Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β
Abstract
:1. Introduction
2. Results
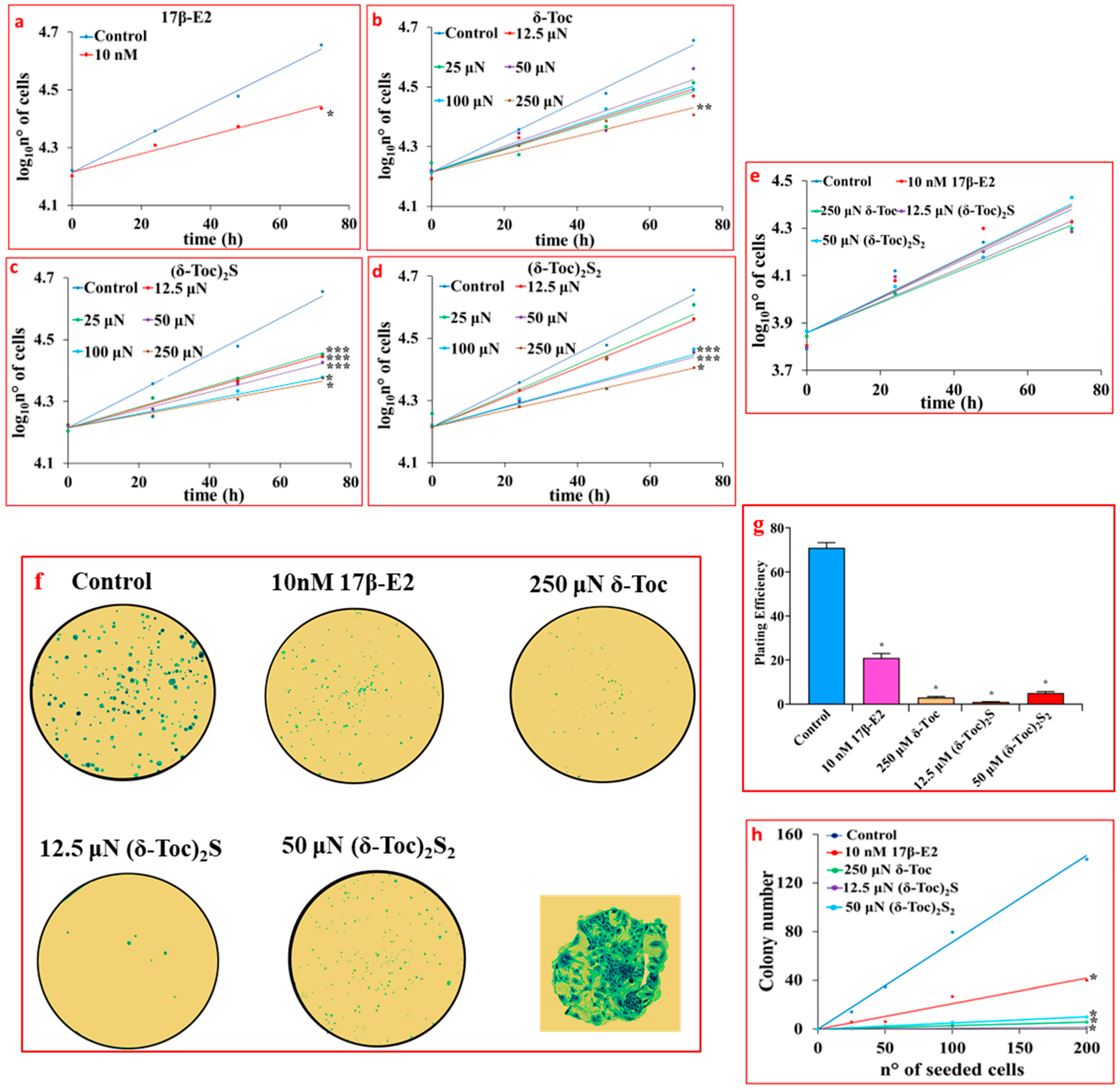
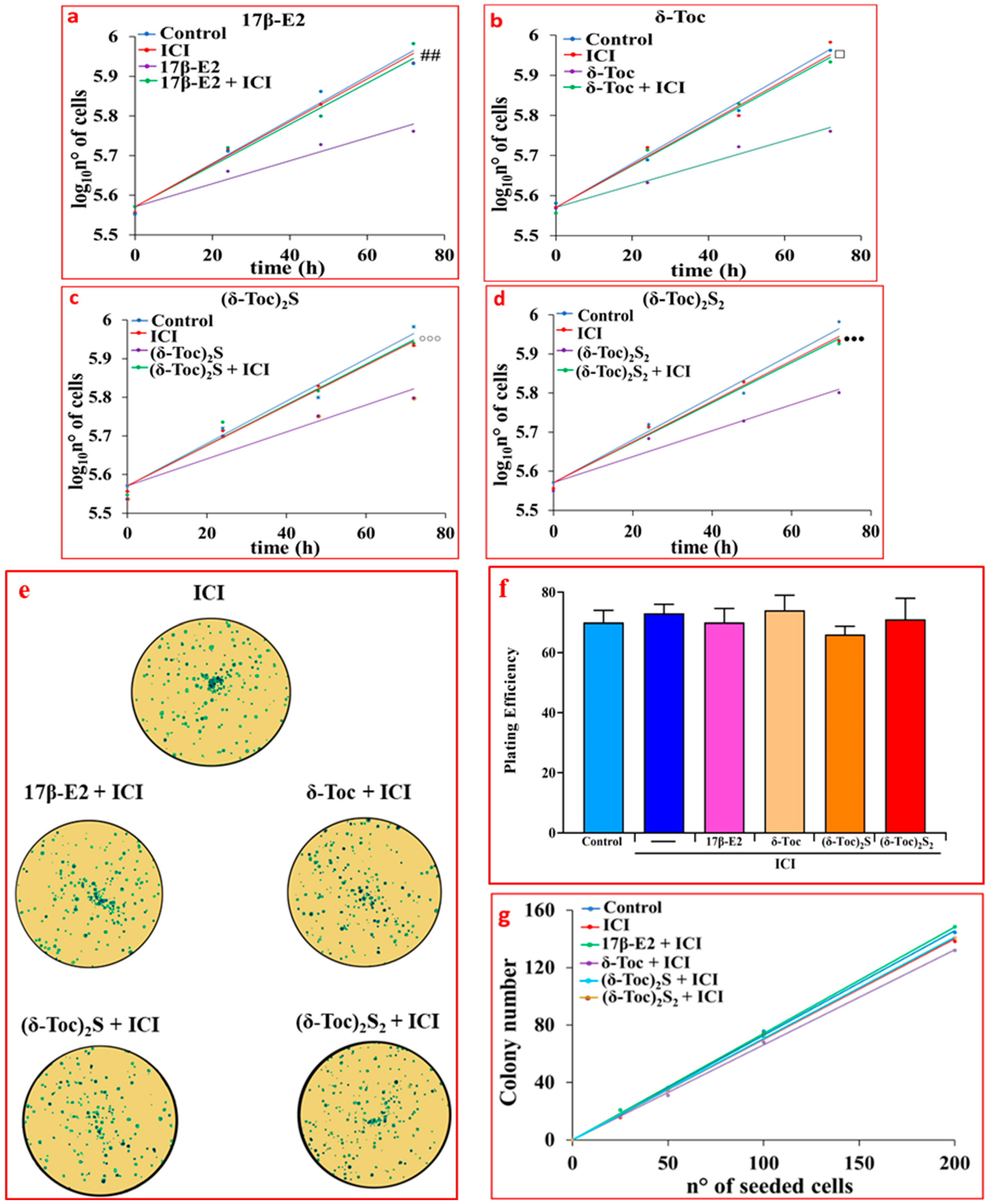
2.1. Effect of 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S and (δ-Toc)2S2 on Proliferation of HCT8-β8 Cells, Treated or Not with ICI 182,780, and in HCT8-pSV2neo Cells
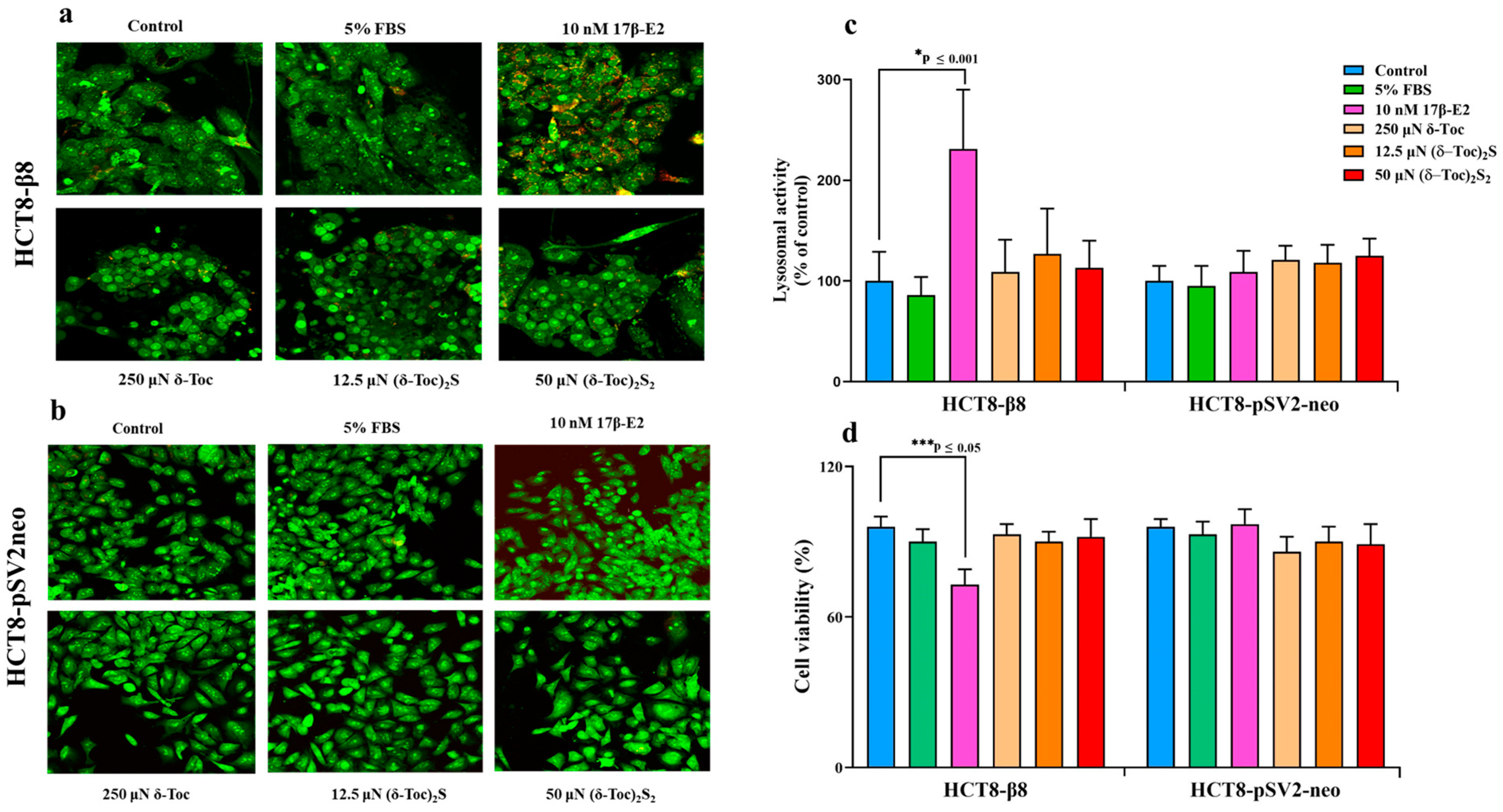
2.2. Effect of 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S and (δ-Toc)2S2 on Viability of HCT8-β8 and HCT8-pSV2neo Cells
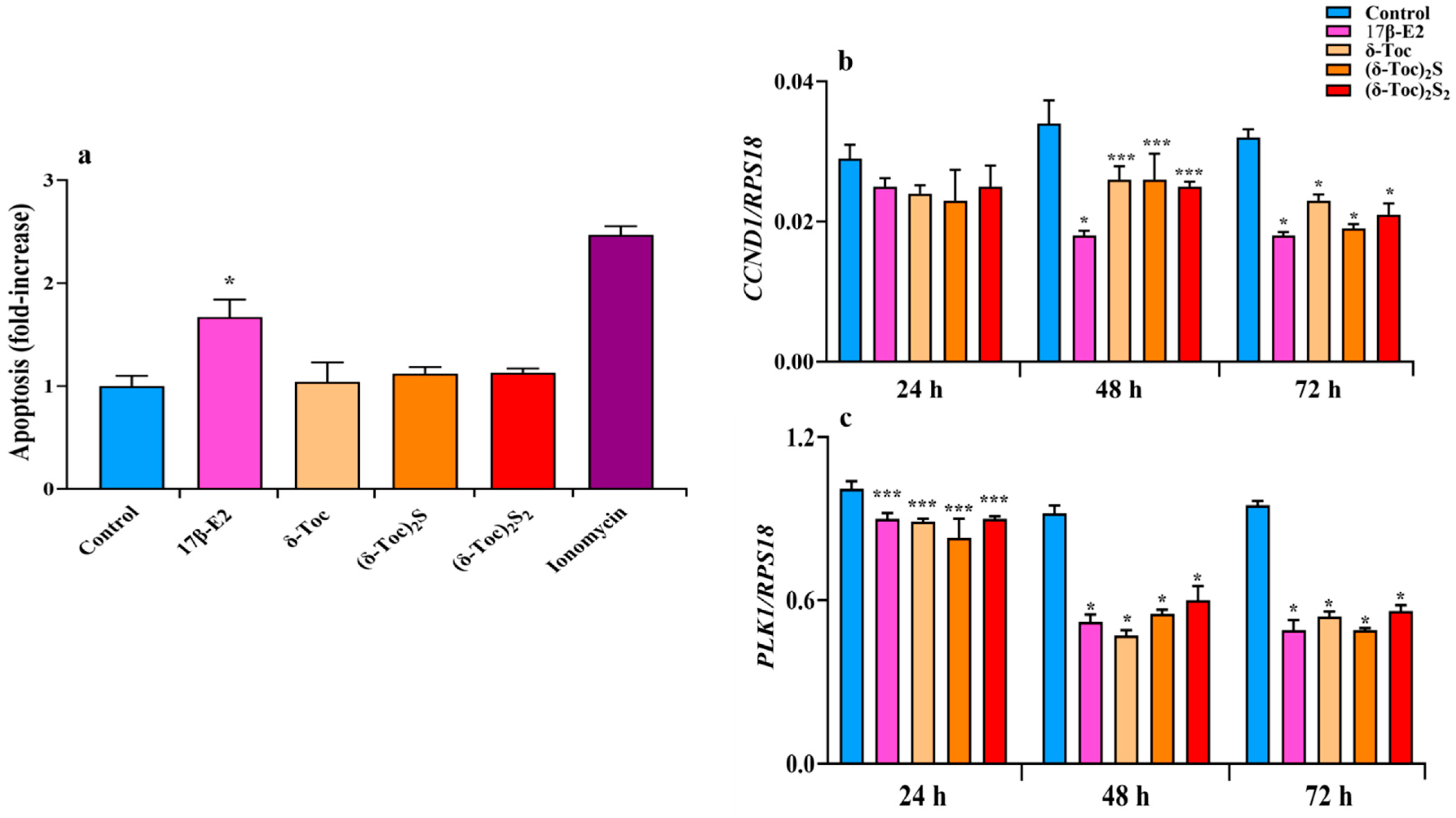
2.3. Effect of 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S and (δ-Toc)2S2 on Apoptosis and Expression of CCND1 and PLK1 Genes in HCT8-β8 Cells
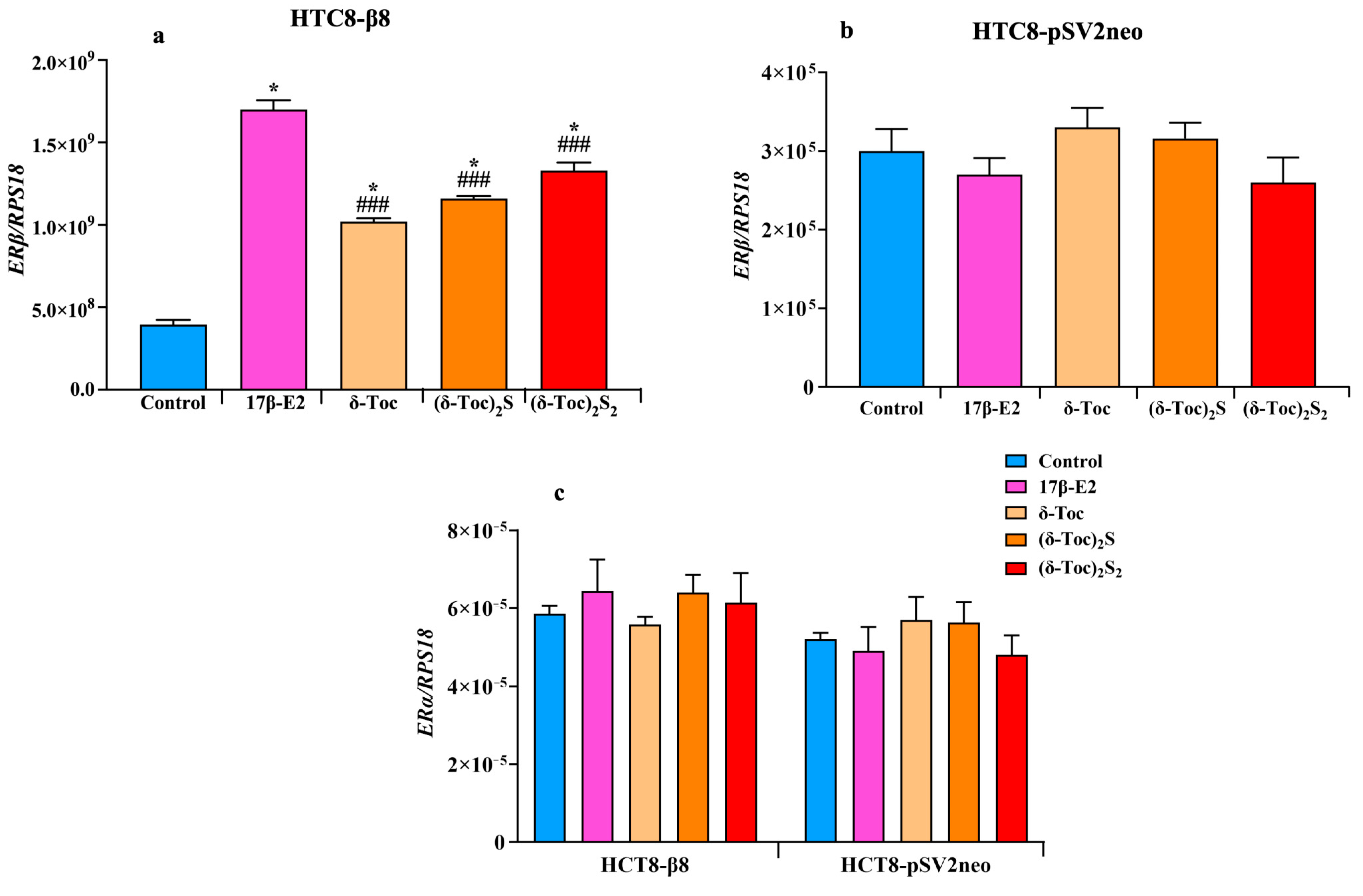
2.4. Effect of 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S and (δ-Toc)2S2 on ERβ and ERα Expression in HCT8-β8 and HCT8-pSV2neo Cells
2.5. Effect of 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S and (δ-Toc)2S2 on Protein ERβ Expression in HCT8-β8 Cells
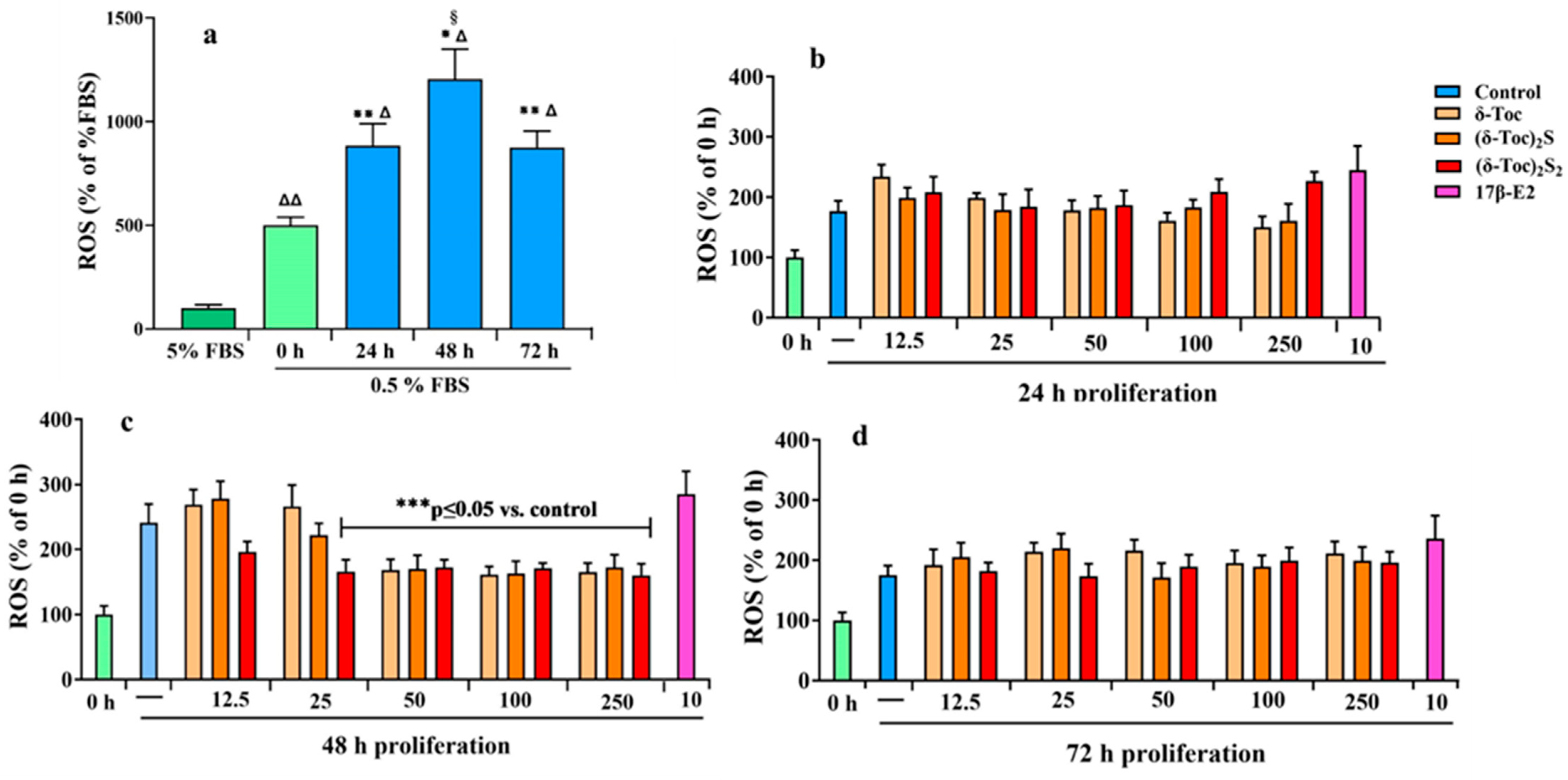
2.6. Intracellular Redox State During Proliferation of HCT8-β8 Treated or Not with 17β-E2, Natural δ-Toc, and Semi-Synthetic (δ-Toc)2S, (δ-Toc)2S2
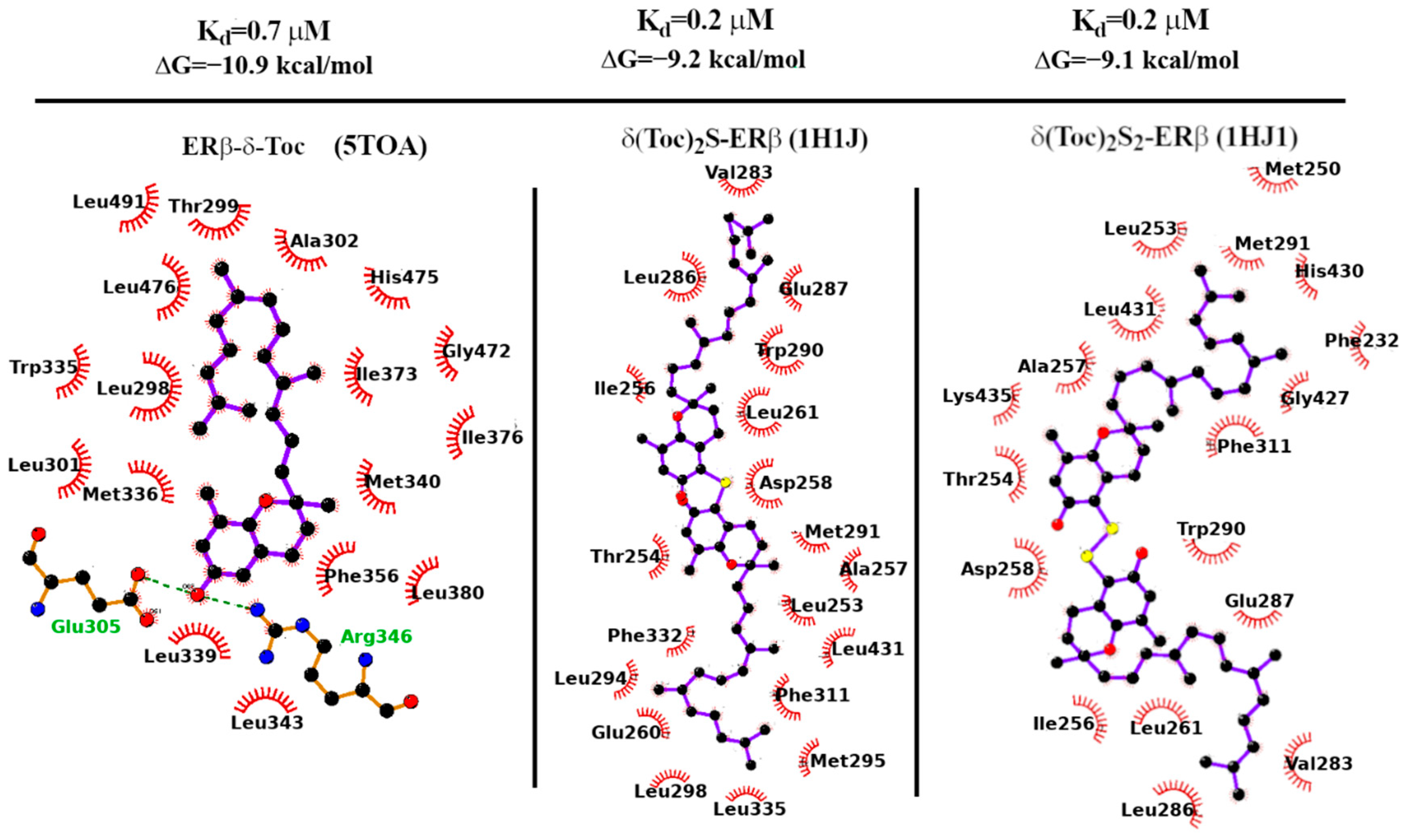
2.7. Docking Calculations
2.8. Binding Strength
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Bis-δ-Tocopheryl Sulfide (δ-Toc)2S and bis-δ-Tocopheryl Disulfide (δ-Toc)2S2 Synthesis
4.3. Cell Proliferation Analysis
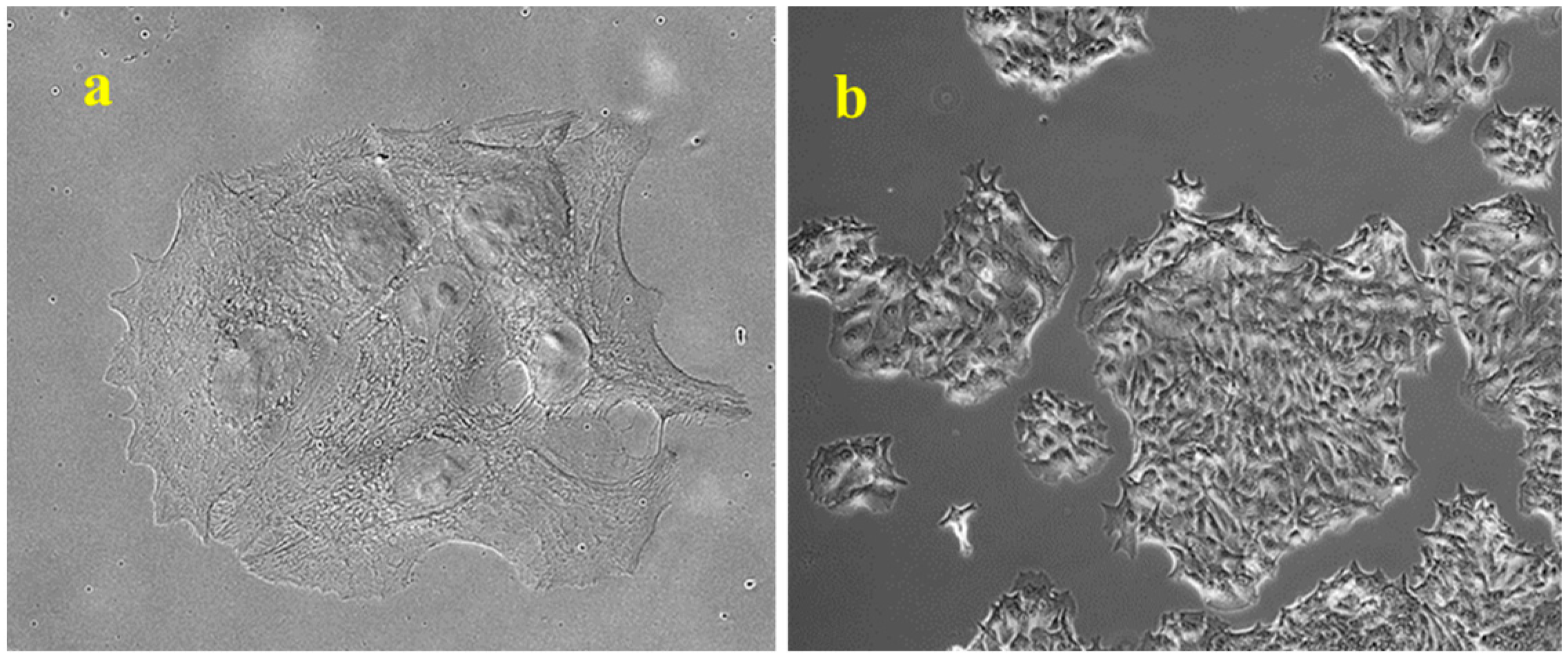
4.4. Colony Formation Assay
4.5. Cell Viability Analysis
4.6. Cell Apoptosis Assay
4.7. RNA Isolation and Real-Time qPCR
4.8. Immunofluorescence Staining of ERβ
4.9. ERβ Protein Assay
4.10. Intracellular ROS Assay
4.11. Protein Assay
4.12. In Silico Approach for the Prediction of the Binding of Tocopherols to ERβ
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The necessary evil for human health, and ways to tame it. Biomed. Pharmacother. 2018, 102, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Caruntu, C.; Mirica, A.; Roşca, A.E.; Mirica, R.; Caruntu, A.; Tampa, M.; Matei, C.; Constantin, C.; Neagu, M.; Badarau, A.I.; et al. The role of estrogens and estrogen receptors in melanoma development and progression. Acta Endocrinol. 2016, 12, 234–241. [Google Scholar] [CrossRef]
- Lewoniewska, S.; Oscilowska, I.; Forlino, A.; Palka, J. Understanding the Role of Estrogen Receptor Status in PRODH/POX-Dependent Apoptosis/Survival in Breast Cancer Cells. Biology 2021, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, R.A.; Hadadi, A.; Lobachev, K.S.; Schwartz, Z.; Boyan, B.D. Estrogen receptor-alpha 36 mediates the anti-apoptotic effect of estradiol in triple negative breast cancer cells via a membrane-associated mechanism. