The Non-Antibacterial Effects of Azithromycin and Other Macrolides on the Bronchial Epithelial Barrier and Cellular Differentiation
Abstract
1. Introduction
2. Results
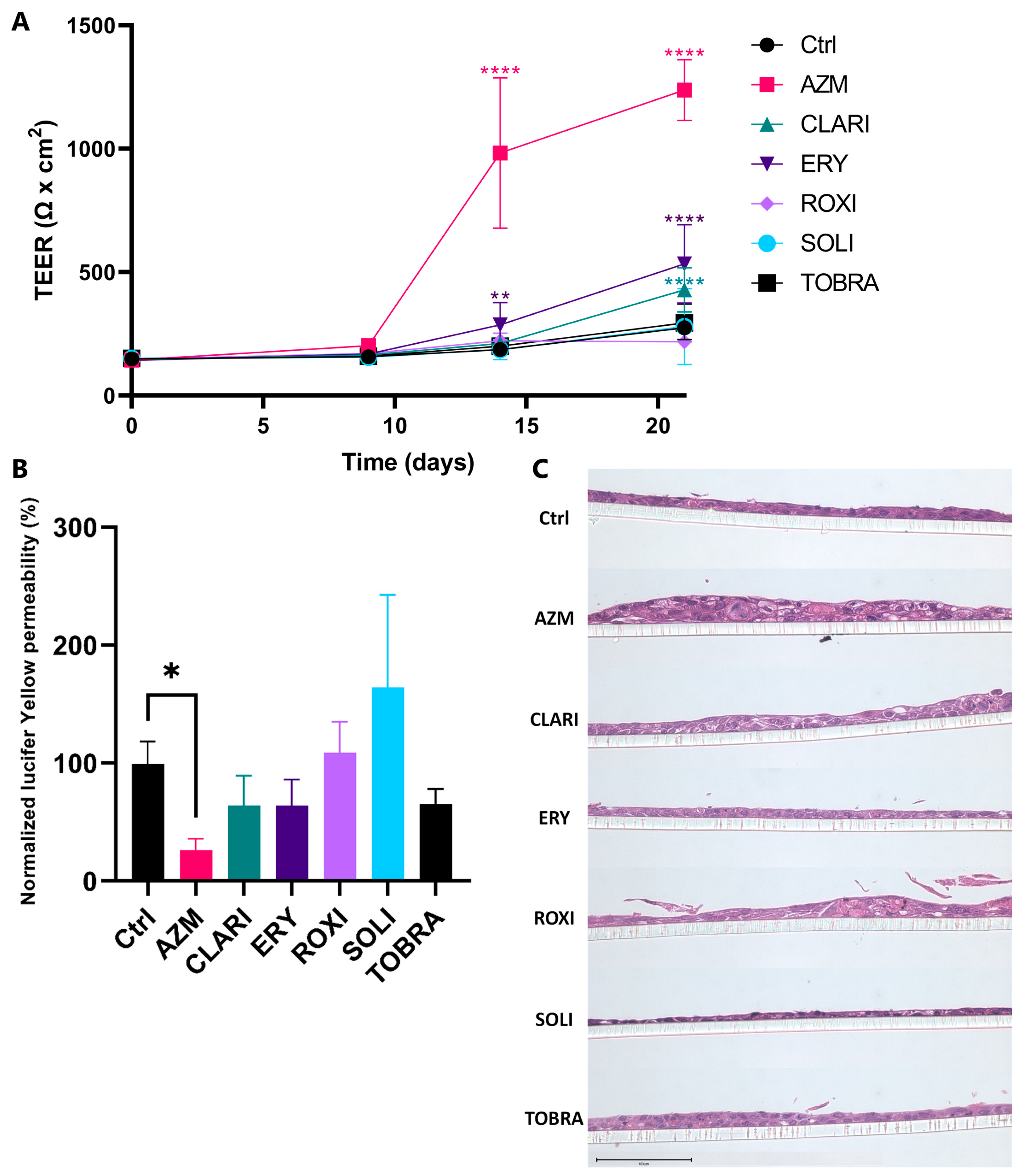
2.1. Azithromycin Enhances the Bronchial Epithelial Barrier Function More than Other Macrolides
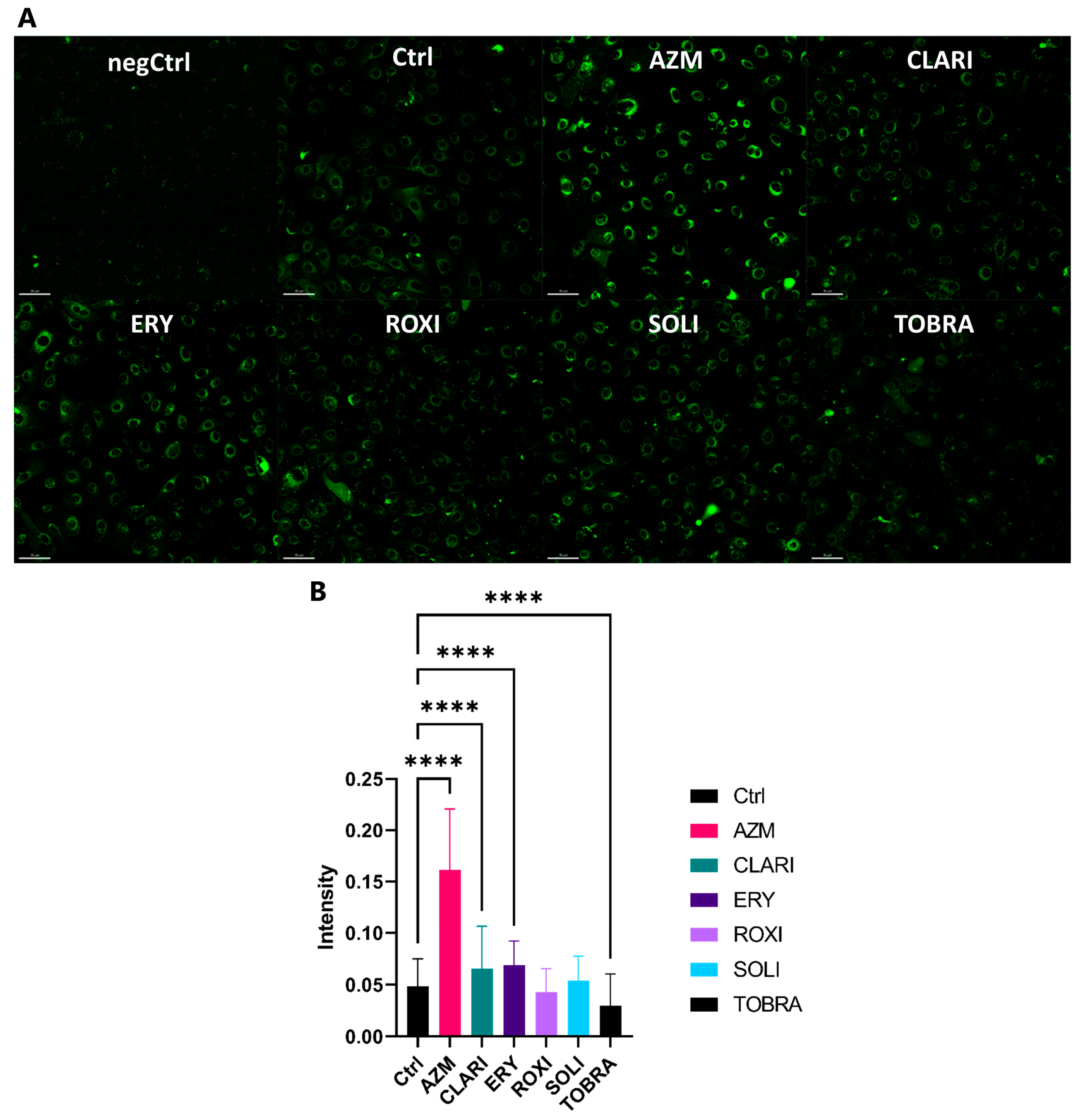
2.2. AZM Affects the Expression and Organization of Factors Participating in Cell Junctions and Tight Junctions
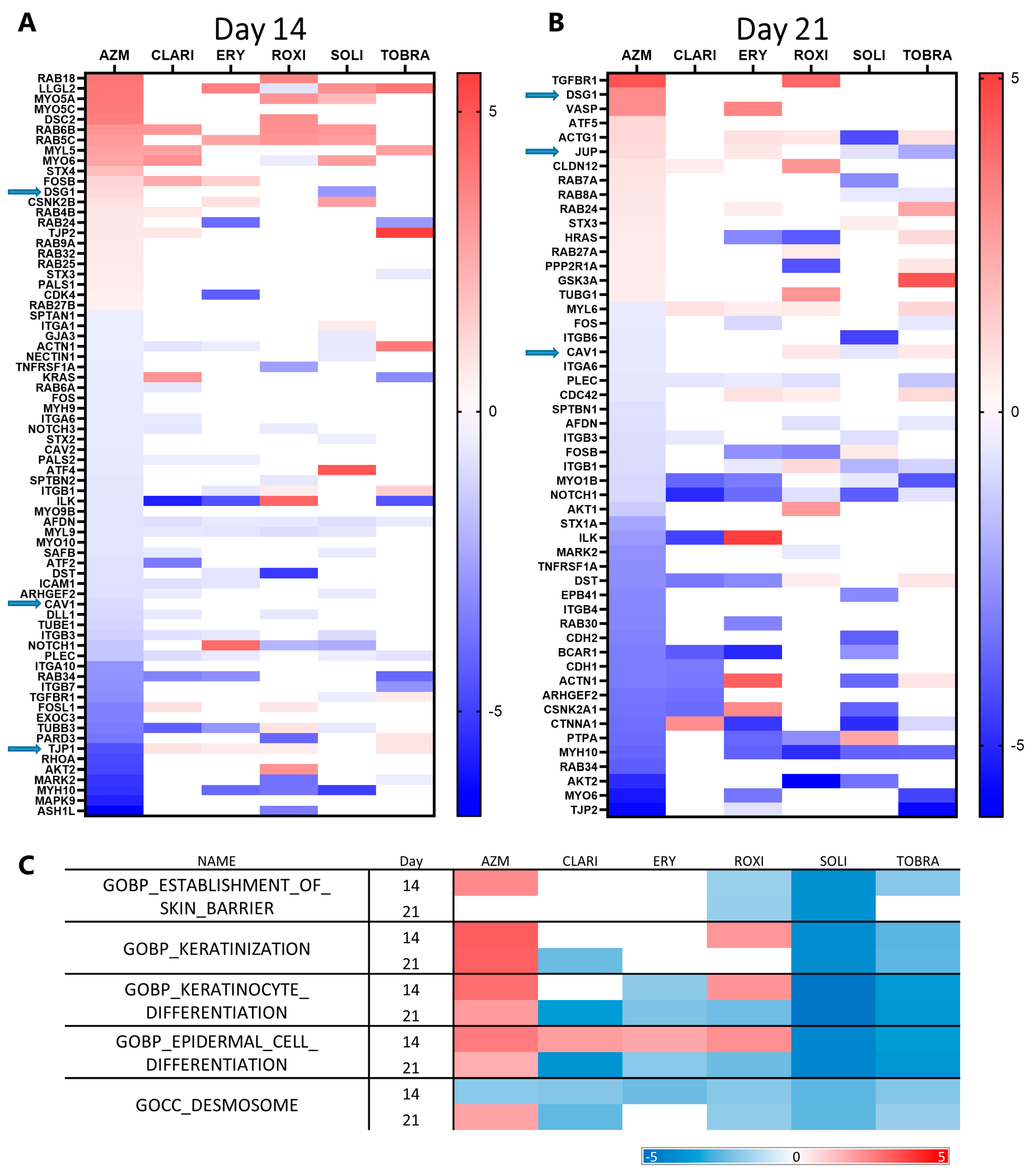
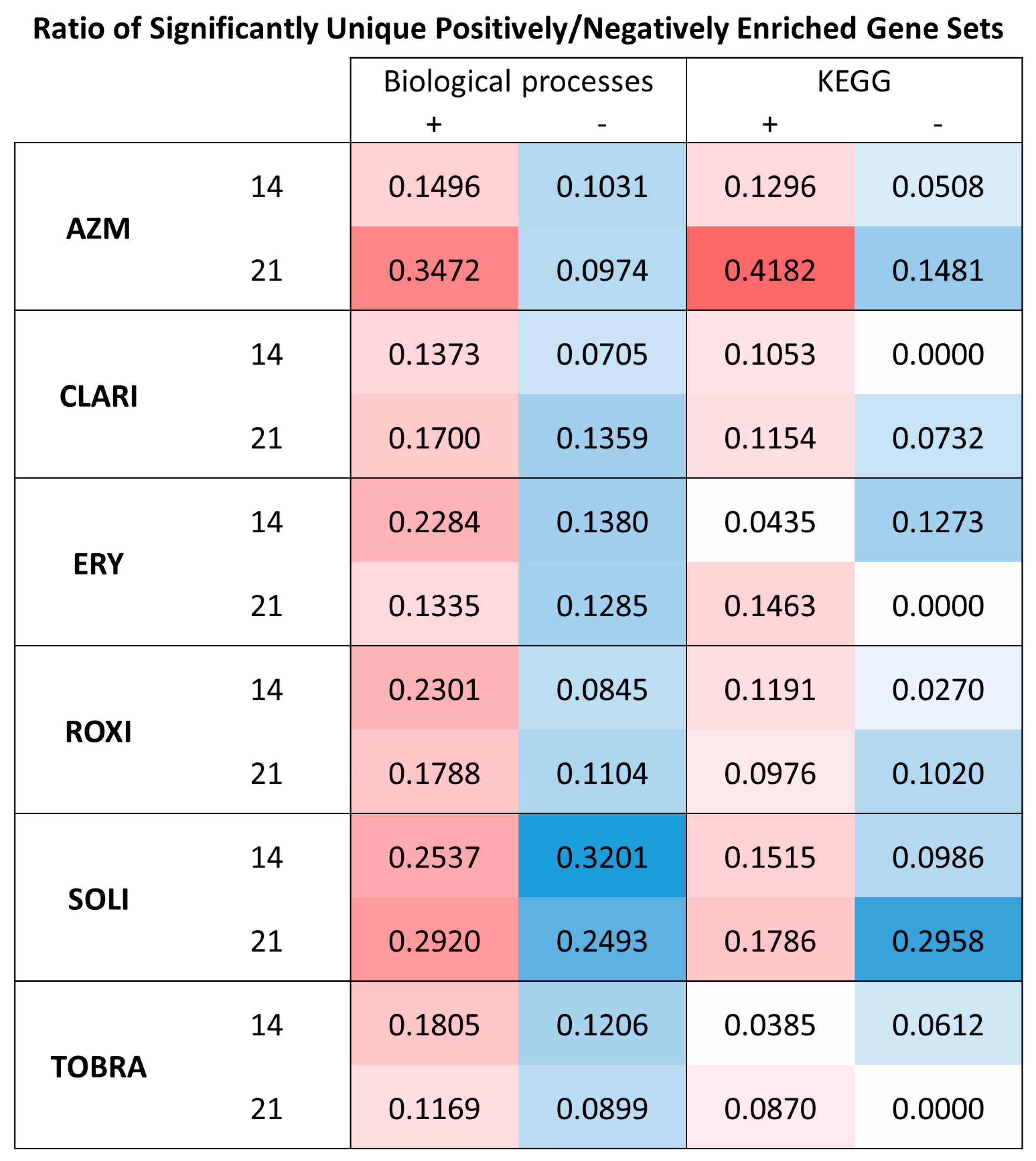
2.3. Gene Set Enrichment Analysis Indicates All Macrolides Have a General Effect on Metabolism and EMT
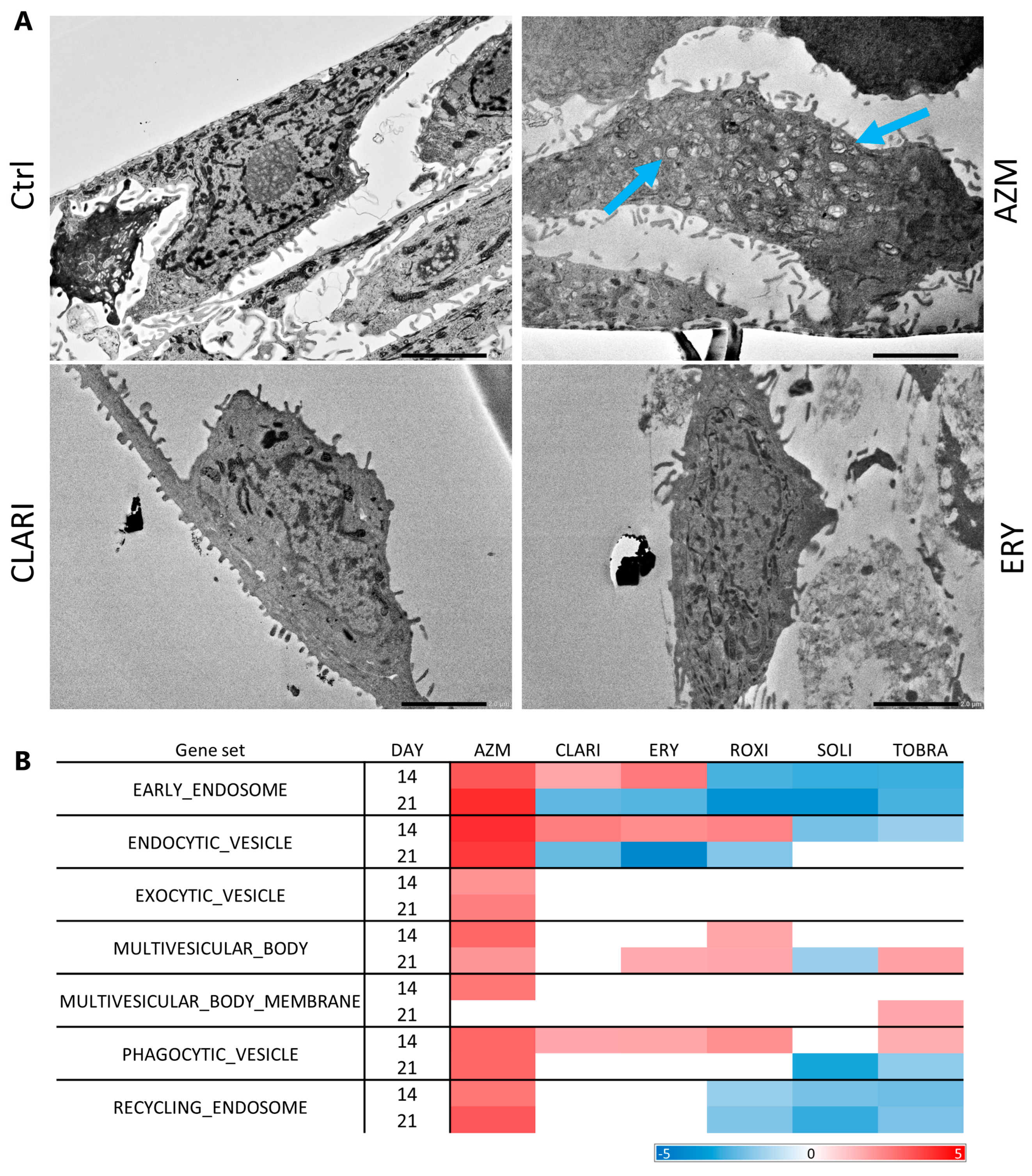
2.4. Azithromycin Treatment Induces a Higher Build-Up of Phospholipids and Vesicles than Other Macrolides
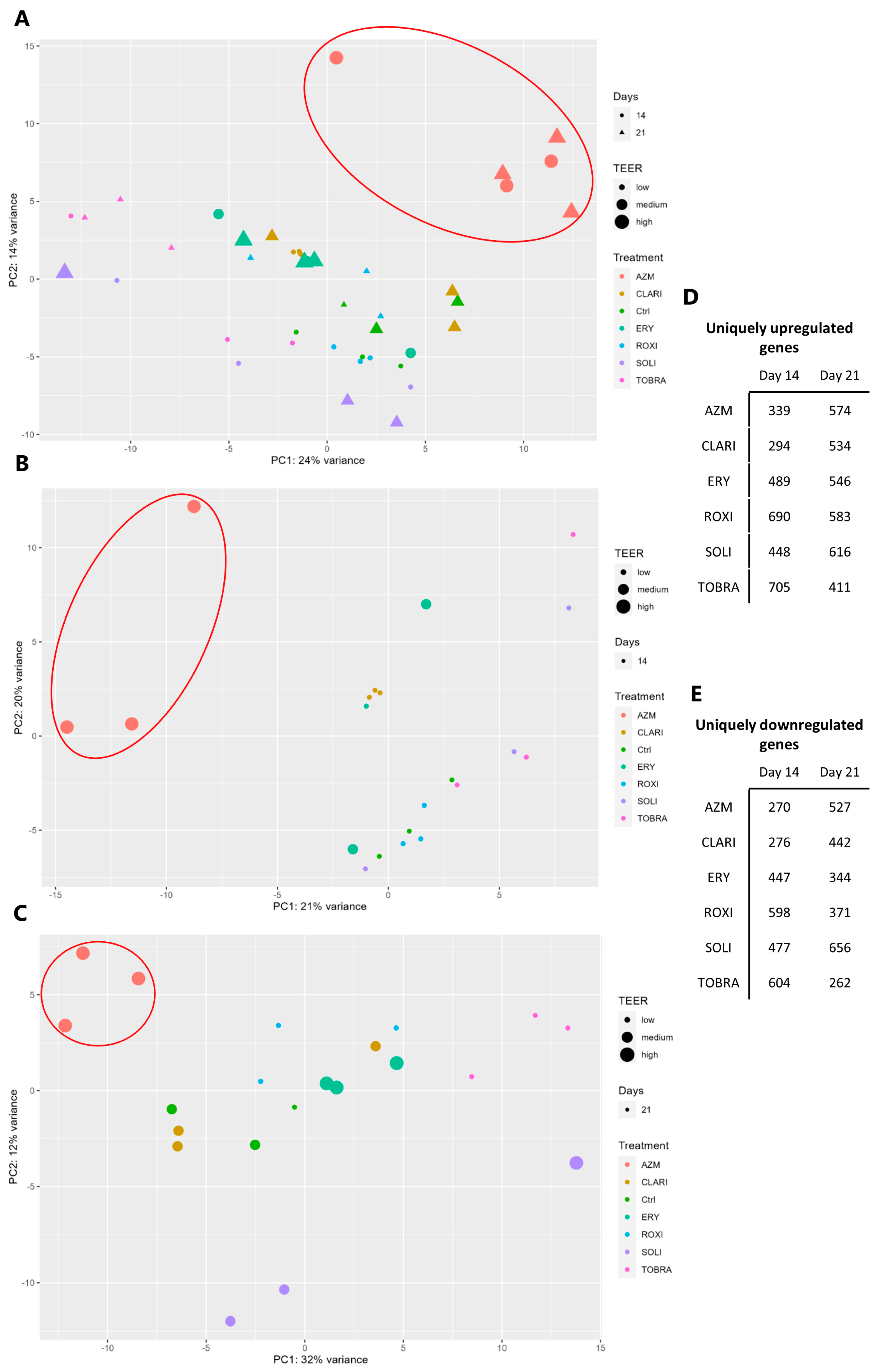
2.5. RNA Sequencing Analyses Show That AZM Has a Distinct Expression Pattern Compared to Other Macrolides
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transepithelial Electrical Resistance
4.3. Paracellular Flux
4.4. RNA Sequencing and Analysis of Gene Expression
4.5. Immunoblotting
4.6. Lipid Retention
4.7. Immunostaining
4.8. Transmission Electron Microscopy
4.9. Venn Diagrams
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Air-liquid interface |
| AZM | Azithromycin |
| CAV-1 | Caveolin-1 |
| CF | Cystic fibrosis |
| CK14 | Cytokeratin 14 |
| CLARI | Clarithromycin |
| COPD | Chronic obstructive pulmonary disease |
| Ctrl | Control |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DSG-1 | Desmoglein-1 |
| EMT | Epithelial-to-mesenchymal transition |
| ERY | Erythromycin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GOCC | Gene ontology cellular component |
| GOBP | Gene ontology biological process |
| GSEA | Gene set enrichment analysis |
| H&E | Hematoxylin and eosin |
| IPF | Idiopathic pulmonary fibrosis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LPS | Lipopolysaccharide |
| NaCac | Sodium-cacodylate buffer |
| PCA | Principal component analysis |
