Lectin-Based Substrate Detection in Fabry Disease Using the Gb3-Binding Lectins StxB and LecA
Abstract
1. Introduction
2. Results
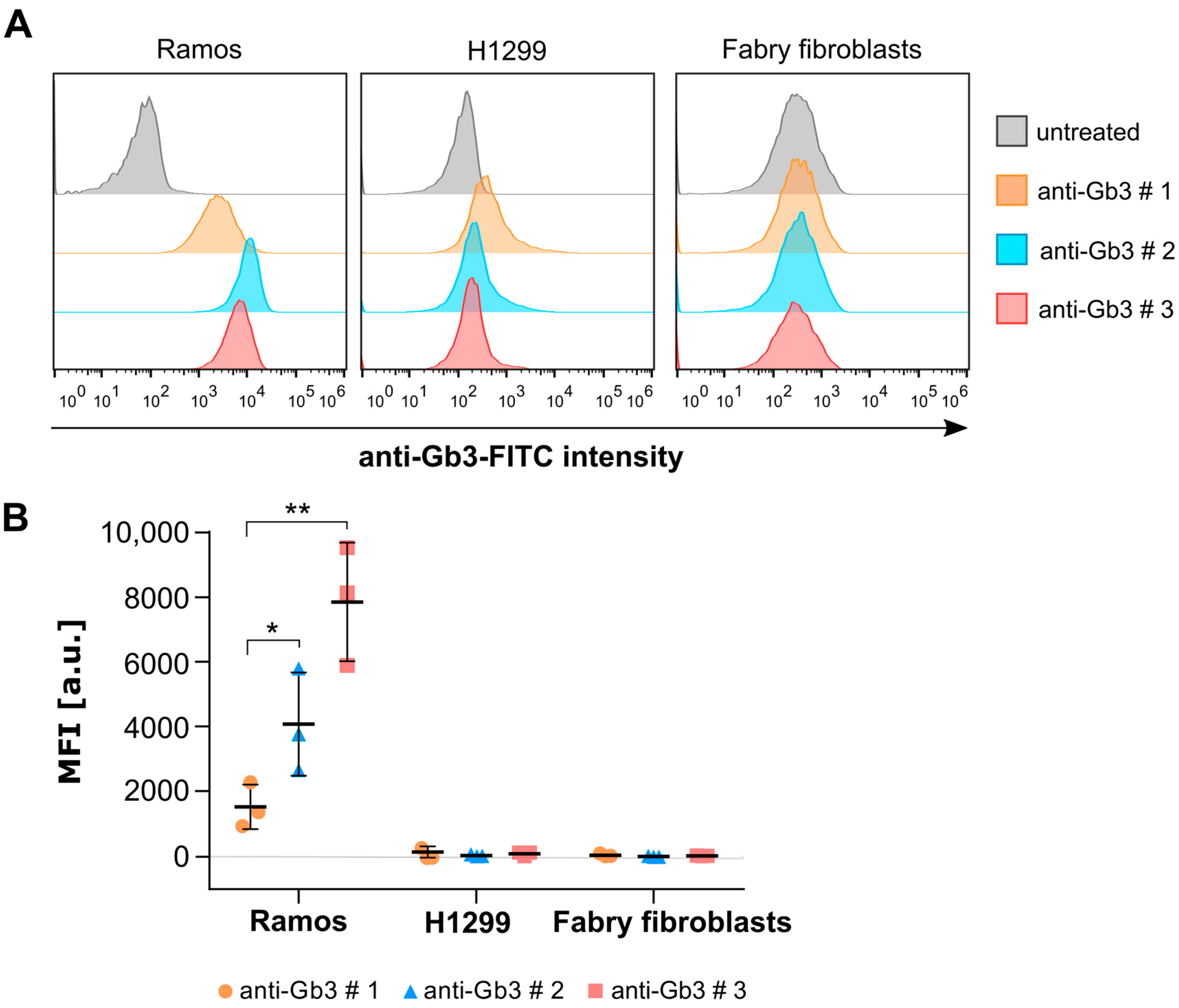
2.1. The Commercially Available Anti-Gb3 Antibodies Tested Do Not Recognize Gb3 in the Plasma Membrane of Fabry Fibroblasts
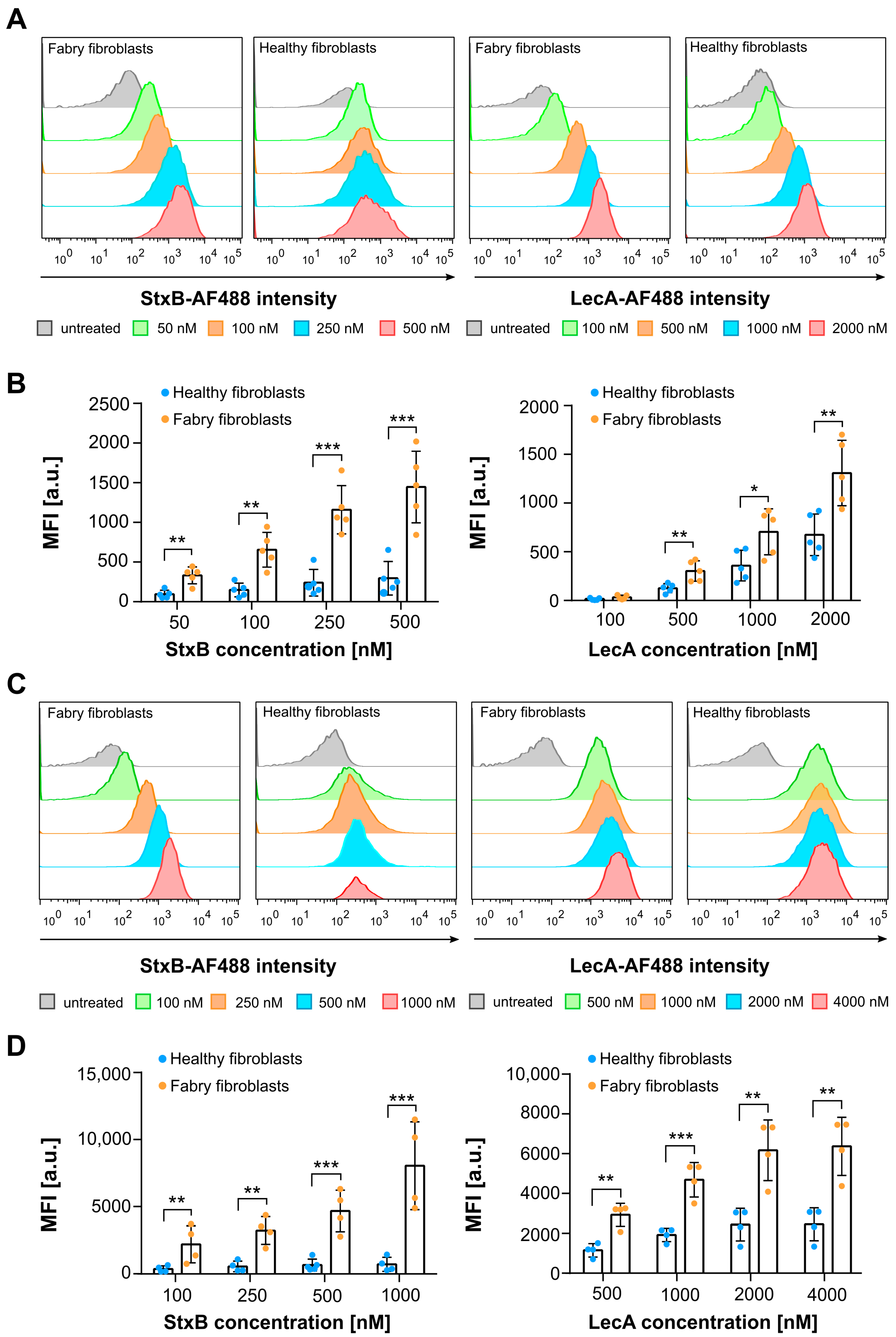
2.2. Plasma Membrane and Whole Cell Gb3 Levels of Fabry and Healthy Fibroblasts Analyzed by Flow Cytometry Using Lectins
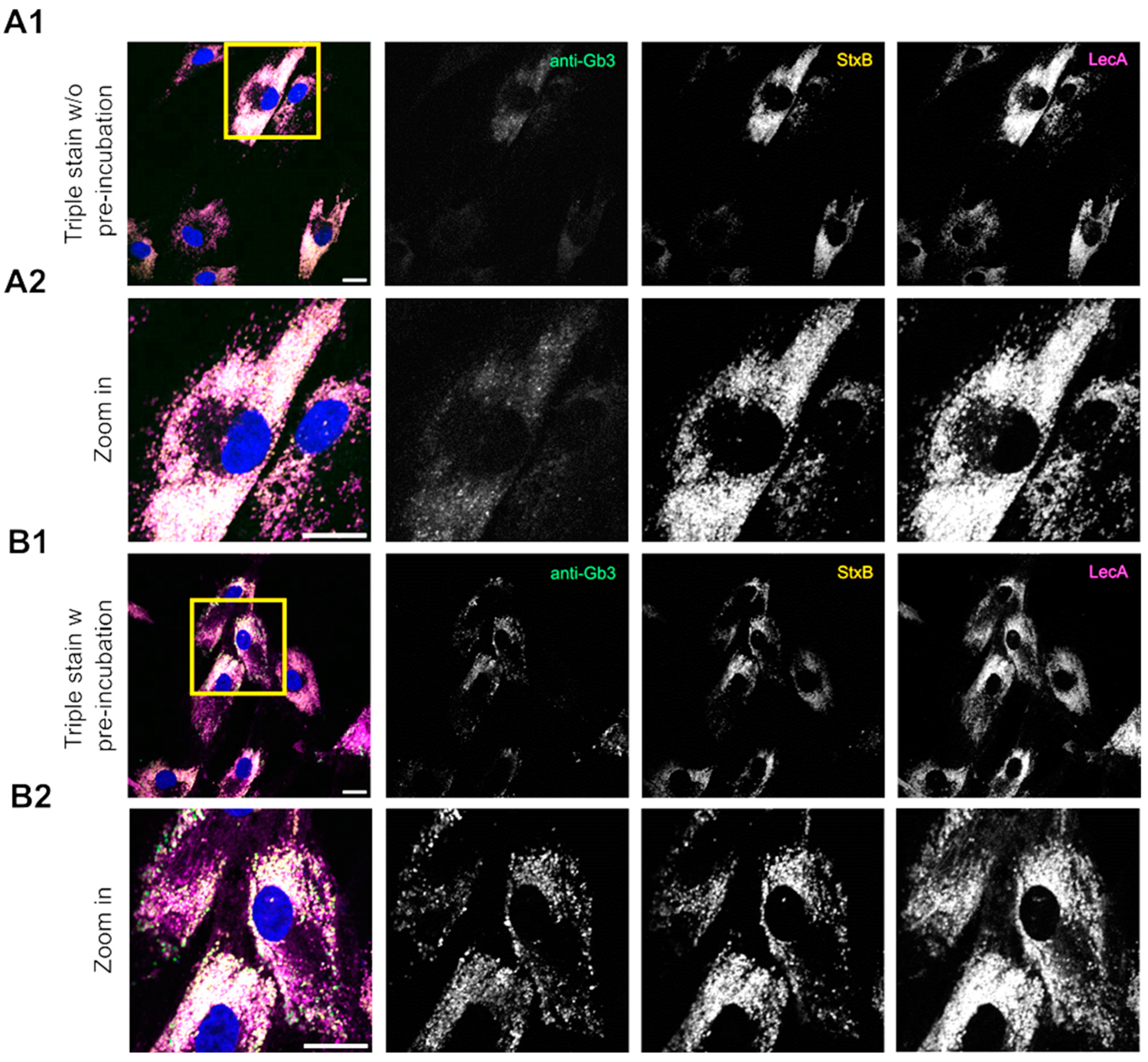
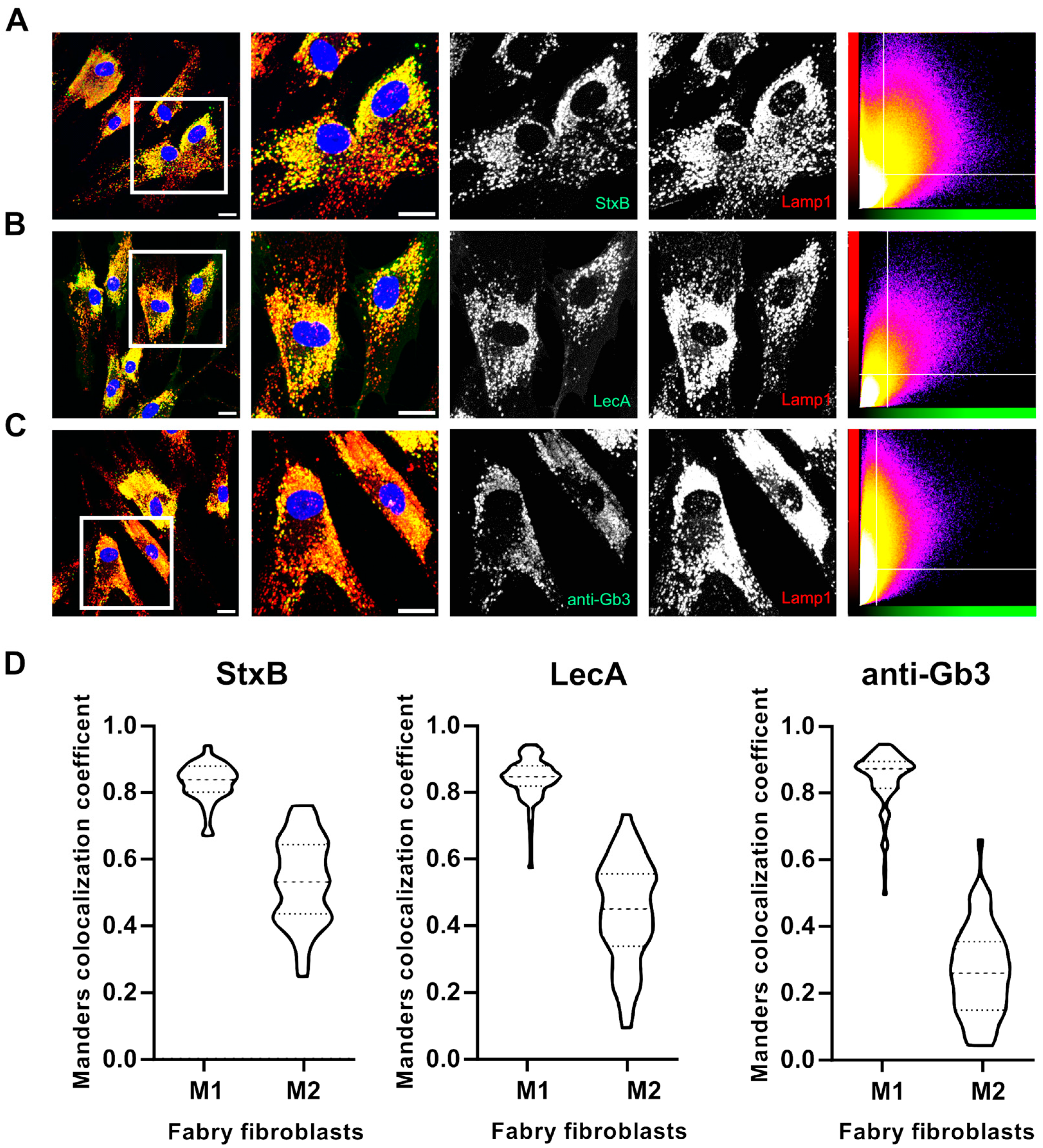
2.3. Lectins Exhibit Advanced Binding Kinetics in Fabry Fibroblasts Compared to an Anti-Gb3 Antibody in Immunofluorescence Assays
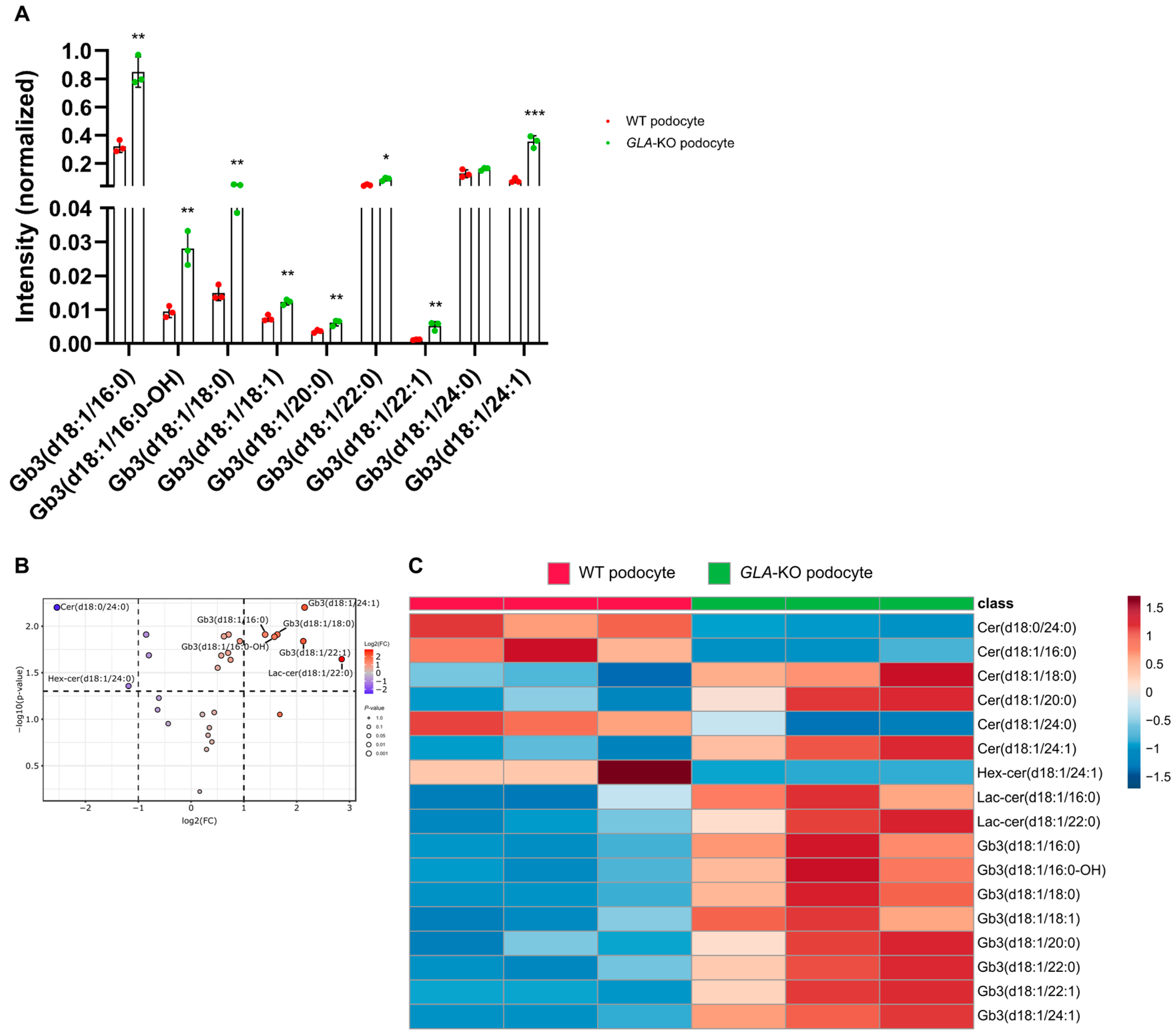
2.4. The Sphingolipid Metabolism Is Disturbed in Fabry Cells as Revealed by Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Antibodies and Chemicals
4.2. Cell Lines
4.3. Cell Culture
4.4. Lectin Labelling
4.5. Immunofluorescence Staining
4.6. Image Acquisition
4.7. Quantitative Colocalization Analysis
4.8. Flow Cytometry
4.9. Liquid Chromatography-Mass Spectrometry (LC/MS) Analysis of Glycosphingolipids
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Brandy, R.O. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967, 276, 2010–2011. [Google Scholar]
- Garman, S.C.; Garboczi, D.N. The Molecular Defect Leading to Fabry Disease: Structure of Human α-Galactosidase. J. Mol. Biol. 2004, 337, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; Van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated Globotriaosylsphingosine Is a Hallmark of Fabry Disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Kanack, A.J.; Dahms, N.M. Progress in the Understanding and Treatment of Fabry Disease. Biochim. Biophys. Acta. Gen. Subj. 2020, 1864, 129437. [Google Scholar] [CrossRef]
