Dihydromyricetin Alleviated Acetaminophen-Induced Acute Kidney Injury via Nrf2-Dependent Anti-Oxidative and Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Results
2.1. DHM Pretreatment Alleviated APAP-Induced AKI in Mice
2.2. DHM Pretreatment Alleviated APAP-Induced Oxidative Stress in Renal Tissue Through Nrf2 Signaling Pathway
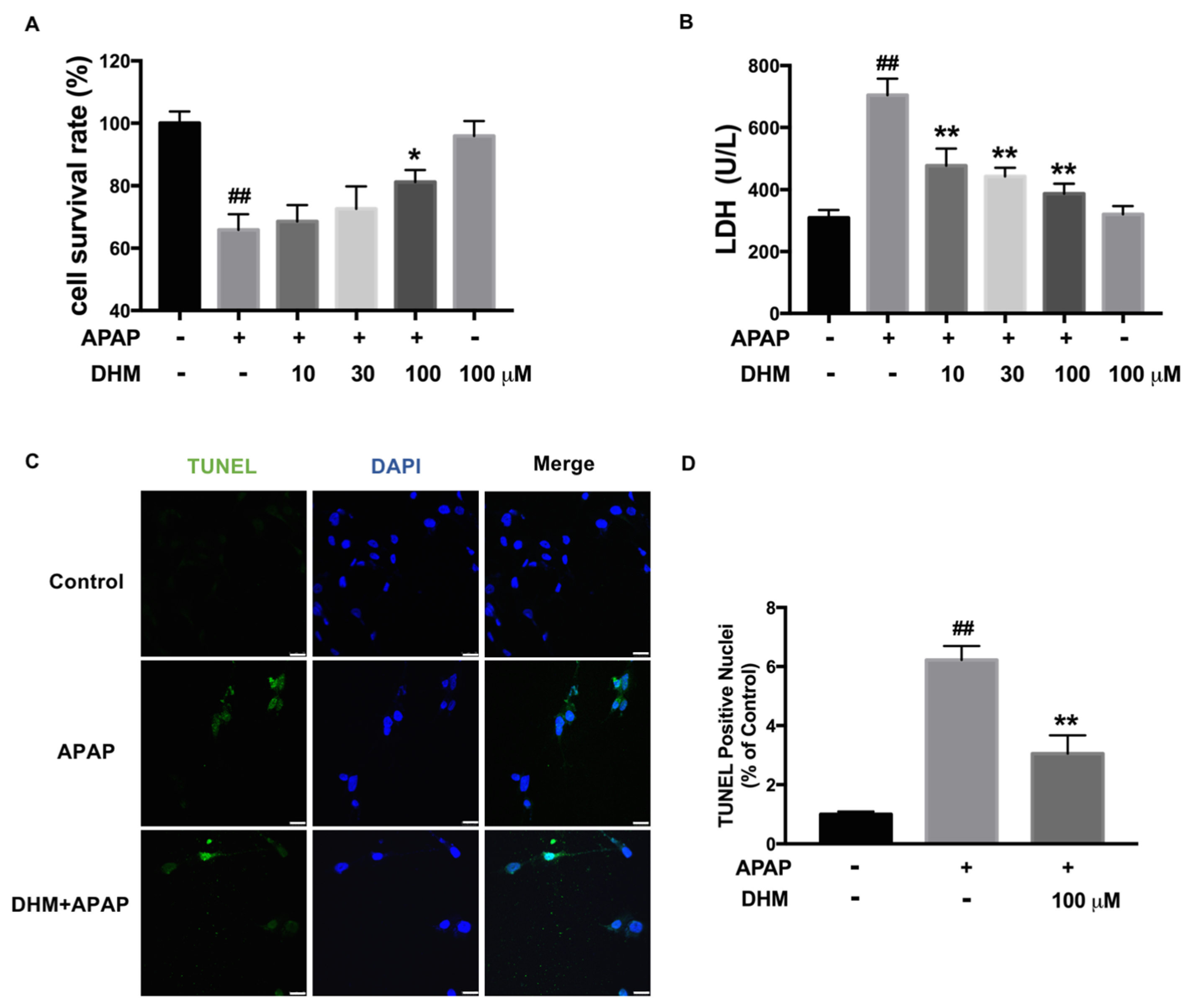
2.3. DHM Alleviated APAP-Induced Cellular Injury in HK-2 Cells
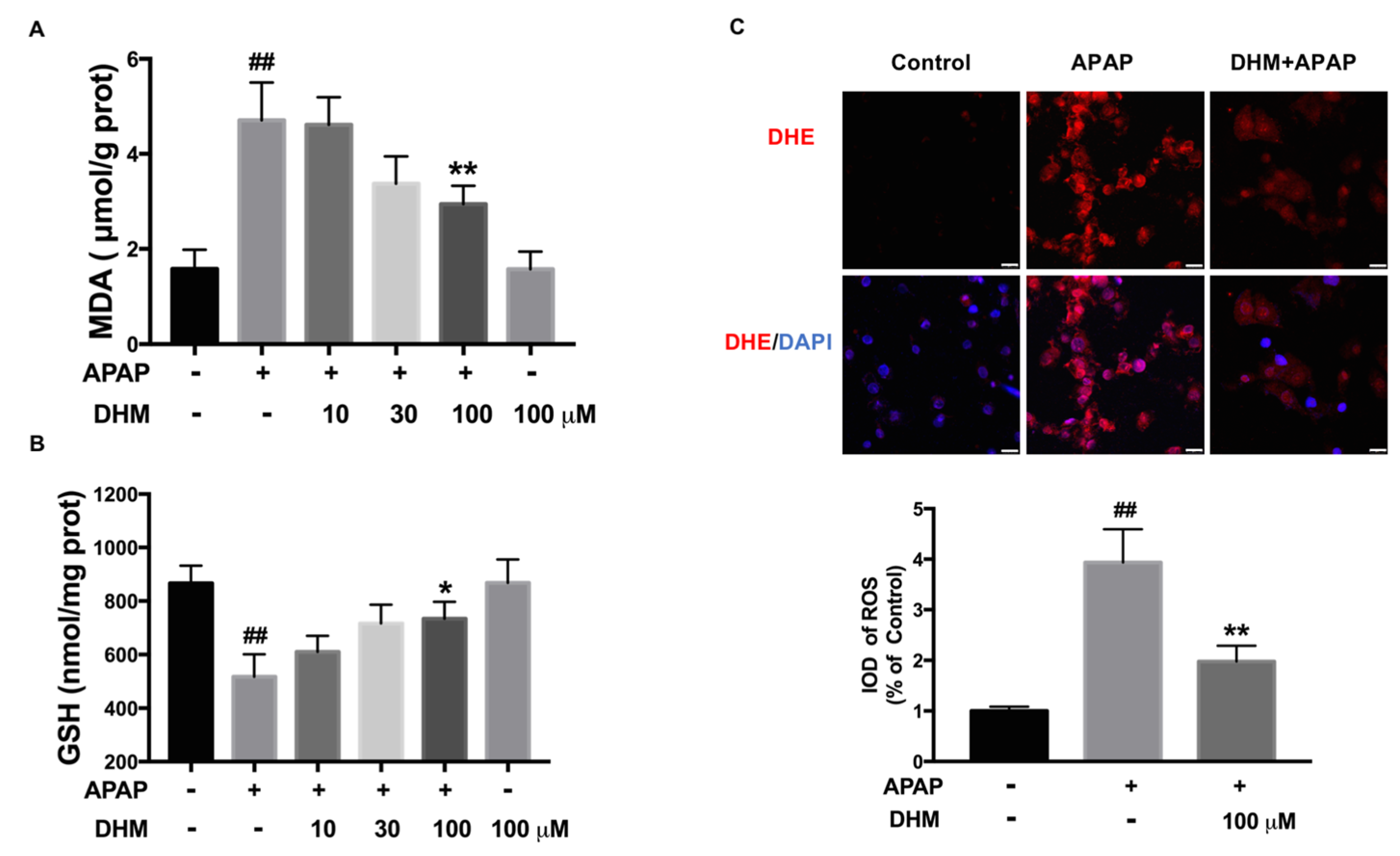
2.4. DHM Alleviated APAP-Induced Intracellular Oxidative Stress in HK-2 Cells
2.5. DHM Inhibited Mitochondrial ROS Production and Mitochondrial Dysfunction in HK-2 Cells
2.6. DHM Improved Antioxidant Capacity of Damaged HK-2 Cells by Activating Nrf2/HO-1 Pathway
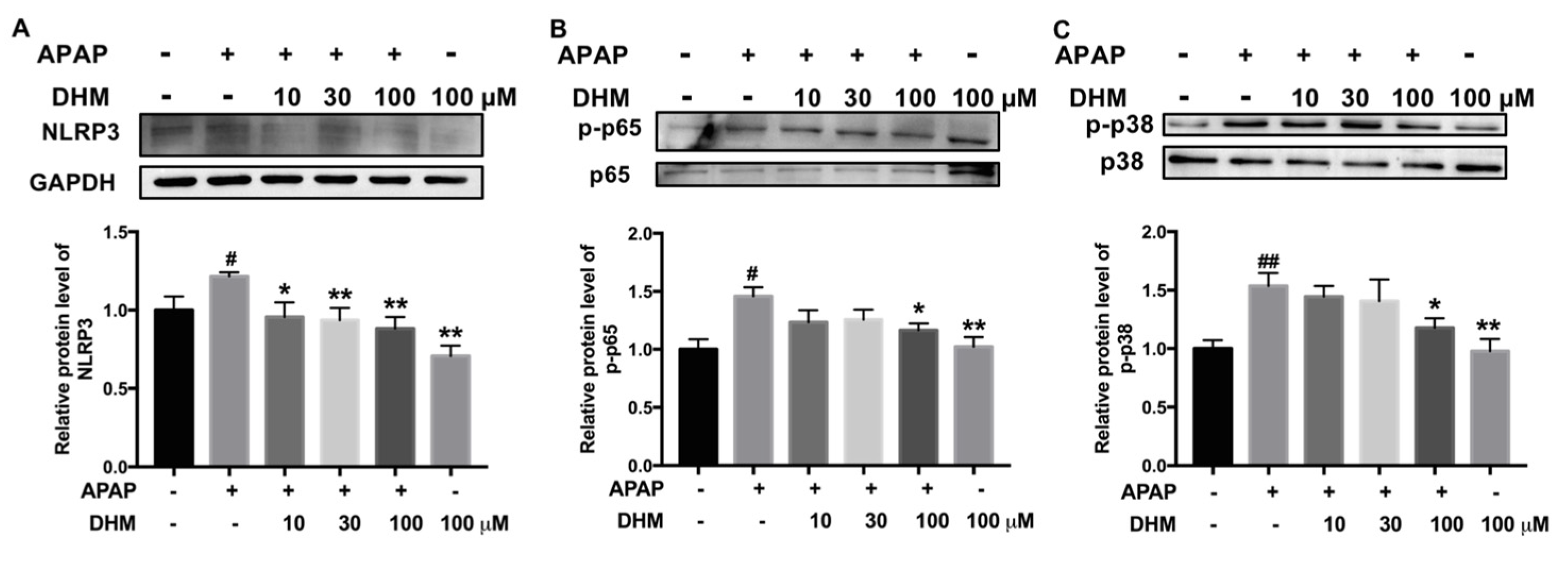
2.7. DHM Inhibited Expression of Inflammation-Related Proteins in HK-2 Cells Treated with APAP
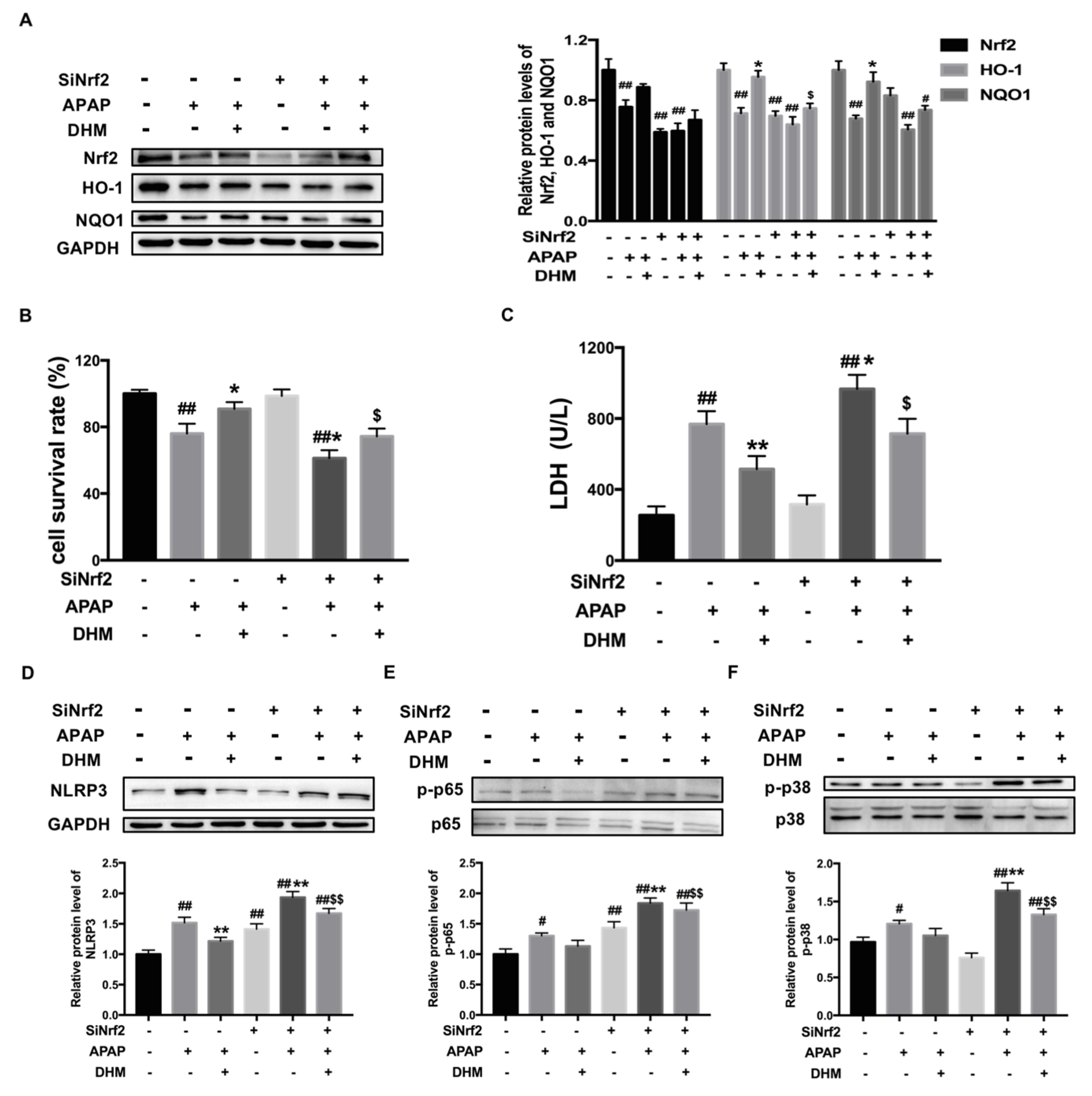
2.8. Nrf2 siRNA Partially Canceled out the Protective Effect of DHM Against APAP-Induced Damage in HK-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Cell Culture and Nrf2 Knockdown
4.3. Histopathological Analysis
4.4. Biochemical Assays
4.5. Determination of ROS Levels
4.6. Cytotoxicity Detection
4.7. Determination of Mitochondrial Membrane Potential (MMP)
4.8. TUNEL Assay
4.9. Western Blot Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DHM | Dihydromyricetin |
| APAP | Acetaminophen |
| AKI | Acute kidney injury |
| NAPQI | N-acetyl-P-benzoquinone |
| Nrf2 | Nuclear factor erythroid-related factor 2 |
