Transcriptome Analysis of Onobrychis viciifolia During Seed Germination Reveals GA3-Inducible Genes Associated with Phenylpropanoid and Hormone Pathways
Abstract
1. Introduction
2. Results
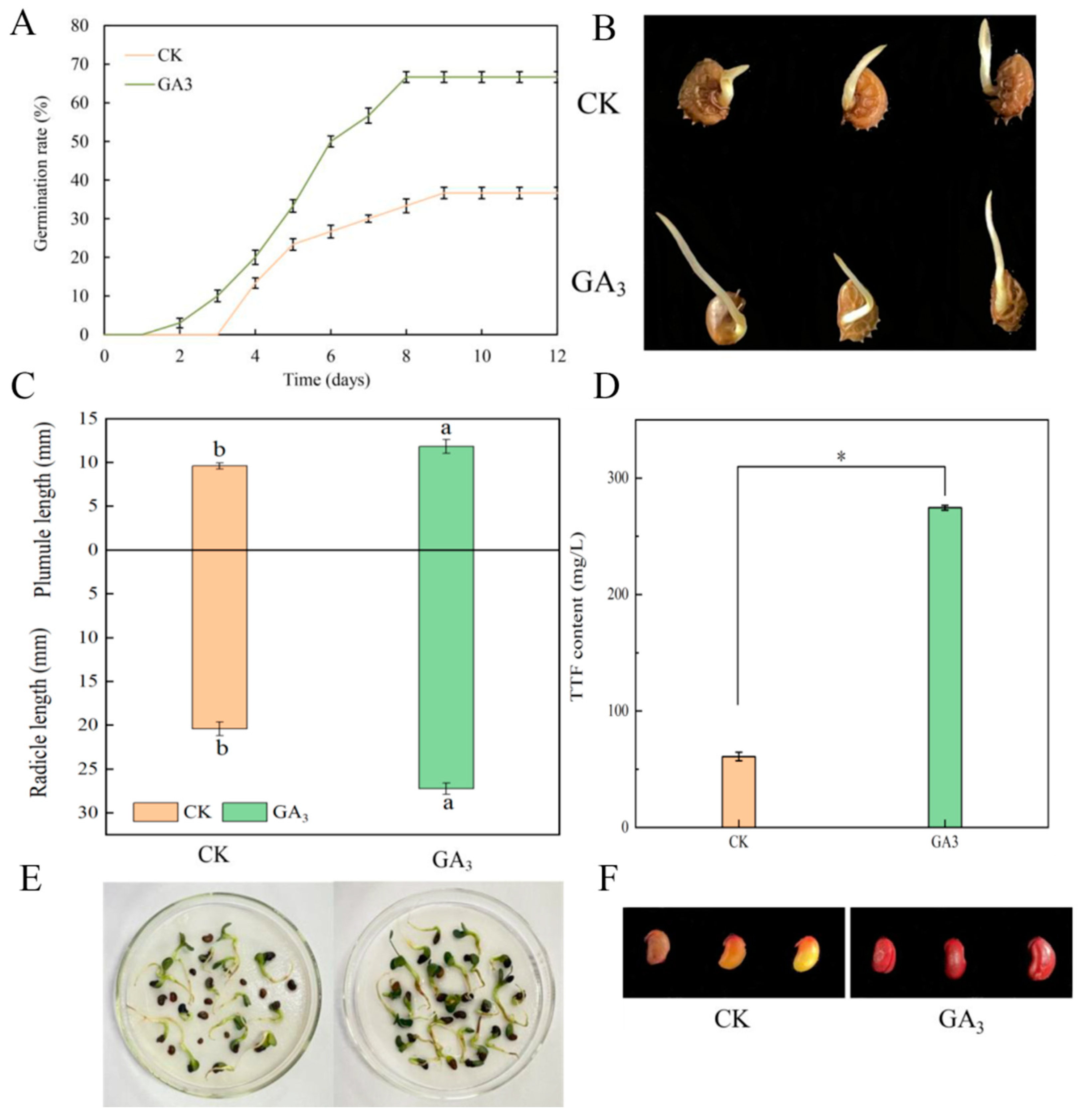
2.1. Effect of GA3 on Germination Rate and Seed Vigor
2.2. Effect of GA3 on Endogenous Inhibitors in Sainfoin Seeds
2.3. RNA-Sequencing Analysis
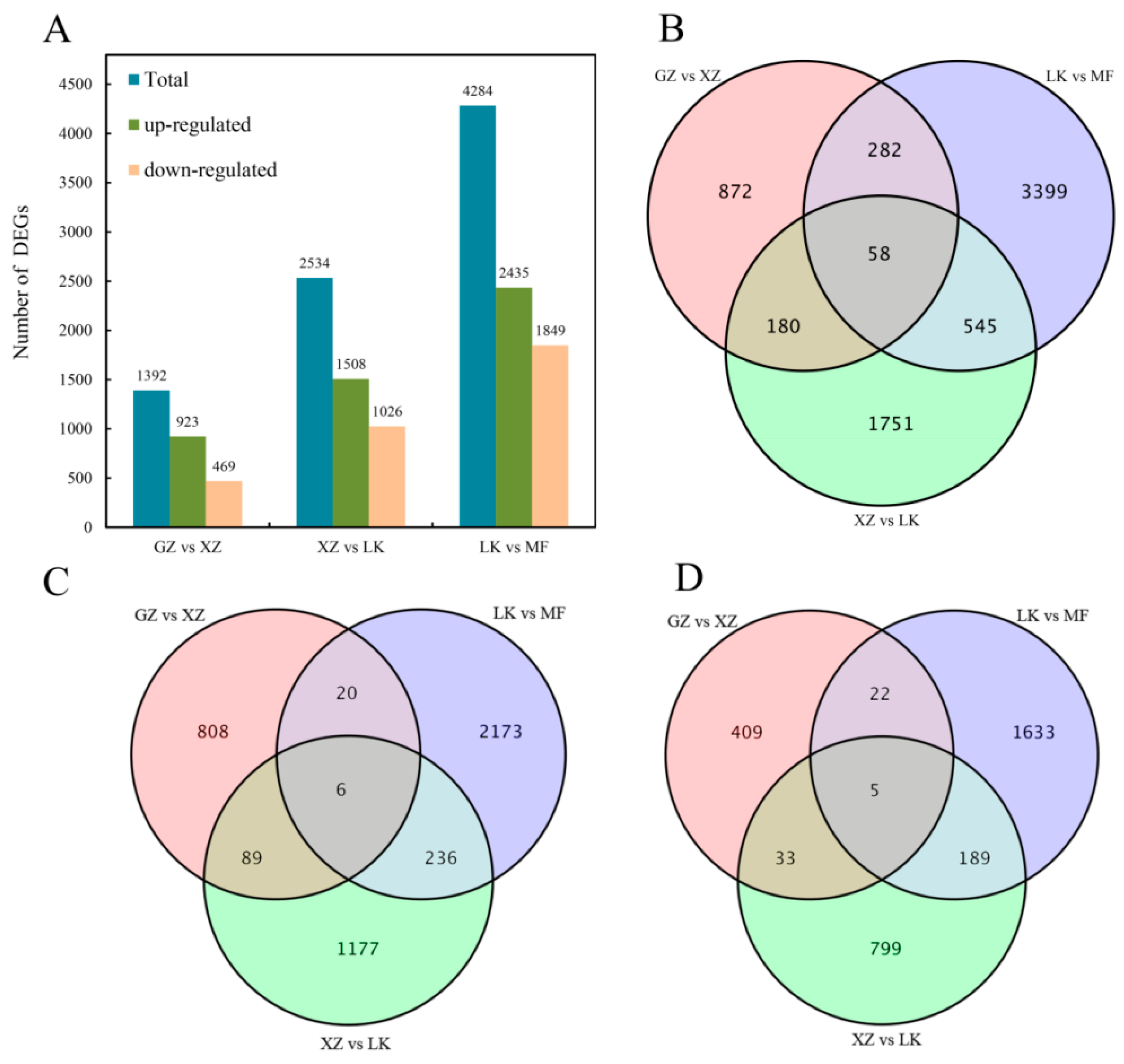
2.4. Identification of DEGs at Different Germination Stages
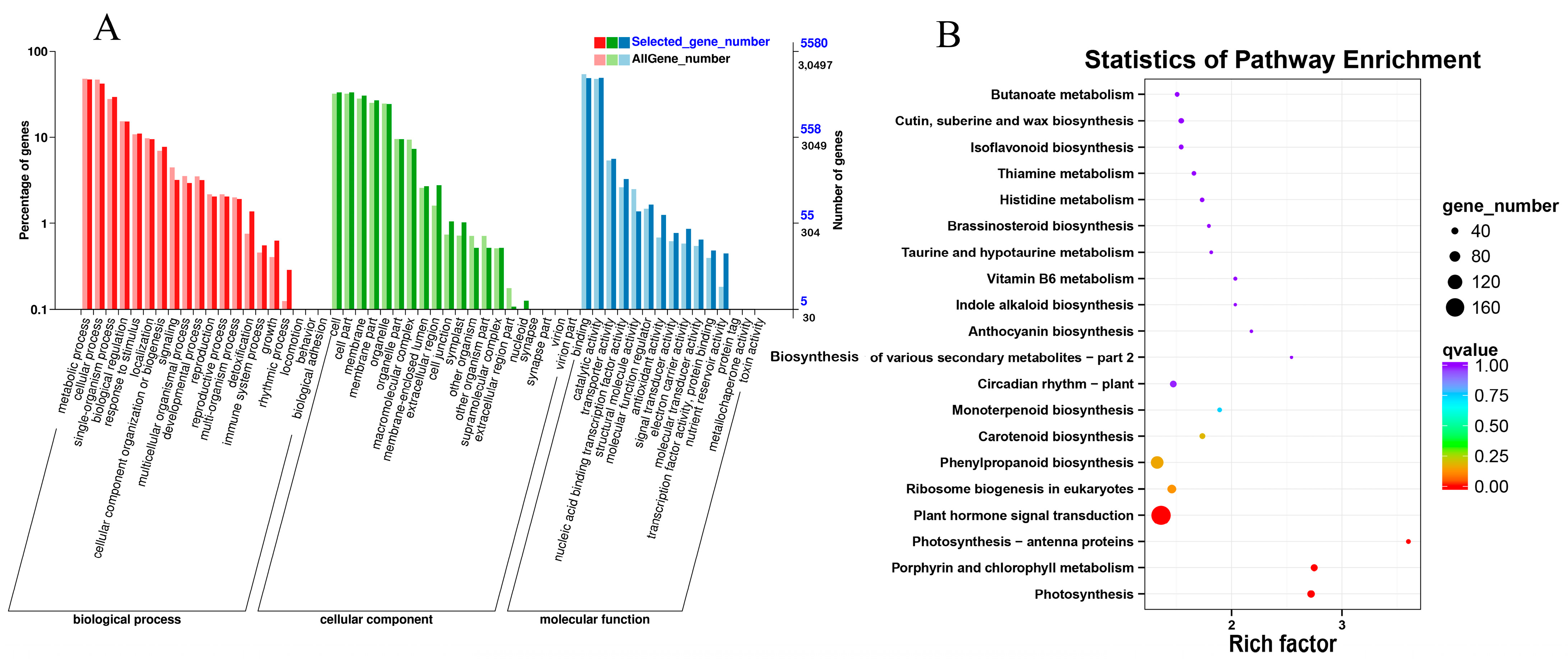
2.5. GO and KEGG Enrichment Analysis
2.6. DEGs Involved in Plant Hormone Signal Transduction
2.7. DEGs Involved in Phenylalanine Biosynthesis Metabolism
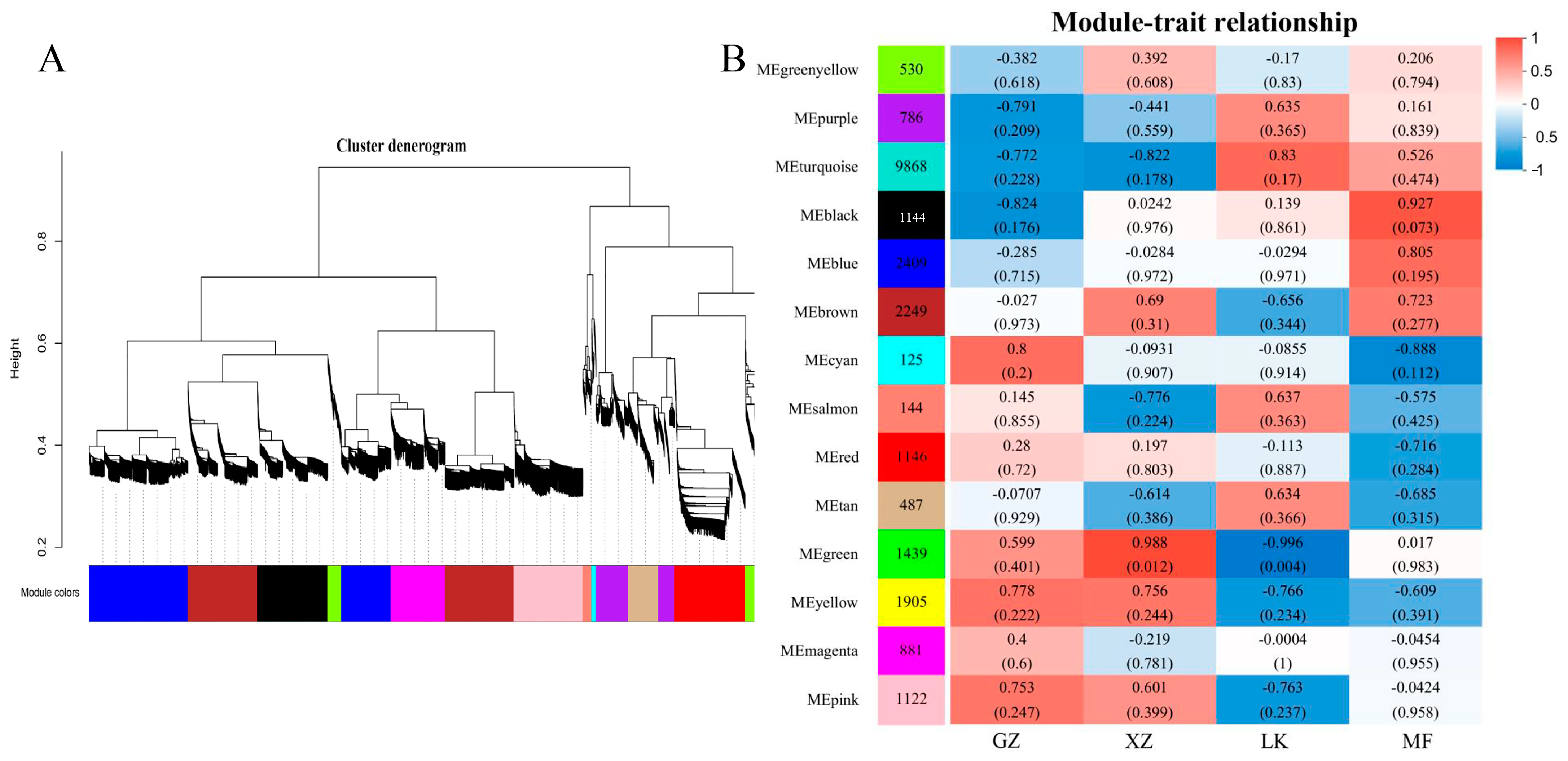
2.8. Weighted Gene Co-Expression Network Construction (WGCNA)
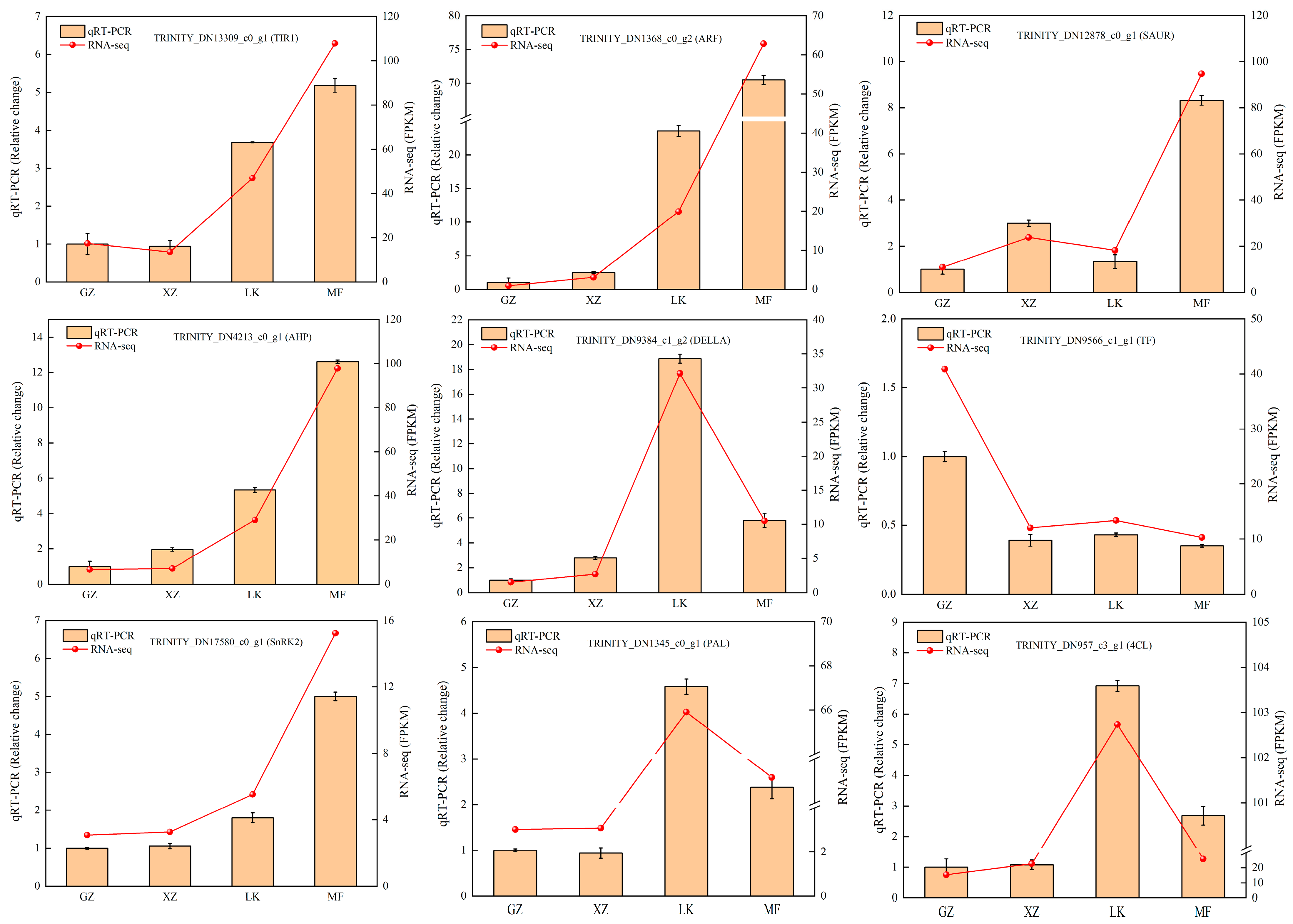
2.9. Analysis of RNA-seq Data Reliability Using qRT-PCR
3. Discussion
3.1. GA3 Promotes Germination and Attenuates the Accumulation of Endogenous Inhibitors
3.2. Plant Hormones Regulate Seed Germination Under GA3-Treatment
3.3. Phenylpropanoid Biosynthesis Involved in Seed Germination of GA3-Treated Seeds
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Seed Vigor, ZT, IAA, GA3, and ABA Contents in Seeds
4.3. Extraction and Determination of Endogenous Inhibitors
4.4. cDNA Library Construction and Transcriptome Sequencing
4.5. De Novo Assembly and Functional Annotation of Unigenes
4.6. Determination of Differential Expression Genes
4.7. qRT-PCR Analysis
4.8. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Z.; Jiang, W.; Luo, Y.; Huang, H.; Yi, D.; Pang, Y. Analyses on flavonoids and transcriptome reveals key MYB gene for proanthocyanidins regulation in Onobrychis Viciifolia. Front. Plant Sci. 2022, 13, 941918. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tian, D.; Li, X.; Wang, X.; Wang, T.; Wang, Z.; Zang, H.; He, X.; Zhang, T.; Yun, Q.; et al. A chromosome-level genome assembly for Onobrychis Viciifolia reveals gene copy number gain underlying enhanced proanthocyanidin biosynthesis. Commun. Biol. 2024, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Wang, X.; Ma, C.; Zhang, F. Effects of intrinsic tannins on proteolysis dynamics, protease activity, and metabolome during sainfoin ensiling. Front. Microbiol. 2022, 13, 976118. [Google Scholar] [CrossRef]
- Huyen, N.T.; Verstegen, M.W.; Hendriks, W.H.; Pellikaan, W.F. Sainfoin (Onobrychis viciifolia) silage in dairy cow rations reduces ruminal biohydrogenation and increases transfer efficiencies of unsaturated fatty acids from feed to milk. Anim. Nutr. 2020, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Rivaroli, D.; Prunier, A.; Meteau, K.; Do Prado, I.N.; Prache, S. Tanninrich sainfoin pellet supplementation reduces fat volatile indoles content and delays digestive parasitism in lambs grazing alfalfa. Animal 2019, 13, 1883–1890. [Google Scholar] [CrossRef]
- Biligetu, B.; Jefferson, P.G.; Lardner, H.A.; Acharya, S.N. Evaluation of sainfoin (Onobrychis viciifolia) for forage yield and persistence in sainfoin–alfalfa (Medicago sativa) mixtures and under different harvest frequencies. Can. J. Plant Sci. 2021, 101, 525–535. [Google Scholar] [CrossRef]
- Qiao, Y.; Cheng, Q.; Zhang, Y.; Yan, W.; Yi, F.; Shi, F. Transcriptomic and chemical analyses to identify candidate genes involved in color variation of sainfoin flowers. BMC Plant Biol. 2021, 21, 61. [Google Scholar] [CrossRef]
- Ries, A.; Benítez, J.V.; Samudio, A.; Armoa, R.; Nakayama, H.D. Germination of bean seeds (Vigna unguiculata L. Walp.) in strong electric fields. MethodsX 2023, 11, 102490. [Google Scholar] [CrossRef] [PubMed]