- Kok, K.; Zwiers, K.C.; Boot, R.G.; Overkleeft, H.S.; Aerts, J.M.F.G.; Artola, M. Fabry Disease: Molecular Basis, Pathophysiology, Diagnostics and Potential Therapeutic Directions. Biomolecules 2021, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Aoki, K.; Moehring, F.; Murphy, C.A.; O’Hara, C.L.; Tiemeyer, M.; Stucky, C.L.; Dahms, N.M. Neuropathic Pain in a Fabry Disease Rat Model. JCI Insight 2018, 3, 99171. [Google Scholar] [CrossRef]
- Lücke, T.; Höppner, W.; Schmidt, E.; Illsinger, S.; Das, A.M. Fabry Disease: Reduced Activities of Respiratory Chain Enzymes with Decreased Levels of Energy-Rich Phosphates in Fibroblasts. Mol. Genet. Metab. 2004, 82, 93–97. [Google Scholar] [CrossRef]
- Schumann, A.; Schaller, K.; Belche, V.; Cybulla, M.; Grünert, S.C.; Moers, N.; Sass, J.O.; Kaech, A.; Hannibal, L.; Spiekerkoetter, U. Defective Lysosomal Storage in Fabry Disease Modifies Mitochondrial Structure, Metabolism and Turnover in Renal Epithelial Cells. J. Inherit. Metab. Dis. 2021, 44, 1039–1050. [Google Scholar] [CrossRef]
- Consolato, F.; De Fusco, M.; Schaeffer, C.; Pieruzzi, F.; Scolari, F.; Gallieni, M.; Lanzani, C.; Feriozzi, S.; Rampoldi, L. α -Gal A Missense Variants Associated with Fabry Disease Can Lead to ER Stress and Induction of the Unfolded Protein Response. Mol. Genet. Metab. Rep. 2022, 33, 1–9. [Google Scholar] [CrossRef]
- Braun, F.; Abed, A.; Sellung, D.; Rogg, M.; Woidy, M.; Eikrem, O.; Wanner, N.; Gambardella, J.; Laufer, S.D.; Haas, F.; et al. Accumulation of α-Synuclein Mediates Podocyte Injury in Fabry Nephropathy. J. Clin. Investig. 2023, 133, 1–14. [Google Scholar] [CrossRef]
- Borisch, C.; Thum, T.; Bär, C.; Hoepfner, J. Human in Vitro Models for Fabry Disease: New Paths for Unravelling Disease Mechanisms and Therapies. J. Transl. Med. 2024, 22, 965. [Google Scholar] [CrossRef]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry Disease Revisited: Management and Treatment Recommendations for Adult Patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yin, L.; Theisen, M.; Zhuo, J.; Siddiqui, S.; Levy, B.; Presnyak, V.; Frassetto, A.; Milton, J.; Salerno, T.; et al. Systemic MRNA Therapy for the Treatment of Fabry Disease: Preclinical Studies in Wild-Type Mice, Fabry Mouse Model, and Wild-Type Non-Human Primates. Am. J. Hum. Genet. 2019, 104, 625–637. [Google Scholar] [CrossRef]
