Differential Myocardial Responses in Male and Female Rats with Uremic Cardiomyopathy
Abstract
1. Introduction
2. Results
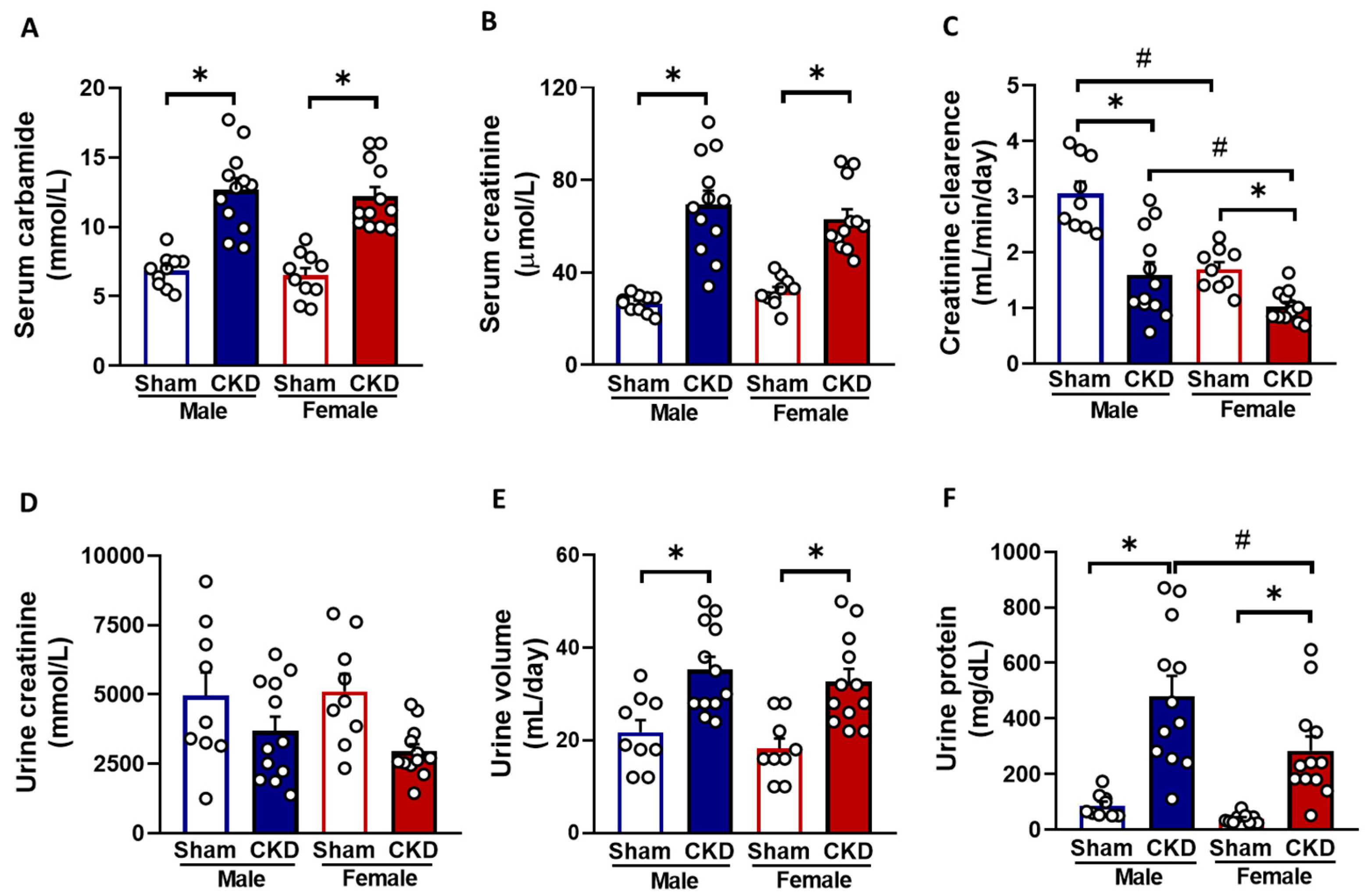
2.1. Males and Females Developed CKD of Similar Severity
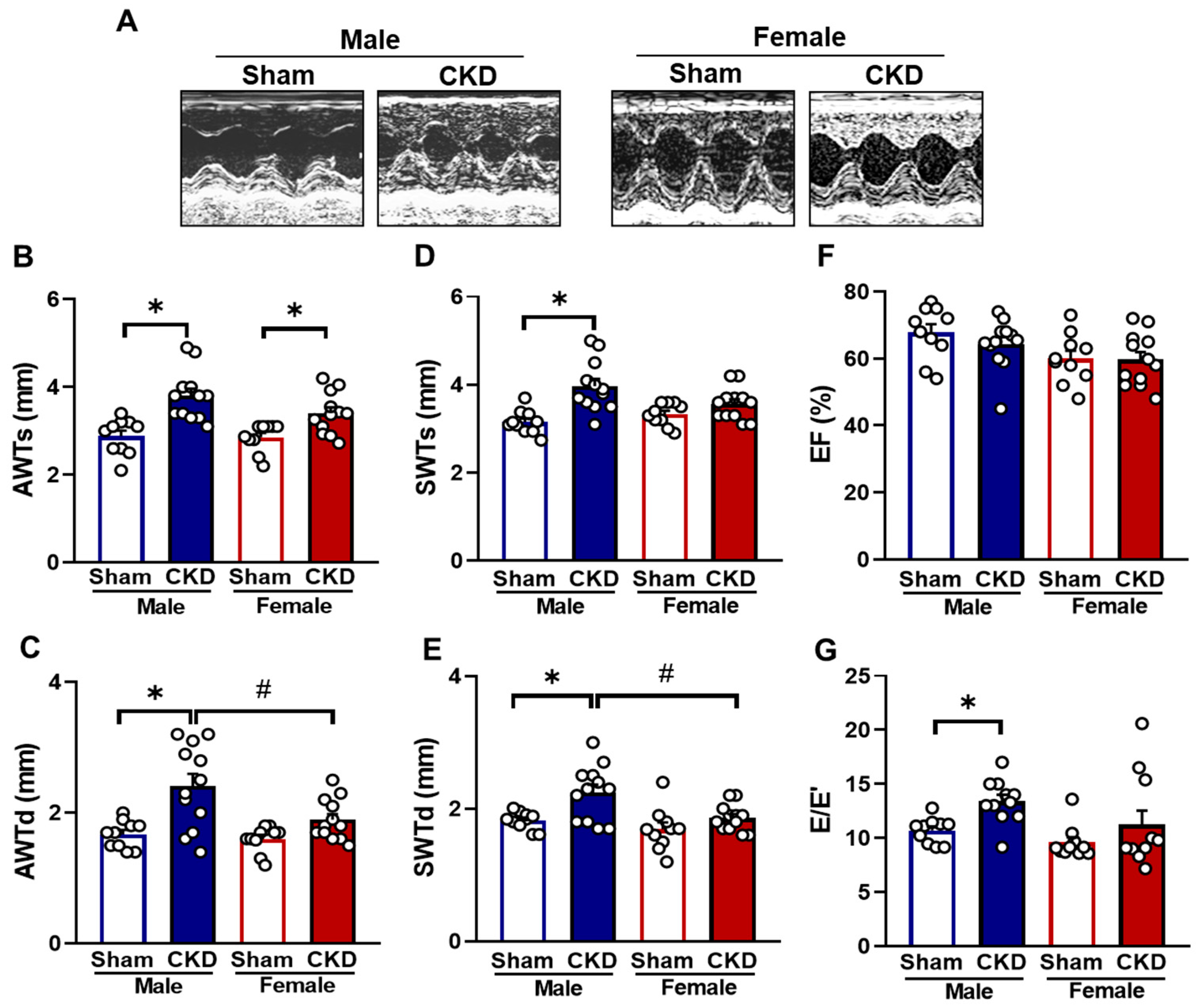
2.2. CKD Males Exhibited More Severe Echocardiographic Signs of LVH than Females
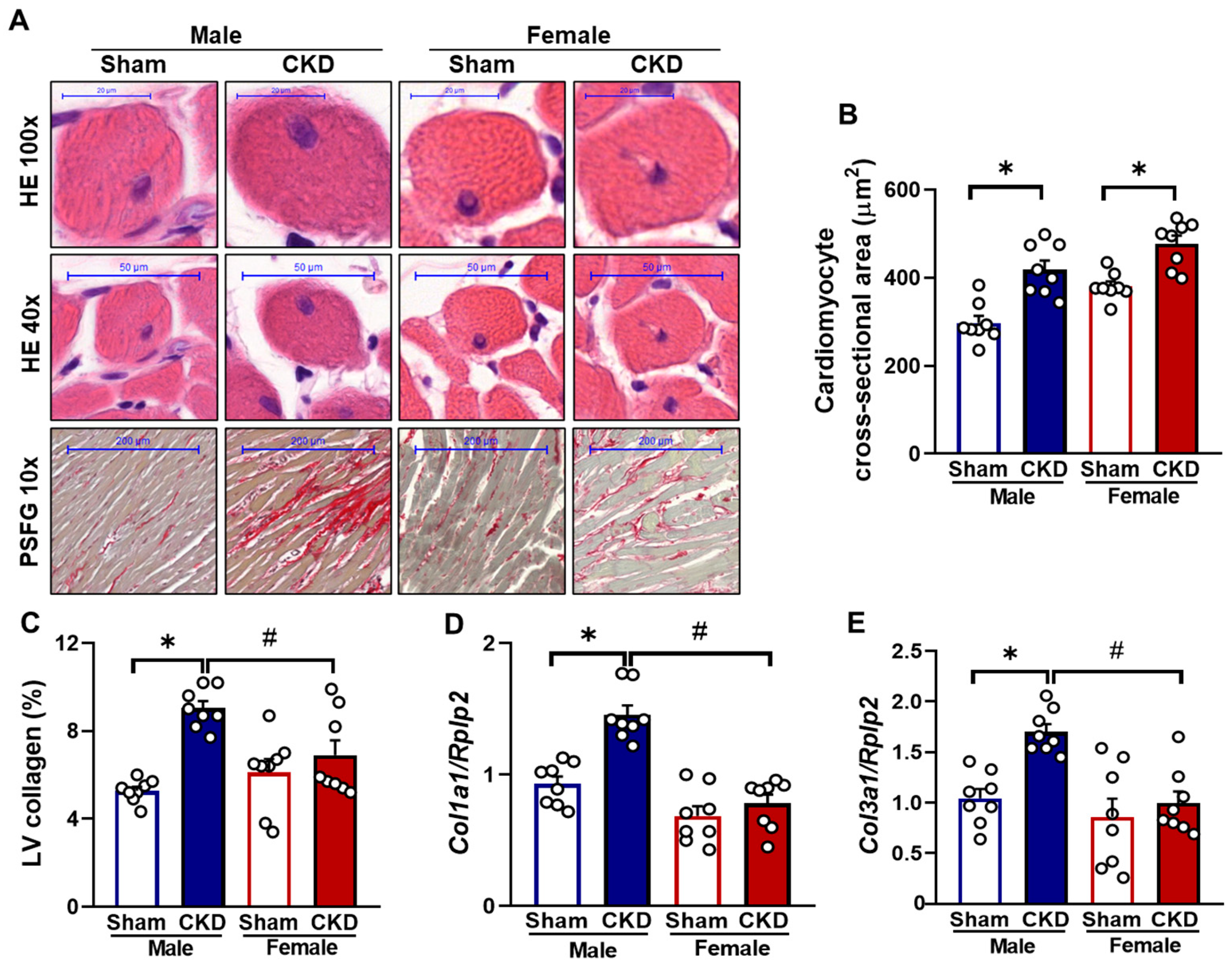
2.3. Both Males and Females Exhibited Ex Vivo Morphological Signs of Hypertrophy in CKD
2.4. CKD Caused Cardiomyocyte Hypertrophy in Both Sexes but Severe Fibrosis Only in Males
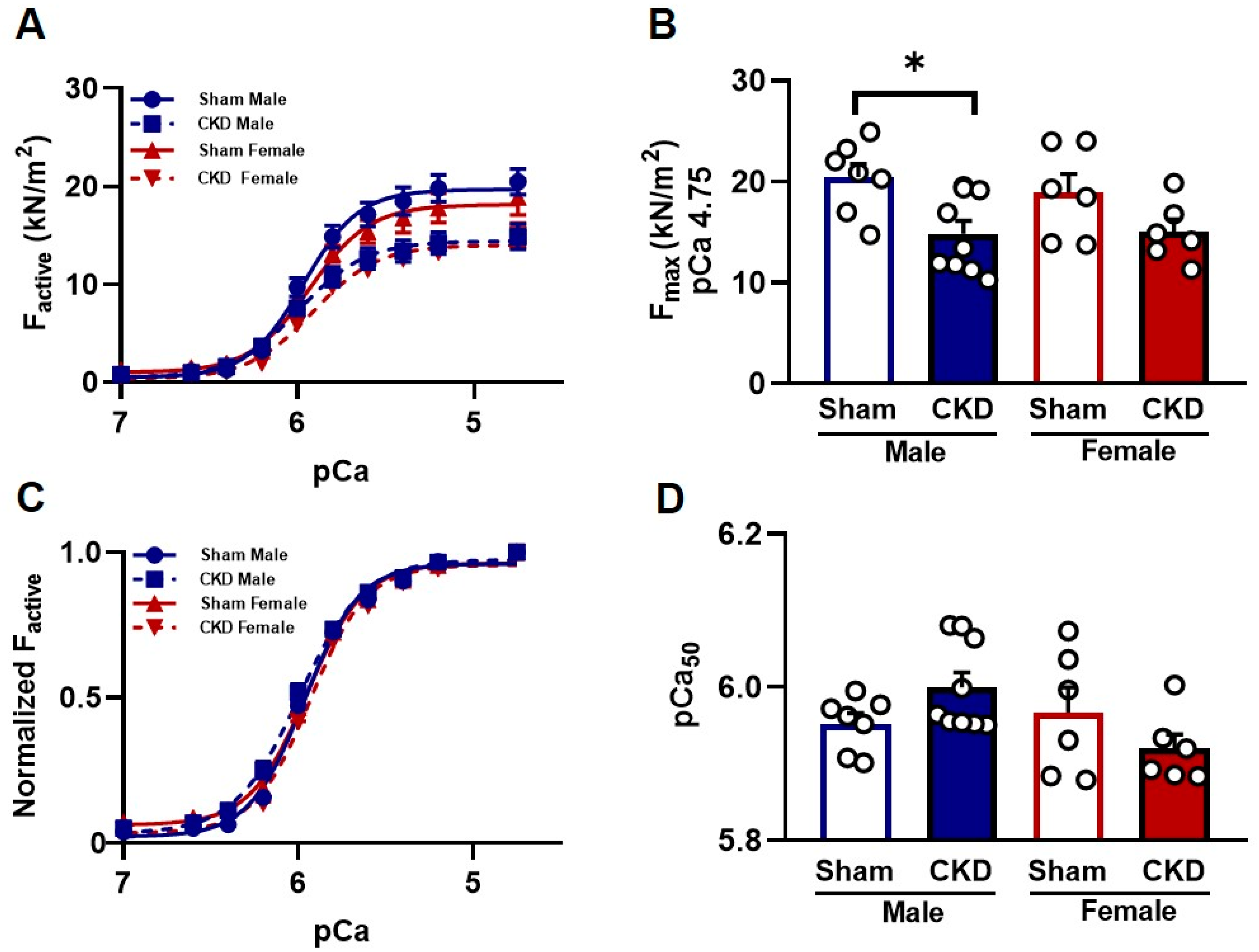
2.5. Myofilament Function
2.5.1. Reduced Ca2+-Activated Force Generation in Single Cardiomyocytes from CKD Male Hearts In Vitro
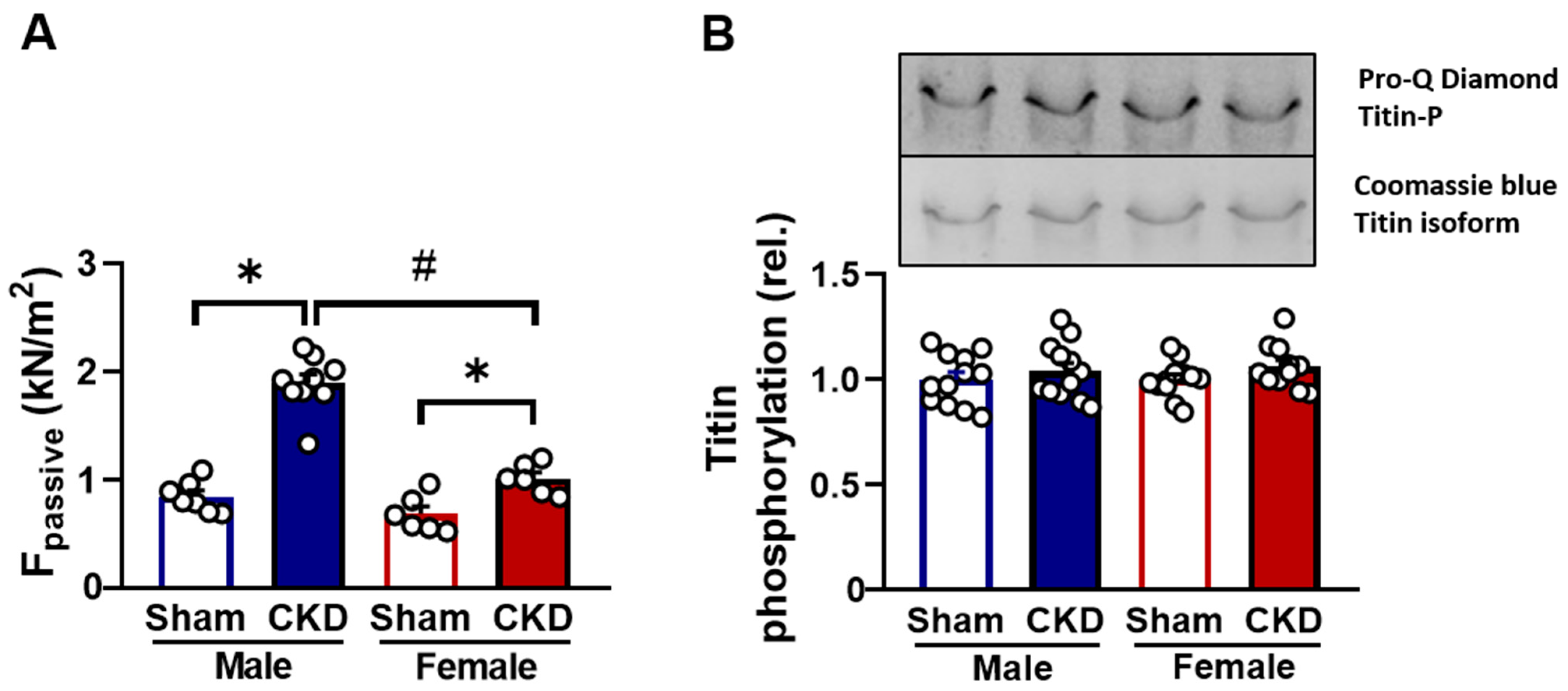
2.5.2. Increased Passive Stiffness in CKD Groups In Vitro
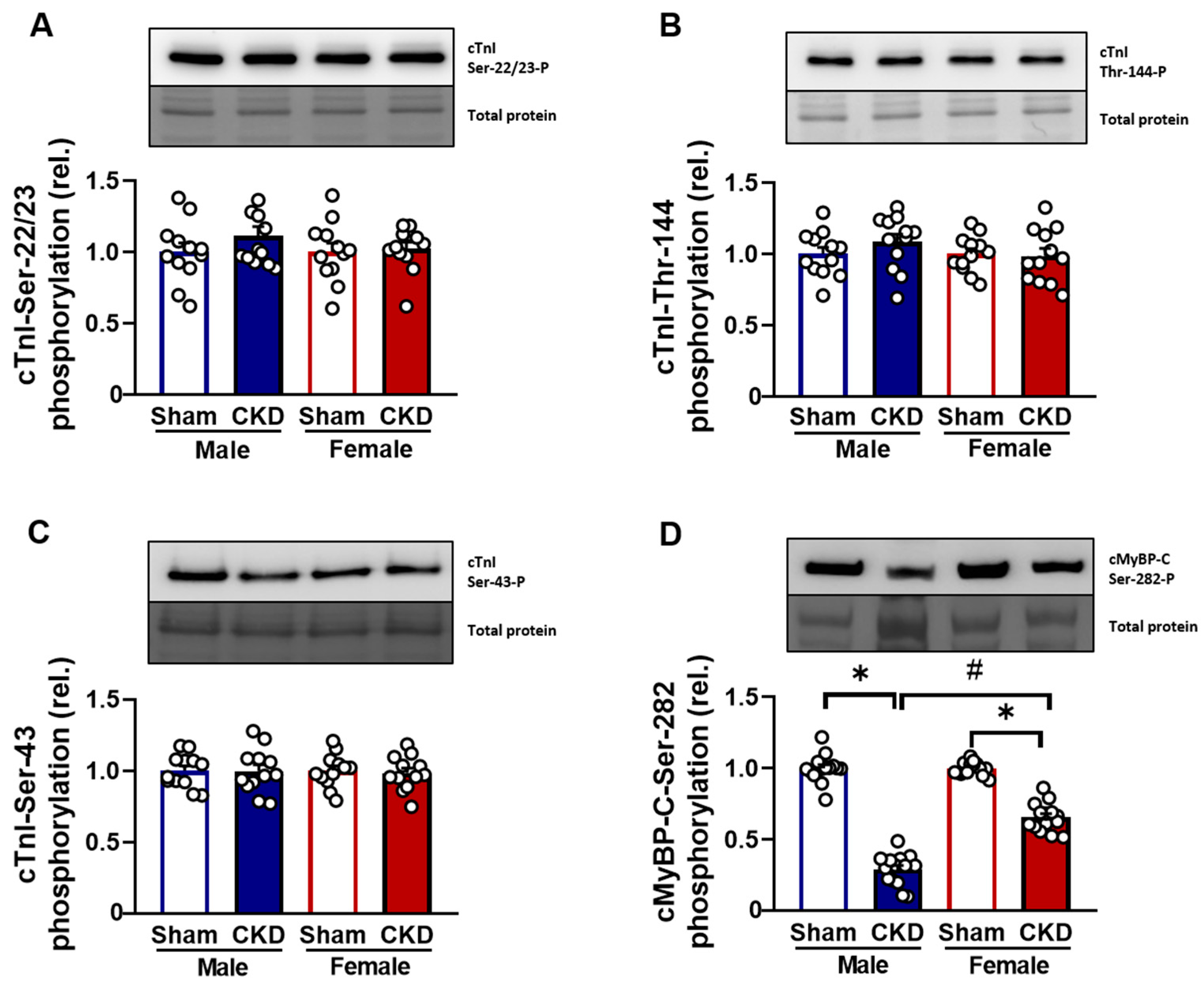
2.5.3. Hypophosphorylation of cMyBP-C Protein in CMs Affected by Uremic Cardiomyopathy
3. Discussion
4. Materials and Methods
4.1. Animals
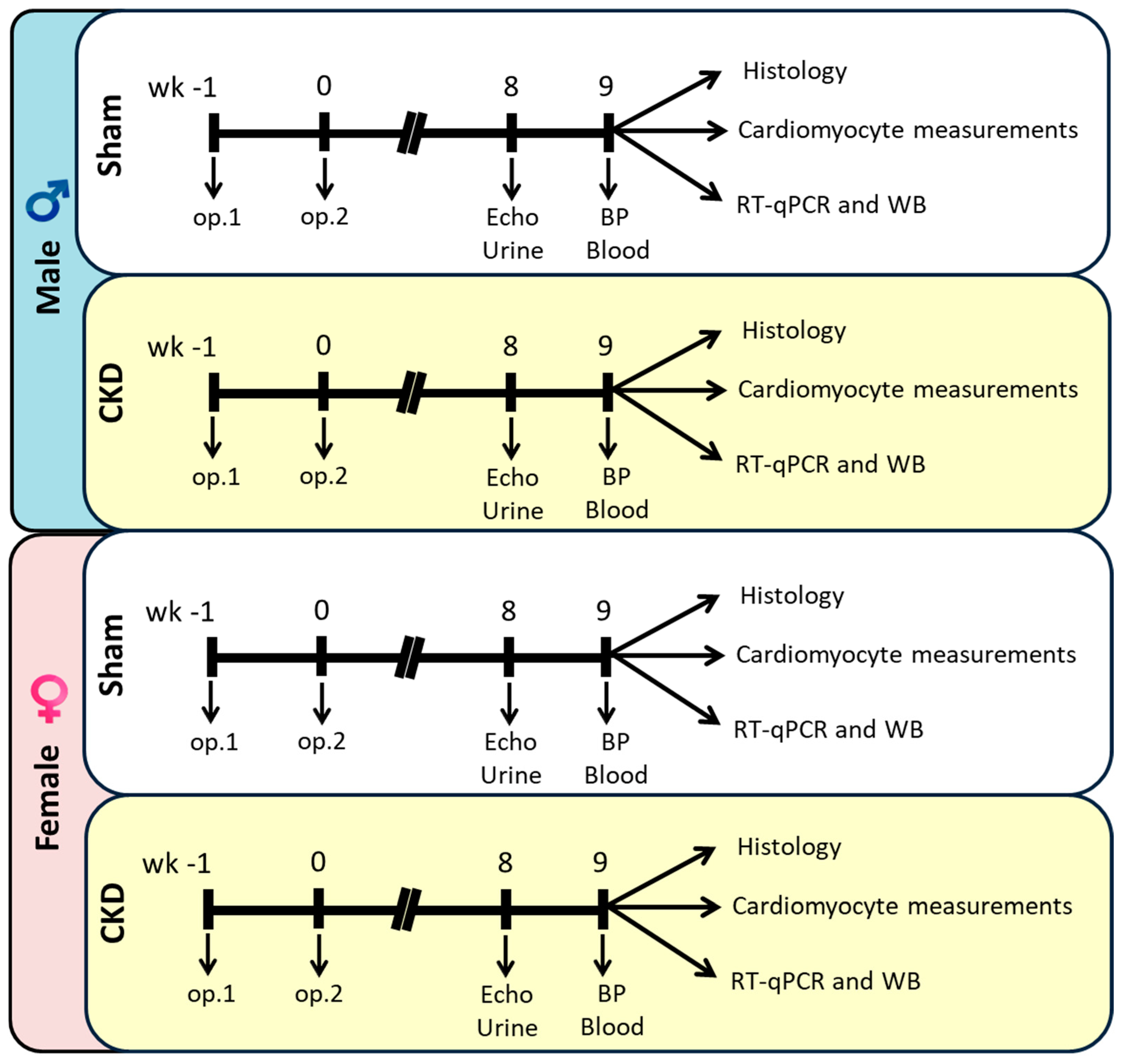
4.2. Experimental Setup
4.3. Subtotal (5/6) Nephrectomy Model
4.4. Transthoracic Echocardiography
4.5. Blood Pressure Measurement
4.6. Urine and Serum Laboratory Parameters
4.7. Hematoxylin-Eosin and Picrosirius Red and Fast Green Stainings
4.8. mRNA Expression Profiling by RT-qPCR
4.9. Force Measurements in Isolated Cardiomyocytes
4.10. Western Immunoblot
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| CKD | chronic kidney disease |
| CM | cardiomyocyte |
| cMyBP-C | cardiac myosin-binding protein C |
| cTnI | cardiac troponin-I |
