Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons
Abstract
1. Introduction
2. The Primary Neurotransmitter of Nociceptive Primary Afferents Is Glutamate
2.1. Vesicular Glutamate Transporter 2
2.2. Glutamate Receptors
2.2.1. Ionotropic Glutamate Receptors
2.2.2. Metabotropic Glutamate Receptors
2.3. Excitatory Amino Acid Transporters
3. Glutamatergic Neurotransmission Can Be Modulated by the Simultaneous Release of Neuropeptides and Neurotrophins
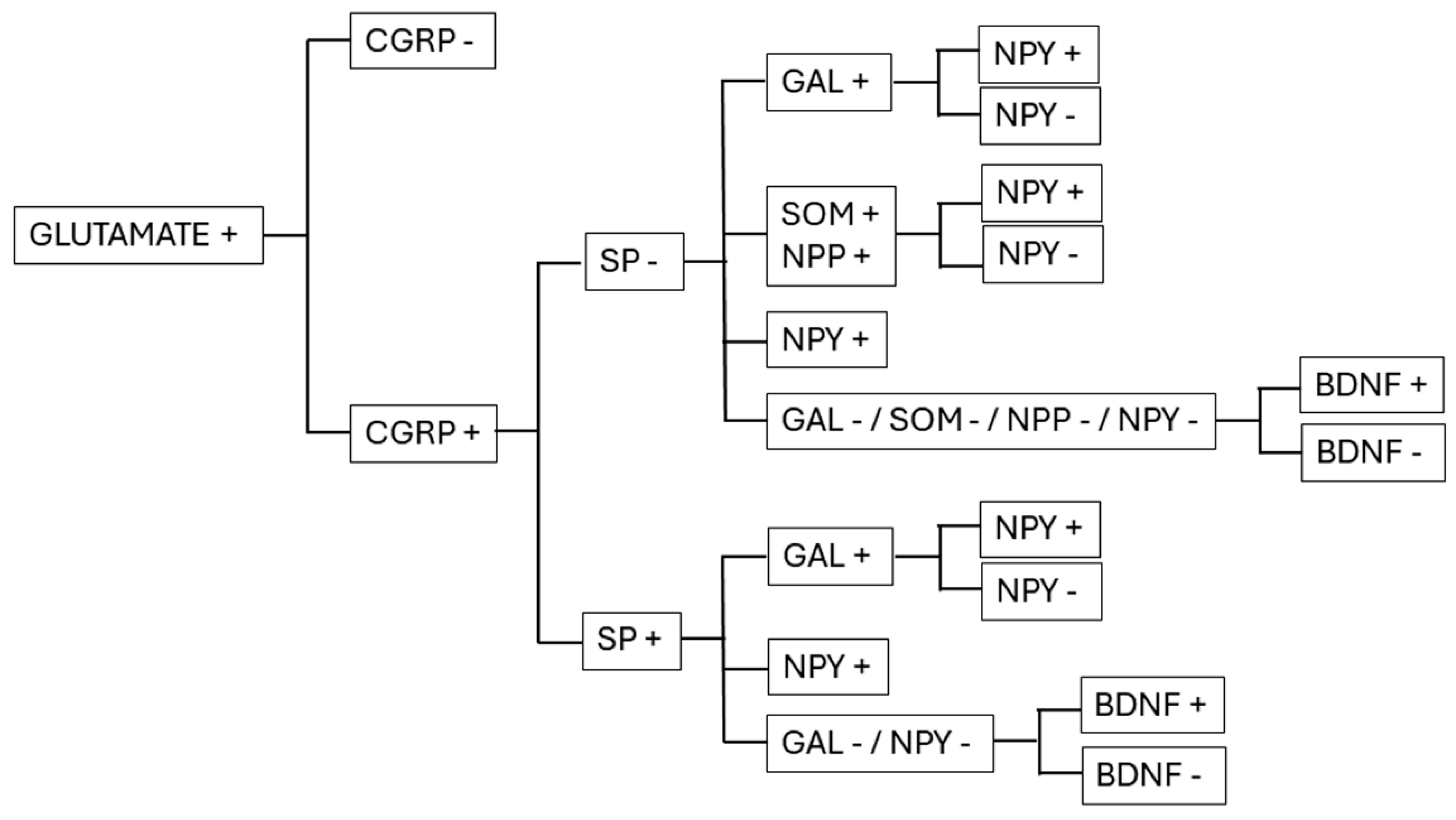
3.1. Neuropeptides
3.1.1. Calcitonin Gene-Related Peptide (CGRP)
3.1.2. Substance P (SP)
3.1.3. Somatostatin (SOM) and Natriuretic Polypeptide (NPP)
3.1.4. Galanin (GAL)
3.1.5. Neuropeptide Y (NPY)
3.1.6. Other Neuropeptides
3.2. Neurotrophins
BDNF
4. Presynaptic Modulation of Neurotransmitter Release from the Axon Terminals of Primary Afferents
4.1. Presynaptic Inhibition via Metabotropic Glutamate Receptors
4.1.1. mGluR4 and mGluR7
4.1.2. mGluR2 and mGluR3
4.1.3. mGluR5
4.1.4. Retrograde Endocannabinoid Signaling
4.2. Roles of Neuropeptides and Neurotrophins in Presynaptic Modulation
4.2.1. CGRP
4.2.2. SP
4.2.3. GAL
4.2.4. NPY
4.2.5. BDNF
4.3. Presynaptic Modulation of Synaptic Transmission by Gamma Aminobutyric Acid and Glycine
4.3.1. GABA
GABAA Receptors
GABAB Receptors
4.3.2. Glycine and Glycine Receptors
4.4. Presynaptic Inhibition of Synaptic Transmission by Endogenous Opioid Peptides
4.5. Presynaptic Inhibition of Synaptic Transmission by the Nociception/Orphanin FQ Peptide and Receptor
4.6. Presynaptic Facilitation of Synaptic Transmission by the Purinergic System
4.7. Presynaptic Modulation of Synaptic Transmission by Monoaminergic Descending Fibers
4.7.1. Noradrenaline
4.7.2. Dopamine
4.7.3. Serotonin
5-HT1 Receptors
5-HT2 Receptors
5-HT7 Receptors
5-HT3 Receptors
5. Conclusions
5.1. Synaptic Versus Volume Transmission
5.2. Corelease of Neurotransmitters
5.3. Presynaptic Modulation of Synaptic Transmission
Funding
Acknowledgments
Conflicts of Interest
References
- Chiechio, S. Modulation of chronic pain by metabotropic glutamate receptors. Adv. Pharmacol. 2016, 75, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Ionotropic glutamate receptors contribute to pain transmission and chronic pain. Neuropharmacology 2017, 112, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Seybold, V.S. The role of peptides in central sensitization. Handb. Exp. Pharmacol. 2009, 194, 451–491. [Google Scholar] [CrossRef]
- Sessler, K.; Blechschmidt, V.; Hoheisel, U.; Mense, S.; Schirmer, L.; Treede, R.D. Spinal cord fractalkine (CX3CL1) signaling is critical for neuronal sensitization in experimental nonspecific, myofascial low back pain. J. Neurophysiol. 2021, 125, 1598–1611. [Google Scholar] [CrossRef]
- Smith, P.A. BDNF in Neuropathic Pain; the Culprit that Cannot be Apprehended. Neuroscience 2024, 543, 49–64. [Google Scholar] [CrossRef]
- Hegyi, Z.; Oláh, T.; Kőszeghy, Á.; Piscitelli, F.; Holló, K.; Pál, B.; Csernoch, L.; Di Marzo, V.; Antal, M. CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018, 8, 10562. [Google Scholar] [CrossRef]
- Lu, Y.; Doroshenko, M.; Lauzadis, J.; Kanjiya, M.P.; Rebecchi, M.J.; Kaczocha, M.; Puopolo, M.J. Presynaptic inhibition of primary nociceptive signals to dorsal horn lamina I neurons by dopamine. J. Neurosci. 2018, 38, 8809–8821. [Google Scholar] [CrossRef]
- Hendrikse, E.R.; Bower, R.L.; Hay, D.L.; Walker, C.S. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019, 39, 403–419. [Google Scholar] [CrossRef]
- Nelson, T.S.; Taylor, B.K. Targeting spinal neuropeptide Y1 receptor-expressing interneurons to alleviate chronic pain and itch. Prog. Neurobiol. 2021, 196, 101894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, Z.; Li, C.; Xing, Z.; Xie, S.; Li, M.; Yao, J. Driving effect of BDNF in the spinal dorsal horn on neuropathic pain. Neurosci. Lett. 2021, 756, 135965. [Google Scholar] [CrossRef] [PubMed]
- Davis, O.C.; Dickie, A.C.; Mustapa, M.B.; Boyle, K.A.; Browne, T.J.; Gradwell, M.A.; Smith, K.M.; Polgár, E.; Bell, A.M.; Kókai, É.; et al. Calretinin-expressing islet cells are a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord. Sci. Rep. 2023, 13, 11561. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Mennit, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Zhang, F.X.; Ge, S.N.; Dong, Y.L.; Shi, J.; Feng, Y.P.; Li, Y.; Li, Y.Q.; Li, J.L. Vesicular glutamate transporter isoforms: The essential players in the somatosensory systems. Prog. Neurobiol. 2018, 171, 72–89. [Google Scholar] [CrossRef]
- Pietrancosta, N.; Djibo, M.; Daumas, S.; El Mestikawy, S.; Erickson, J.D. Molecular, Structural, Functional, and Pharmacological Sites for Vesicular Glutamate Transporter Regulation. Mol. Neurobiol. 2020, 57, 3118–3142. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Villalba, R.M.; Zerda, R.; Schneider, S.P. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J. Comp. Neurol. 2004, 472, 257–280. [Google Scholar] [CrossRef]
- Todd, A.J.; Hughes, D.I.; Polgar, E.; Nagy, G.G.; Mackie, M.; Ottersen, O.P.; Maxwell, D.J. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur. J. Neurosci. 2003, 17, 13–27. [Google Scholar] [CrossRef]
- Landry, M.; Bouali-Benazzouz, R.; El Mestikawy, R.; Ravassard, P.; Nagy, F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J. Comp. Neurol. 2004, 468, 380–394. [Google Scholar] [CrossRef]
- Brumovsky, P.; Watanabe, M.; Hökfelt, T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience 2007, 147, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Abdel Samad, O.; Zhang, L.; Duan, B.; Tong, Q.; Lopes, C.; Ji, R.R.; Lowell, B.B.; Ma, Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 2010, 68, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Osikowicz, M.; Mika, J.; Przewlocka, B. The glutamatergic system as a target for neuropathic pain relief. Exp. Physiol. 2013, 98, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Kang, H.; Sanderson, T.M.; Bortolotto, Z.A.; Georgiou, J.; Zhuo, M.; Kaang, B.K.; Collingridge, G.L. The Role of Calcium-Permeable AMPARs in Long-Term Potentiation at Principal Neurons in the Rodent Hippocampus. Front. Synaptic Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Farrant, M. Ca2+-permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. J. Physiol. 2021, 599, 2655–2671. [Google Scholar] [CrossRef]
- Negrete-Díaz, J.V.; Falcón-Moya, R.; Rodríguez-Moreno, A. Kainate receptors: From synaptic activity to disease. FEBS J. 2022, 289, 5074–5088. [Google Scholar] [CrossRef]
- Gangwar, S.P.; Yelshanskaya, M.V.; Nadezhdin, K.D.; Yen, L.Y.; Newton, T.P.; Aktolun, M.; Kurnikova, M.G.; Sobolevsky, A.I. Kainate receptor channel opening and gating mechanism. Nature 2024, 630, 762–768. [Google Scholar] [CrossRef]
- Ji, R.R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Youn, D.H.; Gerber, G.; Sather, W.A. Ionotropic glutamate receptors and voltage-gated Ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013, 2013, 654257. [Google Scholar] [CrossRef]
- Dahlhaus, A.; Ruscheweyh, R.; Sandkühler, J. Synaptic input of rat spinal lamina I projection and unidentified neurons in vitro. J. Physiol. 2005, 566, 355–368. [Google Scholar] [CrossRef]
- Larsson, M. Ionotropic glutamate receptors in spinal nociceptive processing. Mol. Neurobiol. 2009, 40, 260–288. [Google Scholar] [CrossRef] [PubMed]
- Spike, R.C.; Kerr, R.; Maxwell, D.J.; Todd, A.J. GluR1 and GluR2/3 subunits of the AMPA-type glutamate receptor are associated with particular types of neuron in laminae I-III of the spinal dorsal horn of the rat. Eur. J. Neurosci. 1998, 10, 324–333. [Google Scholar]
- Nagy, G.G.; Al-Ayyan, M.; Andrew, D.; Fukaya, M.; Watanabe, M.; Todd, A.J. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J. Neurosci. 2004, 24, 5766–5777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagy, G.G.; Watanabe, M.; Fukaya, M.; Todd, A.J. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur. J. Neurosci. 2004, 20, 3301–3312. [Google Scholar] [CrossRef]