- Khan, A.; Barber, D.L.; Huang, J.; Rupar, C.A.; Rip, J.W.; Auray-Blais, C.; Boutin, M.; O’Hoski, P.; Gargulak, K.; McKillop, W.M.; et al. Lentivirus-Mediated Gene Therapy for Fabry Disease. Nat. Commun. 2021, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Vedder, A.C.; Linthorst, G.E.; van Breemen, M.J.; Groener, J.E.M.; Bemelman, F.J.; Strijland, A.; Mannens, M.M.A.M.; Aerts, J.M.F.G.; Hollak, C.E.M. The Dutch Fabry Cohort: Diversity of Clinical Manifestations and Gb3 Levels. J. Inherit. Metab. Dis. 2007, 30, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Lidove, O.; Jaussaud, R.; Knebelmann, B.; Barbey, F. The Role of Ceramide Trihexoside (Globotriaosylceramide) in the Diagnosis and Follow-up of the Efficacy of Treatment of Fabry Disease: A Review of the Literaturele. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 289–297. [Google Scholar] [CrossRef]
- Khan, A.; Barber, D.L.; Mckillop, W.M.; Rupar, C.A.; Auray-blais, C.; Fraser, G.; Fowler, D.H.; Berger, A.; Foley, R.; Keating, A.; et al. Lentivirus-Mediated Gene Therapy for Fabry Disease: 5-Year End-of-Study Results from the Canadian FACTs Trial. Clin. Transl. Med. 2025, 15, e70073. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Mehta, A.Y.; Tilton, C.A.; Cummings, R.D. Insights Into Glycobiology and the Protein-Glycan Interactome Using Glycan Microarray Technologies. Mol. Cell. Proteom. 2024, 23, 100844. [Google Scholar] [CrossRef] [PubMed]
- Haab, B.B.; Klamer, Z.; Haab, B.B.; Klamer, Z. Advances in Tools to Determine the Glycan-Binding Specificities of Lectins and Antibodies Authors Advances in Tools to Determine the Glycan- Binding Specificities of Lectins and Antibodies *. Mol. Cell. Proteom. 2020, 19, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Böttger, J.; Henkel, L.; Langjahr, M.; Mayer, C.; Nordbeck, P.; Wanner, C.; Sommer, C. Detection of Blood Gb3 Deposits as a New Tool for Diagnosis and Therapy Monitoring in Patients with Classic Fabry Disease. J. Intern. Med. 2018, 284, 427–438. [Google Scholar] [CrossRef]