| CVD | cardiovascular disease |
| ECL | enhanced chemiluminescence |
| Factive | cardiomyocyte active force |
| Fmax | cardiomyocyte maximal Ca2+-activated force |
| Fpassive | cardiomyocyte passive stiffness |
| ISO | isolating solution |
| LV | left ventricular |
| LVH | left ventricular hypertrophy |
| pCa50 | Ca2+ sensitivity of isometric force production |
| PBS | phosphate-buffered saline |
| SDS | sodium dodecyl sulfate |
| SL | sarcomere length |
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Perico, N.; Remuzzi, G. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bogle, R.; Banerjee, D. Why do young people with chronic kidney disease die early? World J. Nephrol. 2014, 3, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; McCullough, P.A.; Birk, H.-W.; Renker, M.; Brocca, A.; Seeger, W.; Ronco, C. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J. Am. Coll. Cardiol. 2015, 65, 2433–2448. [Google Scholar] [CrossRef]
- Alhaj, E.; Alhaj, N.; Rahman, I.; Niazi, T.O.; Berkowitz, R.; Klapholz, M. Uremic cardiomyopathy: An underdiagnosed disease. Congest. Heart Fail. 2013, 19, E40–E45. [Google Scholar] [CrossRef]
- Di Lullo, L.; Gorini, A.; Russo, D.; Santoboni, A.; Ronco, C. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal Med. 2015, 5, 254–266. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.-P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxid. Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef]
- Middleton, R.J.; Parfrey, P.S.; Foley, R.N. Left ventricular hypertrophy in the renal patient. J. Am. Soc. Nephrol. 2001, 12, 1079–1084. [Google Scholar] [CrossRef]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef]