| PFA | Paraformaldehyde |
| ROXI | Roxithromycin |
| SOLI | Solithromycin |
| TEER | Transepithelial electrical resistance |
| TOBRA | Tobramycin |
| ZO-1 | Zonula occludens-1 |
References
- Raby, K.L.; Michaeloudes, C.; Tonkin, J.; Chung, K.F.; Bhavsar, P.K. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front. Immunol. 2023, 14, 1201658. [Google Scholar] [CrossRef] [PubMed]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflügers Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Gardarsson, F.R.; Kricker, J.A.; Gudjonsson, T.; Norris, V.; Parnham, M.J. Macrolides and Diseases Associated with Loss of Epithelial Barrier Integrity. In Macrolides as Immunomodulatory Agents; Rubin, B.K., Shinkai, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 3–23. [Google Scholar]
- Knight, D.A.; Grainge, C.L.; Stick, S.M.; Kicic, A.; Schuliga, M. Epithelial Mesenchymal Transition in Respiratory Disease: Fact or Fiction. Chest 2020, 157, 1591–1596. [Google Scholar] [CrossRef]
- Halldorsson, S.; Asgrimsson, V.; Axelsson, I.; Gudmundsson, G.H.; Steinarsdottir, M.; Baldursson, O.; Gudjonsson, T. Differentiation potential of a basal epithelial cell line established from human bronchial explant. In Vitro Cell Dev. Biol. Anim. 2007, 43, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Masamune, S.; Bates, G.S.; Corcoran, J.W. Macrolides. Recent progress in chemistry and biochemistry. Angew. Chem. Int. Ed. Engl. 1977, 16, 585–607. [Google Scholar] [CrossRef]
- Woodward, R.B. Struktur und Biogenese der Makrolide. Eine neue Klasse von Naturstoffen. Angew. Chem. 1957, 69, 50–58. [Google Scholar] [CrossRef]
- Mazzei, T.; Mini, E.; Novelli, A.; Periti, P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31 (Suppl. C), 1–9. [Google Scholar] [CrossRef]
- McGuire, J.M.; Bunch, R.L.; Anderson, R.C.; Boaz, H.E.; Flynn, E.H.; Powell, H.M.; Smith, J.W. Ilotycin, a new antibiotic. Antibiot. Chemother. 1952, 2, 281–283. [Google Scholar]