| Keap1 | Kelch-like ECH associated protein 1 |
| HO-1 | Heme Oxygenase-1 |
| NQO1 | NAD (P) H: NADH Quinone Oxidoreductase 1 |
| DAMPs | Damage-associated molecular patterns |
| NF-κB | Nuclear factor kappa B |
| NLRP3 | NOD-like receptor protein 3 |
| NAC | N-acetylcysteine |
| Cre | Creatinine |
| BUN | Urea nitrogen |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| LDH | Lactate dehydrogenase |
| ROS | Reactive oxygen species |
| GSH | Glutathione |
References
- Yan, M.; Hou, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- McCrae, J.C.; Morrison, E.E.; Macintyre, I.M.; Dear, J.W.; Webb, D.J. Long-term adverse effects of paracetamol-a review. Br. J. Clin. Pharmacol. 2018, 84, 2218–2230. [Google Scholar] [CrossRef]
- Chefirat, B.; Zergui, A.; Rahmani, C.; Belmessabih, M.N.; Rezk-Kallah, H. Acute paracetamol poisonings received at the Oran University Hospital. Toxicol. Rep. 2020, 7, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Mazer, M.; Perrone, J. Acetaminophen-Induced Nephrotoxicity: Pathophysiology, Clinical Manifestations, and Management. J. Med. Toxicol. 2008, 4, 2–6. [Google Scholar] [CrossRef]
- Kennon-McGill, S.; McGill, M.R. Extrahepatic toxicity of acetaminophen: Critical evaluation of the evidence and proposed mechanisms. J. Clin. Transl. Res. 2017, 3, 297–310. [Google Scholar]
- Huang, C.; Jiang, S.; Gao, S.; Wang, Y.; Cai, X.; Fang, J.; Yan, T.; Wan, C.; Cai, Y. Sirtuins: Research advances on the therapeutic role in acute kidney injury. Phytomedicine 2022, 101, 154122. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Schepdael, A.V.