- Sárközy, M.; Kovács, Z.Z.A.; Kovács, M.G.; Gáspár, R.; Szűcs, G.; Dux, L. Mechanisms and Modulation of Oxidative/Nitrative Stress in Type 4 Cardio-Renal Syndrome and Renal Sarcopenia. Front. Physiol. 2018, 9, 1648. [Google Scholar] [CrossRef]
- Drummond, C.A.; Buddny, G.; Haller, S.T.; Liu, J.; Yan, Y.; Xie, Z.; Malhotra, D.; Shapiro, J.I.; Tian, J. Gender differences in the development of uremic cardiomyopathy following partial nephrectomy: Role of progesterone. J. Hypertens. 2013, 2. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Animal Models to Study Links between Cardiovascular Disease and Renal Failure and Their Relevance to Human Pathology. Front. Immunol. 2015, 6, 465. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Babler, A.; Floege, J.; Kramann, R. Cardiac Remodeling in Chronic Kidney Disease. Toxins 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.J.; Williams, A.C.; Kriegel, A.J. Comparison of the surgical resection and infarct 5/6 nephrectomy rat models of chronic kidney disease. Am. J. Physiol. Renal Physiol. 2022, 322, F639–F654. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Enoki, Y.; Uchida, S.; Taguchi, K.; Matsumoto, K. A combination of 5/6-nephrectomy and unilateral ureteral obstruction model accelerates progression of remote organ fibrosis in chronic kidney disease. FASEB Bioadv. 2023, 5, 377–394. [Google Scholar] [CrossRef]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Siska, A.; Kővári, B.; Szűcs, G.; Márványkövi, F.; Kovács, M.G.; Diószegi, P.; Bodai, L.; et al. Chronic kidney disease induces left ventricular overexpression of the pro-hypertrophic microRNA-212. Sci. Rep. 2019, 9, 1302. [Google Scholar] [CrossRef]
