Use of Natural Biomolecules in Animal Feed to Enhance Livestock Reproduction
Abstract
1. Introduction
2. Effects of Natural Biomolecules on Female Reproduction and Sperm Quality
2.1. Amino Acids
2.1.1. Essential Amino Acids (EAAs)
2.1.2. Non-Essential Aminoacids (NEAAs)
2.1.3. Combination of Amino Acids
2.2. Fatty Acids
2.3. The Potential of Conjugated Linoleic Acid (CLA) on Embryo Development and Fertility
2.4. Antioxidants
2.4.1. Selenium
2.4.2. Other Antioxidants
2.4.3. Interaction Between Different Antioxidants and Their Cumulative Effects on Reproduction
2.5. Vitamins
2.5.1. Vitamin A
2.5.2. Vitamin E
2.5.3. Vitamin C
2.5.4. Vitamin D
2.5.5. Vitamins B
2.6. Plant Extracts
3. Mechanisms of Action of Biomolecules on Sperm Quality and Female Fertility
4. Challenges and Opportunities
4.1. Challenges
4.2. Opportunities
5. Technological Advances and Innovations
5.1. Nanotechnology
5.2. Microencapsulation
5.3. Nutrigenomics
5.4. Microbiome Modulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Livestock, Engine for Economic Growth and Sustainability. 2022. Available online: https://www.fao.org/cfs/cfs-hlpe/insights/news-insights/news-detail/livestock-engine-for-economic-growth-and-sustainability/en (accessed on 27 February 2025).
- Herrero, M.; Grace, D.; Njuki, J.; Johnson, N.; Enahoro, D.; Silvestri, S.; Rufino, M.C. The roles of livestock in developing countries. Animal 2013, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; El-Hawy, A.S.; El-Bassiony, M.F.; El-Hamid, I.S.A.; Gonzalez-Bulnes, A.; Martinez-Ros, P. Use of GnRH-Encapsulated Chitosan Nanoparticles as an Alternative to eCG for Induction of Estrus and Ovulation during Non-Breeding Season in Sheep. Biology 2023, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G.; Butler, L.D.; Kidd, M.T. Reproductive management of poultry. In Animal Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 349–366. [Google Scholar] [CrossRef]
- Hashem, N.M.; Essawi, W.M.; El-Raghi, A.A. Ovarian activity, hormone profile, pro-inflammatory cytokines and reproductive performance of buffalo cows fed diets with different estrogenicity. J. Anim. Physiol. Anim. Nutr. 2023, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Racewicz, P.; Ludwiczak, A.; Skrzypczak, E.; Składanowska-Baryza, J.; Biesiada, H.; Nowak, T.; Nowaczewski, S.; Zaborowicz, M.; Stanisz, M.; Ślósarz, P. Welfare Health and Productivity in Commercial Pig Herds. Animals 2021, 11, 1176. [Google Scholar] [CrossRef]
- Swelum, A.A.; Hashem, N.M.; Abdelnour, S.A.; Taha, A.E.; Ohran, H.; Khafaga, A.F.; El-Tarabily, K.A.; El-Hack, M.E.A. Effects of phytogenic feed additives on the reproductive performance of animals. Saudi J. Biol. Sci. 2021, 28, 5816–5822. [Google Scholar] [CrossRef]
- Malenica, D.; Kass, M.; Bhat, R. Sustainable Management and Valorization of Agri-Food Industrial Wastes and By-Products as Animal Feed: For Ruminants, Non-Ruminants and as Poultry Feed. Sustainability 2022, 15, 117. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef]
- Escribano, A.J. Organic Feed: A Bottleneck for the Development of the Livestock Sector and Its Transition to Sustainability? Sustainability 2018, 10, 2393. [Google Scholar] [CrossRef]
- Njisane, Y.Z.; Mukumbo, F.E.; Muchenje, V. An outlook on livestock welfare conditions in African communities—A review. Asian-Australasian J. Anim. Sci. 2020, 33, 867–878. [Google Scholar] [CrossRef]
- Pan, S.; Yan, J.; Xu, X.; Chen, Y.; Chen, X.; Li, F.; Xing, H. Current Development and Future Application Prospects of Plants-Derived Polyphenol Bioactive Substance Curcumin as a Novel Feed Additive in Livestock and Poultry. Int. J. Mol. Sci. 2022, 23, 11905. [Google Scholar] [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Hosny, N.S.; El-Desoky, N.; Soltan, Y.A.; Elolimy, A.A.; Sallam, S.M.A.; Abu-Tor, E.-S.M. Alginate Nanoen-capsulated Synbiotic Composite of Pomegranate Peel Phytogenics and Multi-Probiotic Species as a Potential Feed Additive: Physicochemical, Antioxidant, and Antimicrobial Activities. Animals 2023, 13, 2432. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; El-Hawy, A.S.; El-Bassiony, M.F.; Saber, A.; Radwan, M.A.; Ghanem, N. Melatonin administration during the first half of pregnancy improves the reproductive performance of rabbits: Emphasis on ovarian and placental functions. Theriogenology 2023, 205, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and functional characterization of the main bioactive molecules con-tained in hulled Cannabis sativa L. seeds for use as functional ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar]
- Vizzari, F.; Massányi, M.; Knížatová, N.; Corino, C.; Rossi, R.; Ondruška, Ľ.; Tirpák, F.; Halo, M.; Massányi, P. Effects of dietary plant polyphenols and seaweed extract mixture on male-rabbit semen: Quality traits and antioxidant markers. Saudi J. Biol. Sci. 2021, 28, 1017–1025. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef]
- El-Sherbiny, H.R.; Hashem, N.M.; Abdelnaby, E.A. Coat color affects the resilience against heat stress impacts on testicular hemodynamics, reproductive hormones, and semen quality in Baladi goats. BMC Vet.- Res. 2023, 19, 107. [Google Scholar] [CrossRef]
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.G.; Abo-Elezz, Z.R. Improving Rabbit Doe Metabolism and Whole Reproductive Cycle Outcomes via Fatty Acid-Rich Moringa oleifera Leaf Extract Supplementation in Free and Nano-Encapsulated Forms. Animals 2022, 12, 764. [Google Scholar] [CrossRef]
