.; Ge, G.; Zhang, Q.; et al. Ginkgolic acids inhibit SARS-CoV-2 and its variants by blocking the spike protein/ACE2 interplay. Int. J. Biol. Macromol. 2023, 226, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, Q.; Wang, H.; Zhu, G.; Wang, M.; Zhang, Q.; Zhao, Y.; Li, C.; Zhang, Y.; Ge, G. Identification of Vitamin K3 and its analogues as covalent inhibitors of SARS-CoV-2 3CLpro. Int. J. Biol. Macromol. 2021, 183, 182–192. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhai, G.; Zhu, G.; Wang, M.; Gong, X.; Zhang, W.; Ge, G.; Chen, H.; Chen, L. Discovery and mechanism of action of Thonzonium bromide from an FDA-approved drug library with potent and broad-spectrum inhibitory activity against main proteases of human coronaviruses. Bioorg. Chem. 2023, 130, 106264. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, X.; Li, H.; Chen, X.; Sun, D.; Yu, D.; Lu, J.; Xie, Y.; Zhang, Q.; Xu, J. Danshensu inhibits SARS-CoV-2 by targeting its main protease as a specific covalent inhibitor and discovery of bifunctional compounds eliciting antiviral and anti-inflammatory activity. Int. J. Biol. Macromol. 2023, 257, 128623. [Google Scholar] [CrossRef]
- Bepari, A.K.; Reza, H.M. Identification of a novel inhibitor of SARS-CoV-2 3CL-PRO through virtual screening and molecular dynamics simulation. PeerJ 2021, 9, e11261. [Google Scholar] [CrossRef]
- Novak, J.; Potemkin, V.A. A new glimpse on the active site of SARS-CoV-2 3CLpro, coupled with drug repurposing study. Mol Divers 2022, 26, 2631–2645. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Zhu, K.; Borrelli, K.W.; Greenwood, J.R.; Day, T.; Abel, R.; Farid, R.S.; Harder, E. Docking covalent inhibitors: A parameter free approach to pose prediction and scoring. J. Chem. Inf. Model. 2014, 54, 1932–1940. [Google Scholar] [CrossRef]
- Chen, C.C.; Yu, X.; Kuo, C.J.; Min, J.; Chen, S.; Ma, L.; Liu, K.; Guo, R.T. Overview of antiviral drug candidates targeting coronaviral 3C-like main proteases. FEBS J. 2021, 288, 5089–5121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of Covid-19. Top. Curr. Chem. 2021, 379, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, R.; Cai, Y.; Liao, M.; Yuan, W.; Liu, Z. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int. J. Nanomed. 2012, 7, 2077–2086. [Google Scholar]