- Palomar, V.M.; Garciarrubio, A.; Garay-Arroyo, A.; Martínez-Martínez, C.; RosasBringas, O.; Reyes, J.L.; Covarrubias, A.A. The canonical RdDM pathway mediates the control of seed germination timing under salinity. Plant J. 2021, 105, 691–707. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshml, S.; Lingakumar, K. Role of salicylic Acid (SA) in plants-a review. Int. J. Appl. Res. 2017, 3, 33–37. [Google Scholar]
- Song, Q.-L.; Cheng, S.-Y.; Chen, Z.-X.; Nie, G.-P.; Xu, F.; Zhang, J.; Zhou, M.-Q.; Zhang, W.-W.; Liao, Y.-L.; Ye, J.-B. Comparative transcriptome analysis revealing the potential mechanism of seed germination stimulated by exogenous gibberellin in Fraxinus Hupehensis. BMC Plant Biol. 2019, 19, 199. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.-C.; Xie, Y.-L.; Gao, J. Ultrastructure change and transcriptome analysis of GA3 treatment on seed germination of Moso Bamboo (Phyllostachys edulis). Plant Signal. Behav. 2022, 17, 2091305. [Google Scholar] [CrossRef]
- Ge, N.; Jia, J.-S.; Yang, L.; Huang, R.-M.; Wang, Q.-Y.; Chen, C.; Meng, Z.-G.; Li, L.-G.; Chen, J.-W. Exogenous gibberellic acid shortening after-ripening process and promoting seed germination in a medicinal plant Panax notoginseng. BMC Plant Biol. 2023, 23, 67. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of hormonal regulation in rice seed germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Seed dormancy and germination. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar]