- Retana-Marquez, S.; Hernandez, H.; Flores, J.A.; Muñoz-Gutierrez, M.; Duarte, G.; Vielma, J.; Fitz-Rodriguez, G.; Fernandez, I.; Keller, M.; Delgadillo, J.A. Effects of Phytoestrogens on Mammalian Reproductive Physiology. Trop. Subtrop. Agroecosystems 2011, 15, S129–S145. [Google Scholar] [CrossRef]
- Morales Ramírez, M.; Vargas Estrada, D.; Juárez Rodríguez, I.; Sierra Reséndizb, A.; Flores Gonzáleze, H.F.; Cerbón Gutiérrezf, J.L.; Peña-Corona, S.I. Effects of phytoestrogens on the reproductive physiology of productive species. Review. Rev. Mex. De Cienc. Pecu. 2022, 13, 803–829. [Google Scholar] [CrossRef]
- Hashem, N.M.; Soltan, Y.A. Impacts of Phytoestrogens on Livestock Production: A Review. Egypt. J. Nutr. Feed. 2016, 19, 81–89. [Google Scholar] [CrossRef]
- Wyse, J.; Latif, S.; Gurusinghe, S.; McCormick, J.; Weston, L.A.; Stephen, C.P. Phytoestrogens: A Review of Their Impacts on Reproductive Physiology and Other Effects upon Grazing Livestock. Animals 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Debacker, M.; Ďuračka, M.; Kováč, J.; Bučko, O. Quercetin and Naringenin Provide Functional and Antioxidant Protection to Stored Boar Semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef]
- De Assis, S.; Warri, A.; Benitez, C.; Helferich, W.; Hilakivi-Clarke, L. Protective Effects of Prepubertal Genistein Exposure on Mammary Tumorigenesis Are Dependent on BRCA1 Expression. Cancer Prev. Res. 2011, 4, 1436–1448. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Gao, A.; Khawar, M.B.; Gao, F.; Li, W. Food-Derived High Arginine Peptides Promote Spermatogenesis Recovery in Busulfan Treated Mice. Front. Cell Dev. Biol. 2021, 9, 791471. [Google Scholar] [CrossRef]
- Akram, M.; Ali, S.A.; Kaul, G. Probiotic and prebiotic supplementation ameliorates chronic restraint stress-induced male reproductive dysfunction. Food Funct. 2023, 14, 8558–8574. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Nguyen, M.; Pham, T.; Tran, H. The role of prebiotics in improving reproductive performance in small ruminants. Small Rumin. Res. 2019, 174, 16–23. [Google Scholar]
- Artym, J.; Zimecki, M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines 2021, 9, 1940. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, K.; Che, L.; Fang, Z.; Xu, S.; Feng, B.; Zhuo, Y.; Li, J.; Wu, C.; Zhang, J.; et al. The Improvement of Semen Quality by Dietary Fiber Intake Is Positively Related with Gut Microbiota and SCFA in a Boar Model. Front. Microbiol. 2022, 13, 863315. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2008, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional Amino Acids in Growth, Reproduction, and Health. Adv. Nutr. Int. Rev. J. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gilbreath, K.R.; Bazer, F.W.; Satterfield, M.C.; Wu, G. Amino Acid Nutrition and Reproductive Performance in Ruminants. Adv. Exp. Med. Biol. 2021, 1285, 43–61. [Google Scholar]
- Agarwal, A.; Sekhon, L.H. The role of antioxidant therapy in the treatment of male infertility. Hum. Fertil. 2010, 13, 217–225. [Google Scholar] [CrossRef]
- Titi, H.H.; Alnimer, M.A.; Abedal-Majed, M.A. Effect of supplemental rumen-protected methionine on reproduction and production of Awassi ewes. Ital. J. Anim. Sci. 2022, 21, 624–633. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Slevin, J.; Hunter, M.G.; Edwards, S.A.; Ashworth, C.J. Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction 2007, 133, 433–439. [Google Scholar] [CrossRef]
- Süss, D.; Iwersen, M.; Schweinzer, V.; Gusterer, E.; Kanz, P.; Krieger, S.; Pothmann, H.; Wagener, K.; Hoelker, M.; Tesfaye, D.; et al. Supplementing rumen-protected methionine to lactating multiparous dairy cows did not improve reproductive performance. Reprod. Domest. Anim. 2019, 54, 1265–1273. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Fang, Q.; Liu, Q.; Du, R.; Yang, G.; Wang, L.; Hu, J. Supplemental effect of different levels of taurine in Modena on boar semen quality during liquid preservation at 17 °C. Anim. Sci. J. 2017, 88, 1692–1699. [Google Scholar] [CrossRef]
- Bahrami, M.; Morris, M.B.; Day, M.L. Glutamine, proline, and isoleucine support maturation and fertilisation of bovine oocytes. Theriogenology 2023, 201, 59–67. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Jaeger, L.A.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Meininger, C.J.; Spencer, T.E.; Yin, Y.-L. Important roles for the arginine family of amino acids in swine nutrition and production. Livest. Sci. 2007, 112, 8–22. [Google Scholar] [CrossRef]
- Smith, A.; Johnson, B.; Wilson, C. Effects of a Plant Extract on Reproductive Hormones and Sperm Quality in Boars. J. Anim. Sci. 2018, 42, 201–209. [Google Scholar]
- Shakouri, N.; Soleimanzadeh, A.; Rakhshanpour, A.; Bucak, M.N. Antioxidant effects of supplementation of 3,4-dihydroxyphenyl glycol on sperm parameters and oxidative markers following cryopreservation in canine semen. Reprod. Domest. Anim. 2021, 56, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, E.O.; Gruhot, T.R.; Park, S.; Ishak, G.M.; Mote, B.E.; Liao, S.F.; Feugang, J.M. Dietary Arginine Supplementation Modulates the Proteome of Boar Seminal Plasma. Animals 2025, 15, 555. [Google Scholar] [CrossRef]