- Sarukhanyan, E.; Shanmugam, T.A.; Dandekar, T. In Silico Studies Reveal Peramivir and Zanamivir as an Optimal Drug Treatment Even If H7N9 Avian Type Influenza Virus Acquires Further Resistance. Molecules 2022, 27, 5920. [Google Scholar] [CrossRef]
- He, J.-Y.; Li, C.; Wu, G. Discovery of potential drugs for human-infecting H7N9 virus containing R294K mutation. Drug Des. Dev. Ther. 2014, 8, 2377–2390. [Google Scholar] [CrossRef]
- Allain, H.; Bentué-Ferrer, D.; Tribut, O.; Gauthier, S.; Michel, B.-F.; Rochelle, C.D.-L. Alzheimer’s disease: The pharmacological pathway. Fundam. Clin. Pharmacol. 2003, 17, 419–428. [Google Scholar] [CrossRef]
- Gladkova, M.G.; Leidmaa, E.; Anderzhanova, E.A. Epidrugs in the Therapy of Central Nervous System Disorders: A Way to Drive on? Cells 2023, 12, 1464. [Google Scholar] [CrossRef]
- Patnaik, D.; Pao, P.-C.; Zhao, W.-N.; Silva, M.C.; Hylton, N.K.; Chindavong, P.S.; Pan, L.; Tsai, L.-H.; Haggarty, S.J. Exifone Is a Potent HDAC1 Activator with Neuroprotective Activity in Human Neuronal Models of Neurodegeneration. ACS Chem. Neurosci. 2021, 12, 271–284. [Google Scholar] [CrossRef] [PubMed]
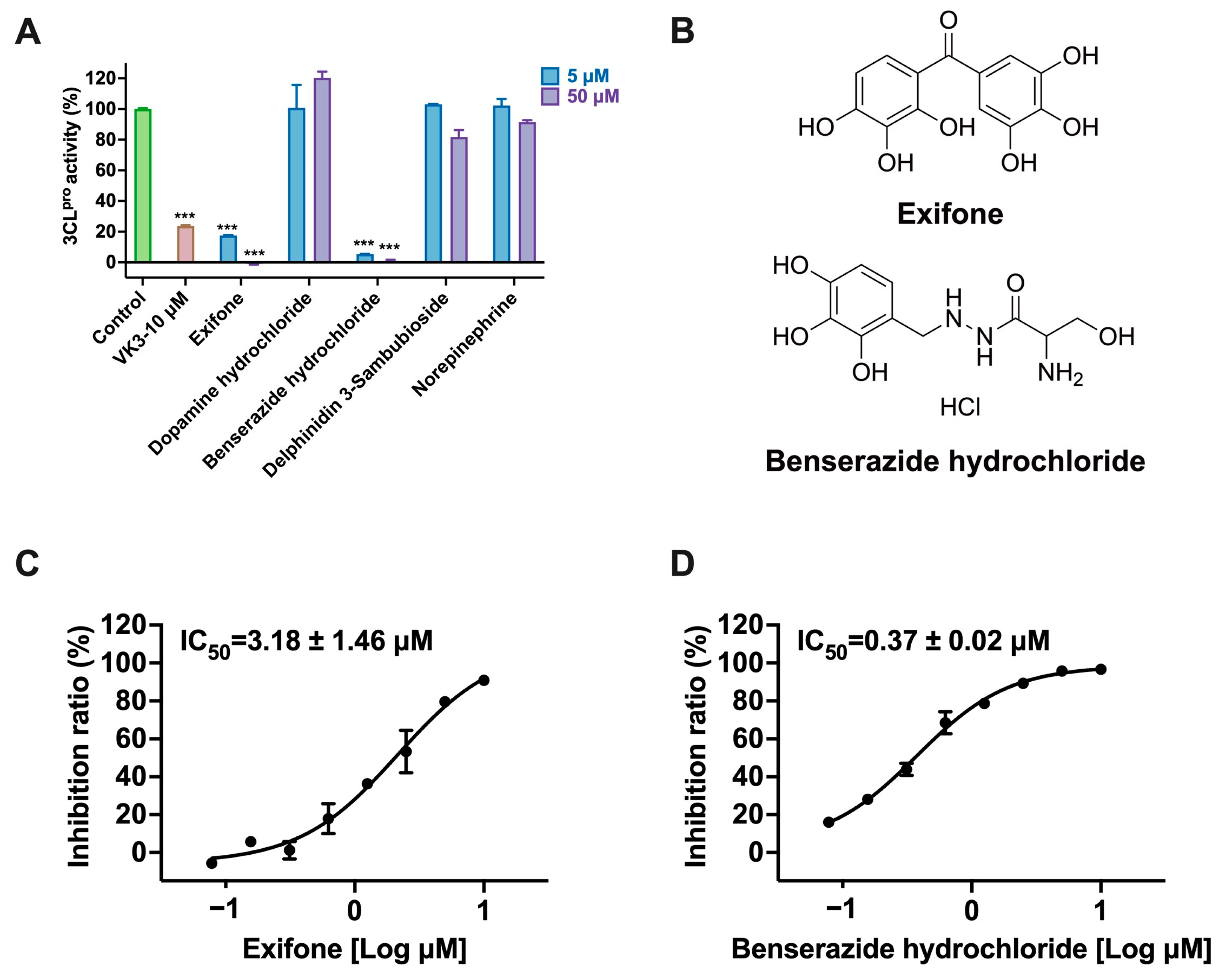


| 3CLpro Inhibition | Pseudovirus Activity Inhibition | Cell Viability Assay | ||
|---|---|---|---|---|
| Type of Experiments | Vero-E6 | hACE2-HEK293T | ||
| Compounds | IC50 (μM) | CC50 (μM) | ||
| Exifone | 3.18 ± 1.46 | 76.48 ± 32.60 | >100 | >200 |
| Benserazide hydrochloride | 0.37 ± 0.02 | >200 | >100 | >200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Tang, Y.; Li, H.; Chen, X.; Qin, P.; Xu, J.; Li, W.; Chen, L. Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds. Int. J. Mol. Sci. 2025, 26, 2243. https://doi.org/10.3390/ijms26052243
Lu J, Tang Y, Li H, Chen X, Qin P, Xu J, Li W, Chen L. Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds. International Journal of Molecular Sciences. 2025; 26(5):2243. https://doi.org/10.3390/ijms26052243
Chicago/Turabian StyleLu, Jiani, Yan Tang, Hongtao Li, Xixiang Chen, Pengcheng Qin, Jianrong Xu, Weihua Li, and Lili Chen. 2025. "Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds" International Journal of Molecular Sciences 26, no. 5: 2243. https://doi.org/10.3390/ijms26052243
APA StyleLu, J., Tang, Y., Li, H., Chen, X., Qin, P., Xu, J., Li, W., & Chen, L. (2025). Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds. International Journal of Molecular Sciences, 26(5), 2243. https://doi.org/10.3390/ijms26052243