- Yan, H.; Chen, H.; Xia, M.; Liao, Q.; Zhao, J.; Peng, L.; Zou, L.; Zhao, G. The impacts of plant hormones on the growth and quality of sprouts. Food Bioprocess Tech. 2024, 17, 2913–2942. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mMechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Cui, N.-H.; Feng, Y.-C.; Zhang, J.; Liu, Y. Effect of gibberellin concentration on seed germination of acer mono maxim. Chin. J. Appl. Environ. Biol. 2021, 3, 555–559. [Google Scholar]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Li, Y.; Gao, R.; Tao, X.; Song, J.; Li, C.; Li, Q. Comparative transcriptome analysis reveals the potential mechanism of GA3-induced dormancy release in Suaeda glauca black seeds. Front. Plant Sci. 2024, 15, 1354141. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Liu, J.-Y.; Zhang, S.-N.; Zhang, Y.-Y. Bioassay of endogenous germination inhibitors in Trillium kamtschaticum seed. Seed Sci. Technol. 2016, 44, 224–232. [Google Scholar] [CrossRef]
- Shi, S.; Cheng, J.; Ahmad, N.; Zhao, W.; Tian, M.; Yuan, Z.; Li, C.; Zhao, C. Effects of potential allelochemicals in a water extract of Abutilon theophrasti Medik. on germination and growth of Glycine max L., Triticum aestivum L., and Zea mays L. J. Sci. Food Agr. 2023, 103, 2155–2165. [Google Scholar] [CrossRef]
- Fu, J.-R. Seed physiology; Agricultural Press: Beijing, China, 1992. [Google Scholar]
- Xu, Y.-X.; Tang, X.-H.; Fu, J.-R. Progress of Research on Seed Physiology; Zhongshan University Press: Guangzhou, China, 1987. [Google Scholar]
- Yu, F.; Li, M.; He, D.-L.; Yang, P.-F. Advances on post-translational modifications involved in seed germination. Front. Plant Sci. 2021, 12, 642979. [Google Scholar] [CrossRef] [PubMed]
- Do, B.H.; Phuong, V.T.B.; Tran, G.B. Emerging functions of chromatin modifications in auxin biosynthesis in response to environmental alterations. Plant Growth Regul. 2019, 87, 165–174. [Google Scholar] [CrossRef]