- Santana, P.D.P.B.; Silva, T.V.G.; da Costa, N.N.; da Silva, B.B.; Carter, T.F.; Cordeiro, M.d.S.; da Silva, B.J.M.; Santos, S.D.S.D.; Herculano, A.M.; Adona, P.R.; et al. Supplementation of bovine embryo culture medium with L-arginine improves embryo quality via nitric oxide production. Mol. Reprod. Dev. 2014, 81, 918–927. [Google Scholar] [CrossRef]
- Gai, Z.; Hu, S.; He, Y.; Yan, S.; Wang, R.; Gong, G.; Zhao, J. L-arginine alleviates heat stress-induced mammary gland injury through modulating CASTOR1-mTORC1 axis mediated mitochondrial homeostasis. Sci. Total. Environ. 2024, 926, 172017. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Al-Sagheer, A.; Noreldin, A.E.; El-Hack, M.E.A.; Khafaga, A.F.; Abdel-Latif, M.A.; Swelum, A.A.; Arif, M.; Salem, A.Z.M. Beneficial effects of rumen-protected methionine on nitrogen-use efficiency, histological parameters, productivity and reproductive performance of ruminants. Anim. Biotechnol. 2019, 32, 51–66. [Google Scholar] [CrossRef]
- Abbasi, I.H.R.; Abbasi, F.; Wang, L.; El Hack, M.E.A.; Swelum, A.A.; Hao, R.; Yao, J.; Cao, Y. Folate promotes S-adenosyl methionine reactions and the microbial methylation cycle and boosts ruminants production and reproduction. AMB Express 2018, 8, 65. [Google Scholar] [CrossRef]
- Kim, W.S.; Nejad, J.G.; Peng, D.Q.; Jo, Y.H.; Kim, J.; Lee, H.G. Effects of different protein levels on growth performance and stress parameters in beef calves under heat stress. Sci. Rep. 2022, 12, 8113. [Google Scholar] [CrossRef]
- Jousan, F.D.; García-Bojalil, C.M.; Satter, L.D. Lysine supplementation of corn-based diets enhances reproductive performance in dairy cows and heifers. J. Dairy Sci. 1992, 75, 1871–1878. [Google Scholar]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N.A.R.S. Nutritional strategies to improve fertility in dairy cows. Anim. Reprod. Sci. 2006, 96, 265–276. [Google Scholar]
- Santos, J.; Thatcher, W.; Chebel, R.; Cerri, R.; Galvão, K. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 2004, 82–83, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Pascal, C.; Nechifor, I.; Florea, M.A.; Pânzaru, C.; Simeanu, D.; Mierliță, D. Diet Influence on Sperm Quality, Fertility, and Reproductive Behavior in Karakul of Botoșani Rams. Agriculture 2023, 13, 2168. [Google Scholar] [CrossRef]
- Bauersachs, S.; Ulbrich, S.E.; Blum, H. The effect of dietary amino acids on the reproductive performance of cattle. J. Anim. Sci. 2009, 87, 2105–2115. [Google Scholar]
- Wu, G.; Bazer, F.W.; Cudd, T.A. The role of amino acids in the regulation of reproduction and fertility in livestock. J. Anim. Sci. 2014, 92, 2991–3000. [Google Scholar]
- Kim, Y.H.; Ahn, J.W.; Lee, S.S. Impact of amino acid supplementation on the fertility of dairy cows. Reprod. Domest. Anim. 2017, 52, 385–392. [Google Scholar]
- Abdulkareem, T.; Ibrahim, F.; Hassan, M.; Mohamad, O.; Lateef, W. Effect of Adding Amino Acids Combinations to Tris Extender for Improving Post Cryopreserved Semen Characteristics of Holstein Bulls. Biochem. Cell. Arch. 2020, 20, 697–701. [Google Scholar]
- Elsaadawy, S.A.; Wu, Z.; Bu, D. Feasibility of Supplying Ruminally Protected Lysine and Methionine to Periparturient Dairy Cows on the Efficiency of Subsequent Lactation. Front. Vet. Sci. 2022, 9, 892709. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Zhao, C.; Malhi, M.; Wang, Y. Effects of dietary arginine supplementation during early pregnancy on embryo survival, fetal development, and hormone concentrations. J. Anim. Sci. 2018, 96, 5245–5256. [Google Scholar]
- Ahmed, K.; Shah, A.A.; Ahmad, S.; Ahmed, Z.; Ahmad, N. Carnitine: A potential supplement to improve semen quality in livestock breeding programs. Anim. Reprod. Sci. 2019, 208, 106–120. [Google Scholar]
- Li, C.; Zhang, J.; Li, Y.; Zhao, X.; Liang, H.; Li, K.; Qu, M.; Qiu, Q.; Ouyang, K. Glutamate Supplementation Improves Growth Performance, Rumen Fermentation, and Serum Metabolites in Heat-Stressed Hu Sheep. Front. Nutr. 2022, 9, 851386. [Google Scholar] [CrossRef]
- Delgado, R.; Abad-Guamán, R.; De la Mata, E.; Menoyo, D.; Nicodemus, N.; García, J.; Carabaño, R. Effect of dietary sup-plementation with arginine and glutamine on the performance of rabbit does and their litters during the first three lacta-tions. Anim. Feed. Sci. Technol. 2017, 227, 84–94. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Sun, W.; Liu, J.; Liu, H.; Wang, Y. Effects of dietary leucine supplementation during early pregnancy on re-productive performance and the mechanistic target of rapamycin signaling pathway in sows. Anim. Reprod. Sci. 2019, 208, 106–124. [Google Scholar]
- Kujoana, T.C.; Mabelebele, M.; Sebola, N.A. Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review. Open Agric. 2023, 9, 20220244. [Google Scholar] [CrossRef]
- Zuidhof, M.J. Precision livestock feeding: Matching nutrient supply with nutrient requirements of individual animals. J. Appl. Poult. Res. 2020, 29, 11–14. [Google Scholar] [CrossRef]
- Castro, T.; Martinez, D.; Isabel, B.; Cabezas, A.; Jimeno, V. Vegetable Oils Rich in Polyunsaturated Fatty Acids Supplementation of Dairy Cows’ Diets: Effects on Productive and Reproductive Performance. Animals 2019, 9, 205. [Google Scholar] [CrossRef]
- Staples, C.; Thatcher, W. Effects of Fatty Acids on Reproduction of Dairy Cows. Recent Adv. Anim. Nutr. 2006, 2005, 229–256. [Google Scholar] [CrossRef]