- Sárközy, M.; Watzinger, S.; Kovács, Z.Z.A.; Acar, E.; Márványkövi, F.; Szűcs, G.; Lauber, G.Y.; Galla, Z.; Siska, A.; Földesi, I.; et al. Neuregulin-1β Improves Uremic Cardiomyopathy and Renal Dysfunction in Rats. JACC Basic Transl. Sci. 2023, 8, 1160–1176. [Google Scholar] [CrossRef]
- Kovács, Z.Z.A.; Szűcs, G.; Freiwan, M.; Kovács, M.G.; Márványkövi, F.M.; Dinh, H.; Siska, A.; Farkas, K.; Kovács, F.; Kriston, A.; et al. Comparison of the antiremodeling effects of losartan and mirabegron in a rat model of uremic cardiomyopathy. Sci. Rep. 2021, 11, 17495. [Google Scholar] [CrossRef]
- Sárközy, M.; Márványkövi, F.M.; Szűcs, G.; Kovács, Z.Z.A.; Szabó, M.R.; Gáspár, R.; Siska, A.; Kővári, B.; Cserni, G.; Földesi, I.; et al. Ischemic preconditioning protects the heart against ischemia-reperfusion injury in chronic kidney disease in both males and females. Biol. Sex Differ. 2021, 12, 49. [Google Scholar] [CrossRef]
- Lemos, C.C.S.; Mandarim-de-Lacerda, C.A.; Carvalho, J.J.; Bregman, R. Gender-related differences in kidney of rats with chronic renal failure. Histol. Histopathol. 2014, 29, 479–487. [Google Scholar] [CrossRef]