- Brittain, D.C. Erythromycin. Med. Clin. N. Am. 1987, 71, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Kricker, J.A.; Page, C.P.; Gardarsson, F.R.; Baldursson, O.; Gudjonsson, T.; Parnham, M.J. Nonantimicrobial Actions of Macrolides: Overview and Perspectives for Future Development. Pharmacol. Rev. 2021, 73, 233–262. [Google Scholar] [CrossRef]
- Parnham, M.J.; Norris, V.; Kricker, J.A.; Gudjonsson, T.; Page, C.P. Prospects for macrolide therapy of asthma and COPD. Adv. Pharmacol. 2023, 98, 83–110. [Google Scholar] [PubMed]
- Girard, A.E.; Girard, D.; English, A.R.; Gootz, T.D.; Cimochowski, C.R.; Faiella, J.A.; Haskell, S.L.; Retsema, J.A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob. Agents Chemother. 1987, 31, 1948–1954. [Google Scholar] [CrossRef]
- Retsema, J.; Girard, A.; Schelkly, W.; Manousos, M.; Anderson, M.; Bright, G.; Borovoy, R.; Brennan, L.; Mason, R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob. Agents Chemother. 1987, 31, 1939–1947. [Google Scholar] [CrossRef]
- Omura, S.; Morimoto, S.; Nagate, T.; Adachi, T.; Kohno, Y. Research and development of clarithromycin. Yakugaku Zasshi 1992, 112, 593–614. [Google Scholar] [CrossRef]
- Chantot, J.F.; Bryskier, A.; Gasc, J.C. Antibacterial activity of roxithromycin: A laboratory evaluation. J. Antibiot. 1986, 39, 660–668. [Google Scholar] [CrossRef]
- Llano-Sotelo, B.; Dunkle, J.; Klepacki, D.; Zhang, W.; Fernandes, P.; Cate, J.H.; Mankin, A.S. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents. Chemother. 2010, 54, 4961–4970. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503. [Google Scholar]
- Umezawa, S. Structures and syntheses of aminoglycoside antibiotics. Adv. Carbohydr. Chem. Biochem. 1974, 30, 111–182. [Google Scholar] [PubMed]
- Mogayzel, P.J., Jr.; Naureckas, E.T.; Robinson, K.A.; Mueller, G.; Hadjiliadis, D.; Hoag, J.B.; Lubsch, L.; Hazle, L.; Sabadosa, K.; Marshall, B. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2013, 187, 680–689. [Google Scholar] [CrossRef]