- Antal, M.; Fukazawa, Y.; Eördögh, M.; Muszil, D.; Molnár, E.; Itakura, M.; Takahashi, M.; Shigemoto, R. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J. Neurosci. 2008, 28, 9692–9701. [Google Scholar] [CrossRef]
- Kopach, O.; Voitenko, N. Spinal AMPA receptors: Amenable players in central sensitization for chronic pain therapy? Channels 2021, 15, 284–297. [Google Scholar] [CrossRef]
- Kopach, O.; Dobropolska, Y.; Belan, P.; Voitenko, N. Ca2+-permeable AMPA receptors contribute to changed dorsal horn neuronal firing and inflammatory pain. Int. J. Mol. Sci. 2023, 24, 2341. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Gasparini, F.; Kuhn, R.; Pin, J.P. Allosteric modulators of group I metabotropic glutamate receptors: Novel subtype-selective ligands and therapeutic perspectives. Curr. Opin. Pharmacol. 2002, 2, 43–49. [Google Scholar] [CrossRef]
- Ren, B.X.; Gu, X.P.; Zheng, Y.G.; Liu, C.L.; Wang, D.; Sun, Y.E.; Ma, Z.L. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology 2012, 116, 122–132. [Google Scholar] [CrossRef]
- Jia, H.; Rustioni, A.; Valtschanoff, J.G. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J. Comp. Neurol. 1999, 410, 627–642. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Villalba, R.M.; Carr, P.A.; Grandes, P.; Somohano, P.M. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J. Comp. Neurol. 2000, 422, 464–487. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Catania, M.V.; Geurts, J.; Yankaya, B.; Troost, D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: Upregulation in reactive astrocytes. Neuroscience 2001, 105, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kew, J.N.C.; Kemp, J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology 2005, 179, 4–29. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, S.R.; Pan, H.L. Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience 2009, 158, 875–884. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Li, H.; Ohishi, H.; Kinoshita, A.; Shigemoto, R.; Nomura, S.; Mizuno, N. Localization of a metabotropic glutamate receptor, mGluR7, in axon terminals of presumed nociceptive, primary afferent fibers in the superficial layers of the spinal dorsal horn: An electron microscope study in the rat. Neurosci. Lett. 1997, 223, 153–156. [Google Scholar] [CrossRef]
- Azkue, J.J.; Mateos, J.M.; Elezgarai, I.; Benítez, R.; Osorio, A.; Díez, J.; Bilbao, A.; Bidaurrazaga, A.; Grandes, P. The metabotropic glutamate receptor subtype mGluR 2/3 is located at extrasynaptic loci in rat spinal dorsal horn synapses. Neurosci. Lett. 2000, 287, 236–238. [Google Scholar] [CrossRef]
- Azkue, J.J.; Murga, M.; Fernández-Capetillo, O.; Mateos, J.M.; Elezgarai, I.; Benítez, R.; Osorio, A.; Díez, J.; Puente, N.; Bilbao, A.; et al. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J. Comp. Neurol. 2001, 430, 448–457. [Google Scholar] [CrossRef]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Cui, L.; Kim, Y.R.; Kim, H.Y.; Lee, S.C.; Shin, H.S.; Szabó, G.; Erdélyi, F.; Kim, J.; Kim, S.J. Modulation of synaptic transmission from primary afferents to spinal substantia gelatinosa neurons by group III mGluRs in GAD65-EGFP transgenic mice. J. Neurophysiol. 2011, 105, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Tzingounis, A.V.; Wadiche, J.I. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 2007, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Gegelashvili, G.; Bjerrum, O.J. Glutamate Transport System as a Novel Therapeutic Target in Chronic Pain: Molecular Mechanisms and Pharmacology. Adv. Neurobiol. 2017, 16, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Liaw, W.J.; Zhang, B.; Yaster, M.; Rothstein, J.D.; Johns, R.A.; Tao, Y.X. Evidence of neuronal excitatory amino acid carrier 1 expression in rat dorsal root ganglion neurons and their central terminals. Neuroscience 2004, 123, 1045–1051. [Google Scholar] [CrossRef]
- Tao, Y.X.; Gu, J.; Stephens, R.L., Jr. Role of spinal cord glutamate transporter during normal sensory transmission and pathological pain states. Mol. Pain 2005, 1, 1744–8069. [Google Scholar] [CrossRef]
- Queen, S.A.; Kesslak, J.P.; Bridges, R.J. Regional distribution of sodium-dependent excitatory amino acid transporters in rat spinal cord. J. Spinal Cord. Med. 2007, 30, 263–271. [Google Scholar] [CrossRef][Green Version]
- Carozzi, V.A.; Canta, A.; Oggioni, N.; Ceresa, C.; Marmiroli, P.; Konvalinka, J.; Zoia, C.; Bossi, M.; Ferrarese, C.; Tredici, G.; et al. Expression and distribution of ‘high affinity’ glutamate transporters GLT1, GLAST, EAAC1 and of GCPII in the rat peripheral nervous system. J. Anat. 2008, 213, 539–546. [Google Scholar] [CrossRef]
- Salio, C.; Lossi, L.; Ferrini, F.; Merighi, A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006, 326, 583–598. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Marderm, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017, 18, 389–403. [Google Scholar] [CrossRef]
- Hay, D.L.; Walker, C.S. CGRP and its receptors. Headache 2017, 57, 625–636. [Google Scholar] [CrossRef]
- Schütz, B.; Mauer, D.; Salmon, A.M.; Changeux, J.P.; Zimmer, A.J. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J. Comp. Neurol. 2004, 476, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. CGRP: Sensory neuropeptide with multiple neurologic implications. Neurology 2011, 77, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Kito, S.; Kubota, Y.; Girgis, S.; Hillyard, C.J.; MacIntyre, I. Autoradiographic localization of calcitonin gene-related peptide binding sites in human and rat brains. Brain Res. 1986, 374, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Yashpal, Y.l.; Kar, S.; Dennis, T.; Quirion, R. Quantitative autoradiographic distribution of calcitonin gene-related peptide (hCGRP alpha) binding sites in the rat and monkey spinal cord. J. Comp. Neurol. 1992, 322, 224–232. [Google Scholar] [CrossRef]
- Oliver, K.R.; Kane, S.A.; Salvatore, C.A.; Mallee, J.J.; Kinsey, A.M.; Koblan, K.S.; Keyvan-Fouladi, N.; Heavens, R.P.; Wainwright, A.; Jacobson, M.; et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci. 2001, 14, 618–628. [Google Scholar] [CrossRef]
- Pokabla, M.J.; Dickerson, I.M.; Papka, R.E. Calcitonin gene-related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides 2002, 23, 507–514. [Google Scholar] [CrossRef]
- Ma, W.; Chabot, J.G.; Powell, K.J.; Jhamandas, K.; Dickerson, I.M.; Quirion, R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 2003, 120, 677–694. [Google Scholar] [CrossRef]
- Gu, X.L.; Yu, L.C. The colocalization of CGRP receptor and AMPA receptor in the spinal dorsal horn neuron of rat: A morphological and electrophysiological study. Neurosci. Lett. 2007, 414, 237–241. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Ma, W.; Chabot, J.G.; Quirion, R. A role for adrenomedullin as a pain-related peptide in the rat. Proc. Natl. Acad. Sci. USA 2006, 103, 16027–16032. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Y.; Chabot, J.G.; Fournier, A.; Quirion, R. Upregulation of adrenomedullin in the spinal cord and dorsal root ganglia in the early phase of CFA-induced inflammation in rats. Pain 2009, 146, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef]
- Hay, D.L. Amylin. Headache 2017, 57 (Suppl. S2), 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Chen, S.; Lutz, T.A.; Parkes, D.G.; Roth, J.D. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol. Rev. 2015, 67, 564–600. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Christopoulos, G.; Christopoulos, A.; Poyner, D.R.; Sexton, P.M. Pharmacological discrimination of calcitonin receptor: Receptor activity-modifying protein complexes. Mol. Pharmacol. 2005, 67, 1655–1665. [Google Scholar] [CrossRef]
- Khoshdel, Z.; Takhshid, M.A.; Owji, A.A. Effects of intrathecal amylin on formalin-induced nociception and on cAMP accumulation in the rat embryonic spinal cells. Neuropeptides 2016, 57, 95–100. [Google Scholar] [CrossRef]