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 2796–2806. [Google Scholar] [CrossRef]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen receptors and their implications in colorectal carcino-genesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Edvardsson, K.; Nguyen-Vu, T.; Kalasekar, S.M.; Pontén, F.; Gustafsson, J.Å.; Williams, C. Estrogen receptor β expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis 2013, 34, 1431–1441. [Google Scholar] [CrossRef]
- Topi, G.; Ehrnström, R.; Jirström, K.; Palmquist, I.; Lydrup, M.-L.; Sjölander, A. Association of the oestrogen receptor beta with hormone status and prognosis in a cohort of female patients with colorectal cancer. Eur. J. Cancer 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Williams, C.; Di Leo, A.; Niv, Y.; Gustafsson, J.-Å. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef]
- Rudolph, A.; Toth, C.; Hoffmeister, M.; Roth, W.; Herpel, E.; Schirmacher, P.; Brenner, H.; Chang-Claude, J. Colorectal Cancer Risk Associated with Hormone Use Varies by Expression of Estrogen Receptor-β. Cancer Res. 2013, 73, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Hases, L.; Indukuri, R.; Birgersson, M.; Nguyen-Vu, T.; Lozano, R.; Saxena, A.; Hartman, J.; Frasor, J.; Gustafsson, J.; Katajisto, P.; et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020, 492, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Maingi, J.W.; Tang, S.; Liu, S.; Ngenya, W.; Bao, E. Targeting estrogen receptors in colorectal cancer. Mol. Biol. Rep. 2020, 47, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Sasso, C.V.; Santiano, F.E.; Arboccó, F.C.V.; Zyla, L.E.; Semino, S.N.; Guerrero-Gimenez, M.E.; Creydt, V.P.; Fontana, C.M.L.; Carón, R.W. Estradiol and progesterone regulate proliferation and apoptosis in colon cancer. Endocr. Connect. 2019, 8, 217–229. [Google Scholar] [CrossRef]
- Mahbub, A.A. Therapeutic Strategies and Potential Actions of Female Sex Steroid Hormones and Their Receptors in Colon Cancer Based on Preclinical Studies. Life 2022, 12, 605. [Google Scholar] [CrossRef]
- Indukuri, R.; Jafferali, M.H.; Song, D.; Damdimopoulos, A.; Hases, L.; Zhao, C.; Archer, A.; Williams, C. Genome-wide estrogen receptor β chromatin binding in human colon cancer cells reveals its tumor suppressor activity. Int. J. Cancer 2021, 149, 692–706. [Google Scholar] [CrossRef]
- Saleiro, D.; Murillo, G.; Benya, R.V.; Bissonnette, M.; Hart, J.; Mehta, R.G. Estrogen receptor-β protects against colitis-associated neoplasia in mice. Int. J. Cancer. 2012, 131, 2553–2561. [Google Scholar] [CrossRef]
- Lozano-Herrera, S.J.; Luna-Bárcenas, G.; Guevara-González, R.G.; Campos-Vega, R.; Solís-Sáinz, J.C.; Hernández-Puga, A.G.; Vergara-Castañeda, H.A. Fermentation extract of naringenin increases the expression of estrogenic receptor β and modulates genes related to the p53 signalling pathway, miR-200c and miR-141 in human colon cancer cells exposed to BPA. Molecules 2022, 27, 6588. [Google Scholar] [CrossRef]