- Bailly, S.; Pocidalo, J.J.; Fay, M.; Gougerot-Pocidalo, M.A. Differential modulation of cytokine production by macrolides: Interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob. Agents Chemother. 1991, 35, 2016–2019. [Google Scholar] [CrossRef]
- Keicho, N.; Kudoh, S. Diffuse panbronchiolitis: Role of macrolides in therapy. Am. J. Respir. Med. 2002, 1, 119–131. [Google Scholar] [CrossRef]
- Keicho, N.; Kudoh, S.; Yotsumoto, H.; Akagawa, K.S. Erythromycin promotes monocyte to macrophage differentiation. J. Antibiot. 1994, 47, 80–89. [Google Scholar] [CrossRef][Green Version]
- Takizawa, H.; Desaki, M.; Ohtoshi, T.; Kikutani, T.; Okazaki, H.; Sato, M.; Akiyama, N.; Shoji, S.; Hiramatsu, K.; Ito, K. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: A potential mechanism of its anti-inflammatory action. Biochem. Biophys. Res. Commun. 1995, 210, 781–786. [Google Scholar] [CrossRef]
- Morikawa, K.; Watabe, H.; Araake, M.; Morikawa, S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 1996, 40, 1366–1370. [Google Scholar] [CrossRef]
- Khan, A.A.; Slifer, T.R.; Araujo, F.G.; Remington, J.S. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int. J. Antimicrob. Agents 1999, 11, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shu, D.; Ying, S.; Dai, Y.; Zhang, Q.; Chen, X.; Chen, H.; Dai, W. Roxithromycin attenuates inflammation via modulation of RAGE-influenced calprotectin expression in a neutrophilic asthma model. Ann. Transl. Med. 2021, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; Wang, R.L.; Dai, Y.R.; Li, F.Q.; Wu, H.Y.; Yan, S.S.; Wang, L.R.; Jin, L.D.; Xia, X.D. Roxithromycin suppresses airway remodeling and modulates the expression of caveolin-1 and phospho-p42/p44MAPK in asthmatic rats. Int. Immunopharmacol. 2015, 24, 247–255. [Google Scholar] [CrossRef]
- Kimura, Y.; Shinoda, M.; Shinkai, M.; Kaneko, T. Solithromycin inhibits IL-13-induced goblet cell hyperplasia and MUC5AC, CLCA1, and ANO1 in human bronchial epithelial cells. PeerJ 2023, 11, e14695. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, Y.; Morinaga, Y.; Kaku, N.; Uno, N.; Kosai, K.; Sakamoto, K.; Hasegawa, H.; Yanagihara, K. A novel macrolide, solithromycin suppresses mucin overexpression induced by Pseudomonas aeruginosa LPS in airway epithelial cells. J. Infect. Chemother. 2020, 26, 1008–1010. [Google Scholar] [CrossRef]