- Diaz, T.; Alberio, R.; Luque, G.M.; Olivera, R.; Becú-Villalobos, D.; Pucciarelli, A.; Hiriart, M.I. Influence of omega-3 and omega-6 polyunsaturated fatty acids on prostaglandin synthesis in ovine granulosa cells. Prostaglandins Leukot. Essent. Fat. Acids 2019, 144, 7–12. [Google Scholar]
- Nazifi, S.; Asadi, E.; Karimi, I.; Khaki, A. Effects of hydrogenated fats (trans-fatty acids) on reproductive system: A review. Vet. Res. Forum 2017, 8, 261–265. [Google Scholar]
- Masoudi, R.; Davachi, N.D. Effect of Dietary fish oil on semen quality and reproductive performance of Iranian Zandi rams. Arch. Razi Inst. 2020, 76, 621–629. [Google Scholar] [CrossRef]
- Abdelatty, A.M.; Noreldin, A.E.; Soliman, M.M. Effect of dietary n-3 polyunsaturated fatty acid supplementation on semen quality and reproductive hormones of rams. J. Adv. Vet. Anim. Res. 2018, 5, 8–15. [Google Scholar]
- Mostek, A.; Dietrich, M.; Janda, T.; Paluszak, J.; Ciereszko, A. Effect of dietary omega-3/omega-6 fatty acid ratios on reproduction-related traits in male broodstock of tench (Tinca tinca L.). Aquac. Nutr. 2016, 22, 1005–1015. [Google Scholar]
- Çoban, S.; Erdoğan, Z. The Effects of Unsaturated fatty acid supplementation to ration on superovulation performance and embryo quality of donor cows. J. Agric. Sci. 2021, 27, 179–186. [Google Scholar] [CrossRef]
- Leal, D.F.; Pugliesi, E.D.M.; Cooke, R.F.; Menezes, R.F.; Nascimento, M.F.A. Effect of omega-6 and omega-3 polyunsaturated fatty acid sources on the reproductive performance of grazing beef cows and preimplantation embryos. J. Anim. Sci. 2017, 95, 1830–1844. [Google Scholar]
- Mattos, R.; Staples, C.; Thatcher, W. Effects of dietary fatty acids on reproduction in ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef]
- Lee, S.S.; Joo, L.P.; Park, Y.S.; Lee, K.S.; Lee, Y.M.; Choi, S.W. Effects of dietary fat sources on reproductive performance and response to gonadotropin treatment in lactating dairy cows. Asian-Australas. J. Anim. Sci. 2011, 24, 1218–1223. [Google Scholar]
- Duckett, S.K.; Neel, J.P.S.; Lewis, R.M.; Fontenot, J.P.; Clapham, W.M. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef]
- Mapiye, C.; Aalhus, J.L.; Turner, T.D.; Rolland, D.C.; Basarab, J.A.; Baron, V.S. Effect of prepartum maternal dietary sup-plementation with calcium salts of fatty acids on postnatal calf growth, carcass, and meat quality traits. J. Anim. Sci. 2018, 96, 1409–1423. [Google Scholar]
- Ribeiro, E.; Lima, F.; Greco, L.; Bisinotto, R.; Monteiro, A.; Favoreto, M.; Ayres, H.; Marsola, R.; Martinez, N.; Thatcher, W.; et al. Prevalence of periparturient diseases and effects on fertility of seasonally calving grazing dairy cows supplemented with concentrates. J. Dairy Sci. 2013, 96, 5682–5697. [Google Scholar] [CrossRef]
- Sharma, A.; Baddela, V.S.; Roettgen, V.; Vernunft, A.; Viergutz, T.; Dannenberger, D.; Hammon, H.M.; Schoen, J.; Vanselow, J. Effects of Dietary Fatty Acids on Bovine Oocyte Competence and Granulosa Cells. Front. Endocrinol. 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G.; Schor, E.; Kopelman, A. Nutritional aspects related to endometriosis. Rev. Assoc. Médica Bras. 2015, 61, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.F.; de Carvalho, R.H.; de Lima, I.C.S.; Batista, A.M.V.; de Oliveira, D.M.; de Macedo Beltrão, N.E. Sperm quality and fatty acid profile of sperm are improved after adding an omega-6-rich nut to the diet of Holstein bulls. Livest. Sci. 2018, 216, 123–126. [Google Scholar]
- Kamal, M.; Youssef, I.M.; Khalil, H.A.; Ayoub, M.A.; Hashem, N.M. Multifunctional Role of Chitosan in Farm Animals: A Comprehensive Review. Ann. Anim. Sci. 2023, 23, 69–86. [Google Scholar] [CrossRef]
- Keane, J.A.; Ealy, A.D. An Overview of Reactive Oxygen Species Damage Occurring during In Vitro Bovine Oocyte and Embryo Development and the Efficacy of Antioxidant Use to Limit These Adverse Effects. Animals 2024, 14, 330. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Hammadi, M.; Tadj, H.; Najarnezhad, V. The effect of different selenium sources on semen quality, glutathione peroxidase activity, and testosterone concentration in ram lambs. Theriogenology 2019, 125, 10–15. [Google Scholar]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Xue, K.; Xu, L.; Jiang, S. Dietary zinc in relation to seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Biol. Trace Elem. Res. 2016, 170, 398–404. [Google Scholar]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2014, 32, 3–16. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Gaughan, J.B. Selenium supplementation alters gene expression profiles associated with innate immunity in whole blood of cattle. Res. Vet. Sci. 2015, 100, 220–225. [Google Scholar]
- Anwar, A.; Fakoorziba, M.R.; Shahidi, F. The effects of dietary selenium supplementation on sperm quality in goats. Vet. Res. Forum 2014, 5, 19–24. [Google Scholar]
- Vonnahme, K.A.; Wilson, M.E.; Ford, S.P. Impacts on conceptus survival in grazing ruminants. J. Anim. Sci. 2002, 80, E24–E31. [Google Scholar]
- Long, Y.; Paengkoum, S.; Lu, S.; Niu, X.; Thongpea, S.; Taethaisong, N.; Han, Y.; Paengkoum, P. Physicochemical properties, mechanism of action of lycopene and its application in poultry and ruminant production. Front. Vet.- Sci. 2024, 11, 1364589. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F. Lycopene counteracts the hepatic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by altering inflammation, oxidative stress, and lipid metabolism. J. Nutr. Biochem. 2013, 24, 1118–1125. [Google Scholar]