- Luceri, C.; Femia, A.P.; Tortora, K.; D’ambrosio, M.; Fabbri, S.; Fazi, M.; Caderni, G. Supplementation with phytoestrogens and insoluble fibers reduces intestinal carcinogenesis and restores ER-β expression in Apc-driven colorectal carcinogenesis. Eur. J. Cancer Prev. 2020, 29, 27–35. [Google Scholar] [CrossRef]
- Giroux, V.; Bernatchez, G.; Carrier, J.C. Chemopreventive effect of ERβ-Selective agonist on intestinal tumorigenesis in ApcMin/+ mice. Mol. Carcinog. 2011, 50, 359–369. [Google Scholar] [CrossRef]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Ström, A.; Gustafsson, J.A. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Martineti, V.; Picariello, L.; Tognarini, I.; Carbonell Sala, S.; Gozzini, A.; Azzari, C.; Mavilia, C.; Tanini, A.; Falchetti, A.; Fiorelli, G.; et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr. Relat. Cancer. 2005, 12, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, B.; Palmini, G.; Mavilia, C.; Zonefrati, R.; Tanini, A.; Brandi, M.L. In vitro effects of polyphenols on colorectal cancer cells. World J. Gastrointest. Oncol. 2014, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Stocker, A. Vitamin E: Non-antioxidant roles. Prog. Lipid Res. 2000, 39, 231–255. [Google Scholar] [CrossRef]
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free. Radic. Biol. Med. 2021, 178, 26–41. [Google Scholar] [CrossRef]
- Germano, B.C.d.C.; de Morais, L.C.C.; Neta, F.I.; Fernandes, A.C.L.; Pinheiro, F.I.; Rego, A.C.M.D.; Filho, I.A.; de Azevedo, E.P.; Cavalcanti, J.R.L.d.P.; Guzen, F.P.; et al. Vitamin E and Its Molecular Effects in Experimental Models of Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 11191. [Google Scholar] [CrossRef]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2020, 53, 1154–1158. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- McIntyre, B.S.; Briski, K.P.; Gapor, A.; Sylvester, P.W. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc. Soc. Exp. Biol. Med. 2000, 224, 292–301. [Google Scholar] [CrossRef]
- Wang, H.; Yan, W.; Sun, Y.; Yang, C.S. δ-Tocotrienol is the most potent vitamin E form in inhibiting prostate cancer cell growth and inhibits prostate carcinogenesis in Ptenp-/- mice. Cancer Prev. Res. 2022, 15, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Weber, T.; Schröder, A.; Lu, M.; Ostermann, G.; Gellert, N.; Mayne, G.C.; Olejnicka, B.; Nègre-Salvayre, A.; Stícha, M.; et al. Induction of cancer cell apoptosis by α-tocopheryl succinate: Molecular pathways and structural requirements. FASEB J. 2001, 15, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Lu, M.; Andera, L.; Lahm, H.; Gellert, N.; Fariss, M.W.; Korinek, V.; Sattler, W.; Ucker, D.S.; Terman, A.; et al. Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (Apo2 ligand) in vivo. Clin. Cancer Res. 2002, 8, 863–869. [Google Scholar] [PubMed]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Anstead, G.M.; Carlson, K.E.; Katzenellenbogen, J.A. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 1997, 62, 268–303. [Google Scholar] [CrossRef]