- Paterson, M.R.; Geurts, A.M.; Kriegel, A.J. miR-146b-5p has a sex-specific role in renal and cardiac pathology in a rat model of chronic kidney disease. Kidney Int. 2019, 96, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Schön, A.; Leifheit-Nestler, M.; Deppe, J.; Fischer, D.-C.; Bayazit, A.K.; Obrycki, L.; Canpolat, N.; Bulut, I.K.; Azukaitis, K.; Yilmaz, A.; et al. Active vitamin D is cardioprotective in experimental uraemia but not in children with CKD Stages 3-5. Nephrol. Dial. Transplant 2021, 36, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Červenka, L.; Škaroupková, P.; Kompanowska-Jezierska, E.; Sadowski, J. Sex-linked differences in the course of chronic kidney disease and congestive heart failure: A study in 5/6 nephrectomized Ren-2 transgenic hypertensive rats with volume overload induced using aorto-caval fistula. Clin. Exp. Pharmacol. Physiol. 2016, 43, 883–895. [Google Scholar] [CrossRef]
- Oneglia, A.; Nelson, M.D.; Merz, C.N.B. Sex Differences in Cardiovascular Aging and Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Salton, C.J.; Chuang, M.L.; O’Donnell, C.J.; Kupka, M.J.; Larson, M.G.; Kissinger, K.V.; Edelman, R.R.; Levy, D.; Manning, W.J. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J. Am. Coll. Cardiol. 2002, 39, 1055–1060. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Kompa, A.R.; Wang, B.H.; Kelly, D.J.; Krum, H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012, 111, 1470–1483. [Google Scholar] [CrossRef]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar] [CrossRef]
- Røe, Å.T.; Aronsen, J.M.; Skårdal, K.; Hamdani, N.; Linke, W.A.; Danielsen, H.E.; Sejersted, O.M.; Sjaastad, I.; Louch, W.E. Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovasc. Res. 2017, 113, 1161–1172. [Google Scholar] [CrossRef]