- Potes, C.S.; Pestana, A.C.; Pontes, M.; Caramelo, A.S.; Neto, F.L. Amylin modulates the formalin-induced tonic pain behaviours in rats. Eur. J. Pain 2016, 20, 1741–1752. [Google Scholar] [CrossRef]
- Almeida, L.S.; Castro-Lopes, J.M.; Neto, F.L.; Potes, C.S. Amylin, a peptide expressed by nociceptors, modulates chronic neuropathic pain. Eur. J. Pain 2019, 23, 784–799. [Google Scholar] [CrossRef]
- Mantyh, P.W. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry 2002, 63 (Suppl. S11), 6–10. [Google Scholar]
- Kestell, G.R.; Anderson, R.L.; Clarke, J.N.; Haberberger, R.V.; Gibbins, I.L. Primary afferent neurons containing calcitonin gene-related peptide but not substance P in forepaw skin, dorsal root ganglia, and spinal cord of mice. J. Comp. Neurol. 2015, 523, 2555–2569. [Google Scholar] [CrossRef]
- Rogoz, K.; Andersen, H.H.; Kullander, K.; Lagerström, M.C. Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol. Pharmacol. 2014, 85, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Seybold, V.S.; McCarson, K.E.; Mermelstein, P.G.; Groth, R.D.; Abrahams, L.G. Calcitonin gene-related peptide regulates expression of neurokinin1 receptors by rat spinal neurons. J. Neurosci. 2003, 23, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp. Physiol. 2002, 87, 245–249. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Lu, C.R.; Wang, H.; Su, C.J.; Chen, L.W.; Zhang, Y.Q.; Ju, G. Two major distinct subpopulations of neurokinin-3 receptor-expressing neurons in the superficial dorsal horn of the rat spinal cord. Eur. J. Neurosci. 2002, 16, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Kawada, T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr. Drug Targets 2006, 7, 963–974. [Google Scholar] [CrossRef]
- Sun, W.; Yang, F.; Zhang, H.; Yuan, Q.; Ling, S.; Wang, Y.; Lv, P.; Li, Z.; Luo, Y.; Liu, D.; et al. Structural insights into neurokinin 3 receptor activation by endogenous and analogue peptide agonists. Cell Discov. 2023, 9, 66. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, J.; Zheng, X.; Hu, X.; He, Y. Prostaglandin and prostaglandin receptors: Present and future promising therapeutic targets for pulmonary arterial hypertension. Respir. Res. 2023, 24, 263. [Google Scholar] [CrossRef]
- Treutlein, E.M.; Kern, K.; Weigert, A.; Tarighi, N.; Schuh, C.D.; Nüsing, R.M.; Schreiber, Y.; Ferreirós, N.; Brüne, B.; Geisslinger, G.; et al. The prostaglandin E2 receptor EP3 controls CC-chemokine ligand 2-mediated neuropathic pain induced by mechanical nerve damage. J. Biol. Chem. 2018, 293, 9685–9695. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef]
- Kawabata, A. Prostaglandin E2 and pain—An update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef]
- Oida, H.; Namba, T.; Sugimoto, Y.; Ushikubi, F.; Ohishi, H.; Ichikawa, A.; Narumiya, S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br. J. Pharmacol. 1995, 116, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, I.; Minami, T.; Watanabe, Y.; Ito, S.; Hayaishi, O. Prostaglandin E2 stimulates glutamate release from synaptosomes of rat spinal cord. Neurosci. Lett. 1995, 196, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Omote, K.; Kawamata, T.; Namiki, A. Role of prostaglandin receptor subtype EP1 in prostaglandin E2-induced nociceptive transmission in the rat spinal dorsal horn. Brain Res. 2004, 1010, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherer, M.M.; Seybold, V.S. A quantitative study of the coexistence of peptides in varicosities within the superficial laminae of the dorsal horn of the rat spinal cord. J. Neurosci. 1989, 9, 195–205. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Li, Z.W.; Wu, B.; Ye, P.; Tan, Z.Y.; Ji, Y.H. Brain natriuretic peptide suppresses pain induced by BmK I, a sodium channel-specific modulator, in rats. J. Headache Pain 2016, 17, 90. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Polgár, E.; Solinski, H.J.; Mishra, S.K.; Tseng, P.Y.; Iwagaki, N.; Boyle, K.A.; Dickie, A.C.; Kriegbaum, M.C.; Wildner, H.; et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 2018, 21, 707–716. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar] [CrossRef]
- Goswami, S.C.; Thierry-Mieg, D.; Thierry-Mieg, J.; Mishra, S.; Hoon, M.A.; Mannes, A.J.; Iadarola, M.J. Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Mol. Pain 2014, 10, 44. [Google Scholar] [CrossRef]
- Bruno, J.F.; Xu, Y.; Song, J.; Berelowitz, M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 11151–11155. [Google Scholar] [CrossRef]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castano, J.P.; Wester, H.J.; et al. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Holloway, S.; Hathway, G.; Woolf, C.J.; Humphrey, P.P.; Emson, P.C. Identification of somatostatin sst2(a) receptor expressing neurones in central regions involved in nociception. Brain Res. 1998, 798, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Ren, X.; Pan, H.; Slade, H.F.E.; Asmar, A.J.; Xiong, C.M.; Shi, A.; Xiong, A.E.; Wang, L.; Duan, B. Spinal somatostatin-positive interneurons transmit chemical itch. Pain 2019, 160, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Hoon, M.A. The cells and circuitry for itch responses in mice. Science 2013, 340, 968–971. [Google Scholar] [CrossRef]
- van den Akker, F.; Zhang, X.; Miyagi, M.; Huo, X.; Misono, K.S.; Yee, V.C. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 2000, 406, 101–104. [Google Scholar] [CrossRef]
- Pandey, K.N. Molecular Signaling Mechanisms and Function of Natriuretic Peptide Receptor-A in the Pathophysiology of Cardiovascular Homeostasis. Front. Physiol. 2021, 12, 693099. [Google Scholar] [CrossRef]
- Aresh, B.; Freitag, F.B.; Perry, S.M. Spinal cord interneurons expressing the gastrin-releasing peptide receptor convey itch through VGLUT2-mediated signaling. Pain 2017, 158, 945–961. [Google Scholar] [CrossRef]
- Haring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lönnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef]
- Freitag, F.B.; Ahemaiti, A.; Jakobsson, J.E.T.; Weman, H.M.; Lagerström, M.C. Spinal gastrin releasing peptide receptor expressing interneurons are controlled by local phasic and tonic inhibition. Sci. Rep. 2019, 9, 16573. [Google Scholar] [CrossRef]
- Liu, H.X.; Hökfelt, T. The participation of galanin in pain processing at the spinal level. Trends. Pharmacol. Sci. 2002, 23, 468–474. [Google Scholar] [CrossRef]
- Zhang, X.; Nicholas, A.P.; Hökfelt, T. Ultrastructural studies on peptides in the dorsal horn of the spinal cord—I. Co-existence of galanin with other peptides in primary afferents in normal rats. Neuroscience 1993, 57, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Bouali-Benazzouz, R.; André, C.; Shi, T.J.; Léger, C.; Nagy, F.; Hökfelt, T. Galanin receptor 1 is expressed in a subpopulation of glutamatergic interneurons in the dorsal horn of the rat spinal cord. J. Comp. Neurol. 2006, 499, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfeld-Hallin, Z.; Xu, X.J. Neuropeptides in neuropathic and inflammatory pain with special emphasis on cholecystokinin and galanin. Eur. J. Pharmacol. 2001, 429, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lemons, L.L.; Wiley, R.G. Galanin receptor-expressing dorsal horn neurons: Role in nociception. Neuropeptides 2011, 45, 377–383. [Google Scholar] [CrossRef]
- Hulse, R.P.; Donaldson, L.F.; Wynick, D. Differential roles of galanin on mechanical and cooling responses at the primary afferent nociceptor. Mol. Pain 2012, 8, 41. [Google Scholar] [CrossRef]
- Mennicken, F.; Hoffert, C.; Pelletier, M.; Ahmad, S.; O’Donnell, D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J. Chem. Neuroanat. 2002, 24, 257–268. [Google Scholar] [CrossRef]