- Khallouki, F.; de Medina, P.; Caze-Subra, S.; Bystricky, K.; Balaguer, P.; Poirot, M.; Silvente-Poirot, S. Molecular and Biochemical Analysis of the Estrogenic and Proliferative Properties of Vitamin E Compounds. Front. Oncol. 2016, 5, 287. [Google Scholar] [CrossRef]
- Comitato, R.; Nesaretnam, K.; Leoni, G.; Ambra, R.; Canali, R.; Bolli, A.; Marino, M.; Virgili, F. A novel mechanism of natural vitamin E tocotrienol activity: Involvement of ERbeta signal transduction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E427–E437. [Google Scholar] [CrossRef]
- Comitato, R.; Leoni, G.; Canali, R.; Ambra, R.; Nesaretnam, K.; Virgili, F. Tocotrienols activity in MCF-7 breast cancer cells: Involvement of ERbeta signal transduction. Mol. Nutr. Food Res. 2010, 54, 669–678. [Google Scholar] [CrossRef]
- Gok, S.; Kuzmenko, O.; Babinskyi, A.; Severcan, F. Vitamin E derivative with modified side chain induced apoptosis by mod-ulating the cellular lipids and membrane dynamics in MCF7 cells. Cell Biochem. Biophys. 2021, 79, 271–287. [Google Scholar] [CrossRef]
- Bak, M.J.; Furmanski, P.; Shan, N.L.; Lee, H.J.; Bao, C.; Lin, Y.; Shih, W.J.; Yang, C.S.; Suh, N. Tocopherols inhibit estrogen-induced cancer stemness and OCT4 signaling in breast cancer. Carcinogenesis 2018, 39, 1045–1055. [Google Scholar] [CrossRef]
- Chen, J.X.; Liu, A.; Lee, M.J.; Wang, H.; Yu, S.; Chi, E.; Reuhl, K.; Suh, N.; Yang, C.S. δ- and γ-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol. Carcinog. 2017, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Li, G.; Liu, A.B.; Lee, M.J.; Yang, Z.; Chen, Y.K.; Lin, Y.; Shih, W.; Yang, C.S. δ- and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. 2012, 5, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.; Jiang, F.; Olsen, C.; Polgar, W.; Toll, L. Small-molecule agonists and antagonists of the opioid receptor-like receptor (ORL1, NOP): Ligand-based analysis of structural factors influencing intrinsic activity at NOP. AAPS J. 2005, 7, E345–E352. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, C.; Vasa, K.; Tanini, D.; Capperucci, A.; Amorati, R.; Valgimigli, L.; Baschieri, A.; Menichetti, S. Ditocopheryl Sulfides and Disulfides: Synthesis and Antioxidant Profile. Chemistry 2019, 25, 9108–9116. [Google Scholar] [CrossRef]