- Haji-Ghassemi, O.; Gagnon, S.M.L.; Müller-Loennies, S.; Evans, S.V. Polyspecificity of Anti-Lipid A Antibodies and Its Relevance to the Development of Autoimmunity. In Protein Reviews; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Lardone, R.D.; Irazoqui, F.J.; Nores, G.A. Most of Anti-Glycolipid IgG-Antibodies Associated to Neurological Disorders Occur without Their IgM Counterpart. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Volynsky, P.; Efremov, R.; Mikhalev, I.; Dobrochaeva, K.; Tuzikov, A.; Korchagina, E.; Obukhova, P.; Rapoport, E.; Bovin, N. Why Human Anti-Galα1–4Galβ1–4Glc Natural Antibodies Do Not Recognize the Trisaccharide on Erythrocyte Membrane? Molecular Dynamics and Immunochemical Investigation. Mol. Immunol. 2017, 90, 87–97. [Google Scholar] [CrossRef]
- Lingwood, C.A. Glycosphingolipid Functions. Cold Spring Harb. Perspect. Biol. 2011, 3, 004788. [Google Scholar] [CrossRef]
- Lingwood, D.; Binnington, B.; Róg, T.; Vattulainen, I.; Grzybek, M.; Coskun, Ü.; Lingwood, C.A.; Simons, K. Cholesterol Modulates Glycolipid Conformation and Receptor Activity. Nat. Chem. Biol. 2011, 7, 260–262. [Google Scholar] [CrossRef]
- Krishnan, P.; Singla, A.; Lee, C.A.; Weatherston, J.D.; Worstell, N.C.; Wu, H.J. Hetero-Multivalent Binding of Cholera Toxin Subunit B with Glycolipid Mixtures. Colloids Surf. B Biointerfaces 2017, 160, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Siukstaite, L.; Imberty, A.; Römer, W. Structural Diversities of Lectins Binding to the Glycosphingolipid Gb3. Front. Mol. Biosci. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Schubert, T.; Sych, T.; Madl, J.; Xu, M.; Omidvar, R.; Patalag, L.J.; Ries, A.; Kettelhoit, K.; Brandel, A.; Mely, Y.; et al. Differential Recognition of Lipid Domains by Two Gb3-Binding Lectins. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Johannes, L.; Römer, W. Shiga Toxins from Cell Biology to Biomedical Applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef]