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Zhou, X.; Song, Y.; Zeng, C.; Zhang, H.; Lv, C.; Shi, M.; Qin, S. Molecular mechanism underlying the regulatory effect of vine tea on metabolic syndrome by targeting redox balance and gut microbiota. Front. Nutr. 2022, 17, 802015. [Google Scholar] [CrossRef]
- Ruan, H.; Luo, J.; Wang, L.; Wang, J.; Wang, Z.; Zhang, J. Sika deer antler protein against acetaminophen-induced nephrotoxicity by activating Nrf2 and inhibition FoxO1 via PI3K/Akt signaling. Int. J. Biol. Macromol. 2019, 141, 961–987. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, K.; Tang, X.; Gao, J.; Wen, B. Phytochemicals protect LO2 cells against hepatotoxicity induced by emodin via the Nrf2 signaling pathway. Toxicol. Res. 2019, 8, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Yu, Q.; Shi, J.; Guo, W.; Zhang, S. Adelmidrol ameliorates liver ischemia-reperfusion injury through activating Nrf2 signaling pathway. Eur. J. Pharmacol. 2024, 964, 176224. [Google Scholar] [CrossRef] [PubMed]
- Wyczanska, M.; Lange-Sperandio, B. DAMPs in unilateral ureteral obstruction. Front. Immunol. 2020, 7, 581300. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Zhang, Y.; Liu, A.; Yang, C.; Wang, H.; Zhu, T.; Fan, Y.; Yang, B. Potent therapy and transcriptional profile of combined erythropoietin-derived peptide cyclic helix B surface peptide and Caspase-3 siRNA against kidney ischemia/reperfusion injury in mice. J. Pharmacol. Exp. Ther. 2020, 375, 92–103. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, X.; Chen, W.; Gao, C.; Bian, Q.; Fan, J.; Luan, J.; Cao, Z.; Gu, Y.; Liu, H.; et al. Interleukin-22 ameliorated acetaminophen-induced kidney injury by inhibiting mitochondrial dysfunction and inflammatory responses. Appl. Microbiol. Biotechnol. 2020, 104, 5889–5898. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Q.; Zhou, Q.; Zhang, L.; Qiu, Y.; Lou, D.; Zhou, L.; Yang, B.; He, Q.; Weng, Q.; et al. Sapidolide A alleviates acetaminophen-induced acute liver injury by inhibiting NLRP3 inflammasome activation in macrophages. Acta Pharmacol. Sin. 2022, 43, 2016–2025. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Morgan, M.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, W.; Xiao, J.; Sun, J.; Ren, X.; Jiang, L. Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. J. Transl. Med. 2024, 22, 309. [Google Scholar] [CrossRef]
- Matouk, A.I.; Awad, E.M.; EI-Tahawy, N.F.G.; EI-Sheikh, A.A.K.; Waz, S. Dihydromyricetin alleviates methotrexate-induced hepatotoxicity via suppressing the TLR4/NF-κB pathway and NLRP3 inflammasome/caspase 1 axis. Biomed. Pharmacother. 2022, 155, 113752. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tan, K.; Luo, Q.; Bai, X. Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn. J. Basic Med. Sci. 2020, 20, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Hua, Y.; Wang, W.; Zhang, H.; Xu, X. Modulation of SIRT1-mediated signaling cascades in the liver contributes to the amelioration of nonalcoholic steatohepatitis in high fat fed middle-aged LDL receptor knockout mice by dihydromyricetin. Biochem. Pharmacol. 