- Wagner, L.; Howe, K.; Philbrick, K.A.; Maddalozzo, G.F.; Kuah, A.F.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Iwaniec, U.T.; Turner, R.T. Effects of Alcohol and Estrogen Receptor Blockade Using ICI 182,780 on Bone in Ovariectomized Rats. Alcohol. Clin. Exp. Res. 2019, 43, 2301–2311. [Google Scholar] [CrossRef]
- Kimmins, S.; Russell, G.L.; Lim, H.C.; Hall, B.K.; MacLaren, L.A. The effects of estrogen, its antagonist ICI 182, 780, and inter-feron-tau on the expression of estrogen receptors and integrin alphaV beta 3 on cycle day 16 in bovine endometrium. Reprod. Biol. Endocrinol. 2003, 1, 38. [Google Scholar] [CrossRef]
- Kavčič, N.; Pegan, K.; Turk, B. Lysosomes in programmed cell death pathways: From initiators to amplifiers. Biol. Chem. 2016, 398, 289–301. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 21, A.3B.1–A.3B.2. [Google Scholar]
- Gil-Parrado, S.; Fernández-Montalván, A.; Assfalg-Machleidt, I.; Popp, O.; Bestvater, F.; Holloschi, A.; Knoch, T.A.; Auerswald, E.A.; Welsh, K.; Reed, J.C.; et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J. Biol. Chem. 2002, 277, 27217–272126. [Google Scholar] [CrossRef]
- Mizushima, S.; Sasanami, T.; Ono, T.; Matsuzaki, M.; Kansaku, N.; Kuroiwa, A. Cyclin D1 gene expression is essential for cell cycle progression from the maternal-to-zygotic transition during blastoderm development in Japanese quail. Dev. Biol. 2021, 476, 249–258. [Google Scholar]
- Liu, Z.; Sun, Q.; Wang, X. PLK1, A Potential Target for Cancer Therapy. Transl. Oncol. 2017, 10, 22–32. [Google Scholar] [CrossRef]
- White, E.Z.; Pennant, N.M.; Carter, J.R.; Hawsawi, O.; Odero-Marah, V.; Hinton, C.V. Serum deprivation initiates adaptation and survival to oxidative stress in prostate cancer cells. Sci. Rep. 2020, 10, 12505. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, J.J.; Kim, T.W.; Kim, B.S.; Lee, M.S.; Yoo, Y.D. Serum deprivation-induced reactive oxygen species production is mediated by Romo1. Apoptosis 2010, 15, 204–218. [Google Scholar] [CrossRef]
- Rivera-Guevara, C.; Pérez-Alvarez, V.; García-Becerra, R.; Ordaz-Rosado, D.; Morales-Ríos, M.S.; Hernández-Gallegos, E.; Cooney, A.J.; Bravo-Gómez, M.E.; Larrea, F.; Camacho, J. Genomic action of permanently charged tamoxifen derivatives via estrogen receptor-α. Bioorganic Med. Chem. 2010, 18, 5593–5601. [Google Scholar] [CrossRef]
- Meyers, M.J.; Sun, J.; Carlson, K.E.; Marriner, G.A.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Estrogen receptor-beta po-tency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001, 44, 4230–4251. [Google Scholar] [CrossRef] [PubMed]