- Hua, X.Y.; Salgado, K.F.; Gu, G.; Fitzsimmons, B.; Kondo, I.; Bartfai, T.; Yaksh, T.L. Mechanisms of antinociception of spinal galanin: How does galanin inhibit spinal sensitization? Neuropeptides 2005, 39, 211–216. [Google Scholar] [CrossRef]
- Yue, H.Y.; Fujita, T.; Kumamoto, E. Biphasic modulation by galanin of excitatory synaptic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. J. Neurophysiol. 2011, 105, 2337–2349. [Google Scholar] [CrossRef]
- Moran, T.D.; Colmers, W.F.; Smith, P.A. Opioid-like actions of neuropeptide Y in rat substantia gelatinosa: Y1 suppression of inhibition and Y2 suppression of excitation. J. Neurophysiol. 2004, 92, 3266–3275. [Google Scholar] [CrossRef]
- Ma, H.; Gao, T.; Jakobsson, J.E.T.; Weman, H.M.; Xu, B.; Larhammar, D.; Lagerström, M.C. The neuropeptide Y Y2 receptor is coexpressed with Nppb in primary afferent neurons and Y2 activation reduces histaminergic and IL-31-induced itch. J. Pharmacol. Exp. Ther. 2020, 372, 73–82. [Google Scholar] [CrossRef]
- Brumovsky, P.; Shi, T.S.; Landry, M.; Villar, M.J.; Hökfelt, T. Neuropeptide tyrosine and pain. Trends Pharmacol. Sci. 2007, 28, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, P.; Stanic, D.; Shuster, S.; Herzog, H.; Villar, M.; Hökfelt, T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J. Comp. Neurol. 2005, 489, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.S.; Fu, W.; Donahue, R.R.; Corder, G.F.; Hökfelt, T.; Wiley, R.G.; Taylor, B.K. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci. Rep. 2019, 9, 7248. [Google Scholar] [CrossRef] [PubMed]
- Acton, D.; Ren, X.; Di Costanzo, S.; Dalet, A.; Bourane, S.; Bertocchi, I.; Eva, C.; Goulding, M. Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch. Cell Rep. 2019, 28, 625–639. [Google Scholar] [CrossRef]
- Brumovsky, P.; Hofstetter, C.; Olson, L.; Ohning, G.; Villar, M.; Hökfelt, T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience 2006, 138, 1361–1376. [Google Scholar] [CrossRef]
- Jakobsson, J.E.T.; Ma, H.; Lagerström, M.C. Neuropeptide Y in itch regulation. Neuropeptides 2019, 78, 101976. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Khakh, B.S.; North, R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Dawbarn, D. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 2006, 110, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.M. Neurotrophins and pain. In Science of Pain; Basbaum, A.I., Bushnell, M.C., Eds.; Oxford Press: Oxford, UK, 2009; pp. 259–278. [Google Scholar]
- Teng, K.K.; Felice, S.; Kim, T.; Hempstead, B.L. Understanding proneurotrophin actions: Recent advances and challenges. Dev. Neurobiol. 2010, 70, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef]
- López-Pérez, A.E.; Nurgali, K.; Abalo, R. Painful neurotrophins and their role in visceral pain. Behav. Pharmacol. 2018, 29, 120–139. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Balkowiec, A.; Katz, D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002, 22, 10399–10407. [Google Scholar] [CrossRef]
- Garraway, S.M.; Huie, J.R. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016, 2016, 9857201. [Google Scholar] [CrossRef]
- Heppenstall, P.A.; Lewin, G.R. BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proc. Natl. Acad. Sci. USA 2001, 98, 8107–8112. [Google Scholar] [CrossRef]
- Michael, G.J.; Averill, S.; Nitkunan, A.; Rattray, M.; Bennett, D.L.; Yan, Q.; Priestley, J.V. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci. 1997, 17, 8476–8490. [Google Scholar] [CrossRef]
- Thompson, S.W.; Bennett, D.L.; Kerr, B.J.; Bradbury, E.J.; McMahon, S.B. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc. Natl. Acad. Sci. USA 1999, 96, 7714–7718. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Bardoni, R.; Salio, C.; Lossi, L.; Ferrini, F.; Prandini, M.; Zonta, M.; Gustincich, S.; Carmignoto, G. Presynaptic functional trkB receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina II (substantia gelatinosa) of postnatal rat spinal cord. Dev. Neurobiol. 2008, 68, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.K.; Hamilton, G.F.; Horner, P.J. Neural stimulation and molecular mechanisms of plasticity and regeneration: A Review. Front. Cell Neurosci. 2020, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Ramer, M.S. Endogenous neurotrophins and plasticity following spinal deafferentation. Exp. Neurol. 2012, 235, 70–77. [Google Scholar] [CrossRef]
- Luo, X.G.; Rush, R.A.; Zhou, X.F. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci. Res. 2001, 39, 377–384. [Google Scholar] [CrossRef]
- Salio, C.; Averill, S.; Priestley, J.V.; Merighi, A. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev. Neurobiol. 2007, 67, 326–338. [Google Scholar] [CrossRef]
- Salio, C.; Lossi, L.; Ferrini, F.; Merighi, A. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur. J. Neurosci. 2005, 22, 1951–1966. [Google Scholar] [CrossRef]
- Merighi, A.; Carmignoto, G.; Gobbo, S.; Lossi, L.; Salio, C.; Vergnano, A.M.; Zonta, M. Neurotrophins in spinal cord nociceptive pathways. Prog. Brain Res. 2004, 146, 291–321. [Google Scholar] [CrossRef]
- Ding, X.; Cai, J.; Li, S.; Liu, X.D.; Wan, Y.; Xing, G.G. BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol. Dis. 2015, 73, 428–451. [Google Scholar] [CrossRef]
- Hildebrand, M.E.; Xu, J.; Dedek, A.; Li, Y.; Sengar, A.S.; Beggs, S.; Lombroso, P.J.; Salter, M.W. Potentiation of Synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-Mediated Disinhibition in Spinal Pain Processing. Cell Rep. 2016, 17, 2753–2765. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Feng, Z.B.; Jin, Z.R.; Liu, B.H.; Zhao, H.Y.; Jing, H.B.; Wei, T.J.; Yang, G.N.; Liu, L.Y.; et al. BDNF Contributes to Spinal Long-Term Potentiation and Mechanical Hypersensitivity Via Fyn-Mediated Phosphorylation of NMDA Receptor GluN2B Subunit at Tyrosine 1472 in Rats Following Spinal Nerve Ligation. Neurochem Res. 2017, 42, 2712–2729. [Google Scholar] [CrossRef]
- Miletic, G.; Miletic, V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain 2008, 137, 532–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Javdani, F.; Holló, K.; Hegedűs, K.; Kis, G.; Hegyi, Z.; Dócs, K.; Kasugai, Y.; Fukazawa, Y.; Shigemoto, R.; Antal, M. Differential expression patterns of K(+)/Cl(−) cotransporter 2 in neurons within the superficial spinal dorsal horn of rats. J. Comp. Neurol. 2015, 523, 1967–1983. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sík, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Guo, D.; Campanelli, D.; Frattini, F.; Mayer, F.; Zhou, L.; Kuner, R.; Heppenstall, P.A.; Knipper, M.; Hu, J. Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction. Nat. Commun. 2014, 5, 5331. [Google Scholar] [CrossRef]
- Chen, S.R.; Zhu, L.; Chen, H.; Wen, L.; Laumet, G.; Pan, H.L. Increased spinal cord Na+-K+-2Cl− cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J. Biol. Chem. 2014, 289, 31111–31120. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Jiang, B.C.; Gao, Y.J. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell. Mol. Life Sci. 2017, 74, 3275–3291. [Google Scholar] [CrossRef]
- Boakye, P.A.; Rancic, V.; Whitlock, K.H.; Simmons, D.; Longo, F.M.; Ballanyi, K.; Smith, P.A. Receptor dependence of BDNF actions in superficial dorsal horn: Relation to central sensitization and actions of macrophage colony stimulating factor 1. J. Neurophysiol. 2019, 121, 2308–2322. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef]
- Dedek, A.; Xu, J.; Lorenzo, L.É.; Godin, A.G.; Kandegedara, C.M.; Glavina, G.; Landrigan, J.A.; Lombroso, P.J.; De Koninck, Y.; Tsai, E.C.; et al. Sexual dimorphism in a neuronal mechanism of spinal hyperexcitability across rodent and human models of pathological pain. Brain 2022, 145, 1124–1138. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Scheyer, A.; Yasmin, F.; Naskar, S.; Patel, S. Endocannabinoids at the synapse and beyond: Implications for neuropsychiatric disease pathophysiology and treatment. Neuropsychopharmacology 2023, 48, 37–53. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 2010, 68, 113–126. [Google Scholar] [CrossRef]