- Badgujar, S.B.; Patel, V.; Bandivdekar, A.H. Fertility enhancement by quercetin in a polycystic ovary syndrome model-rat. Phytother. Res. 2014, 28, 982–986. [Google Scholar]
- Mannerås-Holm, L.; Marklund, A.; Santaniemi, M. Circulating adiponectin concentrations are reduced in women with poly-cystic ovary syndrome and correlate with serum testosterone levels. J. Clin. Endocrinol. Metab. 2004, 89, 956–960. [Google Scholar]
- Danda, R.S.; Habiba, N.M.; Rincon-Choles, H.; Bhandari, B.K.; Barnes, J.L.; Abboud, H.E.; Pergola, P.E. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005, 68, 2562–2571. [Google Scholar] [CrossRef]
- Omar, M.E.A.; Hassanein, E.M.; Shehabeldin, A.M.; Szenci, O.; El-Shereif, A.A. Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows. Pharmaceutics 2025, 17, 274. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Antioxidants in Poultry Nutrition and Reproduction: An Update. Antioxidants 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Romanov, M.N.; Griffin, D.K. Nutritional modulation of the antioxidant capacities in poultry: The case of vitamin E. Poult. Sci. 2019, 98, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wu, H.; Li, G.; Yan, L.; Wang, L.; Zhao, M.; Guan, S.; Xu, S.; Guo, X.; Liu, F.; et al. Melatonin promotes the growth and development of lambs by increasing growth hormone and testosterone, targeting on apoptosis signaling pathway and intestinal microflora. Front. Endocrinol. 2022, 13, 966120. [Google Scholar] [CrossRef]
- Assunção, C.M.; Mendes, V.R.A.; Brandão, F.Z.; Batista, R.I.T.P.; Souza, E.D.; de Carvalho, B.C.; Quintão, C.C.R.; Raposo, N.R.B.; Camargo, L.S.A. Effects of resveratrol in bull semen extender on post-thaw sperm quality and capacity for fertilization and embryo development. Anim. Reprod. Sci. 2021, 226, 106697. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of Dietary Vitamin E on Fertility Functions in Poultry Species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef]
- Abebe, A.; Berhane, G.; Getachew, T.; Gizaw, S.; Haile, A. Reproductive performance and productivity of local and Dorper x local crossbred ewes under community-based management system, Ethiopia. Heliyon 2023, 9, e19906. [Google Scholar] [CrossRef]
- D’occhio, M.J.; Ghuman, S.S.; Neglia, G.; della Valle, G.; Baruselli, P.S.; Zicarelli, L.; Visintin, J.A.; Sarkar, M.; Campanile, G. Exogenous and endogenous factors in seasonality of reproduction in buffalo: A review. Theriogenology 2020, 150, 186–192. [Google Scholar] [CrossRef]
- Murphy, E.; Stanton, C.; Brien, C.; Murphy, C.; Holden, S.; Murphy, R.; Varley, P.; Boland, M.; Fair, S. The effect of dietary supplementation of algae rich in docosahexaenoic acid on boar fertility. Theriogenology 2017, 90, 78–87. [Google Scholar] [CrossRef]
- Azawi, O.I.; Hussein, E.K. Effect of vitamins C or E supplementation to Tris diluent on the semen quality of Awassi rams preserved at 5 °C. Vet. Res. Forum 2013, 4, 157–160. [Google Scholar]
- Ali, S.; Zhao, Z.; Zhen, G.; Kang, J.Z.; Yi, P.Z. Reproductive problems in small ruminants (sheep and goats): A substantial economic loss in the world. Large Anim. Rev. 2019, 25, 215–223. [Google Scholar]
- Hamidian, S.; Talebi, A.R.; Fesahat, F.; Bayat, M.; Mirjalili, A.M.; Ashrafzadeh, H.R.; Rajabi, M.; Montazeri, F.; Babaei, S. The effect of vitamin C on the gene expression profile of sperm protamines in the male partners of couples with recurrent pregnancy loss: A randomized clinical trial. Clin. Exp. Reprod. Med. 2020, 47, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Duran, J.; Bello, J.M.; Ros-Santaella, J.L.; Boersma, B.; del Olmo, A.; Garcia-Ispierto, I. Vitamin D supplementation in dairy cows: A review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 615–630. [Google Scholar]
- Nouri, M.; Ostadhosseini, S.; Sharafi, M.; Ashtiani, R.T.; Zhandi, M.; Tajik, P. In vitro, maturation of ovine oocytes with vitamin B12 improves their developmental competence. Small Rumin. Res. 2019, 173, 7–12. [Google Scholar]
- Alam, M.S.; Szenci, O.; Konyali, A.B.; Asimakopoulos, B.; Cseh, S.; Pal, M.A. Follicular dynamics, oocyte quality, and early embryonic development following supplementation of vitamin B12 during the in vitro maturation of sheep oocytes. Reprod. Domest. Anim. 2021, 56, 452–461. [Google Scholar]
- Khan, M.Z.; Khan, A.; Xiao, J.; Dou, J.; Liu, L.; Yu, Y. Overview of Folic Acid Supplementation Alone or in Combination with Vitamin B12 in Dairy Cattle during Periparturient Period. Metabolites 2020, 10, 263. [Google Scholar] [CrossRef]
- Aboelenain, M.; Balboula, A.Z.; Kawahara, M.; Montaser, A.E.-M.; Zaabel, S.M.; Kim, S.-W.; Nagano, M.; Takahashi, M. Pyridoxine supplementation during oocyte maturation improves the development and quality of bovine preimplantation embryos. Theriogenology 2016, 91, 127–133. [Google Scholar] [CrossRef]
- Lu, J.; Weil, J.; Maharjan, P.; Manangi, M.; Cerrate, S.; Coon, C. The effect of feeding adequate or deficient vitamin B6 or folic acid to breeders on methionine metabolism in 18-day-old chick embryos. Poult. Sci. 2021, 100, 101008. [Google Scholar] [CrossRef]
- Squires, M.W.; Naber, E.C. Vitamin Profiles of Eggs as Indicators of Nutritional Status in the Laying Hen: Riboflavin Study. Poult. Sci. 1993, 72, 483–494. [Google Scholar] [CrossRef]