- Handa, C.; Yamazaki, Y.; Yonekubo, S.; Furuya, N.; Momose, T.; Ozawa, T.; Furuishi, T.; Fukuzawa, K.; Yonemochi, E. Evaluating the correlation of binding affinities between isothermal titration calorimetry and fragment molecular orbital method of es-trogen receptor beta with diarylpropionitrile (DPN) or DPN derivatives. J. Steroid Biochem. Mol. Biol. 2022, 222, 106152. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Ruff, M.; Gangloff, M.; Wurtz, J.M.; Moras, D. Estrogen receptor transcription and transactivation: Structure-function rela-tionship in DNA- and ligand-binding domains of estrogen receptors. Breast Cancer Res. 2000, 2, 353–359. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand inter-actions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Song, C.-H.; Kim, N.; Lee, S.M.; Nam, R.H.; Choi, S.I.; Kang, S.R.; Shin, E.; Lee, D.H.; Lee, H.-N.; Surh, Y.-J. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem. Pharmacol. 2019, 164, 139–151. [Google Scholar] [CrossRef]
- Treeck, O.; Diepolder, E.; Skrzypczak, M.; Schüler-Toprak, S.; Ortmann, O. Knockdown of estrogen receptor β increases pro-liferation and affects the transcriptome of endometrial adenocarcinoma cells. BMC Cancer 2019, 19, 745. [Google Scholar]
- Zhao, X.; Li, X.; Ren, Q.; Tian, J.; Chen, J. Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene 2016, 591, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Kuo, W.-W.; Ju, D.-T.; Yeh, Y.-L.; Tu, C.-C.; Tsai, Y.-L.; Shen, C.-Y.; Chang, S.-H.; Chung, L.-C.; Huang, C.-Y. Estradiol agonists inhibit human LoVo colorectal-cancer cell proliferation and migration through p53. World J. Gastroenterol. 2014, 20, 16665–16673. [Google Scholar] [CrossRef]
- Sotoca, A.M.; van den Berg, H.; Vervoort, J.; van der Saag, P.; Ström, A.; Gustafsson, J.A.; Rietjens, I.; Murk, A.J. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol. Sci. 2008, 105, 303–311. [Google Scholar] [PubMed]
- Ditonno, I.; Losurdo, G.; Rendina, M.; Pricci, M.; Girardi, B.; Ierardi, E.; Di Leo, A. Estrogen Receptors in Colorectal Cancer: Facts, Novelties and Perspectives. Curr. Oncol. 2021, 28, 4256–4263. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Shoulars, K.; Rodriguez, M.A. Luteolin Regulation of Estrogen Signaling and Cell Cycle Pathway Genes in MCF-7 Human Breast Cancer Cells. Int. J. Biomed. Sci. 2011, 7, 101–111. [Google Scholar] [PubMed] [PubMed Central]
- Patel, S.; Kilburn, B.; Imudia, A.; Armant, D.R.; Skafar, D.F. Estradiol Elicits Proapoptotic and Antiproliferative Effects in Human Trophoblast Cells1. Biol. Reprod. 2015, 93, 74. [Google Scholar] [CrossRef]
- Seilicovich, A. Cell Life and Death in the Anterior Pituitary Gland: Role of Oestrogens. J. Neuroendocr. 2010, 22, 758–764. [Google Scholar] [CrossRef]
- Lee, Y.J.; Renaud, R.A.; Friedrich, T.C.; Gorski, J. Estrogen causes cell death of estrogen receptor stably transfected cells via apoptosis. J. Steroid Biochem. Mol. Biol. 1998, 67, 327–332. [Google Scholar]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.; Gopalan, V.; Islam, F. Implications of estrogen and its receptors in colorectal carcinoma. Cancer Med. 2022, 12, 4367–4379. [Google Scholar] [CrossRef]