- Hegyi, Z.; Kis, G.; Holló, K.; Ledent, C.; Antal, M. Neuronal and glial localization of the cannabinoid-1 receptor in the superficial spinal dorsal horn of the rodent spinal cord. Eur. J. Neurosci. 2009, 30, 251–262. [Google Scholar] [CrossRef]
- Nyilas, R.; Gregg, L.C.; Mackie, K.; Watanabe, M.; Zimmer, A.; Hohmann, A.G.; Katona, I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur. J. Neurosci. 2009, 29, 1964–1978. [Google Scholar] [CrossRef]
- Hegyi, Z.; Holló, K.; Kis, G.; Mackie, K.; Antal, M. Differential distribution of diacylglycerol lipase-alpha and N-acylphosphatidylethanolamine-specific phospholipase d immunoreactivity in the superficial spinal dorsal horn of rats. Glia 2012, 60, 1316–1329. [Google Scholar] [CrossRef]
- Araque, A.; Castillo, P.E.; Manzoni, O.J.; Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 2017, 124, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dócs, K.; Hegyi, Z.; Holló, K.; Kis, G.; Hegedűs, K.; Antal, M. Selective axonal and glial distribution of monoacylglycerol lipase immunoreactivity in the superficial spinal dorsal horn of rodents. Brain Struct. Funct. 2015, 220, 2625–2637. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A. The histology, physiology, neurochemistry and circuitry of the substantia gelatinosa Rolandi (lamina II) in mammalian spinal cord. Prog. Neurobiol. 2018, 69, 91–134. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.C.; Pechincha, C.; Luz, L.L.; Kokai, E.; Szucs, P.; Safronov, B.V. Primary afferent-driven presynaptic inhibition of C-fiber inputs to spinal lamina I neurons. Prog. Neurobiol. 2020, 188, 101786. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Fritschy, J.M. Gephyrin: A master regulator of neuronal function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef]
- Choii, G.; Ko, J. Gephyrin: A central GABAergic synapse organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef]
- Lorenzo, L.E.; Godin, A.G.; Wang, F.; St-Louis, M.; Carbonetto, S.; Wiseman, P.W.; Ribeiro-da-Silva, A.; De Koninck, Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABA(A) receptors. J. Neurosci. 2014, 34, 8300–8317. [Google Scholar] [CrossRef]
- Comitato, A.; Bardoni, R. Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits. Int. J. Mol. Sci. 2021, 22, 414. [Google Scholar] [CrossRef]
- Bardoni, R.; Takazawa, T.; Tong, C.K.; Choudhury, P.; Scherrer, G.; Macdermott, A.B. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci. 2013, 1279, 90–96. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Witschi, R.; Punnakkal, P.; Paul, J.; Walczak, J.S.; Cervero, F.; Fritschy, J.M.; Kuner, R.; Keist, R.; Rudolph, U.; Zeilhofer, H.U. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J. Neurosci. 2011, 31, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Zeilhofer, H.U.; Fritschy, J.M. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol. 2012, 520, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hu, J. Spinal presynaptic inhibition in pain control. Neuroscience 2014, 283, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Javdani, F.; Hegedűs, K.; Miranda, C.O.; Hegyi, Z.; Holló, K.; Antal, M. Differential expression of Na+/K+/Cl− cotransporter 1 in neurons and glial cells within the superficial spinal dorsal horn of rodents. Sci. Rep. 2020, 10, 11715. [Google Scholar] [CrossRef]
- Rocha-Gonzalez, H.I.; Mao, S.; Alvarez-Leefmans, F.J. Na+,K+,2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: Thermodynamic and kinetic aspects. J. Neurophysiol. 2008, 100, 169–184. [Google Scholar] [CrossRef]
- Takkala, P.; Zhu, Y.; Prescott, S.A. Combined Changes in Chloride Regulation and Neuronal Excitability Enable Primary Afferent Depolarization to Elicit Spiking without Compromising its Inhibitory Effects. PLoS Comput. Biol. 2016, 12, e1005215. [Google Scholar] [CrossRef]
- Funk, K.; Woitecki, A.; Franjic-Würtz, C.; Gensch, T.; Möhrlen, F.; Frings, S. Modulation of chloride homeostasis by inflammatory mediators in dorsal root ganglion neurons. Mol. Pain 2008, 4, 32. [Google Scholar] [CrossRef]
- Modol, L.; Cobianchi, S.; Navarro, X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain 2014, 155, 1577–1590. [Google Scholar] [CrossRef]
- Galan, A.; Cervero, F. Painful stimuli induce in vivo phosphorylation and membrane mobilization of mouse spina l cord NKCC1 co-transporter. Neuroscience 2005, 133, 245–252. [Google Scholar] [CrossRef]
- Price, T.J.; Cervero, F.; Gold, M.S.; Hammond, D.L.; Prescott, S.A. Chloride regulation in the pain pathway. Brain Res. Rev. 2009, 60, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Cervero, F. Role of the NKCC1 co-transporter in sensitization of spinal nociceptive neurons. Pain 2010, 151, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Goudet, C.; Magnaghi, V.; Landry, M.; Nagy, F.; Gereau, R.W., 4th; Pin, J.P. Metabotropic receptors for glutamate and GABA in pain. Brain Res. Rev. 2009, 60, 43–56. [Google Scholar] [CrossRef]
- Towers, S.; Princivalle, A.; Billinton, A.; Edmunds, M.; Bettler, B.; Urban, L.; Castro-Lopes, J.; Bowery, N.G. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur. J. Neurosci. 2000, 12, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, S.; Peers, C.; Du, X.; Zhang, H.; Gamper, N. GABAB receptors inhibit low-voltage activated and high-voltage activated Ca2+ channels in sensory neurons via distinct mechanisms. Biochem. Biophys. Res. Commun. 2015, 465, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M. GABAB receptors and pain. Neuropharmacology 2018, 136, 102–105. [Google Scholar] [CrossRef]
- Miranda, C.O.; Hegedüs, K.; Wildner, H.; Zeilhofer, H.U.; Antal, M. Morphological and neurochemical characterization of glycinergic neurons in laminae I-IV of the mouse spinal dorsal horn. J. Comp. Neurol. 2022, 530, 607–626. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, H.; Gao, Y.; Gong, Y.; Ren, Y.; Gu, N.; Zhou, S.; Xia, N.; Sun, Y.Y.; Ji, R.R.; et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Investig. 2013, 123, 4050–4062. [Google Scholar] [CrossRef]
- Aubrey, K.R.; Supplisson, S. Heterogeneous Signaling at GABA and Glycine Co-releasing Terminals. Front. Synaptic Neurosci. 2018, 10, 40. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Acuna, M.A.; Gingras, J.; Yévenes, G.E. Glycine receptors and glycine transporters: Targets for novel analgesics? Cell. Mol. Life Sci. 2018, 75, 447–465. [Google Scholar] [CrossRef]
- Miranda, C.O.; Hegedüs, K.; Kis, G.; Antal, M. Synaptic Targets of Glycinergic Neurons in Laminae I-III of the Spinal Dorsal Horn. Int. J. Mol. Sci. 2023, 24, 6943. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Mah, W.; Rah, J.C.; Park, S.K.; Bae, Y.C. Expression of glycine receptor alpha 3 in the rat trigeminal neurons and central boutons in the brainstem. Brain Struct. Funct. 2016, 221, 4601–4613. [Google Scholar] [CrossRef] [PubMed]
- Marvizon, J.C.; Chen, W.; Murphy, N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: An immunofluorescence colocalization study. J. Comp. Neurol. 2009, 517, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.W. Opioids and their receptors: Are we there yet? Neuropharmacology 2014, 76, 198–203. [Google Scholar] [CrossRef]
- Gu, Z.H.; Wang, B.; Kou, Z.Z.; Bai, Y.; Chen, T.; Dong, Y.L.; Li, H.; Li, Y.Q. Endomorphins: Promising Endogenous Opioid Peptides for the Development of Novel Analgesics. Neurosignals 2017, 25, 98–116. [Google Scholar] [CrossRef]
- Martin-Schild, S.; Gerall, A.A.; Kastin, A.J.; Zadina, J.E. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol. 1999, 405, 450–471. [Google Scholar] [CrossRef]
- Boyle, K.A.; Gutierrez-Mecinas, M.; Polgár, E.; Mooney, N.; O’Connor, E.; Furuta, T.; Watanabe, M.; Todd, A.J. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017, 363, 120–133. [Google Scholar] [CrossRef]