- van Loon, T.; Knackstedt, C.; Cornelussen, R.; Reesink, K.D.; La Brunner Rocca, H.-P.; Delhaas, T.; van Empel, V.; Lumens, J. Increased myocardial stiffness more than impaired relaxation function limits cardiac performance during exercise in heart failure with preserved ejection fraction: A virtual patient study. Eur. Heart J. Digit. Health 2020, 1, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; van Veen, T.A.B. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Dinh, H.; Kovács, Z.Z.A.; Márványkövi, F.; Kis, M.; Kupecz, K.; Szűcs, G.; Freiwan, M.; Lauber, G.Y.; Acar, E.; Siska, A.; et al. The kisspeptin-1 receptor antagonist peptide-234 aggravates uremic cardiomyopathy in a rat model. Sci. Rep. 2023, 13, 14046. [Google Scholar] [CrossRef] [PubMed]
- Dinh, H.; Kovács, Z.Z.A.; Kis, M.; Kupecz, K.; Sejben, A.; Szűcs, G.; Márványkövi, F.; Siska, A.; Freiwan, M.; Pósa, S.P.; et al. Role of the kisspeptin-KISS1R axis in the pathogenesis of chronic kidney disease and uremic cardiomyopathy. Geroscience 2024, 46, 2463–2488. [Google Scholar] [CrossRef]
| Parameter (Unit) | Male | Female | ||
|---|---|---|---|---|
| Sham | CKD | Sham | CKD | |
| AWTs (mm) | 2.88 ± 0.13 | 3.80 ± 0.17 * | 2.86 ± 0.10 | 3.40 ± 0.14 * |
| AWTd (mm) | 1.67 ± 0.07 | 2.41 ± 0.19 * | 1.60 ± 0.06 | 1.89 ± 0.10 # |
| IWTs (mm) | 3.10 ± 0.14 | 3.45 ± 0.16 | 3.35 ± 0.12 | 3.64 ± 0.13 |
| IWTd (mm) | 1.85 ± 0.11 | 2.00 ± 0.10 | 1.76 ± 0.12 | 1.98 ± 0.11 |
| SWTs (mm) | 3.15 ± 0.09 | 3.97 ± 0.17 * | 3.33 ± 0.08 | 3.57 ± 0.11 |
| SWTd (mm) | 1.82 ± 0.04 | 2.42 ± 0.12 * | 1.69 ± 0.11 | 1.86 ± 0.06 # |