- Nakamura, S.; Yanagihara, K.; Araki, N.; Yamada, K.; Morinaga, Y.; Izumikawa, K.; Seki, M.; Kakeya, H.; Yamamoto, Y.; Kamihira, S.; et al. High-dose tobramycin inhibits lipopolysaccharide-induced MUC5AC production in human lung epithelial cells. Eur. J. Pharmacol. 2011, 659, 67–71. [Google Scholar] [CrossRef]
- Liu, Y.; Kam, W.R.; Ding, J.; Sullivan, D.A. One man’s poison is another man’s meat: Using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology 2014, 320, 1–5. [Google Scholar] [CrossRef]
- Tyteca, D.; Schanck, A.; Dufrene, Y.F.; Deleu, M.; Courtoy, P.J.; Tulkens, P.M.; Mingeot-Leclercq, M.P. The macrolide antibiotic azithromycin interacts with lipids and affects membrane organization and fluidity: Studies on Langmuir-Blodgett monolayers, liposomes and J774 macrophages. J. Membr. Biol. 2003, 192, 203–215. [Google Scholar] [CrossRef]
- Kosol, S.; Schrank, E.; Krajacic, M.B.; Wagner, G.E.; Meyer, N.H.; Gobl, C.; Rechberger, G.N.; Zangger, K.; Novak, P. Probing the interactions of macrolide antibiotics with membrane-mimetics by NMR spectroscopy. J. Med. Chem. 2012, 55, 5632–5636. [Google Scholar] [CrossRef]
- Arason, A.J.; Joelsson, J.P.; Valdimarsdottir, B.; Sigurdsson, S.; Gudjonsson, A.; Halldorsson, S.; Johannsson, F.; Rolfsson, O.; Lehmann, F.; Ingthorsson, S.; et al. Azithromycin induces epidermal differentiation and multivesicular bodies in airway epithelia. Respir. Res. 2019, 20, 129. [Google Scholar] [CrossRef]