- Lauvrak, S.U.; Torgersen, M.L.; Sandvig, K. Efficient Endosome-to-Golgi Transport of Shiga Toxin Is Dependent on Dynamin and Clathrin. J. Cell Sci. 2004, 117, 2321–2331. [Google Scholar] [CrossRef]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.E.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga Toxin Induces Tubular Membrane Invaginations for Its Uptake into Cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Kociurzynski, R.; Makshakova, O.N.; Knecht, V.; Römer, W. Multiscale Molecular Dynamics Studies Reveal Different Modes of Receptor Clustering by Gb3-Binding Lectins. J. Chem. Theory Comput. 2021, 17, 2488–2501. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.; Nurisso, A.; Hollville, E.; Tétaud, C.; Wiels, J.; Pokorná, M.; Wimmerová, M.; Varrot, A.; Imberty, A. Structural Basis of the Preferential Binding for Globo-Series Glycosphingolipids Displayed by Pseudomonas Aeruginosa Lectin I. J. Mol. Biol. 2008, 383, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Eierhoff, T.; Aigal, S.; Brandel, A.; Thuenauer, R.; de Bentzmann, S.; Imberty, A.; Römer, W. The Pseudomonas Aeruginosa Lectin LecA Triggers Host Cell Signalling by Glycosphingolipid-Dependent Phosphorylation of the Adaptor Protein CrkII. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Brandel, A.; Aigal, S.; Lagies, S.; Schlimpert, M.; Lehmann, A.; Hummel, D.; Fisch, D.; Meléndez, A.V.; Madl, J.; Eierhoff, T.; et al. The Gb3-Enriched CD59/Flotillin Plasma Membrane Domain Regulates Host Cell Invasion by Pseudomonas Aeruginosa. bioRxiv 2020. [Google Scholar] [CrossRef]
- Eierhoff, T.; Bastian, B.; Thuenauer, R.; Madl, J.; Audfray, A.; Aigal, S.; Juillot, S.; Rydell, G.E.; Muller, S.; De Bentzmann, S.; et al. A Lipid Zipper Triggers Bacterial Invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 12895–12900. [Google Scholar] [CrossRef]