- François, A.; Low, S.A.; Sypek, E.I.; Christensen, A.J.; Sotoudeh, C.; Beier, K.T.; Ramakrishnan, C.; Ritola, K.D.; Sharif-Naeini, R.; Deisseroth, K.; et al. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron 2017, 93, 822–839. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.Y.; Ma, J.B.; Li, J.N.; Teng, X.Y.; Chen, Y.B.; Yin, J.B.; Huang, J.; Chen, J.; Zhang, T.; et al. Enkephalinergic Circuit Involved in Nociceptive Modulation in the Spinal Dorsal Horn. Neuroscience 2020, 429, 78–91. [Google Scholar] [CrossRef]
- Brewer, C.L.; Styczynski, L.M.; Serafin, E.K.; Baccei, M.L. Postnatal maturation of spinal dynorphin circuits and their role in somatosensation. Pain 2020, 161, 1906–1924. [Google Scholar] [CrossRef]
- Serafin, E.K.; Burns, R.; Yoo, J.; Baccei, M.L. Gucy2d selectively marks inhibitory dynorphin neurons in the spinal dorsal horn but is dispensable for pain and itch sensitivity. Pain Rep. 2021, 6, e947. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, N.; Garzillo, F.; Polgár, E.; Riddell, J.S.; Todd, A.J. Neurochemical characterization of lamina II inhibitory interneurons that express GFP in the PrP-GFP mouse. Mol. Pain 2013, 9, 56. [Google Scholar] [CrossRef]
- Wu, H.Y.; Mao, X.F.; Tang, X.Q.; Ali, U.; Apryani, E.; Liu, H.; Li, X.Y.; Wang, Y.X. Spinal interleukin-10 produces antinociception in neuropathy through microglial beta-endorphin expression, separated from antineuroinflammation. Brain Behav. Immun. 2018, 73, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Tang, X.Q.; Mao, X.F.; Wang, Y.X. Autocrine Interleukin-10 Mediates Glucagon-Like Peptide-1 Receptor-Induced Spinal Microglial beta-Endorphin Expression. J. Neurosci. 2017, 37, 11701–11714. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Apryani, E.; Wu, H.Y.; Mao, X.F.; Liu, H.; Wang, Y.X. Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial IL-10/beta-endorphin pathway. Biomed. Pharmacother. 2020, 125, 109898. [Google Scholar] [CrossRef]
- Apryani, E.; Ali, U.; Wang, Z.Y.; Wu, H.Y.; Mao, X.F.; Ahmad, K.A.; Li, X.Y.; Wang, Y.X. The spinal microglial IL-10/beta-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of alpha7-nicotinic acetylcholine receptors. J. Neuroinflamm. 2020, 17, 75. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yin, J.B.; Bai, Y.; Li, X.; Zhang, T.; Yang, J.; Yi, X.N.; Zhang, M.M.; Li, Y.Q. Morphological features of endomorphin-2-immunoreactive ultrastructures in the dorsal root ganglion and spinal dorsal horn of the rat. J. Chem. Neuroanat. 2022, 125, 102142. [Google Scholar] [CrossRef]
- Hui, R.; Wang, W.; Chen, T.; Lü, B.C.; Li, H.; Zhang, T.; Wu, S.X.; Li, Y.Q. Origins of endomorphin-2 immunopositive fibers and terminals in the spinal dorsal horn of the rat. Neuroscience 2010, 169, 422–430. [Google Scholar] [CrossRef]
- Yin, J.B.; Lu, Y.C.; Li, F.; Zhang, T.; Ding, T.; Hu, H.Q.; Chen, Y.B.; Guo, H.W.; Kou, Z.Z.; Zhang, M.M.; et al. Morphological investigations of endomorphin-2 and spinoparabrachial projection neurons in the spinal dorsal horn of the rat. Front. Neuroanat. 2022, 16, 1072704. [Google Scholar] [CrossRef]
- Law, P.Y.; Reggio, P.H.; Loh, H.H. Opioid receptors: Toward separation of analgesic from undesirable effects. Trends Biochem. Sci. 2013, 38, 275–282. [Google Scholar] [CrossRef]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Janecka, A.; Fichna, J.; Janecki, T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 2004, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, C.; Stein, C. Opioids. Handb. Exp. Pharmacol. 2007, 177, 31–63. [Google Scholar] [CrossRef]
- Yin, J.B.; Lu, Y.C.; Feng, B.; Wu, Z.Y.; Chen, Y.B.; Zhang, T.; Kou, Z.Z.; Zhang, M.M.; Zhang, H.; Li, J.L.; et al. Endomorphin-2 inhibits the activity of the spinoparabrachial projection neuron through presynaptic mechanisms in the spinal dorsal horn in rats. Neurosignals 2018, 26, 43–57. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhao, B.; Zhong, Y.Q.; Li, K.C.; Li, Z.Y.; Wang, Q.; Lu, Y.J.; Zhang, Z.N.; He, S.Q.; Zheng, H.C.; et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 13117–13122. [Google Scholar] [CrossRef]
- Besse, D.; Lombard, M.C.; Zajac, J.M.; Roques, B.P.; Besson, J.M. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990, 521, 15–22. [Google Scholar] [CrossRef]
- Scherrer, G.; Imamachi, N.; Cao, Y.Q.; Contet, C.; Mennicken, F.; O’Donnell, D.; Kieffer, B.L.; Basbaum, A.I. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009, 137, 1148–1159. [Google Scholar] [CrossRef]
- François, A.; Scherrer, G. Delta Opioid Receptor Expression and Function in Primary Afferent Somatosensory Neurons. Handb. Exp. Pharmacol. 2018, 247, 87–114. [Google Scholar] [CrossRef]
- Wang, D.; Tawfik, V.L.; Corder, G.; Low, S.A.; François, A.; Basbaum, A.I.; Scherrer, G. Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 2018, 98, 90–108.e5. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.R.; Chen, H.; Pan, H.L. mu-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia. J. Physiol. 2019, 597, 1661–1675. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Saez, C.; Mortrud, M.; Bouvier, C.; Williams, J.T.; Low, M.; Grandy, D.K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994, 347, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.C.; Mollereau, C.; Toll, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Butour, J.L.; Guillemot, J.C.; Ferrara, P.; Monsarrat, B.; et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995, 377, 532–535. [Google Scholar] [CrossRef]
- Schröder, W.; Lambert, D.G.; Ko, M.C.; Koch, T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br. J. Pharmacol. 2014, 171, 3777–3800. [Google Scholar] [CrossRef] [PubMed]
- Toll, L.; Bruchas, M.R.; Calo, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef]
- Winters, B.L.; Christie, M.J.; Vaughan, C.W. Electrophysiological Actions of N/OFQ. Handb. Exp. Pharmacol. 2019, 254, 91–130. [Google Scholar] [CrossRef]
- Ding, H.; Kiguchi, N.; Dobbins, M.; Romero-Sandoval, E.A.; Kishioka, S.; Ko, M.C. Nociceptin Receptor-Related Agonists as Safe and Non-addictive Analgesics. Drugs 2023, 83, 771–793. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the entire human opioid receptor family. Cell 2023, 186, 413–427.e17. [Google Scholar] [CrossRef]
- Cox, B.M.; Christie, M.J.; Devi, L.; Toll, L.; Traynor, J.R. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br. J. Pharmacol. 2015, 172, 317–323. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Ko, M.C. Central N/OFQ-NOP Receptor System in Pain Modulation. Adv. Pharmacol. 2016, 75, 217–243. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Ko, M.C. Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J. Neurosci. Res. 2022, 100, 191–202. [Google Scholar] [CrossRef]
- Neal, C.R., Jr.; Mansour, A.; Reinscheid, R.; Nothacker, H.P.; Civelli, O.; Watson, S.J., Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999, 406, 503–547. [Google Scholar] [CrossRef]
- Ozawa, A.; Brunori, G.; Cippitelli, A.; Toll, N.; Schoch, J.; Kieffer, B.L.; Toll, L. Analysis of the distribution of spinal NOP receptors in a chronic pain model using NOP-eGFP knock-in mice. Br. J. Pharmacol. 2018, 175, 2662–2675. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, A.; Brunori, G.; Mercatelli, D.; Wu, J.; Cippitelli, A.; Zou, B.; Xie, X.S.; Williams, M.; Zaveri, N.T.; Low, S.; et al. Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J. Neurosci. 2015, 35, 11682–11693. [Google Scholar] [CrossRef] [PubMed]
- Toll, L.; Ozawa, A.; Cippitelli, A. NOP-Related Mechanisms in Pain and Analgesia. Handb. Exp. Pharmacol. 2019, 254, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Moy, J.K.; Hartung, J.E.; Duque, M.G.; Friedman, R.; Nagarajan, V.; Loeza-Alcocer, E.; Koerber, H.R.; Christoph, T.; Schröder, W.; Gold, M.S. Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain 2020, 161, 1636–1649. [Google Scholar] [CrossRef]