- Asgrimsson, V.; Gudjonsson, T.; Gudmundsson, G.H.; Baldursson, O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob. Agents Chemother. 2006, 50, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Gudjonsson, T.; Gottfredsson, M.; Singh, P.K.; Gudmundsson, G.H.; Baldursson, O. Azithromycin maintains airway epithelial integrity during Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 2010, 42, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Fujita, T.; Yumoto, H.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Shiba, H.; Matsuo, T.; Kurihara, H. Azithromycin recovers reductions in barrier function in human gingival epithelial cells stimulated with tumor necrosis factor-α. Arch. Oral. Biol. 2016, 62, 64–69. [Google Scholar] [CrossRef]
- Slater, M.; Torr, E.; Harrison, T.; Forrester, D.; Knox, A.; Shaw, D.; Sayers, I. The differential effects of azithromycin on the airway epithelium in vitro and in vivo. Physiol. Rep. 2016, 4, e12960. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef]
- Serisier, D.J. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir. Med. 2013, 1, 262–274. [Google Scholar] [CrossRef]
- Mencarelli, A.; Distrutti, E.; Renga, B.; Cipriani, S.; Palladino, G.; Booth, C.; Tudor, G.; Guse, J.-H.; Hahn, U.; Burnet, M.; et al. Development of non-antibiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. Eur. J. Pharmacol. 2011, 665, 29–39. [Google Scholar] [CrossRef]
- Saito, R.; Domon, H.; Hiyoshi, T.; Hirayama, S.; Maekawa, T.; Takenaka, S.; Noiri, Y.; Ikeda, A.; Hirose, T.; Sunazuka, T.; et al. A novel 12-membered ring non-antibiotic macrolide EM982 attenuates cytokine production by inhibiting IKKβ and IκBα phosphorylation. J. Biol. Chem. 2024, 300, 107384. [Google Scholar] [CrossRef]
- Sugawara, A.; Shima, H.; Sueki, A.; Hirose, T.; Matsui, H.; Nakano, H.; Hanaki, H.; Akagawa, K.S.; Ōmura, S.; Sunazuka, T. Non-antibiotic 12-membered macrolides: Design, synthesis and biological evaluation in a cigarette-smoking model. J. Antibiot. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Gardarsson, F.R.; Lehmann, F.; Teodorovic, P. Azithromycin Derivatives with Epithelial Barrier Enhancement Properties. U.S. Patent US11236120B2, 1 February 2022. Available online: https://patentscope.wipo.int/search/en/WO2017085329 (accessed on 26 February 2025).