2020, 175, 113927. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xiao, Y.; San, W.; Chen, Y.; Meng, G. Dihydromyricetin regulates RIPK3-CaMKII to prevent necroptosis in high glucose-stimulated cardiomyocytes. Heliyon 2024, 10, e28921. [Google Scholar] [CrossRef]
- Akakpo, J.; Ramachandran, A.; Orhan, H.; Curry, S.C.; Rumack, B.H.; Jaeschke, H. 4-methylpyrazole protects against acetaminophen-induced acute kidney injury. Toxicol. Appl. Pharmacol. 2020, 409, 115317. [Google Scholar] [CrossRef]
- Ko, J.W.; Shin, J.Y.; Kim, J.W.; Park, S.H.; Shin, N.R.; Lee, I.C.; Shin, I.S.; Moon, C.; Kim, S.H.; Kim, S.H.; et al. Protective effects of diallyl disulfide against acetaminophen-induced nephrotoxicity: A possible role of CYP2E1 and NF-kB. Food Chemical Toxicol. 2017, 102, 156–165. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Akakpo, J.Y.; Ramachandran, A.; Curry, S.C.; Rumack, B.H.; Jaeschke, H. Comparing N-acetylcysteine and 4-methylpyrazole as antidotes for acetaminophen overdose. Arch. Toxicol. 2022, 96, 453–465. [Google Scholar] [CrossRef]
- Rousar, T.; Handl, J.; Capek, J.; Nyvltova, P.; Rousarova, E.; Kubat, M.; Smid, L.; Vanova, J.; Malinak, D.; Musilek, K.; et al. Cysteine conjugates of acetaminophen and p-aminophenol are potent inducers of cellular impairment in human proximal tubular kidney HK-2 cells. Arch. Toxicol. 2023, 97, 2943–2954. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Waguri, S.; Sou, Y.S.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.; Gu, C.; Zhu, T.; Luo, Y.; Pang, M.; Du, W.; Ge, W. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Luo, H.; Xu, M.; Meng, G.; Zhang, W. Ca2+/calmodulin-dependent protein kinase II regulation by inhibitor 1 of T protein phosphatase 1 alleviates necroptosis in high glucose-induced cardiomyocytes injury. Biochem. Pharmacol. 2019, 163, 194–205. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Peng, X.; Huang, J.; Zhang, M.; Wang, Y. Dihydromyricetin Alleviated Acetaminophen-Induced Acute Kidney Injury via Nrf2-Dependent Anti-Oxidative and Anti-Inflammatory Effects. Int. J. Mol. Sci. 2025, 26, 2365. https://doi.org/10.3390/ijms26052365
Shi J, Peng X, Huang J, Zhang M, Wang Y. Dihydromyricetin Alleviated Acetaminophen-Induced Acute Kidney Injury via Nrf2-Dependent Anti-Oxidative and Anti-Inflammatory Effects. International Journal of Molecular Sciences. 2025; 26(5):2365. https://doi.org/10.3390/ijms26052365
Chicago/Turabian StyleShi, Jianan, Xiufang Peng, Junyi Huang, Mengyi Zhang, and Yuqin Wang. 2025. "Dihydromyricetin Alleviated Acetaminophen-Induced Acute Kidney Injury via Nrf2-Dependent Anti-Oxidative and Anti-Inflammatory Effects" International Journal of Molecular Sciences 26, no. 5: 2365. https://doi.org/10.3390/ijms26052365
APA StyleShi, J., Peng, X., Huang, J., Zhang, M., & Wang, Y. (2025). Dihydromyricetin Alleviated Acetaminophen-Induced Acute Kidney Injury via Nrf2-Dependent Anti-Oxidative and Anti-Inflammatory Effects. International Journal of Molecular Sciences, 26(5), 2365. https://doi.org/10.3390/ijms26052365