| PWTs (mm) | 2.98 ± 0.13 | 3.43 ± 0.14 * | 3.29 ± 0.14 | 3.45 ± 0.18 |
| PWTd (mm) | 1.76 ± 0.10 | 1.94 ± 0.11 | 1.86 ± 0.08 | 1.92 ± 0.14 |
| FS (%) | 46.01 ± 1.31 | 56.90 ± 3.90 * | 64.90 ± 3.20 # | 67.71 ± 2.30 # |
| LVEDD (mm) | 7.57 ± 0.36 | 7.15 ± 0.29 | 6.22 ± 0.24 # | 6.06 ± 0.10 # |
| LVESD (mm) | 4.07 ± 0.20 | 3.16 ± 0.32 * | 2.22 ± 0.28 # | 1.97 ± 0.15 # |
| LVEDV (μL) | 106.70 ± 6.50 | 69.51 ± 3.91 * | 82.80 ± 6.61 # | 95.30 ± 6.30 # |
| LVESV (μL) | 34.81 ± 4.61 | 24.01 ± 2.20 | 33.20 ± 3.60 | 38.51 ± 4.11 # |
| SV (μL) | 71.90 ± 4.20 | 45.52 ± 3.61 * | 49.70 ± 4.31 # | 56.81 ± 4.11 # |
| HR (1/min) | 352 ± 10 | 350 ± 10 | 368 ± 14 | 377 ± 11 # |
| CO (mL/min) | 24.41 ± 1.61 | 15.52 ± 1.42 * | 18.61 ± 2.02 # | 22.12 ± 1.71 |
| EF (%) | 67.81 ± 2.52 | 65.75 ± 2.52 | 60.01 ± 2.71 | 59.62 ± 2.52 |
| E-velocity (m/s) | 0.86 ± 0.05 | 0.80 ± 0.04 | 0.86 ± 0.06 | 0.90 ± 0.05 |
| e’-velocity (m/s) | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| E/e’ | 10.71 ± 0.41 | 13.41 ± 0.62 * | 9.62 ± 0.63 # | 11.21 ± 1.34 # |
| IVRT (ms) | 23.92 ± 1.11 | 29.01 ± 1.51 * | 23.13 ± 1.92 | 23.62 ± 1.53 # |
| Parameter (Unit) | Male | Female | ||
|---|---|---|---|---|
| Sham | CKD | Sham | CKD | |
| Systolic blood pressure (mmHg) | 127 ± 6 | 120 ± 4 | 123 ± 6 | 131 ± 7 |
| Diastolic blood pressure (mmHg) | 100 ± 6 | 94 ± 4 | 95 ± 7 | 103 ± 8 |
| Mean blood pressure (mmHg) | 111 ± 6 | 105 ± 4 | 107 ± 7 | 114 ± 8 |
| Body weight | 508 ± 20 | 519 ± 9 | 327 ± 10 # | 324 ± 5 # |
| Tibia length (cm) | 4.29 ± 0.04 | 4.3 ± 0.05 | 3.92 ± 0.04 # | 3.93 ± 0.03 # |