- Qin, G.; Takenaka, T.; Telsch, K.; Kelley, L.; Howard, T.; Levade, T.; Deans, R.; Howard, B.H.; Malech, H.L.; Brady, R.O.; et al. Preselective Gene Therapy for Fabry Disease. Proc. Natl. Acad. Sci. USA 2001, 98, 3428–3433. [Google Scholar] [CrossRef]
- Thomaidis, T.; Relle, M.; Golbas, M.; Brochhausen, C.; Galle, P.R.; Beck, M.; Schwarting, A. Downregulation of α-Galactosidase A Upregulates CD77: Functional Impact for Fabry Nephropathy. Kidney Int. 2009, 75, 399–407. [Google Scholar] [CrossRef][Green Version]
- Meléndez, A.V.; Velasco Cárdenas, R.M.H.; Lagies, S.; Strietz, J.; Siukstaite, L.; Thomas, O.S.; Tomisch, J.; Weber, W.; Kammerer, B.; Römer, W.; et al. Novel Lectin-Based Chimeric Antigen Receptors Target Gb3-Positive Tumour Cells. Cell. Mol. Life Sci. 2022, 79, 1–25. [Google Scholar] [CrossRef]
- Sueoka, H.; Aoki, M.; Tsukimura, T.; Togawa, T.; Sakuraba, H. Distributions of Globotriaosylceramide Isoforms, and Globotriaosylsphingosine and Its Analogues in an α-Galactosidase a Knockout Mouse, a Model of Fabry Disease. PLoS ONE 2015, 10, e0144958. [Google Scholar] [CrossRef]
- Krüger, R.; Tholey, A.; Jakoby, T.; Vogelsberger, R.; Mönnikes, R.; Rossmann, H.; Beck, M.; Lackner, K.J. Quantification of the Fabry Marker LysoGb3 in Human Plasma by Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 883–884, 128–135. [Google Scholar] [CrossRef]
- Alroy, J.; Sabnis, S.; Kopp, J.B. Renal Pathology in Fabry Disease. J. Am. Soc. Nephrol. 2002, 13, S134–S138. [Google Scholar] [CrossRef] [PubMed]
- Lagies, S.; Pichler, R.; Vladimirov, G.; Gawron, J.; Bäzner, F.; Schreiner, A.; Kadena, D.; Plattner, D.A.; Lienkamp, S.S.; Kammerer, B. Metabolic and Lipidomic Assessment of Kidney Cells Exposed to Nephrotoxic Vancomycin Dosages. Int. J. Mol. Sci. 2021, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.D. The Question to Rid Labs of the Reagents That Ruin Experiments. Nat. Featur. 2024, 635, 26–28. [Google Scholar] [CrossRef]