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E.L. Azithromycin, the multidrug-resistant protein, and cystic fibrosis. Lancet 1998, 351, 1286. [Google Scholar] [CrossRef]
- Wolter, J.; Seeney, S.; Bell, S.; Bowler, S.; Masel, P.; McCormack, J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: A randomised trial. Thorax 2002, 57, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Borlak, J. Drug-induced phospholipidosis. FEBS Lett. 2006, 580, 5533–5540. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef]
- Carlier, F.M.; Detry, B.; Lecocq, M.; Collin, A.M.; Planté-Bordeneuve, T.; Gérard, L.; Verleden, S.E.; Delos, M.; Rondelet, B.; Janssens, W.; et al. The memory of airway epithelium damage in smokers and COPD patients. Life Sci. Alliance 2024, 7, e202302341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Q.; Wang, C.; Li, N.; Li, J. Redistribution of tight junction proteins during EPEC infection in vivo. Inflammation 2012, 35, 23–32. [Google Scholar] [CrossRef]
- Buda, A.; Jepson, M.A.; Pignatelli, M. Regulatory function of trefoil peptides (TFF) on intestinal cell junctional complexes. Cell Commun. Adhes. 2012, 19, 63–68. [Google Scholar] [CrossRef]
- Beutel, O.; Maraspini, R.; Pombo-García, K.; Martin-Lemaitre, C.; Honigmann, A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell 2019, 179, 923–936.e11. [Google Scholar] [CrossRef]
- Huang, X.; Shi, X.; Hansen, M.E.; Setiady, I.; Nemeth, C.L.; Celli, A.; Huang, B.; Mauro, T.; Koval, M.; Desai, T.A. Nanotopography Enhances Dynamic Remodeling of Tight Junction Proteins through Cytosolic Liquid Complexes. ACS Nano 2020, 14, 13192–13202. [Google Scholar] [CrossRef]
- Kinoshita, N.; Yamamoto, T.S.; Yasue, N.; Takagi, C.; Fujimori, T.; Ueno, N. Force-dependent remodeling of cytoplasmic ZO-1 condensates contributes to cell-cell adhesion through enhancing tight junctions. iScience 2022, 25, 103846. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhou, J. Phase separation as a therapeutic target in tight junction-associated human diseases. Acta Pharmacol. Sin. 2020, 41, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Dalton, C.M.; Schlegel, C.; Hunter, C.J. Caveolin-1: A Review of Intracellular Functions, Tissue-Specific Roles, and Epithelial Tight Junction Regulation. Biology 2023, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, D.; Sun, G.; Zhang, H.; You, Q.; Shao, M.; Yue, Y. Lipopolysaccharide-induced caveolin-1 phosphorylation-dependent increase in transcellular permeability precedes the increase in paracellular permeability. Drug Des. Dev. Ther. 2015, 9, 4965–4977. [Google Scholar]
- Tiruppathi, C.; Shimizu, J.; Miyawaki-Shimizu, K.; Vogel, S.M.; Bair, A.M.; Minshall, R.D.; Predescu, D.; Malik, A.B. Role of NF-kappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J. Biol. Chem. 2008, 283, 4210–4218. [Google Scholar] [CrossRef]
- Mirza, M.K.; Yuan, J.; Gao, X.P.; Garrean, S.; Brovkovych, V.; Malik, A.B.; Tiruppathi, C.; Zhao, Y.Y. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am. J. Pathol. 2010, 176, 2344–2351. [Google Scholar] [CrossRef]
- Garrod, D.; Chidgey, M. Desmosome structure, composition and function. Biochim. Biophys. Acta 2008, 1778, 572–587. [Google Scholar] [CrossRef]
- Cowin, P.; Burke, B. Cytoskeleton-membrane interactions. Curr. Opin. Cell Biol. 1996, 8, 56–65. [Google Scholar] [CrossRef]
- Saaber, F.; Chen, Y.; Cui, T.; Yang, L.; Mireskandari, M.; Petersen, I. Expression of desmogleins 1–3 and their clinical impacts on human lung cancer. Pathol. Res. Pract. 2015, 211, 208–213. [Google Scholar] [CrossRef]
- Course, C.W.; Lewis, P.A.; Kotecha, S.J.; Cousins, M.; Hart, K.; Watkins, W.J.; Heesom, K.J.; Kotecha, S. Modulation of pulmonary desmosomes by inhaler therapy in preterm-born children with bronchopulmonary dysplasia. Sci. Rep. 2023, 13, 7330. [Google Scholar] [CrossRef]
- Bao, K.; Yuan, W.; Zhou, Y.; Chen, Y.; Yu, X.; Wang, X.; Jia, Z.; Yu, X.; Wang, X.; Yao, L.; et al. A Chinese Prescription Yu-Ping-Feng-San Administered in Remission Restores Bronchial Epithelial Barrier to Inhibit House Dust Mite-Induced Asthma Recurrence. Front. Pharmacol. 2019, 10, 1698. [Google Scholar] [CrossRef]
- Delva, E.; Tucker, D.; Kowalczyk, A. The Desmosome. Cold Spring Harb. Perspect. Biol. 2009, 1, a002543. [Google Scholar] [CrossRef] [PubMed]
- Green, K.J.; Gaudry, C.A. Are desmosomes more than tethers for intermediate filaments? Nat. Rev. Mol. Cell Biol. 2000, 1, 208–216. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Kc, K.; Wu, D.; Djukic, Z.; Caldwell, J.M.; Stucke, E.M.; Kemme, K.A.; Costello, M.S.; Mingler, M.K.; Blanchard, C.; et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal. Immunol. 2014, 7, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Madara, J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998, 60, 143–159. [Google Scholar] [CrossRef]
- Ferruzza, S.; Rossi, C.; Sambuy, Y.; Scarino, M.L. Serum-reduced and serum-free media for differentiation of Caco-2 cells. Altex 2013, 30, 159–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Zucco, F.; Batto, A.F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Montenez, J.P.; Piret, J.; Tulkens, P.M.; Courtoy, P.J.; Mingeot-Leclercq, M.P. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. Eur. J. Pharmacol. 1996, 314, 203–214. [Google Scholar] [CrossRef]
- Montenez, J.-P.; Van Bambeke, F.; Piret, J.; Schanck, A.; Brasseur, R.; Tulkens, P.M.; Mingeot-Leclercq, M.-P. Interaction of the macrolide azithromycin with phospholipids. II. Biophysical and computer-aided conformational studies. Eur. J. Pharmacol. 1996, 314, 215–227. [Google Scholar] [CrossRef]
- Joelsson, J.P.; Kricker, J.A.; Arason, A.J.; Sigurdsson, S.; Valdimarsdottir, B.; Gardarsson, F.R.; Page, C.P.; Lehmann, F.; Gudjonsson, T.; Ingthorsson, S. Azithromycin ameliorates sulfur dioxide-induced airway epithelial damage and inflammatory responses. Respir. Res. 2020, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Birukov, K.G. Oxidized Phospholipids in Control of Endothelial Barrier Function: Mechanisms and Implication in Lung Injury. Front. Endocrinol. 2021, 12, 794437. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Molecular Mechanisms of Lipid Metabolism Disorders in Infectious Exacerbations of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2021, 22, 7634. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin. Immunol. 2008, 126, 67–80. [Google Scholar] [CrossRef]