- Houtani, T.; Nishi, M.; Takeshima, H.; Sato, K.; Sakuma, S.; Kakimoto, S.; Ueyama, T.; Noda, T.; Sugimoto, T. Distribution of nociceptin/orphanin FQ precursor protein and receptor in brain and spinal cord: A study using in situ hybridization and X-gal histochemistry in receptor-deficient mice. J. Comp. Neurol. 2000, 424, 489–508. [Google Scholar] [CrossRef]
- Pettersson, L.M.; Sundler, F.; Danielsen, N. Expression of orphanin FQ/nociceptin and its receptor in rat peripheral ganglia and spinal cord. Brain Res. 2002, 945, 266–275. [Google Scholar] [CrossRef]
- Le Cudennec, C.; Suaudeau, C.; Costentin, J. Evidence for a localization of [3H]nociceptin binding sites on medullar primary afferent fibers. J. Neurosci. Res. 2002, 68, 496–500. [Google Scholar] [CrossRef]
- Murali, S.S.; Napier, I.A.; Rycroft, B.K.; Christie, M.J. Opioid-related (ORL1) receptors are enriched in a subpopulation of sensory neurons and prolonged activation produces no functional loss of surface N-type calcium channels. J. Physiol. 2012, 590, 1655–1667. [Google Scholar] [CrossRef]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Yamamoto, A.; Yoshinori Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Ozono, Y.; Mikuriya, S.; Kohro, Y.; Tozaki-Saitoh, H.; Iwatsuki, K.; Uneyama, H.; Ichikawa, R.; Salter, M.W.; Tsuda, M.; et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 2016, 7, 12529. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef]
- Mori, M.; Heuss, C.; Gähwiler, B.H.; Gerber, U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J. Physiol. 2001, 535, 115–123. [Google Scholar] [CrossRef]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Vesicular release of ATP at central synapses. Pflugers Arch. 2006, 452, 589–597. [Google Scholar] [CrossRef]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Quantal release of ATP in mouse cortex. J. Gen. Physiol. 2007, 129, 257–265. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic mechanisms and pain--an update. Eur. J. Pharmacol. 2013, 716, 24–40. [Google Scholar] [CrossRef]
- Gerevich, Z.; Illes, P. P2Y receptors and pain transmission. Purinergic Signal. 2004, 1, 3–10. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef]
- Nishida, K.; Nomura, Y.; Kawamori, K.; Moriyama, Y.; Nagasawa, K. Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci. Lett. 2014, 579, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hiasa, M.; Ichikawa, R.; Hasuzawa, N.; Kadowaki, A.; Iwatsuki, K.; Shima, K.; Endo, Y.; Kitahara, Y.; Inoue, T.; et al. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc. Natl. Acad. Sci. USA 2017, 114, E6297–E6305. [Google Scholar] [CrossRef] [PubMed]
- Oya, M.; Kitaguchi, T.; Yanagihara, Y.; Numano, R.; Kakeyama, M.; Ikematsu, K.; Tsuboi, T. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem. Biophys. Res. Commun. 2013, 438, 145–151. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. The Role of ATP Receptors in Pain Signaling. Neurochem. Res. 2022, 47, 2454–2468. [Google Scholar] [CrossRef]
- Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Pain and purinergic signaling. Brain Res. Rev. 2010, 63, 222–232. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Mechanisms and Pain. Adv. Pharmacol. 2016, 75, 91–137. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnstock, G.; McMahon, S.B. The expression of P2X3 purinoreceptors in sensory neurons: Effects of axotomy and glial-derived neurotrophic factor. Mol. Cell. Neurosci. 1998, 12, 256–268. [Google Scholar] [CrossRef]
- Vulchanova, L.; Riedl, M.S.; Shuster, S.J.; Stone, L.S.; Hargreaves, K.M.; Buell, G.; Surprenant, A.; North, R.A.; Elde, R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur. J. Neurosci. 1998, 10, 3470–3478. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Gu, J.G. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J. Neurosci. 2001, 21, 6522–6531. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.Z.; Birder, L.A.; de Groat, W.C.; Tai, C.; Roppolo, J.; Buffington, C.A.; Burnstock, G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J. Histochem. Cytochem. 2005, 53, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Artelt, M.; Burnet, M.; Trautmann, K.; Schluesener, H.J. Lesional accumulation of P2X4 receptor+ monocytes following experimental traumatic brain injury. Exp. Neurol. 2006, 197, 252–257. [Google Scholar] [CrossRef]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Purinergic system, microglia and neuropathic pain. Curr. Opin. Pharmacol. 2012, 12, 74–79. [Google Scholar] [CrossRef]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1529. [Google Scholar] [CrossRef]
- Tozaki-Saitoh, H.; Tsuda, M.; Miyata, H.; Ueda, K.; Kohsaka, S.; Inoue, K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 2008, 28, 4949–4956. [Google Scholar] [CrossRef]
- Trang, T.; Salter, M.W. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal. 2012, 8, 621–628. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yamanaka, H.; Fukuoka, T.; Dai, Y.; Obata, K.; Noguchi, K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J. Neurosci. 2008, 28, 2892–2902. [Google Scholar] [CrossRef]
- Jarvis, M.F. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010, 33, 48–57. [Google Scholar] [CrossRef]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Staniland, A.A.; Marchand, F.; Kaan, T.K.; McMahon, S.B.; Malcangio, M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 2010, 30, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Stansberg, C.; Cunningham, C. The interleukin 1 receptor family. Dev. Comp. Immunol. 2004, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Eggen, B.J.; Raj, D.; Hanisch, U.K.; Boddeke, H.W. Microglial phenotype and adaptation. J. Neuroimmune Pharmacol. 2013, 8, 807–823. [Google Scholar] [CrossRef]
- Holló, K.; Ducza, L.; Hegyi, Z.; Dócs, K.; Hegedűs, K.; Bakk, E.; Papp, I.; Kis, G.; Mészár, Z.; Bardóczi, Z.; et al. Interleukin-1 receptor type 1 is overexpressed in neurons but not in glial cells within the rat superficial spinal dorsal horn in complete Freund adjuvant-induced inflammatory pain. J. Neuroinflamm. 2017, 14, 125. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Watanabe, M.; Shimizu, K.; Zou, S.; LaGraize, S.C.; Wei, F.; Dubner, R.; Ren, K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007, 27, 6006–6018. [Google Scholar] [CrossRef]
- Almanza, A.; Simón-Arceo, K.; Coffeen, U.; Fuentes-García, R.; Contreras, B.; Pellicer, F.; Mercado, F. A D2-like receptor family agonist produces analgesia in mechanonociception but not in thermonociception at the spinal cord level in rats. Pharmacol. Biochem. Behav. 2015, 137, 119–125. [Google Scholar] [CrossRef]
- Chakraborty, S.; Elvezio, V.; Kaczocha, M.; Rebecchi, M.; Puopolo, M. Presynaptic inhibition of transient receptor potential vanilloid type 1 (TRPV1) receptors by noradrenaline in nociceptive neurons. J. Physiol. 2017, 595, 2639–2660. [Google Scholar] [CrossRef]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef]
- Ridet, J.L.; Rajaofetra, N.; Teilhac, J.R.; Geffard, M.; Privat, A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience 1993, 52, 143–157. [Google Scholar] [CrossRef]
- Puopolo, M. The hypothalamic-spinal dopaminergic system: A target for pain modulation. Neural Regen. Res. 2019, 14, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Bahar, I. Monoamine transporters: Structure, intrinsic dynamics and allosteric regulation. Nat. Struct. Mol. Biol. 2019, 26, 545–556. [Google Scholar] [CrossRef]
- Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef]
- Hayashida, K.I.; Obata, H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int. J. Mol. Sci. 2019, 20, 822. [Google Scholar] [CrossRef]
- Howorth, P.W.; Teschemacher, A.G.; Pickering, A.E. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J. Comp. Neurol. 2009, 512, 141–157. [Google Scholar] [CrossRef]