- Olkowski, A.A.; Classen, H.L. The study of thiamine requirement in broiler chickens. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Int. J. Vitam. Nutr. Res. J. Int. Vitaminol. Nutr. 1996, 66, 332–341. [Google Scholar]
- Charles, O.; Roland, D.; Edwards, H. Thiamine Deficiency Identification and Treatment in Commercial Turkeys and Coturnix Quail. Poult. Sci. 1972, 51, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Olkowski; Classen, H.L. The Effects of Maternal Thiamine Nutrition on Thiamine Status of the Offspring in Broiler Chickens. Int. J. Vitam. Nutr. Res. 1999, 69, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Akbağ, H.I.; Savaş, T.; Yüceer, Y.K. The effect of fenugreek seed (Trigonella foenum-graecum) supplementation on the performance and milk yield characteristics of dairy goats. Arch. Anim. Breed. 2022, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, Z.; Al-Mola, M. Effect of fenugreek seeds extract on the reproductive performance in male and female rats. Int. J. Pharma Sci. Res. 2012, 3, 229–239. [Google Scholar]
- Ayadi, A.; Kammoun, R.; Mnif, H. Effects of Vitex agnus-castus extract supplementation on estrus synchronization and conception rates in goats. Reprod. Domest. Anim. 2019, 54, 525–533. [Google Scholar]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Zinsstag, J.; Langhans, W.; Hertzberg, H. Effect of sainfoin (Onobrychis viciifolia) silage and hay on established populations of Haemonchus contortus and Cooperia curticei in lambs. Vet.- Parasitol. 2006, 142, 293–300. [Google Scholar] [CrossRef]
- Evans, H.C.; Briggs, E.F.; Burnett, R.H.; Contreras-Correa, Z.E.; Duvic, M.A.; Dysart, L.M.; Gilmore, A.A.; Messman, R.D.; Reid, D.; Ugur, M.R.; et al. Harnessing the value of reproductive hormones in cattle production with considerations to animal welfare and human health. J. Anim. Sci. 2022, 100, skac177. [Google Scholar] [CrossRef]
- Sun, S.; Lv, M.; Niu, H.; Luo, J. Influence of repeated estrus synchronization treatment on hormone secretion, growth, and development of dairy goats. Front. Vet.- Sci. 2024, 10, 1333633. [Google Scholar] [CrossRef]
- Hudson, T. Saw palmetto for men and women. Townsend Lett. Dr. Patients 2003, 238, 40–42. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Malekinejad, H.; Scherpenisse, P.; Bergwerff, A.A. Naturally Occurring Estrogens in Processed Milk and in Raw Milk (from Gestated Cows). J. Agric. Food Chem. 2006, 54, 9785–9791. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.E.; Betts, J.E. The effects of red clover (Trifolium pratense var. redhead), white clover (Trifolium repens var. S 100) or perennial ryegrass (Lolium perenne var. S 23) on the reproductive performance of sheep. J. Agric. Sci. 1973, 80, 323–327. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls. Antioxidants 2024, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Pen, B.; Sar, C.; Mwenya, B.; Kuwaki, K.; Morikawa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed. Sci. Technol. 2006, 129, 175–186. [Google Scholar] [CrossRef]
- Placha, I.; Takacova, J.; Ryzner, M.; Cobanova, K.; Laukova, A.; Strompfova, V.; Venglovska, K.; Faix, S. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 2014, 55, 105–114. [Google Scholar] [CrossRef]
- Boué, S.M.; Wiese, T.E.; Nehls, S.; Burow, M.E.; Elliott, S.; Carter-Wientjes, C.H.; Shih, B.Y.; McLachlan, J.A.; Cleveland, T.E. Evaluation of the Estrogenic Effects of Legume Extracts Containing Phytoestrogens. J. Agric. Food Chem. 2003, 51, 2193–2199. [Google Scholar] [CrossRef]
- Austin, A.R.; Aston, K.; Drane, H.M.; Saba, N. The fertility of heifers consuming red clover silage. Grass Forage Sci. 1982, 37, 101–106. [Google Scholar] [CrossRef]
- Surai, P. Natural Antioxidants in Avian Nutrition and Reproduction; Nottingham University Press: Nottingham, UK, 2002; pp. 5–9. Available online: http://www.feedfood.co.uk/download/Imm_my_2002a.pdf (accessed on 2 March 2025).
- Vasconcelos, J.; Sartori, R.; Oliveira, H.; Guenther, J.; Wiltbank, M. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology 2001, 56, 307–314. [Google Scholar] [CrossRef]
- Akhtar, P.; Rajoriya, J.S.; Ojha, B.K.; Jha, A.K.; Bisen, A.; Bajaj, N.K.; Ahirwar, M.K.; Raje, A.; Singh, A.P.; Peepar, S.S.; et al. Effects of dietary supplementation with omega-3 fatty acid-rich linseed on the reproductive performance of ewes in subtropical climates. Front. Vet.- Sci. 2024, 11, 1398961. [Google Scholar] [CrossRef]
- Kujoana, T.C.; Sehlabela, L.D.; Mabelebele, M.; Sebola, N.A. The potential significance of antioxidants in livestock re-production: Sperm viability and cryopreservation. Anim. Reprod. Sci. 2024, 267, 107512. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kupczyński, R.; Gałęska, E.; Araujo, J.P.; Czerniawska-Piątkowska, E. Clinical Application of Bioextracts in Supporting the Reproductive System of Animals and Humans: Potential and Limitations. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biscarini, F.; Nicolazzi, E.L.; Stella, A.; Boettcher, P.J.; Gandini, G. Challenges and opportunities in genetic improvement of local livestock breeds. Front. Genet. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of Endocrine System in the Regulation of Female Insect Reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef]
- Závorka, L.; Blanco, A.; Chaguaceda, F.; Cucherousset, J.; Killen, S.S.; Liénart, C.; Mathieu-Resuge, M.; Němec, P.; Pilecky, M.; Scharnweber, K.; et al. The role of vital dietary biomolecules in eco-evo-devo dynamics. Trends Ecol. Evol. 2022, 38, 72–84. [Google Scholar] [CrossRef]