- Loffredo, L.F.; Abdala-Valencia, H.; Anekalla, K.R.; Cuervo-Pardo, L.; Gottardi, C.J.; Berdnikovs, S. Beyond epithelial-to-mesenchymal transition: Common suppression of differentiation programs underlies epithelial barrier dysfunction in mild, moderate, and severe asthma. Allergy 2017, 72, 1988–2004. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, Y.; Zhou, Y.; Wan, L.H. Azithromycin suppresses TGF-β1-related epithelial-mesenchymal transition in airway epithelial cells via targeting RACK1. Chem. Biol. Interact. 2023, 370, 110332. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Liu, Y.; Liao, S.; Miao, S.; Zhou, L.; Wan, L. Azithromycin ameliorates OVA-induced airway remodeling in Balb/c mice via suppression of epithelial-to-mesenchymal transition. Int. Immunopharmacol. 2018, 58, 87–93. [Google Scholar] [CrossRef]
- Banerjee, B.; Musk, M.; Sutanto, E.N.; Yerkovich, S.T.; Hopkins, P.; Knight, D.A.; Lindsey-Temple, S.; Stick, S.M.; Kicic, A.; Chambers, D.C. Regional differences in susceptibiity of bronchial epithelium to mesenchymal transition and inhibition by the macrolide antibiotic azithromycin. PLoS ONE 2012, 7, e52309. [Google Scholar] [CrossRef]
- Krempaska, K.; Barnowski, S.; Gavini, J.; Hobi, N.; Ebener, S.; Simillion, C.; Stokes, A.; Schliep, R.; Knudsen, L.; Geiser, T.K.; et al. Azithromycin has enhanced effects on lung fibroblasts from idiopathic pulmonary fibrosis (IPF) patients compared to controls [corrected]. Respir. Res. 2020, 21, 25. [Google Scholar] [CrossRef]
- Sun, B.; Shen, K.; Zhao, R.; Li, Y.; Lin, J. Clarithromycin attenuates airway epithelial-mesenchymal transition in ovalbumin-induced asthmatic mice through modulation of Kv1.3 channels and PI3K/Akt signaling. Int. Immunopharmacol. 2024, 139, 112624. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Gao, S.; Li, S.; Luan, J.; Jiang, Q.; Li, X.; Yin, H.; Zhou, H.; Yang, C. Deglycosylated Azithromycin Attenuates Bleomycin-Induced Pulmonary Fibrosis via the TGF-β1 Signaling Pathway. Molecules 2021, 26, 2820. [Google Scholar] [CrossRef]
- Yew-Booth, L.; Birrell, M.A.; Lau, M.S.; Baker, K.; Jones, V.; Kilty, I.; Belvisi, M.G. JAK-STAT pathway activation in COPD. Eur. Respir. J. 2015, 46, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Caramori, G.; Capelli, A.; Gnemmi, I.; Ricciardolo, F.L.; Oates, T.; Donner, C.F.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. STAT4 activation in smokers and patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2004, 24, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.E.; Harvey, B.G.; Heguy, A.; Hackett, N.R.; Wang, R.; O’Connor, T.P.; Crystal, R.G. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009, 179, 457–466. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Holmes, M.; Flower, R.; Scicchitano, R. Interleukin-4 and tumour necrosis factor-alpha inhibit transforming growth factor-beta production in a human bronchial epithelial cell line: Possible relevance to inflammatory mechanisms in chronic obstructive pulmonary disease. Respirology 2001, 6, 205–211. [Google Scholar] [CrossRef]
- Tam, A.; Hughes, M.; McNagny, K.M.; Obeidat, M.; Hackett, T.L.; Leung, J.M.; Shaipanich, T.; Dorscheid, D.R.; Singhera, G.K.; Yang, C.W.T.; et al. Hedgehog signaling in the airway epithelium of patients with chronic obstructive pulmonary disease. Sci. Rep. 2019, 9, 3353. [Google Scholar] [CrossRef]
- Belgacemi, R.; Luczka, E.; Ancel, J.; Diabasana, Z.; Perotin, J.M.; Germain, A.; Lalun, N.; Birembaut, P.; Dubernard, X.; Mérol, J.C.; et al. Airway epithelial cell differentiation relies on deficient Hedgehog signalling in COPD. EBioMedicine 2020, 51, 102572. [Google Scholar] [CrossRef]
- Schumann, D.M.; Leeming, D.; Papakonstantinou, E.; Blasi, F.; Kostikas, K.; Boersma, W.; Louis, R.; Milenkovic, B.; Aerts, J.; Sand, J.M.B.; et al. Collagen Degradation and Formation Are Elevated in Exacerbated COPD Compared with Stable Disease. Chest 2018, 154, 798–807. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Hu, W.P.; Zuo, Y.H.; Wang, X.R.; Zhang, J. Altered serum levels of type I collagen turnover indicators accompanied by IL-6 and IL-8 release in stable COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 163–168. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Arason, A.J.; Palsson, R.; Franzdottir, S.R.; Gudbjartsson, T.; Isaksson, H.J.; Gudmundsson, G.; Gudjonsson, T.; Magnusson, M.K. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Lab. Investig. 2015, 95, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 9 September 2024).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asbjarnarson, A.; Joelsson, J.P.; Gardarsson, F.R.; Sigurdsson, S.; Parnham, M.J.; Kricker, J.A.; Gudjonsson, T. The Non-Antibacterial Effects of Azithromycin and Other Macrolides on the Bronchial Epithelial Barrier and Cellular Differentiation. Int. J. Mol. Sci. 2025, 26, 2287. https://doi.org/10.3390/ijms26052287
Asbjarnarson A, Joelsson JP, Gardarsson FR, Sigurdsson S, Parnham MJ, Kricker JA, Gudjonsson T. The Non-Antibacterial Effects of Azithromycin and Other Macrolides on the Bronchial Epithelial Barrier and Cellular Differentiation. International Journal of Molecular Sciences. 2025; 26(5):2287. https://doi.org/10.3390/ijms26052287
Chicago/Turabian StyleAsbjarnarson, Arni, Jon Petur Joelsson, Fridrik R. Gardarsson, Snaevar Sigurdsson, Michael J. Parnham, Jennifer A. Kricker, and Thorarinn Gudjonsson. 2025. "The Non-Antibacterial Effects of Azithromycin and Other Macrolides on the Bronchial Epithelial Barrier and Cellular Differentiation" International Journal of Molecular Sciences 26, no. 5: 2287. https://doi.org/10.3390/ijms26052287
APA StyleAsbjarnarson, A., Joelsson, J. P., Gardarsson, F. R., Sigurdsson, S., Parnham, M. J., Kricker, J. A., & Gudjonsson, T. (2025). The Non-Antibacterial Effects of Azithromycin and Other Macrolides on the Bronchial Epithelial Barrier and Cellular Differentiation. International Journal of Molecular Sciences, 26(5), 2287. https://doi.org/10.3390/ijms26052287