| Heart weight (mg) | 1158 ± 35 | 1324 ± 47 * | 946 ± 34 # | 1113 ± 56 *# |
| Kidney weight (mg) | 1330 ± 43 | 1809 ± 79 * | 989 ± 50 # | 1189 ± 75 *# |
| Lung weight (mg) | 1596 ± 55 | 1765 ± 67 * | 1508 ± 36 # | 1464 ± 37 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bódi, B.; Vágó, R.R.; Nagy, L.; Ráduly, A.P.; Gulyás, A.; Kupecz, K.; Azar, L.; Márványkövi, F.M.; Szűcs, G.; Siska, A.; et al. Differential Myocardial Responses in Male and Female Rats with Uremic Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 2259. https://doi.org/10.3390/ijms26052259
Bódi B, Vágó RR, Nagy L, Ráduly AP, Gulyás A, Kupecz K, Azar L, Márványkövi FM, Szűcs G, Siska A, et al. Differential Myocardial Responses in Male and Female Rats with Uremic Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(5):2259. https://doi.org/10.3390/ijms26052259
Chicago/Turabian StyleBódi, Beáta, Rebeka Rita Vágó, László Nagy, Arnold Péter Ráduly, András Gulyás, Klaudia Kupecz, Lilian Azar, Fanni Magdolna Márványkövi, Gergő Szűcs, Andrea Siska, and et al. 2025. "Differential Myocardial Responses in Male and Female Rats with Uremic Cardiomyopathy" International Journal of Molecular Sciences 26, no. 5: 2259. https://doi.org/10.3390/ijms26052259
APA StyleBódi, B., Vágó, R. R., Nagy, L., Ráduly, A. P., Gulyás, A., Kupecz, K., Azar, L., Márványkövi, F. M., Szűcs, G., Siska, A., Cserni, G., Földesi, I., Papp, Z., & Sárközy, M. (2025). Differential Myocardial Responses in Male and Female Rats with Uremic Cardiomyopathy. International Journal of Molecular Sciences, 26(5), 2259. https://doi.org/10.3390/ijms26052259