- Rosato, F.; Pasupuleti, R.; Tomisch, J.; Meléndez, A.V.; Kolanovic, D.; Makshakova, O.N.; Wiltschi, B.; Römer, W. A Bispecific, Crosslinking Lectibody Activates Cytotoxic T Cells and Induces Cancer Cell Death. J. Transl. Med. 2022, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kanack, A.J.; Prodoehl, E.; Ishihara-Aoki, M.; Aoki, K.; Dahms, N.M. Glycosphingolipids and Their Impact on Platelet Activity in a Murine Model of Fabry Disease. Sci. Rep. 2024, 14, 29488. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Grüner, J.; Breyer, M.; Schlegel, J.; Schottmann, N.M.; Hofmann, L.; Gauss, K.; Mease, R.; Erbacher, C.; Finke, L.; et al. Small Fibre Neuropathy in Fabry Disease: A Human-Derived Neuronal in Vitro Disease Model and Pilot Data. Brain Commun. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Hofmann, L.; Hose, D.; Grießhammer, A.; Blum, R.; Döring, F.; Dib-Hajj, S.; Waxman, S.; Sommer, C.; Wischmeyer, E.; Üçeyler, N. Characterization of Small Fiber Pathology in a Mouse Model of Fabry Disease. Elife 2018, 7, e39300. [Google Scholar] [CrossRef] [PubMed]
- Worstell, N.C.; Singla, A.; Saenkham, P.; Galbadage, T.; Sule, P.; Lee, D.; Mohr, A.; Kwon, J.S., II; Cirillo, J.D.; Wu, H.J. Hetero-Multivalency of Pseudomonas Aeruginosa Lectin LecA Binding to Model Membranes. Sci. Rep. 2018, 8, 8419. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Lavoie, P.; Martineau, T.; Rupar, C.A.; Barber, D.L.; Keating, A.; Foley, R.; Khan, A.; West, M.L.; Medin, J.A. Longitudinal Biomarker Evaluation in Fabry Disease Patients Receiving Lentivirus-Mediated Gene Therapy. Rare Dis. Orphan Drugs J. 2024, 3, 18. [Google Scholar] [CrossRef]
- Ohshima, T.; Murray, G.J.; Swaim, W.D.; Longenecker, G.; Quirk, J.M.; Cardarelli, C.O.; Sugimoto, Y.; Pastan, I.; Gottesman, M.M.; Brady, R.O.; et al. α-Galactosidase A Deficient Mice: A Model of Fabry Disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2540–2544. [Google Scholar] [CrossRef]
- Sample Detail: GM00107-Coriell Institute. Available online: https://www.coriell.org/0/Sections/Search/Sample_Detail.aspx?Ref=GM00107 (accessed on 24 February 2025).