- Hickey, L.; Li, Y.; Fyson, S.J.; Watson, T.C.; Perrins, R.; Hewinson, J.; Teschemacher, A.G.; Furue, H.; Lumb, B.M.; Pickering, A.E. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J. Neurosci. 2014, 34, 4148–4160. [Google Scholar] [CrossRef]
- Stone, L.S.; Broberger, C.; Vulchanova, L.; Wilcox, G.L.; Hökfelt, T.; Riedl, M.S.; Elde, R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J. Neurosci. 1998, 18, 5928–5937. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Koblinger, K.; Füzesi, T.; Ejdrygiewicz, J.; Krajacic, A.; Bains, J.S.; Whelan, P.J. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS ONE 2014, 9, e109636. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Yamaguchi, T.; Hamaguchi, S.; Yamaguchi, S.; Ueda, S. Three Types of A11 Neurons Project to the Rat Spinal Cord. Neurochem. Res. 2017, 42, 2142–2153. [Google Scholar] [CrossRef]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Cobacho, N.; de la Calle, J.L.; Paíno, C.L. Dopaminergic modulation of neuropathic pain: Analgesia in rats by a D2-type receptor agonist. Brain Res. Bull. 2014, 106, 62–71. [Google Scholar] [CrossRef]
- Gautier, A.; Geny, D.; Bourgoin, S.; Bernard, J.F.; Hamon, M. Differential innervation of superficial versus deep laminae of the dorsal horn by bulbo-spinal serotonergic pathways in the rat. IBRO Rep. 2017, 2, 72–80. [Google Scholar] [CrossRef]
- Bardoni, R. Serotonergic Modulation of Nociceptive Circuits in Spinal Cord Dorsal Horn. Curr. Neuropharmacol. 2019, 17, 1133–1145. [Google Scholar] [CrossRef]
- Fauss, G.N.K.; Hudson, K.E.; Grau, J.W. Role of descending serotonergic fibers in the development of pathophysiology after spinal cord injury (SCI): Contribution to chronic pain, spasticity, and autonomic dysreflexia. Biology 2022, 11, 234. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Wang, W.; Hou, Y.Y.; Pan, Z.Z. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol. Pain 2014, 10, 70. [Google Scholar] [CrossRef]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108155. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Shi, W.; Liu, W.; Chen, Q.Y.; Zhuo, M. Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Front. Synaptic Neurosci. 2023, 15, 1122381. [Google Scholar] [CrossRef] [PubMed]
- Descarries, L.; Cornea-Herbert, V.; Riad, M. Cellular and subcellular localization of serotonin receptors in the central nervous system. In The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics; Roth, B.L., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 277–317. [Google Scholar]
- Matthys, A.; Haegeman, G.; Van Craenenbroeck, K.; Vanhoenacker, P. Role of the 5-HT7 receptor in the central nervous system: From current status to future perspectives. Mol. Neurobiol. 2011, 43, 228–253. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Heijmans, L.; Mons, M.R.; Joosten, E.A. A systematic review on descending serotonergic projections and modulation of spinal nociception in chronic neuropathic pain and after spinal cord stimulation. Mol. Pain 2021, 17, 17448069211043965. [Google Scholar] [CrossRef]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.X. Spinal microglial beta-endorphin signaling mediates IL-10 and exenatide-induced inhibition of synaptic plasticity in neuropathic pain. CNS Neurosci. Ther. 2021, 27, 1157–1172. [Google Scholar] [CrossRef]
- Hou, M.; Kanje, M.; Longmore, J.; Tajti, J.; Uddman, R.; Edvinsson, L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: Co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001, 909, 112–120. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Noghero, A.; Thibault, K.; Brisorgueil, M.J.; Fischer, J.; Conrath, M. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience 2009, 161, 838–846. [Google Scholar] [CrossRef]
- Doly, S.; Madeira, A.; Fischer, J.; Brisorgueil, M.J.; Daval, G.; Bernard, R.; Vergé, D.; Conrath, M.J. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J. Comp. Neurol. 2004, 472, 496–511. [Google Scholar] [CrossRef]
- Bardoni, R. Serotonergic 5-HT7 Receptors as Modulators of the Nociceptive System. Curr. Neuropharmacol. 2023, 21, 1548–1557. [Google Scholar] [CrossRef]
- Maxwell, D.J.; Kerr, R.; Rashid, S.; Anderson, E. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Exp. Brain Res. 2003, 149, 114–124. [Google Scholar] [CrossRef]
- García-Ramírez, D.L.; Calvo, J.R.; Hochman, S.; Quevedo, J.N. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS ONE 2014, 9, e89999. [Google Scholar] [CrossRef]

| Receptor | Ligand | Localization of the Receptor | Coupled Intracellular Protein | Effect of the Activation of the Receptor |
|---|---|---|---|---|
| mGluR4 mGluR7 | Glutamate | Presynaptic membrane | Gi/o | Attenuation of transmitter release |
| mGluR2 mGluR3 | Glutamate | Extrasynaptic | Gi/o | Attenuation of transmitter release |
| mGluR5 | Glutamate | Perisynaptic | Gq/11 DAG-lipase | Activation of retrograde endocannabinoid signaling |
| CB1 | 2–AG | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Gi/o | Attenuation of transmitter release |
| CLR–RAMP1 CTR-RAMP1 | CGRP | Extrasynaptic on peptidergic terminals | Gs | Enhancement of transmitter release |
| EP1, EP3, EP4 (?) | PGE2 | Extrasynaptic | Gq/11, Gs, Gi (?) | Enhancement of transmitter release |
| GALR2 | GAL | Extrasynaptic on peptidergic terminals | Gi/o, Gq/11 | Enhancement of transmitter release (?) |
| Y2 | NPY | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Gi/o | Attenuation of transmitter release |
| TrkB | BDNF | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Tyrosine residues | Enhancement of transmitter release |
| GABAA | GABA | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Ionotropic | May generate hyperpolarization or PAD depending on the expression of NKCC1 |
| GABAB | GABA | Extrasynaptic on both peptidergic and nonpeptidergic terminals? | Gi/o | Attenuation of transmitter release |
| GlyR | Glycine | Extrasynaptic on nonpeptidergic terminals | Ionotropic | Attenuation of transmitter release |
| MOR | Endomorphin, enkephalin, β-endorphin | Extrasynaptic on peptidergic terminals | Gi/o | Attenuation of transmitter release |
| DOR | Enkephalin, β-endorphin | Extrasynaptic on nonpeptidergic terminals | Gi/o | Attenuation of transmitter release |
| KOR | Dynorphin | Extrasynaptic | Gi/o | Attenuation of transmitter release |
| NOP | N/OFQ peptide | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Gi/o | Attenuation of transmitter release |
| P2X3, P2X2/3 | ATP | Extrasynaptic on nonpeptidergic terminals | Ionotropic | Enhancement of transmitter release |
| α2A | Noradrenaline | Extrasynaptic on peptidergic terminals | Gi/o | Attenuation of transmitter release |
| D2, D3, D4 | Dopamine | Extrasynaptic | Gi/o | Attenuation of transmitter release |
| 5-HT1A 5-HT1B | Serotonin | Extrasynaptic on peptidergic terminals | Gi/o | Attenuation of transmitter release |
| 5–HT2A | Serotonin | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Gq/11 | Enhancement of transmitter release (?) |
| 5–HT3 | Serotonin | Extrasynaptic on both peptidergic and nonpeptidergic terminals | Ionotropic | Enhancement of transmitter release (?) |
| 5–HT7 | Serotonin | Extrasynaptic | Gs | Enhancement of transmitter release (?) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antal, M. Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons. Int. J. Mol. Sci. 2025, 26, 2356. https://doi.org/10.3390/ijms26052356
Antal M. Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons. International Journal of Molecular Sciences. 2025; 26(5):2356. https://doi.org/10.3390/ijms26052356
Chicago/Turabian StyleAntal, Miklós. 2025. "Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons" International Journal of Molecular Sciences 26, no. 5: 2356. https://doi.org/10.3390/ijms26052356
APA StyleAntal, M. (2025). Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons. International Journal of Molecular Sciences, 26(5), 2356. https://doi.org/10.3390/ijms26052356