- Lv, B.; Yu, Q.; Liu, J.; Wen, X.; Yan, Z.; Hu, K.; Li, H.; Kong, X.; Li, C.; Tian, H.; et al. Non-canonical AUX/IAA protein IAA 33 competes with canonical AUX/IAA repressor IAA 5 to negatively regulate auxin signaling. EMBO J. 2020, 39, e101515. [Google Scholar] [CrossRef] [PubMed]
- Sybilska, E.; Daszkowska-Golec, A. A complex signaling trio in seed germination: Auxin-JA-ABA. Trends Plant Sci. 2023, 28, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Qiu, L.; Yang, D.; Zhao, Z.; Gao, Y.; Zhang, S. Genome-wide identification of the SAUR gene family and screening for SmSAURs involved in root development in Salvia Miltiorrhiza. Plant Cell Rep. 2024, 43, 165. [Google Scholar] [CrossRef]
- Wu, M.-J.; Wu, J.-Y.; Gan, Y.-B. The new insight of auxin functions: Transition from seed dormancy to germination and floral opening in plants. Plant Growth Regul. 2020, 91, 169–174. [Google Scholar] [CrossRef]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C.; et al. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Bioch. 2021, 162, 506–516. [Google Scholar] [CrossRef]
- Li, B.; Zhang, P.; Wang, F.; Li, R.; Liu, J.; Wang, Q.; Hu, G. Integrated analysis of the transcriptome and metabolome revealed candidate genes involved in GA3-induced dormancy release in Leymus Chinensis seeds. Int. J. Mol. Sci. 2021, 22, 4161. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Chen, A.; Saleem, N.; Jia, Q.; Zhao, C.; Zhang, M. FUSCA3-induced AINTEGUMENTA-like 6 manages seed dormancy and lipid metabolism. Plant Physiol. 2023, 193, 1091–1108. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Briones-Moreno, A.; Blázquez, M.A. Origin and evolution of gibberellin signaling and metabolism in plants. Semin. Cell Dev. Biol. 2021, 109, 46–54. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep. 2017, 36, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Chen, Y.; Li, T.; Shi, H.; Yu, S.; Gao, Y.; Wang, Z. APETALA2 is involved in ABA signaling during seed germination. Plant Mol. Biol. 2023, 112, 99–103. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Z.; Cheng, C.; Wang, T.; Ji, H.; Zhao, Y.; Deng, Z.; Zhi, L.; Lu, J.; Wu, X.; et al. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Mol. Plant 2020, 13, 1284–1297. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, S.C. Arabidopsis SnRK2.3/SRK2I plays a positive role in seed germination under cold stress conditions. Environ. Exp. Bot. 2023, 212, 105399. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic regulation of ABA and GA in metabolism and signaling pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Miyakawa, T.; Sawano, Y.; Kubota, K.; Kang, H.J.; Asano, A.; Miyauchi, Y.; Takahashi, M.; Zhi, Y.; Fujita, Y.; et al. Structural basis of abscisic acid signaling. Nature 2009, 462, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular mimicry regulates ABA signaling by SnRK2 Kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, W.; Li, Y.; Zhang, X.; Bai, X.; Niu, Z.; Zhang, X.; Li, Z.; Wan, D. Transcriptomic analysis of seed germination under salt stress in two desert sister species (Populus euphratica and P. pruinosa). Front. Genet. 2019, 10, 231. [Google Scholar] [CrossRef]
- Awale, P.; McSteen, P. Hormonal regulation of inflorescence and intercalary meristems in grasses. Curr. Opin. Plant Biol. 2023, 76, 102451. [Google Scholar] [CrossRef]
- Rahim, M.A.; Zhang, X.; Busatto, N. Phenylpropanoid biosynthesis in plants. Front. Plant Sci. 2023, 14, 1230664. [Google Scholar] [CrossRef]