- Thurberg, B.L.; Rennke, H.; Colvin, R.B.; Dikman, S.; Gordon, R.E.; Collins, A.B.; Desnick, R.J.; O’Callaghan, M. Globotriaosylceramide Accumulation in the Fabry Kidney Is Cleared from Multiple Cell Types after Enzyme Replacement Therapy. Kidney Int. 2002, 62, 1933–1946. [Google Scholar] [CrossRef]
- Welford, R.W.D.; Mühlemann, A.; Garzotti, M.; Rickert, V.; Groenen, P.M.A.; Morand, O.; Üçeyler, N.; Probst, M.R. Glucosylceramide Synthase Inhibition with Lucerastat Lowers Globotriaosylceramide and Lysosome Staining in Cultured Fibroblasts from Fabry Patients with Different Mutation Types. Hum. Mol. Genet. 2018, 27, 3392–3403. [Google Scholar] [CrossRef]
- Monticelli, M.; Hay Mele, B.; Allocca, M.; Liguori, L.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Andreotti, G. Curcumin Has Beneficial Effects on Lysosomal Alpha-Galactosidase: Potential Implications for the Cure of Fabry Disease. Int. J. Mol. Sci. 2023, 24, 1095. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, M.; Liguori, L.; Allocca, M.; Bosso, A.; Andreotti, G.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Mele, B.H. Drug Repositioning for Fabry Disease: Acetylsalicylic Acid Potentiates the Stabilization of Lysosomal Alpha-Galactosidase by Pharmacological Chaperones. Int. J. Mol. Sci. 2022, 23, 5105. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Blomberg, L.; Brodesser, S.; Liebau, M.C.; Schermer, B.; Benzing, T.; Kurschat, C.E. Enzyme Replacement Therapy Clears GB3 Deposits from a Podocyte Cell Culture Model of Fabry Disease but Fails to Restore Altered Cellular Signaling. Cell. Physiol. Biochem. 2019, 52, 1139–1150. [Google Scholar] [CrossRef]
- Porto, C.; Pisani, A.; Rosa, M.; Acampora, E.; Avolio, V.; Tuzzi, M.R.; Visciano, B.; Gagliardo, C.; Materazzi, S.; La Marca, G.; et al. Synergy between the Pharmacological Chaperone 1-Deoxygalactonojirimycin and the Human Recombinant Alpha-Galactosidase A in Cultured Fibroblasts from Patients with Fabry Disease. J. Inherit. Metab. Dis. 2012, 35, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, E.; Kleiser, S.; Sengle, G.; Bruckner-Tuderman, L.; Nyström, A. Diversity of Mechanisms Underlying Latent TGF-β Activation in Recessive Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2021, 141, 1450–1460.e9. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Chang, Y.X.; Ni, L.; Mathieson, P.W.; Mundel, P. A Conditionally Immortalized Human Podocyte Cell Line Demonstrating Nephrin and Podocin Expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of Co-localization of Objects in Dual-colour Confocal Images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N.; Smith, P.L.; Reid, D.B.; Environment, C.; Palo, L.; Alto, P.; Smith, P.L. Otsu 1979 Otsu Method. IEEE Trans. Syst. Man. Cybern. 1979, C, 62–66. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A Guided Tour into Subcellular Colocalization Analysis in Light Microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A Practical Guide to Evaluating Colocalization in Biological Microscopy. Am. J. Physiol.-Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elçin-Guinot, S.; Lagies, S.; Avi-Guy, Y.; Neugebauer, D.; Huber, T.B.; Schell, C.; Kammerer, B.; Römer, W. Lectin-Based Substrate Detection in Fabry Disease Using the Gb3-Binding Lectins StxB and LecA. Int. J. Mol. Sci. 2025, 26, 2272. https://doi.org/10.3390/ijms26052272
Elçin-Guinot S, Lagies S, Avi-Guy Y, Neugebauer D, Huber TB, Schell C, Kammerer B, Römer W. Lectin-Based Substrate Detection in Fabry Disease Using the Gb3-Binding Lectins StxB and LecA. International Journal of Molecular Sciences. 2025; 26(5):2272. https://doi.org/10.3390/ijms26052272
Chicago/Turabian StyleElçin-Guinot, Serap, Simon Lagies, Yoav Avi-Guy, Daniela Neugebauer, Tobias B. Huber, Christoph Schell, Bernd Kammerer, and Winfried Römer. 2025. "Lectin-Based Substrate Detection in Fabry Disease Using the Gb3-Binding Lectins StxB and LecA" International Journal of Molecular Sciences 26, no. 5: 2272. https://doi.org/10.3390/ijms26052272
APA StyleElçin-Guinot, S., Lagies, S., Avi-Guy, Y., Neugebauer, D., Huber, T. B., Schell, C., Kammerer, B., & Römer, W. (2025). Lectin-Based Substrate Detection in Fabry Disease Using the Gb3-Binding Lectins StxB and LecA. International Journal of Molecular Sciences, 26(5), 2272. https://doi.org/10.3390/ijms26052272