- Chen, J.; Hou, R.; Zhang, X.; Ye, Y.; Wang, Y.; Tian, J. Calycosin suppresses breast cancer cell growth via ERβ-dependent reg-ulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS ONE 2014, 9, e91245. [Google Scholar]
- Huang, M.; Li, X.; Jia, S.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ. Pollut. 2020, 270, 116051. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Zhang, Y.; Zhang, S.; Zhang, R.; Chen, D.; Shi, J.; Xu, J.; Li, L. Nonivamide inhibits proliferation of human corneal epithelial cells by inducing cell cycle arrest and oxidative stress. Toxicology 2023, 500, 153674. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Domazetovic, V.; Falsetti, I.; Viglianisi, C.; Vasa, K.; Aurilia, C.; Stio, M.; Menichetti, S.; Iantomasi, T. Protective Role of Natural and Semi-Synthetic Tocopherols on TNFα-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells. Antioxidants 2021, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Estrogen inhibits starvation-induced apoptosis in osteocytes by a redox-independent process involving association of JNK and glutathione S-transferase P1-1. FEBS Open Bio. 2017, 7, 705–718. [Google Scholar] [CrossRef]
- Mann, V.; Huber, C.; Kogianni, G.; Collins, F.; Noble, B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone 2006, 40, 674–684. [Google Scholar] [CrossRef]
- Panza, S.; Santoro, M.; De Amicis, F.; Morelli, C.; Passarelli, V.; D’aquila, P.; Giordano, F.; Cione, E.; Passarino, G.; Bellizzi, D.; et al. Estradiol via estrogen receptor beta influences ROS levels through the transcriptional regulation of SIRT3 in human seminoma TCam-2 cells. Tumor Biol. 2017, 39, 1010428317701642. [Google Scholar] [CrossRef]
- Viglianisi, C.; Bonardi, C.; Ermini, E.; Capperucci, A.; Menichetti, S.; Tanini, D. Selenosilane-promoted selective mild trans-formation of N-thiophthalimides into symmetric disulfides. Synthesis 2019, 51, 1819–1824. [Google Scholar]
- Catarzi, S.; Romagnoli, C.; Marcucci, G.; Favilli, F.; Iantomasi, T.; Vincenzini, M.T. Redox regulation of ERK1/2 activation in-duced by sphingosine 1-phosphate in fibroblasts: Involvement of NADPH oxidase and platelet-derived growth factor receptor. Biochim. Biophys. Acta 2011, 1810, 446–456. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [PubMed]
- Pike, A.C.; Brzozowski, A.; Walton, J.; Hubbard, R.E.; Thorsell, A.-G.; Li, Y.-L.; Gustafsson, J.; Carlquist, M. Structural Insights into the Mode of Action of a Pure Antiestrogen. Structure 2001, 9, 145–153. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Textor, L.C.; Melo, D.C.; Nascimento, A.S.; Skaf, M.S.; Polikarpov, I. An alternative conformation of ERβ bound to estradiol reveals H12 in a stable antagonist position. Sci. Rep. 2017, 7, 3509. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The structural basis of estrogen recep-tor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998, 95, 927–937. [Google Scholar]