- Yu, A.-L.; Zhao, J.-F.; Wang, Z.-H.; Cheng, K.; Zhang, P.; Tian, G. Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress. BMC Plant Biol. 2020, 20, 274. [Google Scholar] [CrossRef]
- Saha, P.; Lin, F.; Thibivilliers, S.; Xiong, Y.; Pan, C.; Bartley, L.E. Phenylpropanoid biosynthesis gene expression precedes lignin accumulation during shoot development in lowland and upland switchgrass genotypes. Front. Plant Sci. 2021, 12, 640930. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, H.; Li, Q. Biosynthetic regulatory network of flavonoid metabolites in stems and leaves of Salvia miltiorrhiza. Sci. Rep. 2022, 12, 18212. [Google Scholar] [CrossRef]
- Chen, X.-T.; Zhao, G.-Y.; Li, Y.-L.; Wei, S.-M.; Dong, Y.-H.; Jiao, R.-Z. Integrative analysis of the transcriptome and metabolome reveals the mechanism of Chinese fir seed germination. Forests 2023, 14, 676. [Google Scholar] [CrossRef]
- Tong, Y.; Yi, S.-C.; Liu, S.-Y.; Xu, L.; Qiu, Z.-X.; Zeng, D.-Q. Bruceine D may affect the phenylpropanoid biosynthesis by acting on ADTs thus inhibiting Bidens Pilosa L. seed germination. Ecotox. Environ. Safe. 2022, 242, 113943. [Google Scholar] [CrossRef]
- Ma, Z.-X.; Liu, J.; Dong, J.-J.; Yu, J.-H.; Huang, S.-X.; Lin, H.; Wang, J.-F. Optimized qualitative and quantitative methods for barley viability testing using triphenyl tetrazolium chloride staining. Cereal Chem. 2019, 96, 421–428. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, H.; Hou, L.; Zhang, C.; Bo, W.; Pang, X.; Li, Y. HPLC-MS/MS-based and transcriptome analysis reveal the effects of ABA and MeJA on jujube (Ziziphus jujuba Mill.) cracking. Food Chem. 2023, 421, 136155. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Boil. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Wei, J.; Chen, S.; Xue, S.; Zhu, Q.; Liu, S.; Cui, L.; Wang, Y. Blockade of inflammation and apoptosis pathways by siRNA prolongs cold preservation time and protects donor hearts in a porcine model. Mol. Ther. Nucl. Acids 2017, 9, 428–439. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.-X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato- Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Mortezapour, M.; Tapak, L.; Bahreini, F.; Najafi, R.; Afshar, S. Identification of key genes in colorectal cancer diagnosis by weighted gene co-expression network analysis. Comput. Biol. Med. 2023, 157, 106779. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wang, K.; Cheng, J.; Nan, L. Transcriptome Analysis of Onobrychis viciifolia During Seed Germination Reveals GA3-Inducible Genes Associated with Phenylpropanoid and Hormone Pathways. Int. J. Mol. Sci. 2025, 26, 2335. https://doi.org/10.3390/ijms26052335
Luo Y, Wang K, Cheng J, Nan L. Transcriptome Analysis of Onobrychis viciifolia During Seed Germination Reveals GA3-Inducible Genes Associated with Phenylpropanoid and Hormone Pathways. International Journal of Molecular Sciences. 2025; 26(5):2335. https://doi.org/10.3390/ijms26052335
Chicago/Turabian StyleLuo, Yanyan, Kun Wang, Jiao Cheng, and Lili Nan. 2025. "Transcriptome Analysis of Onobrychis viciifolia During Seed Germination Reveals GA3-Inducible Genes Associated with Phenylpropanoid and Hormone Pathways" International Journal of Molecular Sciences 26, no. 5: 2335. https://doi.org/10.3390/ijms26052335
APA StyleLuo, Y., Wang, K., Cheng, J., & Nan, L. (2025). Transcriptome Analysis of Onobrychis viciifolia During Seed Germination Reveals GA3-Inducible Genes Associated with Phenylpropanoid and Hormone Pathways. International Journal of Molecular Sciences, 26(5), 2335. https://doi.org/10.3390/ijms26052335