- Bó, G.A.; Menchaca, A. Prohibition of hormones in animal reproduction: What to expect and what to do? Anim. Reprod. 2023, 20, e20230067. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdoun, K.A. Safety and concerns of hormonal application in farm animal production: A review. J. Appl. Anim. Res. 2022, 50, 426–439. [Google Scholar] [CrossRef]
- Khan, S.U.; Jamal, M.A.; Su, Y.; Wei, H.-J.; Qing, Y.; Cheng, W. Towards Improving the Outcomes of Multiple Ovulation and Embryo Transfer in Sheep, with Particular Focus on Donor Superovulation. Vet. Sci. 2022, 9, 117. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and reproductive management of farm animals: Challenges and ad-vances. Animals 2021, 11, 1932. [Google Scholar] [CrossRef]
- Choudhary, K.K.; Kavya, K.M.; Jerome, A.; Sharma, R.K. Advances in reproductive biotechnologies. Vet.- World 2016, 9, 388–395. [Google Scholar] [CrossRef]
- Nalla, K.; Manda, N.K.; Dhillon, H.S.; Kanade, S.R.; Rokana, N.; Hess, M.; Puniya, A.K. Impact of Probiotics on Dairy Production Efficiency. Front. Microbiol. 2022, 13, 805963. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Hsieh, Y.C.; Lee, T.-T. The Effects of Fungal Feed Additives in Animals: A Review. Animals 2020, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, W.A.A.; Shaalan, M.; Salem, H.M. Nanoparticles applications in poultry production: An updated review. World’s Poult. Sci. J. 2021, 77, 1001–1025. [Google Scholar] [CrossRef]
- Mekonnen, G. Review on application of nanotechnology in animal health and production. J. Nanomed. Nanotechnol. 2021, 12, 559. [Google Scholar]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78–93. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Sherbiny, H.R.; Fathi, M.; Abdelnaby, E.A. Nanodelivery System for Ovsynch Protocol Improves Ovarian Response, Ovarian Blood Flow Doppler Velocities, and Hormonal Profile of Goats. Animals 2022, 12, 1442. [Google Scholar] [CrossRef]
- Toledano-Díaz, A.; Castaño, C.; Velázquez, R.; Bóveda, P.; López-Sebastián, A.; Martínez-Nevado, E.; Villaverde-Morcillo, S.; Esteso, M.C.; Santiago-Moreno, J. Cryopreservation of ferret (Mustela putorius furo) sperm collected by rectal massage and electroejaculation: Comparison of a decelerating and an accelerating freezing rate protocol. Vet. Med. Sci. 2021, 7, 256–263. [Google Scholar] [CrossRef]
- Bernier, N.J.; Alderman, S.L. Applied aspects of fish. In Conservation Physiology for the Anthropocene—A Systems Approach; Elsevier: Amsterdam, The Netherlands, 2022; p. 253. [Google Scholar]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse Effects of Phytoestrogens on the Reproductive Performance: Cow as a Model. Int. J. Endocrinol. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Gumus, R.; Gelen, S.U. Effects of dietary thyme and rosemary essential oils on performance parameters with lipid oxidation, water activity, pH, colour and microbial quality of breast and drumstick meats in broiler chickens. Arch. Anim. Breed. 2023, 66, 17–29. [Google Scholar] [CrossRef]
- Pavlidis, C.; Patrinos, G.P.; Katsila, T. Nutrigenomics: A controversy. Appl. Transl. Genom. 2015, 4, 50–53. [Google Scholar] [CrossRef]
- Neeha, V.S.; Kinth, P. Nutrigenomics research: A review. J. Food Sci. Technol. 2012, 50, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Haq, Z.U.; Saleem, A.; Alam Khan, A.; Dar, M.A.; Ganaie, A.M.; Beigh, Y.A.; Hamadani, H.; Ahmad, S.M. Nutrigenomics in livestock sector and its human-animal interface-a review. Vet.- Anim. Sci. 2022, 17, 100262. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production pro-grams. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Mendiola, J.; Torres-Cantero, A.M.; Moreno-Grau, J.M.; Ten, J.; Roca, M.; Moreno-Grau, S.; Bernabeu, R. Food intake and its relationship with semen quality: A case-control study. Fertil. Steril. 2008, 91, 812–818. [Google Scholar] [CrossRef]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef]
- Zhang, N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Mostafa, T.H.; Elsayed, F.A.; Ahmed, M.A.E.-S.; Elkholany, M.A. Effect of Using Some Feed Additives (Tw-Probiotics) in Dairy Cow Rations on Production and Reproductive Performance. Egypt. J. Anim. Prod. 2014, 51, 1–11. [Google Scholar]
- Kulkarni, N.A.; Chethan, H.S.; Srivastava, R.; Gabbur, A.B. Role of probiotics in ruminant nutrition as natural modulators of health and productivity of animals in tropical countries: An overview. Trop. Anim. Health Prod. 2022, 54, 110. [Google Scholar] [CrossRef]
- El-Garhi, M.; Soltan, M.; Ahmed, H.; Abdel-Latif, M.A.; Galal, M.; El-Bordeny, N. Assessment impact of using locally produced probiotic bacteria on the productive and reproductive performance of Holstein dairy cows. Assiut Vet. Med. J. 2019, 65, 39–50. [Google Scholar]
- Dou, Y.; Yu, X.; Luo, Y.; Chen, B.; Ma, D.; Zhu, J. Effect of Fructooligosaccharides Supplementation on the Gut Microbiota in Human: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3298. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Mahendra, M.Y.N.; Dadi, T.B.; Kamaludeen, J.; Pertiwi, H. Beneficial Effects of Lactic Acid Bacteria on Animal Reproduction Function. Vet. Med. Int. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Adnane, M.; Whiston, R.; Tasara, T.; Bleul, U.; Chapwanya, A. Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals 2024, 14, 1073. [Google Scholar] [CrossRef]