- Tanenbaum, D.M.; Wang, Y.; Williams, S.P.; Sigler, P.B. Crystallographic comparison of the estrogen and progesterone re-ceptor’s ligand binding domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5998–6003. [Google Scholar] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]


| ERα | ERβ | |||||
|---|---|---|---|---|---|---|
| Ligand | PDB | ΔGcalc | ΔGexp | PDB | ΔGcalc | ΔGexp |
| 17β-E2 | 1A52 | −10.2 | −13.4 | 5TOA | −10.5 | −13.2 |
| DPN | − | − | − | 7XVY | −8.7 | −9.0 |
| δ-Toc | 3ERD | −8.4 | − | 7XVY | −10.9 | − |
| (δ-Toc)2S | − | − | − | 1HJ1 | −9.2 | − |
| (δ-Toc)2S2 | − | − | − | 1HJ1 | −9.0 | − |
| Gene | Oligonucleotides | Sequence (5′ → 3′) | Amplicon Size (bp) | Tann (°C) |
|---|---|---|---|---|
| ERβ | Forward primer Reverse Probe * | TCGCCAGTTATCACATCTGTATGCGG GTGTCTCTCTGTTTACAGGTAAGGTGTG FTCCCGGTGZTGAAGCAAGATCGCTAGAAQ | 95 | 65 |
| ERα | Forward primer Reverse Probe * | GACTATGCTTCAGGCTACCATTA GGCTGGACACATATAGTCGTTAT FTCTCTTGAAZGAAGGCCTTGCAGCCQ | 120 | 60 |
| CCND1 | Forward primer Reverse Probe * | CTGTGCATCTACACCGACAA AGGTTCCACTTGAGCTTGTT FAGCTCCATTZTGCAGCAGCTCCTCQ | 86 | 55 |
| PLK1 | Forward primer Reverse Probe * | CACAGTGTCAATGCCTCCAA AGGCCGTACTTGTCCGAATA FATCTTCTGGZGTCAGCAAGTGGGTGQ | 125 | 55 |
| RPS18 | Forward primer Reverse Probe * | AATCCGTTGACTCCGACCTTC ACAGTACAGCCGCATCTTC FCCACATCGCZTCAGACACCATGGGQ | 179 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falsetti, I.; Palmini, G.; Zonefrati, R.; Vasa, K.; Donati, S.; Aurilia, C.; Baroncelli, A.; Viglianisi, C.; Ranaldi, F.; Iantomasi, T.; et al. Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β. Int. J. Mol. Sci. 2025, 26, 2305. https://doi.org/10.3390/ijms26052305
Falsetti I, Palmini G, Zonefrati R, Vasa K, Donati S, Aurilia C, Baroncelli A, Viglianisi C, Ranaldi F, Iantomasi T, et al. Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β. International Journal of Molecular Sciences. 2025; 26(5):2305. https://doi.org/10.3390/ijms26052305
Chicago/Turabian StyleFalsetti, Irene, Gaia Palmini, Roberto Zonefrati, Kristian Vasa, Simone Donati, Cinzia Aurilia, Allegra Baroncelli, Caterina Viglianisi, Francesco Ranaldi, Teresa Iantomasi, and et al. 2025. "Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β" International Journal of Molecular Sciences 26, no. 5: 2305. https://doi.org/10.3390/ijms26052305
APA StyleFalsetti, I., Palmini, G., Zonefrati, R., Vasa, K., Donati, S., Aurilia, C., Baroncelli, A., Viglianisi, C., Ranaldi, F., Iantomasi, T., Procacci, P., Menichetti, S., & Brandi, M. L. (2025). Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β. International Journal of Molecular Sciences, 26(5), 2305. https://doi.org/10.3390/ijms26052305