- Rani, K.; Kaur, G.; Ali, S.A. Probiotic-prebiotic therapeutic potential: A new horizon of microbial biotherapy to reduce female reproductive complications. PharmaNutrition 2023, 24, 100342. [Google Scholar] [CrossRef]
- Mahalak, K.K.; Firrman, J.; Narrowe, A.B.; Hu, W.; Jones, S.M.; Bittinger, K.; Moustafa, A.M.; Liu, L. Fructooligosaccharides (FOS) differentially modifies the in vitro gut microbiota in an age-dependent manner. Front. Nutr. 2023, 9, 1058910. [Google Scholar] [CrossRef]
- Omondi, V.O.; Bosire, G.O.; Onyari, J.M.; Kibet, C.; Mwasya, S.; Onyonyi, V.N.; Getahun, M.N. Multi-omics analyses reveal rumen microbes and secondary metabolites that are unique to livestock species. mSystems 2024, 9, e0122823. [Google Scholar] [CrossRef]
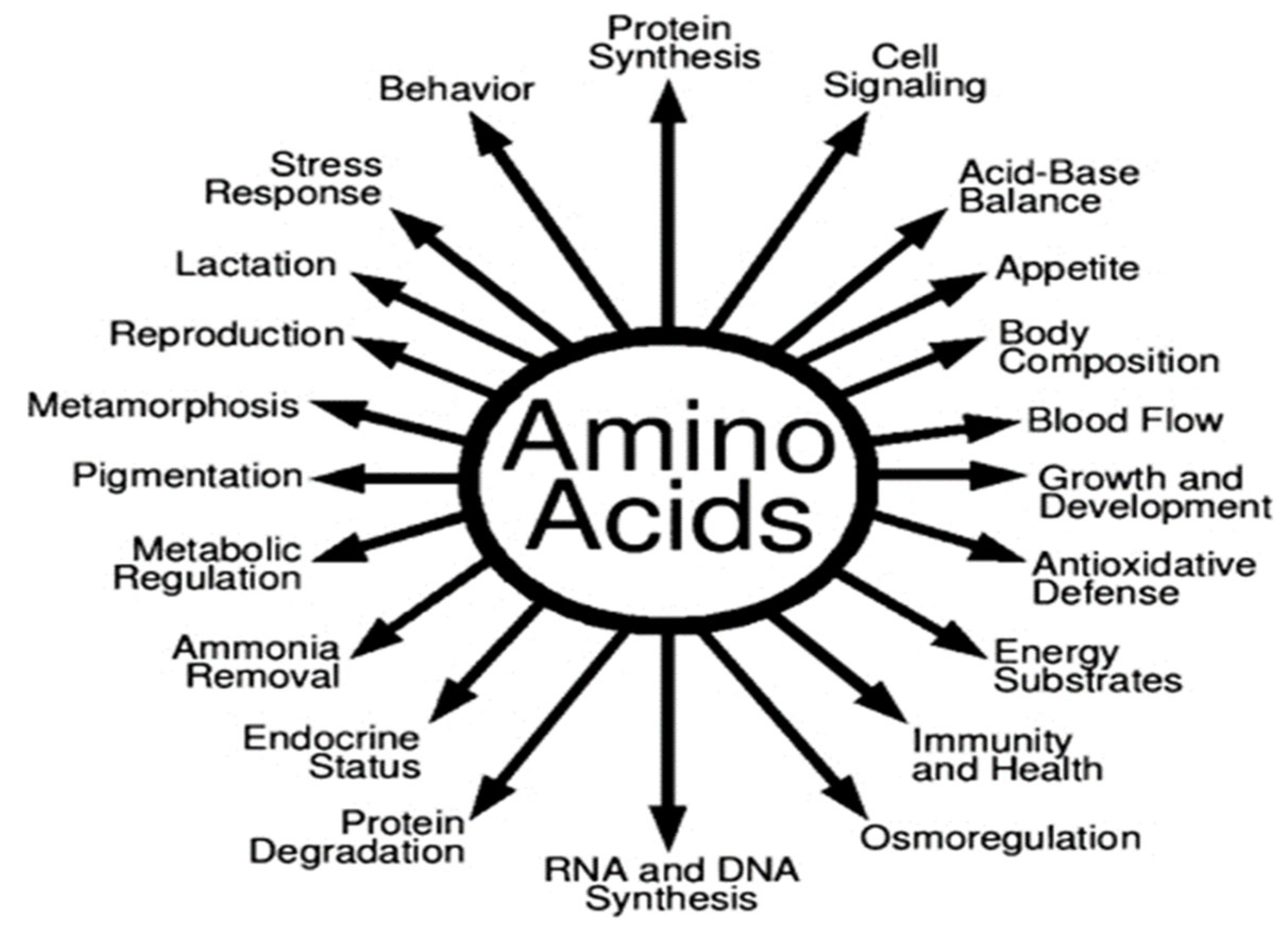
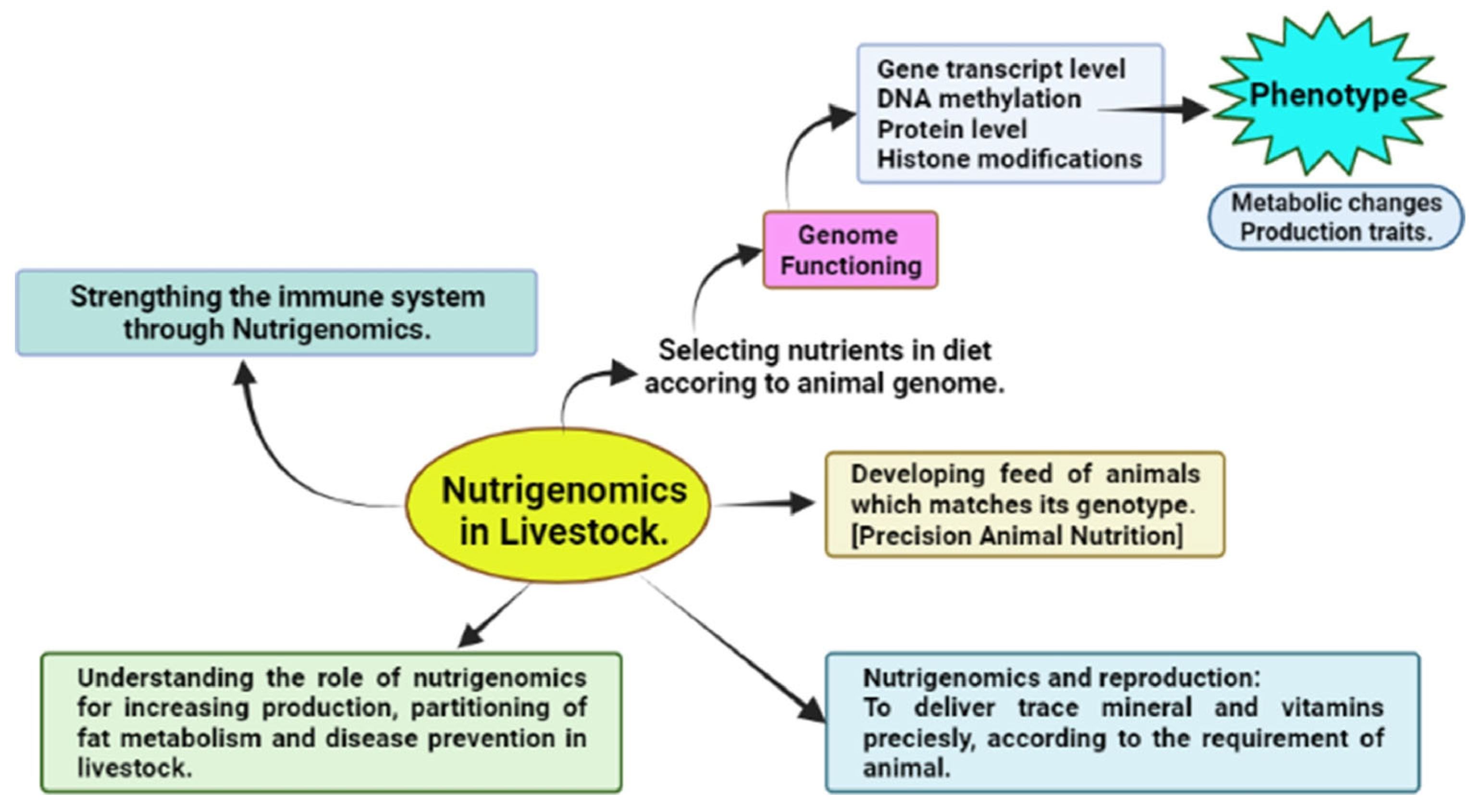
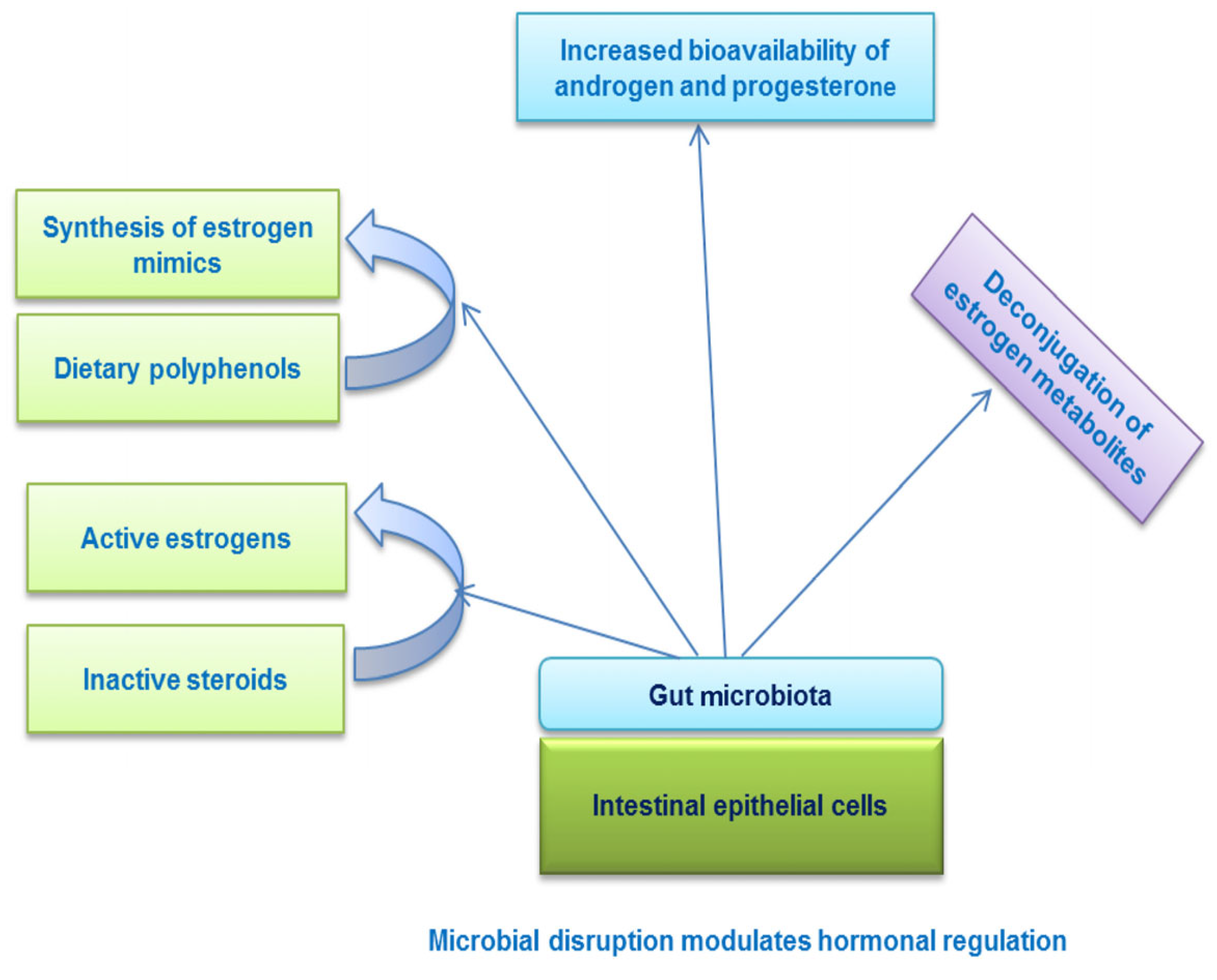
| Amino Acid | Effect on Male Fertility | Effect on Female Fertility | Reference |
|---|---|---|---|
| Arginine | Improved sperm quality | Enhanced ovulation in sheep | [48,49] |
| Methionine | Increased sperm count | Improved embryo quality | [50,51] |
| Lysine | Enhanced sperm motility | Increased conception rate in cows and heifers | [52,53] |
| Histidine | Improved semen parameters | Regulated estrus cycle in dairy cows | [54,55] |
| Tryptophan | Enhanced sperm viability | Improved reproductive health in sheep and goats | [56] |
| Fatty Acid | Effects on Male Fertility | Effects on Female Fertility | Reference |
|---|---|---|---|
| Omega-3 Fatty Acids | Improved sperm quality, increased sperm count, and motility. | Enhanced ovarian function, improved oocyte quality, and increased embryo development. | [77] |
| Omega-6 Fatty Acids | Decreased sperm quality and motility, impaired reproductive performance. | Disrupted ovarian function, decreased oocyte quality, and impaired embryo development. | [78,79] |
| Saturated Fatty Acids | Negative impact on sperm quality, motility reduced reproductive performance. | Adverse effects on ovarian function, oocyte quality, and embryo development. | [78,80] |
| Trans Fatty Acids | Detrimental effects on sperm quality and motility. | Disrupted ovarian function, impaired oocyte maturation, and reduced embryo quality. | [81,82] |
| Monounsaturated Fatty Acids | Mixed effects on sperm quality, moderate influence on fertility. | Variable impact on ovarian function, oocyte quality, and embryo development. | [83,84] |
| Antioxidant | Effect on Male Fertility | Effect on Female Fertility | Reference |
|---|---|---|---|
| Selenium | Improved sperm quality, increased sperm motility, reduced sperm abnormalities. | Improved oocyte quality, enhanced reproductive performance, and reduced embryonic mortality. | [98,99,100] |
| Lycopene | Enhanced sperm quality and improved sperm parameters. | Potential improvement in ovarian function, and reduction in oxidative stress. | [101,102,103] |
| Quercetin | Increased sperm count and improved sperm viability. | Enhanced ovarian function, potential reduction in oxidative stress. | [104,105,106] |
| Omega-3 Fatty Acids | Improved sperm quality and increased libido. | Improved ovarian function, increased conception rates | [86] |
| Biomolecule | Mechanism of Action | Ruminant Species | Reference |
|---|---|---|---|
| Fatty Acids | Enhance ovarian function, regulate reproductive hormones, | Sheep, Goats, | [145] |
| improve oocyte quality, reduce inflammation, | Cows | ||
| and promote embryo development. | |||
| Amino Acids | Serve as building blocks for protein synthesis, | Sheep, Goats, | [146] |
| influencing hormone production and fertility. | Cows | ||
| Antioxidants | Combat oxidative stress, protect gametes and embryos, | Sheep, Goats, | [147] |
| improve sperm quality and reproductive efficiency. | Cows | ||
| Plant Extracts | Modulate hormone secretion, enhance uterine environment, | Sheep, Goats, | [148] |
| regulate ovarian function, and improve sperm motility. | Cows | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
BenSouf, I.; Saidani, M.; Maazoun, A.; Bejaoui, B.; Larbi, M.B.; M’Hamdi, N.; Aggad, H.; Joly, N.; Rojas, J.; Morillo, M.; et al. Use of Natural Biomolecules in Animal Feed to Enhance Livestock Reproduction. Int. J. Mol. Sci. 2025, 26, 2328. https://doi.org/10.3390/ijms26052328
BenSouf I, Saidani M, Maazoun A, Bejaoui B, Larbi MB, M’Hamdi N, Aggad H, Joly N, Rojas J, Morillo M, et al. Use of Natural Biomolecules in Animal Feed to Enhance Livestock Reproduction. International Journal of Molecular Sciences. 2025; 26(5):2328. https://doi.org/10.3390/ijms26052328
Chicago/Turabian StyleBenSouf, Ikram, Mariem Saidani, Asma Maazoun, Bochra Bejaoui, Manel Ben Larbi, Naceur M’Hamdi, Hebib Aggad, Nicolas Joly, Janne Rojas, Marielba Morillo, and et al. 2025. "Use of Natural Biomolecules in Animal Feed to Enhance Livestock Reproduction" International Journal of Molecular Sciences 26, no. 5: 2328. https://doi.org/10.3390/ijms26052328
APA StyleBenSouf, I., Saidani, M., Maazoun, A., Bejaoui, B., Larbi, M. B., M’Hamdi, N., Aggad, H., Joly, N., Rojas, J., Morillo, M., & Martin, P. (2025). Use of Natural Biomolecules in Animal Feed to Enhance Livestock Reproduction. International Journal of Molecular Sciences, 26(5), 2328. https://doi.org/10.3390/ijms26052328









